Abstract
The Melatonin Osteoporosis Prevention Study (MOPS) demonstrated that nightly melatonin resulted in a time-dependent decrease in equilibrium ratios of serum osteoclasts and osteoblasts in perimenopausal women. This study examines mechanisms related to the ratios of osteoblasts and osteoclasts using coculture models (transwell or layered) of human mesenchymal stem cell (MSC) and human peripheral blood monocytes (PBMCs). Human MSC/PBMC cocultures exposed to melatonin in osteogenic (OS+) medium for 21 days induced osteoblast differentiation and mineralization; however, only in layered cocultures did melatonin inhibit osteoclastogenesis. Melatonin effects were mediated through MT2 melatonin receptors, MEK1/2, and MEK5. In layered but not transwell cocultures, melatonin increased OPG:RANKL ratios by inhibiting RANKL, suggesting that contact with osteoclasts during osteoblastogenesis inhibits RANKL secretion. Melatonin modulated expression of ERK1/2, ERK5, β1 integrin, GLUT4, and IRβ that was dependent upon the type of coculture; however, in both cultures, melatonin increased RUNX2 and decreased PPARγ expression, indicating a role for metabolic processes that control osteogenic vs adipogenic cell fates of MSCs. Furthermore, melatonin also has osteoblast-inducing effects on human adipose-derived MSCs. In vivo, one-year nightly melatonin (15 mg/L) given to neu female mice in their drinking water increased pErk1/2, pErk5, Runx2, and Opg and Rankl levels in bone consistent with melatonin’s already reported bone-enhancing effects. Finally, analysis of daily logs from the MOPS demonstrated a significant improvement in mood and perhaps sleep quality in women receiving melatonin vs placebo. The osteoblast-inducing, bone-enhancing effects of melatonin and improvement in quality of life suggest that melatonin is a safe and effective bone loss therapy.
Keywords: adipocytes, GLUT4, MEK1/2, MEK5, melatonin, mesenchymal stem cells, MT2 melatonin receptor, osteoblasts, osteoclasts, PPARγ
1 |. BACKGROUND
Vertebrates have a unique skeletal system where each of the 206 bones undergoes bone remodeling throughout the course of one’s life via a tightly coupled osteoblast-osteoclast bone remodeling process. Coordinated bone remodeling is to maintain good bone density and bone quality allowing bone to carry mechanical load and resist fracture.1 Communication between osteoclasts and osteoblasts is essential to preserve bone remodeling as well as bone quality.2–4 Latent bone-lining osteoblast precursors, including mesenchymal stem cells (MSCs), are activated in response to various stimuli including mechanical load changes causing bone marrow stromal cells and pre-osteoblasts to increase surface expression of receptor activator of nuclear factor κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF). Immature progenitors, such as mononuclear cells, released into the circulation begin fusion with other monocytes eventually forming mature multinucleated osteoclasts in response to RANKL and M-CSF. Following osteoclast-mediated bone resorption through the secretion of tartrate-resistant acid phosphatase (TRAP) and cathepsin K, osteoblast-mediated bone formation begins initiated by the release of osteoprotegerin (OPG) from osteoblasts to inhibit osteoclastogenesis through OPG-RANKL binding; the OPG/RANKL ratio is a critical transition point for bone cell activity. Likewise, osteoclasts can regulate osteoblast function through membrane-bound and secreted factors released within the matrix.3 A disruption in the bone remodeling process resulting in increases in bone-resorbing osteoclastic activity can lead to osteopenia, osteoporosis, and/or fragility fractures.1,5–9
In the geriatric population, increases in the prevalence of osteopenia, osteoporosis, as well as fracture-related morbidity and mortality are occurring despite the abundance of efficacious osteoporosis drugs.10 The lack of efficacy may be due to the fact that many of the osteoporosis therapies target osteoclasts only preventing further bone loss rather than inducing new bone formation.11 Another issue may be due to lack patient compliance due to adverse drug reactions.12 These findings underscore the importance of developing safe osteoporosis therapies targeted at both osteoclasts and osteoblasts to prevent further bone loss, induce bone growth, but also that have minimal adverse effects to improve patient compliance.
A decrease in the nocturnal production of melatonin through aging or by light exposure at night has been associated with increased risk of osteoporosis.13–16 The Melatonin Osteoporosis Prevention Study (MOPS) demonstrated that nightly 3 mg melatonin treatment for 6 mos renormalized bone marker turnover status and improved the physical symptoms of menopause in healthy perimenopausal women.17 These effects of melatonin on bone marker turnover were further supported in the MelaOst trial demonstrating a significant improvement in bone mineral density (BMD) in postmenopausal women with osteopenia following nightly melatonin (3 mg po) for one year.18 In the MelaOst trial, the authors concluded that the melatonin-mediated increases in bone density may be due to increases in bone mineralization as calcium excretion in the group taking melatonin decreased compared to placebo. In the MOPS, melatonin renormalized bone marker turnover no matter what the woman’s osteoblast:osteoclast ratios were at the outset of the study (ie, low osteoblast/normal osteoclast levels; normal osteoblast/high osteoclast levels; low osteoblast/high osteoclast levels). These findings suggest that melatonin is modulating both osteoblasts and osteoclasts to renormalize bone marker turnover. Melatonin’s favorable effects on bone density via stimulatory effects on osteoblasts and inhibitory effects on osteoclasts have been reported in myriad studies.16,19–28 Specifically, melatonin promotes osteogenesis through a variety of mechanisms that include enhancing the proliferation24,25 or differentiation20,29 of osteoblasts. Melatonin induces osteoblast differentiation from hMSCs and preosteoblast cells19–23,29 through an activation of MEK/ERK1/2, RUNX-2, OCN, bone morphogenetic protein-2 (BMP-2), BMP-4, and Wnt signal transduction pathways.19,21,23,29 These findings in vitro correlate with in vivo studies reporting that exogenous administration of melatonin enhances bone cell proliferation,24 increases bone mass,16,26 and induces new bone growth27,28 in rodent models. Melatonin also produces inhibitory effects on osteoclasts26,30; however, how this occurs is not known. Melatonin effects in combination with other bone-preserving agents have also demonstrated a benefit on bone health.31–34
This study extends the findings of the MOPS with a focus on identifying if and how osteoblasts modulate osteoclast activity using a novel coculture model system consisting of MSCs and human peripheral blood monocytes (PBMCs) grown as transwell (indirect) or layered (direct) cocultures to mimic the different ways osteoblasts and osteoclasts interact and communicate in vivo. Melatonin effects on signaling proteins involved in osteoblastogenesis and osteoclastogenesis were also assessed in mouse tibia taken from HER2/neu female mice exposed to nightly melatonin (equivalent to ~5 mg daily melatonin in humans) for one year to confirm the mechanistic findings from the in vitro coculture studies and to identify potential mechanisms underlying the melatonin-mediated increases in bone density already reported in these mice.16 This study also extends the findings of the MOPS, which demonstrated that perimenopausal women taking melatonin had a decrease in the frequency of menstrual bleeding and had an improvement in the physical symptoms of menopause as measured using MENQOL.17 Because the physical domain of the MENQOL measures 19 different variables, including mood, lack of stamina, lack of energy, feeling tired or worn out, and sleep quality, and produces an overall score, it was not possible to identify, specifically, which of the physical symptoms melatonin improved upon. Significant relationships between depression, bone mineral density, and cortisol levels have been reported,35,36 and so any improvement in any of these variables could also contribute to melatonin’s beneficial effects on bone. Therefore, the daily diary logs kept by the MOPS cohort were further analyzed to provide additional data to explain melatonin’s beneficial actions (eg, lifestyle, mood, sleep) on bone as described in other studies.37–41
We present our findings from this study—from in vitro to preclinical to clinical—provide novel mechanistic data to explain the bone density increases induced by melatonin exposure in vivo through its effects on osteoblasts and osteoclasts and show improvements in QOL in a geriatric population with the hopes of advancing the clinical use of melatonin to prevent and treat bone loss.
2 |. MATERIALS AND METHODS
2.1 |. In vitro bone cell cocultures and treatments
Human adult mesenchymal stem cells (MSCs) (Lonza, Walkersville, MD, USA) and PBMCs were used to develop this coculture model system as described previously.42 Mesenchymal stem cells and monocytes, very immature states of osteoblasts and osteoclasts, were used experimentally because the goals of the study were to assess melatonin’s effects on osteoblastogenesis and osteoclastogenesis, respectively. Briefly, MSCs were maintained, grown, and passaged at 80% confluence in mesenchymal stem basal cell growth medium (Os−) (Lonza, MD, USA) at 37°C, 5% CO2, and 90% humidity. Cells were seeded (at passage 3–5) at an initial density of 3 × 103 cells/cm2 in the bottom chamber of a 6-well transwell plate or typical 6-well plate (Corning, NY, USA). Cells were then treated with either basal growth medium (Os−) or osteogenic medium (Os+) (Lonza, USA) with or without 50 nmol/L melatonin (Sigma, USA). Ethanol (0.001%) was used as the vehicle control. For the inhibitor studies, cells were treated with 1 μmol/L of the MT2 melatonin receptor antagonist, 4P-PDOT (Tocris), 3 μmol/L of the MEK1/2 inhibitor, PD898059 (SantaCruz Biotechnology, USA), 10 μmol/L of the MEK5 inhibitor, BIX02189 (SantaCruz Biotechnology, Dallas, TX, USA), or 10 μmol/L of SC-1–151, a MEK1/2 and MEK5 inhibitor (generous gift from Dr. Patrick Flaherty; https://www.google.com/patents/WO2015038743A1?cl=en) either alone or in combination with melatonin. Osteoblastic differentiation of MSCs was induced by the use of osteogenic media (Os+) containing ascorbate, dexamethasone, and β-glycerophosphate.43 Full media changes occurred once every four days. On day 13, blood was taken from a young healthy volunteer via venous puncture using BD Vacutainer Safety-Lok blood collection set with a 23-gauge needle (BD, USA) for monocyte (PBMC) isolation. Freshly collected blood was mixed with Ficoll-Paque™ Plus (Amersham Pharmacia Biotech, Sweden) and centrifuged twice (400 g for 30–40 minutes at 18–20°C without break). Human PBMCs were isolated from centrifuged blood and resuspended in RoboSep™ buffer (StemCell Technologies, USA). Pure human PBMCs were separated magnetically from the cell suspension using EasySep™ negative selection human monocyte enrichment kit without CD16 depletion (StemCell Technologies, Cambridge, MA) and purple EasySep™ magnet (StemCell Technologies, Cambridge, MA) per manufacturer’s instructions. Monocytes (PBMCs) were seeded at a density of 5 × 103 cells/cm2 in the top chamber of the transwell plate or layered directly on top of the MSCs to initiate the transwell or layered cocultures. The permeable membrane present between the two chambers of the transwell allows for the MSCs/osteoblasts and PBMCs/osteoclasts to communicate via passage of factors released into the media and not through contact, whereas in the layered coculture, MSCs/osteoblasts and PBMCs/osteoclasts can communicate via both means. Full media changes continued once every four days until day 21. In addition to the cocultures, MSC monocultures were developed following a similar treatment protocol and timeline, except that no PBMCs were added to the culture on day 13. The 21-day time period was chosen based on the previous studies demonstrating that melatonin-mediated differentiation of MSCs into osteoblasts required this period of time.21 Human PBMCs were added on day 13 because pre-osteoblasts proliferate between days 14 and 2121 releasing enough RANKL, M-CSF, and/or OPG to induce osteoclastogenesis.44,45
2.2 |. Osteoblast differentiation and mineralization
Calcium mineralization was assessed by alizarin red staining according to kit instructions (EMD Millipore, Billerica, MA) on day 21; transwell osteoblasts were located in the bottom chamber of the transwell plate, or the whole plate of the layered cocultures was exposed to alizarin red. Osteogenesis was quantified by spectrophotometry at 405 nm using the Perkin-Elmer Victor 1420 Multilabel plate reader (Waltham, MA, USA) or by qualitative observation using a VistaVision microscope (VWR International, Allison Park, PA) with a progress C3 camera (Jenoptik). Alizarin red levels, which is proportional to osteoblast differentiation, were calculated from standard curves and then normalized against (Os−) treated cultures. Osteoblastic mineralization activity was then compared within and between groups.
2.3 |. Osteoclast differentiation and activity
To evaluate osteoclast activity in response to treatments, tartrate-resistant acid phosphatase (TRAP) expression was measured on day 21 in the top chamber of the transwell coculture or on the whole 6-well plate of the layered coculture using a modified protocol described by Janckila et al,46 Briefly, cell lysates were prepared by scraping cells into TRAP buffer (1 mL/well). TRAP buffer contained 2.5 mmol/L naphthol ASBI phosphate (N-ASBI-P), 1% ethylene glycol monomethyl ether (EGME), 2% NP40, 100 mmol/L Na-acetate, and 50 mmol/L Na-tartrate with pH adjusted at 5.5–6.1. Cells were then incubated at 37°C for 30 minutes using 2.5 mL 0.1 mol/L NaOH containing 0.05% NP-40 to terminate the reaction. Fluorescence readings were then taken using a Perkin-Elmer Victor 1420 Multilabel plate reader at 405-nm excitation and 515-nm emission. Data were normalized against (Os−) treated cultures and then compared between groups. Qualitative TRAP analysis was performed using the Acid Phosphatase, Leukocyte assay kit according to manufacturer’s instructions (Sigma, USA). Following the counterstaining of the cells with hematoxylin and then a rinse in alkaline tap water to visualize the blue nuclei, images were then captured using a VistaVision microscope (VWR international) with progress C3 camera (Jenoptik) under gray setting.
2.4 |. Radioligand binding assay
Melatonin receptor expression in MSCs, PBMCs, or ASCs was determined on day 14 (AMSCs) or on day 21 (MSC:PBMC cocultures) of treatments by total binding analysis using 500pM of the radioligand 2-[125I]-iodomelatonin as described.47 Total specific binding of 2-[125I]-iodomelatonin was calculated by subtracting the nonspecific binding using 10 μmol/L melatonin from the total binding. Data were normalized as fmole per mg of protein. Protein levels were measured using a Bradford assay (Bio-Rad, Hercules, CA, USA).
2.5 |. Enzyme-linked immunosorbent assay (ELISA)
The concentration of osteoprotegerin (OPG) and receptor activator of nuclear factor kappa B ligand (RANKL) secreted by osteoblasts into the culture media was measured using Osteoprotegerin Human ELISA kit (Abcam, Cambridge, MA, USA) and total sRANKL (human) ELISA kit (Enzo Life Science, Farmingdale, NY, USA), respectively, per kit instructions. Mean concentration changes in secreted proteins, sOPG and sRANKL, as well as their ratios (ie, sOPG: sRANKL) were calculated, normalized against Os− treated cultures and then compared between groups.
2.6 |. Western blot
2.6.1 |. Cell lysate preparation
On day 21, cells from the cocultures—transwell or layered—or cells from the MSC monocultures were scraped into Laemmli sample buffer (Bio-Rad, Hercules, CA, USA). Samples were then heated for 5 minutes at 95°C, cooled down, and stored at −20°C until use.
2.6.2 |. Mouse bone lysate preparation
All animal work was approved and conducted according to the IACUC guidelines at Duquesne University. Bone tissue lysates were prepared from MMTV-neu transgenic mice treated for one year with nightly melatonin (15 mg/ mL given in the drinking water) as described previously by Witt-Enderby et al16 Following euthanasia, mouse tibias were collected, snap-frozen immediately in liquid nitrogen, and stored at −80°C until use. Bones were stripped of muscle and then pulverized mechanically into a powder using a mortar and pestle under liquid nitrogen. Samples were then scraped into 2 mL of cell lysis buffer [2% SDS, 2 mol/L urea, 10% glycerol, 10 mmol/L Tris-HCl (pH 6.8), 10 mmol/L DTT, and 1 mmol/L PMSF)] and homogenized using a Tissue Tearor™, where the suspension was subjected to three 10-seconds bursts on ice. Samples were centrifuged at 15 000 g, and the supernatant was stored at −20°C until use.
2.6.3 |. SDS-PAGE
Protein expression of osteoprotegerin (OPG), receptor activator of nuclear factor kappa B ligand (RANKL), extracellular signal-regulated protein kinases 1 and 2 (ERK1/MAPK3 and MAPK1/ERK2), extracellular signal-regulated kinase 5 (ERK5/MAPK7), runt-related transcription factor 2 (RUNX2), integrin Beta-1 (ITGB1), nuclear factor kappa B (NFκB), peroxisome proliferator-activated receptors γ (PPARγ), glucose transporter type 4 (GLUT4/ SLC2A4), and beta subunit of insulin receptor (Insulin Rβ) was measured using the Odyssey® Western Blotting Kit IV RD (LI-COR Bioscience, Lincoln, NE, USA). Protein bands were visualized using the Odyssey Infrared Imager and quantified using Odyssey software (LI-COR). Proteins were normalized against β-actin to control for any variation in protein loading. Protein levels were then normalized against Os−/Mel and compared between groups. Primary antibodies included rabbit anti-OPG/TNFRSF11B (ab73400, Abcam, Cambridge, MA, USA), rabbit anti-RANKL/TNFSF11 (ab9957, Abcam), rabbit anti-phospho ERK1/2 (9101, Cell Signaling, Danvers, MA, USA), rabbit anti-total ERK1/2 (9102, Cell Signaling), rabbit anti-phospho ERK5 (3371, Cell Signaling), rabbit anti-total ERK5 (3372, Cell Signaling), rabbit anti-RUNX2 (sc10758, Santa Cruz Biotech, Dallas, TX, USA), rabbit anti-Integrin β1 (sc8978, Santa Cruz Biotech), rabbit anti-NFκB (sc298, Santa Cruz Biotech), rabbit anti-PPARγ (sc7196, Santa Cruz Biotech), rabbit anti-GLUT4 (sc7938, Santa Cruz Biotech), rabbit anti-insulin Rβ (sc711, Santa Cruz Biotech), and mouse anti–β-actin (926–42212, LI-COR, Lincoln, NE, USA). Secondary antibodies against appropriate IgG included goat anti-rabbit (800 nm) and goat anti-mouse (680 nm). The use of the LI-COR system permits the use of secondary antibodies with different IR spectra to identify protein bands of interest based on color—bands of the appropriate sizes were detected by each antibody and no cross-reactivity occurred between proteins.
2.7 |. Isolation and culture of adipose-derived mesenchymal stem cells (ASCs)
Adipose-derived mesenchymal stem cells (ASCs) were isolated from human adipose tissues obtained after informed donor consent and with approval from the Mayo Clinic Institutional Review Board (IRB), as previously described.48,49 Briefly, adipose tissue was digested with 0.075% collagenase type I (Worthington Biochemicals, Lakewood, NJ, USA) for 90 minutes at 37°C, and adipocytes removed from the stromal vascular fraction by low-speed centrifugation at 400 g for 5 minutes. The resulting cell pellet was washed with PBS followed by sieving through 70-μm and 40-μm cell strainers (BD Biosciences, San Jose, CA, USA). The cell suspension was seeded at densities of 1.0–2.5 × 103 cells/cm2 in T-175 flasks using maintenance media (Advanced MEM) with 5% platelet lysate, PLTMax (MillCreek LifeSciences, USA), 2 U/mL heparin (Novaplus, USA), 2 mmol/L L-glutamine (Invitrogen, Waltham, MA, USA), and antibiotics (100 U/mL penicillin, 100 g/mL streptomycin, Sigma, St Louis, MO, USA) and incubated in standard culture conditions of 37°C and 5% CO2.
2.8 |. Osteogenic differentiation of ASCs
Adipose-derived MSCs (ASCs) from passage 7 were seeded at a density of 5000 cells/cm2 on 6-well culture plates and allowed to adhere overnight. The following day, media were changed to either basal media (Os−) or osteogenic media (Os+) containing either vehicle (0.001% ethanol), melatonin (50 nmol/L; Sigma, USA), or 4P-PDOT (1 μmol/L; Tocris Bioscience, Minneapolis, MN, USA), or a combination of each. Cells were then maintained for up to a maximum of 8 days with a media change occurred every 3–4 days. Mineralization was detected on day 8 post-treatment where the media were removed and ASCs were washed twice with PBS. DNA content per well was quantified using Hoechst dye at a concentration of 0.5 μg/mL, and fluorescence was measured at 490 nm using Infinite® 200 PRO plate reader (Tecan, Switzerland). The cells were fixed in 4% paraformaldehyde (PFA) for 10 minutes and incubated in 0.1% solution of alizarin red S (pH 4.3) for 30 minutes on a shaker at low speed. Following removal of the excess stain by thorough washing with distilled water and air-drying the plates, the presence of mineralization was quantified by measuring the staining intensity at 450 nm using Infinite® 200 PRO plate reader (Tecan, Switzerland). Alizarin red intensity values were normalized to DNA content. A darker stain implies a higher intensity value.
2.9 |. Binding assays of ASCs
2.9.1 |. Melatonin effects on general well-being in healthy perimenopausal women
The effect of melatonin on the general well-being was measured via retrospective analysis of the Melatonin Osteoporosis Prevention Study (MOPS) conducted by Kotlarczyk et al17 Briefly, a total of 18 perimenopausal women were randomized to receive either 3 mg of melatonin (n = 13) or placebo (n = 5) p.o. nightly for six months. To measure the effect of melatonin treatment on general well-being, participants were given a daily diary to record their sleep duration and quality and then were provided an opportunity to write down any other comments they felt pertinent to record. Based on the diary comments written down by the participants in the MOPs, four general categories emerged as being described the most: mood, sleep quality, menopause symptoms, and GI/joint pain. Comments under each category were then substratified into positive and negative comments, where positive comments indicated improvement in physical condition or any positive feeling, and negative comments indicated worsening of any existing condition or emerging of a new problem, as well as negative feelings. Positive and negative comments under each category were then taken as a percentage of the total comments made and then compared within and between groups. Results were reported as the percent of total comments made under each category by each group throughout the study.
2.9.2 |. Statistics
For in vitro assays, all data were normalized against basal media (Os−) containing either Mel, vehicle, or inhibitors. Data analysis was performed using one-way ANOVA followed by Bonferroni’s post hoc multiple comparison t test with significance defined as P < .05. Diary comments for general well-being were analyzed using two-tailed Fisher’s exact test for two categorical outcomes following intention to treat principle. All statistical testing was carried out using JMP versions 12 (SAS Institute Inc., Cary, NC, USA) and GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA) for Macintosh.
3 |. RESULTS
3.1 |. Role of MT2 melatonin receptors, MEK1/2, and MEK5 in osteoblast and osteoclast differentiation in coculture models
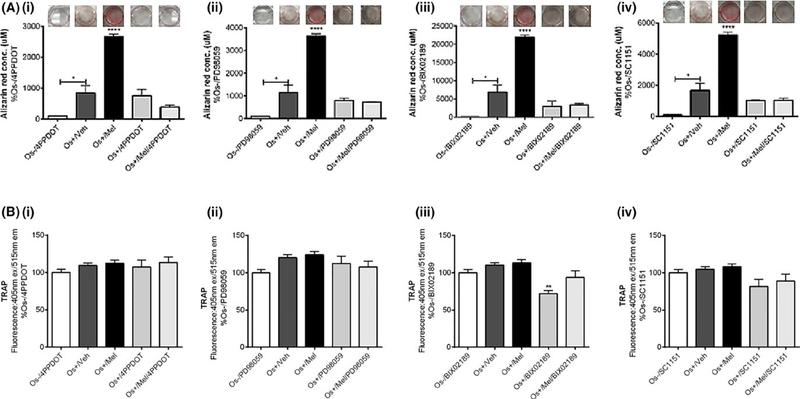
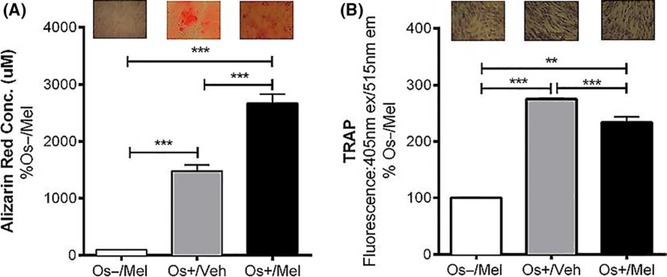
In both the transwell and layered cocultures, our 21-day culturing conditions resulted in osteoblast-mediated differentiation of osteoclasts reflected by increases in alizarin red and TRAP staining (Figure 1). Figure 1A (i–iv) represents calcium deposition activity of the differentiated, mature osteoblasts grown as transwell cocultures. Treatment with the basal growth medium (Os−) did not induce osteoblastic differentiation, revealed by no alizarin red staining in Os− treated cultures. Exposure to osteogenic (Os+) significantly enhanced osteoblast differentiation and mineralization (P < .05 vs Os−) while the addition of melatonin (OS+/Mel) enhanced this further (P < .0001 vs Os+/V). Treatment with the MT2 melatonin receptor antagonist 4P-PDOT significantly blocked melatonin-mediated osteoblast differentiation (Figure 1Ai). The involvement of MEK1/2 and MEK5 in melatonin-mediated osteoblast differentiation from MSCs was evaluated using the selective MEK1/2 inhibitor PD98059, the selective MEK5 BIX02189, or the dual MEK1/2 and MEK5 inhibitor SC-1–151. Coadministration of melatonin with the MEK1/2/ERK1/2 inhibitor PD98059 (Figure 1Aii), MEK5/ERK5 selective inhibitor BIX02189 (Figure 1Aiii), or dual ERK1/2 and 5 inhibitor SC-1–151 (Figure 1Aiv) blocked completely melatonin’s effects on osteoblast differentiation resulting in alizarin red levels similar to control. These data demonstrate that MEK1/2 and MEK5 are essential in mediating melatonin’s effects on osteoblast differentiation from MSCs. Figure 1B demonstrates TRAP releasing activity of transwell osteoclasts following transwell osteoblast exposure to melatonin alone or in combination with 4P-PDOT, PD98059, BIX02189, or SC-1–151. Although Os+-stimulated osteoblastogenesis induced osteoclastogenesis and that melatonin induced osteoblastogenesis even further, this did not translate to changes in osteoclastogenesis. Melatonin combined with these inhibitors did not have any effect on osteoclast differentiation of PBMCs; however, BIX02189 alone inhibited osteoclast differentiation (P < .01; Figure 1B) consistent with its role in osteoclastogenesis.50 In the layered coculture, melatonin induced osteoblast mineralization (P < .001 vs Os+/Veh; Figure 2A) and decreased TRAP expression (P < .001 vs Os+/Veh; Figure 2B).
FIGURE 1.
Effect of melatonin on osteoblast-mediated calcium mineralization and osteoclast-mediated TRAP formation in transwell cocultures. On day 21, calcium deposition by the differentiated osteoblasts (A) and TRAP expression by differentiated osteoclasts (B) were measured via alizarin red or TRAP assays, respectively. Osteoblasts were treated with vehicle, melatonin and/or (i) 4P-PDOT, (ii) BIX02189, (iii) PD98059, and (iv) SC-1–151, respectively. Each bar represents the mean concentration of alizarin red (in μmol/L) or mean fluorescence reading of TRAP (at 405 nm ex, 515 nm em) for respective groups normalized against basal media (Os−) containing the corresponding inhibitor. ****P < .0001 vs all groups, *P < .05 (in transwell coculture); one-way ANOVA followed by Bonferroni’s post hoc multiple comparison t test (n = 3 per group). Os− =basal medium, Os+ =osteogenic medium, Veh = vehicle (ethanol), Mel = melatonin. INSET: representative alizarin red images obtained in response to each treatment are shown above each bar
FIGURE 2.
Effect of melatonin on osteoblast-mediated calcium mineralization and osteoclast-mediated TRAP formation in layered cocultures. On day 21, calcium deposition by the differentiated osteoblasts (A) and TRAP expression by differentiated osteoclasts (B) were measured via alizarin red or TRAP assays, respectively, in layered cocultures treated with melatonin. Each bar represents the mean concentration of alizarin red (in μmol/L) or mean fluorescence reading of TRAP (at 405 nm ex, 515 nm em) for respective groups normalized against basal media containing melatonin (Os−/Mel). **P < .01, ***P < .001. One-way ANOVA followed by Bonferroni’s post hoc multiple comparison t test (n = 3 per group). Os− = basal media, Os+ = osteogenic media, Veh = vehicle (ethanol), Mel = melatonin. INSET: representative alizarin red or TRAP images in response to the treatments are shown above each bar
3.2 |. Treatment effects on melatonin receptor binding undifferentiated and mature osteoblasts and osteoclasts
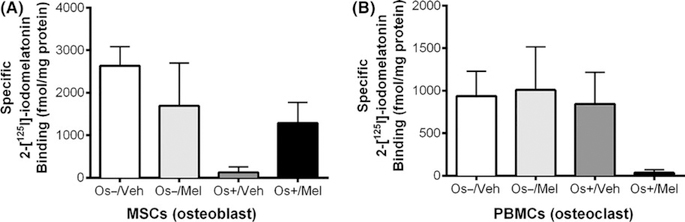
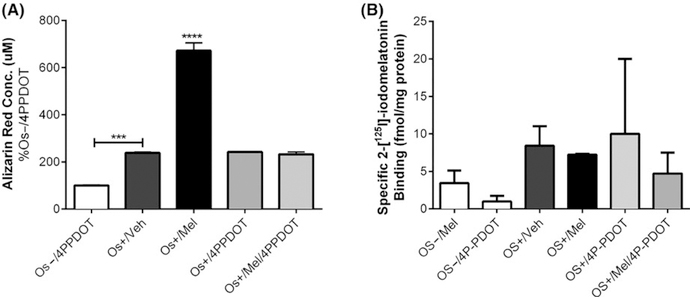
Figure 3 represents the effect of melatonin alone or in combination with growth medium (OS−) or osteogenic medium (OS+) on the expression of melatonin receptors. Specific 2-[125I]-iodomelatonin binding sites were found in both MSCs (range 100 fmol/mg protein-2.5 pmol/mg protein) and PBMCs (range 100 fmol/mg protein-1pmol/mg protein) cocultures grown in transwells, indicating the presence of melatonin receptors in both osteoblasts and osteoclasts. One-way ANOVA did not reveal significant differences between the groups; however, when transwell osteoblasts exposed to OS−/Veh were compared to OS+/Veh by unpaired one-tailed t test, significance (P = .04) was obtained but when compared by unpaired two-tailed t test, a trend (P = .09) was noted. When transwell osteoblasts exposed to OS−/Veh were compared to OS+/Mel by unpaired one-tailed t test, a trend (P = .07) was observed; however, unpaired two-tailed t test resulted in a P-value of .14 (not significant). For the transwell osteoclasts, comparison between OS−/Veh and OS+/Veh by unpaired one-tailed t test (P = .42) and unpaired two-tailed t test (P = .85) revealed no significance. When transwell osteoclasts exposed to OS−/Veh were compared to OS+/Mel by unpaired one-tailed t test, a trend (P = .04) was observed; however, unpaired two-tailed t test resulted in a trend (P = .08).
FIGURE 3.
Melatonin receptor expression in osteoblasts and osteoclasts. On day 21, melatonin receptor expression in the differentiated osteoblast and osteoclast was evaluated via total 2-[125I]-iodomelatonin binding analysis on the (A) bottom chamber cells and (B) top chamber cells of transwell coculture, respectively. Each bar represents mean ± SEM concentration of bound total 2-[125I]-iodomelatonin (in fmol/mg of protein) for respective groups. One-way ANOVA followed by Bonferroni’s post hoc multiple comparison t test (n = 3 per group), where significance was defined as P < .05. Os− = basal media, Os+ = osteogenic media, Veh = vehicle (ethanol), Mel = melatonin
3.3 |. Treatment effects on osteoprotegerin and RANKL expressed or secreted from osteoblasts
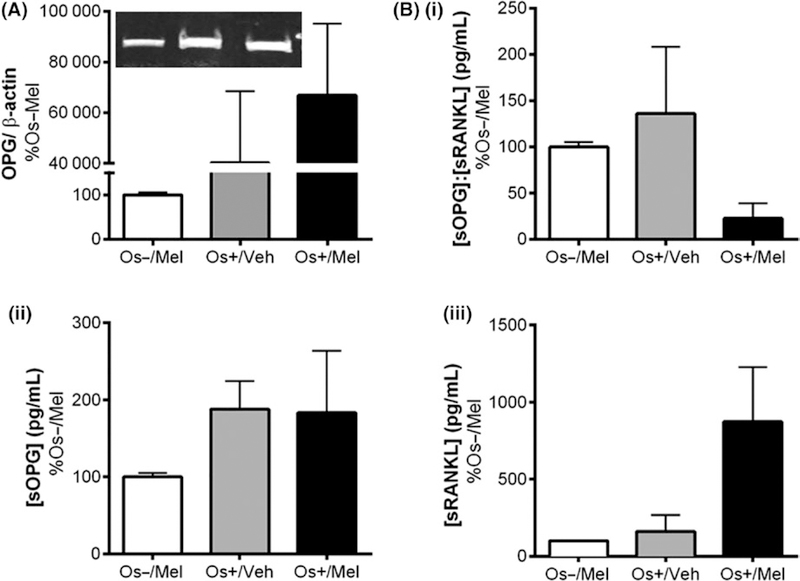
Figure 4 illustrates the expression of OPG in transwell osteoblasts (detected by western blot) and the secretion of OPG (sOPG) and RANKL (sRANKL) into the culture medium in response to melatonin (detected by ELISA). Similar expressions of OPG and secreted OPG and RANKL in layered coculture and hMSC monoculture are shown in Tables 1 and 2, respectively. As shown in Figure 4A and Table 1, melatonin did not induce transwell osteoblastic OPG expression, but a trend (P = .0505) toward an increase in OPG expression was observed in layered osteoblasts treated with osteogenic medium and melatonin (Os+/Mel). In MSCs grown as monocultures (ie, in the absence of PBMCs), the addition of melatonin to osteogenic medium (Os+/Mel) significantly increased OPG expression in osteoblasts (P < .05 vs Os−/ Mel; Table 2). Secreted OPG and RANKL levels were also measured in culture media as this is one of the modes of communication between osteoblasts and osteoclasts to regulate their activity. No melatonin effects on sOPG, or the ratio of sOPG:sRANKL levels, occurred in transwell osteoblasts (Figure 4B); however, melatonin increased sOPG:sRANKL ratios in layered osteoblasts (P < .05 vs Os+/Veh) via decreases in sRANKL (Table 1). The addition of melatonin to the osteogenic medium (Os+/Mel) increased sOPG:sRANKL ratios in hMSCs monoculture (P < .05 vs Os+/Veh) most probably by decreasing sRANKL levels (Table 2).
FIGURE 4.
Effect of melatonin on the expression of (A) OPG expression in transwell osteoblasts and (B) OPG (sOPG) and RANKL (sRANKL) secreted from transwell osteoblasts. (A) After 21 d of melatonin exposure, expressions of OPG in differentiated osteoblasts were measured using Western blot. Osteoblasts lysates were prepared on day 21 from the bottom chamber cells of transwell coculture containing differentiated osteoblasts and stored at −20°C until use. Protein levels were normalized against Os−/Mel following normalization against β-actin. Mean expression of OPG was analyzed and compared between groups (Os−/Mel, Os+/Veh, Os+/Mel). (B) OPG and RANKL secreted from osteoblast into the media, named as sOPG and sRANKL, respectively, were measured via sandwich ELISA assay. Culture media were collected on day 21 from transwell and stored at −20°C until use. Mean concentrations (pg/mL) of (i) sOPG: sRANKL, (ii) sOPG, and (iii) sRANKL were analyzed, normalized against Os−/Mel, and compared between groups (Os−/Mel, Os+/Veh, Os+/Mel). One-way ANOVA followed by Bonferroni’s post hoc multiple comparison t test (n = 6 per group) revealed no significant differences between groups
TABLE 1.
Effect of melatonin on osteogenic and metabolic protein expression in osteoblasts and osteoclasts grown as layered cocultures. Cell lysates (containing both osteoblasts and osteoclasts) were prepared from layered cocultures following 21 d of treatment
| Quantitative (% Os−/Mel) |
||||
|---|---|---|---|---|
| Qualitative | Os−/Mel | Os+/Veh | Os+/Mel | |
| OPG | 100.0 (±2.04) | 178.7 (±32.75) | 225.1 (±12.56) | |
| sOPG | 100.0 (±2.04) | 23.81 (±5.44) | 23.30 (±3.9) | |
| sRANKL | 100.0 (±2.04) | 224.7 (±79.41)a* | 169.1 (±50.21) | |
| sOPG:sRANKL | 100.0 (±2.04) | 32.87 (±5.95) | 53.65 (±1.15)b* | |
| pERK1/2 | 100.0 (±5.78) | 16.76 (± 1.47)a** | 35.92 (±16.82)a** | |
| pERK5 | 100.0 (±5.78) | 1241 (±407.2) | 1129 (±321.6) | |
| tERK5 | 100.0 (±5.78) | 3805 (± 1601) | 6444 (± 3478) | |
| pERK5:tERK5 | 100.0 (±5.78) | 41.16 (± 18.93)a* | 23.14 (±5.13)a* | |
| RUNX2 | 100.0 (±3.65) | 876.1 (±96.16)a** | 1361 (±237.9)a*** | |
| INTEGRIN β1 | 100.0 (±0.36) | 12 965 (±5103)a* | 3077 (±2796)b* | |
| NFκB | 100.0 (±5.77) | 222.4 (±73.57) | 105.5 (±24.56) | |
| PPARγ | 100.0 (±4.08) | 326.5 (±47.93)a*** | 105.0 (±5.78)b** | |
| GLUT4 | 100.0 (±4.08) | 631.7 (±104.2)a*** | 91.37 (±22.74)b*** | |
| INSULIN Rβ | 100.0 (±4.08) | 1015 (±607.8) | 1021 (±171.3) | |
Mean protein levels were analyzed and compared between groups (Os−/Mel, Os+/Veh, Os+/Mel) and expressed as mean (±SEM).
= vs Os−/Mel
= vs Os+/Veh
P < .05
P < .01
P < .001
One-way ANOVA followed by Bonferroni’s post hoc multiple comparison t test (n = 3 per group).
TABLE 2.
Effect of melatonin on the expression of OPG, RANKL, ERK1/2, and ERK5 in hMSC monocultures. Cell lysates containing osteoblasts only were prepared following 21 d of treatment
| Quantitative (% Os−/Mel) |
||||
|---|---|---|---|---|
| Qualitative | Os−/Mel | Os+/Veh | Os+/Mel | |
| OPG | 100.0 (±2.89) | 1985 (±349) | 4133 (±1505)a* | |
| sOPG | 100.0 (±2.04) | 119.8 (±17.37)a*** | 119 (±24.07)a*** | |
| sRANKL | 100.0 (±2.04) | 333.3 (±71.97) | 162.1 (±27.08) | |
| sOPG:sRANKL | 100.0 (±2.24) | 57.88 (±15.91)a*** | 135.3 (±28.18)a***b* | |
| pERK1/2 | Taken from ref. no.20 | 100 (±6.67) | 160 (±28)* | |
| pERK5 | 100.0 (±5.77) | 103.4 (±24.98) | 69.11 (±15.65) | |
| tERK5 | 100.0 (±5.77) | 134.7 (±30.22) | 31.76 (±12.10)a* | |
| pERK5:tERK5 | 100.0 (±5.77) | 120.6 (±20.44) | 298.7 (±50.60)a*b* | |
Mean protein levels were analyzed, compared between groups (Os−/Mel, Os+/Veh, Os+/Mel), and expressed as mean (±SEM).
= vs Os−/Mel
= vs Os+/Veh
P < .05
P < .01
P < .001
one-way ANOVA followed by Bonferroni’s post hoc multiple comparison t test (n = 3 per group).
3.4 |. Role of MT2 melatonin receptors, MEK1/2, and/or MEK5 on melatonin-mediated modulation of pERK1/2 in cocultures
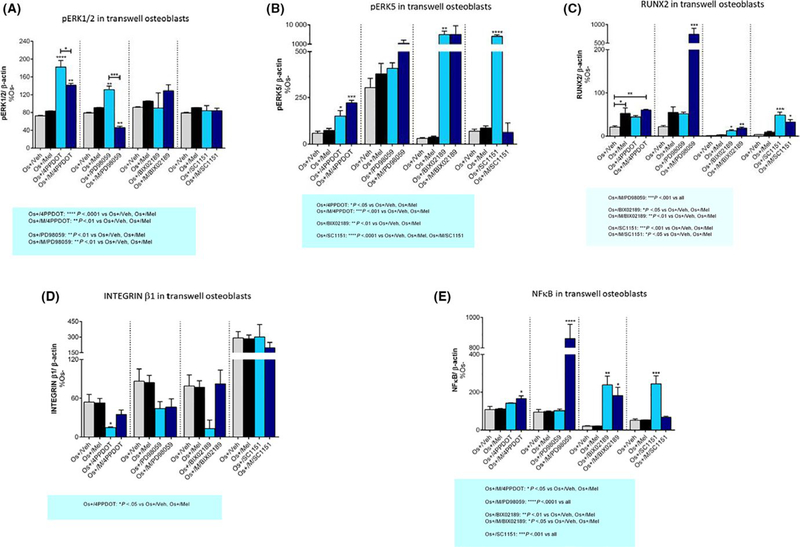
Past studies have demonstrated that ERK1/2 is associated with an increase in cellular differentiation51–53 and melatonin-mediated osteoblast differentiation.20,21 These past studies as well as the findings from Figure 1 where melatonin-mediated osteoblast differentiation and activity were inhibited in the presence of MEK1/2 inhibitors prompted the evaluation of these proteins in this coculture system. As shown in Figure 5A, transwell osteoblasts exposed to osteogenic medium (Os+) containing melatonin had significant increases in pERK1/2 expression (Figure 9A) compared to control. Both the MT2 melatonin receptor antagonist 4P-PDOT and the MEK1/2 inhibitor PD98059 but not the MEK5 inhibitor BIX02189, or the dual MEK1/2 and 5 inhibitor SC-1–151, increased phospho-ERK1/2 expression (4P-PDOT: P < .0001; PD98059: P < .01). Cotreatment of 4P-PDOT and PD98059 with melatonin decreased pERK1/2; no effect of melatonin was observed when coadministered with BIX02189 or SC-1–151. In contrast, an inhibitory effect of osteogenic medium (Os+) on pERK1/2 expression was observed for layered coculture (Table 1), whereas no further change occurred in the presence of melatonin. Because the layered coculture contains both osteoblasts and osteoclasts and that ERK1/2 plays an essential role in both osteoblastogenesis and osteoclastogenesis,53 it was not possible to conclude whether this increase in ERK1/2 activity was solely attributed to osteoblasts.
FIGURE 5.
Effect of melatonin and inhibitors on ERK1/2, ERK5, RUNX2, integrin ß1, and NFκB expression in transwell osteoblasts. After 21 d of melatonin transwell and inhibitor exposure, Western blot analysis was performed to determine the expression of (A) pERK1/2, (B) pERK5, (C) RUNX2, (D) INTEGRIN β1, (E) NFκB, respectively, in the osteoblasts grown in transwell coculture. Cell lysates were prepared separately from the bottom chamber (containing osteoblasts) and stored at −20°C until use. Protein levels were normalized against Os−/Mel or inhibitor (where indicated) following normalization against β-actin. Mean expression of expression levels was compared between groups (Os−/Mel, Os+/Veh, Os+/Mel). *P < .05, **P < .01, ***P < .001, and ****P < .0001; One-way ANOVA followed by Bonferroni’s post hoc multiple comparison t test (n = 3 per group). Statistical interpretation: (A) Os+/4P-PDOT = ****P < .0001 vs Os+/Veh, Os+/Mel; Os+/M/4P-PDOT = **P < .01 vs Os+/Veh, Os+/Mel; Os+/PD98059 = **P < .01 vs Os+/Veh, Os+/Mel; Os+/M/PD98059 = **P < .01 vs Os+/Veh, Os+/ Mel. (B) Os+/4P-PDOT = *P < .05 vs Os+/Veh, Os+/Mel; Os+/M/4P-PDOT = ***P < .001 vs Os+/Veh, Os+/Mel; Os+/BIX02189 = **P < .01 vs Os+/Veh, Os+/Mel; Os+/SC1151 = ****P < .0001 vs Os+/Veh, Os+/Mel, Os+/M/SC1151. (C) Os+/M/PD98059 = ***P < .001 vs all; Os+/BIX02189 = *P < .05 vs Os+/Veh, Os+/Mel; Os+/M/BIX02189 = **P < .01 vs Os+/Veh, Os+/Mel; Os+/SC1151 = ***P < .001 vs Os+/Veh, Os+/Mel; Os+/M/SC1151: *P < .05 vs Os+/Veh, Os+/Mel. (D) Os+/4P-PDOT = *P < .05 vs Os+/Veh, Os+/Mel. (E) Os+/M/4P-PDOT = *P < .05 vs Os+/Veh, Os+/Mel; Os+/M/PD98059 = ****P < .0001 vs all; Os+/BIX02189 = **P < .01 vs Os+/Veh, Os+/Mel; Os+/M/BIX02189 = *P < .05 vs Os+/Veh, Os+/Mel; Os+/SC1151 = ***P < .001 vs all
FIGURE 9.
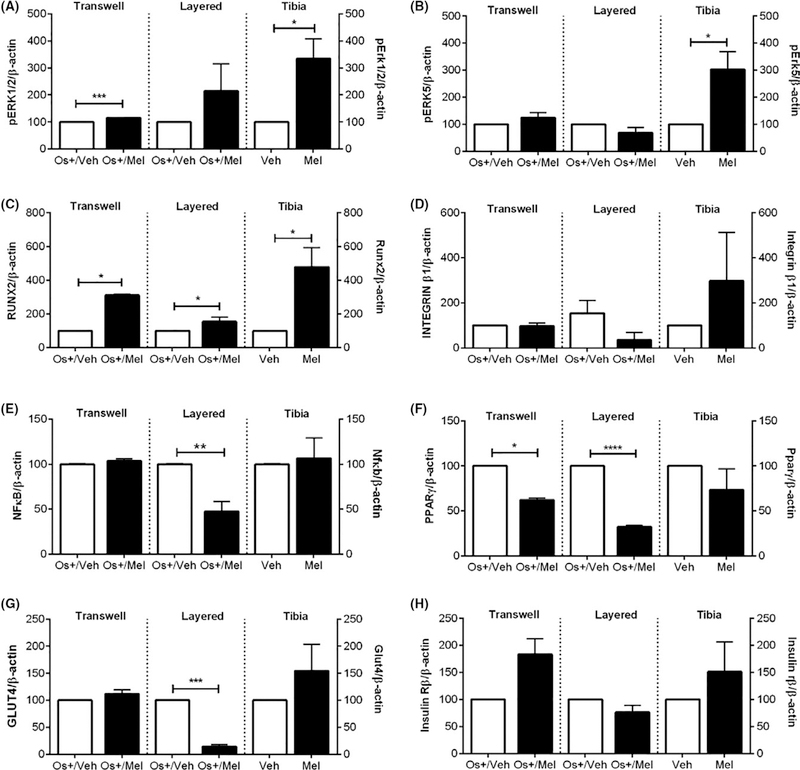
Comparison between in vitro and in vivo effects of melatonin on (A) pERK1/2, (B) pERK5, (C) RUNX2, (D) INTEGRIN β1, (E) NFκB, (F) PPARγ, (G) GLUT4 and (H) insulin receptor β expression. Effect of melatonin exposure on osteogenic and metabolic protein expression in transwell osteoblasts, layered osteoblast-osteoclast cocultures, and female mouse tibia. All proteins were normalized against β-actin and then compared by unpaired one- or two-tailed t test where significance was defined as follows: *P < .05, **P < .01, and ***P < .001. Each bar represents the mean ± SEM of 3–6 independent experiments
3.5 |. Role of MT2 melatonin receptors, MEK1/2, and/or MEK5 on melatonin-mediated modulation of pERK5 in cocultures
The effect of melatonin on ERK5 was also evaluated because this kinase plays an essential role in osteoblast and osteoclast differentiation and proliferation54–56 as shown in Figure 1. Figure 5B illustrates the effect of melatonin on ERK5 expression in transwell osteoblasts. Similar to pERK1/2, 4P-PDOT alone increased pERK5 expression; however, melatonin was without effect (Figure 9B). In layered cocultures (Table 1), exposure to the osteogenic medium (Os+) decreased pERK5 activity; however, the addition of melatonin produced no further effect on pERK5 activity. In MSC monocultures (Table 2), melatonin added in combination with osteogenic medium (Os+/Mel) increased the ratio of pERK5:tERK5 due to a decrease in total ERK5 levels. These findings demonstrate a strong influence of the osteoclast on ERK5 activity in osteoblasts. Melatonin added in combination with osteogenic medium (Os+/Mel) trended toward an increase in pERK5 in transwell osteoclasts (P = 07 vs Os+/Veh; data not shown).
3.6 |. Role of MT2 melatonin receptors, MEK1/2, and/or MEK5 on melatonin-mediated modulation of RUNX2 expression in cocultures
As shown previously, melatonin induced osteoblast formation, and this was associated with increases in RUNX2.21,23 In this study and consistent with these past studies, melatonin increased RUNX2 expression in transwell osteoblasts (Figure 5C) beyond that induced by osteogenic medium containing vehicle (P < .01 vs Os+/Veh) (Figure 9C); in layered cocultures, however, this was not observed (Table 1). In transwell osteoblasts, 4P-PDOT alone was without effect. The MEK5 inhibitor, BIX02189, alone increased RUNX2 expression vs OS+/Veh and OS+/Mel; however, the addition of melatonin to BIX02189 was without effect. Similar effects were observed for the SC-1–151. RUNX2 expression levels were not modulated by PD98059 alone; however, dramatic increases (~200% increase) in RUNX2 expression occurred when melatonin was combined with PD98059 suggesting some synergy between melatonin and MEK1/2.
3.7 |. Role of MT2 melatonin receptors, MEK1/2, and/or MEK5 on melatonin-mediated modulation of INTEGRIN β1 expression in cocultures
Figure 5D illustrates the effect of melatonin and the MEK1/2 and 5 inhibitors on integrin β1 expression in transwell osteoblasts. Integrin β1 plays a critical role in bone cell growth and activity by regulating the interaction between bone cells and the extracellular matrix.57 As expected, no effect of OS+ alone or in combination with melatonin affected integrin β1 levels in transwell osteoblasts because no cell-to-cell contact was made (Figure 9D); however, in layered cocultures, integrin β1 levels increased in response to OS+/Veh which decreased in the presence of melatonin (Table 1). Except for 4-P-PDOT, which decreased integrin β1 levels, no other effects of the MEK1/2 or 5 inhibitors were observed (Figure 5D).
3.8 |. Role of MT2 melatonin receptors, MEK1/2, and/or MEK5 on melatonin-mediated modulation of NFκB expression in cocultures
NFκB levels were assessed in both cocultures even though NFκB is an important regulator of osteoclastogenesis.58,59 As shown in Figure 5E, no effect of melatonin was observed on NFκB expression in transwell osteoblasts (Figure 9E); however, melatonin decreased NFκB expression in layered cocultures (Table 1). Alone, BIX02189 and SC-1–151 increased NFκB levels in transwell osteoblasts; however, increases in NFκB levels occurred when melatonin was combined with 4P-PDOT, BIX02189, or PD98059 in transwell osteoblasts (Figure 5E).
3.9 |. Role of MT2 melatonin receptors, MEK1/2, and/or MEK5 on melatonin-mediated modulation of metabolic protein expression in cocultures
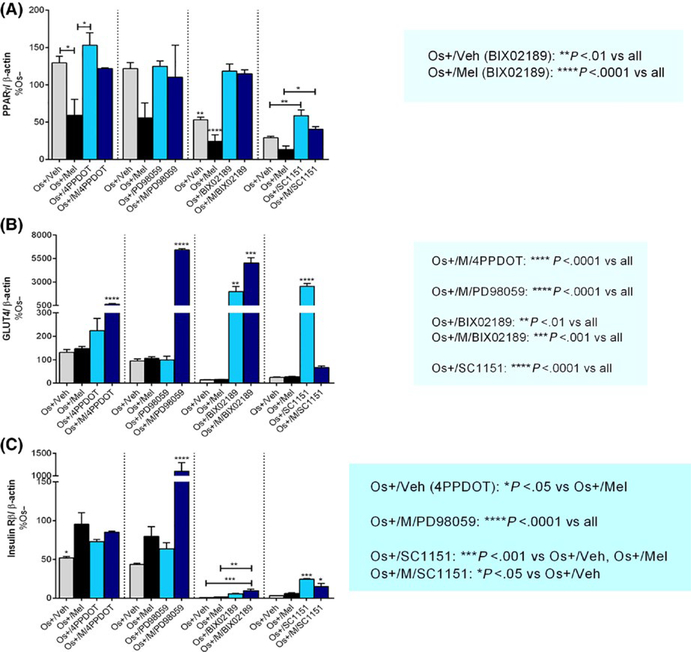
Figure 6 illustrates the effect of melatonin on the expression of the metabolic proteins, PPARγ, GLUT4, and insulin Rß in transwell osteoblasts, due to their potential impact on bone cell differentiation and activity.60–62 Exposure to melatonin in osteogenic media (Os+/Mel) significantly reduced PPARγ expression in both cocultures—transwell and layered (Figure 6A, Table 1 and Figure 9F, respectively). In transwell osteoblasts, the addition of 4P-PDOT trended toward blocking melatonin’s inhibitory effect on PPARγ. Except for SC-1–151, which increased PPARγ expression in transwell osteoblasts, no other effects of the inhibitors alone—MEK1/2 or MEK5—were observed. When coadministered with SC-1–151, melatonin trended toward a decrease in PPARγ expression (Figure 6A).
FIGURE 6.
Effect of melatonin and inhibitors on metabolic proteins PPARγ, GLUT4, and insulin Rß in transwell osteoblasts. After 21 d of melatonin transwell and inhibitor exposure, Western blot analysis was performed to determine melatonin effects on (A) PPARγ, (B) GLUT4, and (C) INSULIN Rß expression in transwell osteoblasts. Cell lysates were prepared separately from the bottom chamber (containing osteoblasts) and stored at −20°C until use. Protein levels were normalized against Os−/Mel or inhibitor (where indicated) following normalization against β-actin. Mean protein level in each coculture were analyzed and compared between groups (Os−/Mel, Os+/Veh, Os+/Mel). *P < .05, **P < .01, ***P < .001, and ****P < .0001; One-way ANOVA followed by Bonferroni’s post hoc multiple comparison t test (n = 3 per group). Statistical interpretation: (A) Os+/Veh (BIX02189) = **P < .01 vs all; Os+/Mel (BIX02189) = ****P < .0001 vs all. (B) Os+/M/4P-PDOT = ****P < .0001 vs all; Os+/M/PD98059 = ****P < .0001 vs all; Os+/BIX02189 = **P < .01 vs all; Os+/M/BIX02189 = *** P < .001 vs all; Os+/SC1151 = ****P < .0001 vs all. (C) Os+/Veh (4P-PDOT) = *P < .05 vs Os+/Mel; Os+/M/PD98059 = ****P < .0001 vs all; Os+/SC1151 = ***P < .001 vs Os+/Veh, Os+/Mel; Os+/M/SC1151 = *P < .05 vs Os+/Veh
Regarding GLUT4 expression, melatonin was without effect on its expression in transwell osteoblasts (Figures 6B and 9G); however, when added in combination with 4P-PDOT, BIX02189, or PD98959, GLUT4 levels increased. Both BIX02189 and SC-1–151 increased GLUT4 levels on their own in transwell osteoblasts. In layered cocultures, melatonin decreased GLUT4 expression (Table 1; Figure 9G).
For insulin receptor ß, melatonin was without effect in either coculture (Figure 6C, Table 2 and Figure 9H, respectively); however, when combined with BIX02189, PD98059, or SC-1–151, insulin receptor ß levels increased in transwell osteoblasts. Except for SC-1–151, which increased insulin receptor ß expression alone, no other effects of the inhibitors were observed (Figure 6C).
3.10 |. Role of MT2 melatonin receptors on melatonin-mediated differentiation of AMSCs into osteoblasts
We next evaluated the effect of melatonin on MSC differentiation isolated from human adipocytes (ASCs). Similar to bone marrow-derived MSCs, melatonin induced the differentiation of ASCs into osteoblasts reflected by a nearly 4-fold increase in mineralization (P < .0001 vs Os+/Veh) (Figure 7A). The addition of the MT2 melatonin receptor antagonist, 4P-PDOT, blocked these effects of melatonin on osteoblast differentiation from ASCs. Supporting this finding was the detection of melatonin receptors using 2-[125I]-iodomelatonin, which were not changed in response to any of the treatments (Figure 7B).
FIGURE 7.
Effect of melatonin on adipose-derived mesenchymal stem cell differentiation into osteoblasts and melatonin receptor expression. Adipose-derived mesenchymal stem cells (ASCs) were exposed to melatonin (±4P-PDOT) for 8 d (A) or 14 d (B) to assess its effects on alizarin red staining (A) or melatonin receptor expression (B). Each bar represents the mean absorbance of alizarin red at 490 nm (n = 3 per group). ***P < .001 vs Os−/4PPDOT; ****P < .0001 vs all
3.11 |. Effect of chronic melatonin exposure on osteogenic and metabolic protein expression in HER2/neu female mouse tibia
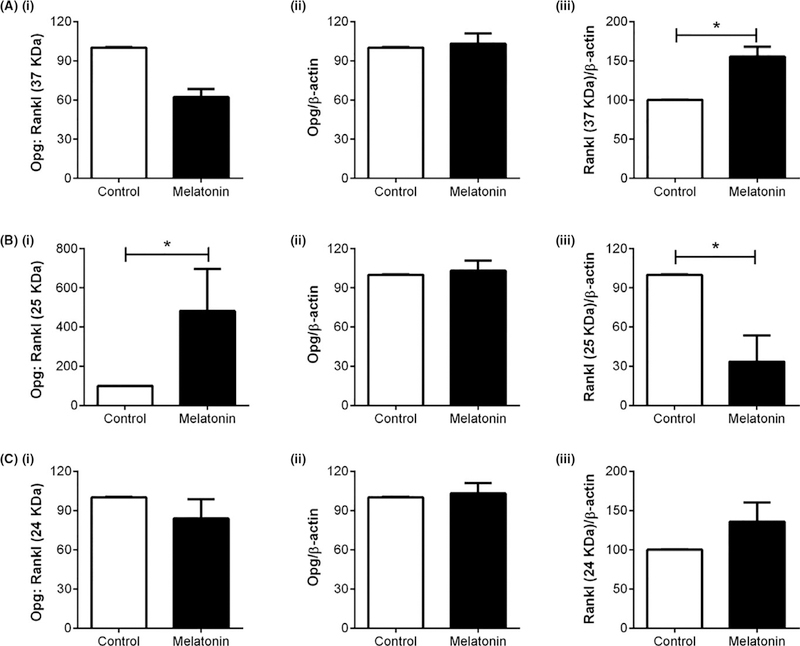
To determine whether the in vitro findings translated to in vivo, melatonin effects on osteogenic and metabolic proteins were examined in female mice. As demonstrated previously, melatonin (15 mg/L po in water) given nightly to HER2/neu transgenic female mice for one year increased bone mineral density (BMD) vs control, which was equal in efficacy to hormone therapy.16 The mechanisms underlying these effects were unclear, and so the same proteins demonstrated to be modulated by melatonin in both the transwell and layered cocultures were also assessed in these bones. As shown in Figure 8, melatonin modulated Opg:Rankl ratios uniquely dependent upon which Rankl fragment was being analyzed (ie, 37 kDa, 25 kDa or 24 kDa Rankl fragment). For example, in mice exposed to melatonin, levels of the 37 kDa Rankl fragment increased, whereas the 25 kDa Rankl fragment decreased. This decrease in the 25 kDa Rankl resulted in an increase in the Opg:Rankl (25 kDa) ratio in mouse bone, favoring osteogenesis.44,63 The increase in the 37 kDa Rankl fragment, when compared against Opg levels, did not affect the ratio of Opg:Rankl (37 kDa). Regarding pErk1/2, pErk5, and Runx2, melatonin induced their expression in mouse bone when compared to control bone; however, no change in NFκB, β1 integrin, Pparγ, Irβ, and Glut4 occurred—these mixed effects are similar to those observed for transwell osteoblasts and layered cocultures (Figure 9).
FIGURE 8.
Effect of melatonin on osteoprotegerin and Rankl protein expression in Her2/neu female mouse bone. Effect of one-year nightly melatonin (15 mg/L) exposure on Opg (Aii, Bii, Cii), Rankl (37 kDa; Aiii), Rankl (25 kDa; Biii), Rankl (24 kDa; Ciii), and Opg:Rankl ratios for Opg:37 kDa Rankl (Ai), Opg:25 kDa Rankl (Bi), and Opg:24 kDa Rankl (Ci) protein expression in mouse tibia. All proteins were normalized against β-actin and then compared by unpaired two-tailed t test where significance was defined as follows: *P < .05, **P < .01, and ***P < .001. Each bar represents the mean ± SEM of 3–6 mice
3.12 |. Effect of melatonin on subject-reported measures in perimenopausal women
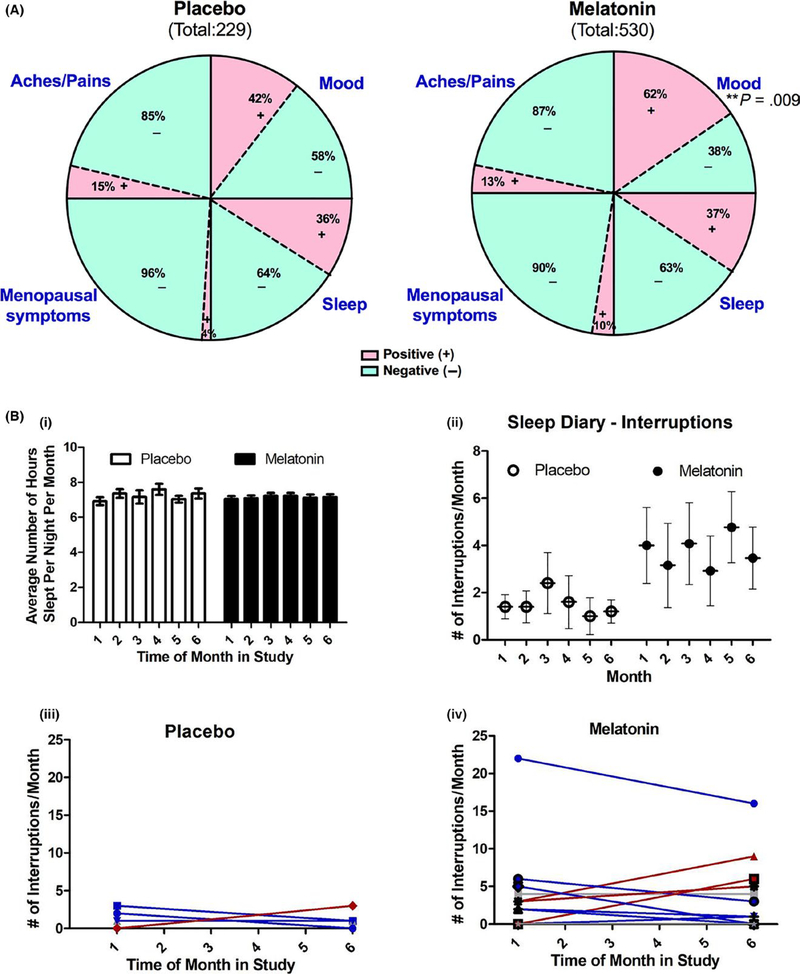
The melatonin osteoporosis prevention study (MOPS) demonstrated previously that melatonin (3 mg) given nightly for 6 months renormalized bone marker turnover in healthy perimenopausal women.17 Melatonin also increased BMD in postmenopausal osteopenic women in the MelaOst study.18 It was also shown in the MOPS trial that melatonin improved the physical symptoms of menopause as revealed by the MENQOL and reduced menstrual cycling by increasing the time between cycles.17 To identify which items melatonin improved, women were given daily diary logs. As shown in Figure 10A, melatonin treatment was found to improve mood in the perimenopausal women vs placebo (P = .009). Although not significant, a 6% decrease in menstrual complaints and a 1% decrease in sleep complaints also occurred. Further analysis of sleep quality revealed that sleep duration was not significantly different than placebo (Figure 10Bi); however, interrupted sleep may have improved for some in the melatonin group where 8 of 13 (62%) women in the melatonin group showed a decrease in the number of times they got up in the middle of the night compared to placebo (2/5 or 60%) (Figure 10B iii, iv). This is an important finding because although we block-randomized our participants as they enrolled in the MOPS, our melatonin cohort was older and reported having more vasomotor symptoms at baseline compared to placebo17; this may also explain the higher number of sleep interruptions reported by women in the melatonin group vs placebo (Figure 10Bii). These improvements in QOL in response to melatonin may result in improved patient compliance when taking melatonin to prevent bone loss in an aging population.
FIGURE 10.
Effect of melatonin on quality of life in perimenopausal women using daily logs. (A) Melatonin effects on self-reported sleep quality, mood, menopausal symptoms, and general aches/pains in perimenopausal women. Total diary comments made by the participants in each group were stratified into four categories (ie, sleep, mood, menopausal symptoms, and general aches and pains) based on what was written in their diary logs as illustrated by the four segments in the pie diagram. Each category was substratified as positive (pink) or negative (green) comments. Each portion represents the percent of total comments made under each category. Diary comments were analyzed using two-tailed Fisher’s exact test for two categorical outcomes where significance was defined as follows: *P < .05, **P < .01, and ***P < .001. (B) Melatonin effects on (i) sleep duration; (ii) sleep interruptions in total; (iii, iv) individual sleep interruptions in placebo; (iii) and individual sleep interruptions in melatonin-treated (iv) perimenopausal women taken from daily diary logs (n = 5 placebo and 13 melatonin)
4 |. DISCUSSION
Bone remodeling substitutes primary juvenile bones, as well as stress and age-related microfractured bones, with new, healthy, more mechanically competent bones to preserve mass, strength, integrity, and mineral homeostasis.4,64,65 The complex relationship between osteoblasts and osteoclasts and their regulation of each other’s activity during bone remodeling are still not clear but depend upon multiple modes of communication. Osteoblast and osteoclast communication can occur via direct cell-to-cell contact to allow interaction between membrane-bound ligands and initiation of intracellular signaling; via releasing diffusible paracrine factors such as growth factors, cytokines, chemokines, and other small molecules from either cell types to control each other’s activities; or by forming gap junctions through which small molecules can pass between the two cells.2,3 The present study used MSCs as osteoblast precursor and PBMCs as osteoclast precursors—grown in contact or not in contact with each other—to represent the different intercellular communication pathways utilized by osteoblasts and osteoclasts as they move through different stages of differentiation.
The findings from this study demonstrate that the mode of communication between osteoblasts and osteoclasts is important in regulating osteoclastogenesis in a “general sense” but particularly so for melatonin. For example, in both transwell and layered cocultures, melatonin induced the differentiation of osteoblasts; however, only in layered cocultures did melatonin inhibit osteoclast differentiation. Osteoblast contact with the osteoclast also modified OS+-mediated actions on osteoclastogenesis and proteins. Specifically, in layered cocultures but not transwells, OS+ media increased osteoclast differentiation reflected by significant increases in TRAP staining vs cultures grown in basal growth (OS−) medium.
This may be due to osteoblast-derived osteoclast inhibitory lectin (OCIL), a type II transmembrane C-type lectin that can suppress osteoclast differentiation2,66 or modulation of OPG and RANKL expression and secretion from the osteoblast. Regarding OPG and RANKL, osteogenic (OS+) medium alone was without effect on OPG or sOPG:sRANKL expression in either coculture—transwell or layered—but decreased sOPG:sRANKL levels in osteoblast monocultures—opposite to what occurred in the presence of melatonin. In the presence of melatonin, OPG levels were unchanged in transwell osteoblasts; they trended toward an increase in layered cocultures and significantly increased in monocultures exposed to melatonin. These increases in OPG in the osteoblast altered the ratio of secreted sOPG:sRANKL levels to favor osteoblastogenesis and, in layered cocultures, to inhibit osteoclastogenesis. The relative expression and release of OPG and RANKL from osteoblasts are a critical transition point for balancing bone mineralization by inhibiting osteoclast-mediated bone resorption to allow for osteoblast-mediated bone formation.44 Although melatonin-mediated induction of OPG in osteoblast monocultures has been shown,11,26 the presence of the osteoclast was essential in modulating melatonin’s effect on OPG expression and release from the osteoblast.
It is known that osteoblasts regulate osteoclast differentiation and activity via the OPG/RANKL/RANK pathway osteoblasts45; however, it is not clear how osteoclasts can regulate osteoblasts and the production of OPG. Cellular fos and macrophage colony-stimulating factor may play a role as shown in knockout mice where the absence of osteoclasts leads to defective bone formation.64,65 Paracrine factors [eg, insulin-like growth factor (IGF) I and II, fibroblast growth factor (FGF), transforming growth factor (TGF) 1 and 2, bone morphogenetic proteins (BMPs) 2, 3, 4, 6, and 7, and platelet-derived growth factor (PDGF)] embedded in the bone matrix and released following osteoclast-mediated bone resorption are other possibilities. In addition, the ephrin signaling pathway, which involves the interaction between osteoclast-derived ephrinB2 and osteoblast-derived EphB4, can initiate osteoblastogenesis and, in turn, suppress osteoclastogenesis when osteoclasts and osteoblasts contact one another3,64,65; this may explain the differences between the transwell and layered cocultures with respect to melatonin’s effects on osteoclastogenesis.
Modulation of ERK1/2 and ERK5 may have also contributed to the differential effects of melatonin on osteoclastogenesis between cocultures by increasing the state of osteoblast differentiation; mature osteoblasts produce OPG to negatively regulate osteoclastogenesis. ERK1/2 is one of the key regulatory pathways involved in osteoblast differentiation.51–53,67 Specific deletion of ERK1 and ERK2 in limb mesenchyme of mice results in low bone mineralization.53 Dominant-negative mutants of MEK1 in mouse osteoblasts exhibit low clavicular and calvarial bone mass and hypomineralization,51 which can be restored following the introduction of constitutively active MEK1.52 Regarding ERK5 in osteoblast physiology, Kaneshiro et al. recently demonstrated that MEK5-ERK5 pathway suppresses osteoblast differentiation and promotes osteoblastic cell proliferation in pre-osteoblastic MC3T3-E1 and bone marrow stromal cells.55 MEK5/ERK5 are also involved in fluid shear stress-mediated proliferation of osteoblasts.54,56 In cultures exposed to osteogenic (OS+) medium alone, pERK1/2 or pERK5 levels were not changed in transwell osteoblasts but were decreased in layered cocultures; this correlated with increases in both osteoblastogenesis and osteoclastogenesis. When examining the effect of melatonin on these kinases, pERK1/2 levels increased in transwell osteoblasts (Figure 5A) and monocultures20,21 but remained unchanged in layered cocultures (Table 1). With respect to pERK5, levels were unaffected in both transwell and layered cocultures but increased in osteoblast monocultures. These findings suggest that melatonin-mediated increases in osteoblastogenesis and decreases in osteoclastogenesis are associated with high pERK1/2 and low pERK5—consistent with the role of these kinases and melatonin in osteoblasts and osteoclasts.20,21,51–56,67
It should also be noted that the type of culturing condition—transwell vs layered—played significant roles in OS+-induced expression patterns of pERK1/2, pERK5, integrin β1, PPARγ, GLUT4, and INRβ when compared to growth (OS−) medium. For example, in transwell osteoblasts, OS+/V was without effect on pERK1/2, pERK5, integrin β1, PPARγ, and GLUT4 and decreased INRβ; however, in layered cocultures, levels of pERK1/2 and pERK5 decreased, levels of integrin β1, PPARγ, and GLUT4 increased, and INRβ was unchanged. These findings suggest that osteogenic and metabolic proteins cooperate and drive osteoblastogenesis and osteoclastogenesis when osteoblasts and osteoclasts, respectively, are in contact with one another. When comparing the influence of melatonin between cocultures, the most notable differences occurred with respect to pERK1/2, NFκB, and GLUT4; in transwell osteoblasts, melatonin induced pERK1/2 expression and was without effect on NFκB and GLUT4 while in layered cocultures, melatonin decreased NFκB and GLUT4 expression and was without effect on pERK1/2. These findings suggest that melatonin-mediated increases in osteoblastogenesis and decreases in osteoclastogenesis involve modulation of pERK1/2 pERK5, integrin β1, NFκB, and GLUT4, which is consistent with what has been reported for MEK5/ERK5,54–56 GLUT4,61 NFκB,44,58,68–70 and integrin β1 matrix57 in osteoblasts and osteoclasts.
The use of the transwell coculture model system, and particularly MSCs and PBMCs, allowed for us to more closely model osteoblastic and osteoclastic differentiation by assessing the effect of melatonin on immature rather than on mature cells. This model also allowed for the study of osteoblast/ osteoclast comodulation through the release of diffusible factors and enabled us to identify those proteins underlying melatonin-mediated osteoblastogenesis. As stated previously, melatonin added to transwell osteoblasts did not affect osteoclastogenesis and this held true for the inhibitors. Except for BIX02189 alone, which inhibited osteoclastogenesis, blockade of MT2 melatonin receptors, MEK1/2, or MEK5 in osteoblasts did not affect osteoclast differentiation even though they significantly inhibited osteoblastogenesis. These findings suggest that osteoblasts do not modulate osteoclast differentiation in a significant way by the release of paracrine factors in this model system but through direct contact.
MT2 melatonin receptors expressed in osteoblasts were essential for mediating melatonin’s effects on osteoblastogenesis; this was true for osteoblasts differentiated from MSCs derived from bone marrow or adipose tissue. This was further supported in the 2-[125I]-iodomelatonin binding assays. For the first time, melatonin receptor protein expression was also detected in osteoclasts derived from PBMCs and, like the osteoblasts, their expression may be regulated by melatonin. These findings in osteoblasts are consistent with other studies demonstrating that MT2 melatonin receptors mediate melatonin’s effects on osteoblast differentiation and osteogenic (ie, Runx2, Bmp2, and Bglap) gene expression.20,21
Chronic 21-day exposure to 4P-PDOT alone enhanced the expression of pERK1/2 and pERK5 and decreased the expression of beta 1 integrin vs OS+; however, these changes in the osteoblast in response to 4P-PDOT did not translate to changes in osteoblast differentiation as no significant increases in alizarin red staining occurred in response to 4P-PDOT vs OS+/Veh (Figure 1A). Coadministration of 4P-PDOT with melatonin, which blocked calcium mineralization, also decreased pERK1/2, further enhanced pERK5, and attenuated 4P-PDOT’s inhibitory actions on integrin β1 suggesting that MT2 melatonin receptor-mediated modulation of pERK1/2, pERK5, and integrin β1 may underlie melatonin’s effects on osteoblast differentiation (Figure 1A). These findings are consistent with previous in vitro studies, which show stimulatory roles of melatonin in osteoblast differentiation and mineralization from MSCs and pre-osteoblasts through MT2 melatonin receptors and MAPKs.11,19–23 For integrin β1, these effects of melatonin/MT2 melatonin receptors are new and may involve α1β1, α2β1, and α5β1—the most commonly expressed integrins in osteoblasts71 that also play an important role in osteoblast organization on the bone surface during osteoid synthesis.72 Our findings that melatonin treatment decreased integrin β1 expression in layered cocultures suggest that melatonin may work through an integrin-dependent mechanism when osteoblasts are in direct contact with osteoclasts during their differentiation process. The role of integrin in cell-cell and cell-matrix interactions between osteoblasts and osteoclasts via cytoskeletal organization is known to be a key requirement for bone cell proliferation, differentiation, migration, and apoptosis as well as skeletal development and homeostasis57,73–77 and may also involve αvβ3 and α2β1 integrins expressed in osteoclasts.78,79
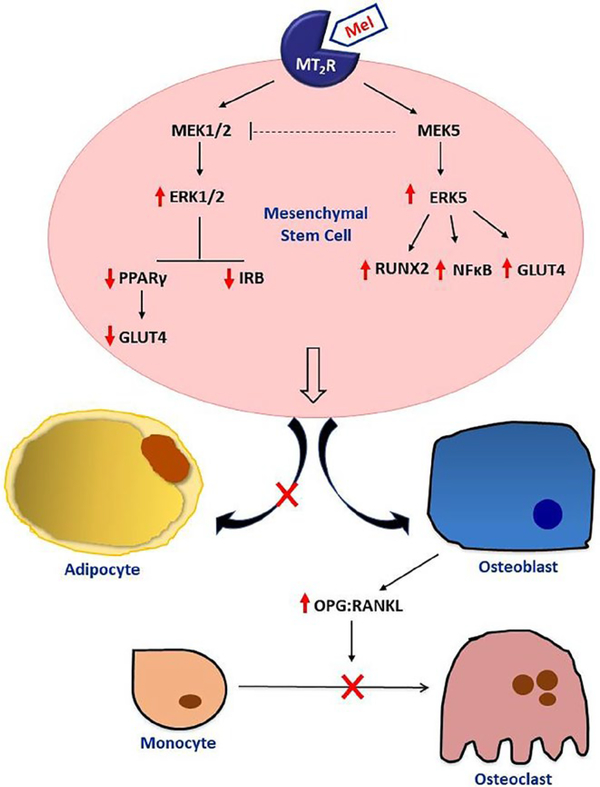
The role of MEK1/2 and MEK5 was further supported in studies using selective inhibitors to MEK1/2 and MEK5 in combination with melatonin. Here, it was shown that PD98059, BIX02189, or SC-1–151 given simultaneously with melatonin at the outset of the treatments inhibited melatonin-induced osteoblast differentiation suggesting the involvement of MEK1/2 and MEK5, respectively. Besides helping to identify the signaling cascades involved in melatonin-mediated effects on osteoblast differentiation, the use of these inhibitors helped to tease out the complicated relationships that exist between MEK1/2 and MEK5 and the proteins that lay downstream from them. With respect to comodulation of each other, inhibition of either kinase separately (ie, PD98059 effects on MEK5 and BIX02189 effects on MEK1/2) produced no cross talk between kinases; however, both inhibitors alone upregulated their own kinase activity—PD98059 increased pERK1/2 expression and BIX02189 increased pERK5 expression, which may be due to a compensatory increase in their activity due to chronic blockade. When both kinases were inhibited at the same time, however, using SC-1–151 (MEK1/2/5 inhibitor), only pERK5 increased; no effect occurred on pERK1/2. These findings suggest that cross talk between MEK5 and MEK1/2 may have occurred whereby simultaneous inhibition of both kinases leads to a MEK5 dominant effect, which was also observed for RUNX2, NFκB, and GLUT4 in the presence of SC-1–151. This result and the fact that BIX20189 but not PD98059 exposure led to increases in ERK5, RUNX2, NFκB, and GLUT4 also suggest that RUNX2, NFκB, and perhaps GLUT4 lay downstream of ERK5. For GLUT4, though, these relationships were not so clear as GLUT4 expression can be modulated by PPARγ,80,81 which appears to also lie downstream from MEK/ERK1/2 in this model (Figure 11).
FIGURE 11.
Proposed mechanisms underlying melatonin’s actions on osteoblastogenesis and osteoclastogenesis. As shown, melatonin, via MT2 melatonin receptors, modulates osteoblastogenesis by coupling to both MEK1/2 and MEK5. Stimulation of MT2 melatonin receptors by melatonin in osteoblasts leads to activation of MEK5 resulting in increases in pERK5, RUNX2, NFκB, and GLUT4. MT2 melatonin receptor-mediated activation of MEK1/2 leads to increases in pERK1/2 and decreases in PPARγ, GLUT4, and IRβ. It is proposed that cross talk between MEK1/2 and MEK5 may occur where MEK5 negative regulates MEK1/2 only when both kinases are inhibited simultaneously. In MSCs, melatonin/MT2R-mediated decreases in PPARγ levels shift MSCs away from adipogenesis and toward osteogenesis. Following MSC differentiation into osteoblasts by melatonin, the mature osteoblasts begin secreting OPG to inhibit osteoclastogenesis from PBMCs
Chronic exposure to the MEK1/2 inhibitor, PD98059, increased pERK1/2 (vs OS+/Veh) which was inhibited in the presence of melatonin (even below that of OS+/Veh). Interestingly, although OS+/Veh, OS+/Mel, and OS+/PD98059 alone were without effect on NFκB, GLUT4, and IRβ expression, melatonin added in combination with the MEK1/2 inhibitor, PD98059, substantially increased their expression; this pattern of expression was also associated with decreased alizarin red staining. These data suggest that low ERK1/2, high NFκB, GLUT4, and IRβ were associated with low osteoblast differentiation and calcium mineralization. For RUNX2, melatonin alone increased its expression, which was further enhanced when MEK1/2 was inhibited. These findings suggest that MEK1/2 is negatively coupled to RUNX2.
The dual MEK1/2 and MEK5 inhibitor further confirmed these relationships as blockade of both MEK1/2 and MEK5 by SC-1–151 alone increased pERK5, RUNX2, NFκB, PPARγ, GLUT4, and IRβ. For ERK5, RUNX2, NFκB, and GLUT4, these effects of SC-1–151 mirrored those induced by BIX02189 alone (compare BIX02189 alone to SC-1–151) again, suggesting that RUNX2, NFκB, and GLUT4 are positively coupled to MEK5/ERK5. For PPARγ and IRβ, these proteins are likely to be negatively coupled to MEK/ERK1/2 due to the fact that their patterns of expression did not mirror that induced by BIX02189 exposure alone and also that their levels increased when exposed to PD98059 and melatonin.
In the MelaOst study, it was observed that postmenopausal women with osteopenia who consumed melatonin for one year not only had an improvement in bone density18 but also had a reduction in total fat mass and a trend toward an increase in lean body mass.82 These findings suggested that melatonin may be producing effects on metabolic proteins such as PPARγ, GLUT4, and INRB in osteoblasts. High levels of PPARγ switches the fate of MSCs toward adipogenesis at the expense of osteoblastogenesis and stimulates RANKL production contributing to bone loss by increasing the fat content in bone marrow and by increasing osteoclast activity60,83; whereas low PPARγ levels increase bone mass by enhancing osteoblastogenesis.60,62,84 In this study, the metabolic protein, PPARγ, was modulated by the various treatments in a manner that was consistent with its reported role in osteoblast differentiation60,62,84; that is, when calcium mineralization was high (ie, in the presence of melatonin), PPARγ levels were low, and when calcium mineralization was low (ie, in the presence of melatonin plus inhibitors), PPARγ expression was high. These findings suggest that melatonin, by decreasing PPARγ in MSCs, moves MSCs down an osteogenic rather than adipogenic pathway forming osteoblasts rather than adipocytes—a PPARγ insufficiency has been shown to enhance osteoblastogenesis leading to increases in bone mass.23,60,62,84 This is supported in other studies showing that melatonin directly inhibits adipogenic differentiation of hMSCs and mMSCs by suppressing PPARγ expression in favor of osteoblastogenesis.23,85
This may occur through PPARγ-mediated regulation of the GLUT4 expression in cells (eg, adipocytes and osteoclasts) modulating their states of viability through energetics. Support for this comes from studies demonstrating that PPARγ agonists such as rosiglitazone and pioglitazone enhance GLUT4 mRNA in muscle tissue, whereas a loss in PPARγ decreases GLUT1 and GLUT4 function in adipocytes.80,81 GLUT4 mRNA levels increase during osteoblast differentiation.61 Melatonin decreased GLUT4 expression in the layered cocultures, and this correlated with PPARγ and NFκB expression. Perhaps the proximity and actual contact of MSCs with PBMCs during the differentiation process enhanced the inhibitory effects of melatonin on osteoclastogenesis by downregulating NFκB and lowering energetics within osteoclasts. These areas will be pursued in the future.
The effect of melatonin on these same proteins was also examined in vivo using a preclinical mouse model exposed to melatonin (15 mg/L) nightly for one year; this dose is equivalent to ~5 mg melatonin in humans. As already reported, tibia bone density increased in mice ingesting melatonin in their drinking water compared to control, which was equal in efficacy to a therapeutically relevant estrogen/progesterone hormone therapy given for one year in food.16 The patterns of modulation of Opg, Rankl, pErk1/2, Erk5, Runx2, and Pparγ in melatonin-exposed bone are consistent with melatonin-induced effects on osteoblast differentiation in vitro and provide important mechanistic information to explain the increases in bone density observed in melatonin-treated bone.16 The other interesting observation that came out of the bone analyses was the differential effect of melatonin on the 24 kDa, 25 kDa, and 37 kDa Rankl proteins where melatonin decreased the 25 kDa Rankl fragment, increased the mature 37 kDa Rankl protein, and was without effect on the 24 kDa fragment. The decrease in the smaller 25 kDa Rankl fragment and increase in the 37 kDa protein may reflect a melatonin inhibitory effect on ectodomain shedding of membrane-bound Rankl via the action of matrix metalloproteases 3 and 7 and ADAM 17 (or TACE) and 19.63,68 This may explain the melatonin-mediated decreases in sRANKL that occurred in layered cocultures and provide a mechanism underlying melatonin’s inhibitory effect on osteoclastogenesis that was observed only in the layered cocultures.
The significance of the clinical findings obtained from the MOPS daily diaries demonstrates that melatonin primarily improved subject-reported mood, menopausal symptoms (eg, vasomotor symptoms and bloating), and sleep quality (mostly on frequency of getting up in the middle of the night)—these findings provide context to the MOPS findings showing a significant improvement in MENQOL physical domain scores in perimenopausal women taking melatonin17 and describe other benefits of melatonin on bone health. Significant relationships between depression, bone mineral density, and cortisol levels have been reported where depressed patients were shown to have higher cortisol levels and lower bone mineral density compared to placebo controls.35,36 Also, lifestyle that includes sleep disruption through shift work,16,17,27,86–89 sleep deprivation,37–40 physical strength,90 weight gain,91 and stress levels41 can adversely affect bone. Besides improving compliance, the positive effect of melatonin on mood and sleep may show benefits to bone by reducing cortisol levels and re-entraining sleep/wake cycles to coincide with the light/ dark cycle and natural bone rhythms. A realignment of sleep cycles with melatonin and bone rhythms may help to maintain and restore bone through effects on osteoblasts; this is supported in a recent study demonstrating decreases in P1NP levels but not CTx levels in sleep-deprived males.37
In summary, this translational study describes novel mechanisms underlying melatonin’s effects on osteoblastogenesis and osteoclastogenesis that include MT2 melatonin receptors, MEK1/2, and MEK5. These findings that inhibition of MEK1/2 alone, MEK5 alone, or both early on in immature, undifferentiated MSCs completely inhibited melatonin’s effects on osteoblast differentiation demonstrate that these pathways are essential. The findings that melatonin modulation of PPARγ may be the switch for MSCs to move down osteogenesis rather than adipogenesis and may explain the findings by Amstrup et al.18 demonstrating that postmenopausal women with osteopenia who took melatonin not only showed improvements in bone density but also had reductions in total fat mass and a trend toward increases in lean body mass.82 Melatonin’s effects on OPG and RANKL and RANKL fragments also open up novel areas for research especially in the role of MMPs and ADAMs in mediating melatonin’s effects on osteoclastogenesis. The bone-protective effects of melatonin through regulation of osteoblasts and osteoclasts coupled with subjective improvements in mood and sleep make melatonin and, perhaps, MT2 melatonin receptor agonists attractive alternatives to prevent and/or treat bone loss in an aging population.
ACKNOWLEDGEMENTS
We thank the National Institute for Arthritis and Musculoskeletal Diseases NIAMS R15 AR068605 to PAWE and NIAMS R01 AR049069 to AVW for funding the in vitro work; Susan G. Komen for the Cure to PAWE and VLD for funding the mouse study; and Duquesne University Translational Research grant to PAWE for funding of the MOPS.
Funding information National Institute for Arthritis and Musculoskeletal Diseases, Grant/Award Number: NIAMS R15 AR068605 and NIAMS R01 AR049069; Susan G. Komen for the Cure Duquesne University Translational Research
REFERENCES
- 1.Gueldner SH, Fgsa F, Grabo TN, Newman ED, Cooper DR. Osteoporosis: Clinical Guidelines for Prevention, Diagnosis, and Management. New York, NY: Springer Publishing Company; 2007. [Google Scholar]
- 2.Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473:201–209. [DOI] [PubMed] [Google Scholar]
- 3.Sims NA, Gooi JH. Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19:444–451. [DOI] [PubMed] [Google Scholar]
- 4.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285:25103–25108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng X, Mcdonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson JE, Goltzman D. The Osteoporosis Primer. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 7.Zebaze RM, Ghasem-Zadeh A, Bohte A, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–1736. [DOI] [PubMed] [Google Scholar]
- 8.Sroga GE, Vashishth D. Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr Osteoporos Rep. 2012;10:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. [DOI] [PubMed] [Google Scholar]
- 10.Reginster J-Y, Burlet N. Osteoporosis: a still increasing prevalence. Bone. 2006;38:4–9. [DOI] [PubMed] [Google Scholar]
- 11.Maria S, Witt-Enderby PA. Melatonin effects on bone: potential use for the prevention and treatment for osteopenia, osteoporosis, and periodontal disease and for use in bone-grafting procedures. J Pineal Res. 2014;56:115–125. [DOI] [PubMed] [Google Scholar]
- 12.Caro JJ, Ishak KJ, Huybrechts KF, Raggio G, Naujoks C. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004;15:1003–1008. [DOI] [PubMed] [Google Scholar]
- 13.Cardinali DP, Ladizesky MG, Boggio V, Cutrera RA, Mautalen C. Melatonin effects on bone: experimental facts and clinical perspectives. J Pineal Res. 2003;34:81–87. [DOI] [PubMed] [Google Scholar]
- 14.Ostrowska Z, Kos-Kudla B, Marek B, Kajdaniuk D. Influence of lighting conditions on daily rhythm of bone metabolism in rats and possible involvement of melatonin and other hormones in this process. Endocr Regul. 2003;37:163–174. [PubMed] [Google Scholar]
- 15.Sandyk R, Awerbuch GI. Nocturnal plasma melatonin and alpha-melanocyte stimulating hormone levels during exacerbation of multiple sclerosis. Int J Neurosci. 1992;67:173–186. [DOI] [PubMed] [Google Scholar]
- 16.Witt-Enderby PA, Slater JP, Johnson NA, et al. Effects on bone by the light/dark cycle and chronic treatment with melatonin and/or hormone replacement therapy in intact female mice. J Pineal Res. 2012;53:374–384. [DOI] [PubMed] [Google Scholar]
- 17.Kotlarczyk MP, Lassila HC, O’neil CK, et al. Melatonin osteoporosis prevention study (MOPS): a randomized, double-blind, placebo-controlled study examining the effects of melatonin on bone health and quality of life in perimenopausal women. J Pineal Res. 2012;52:414–426. [DOI] [PubMed] [Google Scholar]
- 18.Amstrup AK, Sikjaer T, Heickendorff L, Mosekilde L, Rejnmark L. Melatonin improves bone mineral density at the femoral neck in postmenopausal women with osteopenia: a randomized controlled trial. J Pineal Res. 2015;59:221–229. [DOI] [PubMed] [Google Scholar]
- 19.Park KH, Kang JW, Lee EM, et al. Melatonin promotes osteoblastic differentiation through the BMP/ERK/Wnt signaling pathways. J Pineal Res. 2011;51:187–194. [DOI] [PubMed] [Google Scholar]
- 20.Radio NM, Doctor JS, Witt-Enderby PA. Melatonin enhances alkaline phosphatase activity in differentiating human adult mesenchymal stem cells grown in osteogenic medium via MT2 melatonin receptors and the MEK/ERK (1/2) signaling cascade. J Pineal Res. 2006;40:332–342. [DOI] [PubMed] [Google Scholar]
- 21.Sethi S, Radio NM, Kotlarczyk MP, et al. Determination of the minimal melatonin exposure required to induce osteoblast differentiation from human mesenchymal stem cells and these effects on downstream signaling pathways. J Pineal Res. 2010;49: 222–238. [DOI] [PubMed] [Google Scholar]
- 22.Zaminy A, Ragerdi Kashani I, Barbarestani M, Hedayatpour A, Mahmoudi R, Farzaneh Nejad A. Osteogenic differentiation of rat mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells: melatonin as a differentiation factor. Iran Biomed J. 2008;12:133–141. [PubMed] [Google Scholar]
- 23.Zhang L, Su P, Xu C, et al. Melatonin inhibits adipogenesis and enhances osteogenesis of human mesenchymal stem cells by suppressing PPARγ expression and enhancing Runx2 expression. J Pineal Res. 2010;49:364–372. [DOI] [PubMed] [Google Scholar]
- 24.Nakade O, Koyama H, Ariji H, Yajima A, Kaku T. Melatonin stimulates proliferation and type I collagen synthesis in human bone cells in vitro. J Pineal Res. 1999;27:106–110. [DOI] [PubMed] [Google Scholar]
- 25.Satomura K, Tobiume S, Tokuyama R, et al. Melatonin at pharmacological doses enhances human osteoblastic differentiation in vitro and promotes mouse cortical bone formation in vivo. J Pineal Res. 2007;42:231–239. [DOI] [PubMed] [Google Scholar]
- 26.Koyama H, Nakade O, Takada Y, Kaku T, Lau KHW. Melatonin at pharmacologic doses increases bone mass by suppressing resorption through down-regulation of the RANKL-mediated osteoclast formation and activation. J Bone Miner Res. 2002;17:1219–1229. [DOI] [PubMed] [Google Scholar]
- 27.Clafshenkel WP, Rutkowski JL, Palchesko RN, et al. A novel calcium aluminate-melatonin scaffold enhances bone regeneration within a calvarial defect. J Pineal Res. 2012;53:206–218. [DOI] [PubMed] [Google Scholar]
- 28.Cutando A, Gómez-Moreno G, Arana C, et al. Melatonin stimulates osteointegration of dental implants. J Pineal Res. 2008;45:174–179. [DOI] [PubMed] [Google Scholar]
- 29.Roth JA, Kim B-G, Lin W-L, Cho M-I. Melatonin promotes osteoblast differentiation and bone formation. J Biol Chem. 1999;274:22041–22047. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki N, Hattori A. Melatonin suppresses osteoclastic and osteoblastic activities in the scales of goldfish. J Pineal Res. 2002;33:253–258. [DOI] [PubMed] [Google Scholar]
- 31.Witt-Enderby PA, Radio NM, Doctor JS, Davis VL. Therapeutic treatments potentially mediated by melatonin receptors: potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J Pineal Res. 2006;41:297–305. [DOI] [PubMed] [Google Scholar]
- 32.Witt-Enderby P, Clafshenkel WP, Kotlarczyk M, Sethi S. Melatonin in bone health In: Watson RR, ed. Melatonin in the Promotion of Health. Boca Raton, FL: CRC Press; 2011:261–270. [Google Scholar]
- 33.Ladizesky MG, Boggio V, Albornoz LE, Castrillon PO, Mautalen C, Cardinali DP. Melatonin increases oestradiol-induced bone formation in ovariectomized rats. J Pineal Res. 2003;34:143–151. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Martinez F, Olivares Ponce PN, Guerra Rodriguez M, Martinez Pedraza R. Melatonin: bone metabolism in oral cavity. Int J Dent. 2012;2012:628406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furlan PM, Ten Have T, Cary M, et al. The role of stress-induced cortisol in the relationship between depression and decreased bone mineral density. Biol Psychiatry. 2005;57:911–917. [DOI] [PubMed] [Google Scholar]
- 36.Altindag O, Altindag A, Asoglu M, Gunes M, Soran N, Deveci Z. Relation of cortisol levels and bone mineral density among premenopausal women with major depression. Int J Clin Pract. 2007;61:416–420. [DOI] [PubMed] [Google Scholar]
- 37.Swanson C, Shea SA, Wolfe P, et al. Bone turnover markers after sleep restriction and circadian disruption: a mechanism for sleep-related bone loss in humans. J Clin Endocrinol Metab. 2017;102:3722–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Everson CA, Folley AE, Toth JM. Chronically inadequate sleep results in abnormal bone formation and abnormal bone marrow in rats. Exp Biol Med. 2012;237:1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu X, Wang L, Chen L, et al. Effects of chronic sleep deprivation on bone mass and bone metabolism in rats. J Orthop Surg Res. 2016;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucassen EA, Coomans CP, Van Putten M, et al. Environmental 24-hr cycles are essential for health. Curr Biol. 2016;26:1843–1853. [DOI] [PubMed] [Google Scholar]
- 41.Maria S, Witt-Enderby PA. Circadian regulation of bone In: Jazwinski SM, Belancio VP, Hill SM, eds. Circadian Rhythms and Their Impact on Aging. Cham: Springer Publishing; 2017:65–82. [Google Scholar]
- 42.Maria S, Swanson MH, Enderby LT, et al. Melatonin-micronutrients Osteopenia Treatment Study (MOTS): a translational study assessing melatonin, strontium (citrate), vitamin D3 and vitamin K2 (MK7) on bone density, bone marker turnover and health related quality of life in postmenopausal osteopenic women following a one-year double-blind RCT and on osteoblast-osteoclast co-cultures. Aging (Albany NY). 2017;9:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langenbach F, Handschel J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res Ther. 2013;4:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atkins GJ, Kostakis P, Pan B, et al. RANKL expression is related to the differentiation state of human osteoblasts. J Bone Miner Res. 2003;18:1088–1098. [DOI] [PubMed] [Google Scholar]
- 46.Janckila AJ, Takahashi K, Sun SZ, Yam LT. Naphthol-ASBI phosphate as a preferred substrate for tartrate-resistant acid phosphatase isoform 5b. J Bone Miner Res. 2001;16:788–793. [DOI] [PubMed] [Google Scholar]
- 47.Suofu Y, Li W, Jean-Alphonse FG, et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc Natl Acad Sci. 2017;114:E7997–E8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crespo-Diaz R, Behfar A, Butler GW, et al. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant. 2011;20:797–811. [DOI] [PubMed] [Google Scholar]
- 49.Mader EK, Butler G, Dowdy SC, et al. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. J Transl Med. 2013;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amano S, Chang Y-T, Fukui Y. ERK5 activation is essential for osteoclast differentiation. PLoS ONE. 2015;10:e0125054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ge C, Xiao G, Jiang D, Franceschi RT. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J Cell Biol. 2007;176: 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ge C, Xiao G, Jiang D, Yang Q, Hatch NE, Franceschi RT. Identification and functional characterization of extracellular-regulated kinase/MAPK phosphorylation sites in the Runx2 transcription factor. J Biol Chem. 2009;284:32533–32543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsushita T, Chan YY, Kawanami A, Balmes G, Landreth GE, Murakami S. Extracellular signal-regulated kinase 1 (ERK1) and ERK2 play essential roles in osteoblast differentiation and in supporting osteoclastogenesis. Mol Cell Biol. 2009;29:5843–5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bo Z, Bin G, Jing W, et al. Fluid shear stress promotes osteoblast proliferation via the Gαq-ERK5 signaling pathway. Connect Tissue Res. 2016;57:299–306. [DOI] [PubMed] [Google Scholar]
- 55.Kaneshiro S, Otsuki D, Yoshida K, Yoshikawa H, Higuchi C. MEK5 suppresses osteoblastic differentiation. Biochem Biophys Res Commun. 2015;463:241–247. [DOI] [PubMed] [Google Scholar]
- 56.Li P, Ma Y-C, Sheng X-Y, et al. Cyclic fluid shear stress promotes osteoblastic cells proliferation through ERK5 signaling pathway. Mol Cell Biochem. 2012;364:321–327. [DOI] [PubMed] [Google Scholar]
- 57.Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-α2β1 integrin interaction. J Cell Physiol. 2000;184:207–213. [DOI] [PubMed] [Google Scholar]
- 58.Jimi E, Aoki K, Saito H, et al. Selective inhibition of NF-κB blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10:617–624. [DOI] [PubMed] [Google Scholar]
- 59.Hikita A, Yana I, Wakeyama H, et al. Negative regulation of osteoclastogenesis by ectodomain shedding of receptor activator of NF-κB ligand. J Biol Chem. 2006;281:36846–36855. [DOI] [PubMed] [Google Scholar]
- 60.Akune T, Ohba S, Kamekura S, et al. PPAR γ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Investig. 2004;113:846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Z, Leslie J, Wong GW, Kahn B, Riddle R, Clemens T. Expression of Glucose Transporter-4 by the Osteoblast is Required for Global Glucose Metabolism In: J Bone Miner Res. Hoboken, NJ: Wiley-Blackwell; 2013. [Google Scholar]
- 62.Takada I, Suzawa M, Matsumoto K, Kato S. Suppression of PPAR transactivation switches cell fate of bone marrow stem cells from adipocytes into osteoblasts. Ann N Y Acad Sci. 2007;1116:182–195. [DOI] [PubMed] [Google Scholar]
- 63.Nakashima T, Kobayashi Y, Yamasaki S, et al. Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-κB ligand: modulation of the expression by osteotropic factors and cytokines. Biochem Biophys Res Commun. 2000;275:768–775. [DOI] [PubMed] [Google Scholar]
- 64.Eriksen EF. Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord. 2010;11:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA. Local communication on and within bone controls bone remodeling. Bone. 2009;44:1026–1033. [DOI] [PubMed] [Google Scholar]
- 66.Kartsogiannis V, Sims NA, Quinn JM, et al. Osteoclast inhibitory lectin, an immune cell product that is required for normal bone physiology in vivo. J Biol Chem. 2008;283:30850–30860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greenblatt MB, Shim J-H, Glimcher LH. Mitogen-activated protein kinase pathways in osteoblasts. Annu Rev Cell Dev Biol. 2013;29:63–79. [DOI] [PubMed] [Google Scholar]
- 68.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. [DOI] [PubMed] [Google Scholar]
- 69.Chang J, Wang Z, Tang E, et al. Inhibition of osteoblastic bone formation by nuclear factor-κB. Nat Med. 2009;15:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marie PJ. Osteoblast dysfunctions in bone diseases: from cellular and molecular mechanisms to therapeutic strategies. Cell Mol Life Sci. 2015;72:1347–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Helfrich MH, Stenbeck G, Nesbitt SA, Horton MA. Integrins and other cell surface attachment molecules of bone cells In: Bilezikian JP, Raisz LG, JohnMartin T, eds. Principles of Bone Biology (Third Edition). San Diego, CA: Elsevier; 2008:385–424. [Google Scholar]
- 72.Zimmerman D, Jin F, Leboy P, Hardy S, Damsky C. Impaired bone formation in transgenic mice resulting from altered integrin function in osteoblasts. Dev Biol. 2000;220:2–15. [DOI] [PubMed] [Google Scholar]
- 73.Clover J, Dodds R, Gowen M. Integrin subunit expression by human osteoblasts and osteoclasts in situ and in culture. J Cell Sci. 1992;103:267–271. [DOI] [PubMed] [Google Scholar]
- 74.Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ. Integrin expression and function on human osteoblast-like cells. J Bone Miner Res. 1997;12:1189–1197. [DOI] [PubMed] [Google Scholar]
- 75.Horton MA, Helfrich MH. Integrins and Development: Integrins in Skeletal Cell Function and Development; 2000.
- 76.Rao H, Lu G, Kajiya H, et al. α9β1: a novel osteoclast integrin that regulates osteoclast formation and function. J Bone Miner Res. 2006;21:1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Helfrich M, Nesbitt S, Lakkakorpi P, et al. β 1 integrins and osteoclast function: involvement in collagen recognition and bone resorption. Bone. 1996;19:317–328. [DOI] [PubMed] [Google Scholar]
- 78.Florencio-Silva R, Sasso GRDS, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int. 2015;2015:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Helfrich M, Nesbitt S, Lakkakorpi P, et al. Integrins and osteoclast function: involvement in collagen recognition and bone resorption. Bone. 1996;19:317–328. [DOI] [PubMed] [Google Scholar]
- 80.Liao W, Nguyen MA, Yoshizaki T, et al. Suppression of PPAR-γ attenuates insulin-stimulated glucose uptake by affecting both GLUT1 and GLUT4 in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2007;293:E219–E227. [DOI] [PubMed] [Google Scholar]
- 81.Armoni M, Harel C, Karnieli E. Transcriptional regulation of the GLUT4 gene: from PPAR-γ and FOXO1 to FFA and inflammation. Trends Endocrinol Metab. 2007;18:100–107. [DOI] [PubMed] [Google Scholar]
- 82.Amstrup AK, Sikjaer T, Pedersen SB, Heickendorff L, Mosekilde L, Rejnmark L. Reduced fat mass and increased lean mass in response to 1 year of melatonin treatment in postmenopausal women: a randomized placebo-controlled trial. Clin Endocrinol (Oxf). 2015;84:342–347. [DOI] [PubMed] [Google Scholar]
- 83.Lecka-Czernik B Bone loss in diabetes: use of antidiabetic thiazolidinediones and secondary osteoporosis. Curr Osteoporos Rep. 2010;8:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lecka-Czernik B, Suva LJ. Resolving the two “bony” faces of PPAR-γ. PPAR Res. 2006;2006:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saidak Z, Haÿ E, Marty C, Barbara A, Marie PJ. Strontium ranelate rebalances bone marrow adipogenesis and osteoblastogenesis in senescent osteopenic mice through NFATc/Maf and Wnt signaling. Aging Cell. 2012;11:467–474. [DOI] [PubMed] [Google Scholar]
- 86.Feskanich D, Hankinson SE, Schernhammer ES. Nightshift work and fracture risk: the Nurses’ Health Study. Osteoporos Int. 2009;20:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang K, Wu Y, Yang Y, et al. The associations of bedtime, nocturnal, and daytime sleep duration with bone mineral density in pre- and post-menopausal women. Endocrine. 2015;49:538–548. [DOI] [PubMed] [Google Scholar]
- 88.Quevedo I, Zuniga AM. Low bone mineral density in rotating-shift workers. J Clin Densitom. 2010;13:467–469. [DOI] [PubMed] [Google Scholar]
- 89.Kim BK, Choi YJ, Chung Y-S. Other than daytime working is associated with lower bone mineral density: The Korea National Health and Nutrition Examination Survey 2009. Calcif Tissue Int. 2013;93:495–501. [DOI] [PubMed] [Google Scholar]
- 90.Emaus N, Wilsgaard T, Ahmed LA. Impacts of body mass index, physical activity, and smoking on femoral bone loss: the Tromsø study. J Bone Miner Res. 2014;29:2080–2089. [DOI] [PubMed] [Google Scholar]
- 91.Labouesse MA, Gertz ER, Piccolo BD, et al. Associations among endocrine, inflammatory, and bone markers, body composition and weight loss induced bone loss. Bone. 2014;64:138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]