Abstract
Selectins are vascular adhesion molecules that mediate physiological responses such as inflammation, immunity and hemostasis. During cancer progression selectins promote various steps enabling the interactions between tumor cells and the blood constituents, including platelets, endothelial cells, and leukocytes. Selectins are carbohydrate-binding molecules that bind to sialylated, fucosylated glycan structures. The increased selectin ligand expression on tumor cells correlates with enhanced metastasis and poor prognosis for cancer patients. While, many studies focused on the role of selectin as a mediator of tumor cell adhesion and extravasation during metastasis, there is evidence for selectins to activate signaling cascade that regulates immune responses within a tumor microenvironment. L-selectin binding induces activation of leukocytes, which can be further modulated by selectin-mediated interactions with platelets and endothelial cells. Selectin ligand on leukocytes, PSGL-1, triggers intracellular signaling in leukocytes that are induced through platelet’s P-selectin or endothelial E-selectin binding. In this review I summarize the evidence for selectin-induced immune modulation in cancer progression that represents a possible target for controlling tumor immunity.
Introduction
Changes in cell-surface glycosylation is one of the hallmarks of malignant transformation and cancer progression (reviewed in Boligan et al. 2015, Hauselmann and Borsig 2014, Pinho and Reis 2015). Glycans are oligosaccharides that are covalently bound to the protein either through Asn (N-linked glycan) or through Ser or Thr (O-linked glycan) or to lipids. Altered cancer glycosylation is characterized by two principal modifications: a) enhanced branching and sialylation and; b) incomplete synthesis of glycans (Hauselmann and Borsig 2014, Pinho and Reis 2015). Upregulation of glycosyltransferases has been detected virtually in every cancer type and is associated with expression of common tumor cell epitopes such sialyl-Lewisx and sialyl-Lewisa (sLex/sLea), Thomsen-nouvelle antigen (Tn) and sialyl-Tn (sTn) (Fuster and Esko 2005, Hauselmann and Borsig 2014, Pinho and Reis 2015).
Cell-cell recognition, cell adhesion and mobility; and host-pathogen interactions are facilitated by lectins in healthy organisms. There is accumulating evidence that glycans also modulate immune responses during inflammation, infection and malignancy (Boligan et al. 2015, Pearce and Laubli 2016). The ubiquitous expression of lectins on immune cells, endothelial cells or as soluble molecules enables them to bind to glycans on tumors cells and thereby regulate tumor cell progression. Cancer-associated glycosylation is recognized by the lectin family of Siglecs (covered by a separate article in this issue) and Galectins, but there is increasing evidence that also selectins and their ligands actively contribute to tumor immunity.
Cancer immunity
Recent progress in cancer immunotherapy showed that stimulation of the immune system can lead to anti-tumor response and elimination of tumor cells. The so-called cancer immunity cycle describes the steps leading to and effective anti-cancer immune response (Chen and Mellman 2013). This cycle begins by tumor antigens release, their effective presentation on antigen-presenting cells, T cell priming, effective T-cell recruitment to tumor sites, recognition of the tumor cells and finally their elimination. In addition, cancer progression induces an immunosuppressive tumor microenvironment. Selectins were shown to mediate T cell recruitment both to the lymph node and to tumor sites, but also myeloid-derived cells to tumors (Chen and Mellman 2013, Läubli and Borsig 2010, Ley 2003, Rosen 2004). Thus, selectins represent another potential arm to modulate immune responses during cancer progression.
Selectins – vascular cell adhesion molecules
The selectins are a family of three C-type lectins expressed by bone-marrow-derived cells and endothelial cells. These vascular cell adhesion molecules are identified as L-selectin expressed on leukocytes, E-selectin expressed on endothelial cells ad P-selectin expressed on platelet and endothelial cells. All three selectins have a similar structure consisting of an amino-terminal lectin domain, one EGF-like domain, several consensus repeats, a single transmembrane domains and a carboxy-terminal cytoplasmic domain (Kansas 1996, Ley 2003). The main physiological function of all selectins is to mediate leukocyte recruitment to sites of inflammation or to lymphoid tissues. Importantly, there is evidence that selectins and selectin ligands also have signaling functions (Crockett-Torabi 1998, Läubli and Borsig 2010, Zarbock et al. 2009). P-selectin is found in secretory granules of platelets (α-granules) and endothelial cells (Weibel-Palade bodies) and upon activation is present on the surface of platelets and endothelial cells. E-selectin expression on activated endothelial cells requires de novo transcription, thus occurs several hours after stimulation and persists for a longer time period as observed in chronic inflammation. In addition, E-selectin is expressed in quiescent, non-inflamed, skin microvessels and in parts of the bone marrow microvasculature (Keelan et al. 1994, Sipkins et al. 2005). L-selectin is expressed by all myeloid-derived cells, naïve T cells, and some memory T cells (Kansas 1996, Sallusto et al. 1999). In addition, L-selectin has been detected in a non-vascular compartment on trophoblasts, mediating the adhesion in the uterus during placental development (Prakobphol et al. 2006).
Selectins mediate interactions among leukocytes, platelets and the endothelial cells in various physiological and pathophysiological situations, including inflammation and cancer. All selectins have been shown to contribute to metastasis through the recruitment of myeloid-derived cells (Borsig et al. 2002, Hauselmann et al. 2016, Läubli et al. 2006). P-selectin-mediated platelet-tumor cell interactions promote tumor cell adhesion, L-selectin facilitates myeloid-derived cells recruitment, and together with E-selectin contribute to efficient tumor cell extravasation (reviewed in Läubli and Borsig 2010). The extent of selectin contribution to cancer progression is defined by temporal and special presence not only of selectin but also selectin ligands.
Selectin ligands in cancer
Selectin ligands are glycan structures that are formed by post-translational modification of scaffold proteins by glycosyltransferases (Kansas 1996, Rosen 2004, Varki 1997). The minimal recognition motif recognized by all three selectins is a terminal tetrasaccharide sialyl-Lewisx (sLex): Siaα2,3-Galβ1,4-(Fucα1,3-)GlcNAcβ1-, and its isomer sialyl-Lewisa (sLea): Siaα2,3-Galβ1,3-(Fucα1,4-)GlcNAcβ1- that are synthesized by α1,3-fucosyltransferases, FucTIV and VII; α2,3-sialyltransferases, β1,4-galactosyltransferase GalT-I, and β1-glucosaminyltransferases (Kansas 1996, Rosen 2004). Carbohydrates must be presented on specialized protein scaffolds that enhance ligand clustering, which enables effective selectin binding (Varki 1997). P-selectin glycoprotein ligand- 1 (PSGL-1) is the best characterized selectin ligand for all three selectins. For P-selectin high-affinity binding, sulfated tyrosine are required in a close proximity to the glycan structure carrying sLex structure. Further modification of the sLex structure by sulfation either on galactose or N-acetylglucosamine, 6-sulfo-SLex, increases the L-selectin affinity. Natural ligands for L-selectin are presented in the lymph nodes and belong to the family of peripheral node addressins (Ley and Kansas 2004, Rosen 2004). In addition, L-selectin binds to endothelial heparan sulfate and mediates neutrophil recruitment during inflammation (Wang, L. et al. 2005). E-selectin binds to various ligands, including PSGL-1, ESL-1, CD44 and CD34.
There is compelling clinical and experimental evidence that enhanced expression of sLex and sLea correlates with poor prognosis due to enhanced metastasis in a number of tumor types, including colon, gastric, prostate, renal, pancreatic and lung cancer (reviewed in Hauselmann and Borsig 2014, Kannagi 2004, Kim and Varki 1997). Major carriers of altered glycosylation in tumors and particularly carcinomas are mucins. Mucins are large glycoproteins carrying O-glycans attached to Ser or Thr in tandem repeat sequences. Mucins as carriers of selectin ligands associated with cancer progression are MUC1, MUC2 MUC4 and MUC16 (Hollingsworth and Swanson 2004). Also other selectin ligands has been detected during cancer progression on properly glycosylated proteins including CD44, CD24, ESL-1, PSGL-1, death receptor-3 and podocalyxin-like proteins while this list is still growing (Aigner et al. 1997, Burdick et al. 2006, Dimitroff et al. 2005, Gout et al. 2006, Thomas et al. 2009).
Enhanced selectin ligand expression on tumor cells is clearly associated with cancer progression and enables the contact with blood constituents (platelets, leukocytes and the endothelium), particularly during metastatic dissemination (Läubli and Borsig 2010, Witz 2008). However; recent findings indicate that endogenous selectin ligands on non-malignant cells are required for metastasis (Hauselmann et al. 2016, Hoos et al. 2014). Hence, the normal physiological selectin function seems to be “hijacked” during cancer progression and represents an additional mechanism how selectin modulate to cancer progression.
Selectins shape up the tumor microenvironment
Selectin-dependent recruitment of leukocytes to tumor sites enhances different steps of metastasis including immune invasion, dissemination, extravasation and formation of a metastatic niche (Borsig et al. 2002, Hauselmann et al. 2016, Hoos et al. 2014, Ku et al. 2016, Läubli et al. 2006). The use of selectin-deficient mice in a number of different cancer models have shown that selectin-mediated recruitment of myeloid-derived cells (CD11b+Ly6C+Ly6G+) supports tumor metastasis (Borsig et al. 2002, Hauselmann et al. 2016, Läubli et al. 2006). Elevated levels of selectin ligands by carcinomas are associated with poor prognosis (reviewed in Hauselmann and Borsig 2014, Kim and Varki 1997, Pinho and Reis 2015). Selectin-based cell-cell interactions of tumor cells with leukocytes, platelet and endothelial cells have been observed in different animal models and appear to be critical for cancer progression (Hauselmann and Borsig 2014, Krause and Turner 1999, Pinho and Reis 2015). These findings suggest that selectins support tumor progression mainly through the cooption of the inflammatory pathways (Läubli and Borsig 2010). Thus, selectins significantly contribute to the formation and maintenance of the tumor microenvironment (Figure 1).
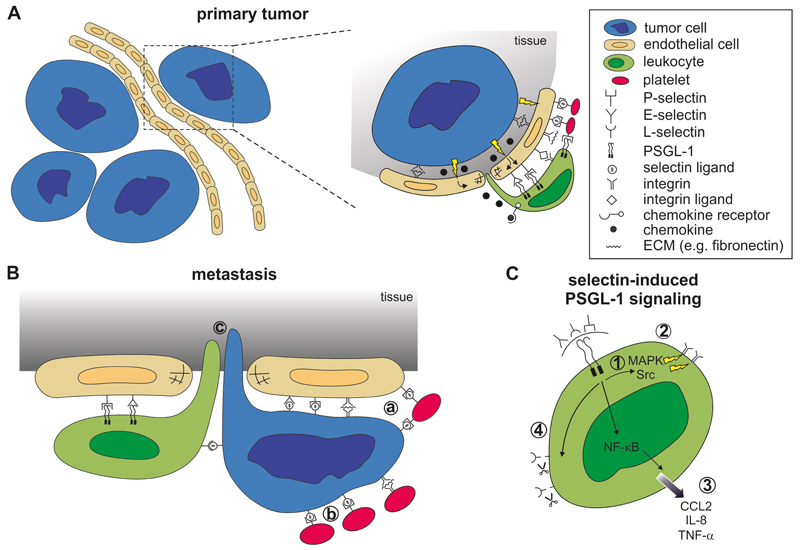
Figure 1. Selectin-dependent signaling during cancer progression.
A) Primary tumor sites: Tumor cells produce various cytokines leading to endothelial activation that result in a leaky vasculature. Tumor-derived chemokines facilitate leukocyte recruitment and their extravasation. Leukocytes expressing PSGL-1 infiltrate tumors through binding to vascular selectins (P- and E-selectin). Platelets further promote leukocyte adhesion through P-selectin-mediated interactions. B) Metastatic sites: Selectins promote tumor cell-endothelial interaction and the recruitment of leukocytes. a: Platelet binding to tumor cells and to the endothelium promote tumor cell adhesion; b: Platelet-tumor cell interactions are largely facilitated by P-selectin; c: Selectin-triggered endothelial activation leads to leukocyte-assisted tumor cell extravasation. C) The intracellular signaling in leukocytes is initiated through selectin-binding to PSGL-1 on leukocytes resulting in 1: activation of MAPK and src kinase pathways; 2: activation of integrins; 3: activation of NF-κB pathways and secretion of cytokines (e.g. CCL2, IL-8 or TNF-α; 4: shedding of cell surface L-selectin.
Local activation of the metastatic niche by tumor cells, together with all blood components, resulted in enhanced expression of E-selectin and increased production of CCL5 chemokine (Läubli and Borsig 2009). CCL5 enhanced monocyte recruitment to the site of metastatic extravasation, indicating that the selectin-mediated leukocyte recruitment is further supported by chemokine-driven mechanisms. This finding is in line with previous observations that the neutrophil recruitment was completely blocked only by the combination of E-selectin inhibitor and an inhibitor of Gα protein signaling used by most chemokine receptors (Smith et al. 2004). Tumor-induced endothelial activation during metastasis induces selectin ligand expression that contributes to L-selectin dependent recruitment of myeloid-derived cells CD11b+ (Läubli et al. 2006). The absence of selectin ligands in non-hematopoietic cells resulted in decreased chemokine levels, reduced inflammatory monocytes (Ly6Chi cells), and decreased lung metastasis (Hoos et al. 2014). Further investigations showed that an engagement of E-selectin on endothelial cells by Ly6Chi cells is essential for loosening of endothelial VE-cadherin-based junctions and monocyte-assisted transendothelial migration of tumor cells in the lungs (Hauselmann et al. 2016). In addition, activation of extracellular signal-regulated kinase (ERK) by E-selectin facilitates the opening of endothelial junctions by Src kinase activation and dissociation of VE-cadherin/β-catenin complex (Tremblay et al. 2006). Neutrophil-derived TNF-α significantly up-regulate selectin ligand expression on non-small cell lung cancer cells (St Hill et al. 2011). As a result, TNF-α stimulation also increased tumor cell adhesion to E-selectin. Taken together, these data support the essential role of selectins in the recruitment of innate immune cells during metastatic colonization.
Selectins modulate immune responses
Selectin-mediated interactions promote leukocyte recruitment to the site of inflammation or cancer progression. The involvement of selectin-induced signal transduction in immune modulation has just been proposed (Läubli and Borsig 2010, Ley 2003, Rosen 2004, Zarbock et al. 2009). There are two major pathways regulating immune responses: a) the engagement of PSGL-1 on leukocytes; b) the L-selectin-dependent leukocyte activation.
PSGL-1
Selectin binding to PSGL-1 on leukocytes induces activation of several signaling pathways (Figure 1C), which is mediated through the cytoplasmic domain of PSGL-1 interacting both with cytoskeletal and signaling proteins (reviewed in Zarbock et al. 2009). Deletion of cytoplasmic domain of PSGL-1 did not alter leukocyte rolling, but was found to be essential for activation of β2 integrins (Miner et al. 2008). The ligation of the extracellular domain of PSGL-1 induces downstream signaling through the 69 amino acid long cytoplasmic tail, which interacts with the ezrin-radixin-moesin (ERM) protein complex and activate spleen tyrosine kinase (Syk) (Urzainqui et al. 2002). Syk is required for integrin activation after E-selectin or P-selectin binding to PSGL-1 on neutrophils (Zarbock et al. 2007). In addition, TNF-α triggers neutrophil adhesion on E-selectin through CXCR2 expression. Leukocyte-derived CXCL1 actively induces neutrophils arrest, while the inhibition of CXCR2 actively inhibited this process (Zarbock et al. 2007). The ERM-proteins contain an immunoreceptor tyrosine-like activation motif (ITAM)-like domain, which leads to activation of a transcriptional activity (Urzainqui et al. 2002). Mutation of the Syk binding site on moesin resulted in reduced transcriptional activity upon ligation of PSGL-1.
In other studies, extracellular activation of PSGL-1 has led to tyrosine phosphorylation which further activated MAPK and Src kinase signaling pathways in neutrophils (Hidari et al. 1997, Wang, H. B. et al. 2007). Adhesion of human monocytes to P-selectin enhanced nuclear translocation of nuclear factor-kappa B (NF-κB), a transcription factor required for expression of CCL2, TNF-α and IL-8 (Weyrich et al. 1995). Blocking of PSGL-1 on monocytes resulted in inhibition of cytokine secretion. P-selectin binding to PSGL-1 on leukocytes activates their integrins through the Src kinase family, Nef-associated factors 1 and phosphoinositide-3-OH-kinase (PI3-kinase) signal transduction pathways (Evangelista et al. 2007, Wang, H. B. et al. 2007).
Recent studies confirmed the PSGL-1-induced signaling in vivo (Sreeramkumar et al. 2014, Zuchtriegel et al. 2016). During inflammation, platelets through P-selectin bind to PSGL-1 on leukocytes that promote the recruitment of neutrophils and inflammatory monocytes to the extravasation sites in the venular microvessels (Zuchtriegel et al. 2016). PSGL-1-transduced signals resulted in a redistribution of adhesion receptors that drive neutrophil migration (Sreeramkumar et al. 2014). In the absence of PSGL-1, neutrophils are unable to polarize their adhesion receptors (e.g. integrins) and display aberrant crawling with a subsequent extravasation blockade. Specifically, ligation of PSGL-1 by P-selectin induced the high-affinity conformation of β2 integrins on the surface of neutrophils and inflammatory monocytes via ERK1/2 MAPK-dependent signaling events (Zuchtriegel et al. 2016). The binding of platelets to monocytes via P-selectin-PSGL-1 interactions was shown to increase expression and activity of α4β1- and αMβ2-integrins, which increases the binding to the endothelium and transendothelial migration of monocytes (da Costa Martins et al. 2006). Importantly, PSGL-1 mediated monocyte activation correlated with a concomitant decrease of L-selectin expression. Proteolytic cleavage of L-selectin is relevant for the physiologic leukocyte rolling, then the inhibition of L-selectin shedding significantly decreased the rolling velocity (Hafezi-Moghadam and Ley 1999). Blocking of homeostatic L-selectin shedding resulted in an increased recruitment of neutrophils to the inflamed tissues, thereby affecting migration patterns in vivo (Venturi et al. 2003).
L-selectin controls the capacity for naïve T cells to migrate to the lymph nodes (LN), whereas P- and E-selectin capture activated T cells on activated inflamed endothelium to initiate their migration into non-lymphoid tissues (Hobbs and Nolz 2017). The capacity of T cells to interact with vascular selectins is dependent on the enzymatic synthesis of complex O-glycans, thus making this cell surface modification indispensable for T cell homing (Ebel et al. 2015). Six different cytokines that affect T cell differentiation, including IL-12 IL-18, IL-25 and TGF-β, were shown to induce selectin ligand expression above the levels induced by TCR engagement. The selectin ligand expression was blocked by inhibition of p38 MAPK, which has been identified to be required for Fut7 and CgNt1 expression, both enzymes critical for production of sLex (Ebel et al. 2015). This highly flexible O-glycan synthesis in T cells is in contrast to myeloid cells with minimal changes in glycosylation (Hobbs and Nolz 2017) and represents yet another level of selectin-dependent leukocyte activation in T-cells.
PSGL-1 has several functions beyond mediating selectin-dependent leukocyte recruitment, including modulation of T cells and interactions with CCL19 and CCL21 chemokines (Tinoco et al. 2016, Veerman et al. 2007). T cell entry to secondary lymphoid organs is promoted by PSGL-1 interaction with CCL19 and CCL21. The recent evidence shows that PSGL-1 is a check-point regulator of T cells where PSGL-1 engagement leads to T-cell exhaustion (Tinoco et al. 2016). Ligation of PSGL-1 on CD8+ cells inhibited IL-2 and T cell receptor signaling and upregulated programmed cell death expression protein 1 (PD-1). PSGL-1 deficiency in a melanoma model led to improved T cell responses and tumor control due to downregulation of PD-1 (Tinoco et al. 2016). Tumor infiltrating effector CD8+ and CD4+ T cells were significantly increased in PSGL-1-deficient mice. Interestingly, neither selectin blockade nor deficiency in CCL19 and CCL21 alter CD8+ T cell numbers or PD-1 expression, thus the immune regulation through PSGL-1 is mediated by a presently unknown ligand.
L-selectin
Initial observations that antibody-based cross-linking of L-selectin on neutrophils primed these cells and induced an up-regulation of cell surface expression of Mac-1 epitope, a αMβ2 integrin (Crockett-Torabi et al. 1995) provided the first evidence that L-selectin activation may induce intracellular signaling cascade. Another study demonstrated that L-selectin cross-linking increased L-selectin adhesion and transmigration efficiency of neutrophil through endothelial cells (Simon et al. 1995). Further analysis of neutrophils revealed that L-selectin activation increased intracellular calcium levels in a dose dependent manner (Crockett-Torabi et al. 1995, Laudanna et al. 1994, Waddell et al. 1994) and led to respiratory burst and O2- generation (Crockett-Torabi et al. 1995, Waddell et al. 1994). Induced expression of TNF-α and IL-8 cytokines was observed in L-selectin-activated neutrophils with a sulfatide; 3-O-sulfogalactosylceramide (Laudanna et al. 1994). Finally, L-selectin clustering on the cell surface of neutrophils activates p38 mitogen-activated protein kinase to induce shape change, integrin activation and release of secretory granules (Smolen et al. 2000). Further study showed that L-selectin triggering on human T cell line, Jurkat cells, stimulated Ras pathway that required presence of a functional tyrosin kinase p56 (Brenner et al. 1996). Phosphorylation of the cytoplasmic tail of L-selectin leads to a release of calmodulin that occurs during shedding of the L-selectin (Kahn et al. 1998). Taken together, these in vitro studies have shown that L-selectin triggers functional responses (Crockett-Torabi 1998, Ivetic 2013), however; L-selectin activation in these studies was not triggered by selectin ligands.
The biological relevance of L-selectin endoproteolytic cleavage has been studies in transgenic mice, with mutation in the cleavage site that was blocking L-selectin shedding or using defective in tumor necrosis factor α-converting enzyme (ADAM 17) deficient mice (Venturi et al. 2003, Walcheck et al. 2003). Although upon stimulation L-selectin shedding occurred also in ADAM-17-deficient leukocytes, the ADAM-17 reconstitution significantly increased the turnover of L-selectin (Walcheck et al. 2003). Blocking the cleavage of T cells on antigen-stimulated T cells resulted in their accumulation in the LN, while activation in wild type cells showed a significant decrease in L-selectin (Venturi et al. 2003). L-selectin cleavage regulates activation-induced changes in L-selectin density. Neutrophils in cleavage-resistant mice centered the inflamed peritoneum in greater numbers and for longer period that wt neutrophils, which shed off L-selectin rapidly upon migration (Venturi et al. 2003). Rapid metalloproteinase-dependent shedding of L-selectin occurred upon TCR engagement on antigen-activated T cells (Galkina et al. 2003). While shedding-resistant cells continued to migrate to LNs, L-selectin shedding from antigen-activated T cells prevented reentry to LNs. Thus, L-selectin shedding controls the capacity of activated T cells to migrate to peripheral LNs (Galkina et al. 2003, Venturi et al. 2003). L-selectin shedding is directly linked to trans-endothelial migration of monocytes, since L-selectin is effectively removed from the transmigrating pseudopods of migrating cells (Rzeniewicz et al. 2015). Calmodulin/L-selectin interaction, which acts to block shedding, is lost through Ser phosphorylation of the L-selectin cytoplasmic tail, occurring specifically within transmigrating pseudopods. Hence, L-selectin shedding directly regulates polarity in transmigrated monocytes through activated endothelial cells (Rzeniewicz et al. 2015). L-selectin expression is reduced in lymphatic cells, upon arrival to the LNs, but CD8+ T-cells re-express L-selectin upon reentering the circulation and the recruitment of CD8+ cells to infected tissues is L-selectin dependent (Mohammed et al. 2016). Importantly, L-selectin expression of T cells confers protective immunity.
The current evidence for the L-selectin involvement in immune modulation is mostly based on various inflammatory and infectious models. While many parallels between inflammation and cancer progression has been described, it remains to be tested to which extent selectin-mediated modulation of immune responses contribute to cancer progression.
L-selectin modulate adaptive and innate immunity in cancer
The capacity of leukocyte to either promote or control cancer progression largely depends on spatial and temporal stimulation of the tumor microenvironment (Coussens and Werb 2002, Mantovani et al. 2008). This modulation of cancer progression depends on the recruitment of leukocytes and their activation status. While several adhesion mechanisms have been implicated in leukocyte recruitment (e.g. chemokine, integrins, and cell-surface ligand presentation), selectin-mediated recruitment has been observed in many preclinical models. L-selectin-mediated leukocyte recruitment to tumor or to metastatic sites represents one possible mechanism that has been explored mostly using L-selectin deficient mice. Several studies have shown that L-selectin dependent monocytes recruitment promoted lung metastasis through enhancement of tumor cell extravasation (Borsig et al. 2002, Hoos et al. 2012, Läubli et al. 2006). Recently, the use of spontaneous metastatic model resulted in reduced lung metastasis, but an increase in metastasis to other organs (L.B. manuscript in preparation). The apparent tissue-dependent differences in modulation of cancer progression through L-selectin have been linked to recruitment of distinct leukocyte subpopulation.
Myeloid-derived suppressor cells (MDSCs) accumulate in most cancer patients and also in experimental animals with cancer. MDSCs suppress both innate and adaptive antitumor immunity by various mechanisms including sequestration of cysteine and down-regulation of L-selectin (Ostrand-Rosenberg 2010). Antigen-naïve T cells upon encounter of antigens become activated and are directed to the LN in an L-selectin-dependent manner. During cancer, MDSCs actively suppress L-selectin presence on T cells as has been shown by co-culture of CD4+ and CD8+ cells with MDSCs (Hanson et al. 2009). In a mouse model surgical removal of a tumor is associated with a reduction of MDSC levels and an increased expression of L-selectin on circulating T cells. Initial investigations suggested that L-selectin shedding is dependent on surface expression of ADAM17 on MDSCs (Hanson et al. 2009, Ostrand-Rosenberg 2010). The use of ADAM17-deficient mice showed reduced L-selectin levels in tumor-bearing nice when compared to naïve animals, thus providing evidence that down-regulation of L-selectin on T and B cells occurs also in a ADAM17 independent manner (Ku et al. 2016). Importantly, the loss of L-selectin on T cells occurs within the MDSC-enriched blood compartment and in a contact-dependent manner, although the exact mechanism remains unclear. Even moderate reduction in L-selectin expression impaired T-cell trafficking to peripheral LNs (Ku et al. 2016). Consequently, T cells preconditioned with MDSCs have diminished responses to subsequent antigen exposure, thereby severely restricting antigen-driven cell expansion in the LNs.
The regulation of L-selectin expression is essential for controlling the traffic of T lymphocytes to and from the peripheral LNs. To evaluate the relationship between L-selectin shedding and T cell activation, an in vitro anti-tumor antigen generated T cells were assessed with a panel of melanoma cells (Yang et al. 2011). In the human system, the encounter of T cell with tumor cells has led to a rapid decrease of L-selectin from the cell surface, which correlated with an increased expression of a lysosomal-associated membrane protein-1 (CD107a). CD107a serves as a marker of T-cell degranulation and release of granzyme B and perforin, reflecting the lytic potential of these cells. This observation suggests that antigen-dependent T cell stimulation leads to L-selectin shedding. In another study, ex vivo cultured murine lymphocytes used for adoptive immunotherapy to treat B16 melanoma in mice showed that early effector T cells controlled much better the melanoma growth than more differentiated effector T cells with enhanced in vitro antitumor properties (Gattinoni et al. 2005). There was a strong difference in L-selectin expression, which was significantly reduced in more differentiated, but less potent CD8 T cells in vivo. While the immune system balances T cell homing and effector function of T cells, these data suggest that L-selectin is involved in acquisition of an effector function, although the exact mechanism remains to be defined.
In hematogenous malignancies, tumor cells not only express selectin ligands, but also express L-selectin itself. L-selectin on the surface of leukemia cells can affect their trafficking and distribution. In patients with chronic lymphocytic leukemia (CLL), tumor cells are re-circulating to LN through L-selectin mediated rolling, which promotes cancer progression (Lafouresse et al. 2015). Interestingly, a clinically approved inhibitor for treatment of CLL, a PI3-kinase delta inhibitor, Idelalisib, decreased L-selectin expression. This might be one possible mechanism how this drug is interfering with CLL progression (Lafouresse et al. 2015). However, L-selectin expression on B-cell lymphoma cell line was not associated with LN metastasis (Aviram et al. 2001). Further studies will be required to assess the involvement of L-selectin in hematogenous malignancies.
The newly described mechanism of adoptive immunity modulation through MDSCs that is dependent on down regulation of L-selectin has further implications for systemic cancer immunity. Since virtually all leukocyte population express L-selectin, the involvement of L-selectin in regulation of innate and adoptive immune cells guarantees further investigations.
Conclusions and outlook
Selectins expressed on leukocytes, platelets and endothelial cells are among the first adhesion molecules that induce intracelullar signaling once binding to selectin ligands. The virtual omnipresence of selectin ligands on tumor cells is linked to cancer-associated aberrant glycosylation (Boligan et al. 2015, Hauselmann and Borsig 2014, Pinho and Reis 2015) and makes selectins potential targets for cancer therapy. In addition, other lectin binding molecules, such as Siglecs, have been described to modulate immune responses during cancer and are covered in this issue (Läubli and von Gunten article in this issue). Cancer immunotherapy, particularly focusing on PD-1 and CTLA-4 pathways, is actively being pursued also in the clinical settings (reviewed in Boussiotis 2016, Burugu et al. 2017). However; the potential to target tumor glycans-induced interactions, e.g. through selectins, has not be explored. Yet, several studies describe mechanisms how selectins modulate immune responses that have the potential to be therapeutically targeted (Dinkla et al. 2016, Shamay et al. 2016, Tian et al. 2016). Differentiation of regulatory T cells (Treg) into IL-17 producing cells was inhibited by presence of platelet microparticles (Dinkla et al. 2016). This inhibition of Treg plasticity was dependent on P-selectin binding to PSGL-1 specifically on memory-like Treg cells. Blocking of P-selectin restored Treg differentiation and L-selectin expression. In addition, platelets microparticles further modulated Treg differentiation through platelet-derived CXCL4 binding to CXCR3 on leukocytes (Dinkla et al. 2016). Natural killer cells expressing L-selectin has been linked to persistence of these cells in tumors, where they control tumor progression (Tian et al. 2016). Thus, an enrichment of natural killer T cells expressing L-selectin is a target for an efficient cancer immunotherapy. Finally, P-selectin ligands have been used for specific targeting of MEK kinase inhibitor to metastatic tumors that resulted in tumor cell apoptosis (Shamay et al. 2016). Interestingly, tumors are specifically targeted by fucosylated polysaccharide coated nanoparticles carrying the inhibitor through an ionizing radiation that induces the local endothelial activation, associated with P-selectin expression.
Selectin has been studied in a number of pathological situations, e.g. ischemia-reperfusion injury, inflammation etc. (Ivetic 2013, Ley 2003, McEver 2015), providing the rationale for development of selectin inhibitors. Abundant experimental evidence suggests that selectin targeting inhibits metastasis (Barthel et al. 2007, Läubli and Borsig 2010, St Hill 2011). To which extent the observed inhibition of metastasis is mediated by alteration of immune responses through selectin remains to be further evaluated. Interestingly, the treatment of cancer patients with heparin, as an anticoagulant, inadvertently inhibited also P- and L-selectins (Borsig et al. 2007). The inherited tumor heterogeneity includes also the composition of differentially activated immune cells represents the major challenge for development of an approach to modulate immune responses. Since altered cancer glycosylation is a common culprit in tumors, the understanding of lectin-mediated mechanisms involved in immune responses, including selectins and Siglecs, represents another strategy to target cancer progression.
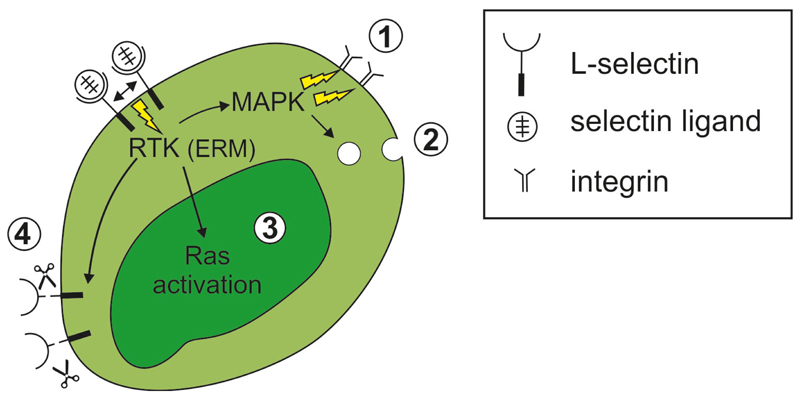
Figure 2. L-selectin-triggered intracellular signaling in leukocytes.
L-selectin binding to ligands causes selectin clustering, which induces receptor tyrosine and serine phosphorylation and activation of the ERM proteins. This leukocyte activation further results in: 1 integrin activation through MAPK kinase; 2 leukocyte degranulation, particularly by neutrophils; 3 activation of the ras pathway; 4 L-selectin shedding, which modulates immune responsiveness of leukocytes.
Acknowledgements
I would like to thank C. Gasser for graphical preparation of figures. This work was supported by the SNF grant #310030-173076.
References
- Aigner S, Sthoeger ZM, Fogel M, Weber E, Zarn J, Ruppert M, Zeller Y, Vestweber D, Stahel R, Sammar M, et al. CD24, a mucin-type glycoprotein, is a ligand for P-selectin on human tumor cells. Blood. 1997;89:3385–3395. [PubMed] [Google Scholar]
- Aviram R, Raz N, Kukulansky T, Hollander N. Expression of L-selectin and efficient binding to high endothelial venules do not modulate the dissemination potential of murine B-cell lymphoma. Cancer Immunol Immunother. 2001;50:61–68. doi: 10.1007/PL00006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets. 2007;11:1473–1491. doi: 10.1517/14728222.11.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boligan KF, Mesa C, Fernandez LE, von Gunten S. Cancer intelligence acquired (CIA): tumor glycosylation and sialylation codes dismantling antitumor defense. Cell Mol Life Sci. 2015;72:1231–1248. doi: 10.1007/s00018-014-1799-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsig L, Stevenson JL, Varki A. Heparin in Cancer: Role of Selectin Interactions. In: Khorana AA, Francis CW, editors. Cancer-Associated Thrombosis. New York: Informa Healthcare; 2007. pp. 97–113. [Google Scholar]
- Borsig L, Wong R, Hynes RO, Varki NM, Varki A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc Natl Acad Sci U S A. 2002;99:2193–2198. doi: 10.1073/pnas.261704098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med. 2016;375:1767–1778. doi: 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B, Gulbins E, Schlottmann K, Koppenhoefer U, Busch GL, Walzog B, Steinhausen M, Coggeshall KM, Linderkamp O, Lang F. L-selectin activates the Ras pathway via the tyrosine kinase p56lck. Proc Natl Acad Sci U S A. 1996;93:15376–15381. doi: 10.1073/pnas.93.26.15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick MM, Chu JT, Godar S, Sackstein R. HCELL is the major E- and L-selectin ligand expressed on LS174T colon carcinoma cells. J Biol Chem. 2006;281:13899–13905. doi: 10.1074/jbc.M513617200. [DOI] [PubMed] [Google Scholar]
- Burugu S, Dancsok AR, Nielsen TO. Emerging targets in cancer immunotherapy. Semin Cancer Biol. 2017 doi: 10.1016/j.semcancer.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett-Torabi E. Selectins and mechanisms of signal transduction. J Leukoc Biol. 1998;63:1–14. [PubMed] [Google Scholar]
- Crockett-Torabi E, Sulenbarger B, Smith CW, Fantone JC. Activation of human neutrophils through L-selectin and Mac-1 molecules. J Immunol. 1995;154:2291–2302. [PubMed] [Google Scholar]
- da Costa Martins PA, van Gils JM, Mol A, Hordijk PL, Zwaginga JJ. Platelet binding to monocytes increases the adhesive properties of monocytes by up-regulating the expression and functionality of beta1 and beta2 integrins. J Leukoc Biol. 2006;79:499–507. doi: 10.1189/jlb.0605318. [DOI] [PubMed] [Google Scholar]
- Dimitroff CJ, Descheny L, Trujillo N, Kim R, Nguyen V, Huang W, Pienta KJ, Kutok JL, Rubin MA. Identification of leukocyte E-selectin ligands, P-selectin glycoprotein ligand-1 and E-selectin ligand-1, on human metastatic prostate tumor cells. Cancer Res. 2005;65:5750–5760. doi: 10.1158/0008-5472.CAN-04-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkla S, van Cranenbroek B, van der Heijden WA, He X, Wallbrecher R, Dumitriu IE, van der Ven AJ, Bosman GJ, Koenen HJ, Joosten I. Platelet microparticles inhibit IL-17 production by regulatory T cells through P-selectin. Blood. 2016;127:1976–1986. doi: 10.1182/blood-2015-04-640300. [DOI] [PubMed] [Google Scholar]
- Ebel ME, Awe O, Kaplan MH, Kansas GS. Diverse inflammatory cytokines induce selectin ligand expression on murine CD4 T cells via p38alpha MAPK. J Immunol. 2015;194:5781–5788. doi: 10.4049/jimmunol.1500485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista V, Pamuklar Z, Piccoli A, Manarini S, Dell'elba G, Pecce R, Martelli N, Federico L, Rojas M, Berton G, et al. Src family kinases mediate neutrophil adhesion to adherent platelets. Blood. 2007;109:2461–2469. doi: 10.1182/blood-2006-06-029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5:526–542. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- Galkina E, Tanousis K, Preece G, Tolaini M, Kioussis D, Florey O, Haskard DO, Tedder TF, Ager A. L-selectin shedding does not regulate constitutive T cell trafficking but controls the migration pathways of antigen-activated T lymphocytes. J Exp Med. 2003;198:1323–1335. doi: 10.1084/jem.20030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout S, Morin C, Houle F, Huot J. Death receptor-3, a new E-Selectin counter-receptor that confers migration and survival advantages to colon carcinoma cells by triggering p38 and ERK MAPK activation. Cancer Res. 2006;66:9117–9124. doi: 10.1158/0008-5472.CAN-05-4605. [DOI] [PubMed] [Google Scholar]
- Hafezi-Moghadam A, Ley K. Relevance of L-selectin shedding for leukocyte rolling in vivo. J Exp Med. 1999;189:939–948. doi: 10.1084/jem.189.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–944. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauselmann I, Borsig L. Altered tumor-cell glycosylation promotes metastasis. Front Oncol. 2014;4:28. doi: 10.3389/fonc.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauselmann I, Roblek M, Protsyuk D, Huck V, Knopfova L, Grassle S, Bauer AT, Schneider SW, Borsig L. Monocyte Induction of E-Selectin-Mediated Endothelial Activation Releases VE-Cadherin Junctions to Promote Tumor Cell Extravasation in the Metastasis Cascade. Cancer Res. 2016;76:5302–5312. doi: 10.1158/0008-5472.CAN-16-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidari KI, Weyrich AS, Zimmerman GA, McEver RP. Engagement of P-selectin glycoprotein ligand-1 enhances tyrosine phosphorylation and activates mitogen-activated protein kinases in human neutrophils. J Biol Chem. 1997;272:28750–28756. doi: 10.1074/jbc.272.45.28750. [DOI] [PubMed] [Google Scholar]
- Hobbs SJ, Nolz JC. Regulation of T Cell Trafficking by Enzymatic Synthesis of O-Glycans. Front Immunol. 2017;8:600. doi: 10.3389/fimmu.2017.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Hoos A, Protsyuk D, Borsig L. Metastatic growth progression caused by PSGL-1-mediated recruitment of monocytes to metastatic sites. Cancer Res. 2014;74:695–704. doi: 10.1158/0008-5472.CAN-13-0946. [DOI] [PubMed] [Google Scholar]
- Hoos A, Wolf MJ, Bauer J, Borsig L, Heikenwalder M. Endothelial chemokine receptors as facilitators of tumor cell extravasation? Oncotarget. 2012;3:919–920. doi: 10.18632/oncotarget.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivetic A. Signals regulating L-selectin-dependent leucocyte adhesion and transmigration. Int J Biochem Cell Biol. 2013;45:550–555. doi: 10.1016/j.biocel.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Kahn J, Walcheck B, Migaki GI, Jutila MA, Kishimoto TK. Calmodulin regulates L-selectin adhesion molecule expression and function through a protease-dependent mechanism. Cell. 1998;92:809–818. doi: 10.1016/s0092-8674(00)81408-7. [DOI] [PubMed] [Google Scholar]
- Kannagi R. Molecular mechanism for cancer-associated induction of sialyl Lewis X and sialyl Lewis A expression-The Warburg effect revisited. Glycoconj J. 2004;20:353–364. doi: 10.1023/B:GLYC.0000033631.35357.41. [DOI] [PubMed] [Google Scholar]
- Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- Keelan ET, Licence ST, Peters AM, Binns RM, Haskard DO. Characterization of E-selectin expression in vivo with use of a radiolabeled monoclonal antibody. Am J Physiol. 1994;266:H278–290. doi: 10.1152/ajpheart.1994.266.1.H279. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj J. 1997;14:569–576. doi: 10.1023/a:1018580324971. [DOI] [PubMed] [Google Scholar]
- Krause T, Turner GA. Are selectins involved in metastasis? Clin Exp Metastasis. 1999;17:183–192. doi: 10.1023/a:1006626500852. [DOI] [PubMed] [Google Scholar]
- Ku AW, Muhitch JB, Powers CA, Diehl M, Kim M, Fisher DT, Sharda AP, Clements VK, O'Loughlin K, Minderman H, et al. Tumor-induced MDSC act via remote control to inhibit L-selectin-dependent adaptive immunity in lymph nodes. Elife. 2016;5 doi: 10.7554/eLife.17375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafouresse F, Bellard E, Laurent C, Moussion C, Fournie JJ, Ysebaert L, Girard JP. L-selectin controls trafficking of chronic lymphocytic leukemia cells in lymph node high endothelial venules in vivo. Blood. 2015;126:1336–1345. doi: 10.1182/blood-2015-02-626291. [DOI] [PubMed] [Google Scholar]
- Läubli H, Borsig L. Heparins attenuate cancer metastasis: are selectins the link? Cancer Invest. 2009;27:474–481. doi: 10.1080/07357900802647136. [DOI] [PubMed] [Google Scholar]
- Läubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol. 2010;20:169–177. doi: 10.1016/j.semcancer.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Läubli H, Stevenson JL, Varki A, Varki NM, Borsig L. L-selectin facilitation of metastasis involves temporal induction of fut7-dependent ligands at sites of tumor cell arrest. Cancer Res. 2006;66:1536–1542. doi: 10.1158/0008-5472.CAN-05-3121. [DOI] [PubMed] [Google Scholar]
- Laudanna C, Constantin G, Baron P, Scarpini E, Scarlato G, Cabrini G, Dechecchi C, Rossi F, Cassatella MA, Berton G. Sulfatides trigger increase of cytosolic free calcium and enhanced expression of tumor necrosis factor-alpha and interleukin-8 mRNA in human neutrophils. Evidence for a role of L-selectin as a signaling molecule. J Biol Chem. 1994;269:4021–4026. [PubMed] [Google Scholar]
- Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 2015;107:331–339. doi: 10.1093/cvr/cvv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Xia L, Yago T, Kappelmayer J, Liu Z, Klopocki AG, Shao B, McDaniel JM, Setiadi H, Schmidtke DW, et al. Separable requirements for cytoplasmic domain of PSGL-1 in leukocyte rolling and signaling under flow. Blood. 2008;112:2035–2045. doi: 10.1182/blood-2008-04-149468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed RN, Watson HA, Vigar M, Ohme J, Thomson A, Humphreys IR, Ager A. L-selectin Is Essential for Delivery of Activated CD8(+) T Cells to Virus-Infected Organs for Protective Immunity. Cell Rep. 2016;14:760–771. doi: 10.1016/j.celrep.2015.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce OM, Laubli H. Sialic acids in cancer biology and immunity. Glycobiology. 2016;26:111–128. doi: 10.1093/glycob/cwv097. [DOI] [PubMed] [Google Scholar]
- Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- Prakobphol A, Genbacev O, Gormley M, Kapidzic M, Fisher SJ. A role for the L-selectin adhesion system in mediating cytotrophoblast emigration from the placenta. Dev Biol. 2006;298:107–117. doi: 10.1016/j.ydbio.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- Rzeniewicz K, Newe A, Rey Gallardo A, Davies J, Holt MR, Patel A, Charras GT, Stramer B, Molenaar C, Tedder TF, et al. L-selectin shedding is activated specifically within transmigrating pseudopods of monocytes to regulate cell polarity in vitro. Proc Natl Acad Sci U S A. 2015;112:E1461–1470. doi: 10.1073/pnas.1417100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Shamay Y, Elkabets M, Li H, Shah J, Brook S, Wang F, Adler K, Baut E, Scaltriti M, Jena PV, et al. P-selectin is a nanotherapeutic delivery target in the tumor microenvironment. Sci Transl Med. 2016;8:345ra387. doi: 10.1126/scitranslmed.aaf7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SI, Burns AR, Taylor AD, Gopalan PK, Lynam EB, Sklar LA, Smith CW. L-selectin (CD62L) cross-linking signals neutrophil adhesive functions via the Mac-1 (CD11b/CD18) beta 2-integrin. J Immunol. 1995;155:1502–1514. [PubMed] [Google Scholar]
- Sipkins DA, Wei X, Wu JW, Runnels JM, Cote D, Means TK, Luster AD, Scadden DT, Lin CP. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Olson TS, Ley K. CXCR2- and E-selectin-induced neutrophil arrest during inflammation in vivo. J Exp Med. 2004;200:935–939. doi: 10.1084/jem.20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen JE, Petersen TK, Koch C, O'Keefe SJ, Hanlon WA, Seo S, Pearson D, Fossett MC, Simon SI. L-selectin signaling of neutrophil adhesion and degranulation involves p38 mitogen-activated protein kinase. J Biol Chem. 2000;275:15876–15884. doi: 10.1074/jbc.M906232199. [DOI] [PubMed] [Google Scholar]
- Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, Nacher M, Pitaval C, Radovanovic I, Fukui Y, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346:1234–1238. doi: 10.1126/science.1256478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CA. Interactions between endothelial selectins and cancer cells regulate metastasis. Front Biosci. 2011;17:3233–3251. doi: 10.2741/3909. [DOI] [PubMed] [Google Scholar]
- Hill CA, Krieser K, Farooqui M. Neutrophil interactions with sialyl Lewis X on human nonsmall cell lung carcinoma cells regulate invasive behavior. Cancer. 2011;117:4493–4505. doi: 10.1002/cncr.26059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SN, Schnaar RL, Konstantopoulos K. Podocalyxin-like protein is an E-/L-selectin ligand on colon carcinoma cells: comparative biochemical properties of selectin ligands in host and tumor cells. Am J Physiol Cell Physiol. 2009;296:C505–513. doi: 10.1152/ajpcell.00472.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G, Courtney AN, Jena B, Heczey A, Liu D, Marinova E, Guo L, Xu X, Torikai H, Mo Q, et al. CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J Clin Invest. 2016;126:2341–2355. doi: 10.1172/JCI83476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco R, Carrette F, Barraza ML, Otero DC, Magana J, Bosenberg MW, Swain SL, Bradley LM. PSGL-1 Is an Immune Checkpoint Regulator that Promotes T Cell Exhaustion. Immunity. 2016;44:1190–1203. doi: 10.1016/j.immuni.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay PL, Auger FA, Huot J. Regulation of transendothelial migration of colon cancer cells by E-selectin-mediated activation of p38 and ERK MAP kinases. Oncogene. 2006;25:6563–6573. doi: 10.1038/sj.onc.1209664. [DOI] [PubMed] [Google Scholar]
- Urzainqui A, Serrador JM, Viedma F, Yanez-Mo M, Rodriguez A, Corbi AL, Alonso-Lebrero JL, Luque A, Deckert M, Vazquez J, et al. ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity. 2002;17:401–412. doi: 10.1016/s1074-7613(02)00420-x. [DOI] [PubMed] [Google Scholar]
- Varki A. Selectin ligands: will the real ones please stand up? J Clin Invest. 1997;99:158–162. doi: 10.1172/JCI119142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerman KM, Williams MJ, Uchimura K, Singer MS, Merzaban JS, Naus S, Carlow DA, Owen P, Rivera-Nieves J, Rosen SD, et al. Interaction of the selectin ligand PSGL-1 with chemokines CCL21 and CCL19 facilitates efficient homing of T cells to secondary lymphoid organs. Nat Immunol. 2007;8:532–539. doi: 10.1038/ni1456. [DOI] [PubMed] [Google Scholar]
- Venturi GM, Tu L, Kadono T, Khan AI, Fujimoto Y, Oshel P, Bock CB, Miller AS, Albrecht RM, Kubes P, et al. Leukocyte migration is regulated by L-selectin endoproteolytic release. Immunity. 2003;19:713–724. doi: 10.1016/s1074-7613(03)00295-4. [DOI] [PubMed] [Google Scholar]
- Waddell TK, Fialkow L, Chan CK, Kishimoto TK, Downey GP. Potentiation of the oxidative burst of human neutrophils. A signaling role for L-selectin. J Biol Chem. 1994;269:18485–18491. [PubMed] [Google Scholar]
- Walcheck B, Alexander SR, Hill CA, Matala E. ADAM-17-independent shedding of L-selectin. J Leukoc Biol. 2003;74:389–394. doi: 10.1189/jlb.0403141. [DOI] [PubMed] [Google Scholar]
- Wang HB, Wang JT, Zhang L, Geng ZH, Xu WL, Xu T, Huo Y, Zhu X, Plow EF, Chen M, et al. P-selectin primes leukocyte integrin activation during inflammation. Nat Immunol. 2007;8:882–892. doi: 10.1038/ni1491. [DOI] [PubMed] [Google Scholar]
- Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6:902–910. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- Weyrich AS, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Monocyte tethering by P-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-alpha secretion. Signal integration and NF-kappa B translocation. J Clin Invest. 1995;95:2297–2303. doi: 10.1172/JCI117921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witz IP. The selectin-selectin ligand axis in tumor progression. Cancer Metastasis Rev. 2008;27:19–30. doi: 10.1007/s10555-007-9101-z. [DOI] [PubMed] [Google Scholar]
- Yang S, Liu F, Wang QJ, Rosenberg SA, Morgan RA. The shedding of CD62L (L-selectin) regulates the acquisition of lytic activity in human tumor reactive T lymphocytes. PLoS One. 2011;6:e22560. doi: 10.1371/journal.pone.0022560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced alpha(L)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 2007;26:773–783. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Muller H, Kuwano Y, Ley K. PSGL-1-dependent myeloid leukocyte activation. J Leukoc Biol. 2009;86:1119–1124. doi: 10.1189/jlb.0209117. [DOI] [PubMed] [Google Scholar]
- Zuchtriegel G, Uhl B, Puhr-Westerheide D, Pornbacher M, Lauber K, Krombach F, Reichel CA. Platelets Guide Leukocytes to Their Sites of Extravasation. PLoS Biol. 2016;14:e1002459. doi: 10.1371/journal.pbio.1002459. [DOI] [PMC free article] [PubMed] [Google Scholar]