Abstract
Long-term space mission exposes astronauts to a radiation environment with potential health hazards. High-energy charged particles (HZE), including 28Si nuclei in space, have deleterious effects on cells due to their characteristics with high linear energy transfer and dense ionization. The influence of 28Si ions contributes more than 10% to the radiation dose equivalent in the space environment. Understanding the biological effects of 28Si irradiation is important to assess the potential health hazards of long-term space missions. The hematopoietic system is highly sensitive to radiation injury and bone marrow (BM) suppression is the primary life-threatening injuries after exposure to a moderate dose of radiation. Therefore, in the present study we investigated the acute effects of low doses of 28Si irradiation on the hematopoietic system in a mouse model. Specifically, 6-month-old C57BL/6 J mice were exposed to 0.3, 0.6 and 0.9 Gy 28Si (600 MeV) total body irradiation (TBI). The effects of 28Si TBI on BM hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) were examined four weeks after the exposure. The results showed that exposure to 28Si TBI dramatically reduced the frequencies and numbers of HSCs in irradiated mice, compared to non-irradiated controls, in a radiation dose-dependent manner. In contrast, no significant changes were observed in BM HPCs regardless of radiation doses. Furthermore, irradiated HSCs exhibited a significant impairment in clonogenic ability. These acute effects of 28Si irradiation on HSCs may be attributable to radiation-induced apoptosis of HSCs, because HSCs, but not HPCs, from irradiated mice exhibited a significant increase in apoptosis in a radiation dose-dependent manner. However, exposure to low doses of 28Si did not result in an increased production of reactive oxygen species and DNA damage in HSCs and HPCs. These findings indicate that exposure to 28 Si irradiation leads to acute HSC damage.
Keywords: Space irradiation, Silicon irradiation, Hematopoietic stem cells, Hematopoietic progenitor cells, Apoptosis
1. Introduction
Space irradiation is an unavoidable complication for astronauts during long-term space missions. Space irradiation is mainly composed of photons, protons, helium and high-energy charged particles (HZE). Eighty-eight percent of the space radiation dose is ascribed to HZE particles, such as 56Fe, 28Si, 16O and 12C (Cucinotta et al., 2003). Depending on dose, dose rate, track structure and fluency, different radiation sources have distinct biological effects on normal tissues. Among HZE particles, the influence of 28 Si ions contribute more than 10% to the radiation dose equivalent in space irradiation. Understanding the biological effects of 28Si irradiation is needed and can benefit the development of new strategies to prevent and/or mitigate space radiation-induced injury.
The sensitivities of tissues to radiation progress from hematopoietic to gastrointestinal tissues and finally to neural and cardiovascular tissues as a function of increasing radiation doses. This indicates that the hematopoietic system is the most radiosensitive tissue of the body (Shao et al., 2014b). For example, low doses of 0.25–3 Gy proton irradiation significantly decreased the number of whole blood cells (WBCs) as early as 4 hours post exposure (Luo-Owen et al., 2012). Our recent data also showed that 1.0 Gy of proton total body irradiation (TBI) dramatically reduced the number of hematopoietic stem cells (HSCs) in bone marrow (BM) 22 weeks after irradiation (Chang et al., 2015). These results suggest that proton radiation induces both the acute and long-term damage to BM and BM HSCs. However, the effects of HZE particles (such as 28 Si) on the hematopoietic system, particularly BM HSCs, have not been reported.
A few studies reported the toxicities of 28Si irradiation in cultured cells and animals. Daila et al. evaluated the effects of 28Si irradiation on immune cells at 113 days after exposure, showing that 2.0 Gy of 28Si irradiation decreased the numbers of nature killer cells and DNA synthesis (Gridley et al., 2002). Montree et al. recently reported that various doses of 28 Si irradiation triggered persistent cellular apoptosis in total bone marrow and heart tissues (Tungjai et al., 2013). Other in vivo studies have reported that low doses (0.25–1.0 Gy) of 28Si irradiation adversely affected the central nervous system in mice, which led to the abnormalities in synaptic plasticity and cognitive function (Raber et al., 2014). In addition, using in vitro cell culture models, a few reports have shown that different doses and energies of 28Si (0.07–6.0 Gy, 300–1000 Mev/nucleon) caused cellular chromosomal instability, cell transformation, decreased cell clonogenic survival and increased DNA damage in bronchial epithelial cells, esophageal epithelial cells, skin fibroblast and hamster embryo cells (Asaithamby et al., 2008; Ding et al., 2013; Tsuruoka et al., 2008, 2005). Both in vitro and in vivo data displayed the deleterious effects of 28Si irradiation exposure on various types of cells and tissues. As the effects of 28Si irradiation on HSCs and hematopoietic progenitor cells (HPCs) have not previously been reported, they were investigated in the present study.
2. Materials and methods
2.1. Animals and irradiation
Six-month-old male C57BL/6 J mice purchased from the Jackson Laboratory (Bar Harbor, ME) were shipped to Brookhaven National Lab-oratories (BNL) in Upton, NY. After a one-week acclimation period, the mice were either sham irradiated or received whole-body irradiation (600 MeV/n; 0.3, 0.6 0.9 Gy). One week after irradiation, the mice were shipped to Oregon Health and Science University (OHSU). At BNL and OHSU, the mice were housed under a constant 12 h light: dark cycle. Food (PicoLab Rodent Diet 20, no. 5053; PMI Nutrition International, St. Louis, MO) and water were provided ad libitum. Behaviorally naïve mice were used for experiments and analyzed at four weeks after irradiation. All procedures were approved by the Institutional Animal Care and Use Committee at OHSU and BNL.
2.2. Isolation of BM mononuclear cells (BM-MNCs), analysis of the frequencies and numbers of different hematopoietic cell populations by flow cytometry
The femora and tibiae were harvested from mice immediately after they were killed by cervical dislocation. BM cells were flushed from the bones into HBSS containing 2% FCS using a 21-gauge needle and syringe. Cells were then incubated with biotin-conjugated anti-CD3e, anti-CD45R/B220, anti-Gr-1, anti-CD11b, and anti-Ter-119 antibodies and with anti-CD16/32 (Fcγ II/III Receptor or FcγR) antibody to block the Fcγ receptors. They were labeled with streptavidin–FITC, anti-Sca- 1–PE-Cy7, anti-c-Kit–APC –Cy7 for HPCs (Lin − Sca1 − c-kit + cells), LSK cells (Lin − Sca1 + c-kit + cells), and HSCs (Lin − Sca1 + c-kit + CD150 + CD48 − cells). Bone marrow mononuclear cells (BM-MNCs) were isolated by Histopaque 1083 separation solution (Sigma, St. Louis, MO). For the isolation of lineage negative cells (Lin − cells), BM-MNCs were incubated with purified rat antibodies specific for murine CD3e, Mac-1, CD45R/B220, Ter-119, and Gr-1. The labeled mature lymphoid and myeloid cells were depleted by incubating with goat anti-rat IgG paramagnetic beads (Life Technologies, Grand Island, NY) at a bead: cell ratio of approximately 4:1. Cells binding the paramagnetic beads were removed with a magnetic field. Lin − cells were washed twice with 2% FBS/HBSS and re-suspended in complete medium (RPMI1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 10 μM HEPES buffer, and 100 U/mL penicillin and streptomycin) at 1 ×107 cells/mL. Subsequently, cells were blocked by Fcγ receptors anti-CD16/32 antibody then stained with anti-Sca1-PE-Cy7, c-Kit-APC –Cy7. All flow antibodies were purchased from eBioscience (San Jose, CA). The frequencies of HPCs and HSCs were analyzed with an Aria II cell sorter. Dead cells were excluded by gating out the cells stained positive with propidium iodide (PI). For each sample, approximately 8 ×105 to 1 ×106 BM cells were acquired and the data were analyzed using BD FACSDiva 6.0 (BD Biosciences) and FlowJo (FlowJo, Ashland, OR) software.
2.3. Colony-forming cell (CFC) assay and cobblestone area-forming cell (CAFC) assay
The CFC assay was performed by culturing BM-MNCs in MethoCult GFM3434 methylcellulose medium (Stem Cell Technologies, Vancouver, BC). Colonies of CFU–granulocyte macrophage (GM) were scored on day 7 and those of CFU-granulocyte, -erythrocyte, -monocyte, and -megakaryocyte (GEMM) on day 12 of the incubation according to the manufacturer’s protocol. The CAFC assay was performed as described elsewhere (Li et al., 2011).
2.4. Analysis of the levels of intracellular reactive oxygen species (ROS)
Briefly, after staining with the appropriate cell surface marker antibodies, Lin − cells (1 ×107 /mL) were suspended in PBS supplemented with 5 mM glucose, 1 mM CaCl2 , 0.5 mM MgSO4 , and 5 mg/ml BSA and then incubated with 10 μM 2′,7′-dichlorofluorescin diacetate (DCFDA) (Life Technologies) for 30 minutes at 37 °C. The levels of ROS in HPCs and HSCs were analyzed by measuring the mean fluorescence intensity (MFI) of 2′,7′-dichlorofluorescein (DCF) with an Aria II cell sorter. For each sample, a minimum of 200,000 lineage negative cells was acquired and the data were analyzed as we previously described (Shao et al., 2014a).
2.5. DNA damage analysis
Lin− cells were first stained with antibodies against various cell-surface markers and fixed and permeabilized using the Fixation/Permeabilization Solution from BD Biosciences (San Diego, CA) followed by 0.2% Triton-X-100 incubation for 10 min. Cells were then stained with Alexa Fluor 488 conjugated anti-phospho-Histone 2AX (Ser139) antibody for 1.5 h at 4 °C and analyzed by flow cytometry. The levels of DNA damage were expressed by the mean fluorescence intensity of phospho-Histone 2AX or γ-H2AX with an Aria II cell sorter.
2.6. Cell cycle analysis
Lin− cells were first stained with antibodies against various cell-surface markers and fixed and permeabilized using the Fixation/Permeabilization Solution (BDBiosciences, San Diego, CA). Subsequently, they were stained with anti-Ki67-FITC antibody (BDBiosciences, San Diego, CA) and 7-AAD (Sigma, St. Louis, MO) and then analyzed by flow cytometer.
2.7. Statistical analysis
All data are presented as mean ± standard derivation of at least five independent biological samples per radiation dose. The differences between sham-irradiated and irradiated groups were examined by one way ANOVA, followed up by post-hoc test as indicated. Differences were considered significant at p < 0.05. Statistical analysis was performed using GraphPad Prism software (GraphPad Software Inc. LaJolla, CA).
3. Results
3.1. 28Si TBI-induced BM HSC injury
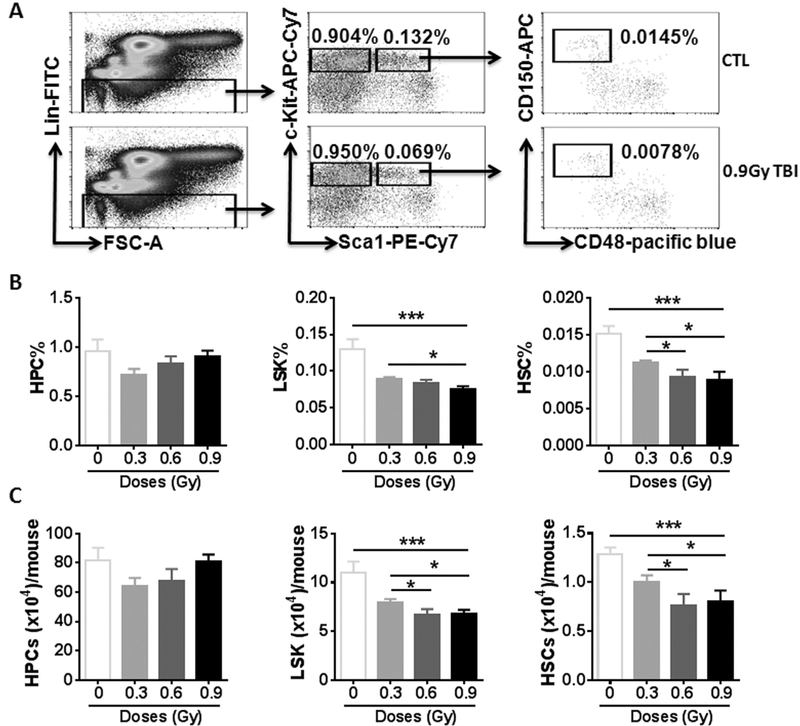
To assess the effects of 28 Si TBI on the hematopoietic system, particularly on BM HSCs, we harvested BM from irradiated mice and shamirradiated controls and analyzed the frequencies and numbers of different hematopoietic cell populations in BM cells by flow cytometry, as shown in Fig. 1A. The results from this assay revealed that the frequencies and numbers of HPCs (Lin − Sca1 − c-kit + cells) in the irradiated mice had reached normal levels (Fig. 1B and C). Compared to the sham- irradiated controls, the frequencies of LSK cells (Lin − Sca1 + c-kit + cells) and HSCs (Lin − Sca1 + c-kit + CD150 + CD48 − cells) in irradiated mice were significantly decreased in a dose-dependent manner after 0.3 and 0.6 Gy 28 Si irradiation (Fig. 1B, p < 0.05–0.001). However, there are comparable frequencies of LSK cells and HSCs between 0.6 and 0.9 Gy 28Si irradiation without statistical significance. The negative effects of 28Si irradiation on the numbers of LSK cells and HSCs were the same as those of the frequencies of LSK cells and HSCs (Fig. 1C, p < 0.05–0.001).
Fig. 1.
28Si TBI induces the reduction of HSCs in a dose-dependent manner. C57BL/6 J mice were exposed to 0.3, 0.6 and 0.9 Gy 28Si TBI or sham irradiated (CTL). Two weeks after TBI, BM cells (BMCs) were harvested from the two hind legs of individual mice for analysis. (A) Representative gating strategy of flow cytometric analysis for HPCs (Lin–Sca1–c-kit- cells), LSK cells (Lin –Sca1 + c-kit + cells) and HSCs (Lin –Sca1 + c-kit + CD150 + CD48 − cells) in BMCs is shown. (B and C) Frequencies (panel B) and total numbers (panel C) of HPCs, LSK cells and HSCs in BMCs from each mouse are presented as mean ± SD (n = 5). The statistical significance for the difference between the control groups and each of irradiated groups is indicated by asterisks. * p < 0.05, ** p < 0.01, *** p < 0.001 by one-way ANOVA analysis.
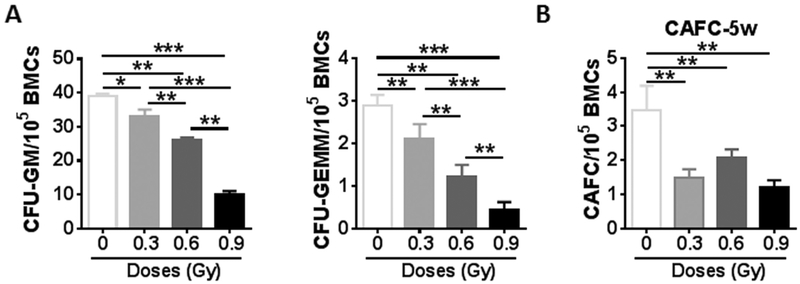
28Si irradiation not only decreased HSC numbers in BM but also resulted in alterations in HSC functions. We utilized colony forming assay to evaluate the differentiation ability of HSCs. The frequencies of CFU- GMs, and CFU-GEMMs were significantly reduced in a dose-dependent manner in irradiated mice, compared to those in sham-irradiated controls ( Fig. 2A, p < 0.05–0.001), indicating that the abilities of HPCs and HSCs to differentiate into granulocytes, erythrocytes, monocytes and/or megakaryocytes was dramatically impaired in irradiated mice compared to those in sham-irradiated mice. We also conducted a cobblestone area forming assay (CAFC), a widely used in vitro surrogate assay for HSCs, to assess the function of HSCs. The frequencies of 5-week CAFCs were significantly lower in BM cells from all irradiated mice than those from non-irradiated controls (Fig. 2B, p < 0.05). These findings clearly demonstrate that exposure to 28Si irradiation causes acute damage to HSCs.
Fig. 2.
28Si TBI decreases HSC colongenic function in a dose-dependent manner. (A) At four weeks after 28 Si TBI, BM-MNCs were isolated from irradiated and sham-irradiated mice. A CFC assay was performed as described in materials and methods. The results are presented as mean CFUs per 100,000 BM-MNCs (n = 3). * p < 0.05, ** p < 0.01, and *** p < 0.001. (B) Four weeks after the indicated doses of 28Si TBI, total BM cells (BMCs) were harvested from sham-irradiated (CTL) and irradiated (TBI) mice and were analyzed by CAFC assays. The numbers of 5-week CAFCs were counted and expressed as mean ± SD (n = 3–4 mice per group) of CAFCs per 100,000 BMCs. ** p < 0.01 TBI vs. CTL.
3.2. 28Si TBI-induced cellular apoptosis selectively in HSCs
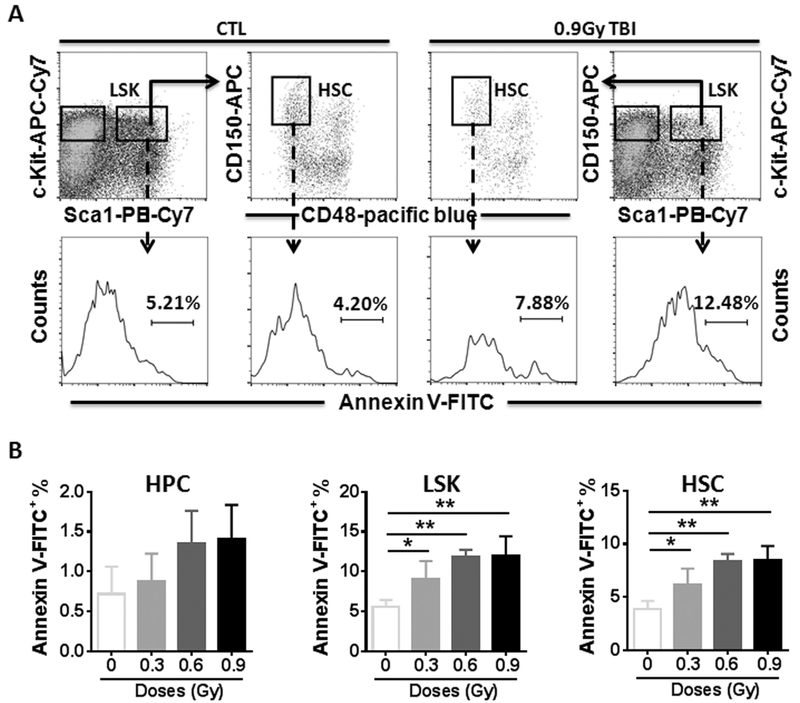
Irradiation causes acute injuries to cells and tissues primarily via induction of cellular apoptosis (Yu et al., 2010). We thus measured apoptosis in different populations using Annexin V staining (Fig. 3A). As shown in Fig. 3B, low levels of apoptotic cells could be detected in HPCs before and after 28Si irradiation. However, significantly higher levels of apoptotic cells were detected in irradiated LSK and HSC populations regardless of radiation doses compared to those in non-irradiated controls (Fig. 3B, p < 0.05–0.01).
Fig. 3.
28Si TBI increases cellular apoptosis in HSCs. Lin− cells were isolated from control (CTL) and irradiated (TBI) mice two weeks after 28Si TBI as described. (A). Representative analysis of apoptosis by flow cytometry using Annexin V staining in BM HPCs, LSK cells and HSCs from control and irradiated mice. The numbers presented in the histograms are percentages of Annexin V-FITC positive cells in the indicated populations from a representative experiment. (B) The percentages of Annexin V positive cells in BM HPCs, LSK cells and HSCs after TBI are presented as mean ± SD (n = 5). * p < 0.05, ** p < 0.01 TBI vs. CTL.
Irradiation induces not only cellular apoptosis but also oxidative stress and reactive oxygen species (ROS), cell cycling and DNA damage (Shao et al., 2014a). To elucidate the role of ROS in 28 Si irradiation-induced HSC damage, intracellular production of ROS in different populations of BM hematopoietic cells from 28 Si-irradiated mice was compared with that from sham-irradiated mice. As shown in Supplemental Figure 1A, ROS production in HPCs, LSK cells and HSCs from irradiated mice was comparable to that in cells from sham-irradiated mice (Supplemental Figure 1A, p > 0.05). Moreover, we utilized Ki-67 and 7-AAD double staining to investigate the cell cycle status of HPCs and HSCs. The results showed that the same percentages of these cells in G 0 phase were detected in the tested populations from irradiated and non-irradiated mice (Supplemental Figure 1B, p > 0.05). In addition, we used flow cytometry to analyze mean fluorescence intensity (MFI) of γ-H2AX immunostaining in HPCs and HSCs to measure DNA damage or DNA double strand breaks, and found that the MFI in HPC and HSCs from irradiated mice were the same as that from non-irradiated controls (Supplemental Figure 1C, p > 0.05). Therefore, these data demonstrate that 28Si irradiation induces cellular apoptosis in irradiated HSCs, which may contribute to 28Si irradiation-induced acute damage to HSCs. However, the induction of HSC apoptosis was not associated with increased production of ROS, DNA DSBs and cell cycling. The mechanisms by which 28Si irradiation induces HSC damage remains to be investigated.
4. Discussion
The present study evaluated the effects of different doses of 28Si TBI on BM HSCs and HPCs and provides new information about the biological effects of 28Si radiation on the hematopoietic system. The results from this study demonstrate that exposure to 28Si irradiation significantly decreases the numbers and functions of HSCs but not HPCs four weeks post irradiation. Consistently, low doses of proton and oxygen ion radiation also significantly decreased the numbers and function of HSCs two weeks after radiation exposure (Chang et al., 2016a). All of three different sources of radiation (0.9 Gy 28Si (600 MeV), 1.0 Gy protons (150 MeV) and 1.0 Gy 16O (600 MeV)) reduced 50% HSCs compared to those of HSCs from non-irradiated animals. Interestingly, the five-week cobblestone area-forming (CAFC) abilities of HSCs post 28 Si TBI were dramatically reduced in a dose-independent manner. A similar finding was also observed in our previous study in which mice were exposed to 3 different doses of oxygen ion (16O, 0.1, 0.25 and 1.0 Gy)( Chang et al., 2016a ). In addition, the data from the apoptosis assay also shows that the induction of LSK and HSC apoptosis by 28 Si irradiation reaches plateau after 0.6 Gy 28Si radiation. These findings suggest that HSCs are highly sensitive to space irradiation but that there is no simple dose- response relationship. We plan to investigate the mechanisms by which 28Si irradiation induces damage to HSCs in a dose-independent manner in a future study. On the other hand, HPCs exhibited different responses to the three sources of radiation. One Gy protons and 16O radiation decreased 50% and 30% of numbers of HPCs at two weeks post exposure, respectively (Chang et al., 2016a). However, the numbers of HPCs in BM were comparable in 0.9 Gy of 28Si-radiated mice and sham-irradiated controls. These unusual dose-response curves, even inverted ones, have also been reported in other space radiation studies for different outcome measures, such as effects of 28Si irradiation on synaptic plasticity and contextual fear memory (Raber et al., 2015; Raber et al., 2014).
As shown in our previous studies, exposure of HPCs to irradiation primarily induces apoptosis while exposure of HSCs to irradiation mainly induces senescence (Meng et al., 2003; Shao et al., 2014a; Wang et al., 2006). In addition, a few numbers of HSCs may have the ability to repair radiation-induced damage or are spared from the damage and able to repopulate HPCs after irradiation via proliferation and differentiation. Therefore, the number of HPCs can be restored to a relatively normal level (Fig. 1B and C) but their function remains suppressed (Fig. 2A) 4 weeks after irradiation. In contrast, senescent HSCs induced by radiation have reduced ability to self-renew and cannot resume normal function after irradiation (Fig. 1B and C and Fig. 2B). We have shown recently that clearance of senescent HSCs can rejuvenate ionizing radiation-induced long-term HSC injury and bone marrow suppression (Chang et al., 2016b). It remains to be determined whether clearance of senescent HSCs can also rejuvenate long-term HSC injury and bone marrow suppression induced by 28Si irradiation.
Radiation-induced HSC damage can be mediated by (1) induction of cellular apoptosis via the p53 signaling pathway; (2) induction of ROS production; (3) induction of DNA damage, particularly DNA DSBs; and (4) stimulation of HSC cycling. These events may lead to the defect of HSC self-renewal and HSC exhaustion. In the current study, a significant increase of cellular apoptosis in HSCs was observed in mice exposed to various doses of 28Si, even though there was an increasing trend of apoptosis in irradiated HPCs without statistical significance. The cellular apoptosis induced by 28Si exposure might result in the decrease of in vitro colony forming ability in irradiated HPCs and HSCs. However, we did not detect any changes in ROS production, DNA damage and cell cycling in HSCs after exposure to three different doses of 28Si TBI. The lack changes in ROS production and DNA damage in HSCs 4 weeks after 28Si irradiation may be attributable to the clearance of apoptotic HSCs induced by increased production of ROS and DNA damage. Data from in vitro experiments have shown that 28Si-induced cellular chromosomal instability and increased cell DNA damage (Asaithamby et al., 2008; Ding et al., 2013; Tsuruoka et al., 2008; Tsuruoka et al., 2005 ), which may be potential factors in HSC injury after 28Si ion exposure.
Compared to 28Si radiation, the similar doses of proton and oxygen ion radiation increased the induction of cellular apoptosis and production of ROS in irradiated HSC two weeks after the exposure (Chang et al., 2016a). 16 O irradiated HSCs induced high levels of un-repaired DNA damage at 2 weeks post exposure. Therefore, although all of three different sources of radiation can acutely decrease the numbers and function of HSCs in BM, the induction of the HSC damage is mediated via different mechanisms.
In summary, our data indicate the potential risk to the hematopoietic system following exposure of 28Si ions. The apoptosis pathway involved in this injury could be targeted for the development of medical counter- measures to protect astronauts during and following space travel.
Supplementary Material
Acknowledgements
The study was supported in part by NASA grant NNJ12ZSA001N (JR), Center of Biomedical Research Excellence (P20, GM109005, MHJ, DZ), an Arkansas Research Alliance Scholarship (DZ), and the Rockefeller Leukemia and Lymphoma Research Endowment (DZ).
Footnotes
Conflict of interests
The authors declare no conflict of interests.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lssr.2017.03.003.
References
- Asaithamby A, Uematsu N, Chatterjee A , Story MD , Burma S , Chen DJ , 2008. Repair of hze-particle-induced DNA double-strand breaks in normal human fibroblasts. Radiat Res. 169, 437–446. [DOI] [PubMed] [Google Scholar]
- Chang J , Feng W , Wang Y , Luo Y , Allen AR , Koturbash I , Turner J , Stewart B , Raber J , Hauer-Jensen M , Zhou D , Shao L , 2015. Whole-body proton irradiation causes long-term damage to hematopoietic stem cells in mice. Radiat Res. 183, 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J , Luo Y , Wang Y , Pathak R , Sridharan V , Jones T , Mao XW , Nelson G , Boerma M , Hauer-Jensen M , Zhou D , Shao L , 2016a. Low doses of oxygen ion irradiation cause acute damage to hematopoietic cells in mice. PLoS One. 11, e0158097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J , Wang Y , Shao L , Laberge RM , Demaria M , Campisi J , Janakiraman K , Sharpless NE , Ding S , Feng W , Luo Y , Wang X , Aykin-Burns N , Krager K , Ponnappan U , Hauer-Jensen M , Meng A , Zhou D , 2016b. Clearance of senescent cells by abt263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 22, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta FA, Wu H, Shavers MR, George K, 2003. Radiation dosimetry and biophysical models of space radiation effects. Gravit. Space Biol. Bull 16, 11–18. [PubMed] [Google Scholar]
- Ding LH, Park S, Peyton M, Girard L, Xie Y, Minna JD, Story MD, 2013. Distinct transcriptome profiles identified in normal human bronchial epithelial cells after exposure to gamma-rays and different elemental particles of high z and energy. BMC Genomics. 14, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley DS, Pecaut MJ, Nelson GA, 2002. Total-body irradiation with high-let particles: Acute and chronic effects on the immune system. Am. J. Physiol. Regul. Integr. Comp. Physiol 282, R677–R688. [DOI] [PubMed] [Google Scholar]
- Li H, Wang Y , Pazhanisamy SK , Shao L , Batinic-Haberle I , Meng A , Zhou D , 2011. Mn(iii) meso-tetrakis-(n-ethylpyridinium-2-yl) porphyrin mitigates total body irradiation-induced long-term bone marrow suppression. Free Radic. Biol. Med 51, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo-Owen X, Pecaut MJ, Rizvi A, Gridley DS, 2012. Low-dose total-body gamma irradiation modulates immune response to acute proton radiation. Radiat Res 177, 251–264. [DOI] [PubMed] [Google Scholar]
- Meng A, Wang Y, Brown SA, Van Zant G, Zhou D, 2003. Ionizing radiation and busulfan inhibit murine bone marrow cell hematopoietic function via apoptosis-dependent and -independent mechanisms. Exp Hematol. 31, 1348–1356. [DOI] [PubMed] [Google Scholar]
- Raber J, Marzulla T, Stewart B, Kronenberg A, Turker MS, 2015. 28silicon irradiation impairs contextual fear memory in b6d2f1 mice. Radiat. Res 183, 708–712. [DOI] [PubMed] [Google Scholar]
- Raber J , Rudobeck E , Campbell-Beachler M , Allen AR , Allen B , Rosi S , Nelson GA , Ramachandran S , Turner J , Fike JR , Vlkolinsky R , 2014. (28) silicon radiation-induced enhancement of synaptic plasticity in the hippocampus of naive and cognitively tested mice. Radiat. Res 181, 362–368. [DOI] [PubMed] [Google Scholar]
- Shao L, Feng W, Li H , Gardner D , Luo Y, Wang Y, Liu L, Meng A, Sharpless NE , Zhou D , 2014a. Total body irradiation causes long-term mouse bm injury via induction of hsc premature senescence in an ink4a- and arf-independent manner. Blood. 123, 3105–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Luo Y, Zhou D, 2014b. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid. Redox Sig 20, 1447–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruoka C, Suzuki M, Hande MP, Furusawa Y, Anzai K, Okayasu R, 2008. The difference in let and ion species dependence for induction of initially measured and non-rejoined chromatin breaks in normal human fibroblasts. Radiat. Res 170, 163–171. [DOI] [PubMed] [Google Scholar]
- Tsuruoka C, Suzuki M, Kanai T, Fujitaka K, 2005. Let and ion species dependence for cell killing in normal human skin fibroblasts. Radiat. Res 163, 494–500. [DOI] [PubMed] [Google Scholar]
- Tungjai M, Whorton EB, Rithidech KN, 2013. Persistence of apoptosis and inflammatory responses in the heart and bone marrow of mice following whole-body exposure to 28silicon (28si) ions. Radiat. Environ. Biophys 52, 339–350. [DOI] [PubMed] [Google Scholar]
- Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D, 2006. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 107, 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H , Shen H , Yuan Y , XuFeng R , Hu X , Garrison SP , Zhang L , Yu J , Zambetti GP , Cheng T , 2010. Deletion of puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood. 115, 3472–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.