Abstract
In the effort to develop cell-based therapies to treat salivary gland dysfunction, many different populations of cells in the adult salivary glands have been proposed as stem cells. These cell populations vary, depending on the assay used, and are often nonoverlapping, leading to the conclusion that salivary glands harbor multiple stem cells. The goal of this review is to critically appraise the assays and properties used to identify stem cells in the adult salivary gland, and to consider the caveats of each. Re-evaluation of the defining criteria may help to reconcile the many potential stem cell populations described in the salivary gland, in order to increase comparability between studies and build consensus in the field.
Keywords: Stem cell, Salivary gland, Progenitor cell, Lineage-restricted, Acinar
Graphical Abstract
Introduction
Salivary gland dysfunction, as a result of radiation therapy for head and neck cancer, or of disease, such as Sjögren’s Syndrome, is a permanent and debilitating condition. Regenerative approaches are focused on cell-based strategies, which require identification of cells with the potential to replace the salivary gland duct and secretory acinar cell types. Salivary gland maintenance and regeneration has been widely held to depend on adult stem cells [1]. Many studies have reported the identification of often nonoverlapping, potential stem cell populations in mouse, rat, and human salivary glands [2]. To reconcile the various reports, it is often concluded that the salivary glands harbor multiple stem cell populations [1, 2].
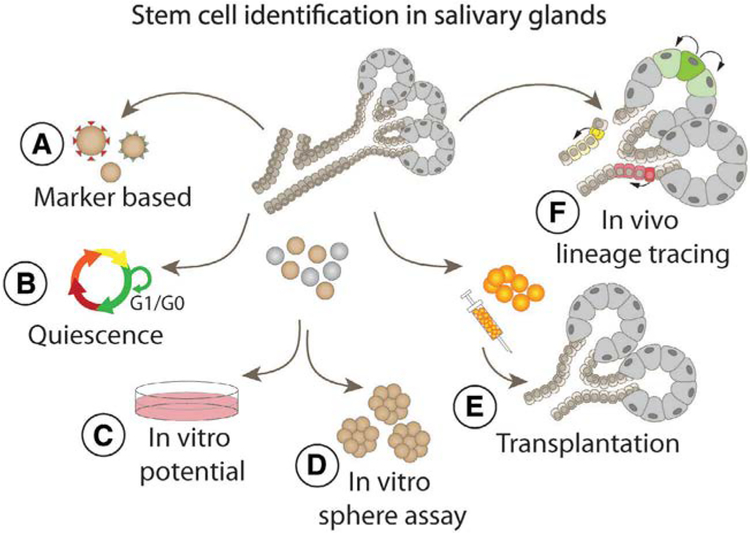
No clear consensus exists on what criteria should be applied for the identification of putative salivary gland stem cells. Those used have included expression of stem cell-associated markers, ability to proliferate or differentiate in vitro, ability to form spheres, rescue of salivary function following transplantation into irradiated glands, and in vivo lineage tracing (Fig. 1). Although several of these features are consistent with the definition of a stem cell, singly each of these assays has caveats and are open to alternative interpretations. We propose that the number of potential stem cell populations identified in the salivary glands may reflect the uneven application of criteria used to define a stem cell. The purpose of this review is to critically evaluate the properties and assays on which salivary gland stem cell identification has been based, with the goal of reconciling the various reports and building a consensus in the field.
Figure 1.
Assays used for the identification of potential stem cells in adult salivary glands have included (A) expression of stem cell markers, (B) proliferation or quiescence, (C) in vitro differentiation, (D) sphere formation, (E) rescue of salivary gland function following transplantation, and (F) in vivo lineage tracing.
Defining and Distinguishing Stem and Progenitor Cells
Classically, there are two key properties that define a stem cell: (a) the unlimited ability to self-renew, and (b) the ability to differentiate into more than one mature cell type [3]. To date, adult stem cells that meet these criteria have been found in only a few tissues [4, 5], such as the intestine and hematopoietic system [6, 7]. It is now recognized that adult stem cells from different tissues do not share identical properties [8]. For example, quiescence is a defining characteristic of hematopoietic, satellite muscle, and neural stem cells [8], while hair follicle and intestinal stem cells undergo rapid and continuous proliferation [9]. This variability in stem cell characteristics has made it difficult to establish rigorous criteria for defining adult stem cells.
It is critical to recognize the difference between stem cells and progenitor cells, which although frequently mentioned interchangeably, are not equivalent and exhibit distinct properties [10]. Stem cells can replicate indefinitely and produce both undifferentiated and differentiated progeny. Progenitor cells undergo only a finite number of cell divisions, do not selfrenew, and are often limited in the number of cell types they can generate [11]. This difference is difficult to experimentally distinguish, but critical to recognize. Long-term self-renewal and multipotent differentiation capacity are functional properties that require rigorous analysis of the cells within their native tissue niche. Because it is difficult to identify stem cells meeting these criteria in vivo, the trend has been toward loosening the criteria to those that describe progenitor cells. However, the removal of stem cells from their niche for in vitro analysis can result in alteration of cell properties [10], leading to observations, which may not reflect in vivo behavior. Ultimately, in vitro evidence of stem cell properties must be corroborated in vivo to unambiguously identify a stem cell. The ability to contribute to salivary gland repair may not require a bona fide stem cell, but the fundamental differences between stem and progenitor cells could be important when using cells that should ideally last the lifespan of the patient. In theory, and very likely in practice, stem and progenitor cells will not be equal in their long-term capacity to repair damaged or diseased tissue. Thus, a common consensus on the criteria used to define stem cells is needed.
Stem Cell Marker Expression
The identification of stem cells in the salivary gland has often been based on the expression of specific markers associated with stem cells in other organs [2]. c-KIT (CD117) and stem cell antigen 1 (SCA-1), surface markers of hematopoietic stem cells [12], are both expressed by subpopulations of cells in the salivary glands [13]. Cells expressing these markers were observed to increase in number after duct ligation injury, supporting the idea that they are stem cells involved in gland repair [13]. Keratin 5 (K5) and Keratin 14 (K14) are cytoskeletal proteins expressed in basal epithelial cells of many adult tissues, which in trachea and olfactory epithelium act as stem cells [14, 15]. In the developing salivary glands, K5 and K14 positive cells are embryonic progenitors of acinar and duct cells [16, 17], leading to the proposal that they act as stem cells in adult glands [16, 17]. The leucinerich repeat containing G-protein coupled receptor 5 (LGR5), is an established marker of adult stem cells in numerous tissues including the intestine [6] and hair follicles [18]. LGR5-expressing cells in human parotid and submandibular glands have been proposed to be stem cells [19].
Although there is some overlap, the salivary gland cell populations expressing general stem cell markers such as c-KIT, K5, or LGR5 are diverse. Notably, all these proposed stem cells are located in the salivary gland ducts. The assumption that salivary gland stem cells are localized in the ducts originated with early thymidine labeling studies and was based primarily on anatomical proximity to labeled acinar cells [20, 21]. The expression of stem cell markers such as SCA-1 by duct cells supported this hypothesis [1]. However, identification of a stem cell based on gene expression has several caveats. No universal stem cell marker has been identified [5, 22], and expression of general stem cell markers is not strictly limited to stem cells. For example, LGR5, well-recognized as a stem cell marker, is also expressed in the olfactory bulb in a large subset of postmitotic neurons [23]. In support of these arguments, recent assays have shown that duct cells expressing c-KIT or K14 do not function as multipotent stem cells in adult salivary glands [24, 25].
The discovery of cells in adult salivary glands that express mesenchymal stem cell (MSC) surface antigens, including CD44, CD49f (integrin), CD90, and CD105, prompted suggestions that they are stem cells [19, 26–28]. These cells can differentiate into chondrocytes, osteoblasts, and adipocytes in vitro and have been analyzed for their ability to contribute to salivary gland acinar and duct cell lineages [19, 26, 27]. Transplantation of the MSC-like cells partially rescues radiation-induced salivary gland dysfunction [19, 27], but in vivo lineage tracing to acinar and duct cells remains to be explored.
Quiescent or Proliferative Stem Cells
Due to uncertainty over their identity, the search for salivary gland stem cells has focused on both quiescent and rapidly dividing cell populations. Label-retaining assays are based on the idea that quiescent stem cells are slowly cycling and retain a DNA label over time in pulse-chase experiments, whereas continued division of nonquiescent cells will eventually dilute the label. Label retaining cells (LRCs) identified in the salivary glands have been suggested to be quiescent stem cells [29–31]. The advantage of label retaining assays is the unbiased approach to stem cell identification, independent of protein markers. A caveat is that the DNA label will be retained by cells undergoing terminal differentiation, as well as by potential quiescent stem cells [32, 33]. Consistent with this, LRCs are found in all parenchymal compartments of the salivary glands [29–31, 34], and can colocalize with markers of differentiated acinar or duct cells [30, 31, 34, 35]. Due to the low rate of cell turnover in the adult salivary gland, cells labeled during an earlier proliferative phase will be retained for long periods. Another limitation of stem cell identification based on label-retaining assays is that the outcome varies with the labeling strategy (age of animal at labeling) and the experimental design (length of chase time). Labeling done during embryonic development identified LRCs after long-term chase that had low proliferative potential in vitro and did not actively proliferate following injury [34]. In contrast, labeling postnatally followed by a shorter chase identified LRCs that showed self-renewal capacity in vitro [31] and in vivo proliferation in response to injury [30, 35]. Labeling at embryonic or postnatal stages likely marked different populations of dividing cells. For unambiguous classification of the LRCs as stem cells, characterization of their in vivo lineage potential needs to be conducted.
In contrast to LRCs, many studies have searched for rapidly dividing stem cells in the salivary gland. Several populations of potential stem cells have been designated based on in vitro proliferation of dissociated primary cells [19, 26–28, 36, 37]. These cells are a heterogeneous population that often expresses c-KIT, K5, or K14, suggesting a ductal cell origin. The combination of in vitro proliferation and expression of these general stem cell markers is taken as proof that they are stem cells. However, in vitro proliferation is not a characteristic unique to stem cells [10]. Many differentiated cell types, including primary salivary gland cells, continue to proliferate in vitro for several cell divisions [38]. Long-term proliferative potential should be confirmed in vivo within the native niche environment of the potential stem cell.
Assessing In Vitro Potential
Several potential salivary gland stem cells have been identified based on the in vitro potential to generate acinar, duct, and myoepithelial cell types [19, 36, 39–43]. A major caveat of using in vitro assays to determine stem cell potential is the removal of a cell from its native location [44]. Most cells are capable of changing their phenotypic properties in response to the surrounding microenvironment [5], and particularly under stress, may exhibit the plasticity to transition to intermediate, dedifferentiated, or alternate cell types [45]. For example, the stem cell marker LGR5 is not expressed in the adult pancreas, but is induced when pancreatic duct cells are cultured in vitro [46]. Thus, stem cell-like characteristics may be an artifact of in vitro culture [5]. Studies in which the stem cell properties appear only after several passages in culture [47] are particularly suspect, as more time in culture introduces the likelihood of alterations in cellular properties. The use of in vitro assays to define stem cell potential should involve rigorous demonstration that cell differentiation is accompanied by a decrease in stem cell marker expression, and increased expression of differentiation markers. Differentiated cells generated in vitro should not express markers of more than one cell type, and should not continue to express stem cell markers.
Some potential stem cell populations isolated from salivary glands have the capacity to differentiate into chondrogenic, osteogenic, and adipogenic cell types, a characteristic of MSCs [19, 26, 27]. Culture of these cells in Matrigel yields branched and aggregated structures resembling native salivary gland acini and ducts [28, 48]. Similar structures have been generated by mouse and human salivary gland cells cultured in Matrigel [36, 40]. However, it is necessary to determine if cells in these structures express acinar and duct cell-specific markers.
Sphere Assays to Determine Self-Renewal
The ability to form spheres in vitro has been used to evaluate stem cell self-renewal and multipotency [49]. The use of this assay assumes that each sphere originates from a single cell, and that only self-renewing stem cells can form spheres [49]. In vitro sphere formation by salivary gland cells is well established [37, 40–43, 50, 51]. However, in many cases, sphere assays have been performed using the heterogeneous mixture of cells obtained after dissociation of an entire gland [36, 37, 42, 43, 50, 51]. Video analysis of dissociated salivary gland cells cultured under nonadherent conditions showed that the cells rapidly aggregated within 24–48 hours [52]. The cell aggregates formed included differentiated cells, and did not originate from single stem cells. To insure that spheres are clonal and derived from a single initiating cell, it is necessary to exclude the possibility of random cellular aggregation [40, 41, 49]. This has been done using flow cytometry followed by plating single cells in Matrigel [40, 41, 50]. Even so, in one study, four isolated and distinct populations of mouse salivary gland cells each formed spheres [50], suggesting that sphere formation may be a general property of many cell types in vitro.
In theory, if derived from a stem cell, salivary spheres should include cells expressing acinar, duct, and myoepithelial markers, as well as stem cell markers. However, expression of the differentiated cell markers should be mutually exclusive, such that single cells do not express markers of more than one cell type. Continued expression of a general stem cell marker by all cells in a sphere raises doubts about the nature of the spheres, and the conclusion that they represent stem cells.
Secondary sphere formation is also used as proof of stem cells, but has similar caveats. Dissociation of a primary sphere will generate a heterogeneous population of cells that includes differentiated cell types, which can continue in vitro proliferation for several generations [38]. As with primary spheres, it is therefore necessary to establish clonality and rule out cell aggregation. In addition, once generated, secondary and tertiary spheres formed from potential stem cells should be reanalyzed for evidence of differentiation to acinar, duct, and myoepithelial cells.
In Vivo Transplantation
The ability of a single cell to repopulate a tissue provides definitive proof of self-renewal and multilineage potential. Evidence that a single cell could permanently repopulate the entire hematopoietic system established the identity of the HSCs [53]. Similarly, transplantation of a single basal mammary stem cell led to generation of a fully functional mammary gland [54]. Interestingly, additional studies have uncovered more than one cell population with this multipotency [55].
Transplantation has frequently been used as an assay for defining salivary gland stem cells by testing whether saliva secretion can be restored following cell injection into irradiated glands [27, 40, 41, 43, 48, 50, 56–61]. However, in most studies, cell populations rather than single cells were injected and trans-plantation protocols vary widely. Potential stem cells have been transplanted as early as one day [27], or as much as 90 days after irradiation [43], and in one study, cells were transplanted multiple times [60]. Such variability in the experimental time-lines compounds the difficulty of comparing studies. Additional discrepancies include the radiation dose used for the transplantation recipients, which ranges from 2 Gy to 18 Gy [27, 60], resulting in widely different degrees of salivary gland damage [62]. To demonstrate that potential stem cells can rescue irradiated salivary glands, it should first be established that the radiation dose used has caused measurable damage to the gland, including a sustained decrease in saliva secretion.
A frequent expectation in these studies is that the transplanted cells survive, engraft in the target tissue, and differentiate into acinar and duct cells. Although most studies show improved saliva secretion and reduced acinar cell loss, there is limited evidence for cell engraftment or for significant contribution of the transplanted cells to restore salivary gland tissue. An alternative possibility is that the transplanted cells promote the survival or regeneration of irradiated endogenous cells through paracrine signaling. In support of this, a recent study demonstrated that MSCs, immobilized through encapsulation in hydrogel and transplanted into irradiated glands, could improve functional saliva secretion and restore acinar cell mass [63]. Not surprisingly, one potential stem cell population identified in the salivary glands expresses elevated levels of at least seven growth factors, including glial cell line-derived neurotrophic factor (GDNF) [41]. Direct injection of these cells into irradiated salivary glands promoted saliva secretion and preserved acinar cells, as did injection of the GDNF factor alone [41]. Other studies have reported that injection of keratinocyte growth factor (KGF) or insulin-like growth factor 1 (IGF1) can rescue salivary glands from radiation damage [64–67]. These results suggest that irradiated glands can respond to paracrine signals and that endogenous cells may be induced to repair and restore gland function. Given these findings, stem cells identified through rescue of salivary gland function after transplantation should be re-evaluated to explore potential paracrine activity.
In Vivo Lineage Tracing
Heritable genetic labeling reveals the in vivo relationship between precursor and progeny, and is considered the gold standard for establishing stem cell potential [5]. Typically, lineage tracing experiments rely on Cre recombinase activity to drive heritable expression of a reporter in progeny cells. Although this powerful tool has offered a better understanding of in vivo line-age relationships, lineage tracing hinges on the specific Cre used. Caution must therefore be used when analyzing the outcome of such experiments, as Cre drivers may have dynamic expression patterns or label heterogenous cell populations.
Lineage tracing has been used to determine the in vivo potential of several proposed stem cell populations in the adult salivary glands [24, 25, 68–74]. Under normal physiological conditions, the proposed K5-expressing and K14-expressing stem cell populations labeled only duct cells [16, 17, 25, 71, 73]. However, K14 cells undergo continuous cycling in vivo, contribute to differentiated cells in the granular ducts, and retain label after 28 weeks, indicating that they are a lineage-restricted unipotent stem cell population [25]. Lineage tracing also revealed that potential stem cells expressing c-KIT [24, 70, 73] and Wntresponsive Axin2 [71] are restricted to generating duct cells in adult. In contrast to these results, lineage tracing of p63-positive cells, which contribute to all salivary gland cell lineages during embryonic development, labeled a small number of acinar cells, in addition to duct and myoepithelial cells [72]. Multiple signaling pathways are known to control p63 expression [75] and dynamic extracellular signals may influence lineage-tracing outcomes. The role of p63-expressing cells in salivary gland cell maintenance and injury repair therefore requires further investigation.
In an alternative approach, lineage tracing was used to determine how much potential stem cells in the ducts contribute to acinar cell replacement [76]. Labeling and long-term chase of all mature acinar cells showed no evidence that new cells are generated from potential duct stem cells under normal homeostasis. Furthermore, use of the versatile R26Brainbow2.1 reporter, to label single acinar cells in distinct colors, demonstrated that individual acinar cells generate clones, indicating that the acinar cell lineage is maintained by acinar cell self-duplication [76]. Consistent with this finding, lineage tracing showed that SOX2-expressing acinar cells in murine sublingual glands generate more acinar cells [70]. In combination with the lineage tracing results of proposed duct stem cells, current evidence suggests that in adult glands, acinar and duct cell line-ages are maintained separately and not through a multipotent stem cell.
Conclusion
The number of proposed stem cell populations in the adult salivary glands suggests a broad interpretation of the criteria used to define a stem cell. The purpose of this review is to highlight the caveats associated with assays used to define stem cells (Table 1). Proposed stem cells, originally identified through marker gene expression, in vitro proliferation, or sphere formation, have more recently been shown through lineage tracing to be lineage-restricted, demonstrating that they do not meet the basic stem cell criteria. Thus, the outcomes vary depending on the assays used and suggest caution when comparing published results. To date, using the assays described, no single cell meeting the stringent definition of a stem cell has been unambigu-ously identified in adult salivary glands.
Table 1.
Assays used for the identification of potential salivary gland stem cells are listed with expected outcomes, the caveats associated with each assay, and possible alternative explanations
| Stem cell assay | Expected outcome | Caveat | Alternative outcomes |
|---|---|---|---|
| Expression of stem cell markers | Cells expressing stem cell markers found in other tissues are considered stem cells | No universal marker for stem cells is known | Stem cell marker expression is not always limited to stem cells |
| Morphology and localization | Proliferating intercalated duct cells located in appropriate proximity to acini are designated as stem cells | Static analysis of cell proximity does not demonstrate a lineage relationship | Rapid division of duct cells generates only duct cells |
| Label-retaining assay | LRCs are slow cycling stem cells | Label can also be retained by postmitotic cells | LRCs can be long-lived terminally differentiated cells |
| In vitro proliferation | In vitro proliferation for more than one passage is an exclusive capability of stem cells |
|
Rapidly dividing and expanding cells may be an in vitro artifact |
| In vitro differentiation potential | Evidence of acinar, ductal, and myoepithelial markers or morphology indicates differentiation of a stem cell |
|
|
| Sphere formation | The formation of spheres in culture is evidence of stem cell activity |
|
Cells aggregated into spheres can include differentiated cells from original dissociation, and dividing cells that may not be stem cells |
| Transplantation | Rescue of salivary gland function by transplanted cells is due to regeneration by stem cells |
|
Rescue of salivary gland function may be due to paracrine activity of injected cells |
| In vivo lineage tracing | Tracing a multipotent stem cell lineage should produce acinar, duct, and even myoepithelial cells | Cell labeling is based on the promoter chosen to drive Cre, which may be dynamically regulated or expressed in multiple cell types | Lineage tracing shows that acinar and duct cells are lineage restricted, except under conditions of extreme injury |
Abbreviation: LRCs, label retaining cells.
Collectively, the evidence indicates that there are several cell populations in adult glands with varying degrees of potential, to proliferate, to differentiate, or to stimulate in a paracrine fashion. Results of lineage tracing show that acinar and duct cell lin-eages are maintained separately in the adult glands, but under conditions of severe cell loss, both cell lineages can contribute to acinar cell regeneration [71]. Evidence that differentiated cells can display cellular plasticity, particularly under stressful conditions, may help in reconciling the various stem cell populations proposed in the salivary glands. Perhaps, therapeutic approaches should be less focused on the identity of a specific stem cell and more on a cellular state that may be manipulated. Understanding cell–cell interactions that lead to plasticity, and whether this is a process that can be applied to salivary gland regeneration are critical areas for investigation.
Significance Statement.
A number of diverse and nonoverlapping cell populations have been designated as adult stem cells in the salivary glands. The present study is focused on the criteria used to define these cell populations and highlights the limitations associated with each. A critical re-evaluation of how the various cell populations were characterized may serve to clarify which cells may be useful for regenerative therapy.
Acknowledgments
We thank Dr. Dirk Bohmann for critical reading of the article. This work was supported by National Institute of Dental and Craniofacial Research (NIDCR/NIH) grants R01 DE022949 and R56DE025098 (C.E.O.); and by the Sjögren’s Syndrome Foundation 002756 (M.H.A.).
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Pringle S, Van Os R, Coppes RP. Concise review: Adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells 2013;31:613–619. [DOI] [PubMed] [Google Scholar]
- 2.Lombaert I, Movahednia MM, Adine C et al. Concise review: Salivary gland regeneration: Therapeutic approaches from stem cells to tissue organoids. Stem Cells 2017;35:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potten CS, Loeffler M. Stem cells: Attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 1990;110:1001–1020. [DOI] [PubMed] [Google Scholar]
- 4.Grompe M Tissue stem cells: New tools and functional diversity. Cell Stem Cell 2012; 10:685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopp JL, Grompe M, Sander M. Stem cells versus plasticity in liver and pancreas regeneration. Nat Cell Biol 2016;18:238–245. [DOI] [PubMed] [Google Scholar]
- 6.Barker N, van Es JH, Kuipers J et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449: 1003–1007. [DOI] [PubMed] [Google Scholar]
- 7.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science 1988;241: 58–62. [DOI] [PubMed] [Google Scholar]
- 8.Visvader JE, Clevers H. Tissue-specific designs of stem cell hierarchies. Nat Cell Biol 2016;18:349–355. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science 2010;327:542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seaberg RM, van der Kooy D. Stem and progenitor cells: The premature desertion of rigorous definitions. Trends Neurosci 2003;26: 125–131. [DOI] [PubMed] [Google Scholar]
- 11.Smith A A glossary for stem-cell biology. Nature 2006;441:1060. [Google Scholar]
- 12.Ogawa M, Matsuzaki Y, Nishikawa S et al. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med 1991;174: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hisatomi Y, Okumura K, Nakamura K et al. Flow cytometric isolation of endodermal progenitors from mouse salivary gland differentiate into hepatic and pancreatic line-ages. Hepatology 2004;39:667–675. [DOI] [PubMed] [Google Scholar]
- 14.Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci 2007;10:720–726. [DOI] [PubMed] [Google Scholar]
- 15.Rock JR, Onaitis MW, Rawlins EL et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 2009;106:12771–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knox SM, Lombaert IM, Reed X et al. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 2010;329:1645–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lombaert IM, Abrams SR, Li L et al. Combined KIT and FGFR2b signaling regulates epithelial progenitor expansion during organogenesis. Stem Cell Rep 2013;1:604–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaks V, Barker N, Kasper M et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 2008;40:1291–1299. [DOI] [PubMed] [Google Scholar]
- 19.Yi T, Lee S, Choi N et al. Single cell clones purified from human parotid glands display features of multipotent epitheliomesenchymal stem cells. Sci Rep 2016;6:36303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Man YG, Ball WD, Marchetti L et al. Contributions of intercalated duct cells to the normal parenchyma of submandibular glands of adult rats. Anat Rec 2001;263:202–214. [DOI] [PubMed] [Google Scholar]
- 21.Denny PC, Denny PA. Dynamics of parenchymal cell division, differentiation, and apoptosis in the young adult female mouse submandibular gland. Anat Rec 1999;254: 408–417. [DOI] [PubMed] [Google Scholar]
- 22.Pevny L, Rao MS. The stem-cell menagerie. Trends Neurosci 2003;26:351–359. [DOI] [PubMed] [Google Scholar]
- 23.Yu Y, Moberly AH, Bhattarai JP et al. The stem cell marker Lgr5 defines a subset of postmitotic neurons in the olfactory bulb. J Neurosci 2017;37:9403–9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwak M, Ninche N, Klein S et al. c-Kit(+) cells in adult salivary glands do not function as tissue stem cells. Sci Rep 2018;8:14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwak M, Alston N, Ghazizadeh S. Identification of stem cells in the secretory complex of salivary glands. J Dent Res 2016;95:776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rotter N, Oder J, Schlenke P et al. Isolation and characterization of adult stem cells from human salivary glands. Stem Cells Dev 2008;17:509–518. [DOI] [PubMed] [Google Scholar]
- 27.Jeong J, Baek H, Kim Y-J et al. Human salivary gland stem cells ameliorate hyposalivation of radiation-damaged rat salivary glands. Exp Mol Med 2013;45:e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato A, Okumura K, Matsumoto S et al. Isolation, tissue localization, and cellular characterization of progenitors derived from adult human salivary glands. Cloning Stem Cells 2007;9:191–205. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y-J, Kwon H-J, Shinozaki N et al. Comparative analysis of ABCG2-expressing and label-retaining cells in mouse submandibular gland. Cell Tissue Res 2008;334:47–53. [DOI] [PubMed] [Google Scholar]
- 30.Kimoto M, Yura Y, Kishino M et al. Label-retaining cells in the rat submandibular gland. J Histochem Cytochem 2008;56:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chibly AM, Querin L, Harris Z et al. Label-retaining cells in the adult murine salivary glands possess characteristics of adult progenitor cells. PLoS One 2014;9:e107893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiel MJ, He S, Ashkenazi R et al. Haematopoietic stem cells do not asymmetri-cally segregate chromosomes or retain BrdU. Nature 2007;449:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Humphreys BD. Cutting to the chase: Taking the pulse of label-retaining cells in kidney. Am J Physiol Renal Physiol 2015;308: F29–F30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwak M, Ghazizadeh S. Analysis of histone H2BGFP retention in mouse submandibular gland reveals actively dividing stem cell populations. Stem Cells Dev 2015;24:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chibly AM, Wong WY, Pier M et al. aPKCζ-dependent repression of Yap is necessary for functional restoration of irradiated salivary glands with IGF-1. Sci Rep 2018;8: 6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pringle S, Maimets M, van der Zwaag M et al. Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells 2016;34:640–652. [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan PP, Patel VN, Liu S et al. Primary salivary human stem/progenitor cells undergo microenvironment-driven acinar-like differentiation in hyaluronate hydrogel culture. Stem Cells Translational Medicine 2017;6: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gout J, Pommier RM, Vincent DF et al. Isolation and culture of mouse primary pan-creatic acinar cells. J Vis Exp 2013;50514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kishi T, Takao T, Fujita K et al. Clonal proliferation of multipotent stem/progenitor cells in the neonatal and adult salivary glands. Biochem Biophys Res Commun 2006;340:544–552. [DOI] [PubMed] [Google Scholar]
- 40.Maimets M, Rocchi C, Bron R et al. Long-term in vitro expansion of salivary gland stem cells driven by Wnt signals. Stem Cell Rep 2016;6:150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao N, Lin Y, Cao H et al. Neurotrophic factor GDNF promotes survival of salivary stem cells. J Clin Invest 2014;124:3364–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rugel-Stahl A, Elliot M, Ovitt CE. Ascl3 marks adult progenitor cells of the mouse salivary gland. Stem Cell Res 2012;8:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lombaert IM, Brunsting JF, Wierenga PK et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One 2008;3:e2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joseph NM, Morrison SJ. Toward an understanding of the physiological function of mam-malian stem cells. Dev Cell 2005;9:173–183. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Wong PP, Sjeklocha L et al. Mature hepatocytes exhibit unexpected plas-ticity by direct dedifferentiation into liver pro-genitor cells in culture. Hepatology 2012;55: 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huch M, Dorrell C, Boj SF et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013;494:247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim J-Y, Yi T, Lee S et al. Establishment and characterization of mesenchymal stem cell-like clonal stem cells from mouse salivary glands. Tissue Eng Part C Methods 2014;21: 447–457. [DOI] [PubMed] [Google Scholar]
- 48.Feng J, van der Zwaag M, Stokman MA et al. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother Oncol 2009;92:466–471. [DOI] [PubMed] [Google Scholar]
- 49.Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: A critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 2011;8:486–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nanduri LSY, Baanstra M, Faber H et al. Purification and ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Rep 2014;3:957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shubin AD, Felong TJ, Schutrum BE et al. Encapsulation of primary salivary gland cells in enzymatically degradable poly(ethylene glycol) hydrogels promotes acinar cell characteristics. Acta Biomater 2017;50:437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varghese JJ, Hansen ME, Sharipol A et al. Salivary gland cell aggregates are derived from self-organization of acinar lineage cells. Arch Oral Biol 2019;97:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 1961; 14:213–222. [PubMed] [Google Scholar]
- 54.Shackleton M, Vaillant F, Simpson KJ et al. Generation of a functional mammary gland from a single stem cell. Nature 2006;439:84–88. [DOI] [PubMed] [Google Scholar]
- 55.van Amerongen R, Bowman AN, Nusse R. Developmental stage and time dic-tate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell Stem Cell 2012;11:387–400. [DOI] [PubMed] [Google Scholar]
- 56.Nelson DA, Manhardt C, Kamath V et al. Quantitative single cell analysis of cell population dynamics during submandibular salivary gland development and differentiation. Biol Open 2013;2:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Luijk P, Pringle S, Deasy JO et al. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci Transl Med 2015;7:305ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nanduri LS, Maimets M, Pringle SA et al. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol 2011;99:367–372. [DOI] [PubMed] [Google Scholar]
- 59.Lim JY, Yi T, Choi JS et al. Intraglandular transplantation of bone marrow-derived clonal mesenchymal stem cells for amelioration of post-irradiation salivary gland damage. Oral Oncol 2013;49:136–143. [DOI] [PubMed] [Google Scholar]
- 60.Sumita Y, Liu Y, Khalili S et al. Bone marrow-derived cells rescue salivary gland function in mice with head and neck irradiation. Int J Biochem Cell Biol 2011;43:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamamura Y, Yamada H, Sakurai T et al. Treatment of salivary gland hypofunction by transplantation with dental pulp cells. Arch Oral Biol 2013;58:935–942. [DOI] [PubMed] [Google Scholar]
- 62.Vissink A, Down JD, Konings AW. Contrasting dose-rate effects of gamma-irradiation on rat salivary gland function. Int J Radiat Biol 1992;61:275–282. [DOI] [PubMed] [Google Scholar]
- 63.Choi JS, An HY, Shin HS et al. Enhanced tissue remodelling efficacy of adipose-derived mesenchymal stem cells using injectable matrices in radiation-damaged salivary gland model. J Tissue Eng Regen Med 2018;12: e695–e706. [DOI] [PubMed] [Google Scholar]
- 64.Tran SD, Liu Y, Xia D et al. Paracrine effects of bone marrow soup restore organ function, regeneration, and repair in salivary glands damaged by irradiation. PLoS One 2013; 8:e61632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi JS, Shin HS, An HY et al. Radioprotective effects of keratinocyte growth factor-1 against irradiation-induced salivary gland hypo-function. Oncotarget 2017;8:13496–13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lombaert IM, Brunsting JF, Wierenga PK et al. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells 2008;26:2595–2601. [DOI] [PubMed] [Google Scholar]
- 67.Limesand KH, Said S, Anderson SM. Suppression of radiation-induced salivary gland dysfunction by IGF-1. PLoS One 2009;4:e4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bullard T, Koek L, Roztocil E et al. Ascl3 expression marks a progenitor population of both acinar and ductal cells in mouse salivary glands. Dev Biol 2008;320:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arnold K, Sarkar A, Yram MA et al. Sox2 (+) adult stem/progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 2011;9:317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Emmerson E, May AJ, Berthoin L et al. Salivary glands regenerate after radiation injury through SOX2-mediated secretory cell replacement. EMBO Mol Med 2018;10:e8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weng PL, Aure MH, Maruyama T et al. Limited regeneration of adult salivary glands after severe injury involves cellular plasticity. Cell Rep 2018;24:1464.e1463–1470.e1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song EC, Min S, Oyelakin A et al. Genetic and scRNA-seq analysis reveals distinct cell populations that contribute to salivary gland development and maintenance. Sci Rep 2018; 8:14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.May AJ, Cruz-Pacheco N, Emmerson E et al. Diverse progenitor cells preserve salivary gland ductal architecture after radiation-induced damage. Development 2018;145:dev166363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Emmerson E, May AJ, Nathan S et al. SOX2 regulates acinar cell development in the salivary gland. Elife 2017;6:e26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoh K, Prywes R. Pathway regulation of p63, a director of epithelial cell fate. Front Endocrinol 2015;6:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aure Marit H, Konieczny Stephen F, Ovitt Catherine E. Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev Cell 2015;33:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]