Abstract
Bacteriophage therapy (BT) employs bacteriophages to treat pathogenic bacteria and is an emerging strategy against multidrug-resistant (MDR) infections. Experience in solid organ transplant is limited. We describe BT in three lung transplant recipients (LTR) with life-threatening MDR infections caused by Pseudomonas aeruginosa (n=2) and Burkholderia dolosa (n=1). For each patient, lytic bacteriophages were selected against their bacterial isolates. BT was administered for variable durations under emergency Investigational New Drug applications and with patient informed consent. Safety was assessed using clinical/laboratory parameters and observed clinical improvements described as appropriate. All patients received concurrent antibiotics. Two ventilator-dependent LTR with large airway complications and refractory MDR P. aeruginosa pneumonia received BT. Both responded clinically and were discharged from the hospital off ventilator support. A third patient had recurrent B. dolosa infection following transplant. Following BT initiation, consolidative opacities improved and ventilator weaning was begun. However, infection relapsed on BT and the patient expired. No BT-related adverse events were identified in the three cases. BT was well tolerated and associated with clinical improvement in LTRs with MDR bacterial infection not responsive to antibiotics alone. BT may be a viable adjunct to antibiotics for patients with MDR infections.
INTRODUCTION
Lytic bacteriophages are viruses that attach to bacterial surface receptors and inject their genomic DNA/RNA into host cytoplasm triggering bacterial lysis. Most bacteriophages are very specific to one or a few related strains/ species of bacteria and the large differences between bacterial prokaryotic and human eukaryotic cells prevent cross-infection. (1) Bacteriophage therapy (BT) involves the identification and administration of bacteriophages to target specific pathogenic bacteria. Experience with BT is over a century old but early clinical potential largely forgotten.(2) Limited antibiotic options for multidrug resistant (MDR) bacteria and technical advances in bacteriophage purification have led to renewed interest. Case reports have highlighted the use of BT for MDR organisms including Acinetobacter baumannii, Pseudomonas aeruginosa, and Staphylococcus aureus.(3–6). Recent case reports describe the successful use of genetically engineered BT in the treatment of disseminated Mycobacterium abscessus infection in a lung transplant recipient (LTR) (7) as well as use of BT to treat MDR P. aeruginosa infection in a CF patient that went on to successful lung transplant.(8)
LTR have uniquely high rates of MDR infections, representing an attractive population to assess the safety, tolerability and potential efficacy of adjunctive BT. In addition to the pre-transplant prevalence of MDR organisms and the risk of post-transplant re-colonization, uncontrolled allograft infection can cause anastomotic and large airway complications, including dehiscence and stenosis (9, 10). When treating MDR bacterial infections, there are limited antibiotic options, many of which have adverse events (AE), including nephrotoxicity and marrow suppression, (11) which are especially significant in LTRs given ongoing need for calcineurin and cell cycle inhibitors. (12)
We describe our clinical experience regarding BT to treat MDR infections in three LTRs.
METHODS
Three LTRs with lower respiratory tract infections were identified at the University of California San Diego, the Hospital of the University of Pennsylvania, and the Cleveland Clinic as potential candidates for BT. Two were infected with MDR P. aeruginosa and one with MDR B. dolosa.
Phages were obtained from the following sources: AmpliPhi Biosciences Corporation (San Diego, CA), Naval Medical Research Center (Fort Detrick, Maryland) and Adaptive Phage Therapeutics (Gaithersburg, MD).
BT administration: Table 1 describes details of the phages used including concentration, frequency of dosing, and route of administration. Endotoxin concentration refers to the amount of bacterial endotoxin lipopolysaccharide produced during phage mediated lysis of the host bacteria during phage propagation for production. The U.S. Food and Drug Administration (FDA) mandates a maximum administered dose of endotoxin to be less than 5 EU/kg body weight per hour.
Table 1.
Details of the bacteriophages used in the case series.
| Patient | Bacteriophage cocktail | Component Bacteriophages | Concentration per unit IV dose | Endotoxin concentration per unit IV dose | Dosing route and frequency |
|---|---|---|---|---|---|
| Case 1 | *AB-PA01 | Pa193, Pa204, Pa222, and Pa223 | 4×109 PFU/ml | 0.2 EU/ml | IV every 6 hours. ∞Nebulized every 12 hours |
| Case 1 | *AB-PA01 m1 | Pa193, Pa204, Pa222, Pa223, and Pa176 | 5×109 PFU/ml | 8.3 EU/ml | IV every 6 hours. ∞Nebulized every 12 hours |
| Case 1 | **Navy phage cocktail 1 | Paϕ1, PaSKWϕ17, and PaSKWϕ22 | 1×109 PFU/ml | 7.3×103 EU/ml | IV every 2 hours. ∞Nebulized every 6 hours |
| Case 1 | **Navy phage cocktail 2 | PaATFϕ1 and PaATFϕ3 | 5×107 PFU/ml | 1.5×103 EU/ml | IV every 4 hours |
| Case 2 | *AB-PA01 | Pa193, Pa204, Pa222, and Pa223 | 4×109 PFU/ml | 22.0 EU/ml | IV every 12 hours |
| Case 3 | Single lytic phage | ***BdPF16phi4281 | 5.3 × 106 PFU/ml – 3.5 × 107 PFU/ml† | 200 EU/ml | IV once daily for the first 2 weeks and then every 12 hours for 4 weeks. |
nebulized dose was 4 times the IV dose
Phages were prepared and supplied in five different batches over the course of treatment
EU=endotoxin units; IV=intravenous; PFU=plaque forming units
AB-PA01 is an investigational product, formulated to contain equal concentrations of 4 P. aeruginosa phages targeting ~80% of clinical P. aeruginosa isolates, available as 1 ml sterile solution (). AB-PA01 was produced at AmpliPhi Good Manufacturing Practices (GMP) facility using a proprietary production process consisting of upstream fermentation and several downstream purification steps. AB-PA01 m1 was produced using the AB-PA01 component phages manufactured in the same way, with the addition of Pa176 produced in a non-GMP facility using analogous methods.
Navy Phage cocktail 1 and 2 consisted of bacteriophages isolated from wastewater collected from water treatment facilities in Germantown, Frederick or Laurel, Maryland using common soft agar overlay techniques.(28)
BdPF16phi4281 was produced by Adaptive Phage Therapeutics and was amplified on the patient bacterial strain on solid growth media. Extracted phage lysate was clarified by centrifugation and filtration. The phage was further purified via isopycnic centrifugation on a cesium chloride gradient followed by multiple rounds of dialysis in an appropriate buffer solution to remove residual cesium. The purified phage was diluted with enough excipient to reduce the endotoxin concentration to below the maximal permissible hourly limit of 5 EU/kg (where kg=body weight in kilograms). The final formulated phage was filtered through a 0.22 micrometer filter, aseptically filled into single use vials, and stored at 4o Celsius until use.
Intravenous (IV) bacteriophage solutions were prepared just before administration when possible; however, stability testing was performed. We used the Pari eFlow® rapid nebulizer for respiratory delivery of the phage solution to Patient One.
Susceptibility Testing:
Two methods were used to assess bacterial susceptibility to phages. Susceptibility to AB-PA01 and AB-PA01-m1 was tested using a version of the double agar overlay method with nutrient broth-based liquid and agar media.(13). Isolates were classified as susceptible when individual plaques were observed in drop tests with serial dilutions. . Susceptibility testing to the Navy phage cocktails 1 and 2 as well as B. dolosa phage was assessed by inoculating bacterial suspensions (104 CFU/ml) with bacteriophages individually and in combination in a 96-well microtiter plates containing tryptic soy media in 1% (vol/vol) tetrazolium dye and incubated at 37°C in a Biolog® machine for 24 hours. Bacterial respiration reduced the tetrazolium dye which changed the color of the media to purple. This color change was depicted as relative units of bacterial growth.(14)
Bacteriophage detection in serum and respiratory samples: Glycerol-amended serum and bronchoalveolar lavage (BAL) samples were thawed, serially diluted and spotted onto an agar overlay. BAL samples were passed through a 0.2 µm polyethersulfone syringe filter prior to dilution. Positive controls were prepared by spiking an aliquot of bacteriophage-free serum/ BAL sample from the same patient with a known quantity PFU/mL of each AB-PA01 component phage.
Ethics/ Regulatory Approval:
Administration of BT was conducted at each institution with individual emergency Investigational New Drug applications (eIND) from the FDA, Institutional Review Board notification, Biohazard Use Authorization and patient informed consent.
Safety:
Safety was assessed using clinical and laboratory parameters, including AE log, vital sign monitoring, and serial complete blood count and comprehensive metabolic panels. All patients were treated in the inpatient setting and were followed closely to assess clinical resolution and/ or improvement of infection (i.e. resolution of fever, leukocytosis, sputum production) as well as development of AE.
RESULTS
Clinical Courses
Patient One
Patient One was a 67-year-old male who underwent bilateral lung transplant for hypersensitivity pneumonitis. His post-transplant course was complicated by primary graft dysfunction, prolonged mechanical ventilation, right main stem bronchial stenosis, and multiple episodes of P. aeruginosa pneumonia with antibiotic susceptibilities noted in Table 2. He required serial bronchial dilation and stenting, developed chronic lung allograft dysfunction requiring extracorporeal photopheresis, and progressive kidney injury necessitating hemodialysis. He had two distinct episodes of MDR P. aeruginosa pneumonia that were treated with BT along with concomitant antibiotics and then was maintained on BT as suppression.
Table 2.
Antibiotic susceptibility of baseline clinical bacterial isolates as determined according to Clinical & Laboratory Standards Institute guidelines.(29)
| Organism (Patient #) | Susceptible | Intermediate | Resistant |
|---|---|---|---|
| Pseudomonas aeruginosa (Patient One, Episode One) | Amikacin, tobramycin | Gentamicin, colistin | Aztreonam, pipercillin-tazobactam, meropenem, ceftazidime, cefepime, ciprofloxacin, ceftazidime-avibactam |
| Pseudomonas aeruginosa (Patient One, Episode Two) | Amikacin, tobramycin, pipercillin-tazobactam | Ceftazidime, colistin | Aztreonam, gentamicin, meropenem, doripenem, cefepime, ciprofloxacin |
| Pseudomonas aeruginosa (Patient One, Episode Two) | Tobramycin, colistin | - | Aztreonam, amikacin, gentamicin, pipercillin-tazobactam, meropenem, doripenem, ceftazidime, cefepime, ciprofloxacin |
| Pseudomonas aeruginosa (Patient Two) | Colistin | pipercillin-tazobactam | Amikacin, cefepime, ceftazidime, ceftazidime-avibactam, gentamicin, levofloxacin, meropenem, tobramycin |
| Burkholderia dolosa (Patient Three) | Minocycline | Ceftazidime, ceftazidime/avibactam, ceftolozane/avibactam, levofloxacin, meropenem, ticarcillin/clavulanate, trimethoprim/ sulfamethoxazole |
Episode One:
Patient One received a two-week course of IV and nebulized AB-PA01, a prefixed phage cocktail consisting of four P. aeruginosa phages with a staggered start of the latter (to assess differential bacteriophage BAL concentrations based on mode of delivery) as an adjunct to systemic antibiotics (piperacillin-tazobactam and colistin). At baseline, the patient required supplemental oxygen through tracheostomy at 10 liters/minute, 35% fraction of inspired oxygen and had copious purulent respiratory secretions. By two weeks of BT, he had significantly decreased inflammation and minimal respiratory secretions noted on bronchoscopy. The patient started to ambulate by day 16; and talked via a Passy-Muir valve for several hours on day 18. Concomitant antibiotics were stopped on day 18. Nebulized AB-PA01 was extended by an additional week without systemic antibiotics in an attempt to re-populate the airways with normal respiratory flora. By day 21, BAL cultures included non-P. aeruginosa bacterial species, suggesting re-establishment of respiratory flora (data not shown). The patient completed inhaled AB-PA01 therapy on day 29.
Episode Two:
On day 46, Patient One clinically decompensated following a surveillance bronchoscopy with development of fever, leukocytosis, respiratory failure requiring mechanical ventilation, and septic shock requiring vasopressors. Respiratory cultures grew mucoid MDR P. aeruginosa. Systemic antibiotics (piperacillin-tazobactam, tobramycin and inhaled colistin) were restarted and BT was employed. BT consisted of distinct courses of AB-PA01-m1 (prefixed combination AB-PA01 + one new specific phage) and Navy phage cocktail 1 (personalized phage cocktail), with clinical resolution of pneumonia.
Suppression Phase:
The patient received suppressive BT (Navy phage cocktails 1 and 2, personalized phage combination) from days 93–150. During this period as well as the following three months, there was no active P. aeruginosa pneumonia. The patient’s tracheostomy was de-cannulated, he made progress with physical rehabilitation, and was discharged from the hospital. No AE related to BT occured during treatment.
Patient Two
Patient Two was a 57-year-old female with non-CF bronchiectasis who was colonized with a MDR P. aeruginosa sensitive to only colistin (Table 2). She was bridged to transplant with ambulatory veno-venous extracorporeal membrane oxygenation. Post-transplant, she had significant bilateral airway ischemic injury and developed recurrent MDR P. aeruginosa infections. She underwent tracheostomy to facilitate ventilator weaning but severe airway stenosis limited her progress despite serial balloon dilation. She also developed Mycobacterium abscessus pulmonary infection, initially treated with imipenem, tigecycline and inhaled amikacin, . As a result of nephrotoxic antibiotic exposure, she had progressive renal failure and required hemodialysis. Due to an inability to clear P. aeruginosa from respiratory cultures and concern that ongoing infection was preventing airway healing, BT was pursued.
She was treated with a four week course of IV AB-PA01 and continued only on inhaled colistin concomitantly. There were no BT-related AE and patient clinically responded to treatment. Markers of inflammation (serum sedimentation rate, C-reactive protein and pro-calcitonin) did not change significantly during AB-PA01 administration.
No additional P. aeruginosa was cultured from BAL specimens following the start of BT treatment until 60 days after completion of AB-PA01 treatment. The isolate grown at day 60 and subsequent strains showed improved antibiotic susceptibility. Additional infections were successfully treated with piperacillin-tazobactam. She was successfully weaned from the ventilator by day 90 and discharged from the hospital. She did not grow additional M. abscessus in the seven months following BT administration and mycobacterial therapy was stopped after 90 days of treatment. She was subsequently readmitted with an aspiration pneumonia but did not grow MDR P. aeruginosa. She remains on inhaled colistin for suppressive therapy.
Patient Three
Patient Three was a 28-year-old female with CF colonized with MDR B. dolosa sensitive only to minocycline (Table 2). At the time of bilateral lung transplant, she received perioperative systemic trimethoprim/sulfamethoxazole, minocycline, and meropenem with amikacin irrigation intra-operatively. Four days following transplant, B. dolosa was grown from bilateral pleural spaces and eight days post-transplant grew B. dolosa from an endobronchial culture. The pleural spaces remained infected despite systemic and intra-pleural antimicrobial therapies and effective drainage. Two months after transplant an isolate was sent for identification of a lytic bacteriophage, but a suitable candidate was not identified until three months later. In the interim she developed recurrent episodes of pneumonia due to B. dolosa. She was treated with minocycline, meropenem, and trimethoprim/sulfamethoxazole (based on in vitro susceptibilities) but experienced ongoing drug-related toxicities and remained ventilator-dependent.
She eventually responded favorably to a six week course of combined therapy with extended infusions of ceftazidime-avibactam and piperacillin-tazobactamHowever, pneumonia recurred three weeks after completing that course. Now six months post-transplant, the same antibiotic regimen was resumed and, one week later, BdPF16phi4281 (a single lytic phage with in vitro anti-B. dolosa activity) was added (Table 1). She had no BT-related AE. After BT initiation, she had improvement of her fever and leukocytosis, decreased airway secretions, improved consolidations on imaging, and decreased B. dolosa burden on BAL culture. After six weeks of combined therapies she was ambulating 660 feet and became ventilator free for up to 12 consecutive hours. However, she developed progressive renal failure ascribed to calcineurin inhibitor toxicity, and, two weeks later, had severe hemorrhage from a splenic artery pseudoaneurysm requiring splenectomy. Post-operatively, she developed progressive liver injury thought to be multifactorial (including existing underlying CF hepatopathy, hypotension, and drug induced injury). On week ten of BT, she developed septicemia from B. dolosa bloodstream infection and pneumonia. Based in part on a clinical case report and updated susceptibilities, her antibiotics were changed to extended infusion meropenem, extended infusion ceftazidime-avibactam, minocycline, and inhaled tobramycin (15). Her sepsis resolved, bloodstream infection cleared and her respiratory status improved. However, she developed progressive liver failure with concern for drug-induced toxicity, including from minocycline, dapsone, and posaconazole prophylaxis. She had completed the planned 12-week course of BT at that time and it was decided that the treatment was unlikely of ongoing benefit. Antibiotics were also stopped due to toxicities. A liver biopsy demonstrated severe cholestasis and hepatocyte swelling. After one week off antibiotics, she again developed pneumonia and demonstrated heavy growth of B dolosa from a BAL sample. Despite antibiotic treatment, she clinically worsened, and transitioned to hospice. She died 11 months following lung transplantation. No autopsy was obtained.
Bacteriophage detection in serum and respiratory samples (Patient One)
No bacteriophages were detected prior to BT initiation. Once administration was started, no viable AB-PA01 or AB-PA01-m1 phages were detected in serum samples drawn within 30 minutes of the next IV dose. High recovery of spiked phages from serum samples suggested that serum itself did not interfere with detection of phages. Each IV dose of AB-PA01 consisted of 4×109 plaque forming units (PFU). Approximately 4×107 PFU/milliliters (ml) were recovered in BAL samples obtained three days after initiation of IV AB-PA01 therapy (and prior to administration of inhaled AB-PA01) and day 29 (when the patient had received inhaled AB-PA01 only for the previous week) (Figure 1). Thus, IV and inhaled AB-PA01 each reached similar pulmonary concentrations, with both modalities exhibiting intrapulmonary phage recovery.
Figure 1.
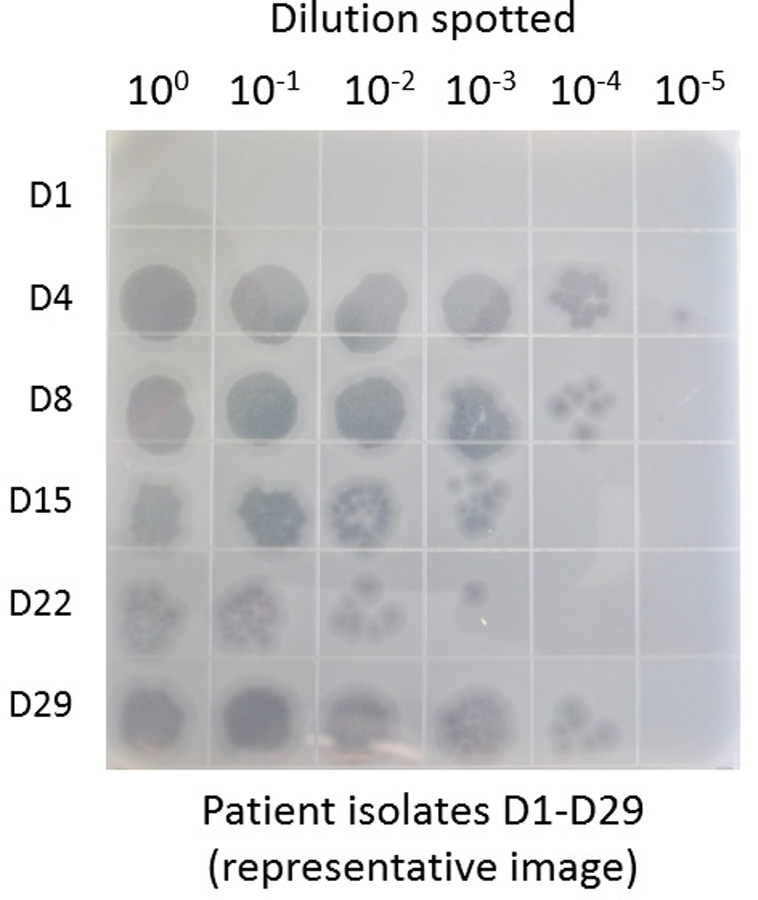
Detection of active bacteriophages in respiratory samples during use of AB-PA01 for Patient One. Collected BAL samples were serially diluted in phage buffer and the phage titer of AB-PA01 assessed in triplicate using the double agar overlay method with nutrient broth-based liquid and agar media Bacteriophage was absent in BAL prior BT (day 1 ) and were measured at high concentrations during therapy (days 4–29).
In vitro bacterial susceptibility to individual phages and phage cocktails
Patient One:
P. aeruginosa isolates were susceptible to all four component bacteriophages of AB-PA01 while on BT (Table 3). Day 36 isolate, cultured seven days after cessation of AB-PA01, was resistant to all 4 component bacteriophages of AB-PA01. Pa176, which was added to create AB-PA01-m1, was chosen to target this isolate. All subsequent P. aeruginosa isolates were susceptible to at least one of the AB-PA01-m1 components.
Table 3.
Susceptibility of serial P. aeruginosa to AB-PA01, AB-PA01-m1, and their component phages [Patient One].*
| Treatment | Timepoint | Susceptibility to Product and Component Phages | |||
|---|---|---|---|---|---|
| AB-PA011 | AB-PA01-m12 | ||||
| 14 days pre-treatment | Susceptible (all 4 phages) | NA | |||
| 9 days pre-treatment | Susceptible (all 4 phages) | NA | |||
| AB-PA01 | IV | Day 13 | Susceptible (all 4 phages) | NA | |
| Day 4 | Susceptible (all 4 phages) | NA | |||
| Day 8 | Susceptible (all 4 phages) | NA | |||
| Neb | Day 15 | Susceptible (all 4 phages) | NA | ||
| Day 22 | Susceptible (Pa222, Pa223) | NA | |||
| Day 29 | Susceptible (Pa193, Pa204) | NA | |||
| 3 week interregnum | Day 36 | Resistant 5 | Susceptible (Pa176) | ||
| Day 46 | Susceptible (all 4 phages) | Susceptible (all 5 phages) | |||
| AB-PA01-m1 | Neb | IV | Day 53 | (no culture) | (no culture) |
| Day 56 | NA | Susceptible (Pa193, Pa204, Pa176) | |||
| Day 60 | NA | Susceptible (Pa193, Pa204, Pa176) | |||
| Day 64 | NA | Susceptible (Pa193, Pa204, Pa176) | |||
| Navy 1 | Neb | IV | |||
| Day 69 | NA | Susceptible (Pa176) | |||
| Day 744 | NA | Susceptible (Pa176) | |||
| Day 77 | NA | No | |||
| Day 78 | (no PA) | (no PA) | |||
| AB-PA01-m1 | Neb | IV | |||
| Day 82 | (no PA) | (no PA) | |||
| Day 88 | (no PA) | (no PA) | |||
| Day 92 | (no PA) | (no PA) | |||
| 1 | IV | Day 110 | NA | Susceptible (Pa222, Pa223) | |
| Navy 2 | Day 114 | NA | Susceptible (all 5 phages) | ||
| Day 1414 | NA | Susceptible (Pa222, Pa223) | |||
Samples were tested in triplicate using independent bacterial cultures and serial dilutions of standardized bacteriophage preparations. Isolates were only classified as “susceptible” when individual plaques were observed in drop tests with serial dilutions..
“Susceptibility to individual phages is indicated in brackets.
AB-PA01 contains four phages
AB-PA01-m1 is AB-PA01 plus one additional phage (total five phages)
Samples was collected prior to first BT
Two isolates were obtained from these samples; both of which yielded similar phage susceptibility patterns.
This isolate, collected during the period when no phage product was being administered, prompted the addition of Pa176 to AB-PA01, leading to \AB-PA01-m1.
NA = not applicable, since the product was neither in use nor being considered for use at this time point
Figure 2 shows bacterial time-kill curves of Patient One’s clinical isolates by P. aeruginosa Navy phage cocktail 1 and 2 (individual components as well as both cocktails). Resistance developed over time to Navy phage cocktail 1 and its individual components as indicated by a shift in the time-kill curves to the left in Panel B versus Panel A. Panel B also shows lytic activity of Navy phage cocktail 2 compared to 1.
Figure 2.
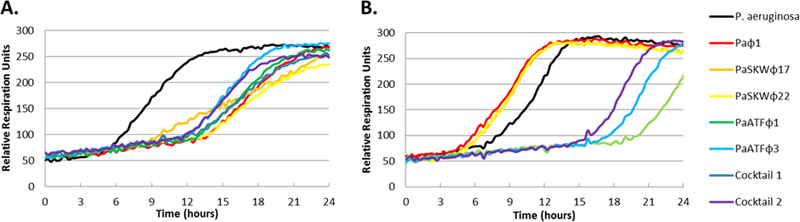
Effects of Navy phage cocktails 1 and 2 on Patient One’s P. aeruginosa isolates. P. aeruginosa isolates (104 CFU/ml) were inoculated with bacteriophage cocktail individually and in combination in a 96-well microtiter plate containing tryptic soy media in 1% (vol/vol) tetrazolium dye and incubated at 37°C in a Biolog® machine for 24 hours to evaluate bacteriophage killing activities. Bacterial respiration reduced the tetrazolium dye which changed the color of the media to purple. This change of color was recorded by a camera and depicted as relative units of bacterial growth. (A) The graphs depict the growth of P. aeruginosa day 29 isolate in presence of individual bacteriophages (Paϕ1, PaSKWϕ17, PaSKWϕ22, PaATFϕ1 and PaATFϕ3) along with Navy phage cocktails 1 and 2. (B) The graphs depict the growth of P. aeruginosa isolate on Day 95 in presence of individual Navy phages (Paϕ1, PaSKWϕ17, PaSKWϕ22, PaATFϕ1 and PaATFϕ3) along with Navy phage cocktails 1 and 2.
Patient Two:
No P. aeruginosa isolates grew for the patient until two months after BT was completed. Two pre-BT isolates were susceptible to AB-PA01 specifically to two of the four component phages (not shown).
Patient Three:
B. dolosa isolates were not tested for phage susceptibility during or after therapy. Two baseline isolates prior to BT were susceptible (not shown).
Adverse Events: IV and nebulized formulations of bacteriophages used in the series were well tolerated without AE attributed to bacteriophage administration.
DISCUSSION
In this case series of LTRs with MDR bacterial infections, BT was well tolerated with no clear BT-related adverse events. When used in combination with systemic or inhaled antibiotics, we noted clinical improvement. In Patient Two, we observed microbiological eradication, which had not been achieved at any prior time point with antibiotics alone. This case series suggests that bacteriophages are a novel adjunct anti-bacterial therapy for the treatment of MDR bacterial infections among LTRs.
Recent clinical use of BT has covered a range of applications, including skin and soft tissue infections, bacteremia, sepsis, osteomyelitis, otitis media, and indwelling medical devices (3, 4, 16–19). There has been growing interest in BT for patients with suppurative advanced lung disease, and animal models have shown BT efficacy for MDR organisms found in CF such as Burkholderia cenocepacia and Klebsiella pneumoniae (20–22). BT may be useful in LTRs for several reasons: 1) to kill specific pathogens while theoretically allowing non-pathogenic microorganisms to re-populate the pulmonary microbiome, 2) to apply directional selection that might render the bacteria more susceptible to antibiotics or less fit in vivo even at cost of developing phage-resistant bacteria (23, 24) and, 3) to penetrate and lyse biofilm-state bacteria that are traditionally less susceptible to antibiotics, such as on stents. Our cases suggest that a combination of the above factors in addition to systemic antibiotics may act together to resolve infection (25, 26). In the two LTRs with MDR P. aeruginosa infections, adjunctive BT may have contributed to clinical improvement through a combination of these mechanisms. Other factors affecting clinical resolution of illness may include phage penetration into the infected site (both P. aeruginosa cases were pneumonias while the B. dolosa pneumonia extended into the pleural cavity), duration of the infection prior to BT treatment, and/or development of phage mediated modulation of immune response directed towards the pathogen.
Development of resistance to the bacteriophage is a concern when utilizing BT (3). In Patient One, one isolate resistant to AB-PA01 appeared after cessation of AB-PA01 and one isolate resistant to AB-PA01-m1 was noted following cessation of AB-PA01m1, but none while on BT. P. aeruginosa isolates also developed resistance while on Navy phage cocktail 1 and this was overcome with the use of a personalized approach and identification of new phages active against the P. aeruginosa isolates. In terms of the B. dolosa case, susceptibility testing of the isolates obtained during BT was not done. We hypothesize that the low concentration titer of this phage (106−7 PFU/ml), less frequent dosing interval, and therapy with a single phage rather than combination, may have led to either inadequate concentration at the site of infection and/ or development of resistance (though we did not test this specifically).
There were no significant BT-related AEs in the three patients. Although Patient Three did not survive, , the sequence of events that led to multiorgan failure are more plausibly ascribed to post-splenectomy complications and liver disease rather than BT. Patient Two did not have clinical evidence of acute cellular or antibody mediated rejection following BT.
Although Patient One subsequently grew P. aeruginosa strains in respiratory specimens, the antibiotic resistance patterns differed from the original isolates (data not shown). This was true for Patient Two as well, whose P. aeruginosa isolates two months after BT cessation had different antimicrobial susceptibility patterns than the original isolates and demonstrated increased in vitro susceptibility to several antibiotic classes.
In this case series, we described two different approaches to the clinical use of BT in a real world setting. One was the use of a pre-existing investigational product (Patients One and Two) and the other was a personalized approach, including the isolation and development of specific bacteriophage(s) against patient’s specific clinical isolates (Patient One and Three). We believe that both approaches may be clinically relevant, depending on the infection being treated. For bacteria that lack susceptibility to pre-existing products, personalization may offer the only feasible option of BT.
In addition to the potential of BT to treat MDR bacterial infections, other potential benefits include avoidance of antibiotic toxicity and potential changes in microbial susceptibility patterns rendering the organism more susceptible to antibiotics (27).
Challenges in obtaining BT for our patients were based mainly on the experimental nature of this form of therapy. Even with the use of a pre-existing phage combination, such as AB-PA01, there was a delay of several weeks from sending patients’ bacterial isolates to a research laboratory for susceptibility testing, development of a treatment plan, approval from the FDA and then the actual logistics of shipping to the hospital (though this timeline has shortened with subsequent cases, as all parties have become more accustomed to the process). The personalized phage approach took longer particularly for the third case as a “phage hunt” needed to be carried out to find lytic phages for a particular bacterial isolate, genotypic characterization, growth in sufficient quantities, and endotoxin removal so that it would be safe to use in a patient. With the growth of extensive phage libraries both in the academic and commercial setting, we hope that the time to match a lytic phage to a bacterial pathogen and subsequent production of suitable safe products will be reduced.
We report early clinical experience of BT in LTRs targeting drug-resistant pathogens that are common in this patient population. Many questions not directly addressed in this study require further investigation. These include optimizing the timing, route, and frequency of administration (inhaled/ intravenous/ both), and duration of therapy; monitoring for the development of phage resistance, impact of dialysis and renal and/or hepatic insufficiency on phage kinetics; bacteriophage-specific antibody responses and complement mediated clearance, interactions with transplant medications including antiviral therapy; surveillance for bacteriophage and allograft directed humoral immune response’ and use of BT as suppressive versus acute treatment.
Conclusions
BT was well tolerated and associated with clinical improvement when used as an adjunct to antibiotics in LTRs with MDR respiratory infections otherwise not responsive to antibiotics alone. BT deserves further evaluation in well-designed clinical trials. in the era of increasing antimicrobial resistance.
ACKNOWLEDGEMENTS
Presented in part at the ISHLT 38th Annual Meeting and Scientific Sessions held in Nice, France from April 11–14, 2018. A portion is accepted for presentation at the ISHLT 39th Annual Meeting and Scientific Sessions to be held in Orlando, FL from April 3–6, 2019.
The views expressed in this article reflect the results of research conducted by the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government. BB, BB, CD, MH, TH, and TL are military service member or federal/contracted employee of the United States government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that copyright protection under this title is not available for any work of the United States Government. Title 17 U.S.C. 101 defines a U.S. Government work as work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Funding information:
BB, MH, TL, BNB, TH, and CD report funding by work unit number A1417. JA reports funding by the Antibacterial Resistance Leadership Group (grant number 5 UM 1AI104681-05) and the National Institutes of Health (grant number K01-AI137317). SS, RTS, and SA report funding by the Center for Innovative Phage Applications and Therapeutics (IPATH) through the University of California San Diego Chancellor’s Campus Innovation Fund.
Abbreviations
- BAL
bronchoalveolar lavage
- BT
Bacteriophage therapy
- CF
cystic fibrosis
- eIND
emergency Investigational New Drug
- EU
endotoxin units
- FDA
Food and Drug Administration
- GMP
Good Manufacturing Practices
- IV
intravenous
- LTR
lung transplant recipients
- mL
milliliters
- MDR
multidrug-resistant
- NA
not applicable
- PFU
plaque forming units
Footnotes
DISCLOSURES
SL, SM, CLLF, and FR are employed by AmpliPhi Biosciences. MJB is the Chief Medical Officer of Adaptive Phage Therapeutics and owns equity in the company. JRF and MS are employed by Adaptive Phage Therapeutics. SAS is a stockholder in Adaptive Phage Therapeutics. RTS is a member of the AmpliPhi Scientific Advisory Board (without compensation).
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Di Giovine M, Salone B, Martina Y, Amati V, Zambruno G, Cundari E et al. Binding properties, cell delivery, and gene transfer of adenoviral penton base displaying bacteriophage. Virology 2001;282(1):102–112. [DOI] [PubMed] [Google Scholar]
- 2.Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S et al. Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol 2010;11(1):69–86. [DOI] [PubMed] [Google Scholar]
- 3.Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob Agents Chemother 2017;61(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duplessis C, Biswas B, Hanisch B, Perkins M, Henry M, Quinones J et al. Refractory Pseudomonas Bacteremia in a 2-Year-Old Sterilized by Bacteriophage Therapy. J Pediatric Infect Dis Soc 2018;7(3):253–256. [DOI] [PubMed] [Google Scholar]
- 5.Hraiech S, Bregeon F, Rolain JM. Bacteriophage-based therapy in cystic fibrosis-associated Pseudomonas aeruginosa infections: rationale and current status. Drug Des Devel Ther 2015;9:3653–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aslam S, Pretorius V, Lehman SM, Morales S, Schooley RT. Novel bacteriophage therapy for treatment of left ventricular assist device infection. J Heart Lung Transplant 2019. [DOI] [PubMed] [Google Scholar]
- 7.Dedrick RM, Guerrero-Bustamante CA, Garlena RA, Russell DA, Ford K, Harris K et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med 2019;25(5):730–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law N, Logan C, Yung G, Furr CL, Lehman SM, Morales S et al. Successful adjunctive use of bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa infection in a cystic fibrosis patient. Infection 2019. [DOI] [PubMed] [Google Scholar]
- 9.Ruttmann E, Ulmer H, Marchese M, Dunst K, Geltner C, Margreiter R et al. Evaluation of factors damaging the bronchial wall in lung transplantation. J Heart Lung Transplant 2005;24(3):275–281. [DOI] [PubMed] [Google Scholar]
- 10.Yserbyt J, Dooms C, Vos R, Dupont LJ, Van Raemdonck DE, Verleden GM. Anastomotic airway complications after lung transplantation: risk factors, treatment modalities and outcome-a single-centre experience. Eur J Cardiothorac Surg 2016;49(1):e1–8. [DOI] [PubMed] [Google Scholar]
- 11.Friedman ND, Temkin E, Carmeli Y. The negative impact of antibiotic resistance. Clin Microbiol Infect 2016;22(5):416–422. [DOI] [PubMed] [Google Scholar]
- 12.Ivulich S, Westall G, Dooley M, Snell G. The Evolution of Lung Transplant Immunosuppression. Drugs 2018;78(10):965–982. [DOI] [PubMed] [Google Scholar]
- 13.Mazzocco A, Waddell TE, Lingohr E, Johnson RP. Enumeration of bacteriophages using the small drop plaque assay system. Methods Mol Biol 2009;501:81–85. [DOI] [PubMed] [Google Scholar]
- 14.Henry M, Biswas B, Vincent L, Mokashi V, Schuch R, Bishop-Lilly KA et al. Development of a high throughput assay for indirectly measuring phage growth using the OmniLog(TM) system. Bacteriophage 2012;2(3):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canton-Bulnes ML, Hurtado Martinez A, Lopez-Cerero L, Arenzana Seisdedos A, Merino-Bohorquez V, Garnacho-Montero J. A case of pan-resistant Burkholderia cepacia complex bacteremic pneumonia, after lung transplantation treated with a targeted combination therapy. Transpl Infect Dis 2018:e13034. [DOI] [PubMed] [Google Scholar]
- 16.Fish R, Kutter E, Bryan D, Wheat G, Kuhl S. Resolving Digital Staphylococcal Osteomyelitis Using Bacteriophage-A Case Report. Antibiotics (Basel) 2018;7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhoads DD, Wolcott RD, Kuskowski MA, Wolcott BM, Ward LS, Sulakvelidze A. Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. J Wound Care 2009;18(6):237–238, 240–233. [DOI] [PubMed] [Google Scholar]
- 18.Wright A, Hawkins CH, Anggard EE, Harper DR. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol 2009;34(4):349–357. [DOI] [PubMed] [Google Scholar]
- 19.Aslam S PV, Lehman SM, Morales S, Schooley RT. Novel Bacteriophage Therapy for Treatment of Left Ventricular Assist Device Infection. Journal of Heart and Lung Transplantation 2019 [DOI] [PubMed] [Google Scholar]
- 20.Semler DD, Goudie AD, Finlay WH, Dennis JJ. Aerosol phage therapy efficacy in Burkholderia cepacia complex respiratory infections. Antimicrob Agents Chemother 2014;58(7):4005–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singla S, Harjai K, Katare OP, Chhibber S. Bacteriophage-loaded nanostructured lipid carrier: improved pharmacokinetics mediates effective resolution of Klebsiella pneumoniae-induced lobar pneumonia. J Infect Dis 2015;212(2):325–334. [DOI] [PubMed] [Google Scholar]
- 22.Carmody LA, Gill JJ, Summer EJ, Sajjan US, Gonzalez CF, Young RF et al. Efficacy of bacteriophage therapy in a model of Burkholderia cenocepacia pulmonary infection. J Infect Dis 2010;201(2):264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coulter LB, McLean RJ, Rohde RE, Aron GM. Effect of bacteriophage infection in combination with tobramycin on the emergence of resistance in Escherichia coli and Pseudomonas aeruginosa biofilms. Viruses 2014;6(10):3778–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glonti T, Chanishvili N, Taylor PW. Bacteriophage-derived enzyme that depolymerizes the alginic acid capsule associated with cystic fibrosis isolates of Pseudomonas aeruginosa. J Appl Microbiol 2010;108(2):695–702. [DOI] [PubMed] [Google Scholar]
- 25.Chan BK, Turner PE, Kim S, Mojibian HR, Elefteriades JA, Narayan D. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol Med Public Health 2018;2018(1):60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harper DR, Enright MC. Bacteriophages for the treatment of Pseudomonas aeruginosa infections. J Appl Microbiol 2011;111(1):1–7. [DOI] [PubMed] [Google Scholar]
- 27.Reindel R, Fiore CR. Phage Therapy: Considerations and Challenges for Development. Clin Infect Dis 2017;64(11):1589–1590. [DOI] [PubMed] [Google Scholar]
- 28.Regeimbal J, Bardwell JC. DsbB catalyzes disulfide bond formation de novo. J Biol Chem 2002;277(36):32706–32713. [DOI] [PubMed] [Google Scholar]
- 29.Hindler JF, Stelling J. Analysis and presentation of cumulative antibiograms: a new consensus guideline from the Clinical and Laboratory Standards Institute. Clin Infect Dis 2007;44(6):867–873. [DOI] [PubMed] [Google Scholar]


