Abstract
Background:
Bipolar disorder (BD) is characterized by cognitive impairments that are known to predict psychosocial functioning and quality of life. While cognitive remediation (CR) was originally developed to directly target cognitive symptoms in traumatic brain injury and psychotic illnesses, the efficacy of CR in BD has begun to emerge only in the last decade. Functional Remediation (FR) is an integrated intervention that has been developed to restore psychosocial functioning by means of ecological neurocognitive techniques that involve psychoeducation about cognitive dysfunctions and their impact on the general functioning. Because of the heterogeneity of treatment targets and mechanisms of actions, here we aim to illustrate the effects induced by existing CR/FR approaches in BD.
Methods:
In this systematic review, we evaluated cognitive and functional outcomes after CR/FR in studies conducted in BD.
Results:
Eleven studies met inclusion criteria: 3 RCTs that compared CR/FR to one or more control condition (n=354), 5 secondary analyses that further examined data from these trials, 2 single-arm studies, and 1 naturalistic study. While features such as the use of computerized training tools and a group-based format recurred across studies, CR/FR paradigms targeting different cognitive and functional domains showed specificity of training focus to outcomes. Effect sizes were in the medium-large range, suggesting that patients with BD respond to treatment at or above the level reported in psychotic patients. Integrated approaches that combined cognitive exercises with group-based experiences were associated with both cognitive and functional improvements.
Conclusions:
In this review, we found support for the use of CR/FR paradigms in patients with BD with evidence of cognitive and functional improvements. The scarcity of currently published RCTs as well as of data examining mechanisms of action and neural correlates limits the generalizability of our findings.
Introduction
Bipolar Disorder (BD) is a mental disorder characterized by recurrent episodes of mania/hypomania and depression and by a series of trait-like neurocognitive impairments in various cognitive domains such as attention, executive functions, verbal memory and learning, and social cognition (Bozikas et al., 2006; Dixon et al., 2004), that are present even during euthymia and periods of symptom remission (Bourne et al., 2013; Carvalho et al., 2015; Robinson et al., 2006). As symptoms of BD arise, cognitive functioning deteriorates, and these cognitive deficits are negatively impacted by the effects of number of episodes, life stress, illness progression, and chronic antipsychotic medication usage (Donaldson et al., 2003; Kapczinski et al., 2009). Because cognitive impairments are critical mediators of adverse psychosocial outcomes and a predictor of unfavorable employment outcomes (Baune and Malhi, 2015; Tse et al., 2014), it is critical to consider them a clinical target in order to improve both psychosocial functioning and quality of life of patients with BD. To date, 14 trials (11 RCTs and 3 open label studies) have tested pharmacological agents as pro-cognitive enhancers for BD. Despite the efforts done so far to identify promising candidates, no drug has shown efficacy in treating cognitive deficits in BD (Sanches et al., 2015). Nonpharmacological approaches such as Cognitive Remediation (CR) – shown to produce moderate, durable effects on cognitive performance in patients with schizophrenia (Wykes et al., 2011) – represent a promising tool to target cognitive deficits in BP. Cognitive remediation (CR) is defined a set of behavioral interventions directly targeting cognitive symptoms with the aim of improving functional outcome (Cognitive Remediation Expert Working Group). Functional remediation (FR) is a variant of standard CR that tackles neurocognitive deficits in an individual and group-based ecologic setting while providing education about cognitive deficits and their impact on daily life situations, providing strategies to manage cognitive deficiencies in different cognitive domains, mainly in attention, memory and executive functions (Martínez-Arán, 2011; Torrent et al., 2013). To date, CR/FR is implemented without knowledge about individual variation that influence therapeutic response. This “one-size-fits-all” approach to CR/FR is problematic, with treatment failure occurring often and incurring substantial cost to the patient, family, and social system. To bend the curve on the individual outcomes of BD and prevent functional deterioration, we must advance our understanding of the mechanisms of action of CR/FR both at a cognitive and neural level. Here, we review the available literature on CR and FR as applied to BD with the goal of better characterizing the cognitive, functional and neural changes induced by these treatments.
Methods
1. Search Strategy
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al., 2009). Peer-reviewed, English-language research articles were selected for the review; relevant review and meta-analytic reports were also selected. We identified studies for inclusion through searching the electronic databases PubMed, PsycINFO, and EMBASE. A 2012 systematic review (Anaya et al., 2012) covered the topic of CR in BD between January 1990 and December 2010. Two sets of keyword search algorithms were used, linked with the Boolean operator AND. The first was related to diagnosis and included the term “bipolar”. The second set of search terms was related to cognitive remediation: “cognitive training” OR “cognitive remediation” OR “cognitive rehabilitation” OR “functional remediation”. Using these criteria, all seven authors screened title and abstract of search results. During this screening phase, we excluded study protocols that clearly failed to meet inclusion criteria (below). Whenever at least one author raised concerns about study inclusion, the full text was inspected and all authors discussed until a consensus was reached. For all search results that passed the first screening, we retrieved and reviewed the full texts. Additionally, at this stage we cross-referenced lists of included studies to gather any papers that the search terms had not identified.
2. Eligibility Criteria and Study Selection
We aimed to evaluate the effects of CR and FR on cognition, functioning, and neural systems in patients with BD. Note that CR is a broad-based term that can be used to refer to range of approaches for the amelioration of cognitive symptoms. We did not specify any parameters regarding the type of CR paradigm employed by the studies, other than the following eligibility criteria. Studies were included if they: 1) presented findings from randomized controlled trials (RCTs), naturalistic, or single-arm studies focusing on CR or FR; 2) exclusively included patients with a diagnosis of BP; 3) were peer-reviewed English language original articles published between January 1990 and February 2019; and 4) were secondary analyses or extended follow-up studies of original trials previously reported. Studies were excluded if: 1) they only provided data on feasibility, acceptability or engagement, single case studies, or no data (e.g. published study protocols); and 2) they did not include participants with diagnoses of BP confirmed by a clinician or through an initial assessment. For articles that were not rated as eligible by all authors, we held a discussion meeting where we analyzed any disagreements until a consensus about study inclusion was reached. All articles matching our eligibility criteria were reviewed in full by all authors.
From each RCT, naturalistic, and single-arm primary study, we extracted within-group effect sizes of measured cognitive or functional outcomes. In addition to cognitive and functional data, two authors independently recorded information concerning demographics, clinical characteristics (diagnosis, age of onset, and duration of illness), as well as potential moderator variables. Risk of bias assessment was in line with guidelines from the Agency for Healthcare Research and Quality Evidence-based Practice Center (Viswanathan et al., 2008). Whenever risk of bias was deemed not low, and/or for every mismatch in extracted data, all authors discussed until a consensus was reached. Given the heterogeneity of study designs and samples, we did not code variables related to medication.
Results
We conducted full database searches in February 2019, with the inclusion and exclusion criteria identified prior to the collection period. Figure 1 shows a PRISMA flowchart of each stage of the search process. Eleven studies were included in the review (see Table 1): three RCTs that compared CR/FR to one or more control conditions were included in the primary results (see Table 1); five secondary analyses that leveraged data from these RCTs, two single-arm studies, and one naturalistic study.
FIGURE 1.
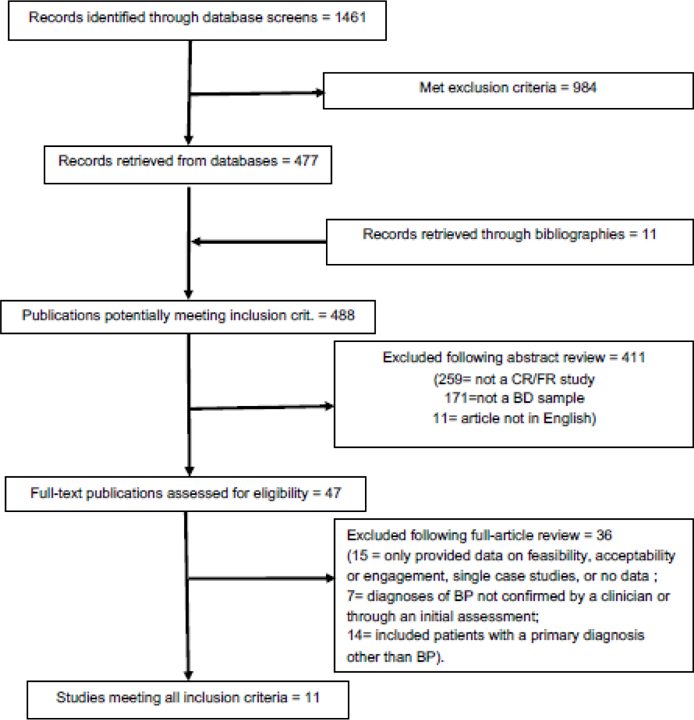
Flow Diagram
Table 1:
Summary of the included studies.
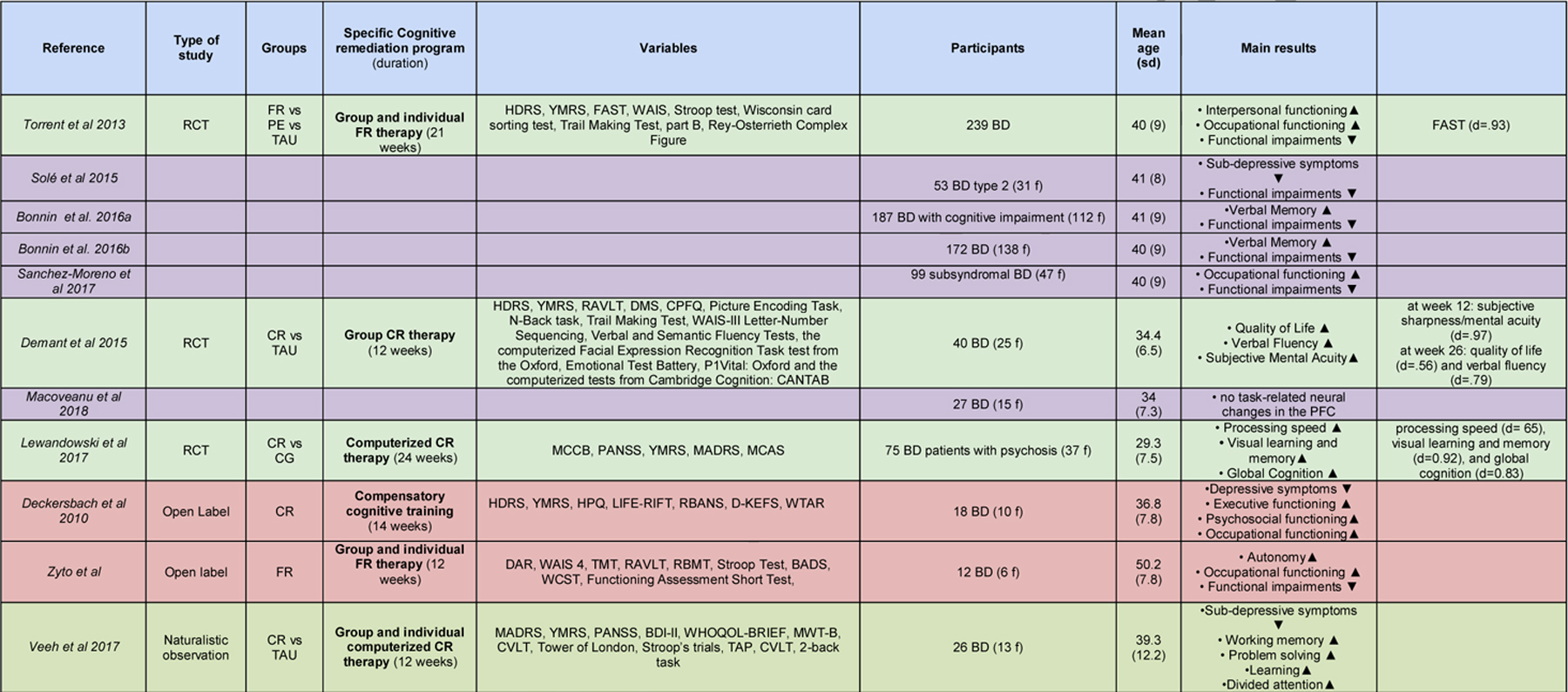 |
NOTE: Green indicates RCTs. Yellow indicates secondary studies of RCTs. Orange indicates open-label studies. Grey indicates observational studies.
LEGEND: (alphabetical order) abCR: action-based cognitive remediation therapy. BD: participants with Bipolar Disorder. BDI-II: Beck Depression Inventory. CG: Computer Games; CPFQ: Cattell’s Personality Factor Questionnaire. CR: cognitive remediation therapy. CVLT: California Verbal Learning Test. DMS: Delayed Matching to Sample test. f: female participants. FAST: Functioning Assessment Short Test, FR: functional remediation therapy. HAM-D: Hamilton Depression Rating Scale. HC: healthy control participants. HPQ: Health Perceptions Questionnaire. HVLT: Hopkins Verbal Learning Test–Revised. LIFE-RIFT: Range of Impaired Functioning Tool. MADRS: Montgomery-Asberg Depression Rating Scale. MCAS:Multnomah Community Ability Scale. MCCB: The MATRICS Consensus Cognitive Battery. MINI-plus: Mini-International Neuropsychiatric Interview. MWT-B: multiple-choice word test. NART: National Adult Reading Test. PANSS: Positive and Negative Syndrome Scale. PE: Psychoeducational therapy. RAVLT: Rey Auditory Verbal Learning Test. RCT: randomized control trial experiment. SCZ: participants with Schizoaffective Disorder. SMI: participants with Serious Mental Illnesses. TAP: Test of Attentional Performance. TAU: treatment as usual. WAIS-R: Wechsler Adult Intelligence Scale - Revised. WAIS: Wechsler Adult Intelligence Scale. WHOQOL-BREF: WORLD HEALTH ORGANIZATION quality of life assessment. YMRS: Young Mania Rating Scale. ▲: increased / improved ▼: reduced / decreased.
RCT studies
We found 3 RCTs evaluating the effects of CR and FR on BD patients (Demant et al., 2015; Lewandowski et al., 2017; Torrent et al., 2013).
In the study by Torrent and colleagues (Torrent et al., 2013), 268 BD patients were randomly assigned to three different groups: (i) Treatment As Usual (TAU) group (n=80), (ii) FR group (n=77) and (iii) Psychosocial Education group (PE, n=82). Patients receiving FR (21 weekly sessions, each lasting 90 minutes) improved the overall psychosocial outcome (specifically, the interpersonal and occupational functioning) compared to TAU but not to PE, as measured the Functioning Assessment Short Test (FAST, Rosa et al., 2007), with a large effect size (d= .93). Interestingly, no differential cognitive improvements were reported in this RCT. Several secondary analyses were carried out on this dataset. In the subsample of BD type 2 patients (n=53), Solé and colleagues found a trend in favor of a better outcome for FR on the FAST total score (but not in the subdomains) when compared to the other two groups, as well as a significant effect of FR in reducing subdepressive symptoms when compared to the PE group, and no effects on manic symptoms(Solé et al., 2015). In the subsample of cognitively impaired patients (n=188), Bonnin and colleagues showed that FR improved verbal memory and the FAST total score (Bonnin et al., 2016a). In the subsample of patients with subsyndromal symptomatology (n=33), Sanchez-Moreno and colleagues showed that FR improved overall psychosocial outcomes, as indexed by the FAST total score, significantly more than PE and TAU at 12-months follow-up (Sanchez-Moreno et al., 2017). Finally, when the whole sample was considered, functional improvements in the domain of autonomy persisted at 1-year follow-up in the FR group compared with the other 2 treatment groups (Bonnin et al., 2016b). Interestingly, an improvement in verbal memory that wasn’t statistically significant in the FR group at post treatment, became significant at 1-year follow-up.
Demant and colleagues analyzed and reported the effects of a 12-week group-based program CR program (n=23) vs TAU (n=23) in a sample of partial remitted bipolar patients with subjective cognitive difficulties (Demant et al., 2015). Each CR session was 2-hour long and consisted of three main components: PE and awareness of cognitive dysfunction in BD for approximately 30 minutes, training of compensatory and strategies for cognitive dysfunction for about one hour and the remaining 30 minutes was spent on computer-assisted cognitive training using RehaCom software (Hasomed, RehaCom version 6.0 and 6.1). The CR program also included some mindfulness exercises to practice at home and at the beginning of each session as a means to enhance the attentional capacity. An extensive clinical, neuropsychological and functional battery administered at baseline, 12 and 26 weeks after treatment showed no significant effect on cognitive or psychosocial function of CR compared with treatment as usual (TAU), although it improved subjective sharpness/mental acuity at week 12 (d=.97), and quality of life (d=.56) and verbal fluency (d=.79) at week 26 follow-up. The authors suggested that a more intensive, individualized, durable intervention may be necessary to improve cognition and psychosocia l functioning in BD patients. In a subgroup of patients from this trial (Macoveanu et al., 2018), changes in prefrontal activation were investigated with functional magnetic resonance imaging during a strategic episodic picture encoding task and a spatial n-back working memory task at baseline and following CR (n=13) and TAU (n=14). While the right dlPFC cortex was activated by both tasks across all patients, it did not show significant activation changes following CR vs TAU, and its activation was not significantly associated with recall accuracy or working memory performance. The authors interpreted these negative findings by hypothesizing that, in order for CR to induce cognitive improvements, it has to critically engage and modulate response in the dlPFC.
Lewandowski and colleagues evaluated the effects of a computerized CR therapy in a group of BD patients with psychotic symptoms, who were randomized to 70 hours of the CR program BrainHQ (n=39), delivered over 6 months with three sessions per week (Posit Science Inc., BrainHQ), or to a dose-matched computer games control condition (n=33). Results showed that: (i) while both groups improved on several domains relative to baseline, CR showed at post-treatment compared to active control medium to large effects on processing speed (d= .65), visual learning and memory (d = 0.92), and global cognition (d = 0.83); (ii) the large CR-induced gains in processing speed and composite were uniquely maintained after 6 months of no study contact; (iii) while cognitive change was associated with functional change across the sample, CR did not specifically improve community functioning (Lewandowski et al., 2017).
Non-RCT studies
Deckersbach and colleagues conducted an open trial in 18 BD subjects with residua depressive symptoms, who received an individual CR therapy called “Compensatory cognitive training” which consisted of 14 sessions of 50 minutes divided into three modules: (i) mood monitoring and treatment of residual depressive symptoms, (ii) organization, planning and time management and (iii) attention and memory training (Deckersbach et al., 2010). The study detected a reduction of depressive symptoms (d=.90), and an increase in executive (d=.57), occupational (d=.56), and psychosocial (d=45) functioning at post-treatment but not at 3-month follow-up, while manic symptoms did not change over time. Interestingly, as authors observed that an history of anxiety interfered with the efficacy of CR, they suggested to add cognitive behavioral therapy in order to provide specific coping tools to handle anxiety during CR.
Zyto and colleagues conducted a small open pilot study assessing the feasibility, acceptability, and preliminary efficacy of a tailored FR 12-weeks program that combined individual and group sessions, in a sample of 12 BD Type 1 and their caregivers (Zyto et al., 2016). The authors assessed functional outcome with the FAST at baseline, immediately post-treatment, and follow-up three months later, and found a significant pre-post improvement on overall psychosocial functioning (d=1.02), driven by gradual and specific gains in autonomy and occupational functioning evolving from baseline to follow-up.
Veeh and colleagues conducted a naturalistic study that evaluated the effects of tailored group-based CR (n=26) vs TAU (n=10) in BD patients with cognitive deficits (Veeh et al., 2017). The CR program consisted of a cognitive skills group and a computer-assisted program for cognitive training (HAPPYneuron Pro). The group sessions took place weekly over 12 weeks and were moderated by a cognitive behavioral psychotherapist. Groups consisted of 4–6 patients, and the sessions lasted 90 min. Each CR session consisted of three main components: coaching on cognitive skills through practical exercises and worksheets, cognitive training, group-based practice of trained skills applied to real-life problems. Throughout the session, the therapist facilitated the acquisition of strategies and provided supportive feedback. After 12 weeks, while significant within-group gains in the CR condition were found for working memory (d=.84), problem solving (d=.80), divided attention (d=.40), delayed recall (d=.84), and depressive symptoms (d=.34), there was no evidence of between-group effects, and no within-group changes induced by CR in quality of life or subjective cognitive complains.
Discussion
The goal of this systematic review was to illustrate existing approaches to remediate cognitive impairments among people with BD. While the literature emerging in the last decade suggests the presence of heterogeneous treatment models, with various targets and mechanisms of action, some recurrent features among these models appear to be the inclusion, at least in some form, of computerized training tools, as well as a group-based format.
Overall, we found some degree of domain specificity between the remediation target and improvements in primary outcomes. Models that prioritized the systematic training of distinct cognitive operations induced medium-to-large effects in many cognitive domains, primarily working memory, problem solving and processing speed. On the other hand, approaches that provided patients with specific cognitive strategies to set goals, engage in real-world activities and navigate workplace situations uniquely induced meaningful improvements in various aspects of functioning, namely occupational, psychosocial, and interpersonal. As a matter of fact, integrated approaches that combined cognitive exercises with ecological, group-based settings designed to provide insights about the importance of cognitive skills to real-world functioning seem to be associated with both cognitive and functional improvements. This is consistent with various studies recently conducted in samples of patients with serious mental illness (SMI) that showed the benefits of supplementing traditional computer-based CR with therapist-led sessions that focused on occupational and/or social skills development (Biagianti et al., 2016; Bowie et al., 2017).
Unfortunately, only one study examined the neural effects of CR, with no evidence of significant activation changes or associations of such changes with cognitive improvements. To date, only one other study conducted in a group of patients suffering from various affective disorders (26/73 had a BD diagnosis) investigated the neural changes induced by CR using fMRI (Meusel et al., 2013). After 10 weeks of a computerized cognitive training (PSSCogRehab), patients showed increased activations in lateral and medial prefrontal and lateral parietal regions that correlated with memory improvements in a 2-back memory fMRI task, as well as increased activation in the left hippocampus that correlated with improvements in a recollection memory fMRI task. This is promising evidence that CR engages critical neural targets where neuroplastic changes occur after CR, and that these changes are likely to be the drivers of significant cognitive gains. Of course, more research is required to systematically investigate the effects of CR/FR models on neural activation patterns, and understand the complex neural correlates of response to CR.
Limitations
The present review has some limitations that should be noted. First, we reviewed studies that exclusively recruited patients with a diagnosis of BP. Therefore, we may have missed a number of studies that included a mixed diagnostic sample of patients with schizophrenia, schizoaffective, and bipolar. At the same time, the dearth of RCTs studying CR/FR in pure BD samples could be ascribed to the challenges of recruiting patients from a single diagnostic category and retaining them in long and intensive trials of CR. Additionally, given the heterogeneity in cognitive, social cognitive, and functional assessments used, and the fact that many studies did not report significance levels and effect sizes for non-significant outcomes, we were not able to compare outcomes across studies and compute an overall effect across studies that includes both significant and non-significant outcomes. Third, because medication regimens, duration of illness, and number of mood episodes were reported inconsistently across studies, we were not able to elucidate the role played by these critical factors on response to CR/FR. Because patients with BD commonly take medications with anticholinergic activity, which is known to lower response to computerized cognitive training (Vinogradov et al., 2009), future studies should rigorously report medications and control the effects of CR for the presence of interfering antipsychotic therapies.
Conclusions
Findings from this review show cognitive and functional improvements as well as some promising reduction of depressive symptoms following CR/FR, and support their use in BD. Effect sizes from individual studies were in the large range, usually higher than what has been reported in meta-analyses of CR in SZ (Wykes et al., 2011), suggesting that patients with BD respond to treatment at or above the level reported in psychotic patients. However, these findings should be interpreted with caution, as few studies to date have examined CR in BD samples.
We believe that, if we are to develop and implement CR/FR interventions for BP that are robust and efficient, predictors of treatment response, including potential subdiagnostic differences and cognitive endophenotypes within BD, should be identified. While this work in CR for psychotic disorders has already identified promising biomarkers (Biagianti et al., 2017; Lindenmayer et al., 2017), research on CR/FR for BD is lagging behind. Once potential predictors, mediators and moderators of response are identified, such elements will guide the refinement of mechanistically informed CR/FR protocols and make possible to personalize them to individual strengths and weaknesses, thus maximizing the efficacy of this promising therapeutic approach.
TABLE 2:
Various forms of Cognitive and Functional Remediation treatments currently described in the literature.
| Specific CR therapy | Effects* | Brief description | |
|---|---|---|---|
| Cognitive Remediation (CR) | Occupational functioning ▲ Psychosocial functioning ▲ Executive functioning ▲ Residual depressive symptoms ▼ |
Standard individualized CR; aimed to improve various cognitive functions. | Deckersbach et al., 2010 |
| Functional Remediation | • Psychosocial functioning ▲ • Interpersonal functioning▲ • Occupational functioning ▲ • Autonomy▲ • Functional impairments ▼ • Sub-depressive symptoms ▼ • Verbal Memory ▲ |
Group-based approach that covers not only cognition but also functioning, providing strategies to manage cognitive impairments in different domains. | Torrent et al., 2013 (and subsequent publications) Zyto et al., 2016 |
| Computerized CR | Processing speed ▲ • Visual learning and memory ▲ • Global Cognition ▲ |
Neuroplasticity-based cognitive training completed individually without assistance or supervision. | Lewandowski et al., 2017 |
| Mixed | • Working memory ▲ • Problem solving ▲ • Verbal Fluency ▲ • Learning▲ • Divided attention▲ • Subjective Mental Acuity▲ • Sub-depressive symptoms ▼ • Quality of Life ▲ |
Combination of two or more of the previously illustrated therapies (f.e. Group-based or individual standard CR + computerized programs (BrainWorks, PSSCogRehab, etc). | Demant et al., 2015 (and subsequent publications) Veeh et al., 2017 |
Indicates the cognitive, psychological, clinical and psychosocial improvements induced by specific treatments (based on included literature).
LEGEND: ▲: increased / improved, ▼: reduced / decreased.
HIGHLIGHTS:
Bipolar disorder is associated with significant impairments in cognitive and functional domains.
Protocol targeting different cognitive domains showed specificity of training focus to outcomes
Cognitive remediation produces cognitive and functional improvements in bipolar disorder
Patients with bipolar disorder respond to treatment at or above the level reported in schizophrenia
Acknowledgments
Role of the Funding source
BB is supported through a grant from the National Institute of Mental Health (R43 MH114765–01). PB is partially supported by grants from the Ministry of Health (RF‐2011‐02352308). CP is partially supported by grants from the Ministry of Health (GR-2016–02361283).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
BB is Senior Scientist at Posit Science, a company that produces cognitive training software. The other authors report no conflict of interest.
Bibliography
- Anaya C, Martinez Aran A, Ayuso-Mateos JL, Wykes T, Vieta E, Scott J, 2012. A systematic review of cognitive remediation for schizo-affective and affective disorders. J. Affect. Disord 142, 13–21. 10.1016/j.jad.2012.04.020 [DOI] [PubMed] [Google Scholar]
- Baune BT, Malhi GS, 2015. A review on the impact of cognitive dysfunction on social, occupational, and general functional outcomes in bipolar disorder. Bipolar Disord 17, 41–55. 10.1111/bdi.12341 [DOI] [PubMed] [Google Scholar]
- Biagianti B, Roach BJ, Fisher M, Loewy R, Ford JM, Vinogradov S, Mathalon DH, 2017. Trait aspects of auditory mismatch negativity predict response to auditory training in individuals with early illness schizophrenia. Neuropsychiatr. Electrophysiol 3 10.1186/s40810-017-0024-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagianti B, Schlosser D, Nahum M, Woolley J, Vinogradov S, 2016. Creating Live Interactions to Mitigate Barriers (CLIMB): A Mobile Intervention to Improve Social Functioning in People With Chronic Psychotic Disorders. JMIR Ment. Health 3, e52 10.2196/mental.6671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin CM, Reinares M, Martínez-Arán A, Balanzá-Martínez V, Sole B, Torrent C, Tabarés-Seisdedos R, García-Portilla MP, Ibáñez A, Amann BL, Arango C, Ayuso-Mateos JL, Crespo JM, González-Pinto A, Colom F, Vieta E, The CIBERSAM Functional Remediation Group, 2016a. Effects of functional remediation on neurocognitively impaired bipolar patients: enhancement of verbal memory. Psychol. Med 46, 291–301. 10.1017/S0033291715001713 [DOI] [PubMed] [Google Scholar]
- Bonnin CM, Torrent C, Arango C, Amann BL, Solé B, González-Pinto A, Crespo JM, Tabarés-Seisdedos R, Reinares M, Ayuso-Mateos JL, García-Portilla MP, Ibañez Á, Salamero M, Vieta E, Martinez-Aran A, CIBERSAM Functional Remediation Group, 2016b. Functional remediation in bipolar disorder: 1-year follow-up of neurocognitive and functional outcome. Br. J. Psychiatry 208, 87–93. 10.1192/bjp.bp.114.162123 [DOI] [PubMed] [Google Scholar]
- Bourne C, Aydemir Ö, Balanzá-Martínez V, Bora E, Brissos S, Cavanagh JTO, Clark L, Cubukcuoglu Z, Dias VV, Dittmann S, Ferrier IN, Fleck DE, Frangou S, Gallagher P, Jones L, Kieseppä T, Martínez-Aran A, Melle I, Moore PB, Mur M, Pfennig A, Raust A, Senturk V, Simonsen C, Smith DJ, Bio DS, Soeiro-de-Souza MG, Stoddart SDR, Sundet K, Szöke A, Thompson JM, Torrent C, Zalla T, Craddock N, Andreassen OA, Leboyer M, Vieta E, Bauer M, Worhunsky PD, Tzagarakis C, Rogers RD, Geddes JR, Goodwin GM, 2013. Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: an individual patient data meta-analysis. Acta Psychiatr. Scand 128, 149–162. 10.1111/acps.12133 [DOI] [PubMed] [Google Scholar]
- Bowie CR, Grossman M, Gupta M, Holshausen K, Best MW, 2017. Action-based cognitive remediation for individuals with serious mental illnesses: Effects of real-world simulations and goal setting on functional and vocational outcomes. Psychiatr. Rehabil. J 40, 53–60. 10.1037/prj0000189 [DOI] [PubMed] [Google Scholar]
- Bozikas V, Tonia T, Fokas K, Karavatos A, Kosmidis M, 2006. Impaired emotion processing in remitted patients with bipolar disorder. J. Affect. Disord 91, 53–56. 10.1016/j.jad.2005.11.013 [DOI] [PubMed] [Google Scholar]
- Carvalho AF, Bortolato B, Miskowiak K, Vieta E, Köhler C, 2015. Cognitive dysfunction in bipolar disorder and schizophrenia: a systematic review of meta-analyses. Neuropsychiatr. Dis. Treat 3111 10.2147/NDT.S76700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckersbach T, Nierenberg AA, Kessler R, Lund HG, Ametrano RM, Sachs G, Rauch SL, Dougherty D, 2010. RESEARCH: Cognitive Rehabilitation for Bipolar Disorder: An Open Trial for Employed Patients with Residual Depressive Symptoms: Cognitive Rehabilitation for Bipolar Disorder. CNS Neurosci. Ther 16, 298–307. 10.1111/j.1755-5949.2009.00110.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demant KM, Vinberg M, Kessing LV, Miskowiak KW, 2015. Effects of Short-Term Cognitive Remediation on Cognitive Dysfunction in Partially or Fully Remitted Individuals with Bipolar Disorder: Results of a Randomised Controlled Trial. PLOS ONE 10, e0127955 10.1371/journal.pone.0127955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon T, Kravariti E, Frith C, Murray RM, McGuire PK, 2004. Effect of symptoms on executive function in bipolar illness. Psychol. Med 34, 811–821. [DOI] [PubMed] [Google Scholar]
- Donaldson S, Goldstein LH, Landau S, Raymont V, Frangou S, 2003. The Maudsley Bipolar Disorder Project: the effect of medication, family history, and duration of illness on IQ and memory in bipolar I disorder. J. Clin. Psychiatry 64, 86–93. [PubMed] [Google Scholar]
- Kapczinski F, Dias VV, Kauer-Sant’Anna M, Frey BN, Grassi-Oliveira R, Colom F, Berk M, 2009. Clinical implications of a staging model for bipolar disorders. Expert Rev. Neurother 9, 957–966. 10.1586/ern.09.31 [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Sperry SH, Cohen BM, Norris LA, Fitzmaurice GM, Ongur D, Keshavan MS, 2017. Treatment to Enhance Cognition in Bipolar Disorder (TREC-BD): Efficacy of a Randomized Controlled Trial of Cognitive Remediation Versus Active Control. J. Clin. Psychiatry 78, e1242–e1249. 10.4088/JCP.17m11476 [DOI] [PubMed] [Google Scholar]
- Lindenmayer J-P, Ozog VA, Khan A, Ljuri I, Fregenti S, McGurk SR, 2017. Predictors of response to cognitive remediation in service recipients with severe mental illness. Psychiatr. Rehabil. J 40, 61–69. 10.1037/prj0000252 [DOI] [PubMed] [Google Scholar]
- Macoveanu J, Demant KM, Vinberg M, Siebner HR, Kessing LV, Miskowiak KW, 2018. Towards a biomarker model for cognitive improvement: No change in memory-related prefrontal engagement following a negative cognitive remediation trial in bipolar disorder. J. Psychopharmacol. (Oxf.) 32, 1075–1085. 10.1177/0269881118783334 [DOI] [PubMed] [Google Scholar]
- Martínez-Arán A, 2011. Functional Remediation for Bipolar Disorder. Clin. Pract. Epidemiol. Ment. Health 7, 112–116. 10.2174/1745017901107010112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusel L-AC, Hall GBC, Fougere P, McKinnon MC, MacQueen GM, 2013. Neural correlates of cognitive remediation in patients with mood disorders. Psychiatry Res. Neuroimaging 214, 142–152. 10.1016/j.pscychresns.2013.06.007 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535–b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, Moore PB, 2006. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J. Affect. Disord 93, 105–115. 10.1016/j.jad.2006.02.016 [DOI] [PubMed] [Google Scholar]
- Rosa AR, Sánchez-Moreno J, Martínez-Aran A, Salamero M, Torrent C, Reinares M, Comes M, Colom F, Van Riel W, Ayuso-Mateos J, Kapczinski F, Vieta E, 2007. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin. Pract. Epidemiol. Ment. Health 3, 5 10.1186/1745-0179-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches M, Bauer IE, Galvez JF, Zunta-Soares GB, Soares JC, 2015. The Management of Cognitive Impairment in Bipolar Disorder: Current Status and Perspectives. Am. J. Ther 22, 477–486. 10.1097/MJT.0000000000000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Moreno J, Bonnín C, González-Pinto A, Amann BL, Solé B, Balanzá-Martínez V, Arango C, Jimenez E, Tabarés-Seisdedos R, Garcia-Portilla MP, Ibáñez A, Crespo JM, Ayuso-Mateos JL, Vieta E, Martinez-Aran A, Torrent C, 2017. Do patients with bipolar disorder and subsyndromal symptoms benefit from functional remediation? A 12-month follow-up study. Eur. Neuropsychopharmacol 27, 350–359. 10.1016/j.euroneuro.2017.01.010 [DOI] [PubMed] [Google Scholar]
- Solé B, Bonnin CM, Mayoral M, Amann BL, Torres I, González-Pinto A, Jimenez E, Crespo JM, Colom F, Tabarés-Seisdedos R, Reinares M, Ayuso-Mateos JL, Soria S, Garcia-Portilla MP, Ibañez Á, Vieta E, Martinez-Aran A, Torrent C, 2015. Functional remediation for patients with bipolar II disorder: Improvement of functioning and subsyndromal symptoms. Eur. Neuropsychopharmacol 25, 257–264. 10.1016/j.euroneuro.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Torrent C, Bonnin C. del M., Martínez-Arán A, Valle J, Amann BL, González-Pinto A, Crespo JM, Ibáñez Á, Garcia-Portilla MP, Tabarés-Seisdedos R, Arango C, Colom F, Solé B, Pacchiarotti I, Rosa AR, Ayuso-Mateos JL, Anaya C, Fernández P, Landín-Romero R, Alonso-Lana S, Ortiz-Gil J, Segura B, Barbeito S, Vega P, Fernández M, Ugarte A, Subirà M, Cerrillo E, Custal N, Menchón JM, Saiz-Ruiz J, Rodao JM, Isella S, Alegría A, Al-Halabi S, Bobes J, Galván G, Saiz PA, Balanzá-Martínez V, Selva G, Fuentes-Durá I, Correa P, Mayoral M, Chiclana G, Merchan-Naranjo J, Rapado-Castro M, Salamero M, Vieta E, 2013. Efficacy of Functional Remediation in Bipolar Disorder: A Multicenter Randomized Controlled Study. Am. J. Psychiatry 170, 852–859. 10.1176/appi.ajp.2012.12070971 [DOI] [PubMed] [Google Scholar]
- Tse S, Chan S, Ng KL, Yatham LN, 2014. Meta-analysis of predictors of favorable employment outcomes among individuals with bipolar disorder. Bipolar Disord 16, 217–229. 10.1111/bdi.12148 [DOI] [PubMed] [Google Scholar]
- Veeh J, Kopf J, Kittel-Schneider S, Deckert J, Reif A, 2017. Cognitive remediation for bipolar patients with objective cognitive impairment: a naturalistic study. Int. J. Bipolar Disord 5 10.1186/s40345-017-0079-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Warm H, Holland C, Kirshner MA, Pollock BG, 2009. The Cognitive Cost of Anticholinergic Burden: Decreased Response to Cognitive Training in Schizophrenia. Am. J. Psychiatry 166, 1055–1062. 10.1176/appi.ajp.2009.09010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M, Santaguida PL, Shamliyan T, Singh K, Tsertsvadze A, Treadwell JR, 2008. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions, in: Methods Guide for Effectiveness and Comparative Effectiveness Reviews, AHRQ Methods for Effective Health Care Agency for Healthcare Research and Quality (US), Rockville (MD). [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P, 2011. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am. J. Psychiatry 168, 472–485. 10.1176/appi.ajp.2010.10060855 [DOI] [PubMed] [Google Scholar]
- Zyto S, Jabben N, Schulte PFJ, Regeer BJ, Kupka RW, 2016. A pilot study of a combined group and individual functional remediation program for patients with bipolar I disorder. J. Affect. Disord 194, 9–15. 10.1016/j.jad.2016.01.029 [DOI] [PubMed] [Google Scholar]


