Abstract
The number of live kidney donors has declined since 2005. This decline parallels the evolving knowledge of risk for biologically related, black, and younger donors. To responsibly promote donation, we sought to identify declining low-risk donor subgroups that might serve as targets for future interventions. We analyzed a national registry of 77,427 donors and quantified the change in number of donors per 5-year increment from 2005–2017 using Poisson regression stratified by donor/recipient relationship and race/ethnicity. Among related donors aged <35, 35–49, and ≥50 years, white donors declined by 21%, 29%, and 3%; black donors declined by 30%, 31%, and 12%; Hispanic donors <35 and 35–49 declined by 18% and 15%; those ≥50 increased by 10%. Conversely, among unrelated donors <35, 35–49, and ≥50, white donors increased by 12%, 4%, 24%; black donors <35 and 35–49 did not change but those ≥50 increased by 34%; Hispanic donors increased by 16%, 21%, and 46%. Unlike unrelated donors, related donors were less likely to donate in recent years across race/ethnicity. While this decline might be understandable for related younger donors, it is less understandable for lower-risk related older donors (≥50 years). Biologically related older individuals are potential targets for interventions to promote donation.
INTRODUCTION
The annual number of live kidney donors in the United States declined from a peak of 6648 donors in 2004 to 5612 in 2012, a 16% drop that has not recovered since despite a two-fold increase in the size of the kidney transplant waiting list over the same period.1 This decline was especially marked among donors who were biologically related to the recipient, those who were black, and the young.1,2 These observed trends might be explained by the evolving knowledge about the familial risk of end-stage renal disease (ESRD), race-specific high-risk genotypes, and the lifetime risk of ESRD for younger donors.3–19 As such, the decline in high-risk subgroups of donors might be justifiable.
What remains unknown is whether there has been a decline in low-risk subgroups of donors, which would be less justified. Unrelated donors may be viewed as lower risk than biologically related donors, and the practice of accepting unrelated donors is now medically and ethically justifiable.20 Subgroups of black donors may not have high-risk genes for kidney disease; for example, some black donors with a family history of ESRD might not have two high-risk APOL1 alleles since 87% of black individuals in the general US population do not carry this high-risk genotype.21 And healthy older black donors who have been screened for kidney disease might be considered low-risk as they have passed the peak age-at-risk for familial and genetic kidney disease. Given uncertainty about the lifetime risk of ESRD in younger donors, and the aging US population of which 32.1% is over 50 years old,22 the transplant community has increasingly accepted older donors for live kidney donation. Older donors are a potentially lower-risk group despite comorbidities such as hypertension and obesity.23–27 Indeed, the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors endorsed increased permissiveness for older donors and less permissiveness for younger donors.28
We hypothesized that we might identify low-risk subgroups of donors with significant declines in donation to serve as potential targets for future interventions. We used a stratified regression framework to identify the characteristics of donors – jointly defined by donor/recipient relationship, race/ethnicity, and donor age – that are associated with the decline in live kidney donation from 2005 to 2017.
METHODS
Data Source and Study Population
This study used data from the Scientific Registry of Transplant Recipients (SRTR) external release made available in June 2018. The SRTR data system includes data on all donors, waitlist candidates, and transplant recipients in the United States, submitted by members of Organ Procurement and Transplantation Network (OPTN), and has been previously described.29,30 The Health Resources and Services Administration under the US Department of Health and Human Services provides federal government oversight to the activities of the OPTN and SRTR contractors. To characterize neighborhood socioeconomic status (SES) of donors, we linked donor residential ZIP codes to the Agency for Healthcare Research and Quality (AHRQ) SES index (range: 0–100), based on the 2010 US Census.4,31
The study population included 77,427 live kidney donors between January 1, 2005, and December 31, 2017.
Outcomes
The outcome of interest was the change in live kidney donation over time. A priori, we stratified the analyses by donor/recipient relationship (biologically related and unrelated) and donor race (white/others, black, and Hispanic) because of the evolving knowledge of risks for specific donor subgroups, including those associated with family history of ESRD and APOL1 high-risk genotypes.11,15,16 By extension, the results were stratified by donor/recipient relationship and race. Age was categorized as <35, 35–49, and ≥50 years to reflect younger, typical, and older donors.
Change in Number of Donors from 2005 to 2017
We used Poisson regression to estimate the change in number of donors per 5-year increment (incidence rate ratio, IRR). The IRR indicates the proportional decline or increase in the number of donors per 5 years. A decline in donor number was initially observed in 2005. Thus data from January 1, 2005 to December 31, 2017 were used for this study analysis. In one sensitivity analysis, we restricted the study population to those who donated to a first-time kidney transplant recipient. A concern was that second-time transplant candidates may have “used up” the only donor available to them, explaining the observed decline in biologically related donors. In a second sensitivity analysis, we excluded pediatric (<18-year-old) recipients. The concern here was that pediatric transplant candidates have received priority for high-quality deceased donor kidneys since 2005,32 potentially explaining the decline in biologically related donors.
We also explored multivariable logistic regression as an alternative framework to compare the odds of live kidney donation in 2012–2017 vs. 2005–2011. While less intuitive than Poisson regression (odds of donation vs. change in number of donors), logistic regression allowed us to adjust for donor demographic and health characteristics including age, sex, renal reserve (estimated glomerular filtration, eGFR), hypertension, obesity (BMI≥30), smoking history, education level, and neighborhood SES index. Poisson regression on the number of donors per year was based on aggregated data and, as such, did not permit adjustment for individual-level demographic and health characteristics. It did, however, estimate the change in number of live donors over time within each stratum of donor type (relationship with recipient, race, and age).
Statistical analysis
All analyses were performed using Stata 14.0/MP for Windows (College Station, Texas). The median and interquartile range (IQR) were used to describe continuous variables. Confidence intervals are reported as per the method of Louis and Zeger.33 We used a 2-sided α of 0.05 to indicate a statistically significant difference.
RESULTS
Study Population
A total of 77,427 live kidney donors were identified within the SRTR between January 1, 2005, and December 31, 2017. In this population, 54% (N=41,494) of donors were biologically related to the recipient (39% were full sibling, 29% were offspring, 16.5% were parents, and 15.5% were non first-degree relative donors) (Supplementary Appendix, Figure S1). Among the remaining 46% (N= 35,933) unrelated donors, 80% were directed donors, 15% were paired donation donors, and 5% were non-directed donors (referred to as anonymous, or altruistic). Compared with unrelated donors, biologically related donors were more likely to be male. Among biologically related donors, 69% were white, 14% were black, and 17% were Hispanic/Latino. Among unrelated donors, 82% were white, 8% were black, and 10% were Hispanic. Overall, black and Hispanic donors were more likely to be younger, have less education, and live in a neighborhood with lower SES index (<50) than white donors (Table 1).
Table 1.
Baseline Characteristics of Live Kidney Donors Between January 1, 2005 and December 31, 2017, Stratified by Donor/Recipient Relationship and Race.
| Biologically related (N= 41,494) | Unrelated (N= 35,933) | |||||
|---|---|---|---|---|---|---|
| Characteristic1 | White (n= 28,488) | Black (n= 5,932) | Hispanic (n= 7,074) | White (n= 29,504) | Black (n= 2,825) | Hispanic (n= 3,604) |
| Age, y median (IQR) | 42 (32, 50) | 35 (27, 44) | 36 (28, 44) | 46 (36, 54) | 40 (32, 47) | 39 (31, 48) |
| ≤34 | 8622 (30%) | 2835 (48%) | 3277 (46%) | 6102 (21%) | 929 (33%) | 1248 (35%) |
| 35–49 | 12203 (43%) | 2350 (40%) | 2878 (41%) | 12390 (42%) | 1352 (48%) | 1647 (46%) |
| ≥50 | 7663 (27%) | 747 (13%) | 919 (13%) | 11012 (37%) | 544 (19%) | 709 (20%) |
| Male sex | 11752 (41%) | 2566 (43%) | 2945 (42%) | 10369 (35%) | 1036 (37%) | 1292 (36%) |
| BMI3, median (IQR) | 26 (24, 29) | 28 (25, 31) | 27 (25, 30) | 26 (24, 29) | 28 (25, 31) | 27 (25, 30) |
| ≤24 | 7873 (29%) | 1192 (21%) | 1439 (21%) | 8297 (29%) | 517 (19%) | 721 (20%) |
| 25–29 | 13762 (50%) | 2758 (49%) | 3637 (53%) | 14556 (51%) | 1367 (50%) | 1853 (52%) |
| ≥30 | 5888 (21%) | 1736 (31%) | 1824 (26%) | 5920 (21%) | 863 (31%) | 965 (27%) |
| Hypertension4 | 853 (3%) | 95 (2%) | 87 (1%) | 1068 (4%) | 63 (2%) | 60 (2%) |
| History of smoking5 | 7841 (28%) | 1167 (20%) | 1249 (18%) | 7722 (27%) | 495 (18%) | 656 (18%) |
| Creatinine6, median (IQR) | 0.8 (0.7, 1.0) | 0.9 (0.8, 1.1) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.9 (0.8, 1.0) | 0.8 (0.7, 0.9) |
| eGFR6, median (IQR) | 97 (84, 108) | 109 (93, 124) | 108 (95, 118) | 94 (82, 106) | 105 (92, 121) | 105 (93, 115) |
| Highest level of education7 | ||||||
| High School or less | 6990 (28%) | 1688 (33%) | 3066 (50%) | 6387 (24%) | 717 (29%) | 1587 (48%) |
| Attended college/Technical school | 6601 (26%) | 1738 (34%) | 1725 (28%) | 7119 (27%) | 812 (32%) | 913 (28%) |
| College/Post-Secondary school | 11332 (45%) | 1681 (33%) | 1386 (22%) | 12951 (49%) | 973 (39%) | 775 (24%) |
| SES index8, median (IQR) | 63 (58, 68) | 59 (53, 65) | 59 (53, 65) | 63 (58, 68) | 60 (54, 66) | 60 (54, 65) |
| Low SES9 | 1001 (4%) | 764 (14%) | 888 (14%) | 933 (3%) | 318 (12%) | 444 (14%) |
Abbreviations: IQR, Interquartile Range; BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; eGFR, estimated glomerular filtration rate. SES, Socioeconomic Status
Characteristics at the time of donation (2005–2017) are shown; age, sex, and race/ethnicity, biological relationship to the recipient were available throughout the study period (0% missing).
The category of “White” included Caucasian, Asian, American Indian or Alaskan Native, Native Hawaiian or other Pacific Islander, and multiracial.
BMI (4.8% missing between 2005–2011; 0.5% missing between 2012–2017)
Hypertension was defined as predonation documented use of antihypertensive therapy/history of hypertension (3.8% missing between 2005–2011; 0.1% missing 2012–2017)
Smoking status (3.4% between 2005–2011; 0% missing 2012–2017)
Creatinine/eGFR (1.7% missing 2005–2011; 0.1% missing 2012–2017)
Education (17.6% missing between 2005–2011; 4.0% missing 2012–2017)
Socioeconomic index (SES) based on the 2010 census (4.0% missing 2005–2011; 3.9% missing 2012–2016; 46.1% missing in Jan-Aug 2017; unavailable after Aug 2017)
Low SES index was defined as SES less than 50
Observed Number of Live Kidney Donors Over the Entire Study Period
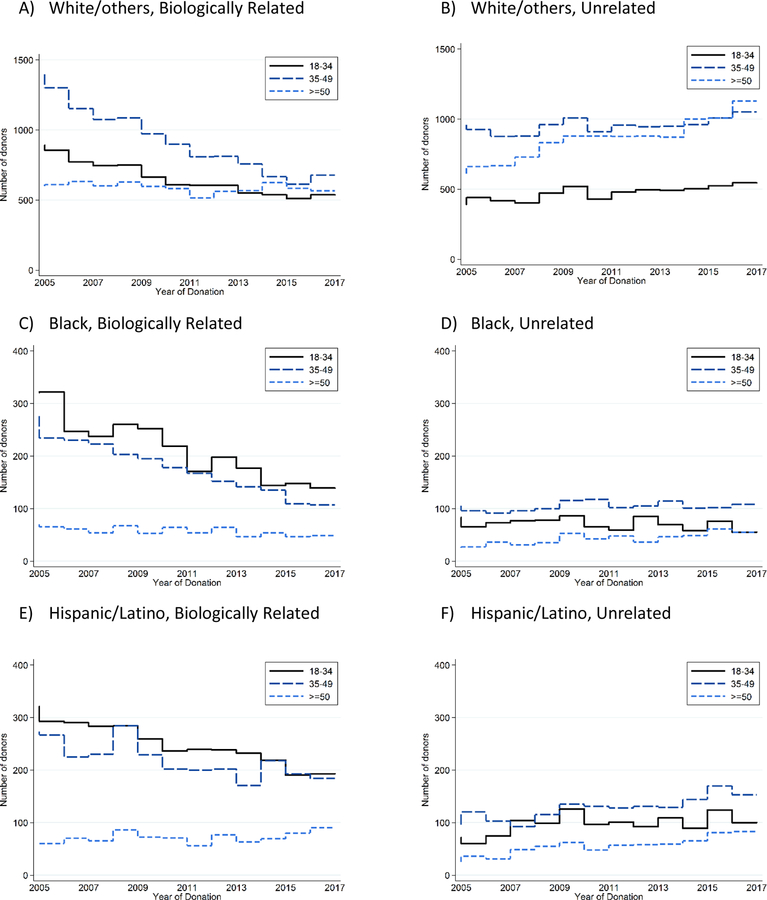
Among biologically related donors from 2005 to 2017, the total number of white donors declined from 891 to 536 for ages under 35 (40% decline), from 1396 to 677 for ages 35–50 (52% decline), and from 598 to 566 for ages over 50 (5% decline). The total number of black donors declined from 321 to 139 for ages under 35 (57% decline), from 276 to 107 for ages 35–49 (61% decline), and from 70 to 49 for ages over 50 (30% decline). The total number of Hispanic donors declined from 321 to 193 for ages under 35 (40% decline), from 273 to 184 for ages 35–49 (33% decline), but increased from 60 to 90 for ages over 50 (50% increase) (Figure 1 and Supplementary Appendix, Figure S2).
Figure 1.
Observed Number of Live Kidney Donors by Age, Stratified by Relationship with the Recipient and Race/Ethnicity.
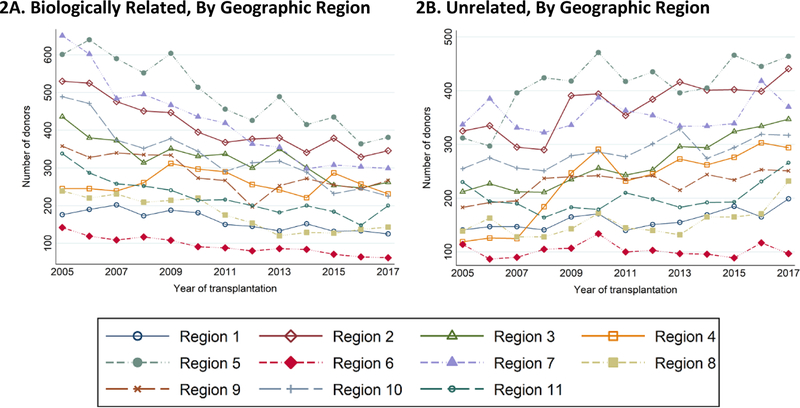
Among unrelated donors from 2005 to 2017, the total number of white donors increased from 391 to 544 for ages under 35 (39% increase), from 959 to 1052 for ages 35–49 (10% increase), from 611 to 1129 for ages over 50 (85% increase). The total number of black donors declined from 83 to 55 for ages under 35 (34% decline) and increased from 105 to 108 for ages 35–49 (3% increase), and from 25 to 55 for ages over 50 (120% increase). The total number of Hispanic increased from 72 to 100 for ages under 35 (39% increase), from 96 to 153 for ages 35–49 (59% increase), and from 25 to 83 for Hispanic (232% increase) (Figure 1). These observed trends were consistent across the organ procurement organization (OPO) geographic regions (Figure 2A–B).
Figure 2.
The Number of Biologically Related (2A) and Unrelated (2B) Live Kidney Donors over Time by the OPO Geographic Regions.
The trends are global across the 11 OPO geographic regions for biologically related and unrelated live kidney donors and are not driven by donor program.
Region 1: Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, Eastern Vermont
Region 2: Delaware, District of Columbia, Maryland, New Jersey, Pennsylvania, West Virginia, Northern Virginia
Region 3: Alabama, Arkansas, Florida, Georgia, Louisiana, Mississippi, Puerto Rico
Region 4: Oklahoma, Texas
Region 5: Arizona, California, Nevada, New Mexico, Utah
Region 6: Alaska, Hawaii, Idaho, Montana, Oregon, Washington
Region 7: Illinois, Minnesota, North Dakota, South Dakota, Wisconsin
Region 8: Colorado, Iowa, Kansas, Missouri, Nebraska, Wyoming
Region 9: New York, Western Vermont
Region 10: Indiana, Michigan, Ohio
Region 11: Kentucky, North Carolina, South Carolina, Tennessee, Virginia
Incident Rate Ratio of Live Kidney Donation From 2005 to 2017
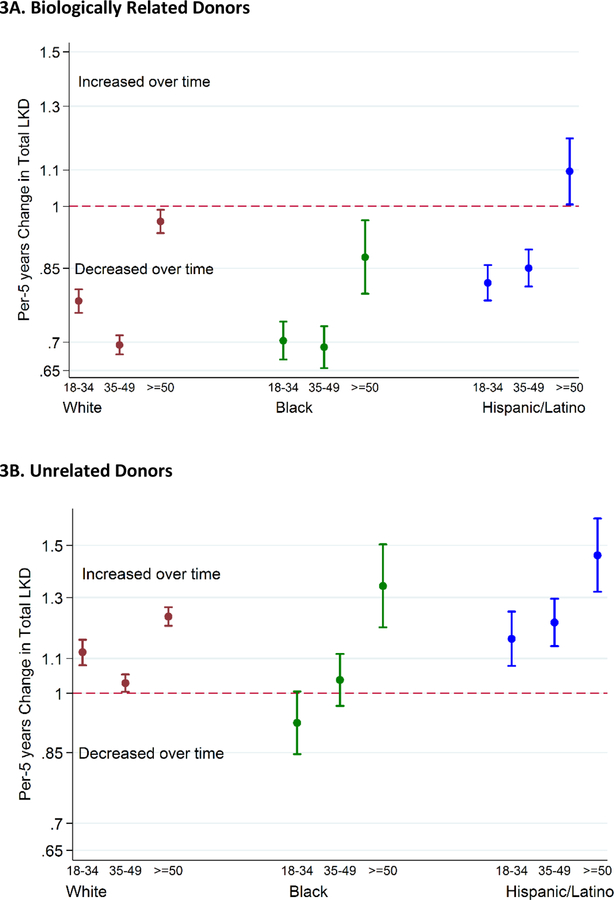
The annual number of biologically related donors declined across all race/ethnicity subgroups. For every 5-year increment, the number of biologically related white donors declined by 21% for ages under 35 (IRR 0.770.790.81), 29% for ages 35–49 (IRR 0.700.710.73), and 3% for ages over 50 (IRR 0.940.971.00). The number of biologically related black donors declined by 30% for ages under 35 (IRR 0.670.700.74), 31% for ages 35–49 (IRR 0.650.690.73), and 12% for ages over 50 (IRR 0.800.880.96). The number of biologically related Hispanic donors declined by 18% for ages under 35 (IRR 0.780.820.86), 15% for ages 35–49 (IRR 0.810.850.89), but increased by 10% for ages over 50 (IRR 1.011.101.20) (Figure 3A).
Figure 3.
Incident Rate Ratio of Live Kidney Donation from 2005 to 2017 by Age, Stratified by Relationship with the Recipient.
Per 5-years change in number of live kidney donors estimated from Poisson regression model. An incidence rate ratio less than one indicates a decline each year in the number of live kidney donors. The number of biologically related white and black donors of all ages and the number of biologically related Hispanic donors under the age of 50 declined after 2005. Conversely, the number of biologically unrelated white and Hispanic donors of all ages and the number of biologically unrelated black donors over the age of 50 increased after 2005.
Conversely, the annual number of unrelated donors increased across all race/ethnicity subgroups. For every 5-year increment, the number of unrelated white donors increased by 12% for ages under 35 (IRR 1.081.121.16), 4% for ages 35–49 (IRR 1.021.041.07), and 24% for ages over 50 (IRR 1.211.241.28). The number of unrelated black donors did not increase significantly for ages under 35 (IRR 0.850.921.01) and ages 35–49 (IRR 0.971.041.11), and increased by 34% for ages over 50 (IRR 1.201.341.50). The number of unrelated Hispanic donors increased by 16% for ages under 35 (IRR 1.081.161.25), 21% for ages 35–49 (IRR 1.141.211.30), and 46% for ages over 50 (IRR 1.321.461.62) (Figure 3B). Our inferences remained unchanged when we restricted the analysis to those who donated to first-time kidney transplant recipients. The inferences also remained unchanged when we excluded those who donated to pediatric recipients. Similarly, when we used the multivariable logistic regression framework to compare the odds of donation in 2012–2017 vs. 2005–2011, the inferences were consistent with those from Poisson regression.
DISCUSSION
In this national study of live kidney donors from 2005 to 2017, we observed an overall decline in the number of biologically related donors across race/ethnicity. Conversely, we observed an overall increase in the number of unrelated donors across race/ethnicity during that same period. The exceptions to these patterns were an increase in biologically related Hispanic donors over 50 years old and no significant change for unrelated black donors under 50 years old. The net effect of these trends in the landscape of live kidney donation was a decline in the number of live donors in the US since the majority of donors were historically biologically related. These trends have paralleled the evolving knowledge of risk for biologically related, black, and younger donors;11,12,34 however, this evolving knowledge may not explain the decline that was observed as early as 2005.
Unlike previous studies that have shown significant declines separately among biologically related donors, black donors, and younger donors,1,2 herein we have demonstrated the interaction among these factors. Our study reaffirms the decline among biologically related donors, but it also reveals growth in donation by unrelated donors of all races. Our study not only reaffirms the reported decline in black donors, but it also reveals significant declines in donation among white and Hispanic donors who are biologically related to the recipient. Furthermore, our study reaffirms the reported decline in younger donors, but also demonstrates a significant decline in older biologically related donors. In other words, the trends we observed were similar across race/ethnicity but differed by donor/recipient relationship.
Our study highlights the fact that declines in donation are mostly restricted to high-risk donor subgroups such as biologically related, black, and younger donors.4,5,10,11,16,17,34 Biologically related donors have familial risk of ESRD that might be two to several orders of magnitude higher than unrelated donors.15,16 Relatives of black individuals with non-diabetes ESRD are enriched for APOL1 high-risk variants,5 and black donors with the sickle cell trait may be at increased risk of ESRD independent of APOL1 risk variants.13,18 Younger donors have a higher residual lifetime risk of ESRD when compared with older donors.34 Moreover, younger donors might develop diseases that could cause ESRD in later life, which might not be present during screening evaluation.24,35 Whether or not the decline in biologically related donors reflects evolving knowledge of long-term risks faced by younger donors, the decline in high-risk subgroups of donors might be justifiable. By extension, the decline in low-risk older donors, who are thought to be beyond the riskiest years in which familial ESRD manifests, is worrisome.23–27 Since the transplant community is looking for opportunities to expand the live kidney donor poor safely,36 older donor candidates might safely be encouraged to donate regardless of their family history of ESRD.23–27
Key strengths of our study include the use of a national registry to capture the entire population of donors and isolate the interdependence among age, race, and biological relationship in the decline in living kidney donation. That said, our study does have several limitations. First, the data available to us cannot explain why the decline in live kidney donation started in 2005 and not in any period earlier or later. Second, we were unable to stratify by health insurance, individual-level socioeconomic status, or genetic risks such as APOL1 high-risk genotypes, which were not reported to the registry but might be associated with decreases in living kidney donation. However, in the secondary analysis where we used an alternative framework accounting for donor demographic and health characteristics including socioeconomic status, we reached similar inferences.37–40 Third, the SRTR database does not have data on donor candidates that were considered ineligible based on risk factors such as obesity, so we cannot investigate this issue. However, we have emphasized the novel lessons from our study: decline in donation is restricted mostly to biologically related donors across race/ethnicity; prior literature describes the decline as a global phenomenon that includes unrelated donors. Thus, importantly, our analysis potentially directs future interventions specifically towards low-risk subgroups with significant declines in living kidney donation. We have identified biologically related older donor-candidates as potential targets.
In conclusion, unlike unrelated donors, biologically related donors were less likely to donate in recent years across race/ethnicity. This decline might be understandable for younger biologically related donors given the uncertainty of their long-term risk. However, it is less understandable for biologically related older donors, a group with lower-risk comparable to unrelated older donors. Our findings call for programs that promote donation by biologically related older individuals.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the following grants from the National Institute of Diabetes and Digestive and Kidney Diseases: K24DK101828 (awarded to Dr Segev), K01DK101677 (awarded to Dr Massie), K01DK114388–01 (awarded to Dr Henderson), 1K23DK115908–01 (awarded to Dr Garonzik-Wang), and grant from the National Heart, Lung, and Blood Institute: T32HL007055 (supporting Mr. Thomas, PI: Wayne Rosamond). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The analyses described herein are the responsibility of the authors alone and do not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US government. The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr. Segev reported institutional grant support from the National Institutes of Health.
ABBREVIATIONS
- AHRQ
Agency for Healthcare Research and Quality
- BMI
body mass index
- CI
confidence interval
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- IQR
interquartile range
- IRR
Incidence Rate ratio
- KDIGO
Kidney Disease: Improving Global Outcomes
- OPO
organ procurement organization regions
- OPTN
Organ Procurement and Transplantation Network
- SES
Socioeconomic Status
- SRTR
Scientific Registry of Transplant Recipients
- UNOS
United Network for Organ Sharing
- US
United States
Footnotes
DISCLOSURE
The other authors have no conflicts of interest to disclose.
DATA AVAILABILITY STATEMENT
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR).
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2017 Annual Data Report: Kidney. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2019;19 Suppl 2:19–123. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigue JR, Schold JD, Mandelbrot DA. The decline in living kidney donation in the United States: random variation or cause for concern? Transplantation 2013;96(9):767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niaudet P Living donor kidney transplantation in patients with hereditary nephropathies. Nature reviews Nephrology 2010;6(12):736–743. [DOI] [PubMed] [Google Scholar]
- 4.Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. The New England journal of medicine 2010;363(8):724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman BI, Langefeld CD, Turner J, et al. Association of APOL1 variants with mild kidney disease in the first-degree relatives of African American patients with non-diabetic end-stage renal disease. Kidney international 2012;82(7):805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hildebrandt F Genetic kidney diseases. Lancet 2010;375:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reese PP, Hoo AC, Magee CC. Screening for sickle trait among potential live kidney donors: policies and practices in US transplant centers. Transplant international : official journal of the European Society for Organ Transplantation 2008;21(4):328–331. [DOI] [PubMed] [Google Scholar]
- 8.Huang E, Samaniego-Picota M, McCune T, et al. DNA testing for live kidney donors at risk for autosomal dominant polycystic kidney disease. Transplantation 2009;87(1):133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niaudet P Genetic forms of nephrotic syndrome. Pediatr Nephrol 2004;19(12):1313–1318. [DOI] [PubMed] [Google Scholar]
- 10.Lentine KL, Schnitzler MA, Garg AX, et al. Race, Relationship and Renal Diagnoses After Living Kidney Donation. Transplantation 2015;99(8):1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA : the journal of the American Medical Association 2014;311(6):579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mjoen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney international 2014;86(1):162–167. [DOI] [PubMed] [Google Scholar]
- 13.Naik RP, Derebail VK, Grams ME, et al. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA : the journal of the American Medical Association 2014;312(20):2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grams ME, Sang Y, Levey AS, et al. Kidney-Failure Risk Projection for the Living Kidney-Donor Candidate. The New England journal of medicine 2016;374(5):411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massie AB, Muzaale AD, Luo X, et al. Quantifying Postdonation Risk of ESRD in Living Kidney Donors. Journal of the American Society of Nephrology : JASN 2017;28(9):2749–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wainright JL, Robinson AM, Wilk AR, Klassen DK, Cherikh WS, Stewart DE. Risk of ESRD in prior living kidney donors. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2018;18(5):1129–1139. [DOI] [PubMed] [Google Scholar]
- 17.Matas AJ, Berglund DM, Vock DM, Ibrahim HN. Causes and timing of end-stage renal disease after living kidney donation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2018;18(5):1140–1150. [DOI] [PubMed] [Google Scholar]
- 18.Naik RP, Irvin MR, Judd S, et al. Sickle Cell Trait and the Risk of ESRD in Blacks. Journal of the American Society of Nephrology : JASN 2017;28(7):2180–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doshi MD, Ortigosa-Goggins M, Garg AX, et al. APOL1 Genotype and Renal Function of Black Living Donors. Journal of the American Society of Nephrology : JASN 2018;29(4):1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Hou S, Bush HL Jr., Kidney transplantation from unrelated living donors. Time to reclaim a discarded opportunity. The New England journal of medicine 1986;314(14):914–916. [DOI] [PubMed] [Google Scholar]
- 21.Friedman DJ, Kozlitina J, Genovese G, Jog P, Pollak MR. Population-based risk assessment of APOL1 on renal disease. Journal of the American Society of Nephrology : JASN 2011;22(11):2098–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Census Bureau. Profile of general population and housing characteristics: 2010 demographic profile data https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk. Accessed September 30, 2018. 2010.
- 23.Steiner RW, Ix JH, Rifkin DE, Gert B. Estimating risks of de novo kidney diseases after living kidney donation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2014;14(3):538–544. [DOI] [PubMed] [Google Scholar]
- 24.Steiner RW. Moving closer to understanding the risks of living kidney donation. Clin Transplant 2016;30(1):10–16. [DOI] [PubMed] [Google Scholar]
- 25.Berger JC, Muzaale AD, James N, et al. Living kidney donors ages 70 and older: recipient and donor outcomes. Clinical journal of the American Society of Nephrology : CJASN 2011;6(12):2887–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reese PP, Bloom RD, Feldman HI, et al. Mortality and cardiovascular disease among older live kidney donors. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2014;14(8):1853–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam NN, Garg AX. Acceptability of older adults as living kidney donors. Current opinion in nephrology and hypertension 2016;25(3):245–256. [DOI] [PubMed] [Google Scholar]
- 28.Lentine KL, Kasiske BL, Levey AS, et al. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation 2017;101(8S Suppl 1):S1–s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2014;14(8):1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine GN, McCullough KP, Rodgers AM, Dickinson DM, Ashby VB, Schaubel DE. Analytical methods and database design: implications for transplant researchers, 2005. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2006;6(5 Pt 2):1228–1242. [DOI] [PubMed] [Google Scholar]
- 31.Adler JT, Hyder JA, Elias N, et al. Socioeconomic status and ethnicity of deceased donor kidney recipients compared to their donors. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2015;15(4):1061–1067. [DOI] [PubMed] [Google Scholar]
- 32.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2011 Annual Data Report: Kidney. American Journal of Transplantation 2013;13(s1):11–46. [DOI] [PubMed] [Google Scholar]
- 33.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics (Oxford, England) 2009;10(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grams ME, Sang Y, Levey AS, et al. Kidney-Failure Risk Projection for the Living Kidney-Donor Candidate. The New England journal of medicine 2015. [DOI] [PMC free article] [PubMed]
- 35.Steiner RW. The Risks of Living Kidney Donation. The New England journal of medicine 2016;374(5):479–480. [DOI] [PubMed] [Google Scholar]
- 36.Al Ammary F, Thomas AG, Massie AB, et al. The landscape of international living kidney donation in the United States. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2019. [DOI] [PMC free article] [PubMed]
- 37.Gill J, Dong J, Rose C, Johnston O, Landsberg D, Gill J. The effect of race and income on living kidney donation in the United States. Journal of the American Society of Nephrology : JASN 2013;24(11):1872–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar K, Tonascia JM, Muzaale AD, et al. Racial differences in completion of the living kidney donor evaluation process. Clin Transplant 2018;32(7):e13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gill J, Joffres Y, Rose C, et al. The Change in Living Kidney Donation in Women and Men in the United States (2005–2015): A Population-Based Analysis. Journal of the American Society of Nephrology : JASN 2018;29(4):1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeves-Daniel A, Adams PL, Daniel K, et al. Impact of race and gender on live kidney donation. Clin Transplant 2009;23(1):39–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.