Abstract
Objective:
To understand cystoscopic surveillance practices among patients with low-risk non-muscle-invasive bladder cancer (NMIBC) within the Department of Veterans Affairs (VA).
Methods:
Using a validated natural language processing algorithm, we included patients newly diagnosed with low-risk (i.e. low-grade Ta) NMIBC from 2005 to 2011 in the VA. Patients were followed until cancer recurrence, death, last contact, or two years after diagnosis. Based on guidelines, surveillance overuse was defined as >1 cystoscopy if followed <1 year, >2 cystoscopies if followed 1 to <2 years, or >3 cystoscopies if followed for 2 years. We identified patient, provider, and facility factors associated with overuse using multilevel logistic regression.
Results:
Overuse occurred in 75% of patients (852/1,135) – with an excess of 1,846 more cystoscopies performed than recommended. Adjusting for 14 factors, overuse was associated with patient race (OR 0.49, 95% CI: 0.28, 0.85 unlisted race vs. White), having two comorbidities (OR 1.60, 95% CI: 1.00, 2.55 vs. no comorbidities), and earlier year of diagnosis (OR 2.50, 95% CI: 1.29, 4.83 for 2005 vs. 2011 and OR 2.03, 95% CI: 1.11, 3.69 for 2006 vs. 2011). On sensitivity analyses assuming all patients were diagnosed with multifocal or large low-grade tumors (i.e. intermediate-risk), overuse would have still occurred in 45% of patients.
Conclusions:
Overuse of cystoscopy among patients with low-risk NMIBC was common, raising concerns about bladder cancer surveillance cost and quality. However, few factors were associated with overuse. Further qualitative research is needed to identify other determinants of overuse not readily captured in administrative data.
Keywords: bladder cancer, cystoscopic surveillance, overuse
INTRODUCTION
Over 80,000 new cases of bladder cancer are diagnosed each year in the United States,1 and approximately 75% of them are non-muscle-invasive.2 About 40% of these non-muscle-invasive bladder cancers (NMIBC) are considered low-risk based on pathology and rarely progress to lethal disease (5-year cancer-specific survival rate 95%).1,2 However, patients with low-risk NMIBC are at risk for tumor recurrence and therefore undergo periodic surveillance cystoscopy.
Historically, urologists were trained to inspect the bladder for tumor recurrence every three months, a recommendation dating back to at least 1936.3 Since 2005, however, multiple national and international panels have advised no more than 3 surveillance cystoscopy procedures in the first two years after diagnosis.4–9 These recommendations reflect the apparent lack of benefit from more intense surveillance in patients with low-risk NMIBC.10,11 Low-intensity surveillance cystoscopy would not only spare patients from potentially unnecessary procedures thereby minimizing anxiety and discomfort related to cystoscopy,12 but also curb health care expenditures in one of the most expensive cancers from diagnosis to death, with spending approaching $100,000 per patient in 2013 dollars.13,14 As the majority of bladder cancer costs in the United States are due to cystoscopic surveillance,13,15 understanding the extent of overuse is important to optimize cancer care.16
We hypothesized that despite recommendations for low-intensity surveillance, many patients with low-risk NMIBC receive too much surveillance. By merging administrative claims data with pathology reports to accurately assign cancer-risk, we examined factors associated with overuse of surveillance. In addition to investigating the extent of overuse, we sought to assess patient, provider, and facility factors contributing to it. Such understanding may identify modifiable targets for future improvement efforts.
METHODS
Overview of Design
We conducted a retrospective cohort study of patients newly diagnosed with low-risk NMIBC from the Department of Veterans Affairs (VA) national database. Our primary interest was to evaluate the extent of surveillance overuse as defined by low-risk NMIBC guidelines and to assess 14 patient, provider, and facility factors for association with overuse. The study was approved by the Veteran’s Institutional Review Board of Northern New England (#897920) and the University of Utah Institutional Review Board (#00079402).
Study Population
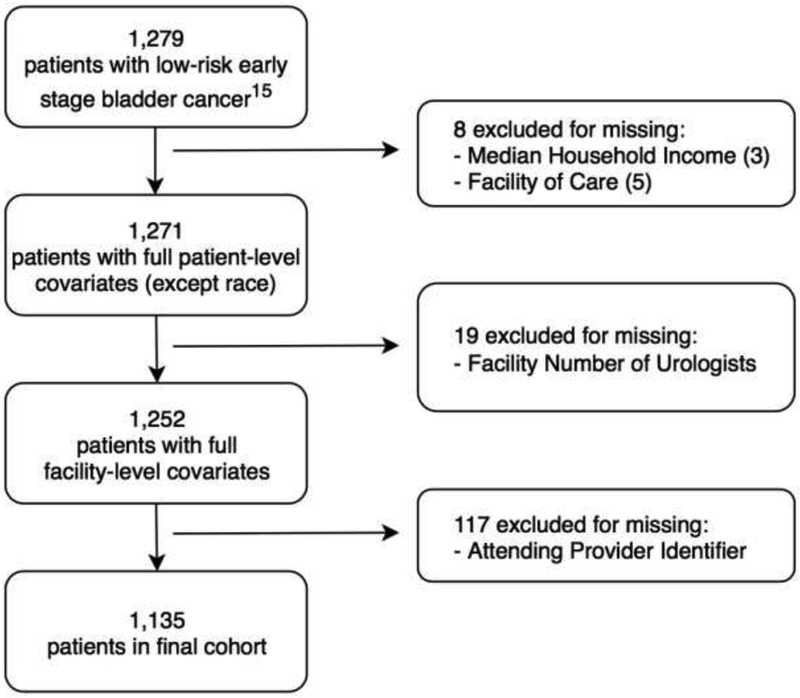
As previously described,17 we combined records from the VA Corporate Data Warehouse with VA Centers for Medicare and Medicaid Services administrative claims to identify Veterans age 66 and older who were diagnosed with low-risk NMIBC between 2005 – when the revised surveillance recommendations first emerged4 – and 2011. Patients were followed only until first cancer recurrence, death, date of last VA encounter, or for two years after diagnosis.18 Patients with low-risk NMIBC (newly diagnosed low-grade Ta) were identified using data extracted from pathology reports via validated natural language processing algorithms.17,19 To handle missing data, we conducted complete case analysis following list-wise deletion for each variable with the exception of patient race, provider age, and provider sex – for which we created “Unlisted” categories. Finally, only Veterans who had their attending provider listed for the majority of their cystoscopy procedures were included (Figure 1).
Figure 1:
Cohort selection flow diagram with inclusion and exclusion criteria.
Outcome
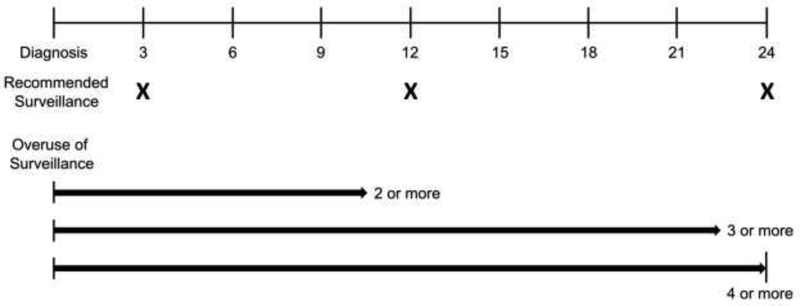
The primary outcome was overuse of cystoscopic surveillance, defined as undergoing more than the recommended number of surveillance cystoscopy procedures during each patient’s respective follow-up period. For each patient, we enumerated cystoscopy procedures using procedure codes.17,18 We then operationalized the outcome as a binary variable – having undergone the recommended surveillance or more than that, i.e. overuse of surveillance. Based on guideline recommendations and length of follow-up, overuse was defined as undergoing two or more cystoscopy procedures if followed for less than one year, three or more procedures if followed between one to less than two years, and four or more procedures if followed for two years after diagnosis (Figure 2). To provide some leeway, we allotted a 90-day grace period centered on the one and two-year follow-up time points. For example, if a patient were to undergo a surveillance cystoscopy at 3 months and subsequently at 10.5 months since initial diagnosis, then this patient would be considered to have received recommended care. Additionally, only one cystoscopy procedure per 30 days was counted as previously described.17
Figure 2: Defining Overuse of Surveillance in Patients with Low-Risk Non-Muscle-Invasive Bladder Cancer:
2 or more cystoscopy procedures if followed for less than one year, 3 or more cystoscopies if followed between one to less than two years, and 4 or more cystoscopies if followed for two years after diagnosis.
Covariates
We considered a number of patient, provider, and facility factors in our analysis. Patient age at time of diagnosis, sex, race, year of diagnosis, and number of comorbidities according to the enhanced Elixhauser Comorbidity Index20 were obtained from the VA Corporate Data Warehouse as were provider-level demographic factors (age and sex). Patient-level socioeconomic status variables (household income and rurality) were obtained from United States census files for the ZIP code of each patient’s residence. Distance from the facility where patients received the majority of their bladder cancer care was estimated using ZIP code to ZIP code distance from the National Bureau of Economic Research.21 Lastly, we retrieved facility factors (size, rurality, complexity level, and number of urologists) from the Veterans Health Administration Support Services Center.22
Statistical Analysis
Data management was performed from December 2015 through April 2018. Data analyses were performed from April 2018 to October 2018. For bivariate analysis, we used the two-sample t-test or the Wilcoxon Rank Sum test to compare continuous variables – stratified by whether patients underwent recommended surveillance or overuse of surveillance. We used the Chi-Square and Fisher’s Exact test to compare categorical variables for patient (sex, race, year of diagnosis, number of comorbidities, rurality), provider (age, sex), and facility factors (rurality, complexity level). After obtaining the number of patients who experienced too much surveillance, we summed the total number of surveillance cystoscopies performed and compared this figure to the recommended number of procedures.
We evaluated 14 patient, provider, and facility factors for association with overuse of surveillance through multilevel logistic regression modeling. Due to the hierarchical nature of the data with patients and providers nested within facilities, we clustered observations at the facility level. Additionally, we evaluated the proportion of variance explained by our model (R2binary) using the latent variable approach.23 All analyses were performed with Stata 15.0.
Sensitivity Analysis
We conducted sensitivity analyses to address the possibility that some patients with low-grade Ta NMIBC may actually be at intermediate-risk due to larger tumor size or multifocality – factors that could not be derived via natural language processing from the full text pathology reports. Because more cystoscopy procedures are recommended for intermediate-risk patients, this could have contributed to an overestimation of the extent of surveillance overuse. In these sensitivity analyses, we assumed an extreme scenario in which all patients were of intermediate-risk. For patients with intermediate-risk NMIBC, cystoscopic surveillance is recommended every 3 to 6 months in the first two years after diagnosis according to the American Urological Association / Society of Urologic Oncology and at 3, 6, 12, 18, and 24 months in the first two years after diagnosis according to the National Comprehensive Cancer Network (NCCN).7,9 The National Institute for Health and Care Excellence advises surveillance cystoscopy even less frequently at 3, 9, and 18 months after diagnosis, while the European Association of Urology recommends a patient-specific surveillance schedule between the low- and high-risk NMIBC guidelines.8,24 Given the variety of intermediate-risk NMIBC surveillance guidelines, we opted to run our sensitivity analyses according to the moderately intensive NCCN recommendations – in which patients should undergo no more than 5 surveillance cystoscopy procedures in the first two years after diagnosis (Supplemental Figure).
RESULTS
We identified 1,135 patients with low-risk NMIBC (mean age 76; 99% male, 84% White) clustered within 84 VA medical centers (average 13.5 patients at each facility, range 1–60). We found overuse of cystoscopy among 75% of patients (852 of 1,135). This included 212 (81%) of 261 patients followed less than 1 year, 182 (85%) of 213 patients followed 1 to less than 2 years, and 458 (69%) of 661 patients followed for 2 years. Across all patients in the cohort, 4,516 cystoscopies were performed although only 2,670 would have been recommended – an excess of 1,846 procedures. Patients who underwent overuse of surveillance were significantly more likely to be diagnosed at an earlier year but were no different in any of the 13 additional factors compared to patients who underwent recommended surveillance (Table 1).
Table 1:
Characteristics of Low Risk Non-Muscle-Invasive Bladder Cancer Patients Undergoing Recommended vs. Overuse of Surveillance
| Recommended Surveillance (n = 283) |
Overuse of Surveillance (n = 852) |
p-value | |
|---|---|---|---|
| Patient Factors | |||
| Age (years), mean (SD) | 76 (7) | 76 (6) | 0.816* |
| Male Sex**, n (%) | >272 (99) | 841 (99) | 1.000Λ |
| Race, n (%) | 0.078Λ | ||
| White | >228 (82) | >727 (85) | |
| African-American | 18 (6) | 60 (7) | |
| Asian** | <11 (1) | <11 (1) | |
| Hispanic** | <11 (1) | 12 (1) | |
| Native American** | <11 (1) | <11 (1) | |
| Unlisted | 26 (9) | 39 (5) | |
| Year of Diagnosis, n (%) | 0.005† | ||
| 2005 | 15 (5) | 93 (11) | |
| 2006 | 20 (7) | 102 (12) | |
| 2007 | 36 (13) | 109 (13) | |
| 2008 | 45 (16) | 131 (15) | |
| 2009 | 49 (17) | 138 (16) | |
| 2010 | 66 (23) | 136 (16) | |
| 2011 | 52 (18) | 143 (17) | |
| Number of Comorbidities§, n (%) | 0.081† | ||
| 0 | 49 (17) | 124 (15) | |
| 1 | 57 (20) | 208 (24) | |
| 2 | 59 (21) | 217 (25) | |
| 3 or more | 118 (42) | 303 (36) | |
| Household Income ($), | 44,750 | 45,927 | 0.454‡ |
| median (IQR) | (38,333–63,050) | (36,704–60,235) | |
| Patient Rurality, n (%) | 0.827† | ||
| Urban | 158 (56) | 482 (57) | |
| Rural | 125 (44) | 370 (43) | |
| Distance to Facility (miles), | 28 (11–58) | 29 (11–63) | 0.643‡ |
| median (IQR) | |||
| Provider Factors | |||
| Provider Age (years), mean (SD) | 0.477† | ||
| <40 | 38 (13) | 100 (12) | |
| ≥40 and <50 | 43 (15) | 116 (14) | |
| ≥50 and <60 | 53 (19) | 173 (20) | |
| ≥60 | 91 (32) | 250 (29) | |
| Unlisted | 58 (20) | 213 (25) | |
| Provider Sex, n (%) | 0.066† | ||
| Male | 217 (77) | 604 (71) | |
| Female | 23 (8) | 64 (8) | |
| Unlisted | 43 (15) | 184 (22) | |
| Facility Factors | |||
| Facility Size||, median (IQR) | 150 (106–218) | 150 (93–199) | 0.157‡ |
| Number of Urologists¶, mean (SD) | 2 (1) | 2 (1) | 0.821* |
| Facility Rurality, n (%) | 0.989† | ||
| Urban | 264 (93) | 795 (93) | |
| Rural | 19 (7) | 57 (7) | |
| Complexity Level#, n (%) | 0.251† | ||
| 1a [most complex] | 131 (46) | 365 (43) | |
| 1b | 44 (16) | 110 (13) | |
| 1c | 28 (10) | 124 (15) | |
| 2 | 68 (24) | 211 (25) | |
| 3 [least complex] | 12 (4) | 42 (5) |
Abbreviation: SD, Standard Deviation | IQR, Interquartile Range
Percentages may not add to 100 due to rounding.
Two-Sample t-Test
Fisher’s Exact Test
Chi-Square Test
Wilcoxon Rank Sum Test
Number of Comorbidities measured by the Elixhauser Comorbidity Index
Facility Size measured by number of operating beds in fiscal year 2008
Number of Urologists measured by full-time equivalents in fiscal year 2010
Complexity level as defined by the VA National Surgery Office. The complexity level reflects the complexity of surgical procedures that can be performed at each facility.
Exact numbers <11 not reported to protect patient confidentiality
In crude analysis, overuse of surveillance was associated with patient race (OR 0.48, 95% CI: 0.28, 0.83 when comparing those with unlisted race to White) and earlier year of diagnosis (OR 2.26, 95% CI: 1.18, 4.31 comparing 2005 to 2011 and OR 1.85, 95% CI: 1.03, 3.33 comparing 2006 to 2011, Table 2). After adjusting for 14 patient, provider, and facility factors, the association of patient race with overuse of surveillance persisted (OR 0.49, 95% CI: 0.28, 0.85 when comparing those with unlisted race to White) and having two comorbidities was associated with overuse (OR 1.60, 95% CI: 1.00, 2.55). The association between earlier year of diagnosis and overuse was stronger in the fully-adjusted model (OR 2.50, 95% CI: 1.29, 4.83 comparing 2005 to 2011 and OR 2.03, 95% CI: 1.11, 3.69 comparing 2006 to 2011, Table 2).
Table 2:
Crude and Adjusted Effect Sizes using Multilevel Logistic Regression for Patient, Provider, and Facility Factor Association with Overuse of Surveillance
| Crude Odds Ratio (95% CI) | Adjusted* Odds Ratio (95% CI) | |
|---|---|---|
| Patient Factors | ||
| Age | 1.00 (0.97, 1.02) | 1.00 (0.98, 1.02) |
| Sex | ||
| Male | Reference | Reference |
| Female | 1.22 (0.33, 4.55) | 1.47 (0.38, 5.66) |
| Race | ||
| White | Reference | Reference |
| African-American | 1.16 (0.65, 2.06) | 1.33 (0.73, 2.43) |
| Asian | 0.92 (0.18, 4.81) | 1.12 (0.21, 6.07) |
| Hispanic | 1.97 (0.42, 9.18) | 1.51 (0.31, 7.34) |
| Native American | 0.53 (0.15, 1.91) | 0.52 (0.14, 1.96) |
| Unlisted | 0.48 (0.28, 0.83) | 0.49 (0.28, 0.85) |
| Year of Diagnosis | ||
| 2011 | Reference | Reference |
| 2010 | 0.74 (0.48, 1.16) | 0.77 (0.49, 1.22) |
| 2009 | 1.03 (0.64, 1.64) | 0.99 (0.61, 1.59) |
| 2008 | 1.11 (0.69, 1.80) | 1.21 (0.74, 1.97) |
| 2007 | 1.12 (0.68, 1.87) | 1.12 (0.66, 1.88) |
| 2006 | 1.85 (1.03, 3.33) | 2.03 (1.11, 3.69) |
| 2005 | 2.26 (1.18, 4.31) | 2.50 (1.29, 4.83) |
| No. of ComorbiditiesΛ | ||
| 0 | Reference | Reference |
| 1 | 1.48 (0.94, 2.33) | 1.57 (0.98, 2.52) |
| 2 | 1.45 (0.92, 2.29) | 1.60 (1.00, 2.55) |
| 3 or More | 1.03 (0.68, 1.54) | 1.12 (0.73, 1.70) |
| Household Income | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) |
| Patient Rurality | ||
| Urban | Reference | Reference |
| Rural | 0.99 (0.74, 1.32) | 0.89 (0.65, 1.23) |
| Distance to Facility | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) |
| Provider Factors | ||
| Provider Age | ||
| <40 | Reference | Reference |
| ≥40 and <50 | 0.95 (0.55, 1.64) | 0.96 (0.54, 1.68) |
| ≥50 and <60 | 1.09 (0.64, 1.87) | 1.17 (0.67, 2.03) |
| ≥60 | 1.03 (0.64, 1.65) | 1.12 (0.68, 1.84) |
| Unlisted | 1.26 (0.75, 2.12) | 1.24 (0.72, 2.11) |
| Provider Sex | ||
| Male | Reference | Reference |
| Female | 0.94 (0.54, 1.62) | 1.12 (0.62, 2.01) |
| Unlisted | 1.47 (0.99, 2.19) | 1.57 (1.00, 2.46) |
| Facility Factors | ||
| Facility Size† | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) |
| Number of Urologists‡ | 0.99 (0.87, 1.12) | 1.08 (0.93, 1.26) |
| Facility Rurality | ||
| Urban | Reference | Reference |
| Rural | 1.03 (0.54, 1.98) | 0.98 (0.46, 2.10) |
| Complexity Level§ | ||
| 1a [most complex] | Reference | Reference |
| 1b | 0.90 (0.54, 1.48) | 0.88 (0.51, 1.50) |
| 1c | 1.66 (0.95, 2.89) | 1.78 (0.99, 3.20) |
| 2 | 1.15 (0.75, 1.76) | 1.08 (0.60, 1.93) |
| 3 [least complex] | 1.31 (0.61, 2.80) | 1.35 (0.49, 3.72) |
Abbreviations: 95% CI, 95% Confidence Interval | No., Number
Adjusted for all variables shown in table
Number of Comorbidities measured by the Elixhauser Comorbidity Index
Facility Size measured by number of operating beds in fiscal year 2008
Number of Urologists measured by full-time equivalents in fiscal year 2010
Complexity level as defined by the VA National Surgery Office. The complexity level reflects the complexity of surgical procedures that can be performed at each facility.
The R2binary for the model when including only patient and provider factors was 0.075, suggesting that patient and provider characteristics in the model account for 7.5% of the observed variance in surveillance overuse. The addition of facility-level factors marginally increased the R2binary to 0.092; therefore, approximately 9.2% of the observed variation was explained in the full model, the minority of which due to facility factors.
On sensitivity analyses assuming all patients in the cohort were newly diagnosed with multifocal or large low-grade tumors (i.e. intermediate-risk NMIBC), 45% of patients (511 of 1,135) would have undergone more cystoscopy procedures than recommended. In adjusted analysis, having one comorbidity (OR 1.85, 95% CI: 1.22, 2.81 compared to no comorbidities), having two comorbidities (OR 1.59, 95% CI: 1.05, 2.42 compared to no comorbidities), unlisted provider sex (OR 1.57, 95% CI: 1.06, 2.31 compared to male sex), and earlier year of diagnosis (OR 2.13, 95% CI: 1.28, 3.56 comparing 2005 to 2011) were associated with overuse.
COMMENT
In this national study of Veterans with low-risk NMIBC, overuse of cystoscopy was common. These findings persisted when considering the possibility that all patients were newly diagnosed with multifocal or large low-grade tumors (i.e. intermediate-risk NMIBC). In both analyses, however, only few patient, provider, and facility factors were associated with overuse – and only a small component of the observed variation in surveillance overuse was explained by the 14 characteristics. The association of earlier year of diagnosis with overuse suggests lack of knowledge or delayed uptake of surveillance recommendations as a potential cause of overuse.
With costs of cancer care projected to increase beyond $170 billion in 2020,25 an effort remains to achieve more affordable and value-oriented care by curbing potentially unnecessary procedures.16,26,27 The subject of overuse has been studied in cancers of the breast, lung, thyroid, and prostate during diagnosis, surveillance, and active treatment.16 However, examining overuse poses several challenges, including professional consensus for standard of care and the ability to accurately measure overuse through claims data or chart review. Indeed, the field of NMIBC remains one such area where overuse has been challenging to estimate. While multiple urologic panels have recommended lower-intensity surveillance schedules for patients with low-risk NMIBC compared to patients with high-risk NMIBC,4–9 the lack of important clinical details in standard administrative and tumor-registry data have so far hindered observational research.18
Our study adds to the current body of literature by using a national sample of Veterans newly diagnosed with low-risk NMIBC to quantify the extent of surveillance cystoscopy use. Using a validated natural language processing algorithm to abstract granular pathology data, we were able to detect recurrent disease for each patient. Recurrent low-grade non-invasive urothelial carcinoma increases the risk of further recurrences and thus renders patients at least intermediate-risk. We thus only followed newly diagnosed patients with low-risk NMIBC until first cancer recurrence. An added strength of using the VA national database is that the VA functions in a capitated system; therefore, one might postulate that financial incentives to perform procedures are less of an influence on practice patterns compared to fee-for-service payment models.28 As such, the extent of surveillance overuse may parallel – if not, underestimate – care outside of the VA.
There are several limitations to our study. First, our cohort includes predominantly male Veterans age 66 and older. Thus, findings may not be generalizable to settings outside of the VA or to younger patients. However, the majority of bladder cancer cases are comprised of older men,1 making our findings relevant to the largest subgroup of bladder cancer patients. Second, the use of claims data routinely poses risk for disease misclassification while deriving the cohort. To address this, we used validated claims and natural language processing algorithms to identify patients newly diagnosed with low-grade Ta NMIBC.18,19 Third, while we ensured all patients were newly diagnosed with low-grade Ta NMIBC and therefore without recurrent disease, we were unable to ascertain tumor size or multifocality. Thus, some patients may have had intermediate-risk cancer despite low-grade Ta disease (e.g., size greater than 3cm or multifocality), and more intense surveillance would have been recommended. Therefore, we performed sensitivity analyses conservatively assuming all patients were of intermediate-risk; still, nearly half of patients would have received more than the recommended number of cystoscopy procedures during follow-up.
In spite of these limitations, our study has important implications for the cost and quality of bladder cancer care. In a Surveillance, Epidemiology, and End Results (SEER)-Medicare study, cystoscopic surveillance was the greatest contributor to costs; and costs across hospital service areas in the first two years after diagnosis among patients with NMIBC ranged from $5,594 to $9,554 per capita.15 Minimizing overuse of cystoscopic surveillance would likely decrease costs, given that up to three quarters of patients with low-risk NMIBC received more cystoscopy procedures than recommended. Bladder cancer patients also experience an overall lower quality of life,29,30 and a component of their anxiety and discomfort appears to stem from the cystoscopy procedure itself.12 Thus, reducing overuse of cystoscopy may improve the patient experience and quality of care.
The substantial magnitude of surveillance overuse implies a disconnect between surveillance guideline recommendations and actual practice. There are at least three potential reasons for this disconnect. First, providers may not be familiar with guideline recommendations. Ongoing endorsement of low-intensity surveillance for low-risk NMIBC patients may improve provider familiarity, and since our study’s observation period, the American Urological Association / Society of Urologic Oncology in 2016 recommended low-intensity surveillance for patients with low-risk NMIBC.7 Second, while consensus exists, guidelines are based on limited evidence.10 As such, some providers may lack confidence in the guideline recommendations. The evidence surrounding cystoscopic surveillance of low-risk NMIBC certainly could be strengthened. For example, there is a need to better understand the relationship between guideline-adherent cystoscopic surveillance and bladder cancer outcomes such as progression of disease. Third, risk-classification schemes and guideline recommendations are complex and hard to remember, which makes implementation in day-today clinical practice challenging.
CONCLUSIONS
Overuse of surveillance was common in patients with low-risk NMIBC. However, few factors were associated with overuse, and only a small proportion of the observed variance was explained by the factors we were able to measure in existing data. Future work should involve qualitative research to assess other determinants of overuse not readily captured in administrative data, including provider knowledge of and trust in the guideline recommendations and other salient barriers to implementation such as complex risk-classification schemes. Additionally, future work should evaluate whether surveillance practice (recommended vs. overuse) is associated with important clinical outcomes such as progression of disease and bladder cancer-specific mortality. We may then be able to develop interventions that improve surveillance care for patients with low-risk NMIBC.
Supplementary Material
Supplemental Figure 1: Defining Overuse of Surveillance in Patients with Intermediate-Risk Non-Muscle-Invasive Bladder Cancer according to the National Comprehensive Cancer Network Guidelines: 2 or more cystoscopy procedures if followed for less than 6 months, 3 or more cystoscopy procedures if followed between 6 months to less than 1 year, 4 or more cystoscopy procedures if followed between 1 to less than 1.5 years, 5 or more cystoscopy procedures if followed between 1.5 to less than 2 years, and 6 or more cystoscopy procedures if followed for 2 years.
Acknowledgement:
VA/Centers for Medicare and Medicaid Services data was received and used with support from the VA Information Resource Center, SDR 02–237.
Funding: FRS is supported by a Conquer Cancer Foundation Career Development Award and by the Dow-Crichlow Award of the Department of Surgery at the Dartmouth-Hitchcock Medical Center. PPG is supported by the Department of Veterans Affairs Health Services Research & Development (IIR 15–085, 1I01HX001880–01A2).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: Opinions expressed in this manuscript are those of the authors and do not constitute official opinions of the Department of Veterans Affairs.
Declarations of interest: JDS, 100 common stock of Johnson and Johnson. All additional coauthors, no conflict of interest.
REFERENCES:
- 1.Noone A, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2015, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. Accessed May 3, 2018. In:2018. [Google Scholar]
- 2.Nielsen ME, Smith AB, Meyer AM, et al. Trends in stage-specific incidence rates for urothelial carcinoma of the bladder in the United States: 1988 to 2006. Cancer. 2014;120(1):86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lokeshwar VB, Merseburger AS, Hautmann SH. Bladder Tumors:: Molecular Aspects and Clinical Management. Springer Science & Business Media; 2010. [Google Scholar]
- 4.Oosterlinck W, Solsona E, Akaza H, et al. Low-grade Ta (noninvasive) urothelial carcinoma of the bladder. Urology. 2005;66(6):75–89. [DOI] [PubMed] [Google Scholar]
- 5.Oosterlinck W, van der Meijden A, Sylvester R, et al. Guidelines on TaT1 (non-muscle invasive) bladder cancer. European Association of Urology. 2006. Available at: http://uroweb.org/wp-content/uploads/EAU-Guidelines-TaT1-Bladder-Cancer-2006.pdf. [accessed 12.14.18]. [Google Scholar]
- 6.Brausi M, Witjes JA, Lamm D, et al. A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. The Journal of urology. 2011;186(6):2158–2167. [DOI] [PubMed] [Google Scholar]
- 7.Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. The Journal of urology. 2016;196(4):1021–1029. [DOI] [PubMed] [Google Scholar]
- 8.Babjuk M, Böhle A, Burger M, et al. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: update 2016. European urology. 2017;71(3):447–461. [DOI] [PubMed] [Google Scholar]
- 9.The National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: bladder cancer Version 3.2018. 2018. Available at: https://www.nccn.org/professionals/physician_gls/PDF/bladder.pdf, accessed May 3, 2018.
- 10.Olsen LH, Genster HG. Prolonging follow-up intervals for non-invasive bladder tumors: a randomized controlled trial. Scandinavian journal of urology and nephrology Supplementum. 1995;172:33–36. [PubMed] [Google Scholar]
- 11.Pruthi RS, Baldwin N, Bhalani V, Wallen EM. Conservative management of low risk superficial bladder tumors. The Journal of urology. 2008;179(1):87–90. [DOI] [PubMed] [Google Scholar]
- 12.Koo K, Zubkoff L, Sirovich BE, et al. The Burden of Cystoscopic Bladder Cancer Surveillance: Anxiety, Discomfort, and Patient Preferences for Decision Making. Urology. 2017;108:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeung C, Dinh T, Lee J. The health economics of bladder cancer: an updated review of the published literature. Pharmacoeconomics. 2014;32(11):1093–1104. [DOI] [PubMed] [Google Scholar]
- 14.Svatek RS, Hollenbeck BK, Holmäng S, et al. The economics of bladder cancer: costs and considerations of caring for this disease. European urology. 2014;66(2):253–262. [DOI] [PubMed] [Google Scholar]
- 15.Skolarus TA, Ye Z, Zhang S, Hollenbeck BK. Regional differences in early stage bladder cancer care and outcomes. Urology. 2010;76(2):391–396. [DOI] [PubMed] [Google Scholar]
- 16.Baxi SS, Kale M, Keyhani S, et al. Overuse of Health Care Services in the Management of Cancer. Medical care. 2017;55(7):723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroeck FR, Lynch KE, won Chang J, et al. Extent of Risk-Aligned Surveillance for Cancer Recurrence Among Patients With Early-Stage Bladder Cancer. JAMA Network Open. 2018;1(5):e183442–e183442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroeck FR, Sirovich B, Seigne JD, Robertson DJ, Goodney PP. Assembling and validating data from multiple sources to study care for Veterans with bladder cancer. BMC urology. 2017;17(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeck FR, Patterson OV, Alba PR, et al. Development of a Natural Language Processing Engine to Generate Bladder Cancer Pathology Data for Health Services Research. Urology. 2017;110:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care. 2005:1130–1139. [DOI] [PubMed] [Google Scholar]
- 21.Roth J ZIP Code Distance Database -- ZIP Code Tabulation Area (ZCTA) Distance Database. The National Bureau of Economic Research. http://www.nber.org/data/zip-code-distance-database.html. Published June 16, 2017 Accessed May 29, 2018. [Google Scholar]
- 22.Department of Veteran Affairs: VHA Support Service Center (VSSC). 2015. Available at: https://vssc.med.va.gov. accessed April 29, 2015.
- 23.Austin PC, Merlo J. Intermediate and advanced topics in multilevel logistic regression analysis. Statistics in medicine. 2017;36(20):3257–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Collaborating Centre for Cancer, National Institute for Health and Care Excellence. “Bladder cancer: diagnosis and management”. 2015. [Google Scholar]
- 25.Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. Journal of the National Cancer Institute. 2011;103(2):117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan DJ, Brownlee S, Leppin AL, et al. Setting a research agenda for medical overuse. The BMJ. 2015;351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levinson W, Kallewaard M, Bhatia RS, Wolfson D, Shortt S, Kerr EA. ‘Choosing Wisely’: a growing international campaign. BMJ Qual Saf. 2015;24(2):167–174. [DOI] [PubMed] [Google Scholar]
- 28.Hillman AL, Pauly MV, Kerstein JJ. How do financial incentives affect physicians’ clinical decisions and the financial performance of health maintenance organizations? New England journal of medicine. 1989;321(2):86–92. [DOI] [PubMed] [Google Scholar]
- 29.Reeve BB, Potosky AL, Smith AW, et al. Impact of cancer on health-related quality of life of older Americans. JNCI: Journal of the National Cancer Institute. 2009;101(12):860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith AB, Jaeger B, Pinheiro LC, et al. Impact of bladder cancer on health-related quality of life. BJU international. 2018;121(4):549–557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Defining Overuse of Surveillance in Patients with Intermediate-Risk Non-Muscle-Invasive Bladder Cancer according to the National Comprehensive Cancer Network Guidelines: 2 or more cystoscopy procedures if followed for less than 6 months, 3 or more cystoscopy procedures if followed between 6 months to less than 1 year, 4 or more cystoscopy procedures if followed between 1 to less than 1.5 years, 5 or more cystoscopy procedures if followed between 1.5 to less than 2 years, and 6 or more cystoscopy procedures if followed for 2 years.