Abstract
Objective:
Children of depressed parents are at increased risk for psychopathology. One putative mechanism of risk appears to be altered processing of emotion-related stimuli. Although prior work has evaluated how adolescent offspring of depressed parents may show blunted reward processing compared to low-risk youth, there has been less attention to how young children with this familial history may differ from their peers during middle childhood, a period of critical socio-affective development.
Method:
The current study evaluated 56 emotionally healthy 6-to 8-year children who were deemed at high-risk (n=25) or low-risk (n=31) for depression based on maternal history of depression. Children completed a behavioral reward seeking task in the laboratory and an fMRI paradigm assessing neural response to happy faces, a social reward.
Results:
Findings demonstrated that high-risk children showed blunted responding to happy faces in the dorsal striatum compared to low-risk children. Further, lower responding in the dorsal striatum and dorsolateral prefrontal cortex was related to lower behavioral reward seeking, but only in high-risk children.
Conclusion:
Function within neural reward regions may be altered in high-risk offspring as young as 6- to 8-years of age. Further, neural reward responding may be linked to lower behavioral response to obtain reward in these high-risk offspring.
Keywords: Affective Stimuli, Reward, Risk, Depression
1. Introduction
Children of depressed parents are at increased risk for developing depression and other psychiatric illnesses relative to their peers (Goodman & Gotlib, 1999; Pilowsky et al., 2006). Although these elevated rates of psychiatric illness are well established, the mechanisms underlying this transmission of risk are less understood. Some evidence suggests that high-risk youth appear to possess neurophysiological differences in the way they process threat and that these differences emerge prior to the onset of psychiatric illness and predict the onset of symptoms (LeMoult, Chen, Foland-Ross, Burley, & Gotlib, 2015; Waugh, Muhtadie, Thompson, Joormann, & Gotlib, 2012). Given that depression is a disorder of altered positive affect (Forbes & Dahl, 2005; 2012), with anhedonia and disrupted social behavior being particularly characteristic of depression, differences in how these youth neurally respond to positive emotion-eliciting events and stimuli may be especially important.
Positive affect has important functions including buffering against stress and facilitating recovery from illness (Fredrickson, 1998; 2001). Importantly, positive affect also promotes approach toward new and social experiences, even in the face of adversity (Fredrickson, 1998; 2001). Indeed, in early and middle childhood, positive affect may assist in building new friendships and in the pursuit of emotional and social learning (Izard, 2002). In that regard, altered responding in reward and positive affective circuitry may interfere with social approach and pursuit in new and challenging situations.
Neural regions implicated in reward and positive affective processing include the ventral and dorsal striatum, orbitofrontal cortex, and the prefrontal cortex (O’Doherty, 2004). The striatum is involved in the subjective pleasure of positive experiences and is implicated in both “wanting” and “liking” these experiences. In particular, the ventral striatum is thought to be involved in pleasure and motivation and the dorsal striatum is implicated in goal-directed behavior, decision-making, and action-contingent learning (Balleine, Delgado, & Hikosaka, 2007). The orbitofrontal cortex appears to play a role in reward learning and valuation (O’Doherty, 2004). The prefrontal cortex has multiple reward-related functions, with medial portions thought to be involved in regulation of reward experiences and lateral regions in reward learning (i.e., vlPFC) and appraisal of unexpected, positive events (dorsolateral prefrontal cortex, dlPFC). The dlPFC is also thought to be involved in promotion of motivated behavior (Ballard et al., 2011). Interestingly, portions of the striatum appear to functionally project to regions within the prefrontal cortex, with more ventral regions of the striatum projecting to ventromedial and orbitofrontal regions of the prefrontal cortex and dorsal regions of the striatum projecting to dorsal regions of the prefrontal cortex (for a review, see Haber & Knutson, 2010). Thus, these regions may be acting in tandem to promote the pursuit of and enjoyment of positive experiences.
A growing body of work has demonstrated that adolescent offspring of depressed parents show altered neural responding to reward in multiple of the aforementioned neural regions (Gotlib et al., 2010, Luking et al., 2016; Monk et al., 2008, Olino et al., 2014, Sharp et al., 2014). Specifically, high-risk adolescents have been demonstrated to show blunted responding in the ventral and dorsal striatum and orbitofrontal cortex in response to anticipating or winning monetary reward or in response to positive stimuli, such as happy faces. These findings mirror similar neural reward patterns observed in clinically depressed adolescents and adults (Forbes et al., 2009, Ng, Alloy, & Smith, 2018; Zhang et al., 2013). Our prior work has also demonstrated that these reward-related alterations preceded increases in depressive symptoms across a 2-year period in community adolescents (Morgan et al., 2013), suggesting that these reward disruptions are important to the pathophysiology of depression.
However, less work has evaluated how young, pre-pubertal children at high familial risk for depression may respond neurally to reward-related stimuli. Behavioral work has demonstrated that high-risk children as young as 3-months to 3-years old show lower positive emotion expression compared to their low-risk peers (Durbin et al., 2005; Field, 2010). Given that neurobehavioral work has found a link between neural responding in reward regions (e.g., ventral striatum) and positive affect expression in healthy adolescents (Forbes et al., 2010), high-risk children may likewise show diminished responding in neural reward regions and this diminished neural responding may be related to the lower behavioral responding previously observed in young children. Two recent studies have demonstrated that prepubertal offspring (ages 6-10) showed blunted striatal and prefrontal response to gain feedback relative to low risk peers (Luking et al., 2016; Wiggins et al., 2017). Likewise, blunted responsivity to reward has been demonstrated in prepubertal children either at high-risk for depression due to family history or increases in depressive symptoms in work using event related potentials (ERP) (Bress, Smith, Foti, Klein, & Hajcak, 2012; Kujawa, Hajcak, & Klein, 2018; Kujawa, Hajcak Proudfit, & Klein, 2014). However, more work is needed to confirm these aberrations and link them to behavioral differences in children of this age and younger.
More specifically, altered function in reward-related regions may already be present in children at familial risk for depression during early to middle childhood and these alterations may be directly related to disruptions in the pursuit of socially rewarding, goal-driven behavior during this developmental point at which social and play behavior is becoming more sophisticated and is important for normative social and emotional learning (Nelson, Jarcho, & Guyer, 2016). Understanding these neurobehavioral differences during early to middle childhood is key, as this is a period during which critical socio-affective foundations (e.g., regulating affect, creating social bonds) are built. Disruptions to these developmental processes could create susceptibility to depression during the vulnerable period of adolescence.
In the current study, we evaluated neural and behavioral reward-related differences in young 6- to −8-year-old emotionally healthy children who were designated to be at high- or low-risk for depression based on maternal history. We chose to evaluate children of this age as middle childhood is a period of vast socio-emotional learning, and changes during this period may be foundational for adolescent vulnerability in the coming years. Similar to prior work (e.g., Kerestes et al., 2015; Monk et al., 2008), we evaluated neural response to happy faces as a measure of social reward in children of this age. We hypothesized that high-risk children would show blunted responding in multiple reward regions (ventral and dorsal striatum, orbitofrontal cortex, prefrontal cortex) and lower behavioral reward seeking relative to low-risk children. We also hypothesized that neural and behavioral reward-related responding would be positively associated with one another.
2. Method
Participants and Procedures
Participants were 56 typically developing 6- to 8-year old (M=6.82 years, SD=.77 years) children with no lifetime history of psychiatric illness or other health problems. Participants were 55% female, and 77% White/Caucasian, 14% Black/African American, and 7% Multiracial. Participants were recruited from existing studies evaluating maternal depression as well as community advertisements. Children were excluded from the study if they met criteria for any Axis I psychiatric disorder (e.g., ADHD, anxiety, depression), developmental disability, or neurological disorder. Children were categorized as high-risk for depression (n=25) if mothers met criteria for two or more lifetime episodes of Major Depressive Disorder or Dysthymia on the Structural Clinical Interview for DSM-IV Disorders (SCID-IV) (i.e., recurrent and/or chronic depression). Mothers were ineligible if they met lifetime criteria for a manic or hypomanic episode, psychotic symptoms, and/or Substance Dependence. In the high-risk group, seven mothers were currently depressed at the time of the study and 17 met lifetime criteria for an Anxiety Disorder (n=9 Panic Disorder, n=8 Social Phobia, n=6 Post-Traumatic Stress Disorder, n=4 Generalized Anxiety Disorder, n=1 Obsessive Compulsive Disorder). Children were categorized as low-risk for depression (n=31) if mothers had no lifetime history of any Axis I disorder.
Originally, 66 participants were enrolled in the study. An additional 4 participants did not complete the fMRI scan (n=1 parent refusal, n=2 child refusal, and n=l scanning contraindication). Of the 62 who completed the entire assessment including the fMRI, n=6 had excessive movement (see fMRI preprocessing), n=1 had low behavioral responding in the scanner (<80% responding), and n=1 was missing behavioral reward seeking data due to a computer error, for a final sample of n=56 (90% of scanned). Children with usable data (n=56; 31 low risk, 25 high risk) did not differ from children removed from the study on child age or mother age (Ms = 6.80 years and 6.70 years, respectively, p=.70; Ms=37.98 years and 36.20 years respectively, p=.36), behavioral reward seeking (Ms= 14.70 and 13.78 tokens won respectively, p=.65) or risk status (χ2 = .80, p=.50, 4 low risk, 6 high risk removed). However, more boys were removed from the study due to unusable scan data than girls (χ2 = 4.24, p=.04; 8 boys vs 2 girls). High risk and low risk children did not differ on study demographic variables including child age, child sex, child race, parent age, parent educational level, or single parenthood (see Table 1).
Table 1.
Participant and Variable Characteristics
| High Risk (n=25) | Low Risk (n=31) | F/t/p | |
|---|---|---|---|
| Child age in years | 6.72 | 6.87 | F=.52, p=47 |
| Number of Females | 15 (60%) | 16 (52%) | Χ2 =.39, p=.60 |
| Number of Minority Race | 4 (16%) | 9 (29%) | X2 =1.60,p=. 45 |
| Number with Single Parent | 4 (16%) | 6 (19%) | X2 =.11,p=.75 |
| Parent age in years | 38.92 | 37.26 | F=1.13, p=.29td |
| Number with Parent with High School education | 4 (16%) | 5 (16%) | X2 =.95,p=.81 |
| Child Tokens Won | 13.96 | 15.29 | F=.90, p=.35 |
| Child Depressive Symptoms | 40.48 | 38.58 | F=5.25,p=.026 |
| Child Anxiety Symptoms | 9.66 | 7.10 | F=2.02, p=.161 |
Children and mothers completed two laboratory visits for the study. At the first visit, mothers and children completed clinical interviews to assess psychiatric history, children completed a computerized reward seeking task with an experimenter, and an experimenter took pictures of mothers using standardized methods (i.e., lighting, backdrop, angle, size). At the second visit, children completed an fMRI scan. The two visits were on average 34.27 days apart (SD=28.79 days, range=l-188 days). The University of Pittsburgh Institutional Review Board approved all research procedures, and written informed consent was obtained from each participant and his/her mother.
Measures
Clinical interviews.
Mothers completed the SCID with a bachelor’s or master’s level interviewer trained to reliability by a licensed clinical psychologist. Children and mothers were interviewed separately using the Kiddie Schedule for Affective Disorders (KSADS) by the same interviewer to assess child present and lifetime psychiatric history. Summary scores on the KSADS were derived of a synthesis of parent and child report. Discrepancies between child and parent report were distinguished via further inquiry with the parent. Once again, none of the children in either group met criteria for any Axis I disorder on the KSADS. Sixteen percent of the SCID clinical interviews and the KSADS clinical interviews (n=12) were double coded by a licensed clinical psychologist and inter-rater reliability was high (97.7% diagnostic agreement for SCID; 98.0% for KSADS).
Progressive ratio schedule.
Children completed a computerized progressive ratio schedule (see Chelonis, Gravelin, & Paule, 2011). In this task, children pressed a button to blow up a virtual balloon. Children were informed that once the balloon was blown all the way up, they would receive a token. Children were also informed that they would be able to exchange their earned tokens at the end of the task for a prize from a prize box (i.e., the more tokens won, the better the prize). The task was set to a progressive ratio of 1:10—the task required increasing levels of effort (i.e., 10 extra presses on each trial relative to the previous trial) to blow up the balloon as the game progressed. Children were able to stop playing the game whenever they wanted (i.e., complete as many trials as they wanted for tokens) for up to 30 minutes. In this regard, number of button presses reflected the number of trials completed. The PR schedule is a widely used measure of reward motivation that has been utilized in both human and animal studies. The task can be used with various reinforcers including money, food, or addictive drugs, is associated with other measures of reward sensitivity, and has been shown to produce similar within-person breakpoints with repeated assessment (Der-Avakian & Markou, 2012, Glover, Roane, Kadey, & Grow, 2008; Miras et al., 2012). The task measured effort a child is willing to exert to achieve a reward and served as a behavioral measure of reward seeking. Similar to other studies (Bell & DeWall, 2019; Chelonis et al., 2011), we used total number of button presses as our measure of behavioral reward seeking in analytical models.
Child affective symptoms.
Parents reported on child depressive and anxiety symptoms using the Mood and Feelings Questionnaire (MFQ; Angold & Costello, 1987) and the Screen for Child Anxiety Related Emotional Disorders (SCARED; Birmaher et al., 1997) respectively. The MFQ has 34 items and includes items such as “s/he felt miserable or unhappy” and “s/he cried a lot”. Parents rated their children’s depressive symptoms within the past two weeks a 3-point Likert scale (0=not true, l=sometimes, 2=true). The SCARED has 41 items such as “my child is nervous” and “my child is a worrier”. Parents rated their children’s anxiety symptoms within the past week on a 3-point Likert scale (0=not true/hardly ever true, l=somewhat true or sometimes true, 2=very true or often true). Both the MFQ and the SCARED had good internal consistency in our study (α=.73 for MFQ and .86 for SCARED).
Faces task.
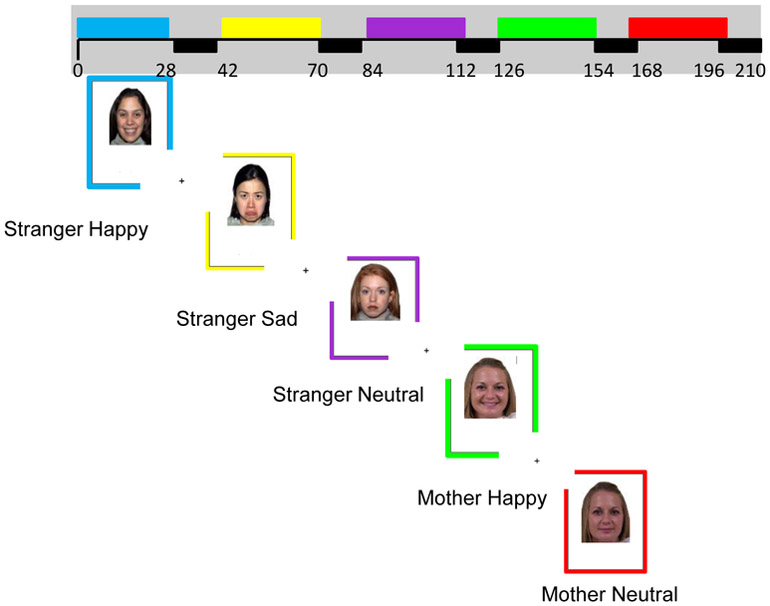
In this 3.5-minute task, adapted from Luby and colleagues (Gaffrey et al., 2011), participants were presented with 5 blocks of unfamiliar adults displaying happy, sad, and neutral expressions and their own mother displaying happy expressions and neutral expressions. Thus, the 5 blocks were unfamiliar happy, unfamiliar sad, unfamiliar neutral, mother happy, and mother neutral blocks. Each block had 7 distinct images and each image was displayed for three seconds with an inter-trial interval of 1s; thus each block lasted 28 seconds long. For both unfamiliar and mother blocks, images were not repeated. Children were asked to respond with a button press each time they saw a face in order to ensure task engagement. Blocks were separated by a 14 second inter-trial interval where children viewed a cross-hair (see Figure 1). To prevent non-standardized carry-over effects, all children viewed blocks in the same order. This task measured implicit emotion processing and contrasts using sad, angry, and fearful faces have been demonstrated to reliably activate multiple regions implicated in emotion processing in prior work (Gaffrey et al, 2011, 2013). To evaluate neural response to happy faces as a measure of positive emotion processing and social reward processing, we evaluated neural response to happy faces > neutral faces using the images of unfamiliar adults (taken from the NimStim; Tottenham et al., 2009) in analyses as they are standardized and have been validated in prior work. Our second contrast of interest was mother happy faces > mother neutral faces, a measure of personally relevant social reward and affective processing.
Figure 1.
fMRI Faces Paradigm
Post-Scan Ratings.
After completing the scan, children were shown the same faces as appeared in the Faces fMRI task in the scanner. Images were not displayed in the same blocks as in the Faces fMRI task (i.e., were mixed by valence and familiarity) but did appear in a standard order across participants. Children were asked to rate the facial expressions viewed during the scan on a 5-point Likert scale (l=very sad, 2=sad, 3=neither happy nor sad, 4=happy, 5=very happy). Across the children in the study, children rated the mother happy faces as 4.78 out of 5, unfamiliar happy faces with an average score of 4.73 out of 5, mother neutral faces with an average score of 3.16 out of 5, unfamiliar neutral faces with an average score of 2.91 out of 5, and unfamiliar sad faces with an average score of 1.51 out of 5.
fMRI Acquisition and Preprocessing
Each participant was scanned using a Siemens 3T TIM Trio scanner. Structural images were acquired using MPRAGE 192 axial slices, 1.0 mm thick (TR/TE = 2200/3.35ms, FOV = 256mm, matrix 256×240, flip angle = 9 degrees). BOLD functional images for the Faces task were acquired in a single run, with a gradient echo planar imaging sequence and covered 39 axial slices, 3.1 mm thick, beginning at the cerebral vertex and encompassing the entire cerebrum and the majority of the cerebellum (TR/TE = 2010/28 ms, FOV = 205mm, matrix = 64×64, flip angle = 90 degrees). All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data. Before the collection of fMRI data for each participant, we acquired and inspected a reference EPI scan to confirm the absence of artifacts and good signal across the entire volume of acquisition.
Preprocessing and analysis of fMRI data were completed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Structural images of each participant were segmented to focus on gray matter. For each functional scan, data were realigned to the first volume to correct for head motion and unwarped to correct for static inhomogeneity interactions. Realigned and unwarped images were then coregistered with the participant’s anatomical image. The anatomical image was then spatially normalized into standard stereostatic space (Montreal Neurological Institute template) using a 12-parameter affine model and smoothed with a 6-mm full-width at half-maximum Gaussian filter based on conventional recommendations (e.g., see Huettel, Song & McCarthy, 2009). Voxels were resampled during preprocessing to 2 mm3. Preprocessed data were analyzed using first-level random effects models that account for scanto-scan variability. First, participants were ensured to have a maximum movement <4mm in each plane for analyzed contrasts,≥ 80% behavioral responding in scanner, and ≥80% signal coverage in ventral regions (e.g., ventral striatum). Further quality control measures revealed that of the 56 participants who met these criteria, 46 had maximum movement less than 2mm in each plane for analyzed contrasts (82% of participants). Of the remaining 10 participants included in analyses, 6 participants had <10% volumes with movement above 2mm. in any plane. Motion parameters from the realignment phase were entered as covariates in first level models, along with estimates of cerebrospinal fluid and white matter, to control for participant movement and non-task related brain activity. This process ensured a balance of retaining as many participants as feasible while still appropriately handling motion in a young child sample. Second level random effects models that account for participant-to-participant variability were then conducted to determine task-specific regional responses.
Data Analytic Strategy
Regression models that included child age, child sex, child risk status, number of tokens received in the PR schedule, and the multiplicative interaction of risk status and tokens (in order to evaluate how the brain-behavior associated varied by risk status) were conducted in SPM8. Whole brain analyses were used to probe significant clusters of interest at a cluster forming threshold of p<.001 and corrected for multiple comparisons using family wise error of p<.05 at the peak-level (Carter, Lesh, & Barch, 2016). We used conjunction analyses for simple slope analyses to probe interactions (Nichols, Brett, Andersson, Wager, & Poline, 2005). We then extracted values from significant clusters and evaluated associations between risk and reward-related brain regions and child’s own depressive and anxiety symptoms.
3. Results
Descriptives
There were no significant risk group differences in child age or sex (ps=.47-.81). Neither child age nor sex were associated with behavioral reward seeking (ps = .13-,65). There was also no significant effect of risk status on behavioral reward seeking (i.e., number of tokens received in the PR Schedule; Mlowrisk = 15.29 tokens, Mhighrisk = 13.96 tokens) (p=.35). High risk children had higher parent ratings of child depressive symptoms but not anxiety symptoms relative to low risk children (MFQ-P: Mhighrisk =40.48; Mlowisk = 38.58, p=.026; SCARED: Mhighrisk =9.66; Mlowisk=7.10, p=.161). Child depressive symptoms and anxiety symptoms were positively correlated (r=.34, p=.011). Child depressive symptoms were also significantly correlated with child behavioral reward seeking, such that higher levels of depressive symptoms were associated with less behavioral reward seeking (r= −.33, p=.014).
Neural Response to Standardized Happy Faces
There was a significant main effect of tokens on neural response to happy faces in the dorsal striatum, posterior cingulate, inferior parietal lobe, and dorsolateral prefrontal cortex, such that greater behavioral reward seeking was associated with greater responding in those regions (see Table 2).
Table 2.
Intercorrelations of Key Study Variables
| Variable | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1. Child age in years | -- | -- | -- | -- |
| 2. Child Behavioral Reward Seeking | .12 | -- | -- | -- |
| 3.Child Depressive Symptoms | −.32* | −.33* | -- | -- |
| 4. Child Anxiety Symptoms | −.15 | −.20 | .34* | -- |
Note.
p<.05
This regression model also revealed a significant main effect of risk status on neural response to happy faces in the dorsal striatum, such that children at high risk for depression showed lower response in the dorsal striatum to happy faces relative to low risk children (see Table 2; 220 voxels, t=5.39, [−16, 2, 12], pFWE=031).
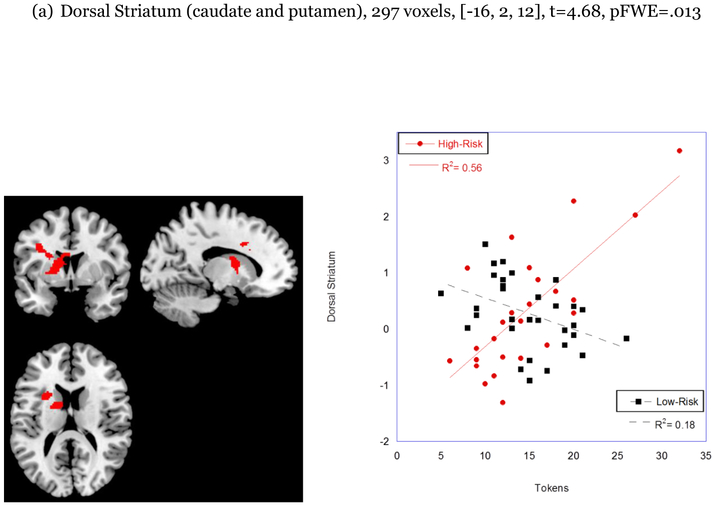
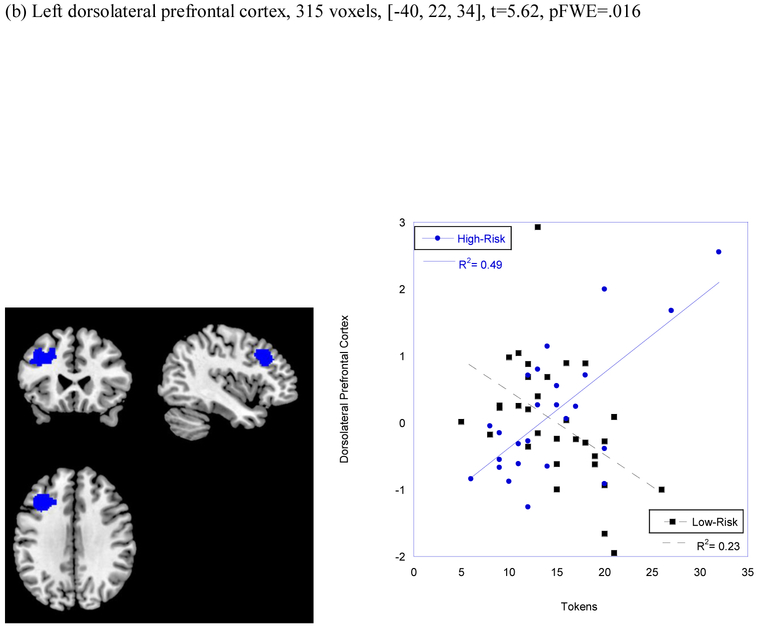
There was also a significant interactive effect of risk status by number of tokens on neural response to happy faces in both the dorsal striatum (297 voxels, t=4.68, [−16, 2, 12], pFWE=013) and in the dlPFC/BA 9 (315 voxels, t=5.62, [−40, 22, 34], pFWE = .016; see Figure 1). Simple slope analyses conducted by group revealed that the association between behavioral reward seeking and neural response to social reward in the dorsal striatum and dorsolateral prefrontal cortex was significant only for children at high risk for depression (276 voxels, t=6.26, [−14, 2, 10], pFWE=002; 144 voxels, t=5.56, [−40, 22, 34] pFWE=.019).1
Neural Response to Mother Happy Faces
There was no association between risk status or behavioral reward seeking and neural response to mother happy faces relative to mother neutral faces. Risk status also did not moderate the association between behavioral reward seeking and neural response to mother happy faces.
Associations with Child Depressive and Anxiety Symptoms
There were no significant associations between task-related activation in the dorsal striatum or dorsolateral prefrontal cortex and child depressive or anxiety symptoms.2
4. Discussion
Our study provided evidence that children as young as 6- to 8- years old at high familial risk for depression show blunted responding to happy faces in the striatum and the dorsolateral prefrontal cortex relative to low-risk children, but this finding was qualified by an interactive effect with child behavioral reward seeking. Our study revealed a unique effect in that lower responding in these regions was associated with less behavioral reward seeking in a task designed to measure effort to pursue reward, but only in high-risk children. Our findings add to the growing body of literature demonstrating that clinically healthy offspring of depressed parents show neurobiological aberrations and illustrate that (1) these neural aberrations are present as early as 6- to 8-years of age and (2) that they relate to differences in behavioral effort to receive reward.
The dorsal striatum is thought to be involved in decision-making, including actionselection and initiation. Further, the caudate nucleus, particularly the left caudate nucleus, has been shown to respond to both social and monetary reward (Izuma, Saito, & Sadato, 2008). In the context of positive affect, the dorsolateral prefrontal cortex, among other functions, is implicated in promotion of motivated behavior and appraisal of unexpected, positive stimuli (Ballard, Murty, Carter, MacInnes, Huettel, & Adcock, 2011). Together, both regions may be involved in pursuit of positive experiences, such as effort to pursue reward. For children with a familial history of depression, lower activity within these regions may be paired with lower approach and effort in situations that typically evoke positive emotions (e.g., playing with friends or engaging in a game). This pattern may be part of an early emerging endophenotype for depression (Hasler et al., 2004) that may place them at greater risk for the onset of depression, a disorder of disrupted positive affect and motivation, later in development.
Prior work had established that neural reward-related disruptions are present in adolescent offspring of depressed parents (Gotlib et al., 2010, Monk et al., 2008, Olino et al., 2014, Sharp et al., 2014). Newly emerging evidence of neurobehavioral reward-related differences during middle childhood is troubling, as this is a developmental period in which vast emotional growth is occurring in the context of positive experiences. In particular, play behavior and its pursuit fosters intellectual, academic, and emotional learning and may serve as a building block through which younger children learn to regulate their emotions and get along with peers (Ginsburg, 2007; Nelson et al., 2016, Rubin & Coplan, 1998). Further, the formation of regulated affect and positive social bonds will be key during the relatively rocky period of adolescence when new stressors emerge, momentary mood becomes less positive in tone (Larson et al, 2002), and vulnerability for depression and other disorders increases (Morgan et al., 2013 a; Morgan et al., 2013b).
In the current study, neural response in the dorsal striatum and dorsolateral prefrontal cortex were not associated with child depressive or anxiety symptoms. This may have been because children in the study were required to be free of Axis I psychiatric disorders. Further, findings remained significant with inclusion of child depressive and anxiety symptom level in our models. Thus, our findings provide critical evidence that these neural alterations in response to affective stimuli and social reward appear to emerge before the onset of psychiatric illness and are not explained by pre-existing sub-threshold symptomatology. Future work should evaluate how these neurobehavioral reward-related alterations during middle childhood predict adolescent reward-related behavior and onset of psychiatric illness. We did not find that activity in other key reward regions, such as the ventral striatum or orbitofrontal cortex, differed by risk group or was differentially associated with behavioral reward seeking. This is surprising given prior findings demonstrating their importance in high-risk adolescent offspring and in depressed adolescents and adults. This may suggest that these regions may be more relevant to normative changes that occur during adolescence, rapid neural changes that are related to social re-orientation (Nelson et al., 2016). However, other recent work using monetary-like rewards (i.e., candy) demonstrated altered responding in the ventral striatum in high-risk young offspring (see Luking et al., 2016). Thus, it may be that these alterations are only apparent in response to “wanting” or anticipatory stimuli (which our paradigm of happy faces did not include), given the ventral striatum’s putative role in anticipatory aspects of reward processing (Haber & Knutson, 2010). Longitudinal research that follows high-risk offspring from early and middle childhood through adolescence and that includes both monetary and social reward paradigms and assessment of both reward anticipation and outcome will be important to elucidate this possibility. Future work should also consider evaluating group differences in circuit-level function (e.g., functional connectivity) to further test how diverse reward-related regions may act in tandem to promote reward-related behavior and how this may differ depending on risk status.
We also did not find that high risk and low risk children responded differently to personally relevant happy faces (i.e., mother faces) nor was neural response to personally relevant happy faces differentially associated with behavioral reward seeking based on risk status in our sample. This may suggest that, at ages 6-8 years, neural differences in affective stimuli and reward-related stimuli may be present only for generalized stimuli, but not for stimuli that is family-related. Another possibility is that findings may have been more pronounced and evident for the unfamiliar faces because of their standardization and prior support of their valence (Tottenham et al., 2009). The use of images of children’s mothers required a tradeoff of personal relevance and standardization, which may have limited their utility.
Other limitations to our study include the use of an emotional faces task as a social reward paradigm rather than a traditional monetary reward paradigm. Although prior work has demonstrated that happy faces elicit activation in primary reward regions (e.g., Kerestes et al., 2015; Monk et al., 2008), our findings may have differed with use of a more traditional incentive-based reward task (e.g., monetary incentive delay task, Wiggins et al., 2017). We chose to focus only on happy faces due to our interest in altered responding in positive affective and rewarding stimuli in children at risk for depression. Future work should evaluate how risk status is related to neural responding to sad faces. Our task was also 3.5 minutes long and included only one run due to need for brevity due to developmental needs of 6- to 8-year old children. Task length may have limited our ability to detect reward-related effects in our sample. All participants viewed the blocks in the same order (unfamiliar happy, sad, neutral then mother happy and neutral) which may have introduced confounds such as subject fatigue and scanner drift.
Our findings build on newly emerging research evaluating reward-related brain function in young children with use of a social reward paradigm. Specifically, our study is unique in 1) evaluation of neural response to social reward stimuli in a very young sample of 6- to 8-year old children, 2) in linking these brain findings to behavioral responding to reward and 3) in use of a sample with a maternal history of recurrent or chronic depression.
Altogether, our findings provide further evidence that function within neural reward regions may be altered in high-risk offspring as young as 6- to 8-years of age, even in the absence of differences in reward-motivated behavior. Furthermore, the level of neural alteration in reward responding may be linked to lower behavioral response to obtain reward in these high-risk offspring. For young children without this familial risk, response in this circuitry does not appear to be related to their effort to seek out rewards. It should be noted that the opposite effect also appears to be true based on our findings: greater neural response to reward in high-risk children is associated with more effort to obtain rewards. In line with a differential susceptibility framework (Belsky et al., 2007), our findings also suggest that stronger response in these reward regions may also uniquely promote pursuit of reward in difficult circumstances for young children with a genetic predisposition for depression. Combined with prior findings that children exposed to depressed parents may be more susceptible to environmental influences (e.g., maternal warmth; Morgan et al., 2014), our findings may provide a hopeful goal—to strengthen neural responding in reward and emotion regulatory regions early in development, perhaps via positive affective support.
Figure 2.
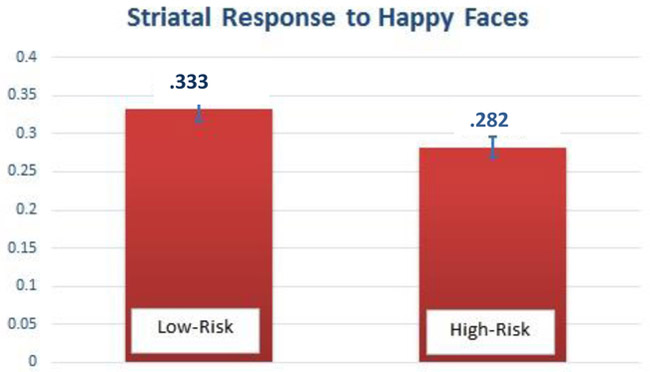
Altered dorsal striatal response in 6- to 8-year old children at high familial risk for depression
Figure 3.
Lower response in the dorsal striatum (a) and dorsolateral prefrontal cortex (b) to happy faces is related to lower behavioral reward seeking, but only in high-risk children.
Table 3.
Regression Model Results
| Variable | Region | k | Coordinates | t, p (peak-level) |
|---|---|---|---|---|
| Age | -- | -- | -- | -- |
| Sex | -- | -- | -- | -- |
| Behavioral Reward Seeking | Dorsal Striatum | 1588 | −14, 2, 10 | t=6.26 (pFWE=.002) |
| Posterior Cingulate | 3658 | −22, −58, 28 | t=6.03 (pFWE =.004) | |
| Inferior Parietal | 387 | 28, 52, 44 | t=5.91 (pFWE=.007) | |
| Dorsolateral Prefrontal Cortex | 197 | −40, 22, 34 | t=5.56 (pFWE=.019) | |
| Risk Status | Dorsal Striatum | 220 | −16, 2, 12 | t=5.39 (pFWE=.031) |
| Dorsolateral Prefrontal Cortex | 219 | −40, 20, 36 | t=5.09 (pFWE=.074) | |
| Behavioral Reward Seeking x Risk Status | Dorsal Striatum | 297 | −16, 2, 12 | t=4.68 (pFWE=.013) |
| Dorsolateral Prefrontal Cortex | 315 | −40, 20, 36 | t=5.62 (pFWE=.016) |
Highlights:
Reward alterations may be a mechanism of risk for children of depressed parents
High risk children showed less striatal response to happy faces
Lower striatal and prefrontal cortex response related to less reward seeking
Altered reward function related to less behavioral effort to obtain reward
Acknowledgements
We thank the study families for their participation.
Role of Funding Source
This research was supported by Grant K01 MH099220 from the National Institutes of Health to Judith K. Morgan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
One participant had neural responding in the dorsal striatum that was greater than 3 standard deviations above the mean. Findings were substantively similar when we removed this participant from the regression model for unfamiliar happy faces > unfamiliar neutral faces. Using a task-based region of interest mask from empirically-identified clusters, there was a significant main effect of risk status on dorsal striatal response [96 voxels, t=4.60 (−16, 2, 16), pFWE=.002] and a significant interactive effect of risk status and behavioral responding on dorsal striatal response and dorsolateral response to unfamiliar happy faces [107 voxels, t=4.75, (−16, 0, 18), pFWE=.001; 69 voxels, t=3.93, (−40, 20, 36), pFWE=.014].
Findings remained substantively similar when we controlled for child depressive and anxiety symptoms on the MFQ and SCARED, respectively, in our regression model for unfamiliar happy faces > unfamiliar neutral faces. Using a whole-brain analysis with cluster p<.001 and corrected for multiple comparisons using family wise error p<.05, high-risk children showed lower dorsal striatal response to unfamiliar happy faces compared to low-risk children [191 voxels, t=5.46, (16, 2, 12), pFWE=.027]. There was also an interactive effect of risk status and behavioral reward seeking on dorsal striatal response and dorsolateral prefrontal cortex response to unfamiliar happy faces [302 voxels, t=5.87, (−16, 2, 12), pFWE=.008; 278 voxels, t=5.50, (−40, 22, 34), pFWE=.024].
5. References
- Angold A & Costello EJ (1987). Mood and feelings questionnaire Durham, NC: Developmental Epidemiology Program, Duke University. [Google Scholar]
- Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA & Adcock RA (2011). Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. Journal of Neuroscience, 31, 10340–10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR & Hikosaka O (2007). The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience, 27, 8161–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SB & DeWall CN (2019). Pressing the rewarding button: The relationship between impulsivity, fatigue, and reward sensitivity. Journal of Research in Personality, 79, 24–29. [Google Scholar]
- Belsky J, Bakersman-Kranenburg MJ, & Van IJzendoorn MH (2007). For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science, 16, 300–304. [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, & Neer SM (1997). The screen for child anxiety related emotional disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 545–553. [DOI] [PubMed] [Google Scholar]
- Bress JN, Smith E, Foti D, Klein DN, & Hajcak G (2012). Neural response to reward and depressive symptoms in late childhood to early adolescence. Biological Psychology, 89, 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Lesh TA, & Barch DM (2016). Thresholds, power, and sample sizes in clinical neuroimaging. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1, 99–100. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, Gravelin CR, & Paule MG (2011). Assessing motivation in children using a progressive ratio task. Behavioral Processes, 87, 203–209. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A & Markou A (2012). The neurobiology of anhedonia and other reward-related deficits. Trends in Neuroscience, 35, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin CE, Klein DN, Buckley ME, & Moerk KC (2005). Temperamental emotionality in preschoolers and parental mood disorders. Journal of Abnormal Psychology, 114, 28. [DOI] [PubMed] [Google Scholar]
- Field T (2010). Postpartum depression effects on early interactions, parenting, and safety practices. Infant Behavior and Development, 33, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon MWJB, & Williams JB (1995). The Structured Clinical Interview for DSM-IV axis I disorders. New York: New York State Psychiatric Institute. [Google Scholar]
- Forbes EE, & Dahl RE (2005). Neural systems of positive affect: Relevance to understanding child and adolescent depression? Development and Psychopathology, 17, 827–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE & Dahl RE (2012). Research review: Altered reward function in adolescent depression: What, when, and how? Journal of Child Psychology and Psychiatry, 53, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, & Dahl RE (2009). Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry, 166, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Tarr JA, Sciarrillo SR, & Dahl RE (2010). Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child and Adolescent PsychiaOy, 49, 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL (1998). What good are positive emotions? Review of General Psychology, 2, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL (2001). The role of positive emotions in positive psychology: The broadenand-build theory of positive emotions. American Psychologist, 58, 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Luby JL, Belden AC, Hirshberg JS, Volsch I, & Barch DM (2011). Association between depression severity and amygdala reactivity during sad face viewing in depressed preschoolers: An fMRI study. Journal of Affective Disorders, 129, 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Barch DM, Singer I, Shenoy R, & Luby JL (2013). Disrupted amygdala reactivity in depressed 4-to-6-year old children. Journal of the American Academy of Child and Adolescent Psychiatiy, 52, 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg KR (2007). The importance of play in promoting healthy child development and maintaining strong parent-child bonds. Pediatrics, 119, 182–1991. [DOI] [PubMed] [Google Scholar]
- Glover AC, Roane HS, Kadey HJ, & Grow LL (2008). Preference for reinforcers under progressive and fixed ratio schedules: A comparison of single and concurrent arrangements. Journal of Applied Behavior Analysis, 41, 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S & Gotlib IH (1999). Risk for psychopathology in the children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychological Review, 106, 458. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, & Joormann J (2010). Neural processing of reward and loss in girls at risk for major depression. Archives of General Psychiatry, 67, 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN & Knutson B (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, & Charney DS (2004). Discovering endophenotypes for major depression. Neuropsychopharmacology, 29, 1765. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, & McCarthy G (2004). Functional magnetic resonance imaging (Vol. 1). Sunderland, MA: Sinauer Associates. [Google Scholar]
- Izard CE (2002). Translating emotion theory and research into preventive interventions. Psychological Bulletin, 128, 795–824. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, & Sadato N (2008). Processing of social and monetary rewards in the human striatum. Neuron, 58, 284–294. [DOI] [PubMed] [Google Scholar]
- Kashdan TB (2007). Social anxiety spectrum and diminished positive experiences: Theoretical synthesis and meta-analysis. Clinical Psychology Review, 27, 348–365. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Segreti AM, Pan LA, Phillips ML, Birmaher B, Brent DA, & Ladouceur CD (2016). Altered neural function to happy faces in adolescents with and at risk for depression. Journal of Affective Disorders, 192, 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, & Klein DN (2014). Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of Abnormal Psychology, 123, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, & Klein DN (2019). Reduced reward responsiveness moderates the effect of maternal depression on depressive symptoms in offspring: Evidence across levels of analysis. Journal of Child Psychology and Psychiatry, 60, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson RW, Moneta G, Richards MH, & Wilson S (2002). Continuity, stability, and change in daily emotional experience across adolescence. Child Development, 73, 1151–1165. [DOI] [PubMed] [Google Scholar]
- LeMoult J, Chen MC, Foland-Ross LC, Burley HW, & Gotlib IH (2015). Concordance of mother-daughter diurnal cortisol production: Understanding the intergenerational transmission of risk for depression. Biological Psychology, 108, 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Pagliaccio D, Luby JL, & Barch DM (2016). Depression risk predicts blunted neural responses to gains and enhanced responses to losses in healthy children. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miras AD, Jackson RN, Jackson SN Goldstone AP, Olbers T, Hackenberg T, Spector AC, & leRoux CW (2012). Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. The American Journal of Clinical Nutrition, 96, 467–473. [DOI] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton III, Guardino M, Masten CL, McClure-Tone EB, Fromm S, & Blair RJ (2008). Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. American Journal of Psychiatry, 165, 90–98. [DOI] [PubMed] [Google Scholar]
- Morgan JK, Olino TM, McMakin DL, Ryan ND, & Forbes EE (2013). Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiology of Disease, 52, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JK, Shaw DS, & Forbes EE (2013). Physiological and behavioral engagement in social contexts as predictors of adolescent depressive symptoms. Journal of Youth and Adolescence, 42, 117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JK, Shaw DS, & Forbes EE (2014). Maternal depression and warmth during childhood predict age 20 neural response to reward. Journal of the American Academy of Child and Adolescent Psychiatiy, 53, 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Jarcho JM, & Guyer AE (2016). Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience, 17, 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T Brett M, Andersson J, Wager T, & Poline JB (2005). Valid conjunction inference with the minimum statistic. Neuroimage, 25, 653–660. [DOI] [PubMed] [Google Scholar]
- Ng TH, Alloy LB, Smith DV (2018). Meta-analysis of reward processing in Major Depressive Disorder: Distinct abnormalities within the reward circuit? BioRxiv, 332981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP (2004). Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Current Opinion in Neurobiology, 14, 769–776. [DOI] [PubMed] [Google Scholar]
- Olino TM, McMakin DL, Morgan JK, Silk JS, Birmaher B, Axelson DA, Williamson DE, Dahl RE, Ryan ND & Forbes EE (2014). Reduced reward anticipation in youth at high-risk for unipolar depression: A preliminary study. Developmental Cognitive Neuroscience, 8, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin KH & Coplan RJ (1998). Social and nonsocial play in childhood: An individual differences perspective. Multiple perspectives on play in early childhood education, 144–170. [Google Scholar]
- Sharp C, Kim S, Herman L, Pane H, Reuter T, & Strathearn L (2014). Major depression in mothers predicts reduced ventral striatum activation in adolescent female offspring with and without depression. Journal of Abnormal Psychology, 123, 298. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus AW, Casey BJ, & Nelson C (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168, 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Muhtadie L, Thompson RJ, Joormann J & Gotlib IH (2012). Affective and physiological responses to stress in girls at elevated risk for depression. Development and Psychopathology, 24, 661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Wickramaratne P, Pilowsky DJ, Poh E, Batten LA, Hernandez M, Flament MF, Stewart JA, McGrath P, Blier P, & Stewart JW (2015). Treatment of maternal depression in a medication clinical trial and its effect on children. American Journal of Psychiatry, 172, 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Schwartz KT, Kryza-Lacombe M, Spechler PA, Blankenship SL, & Dougherty LR (2017). Neural reactivity to reward in school-age offspring of depressed mothers. Journal of Affective Disorders, 214, 81–88. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Chang SH, Guo LY, Zhang KL, & Wang J (2013). The neural correlates of reward-related processing in major depressive disorder: A meta-analysis of functional magnetic resonance imaging studies. Journal of Affective Disorders, 151, 531–539. [DOI] [PubMed] [Google Scholar]