Abstract
Sexual assault (SA) is associated with increased risk for chronic pain, but the mechanisms for this relationship are poorly understood. To explore whether disrupted descending inhibition is involved, this study used a conditioned pain modulation (CPM) task to study inhibition of pain and the nociceptive flexion reflex (NFR; a correlate of spinal nociception) in 32 pain-free SA survivors. This group was compared to 32 pain-free, trauma-exposed persons without SA (no- SA group) and a group of 40 pain-free persons who reported no trauma exposure (no-TE). CPM was assessed from painful electric stimulations (test stimulus) delivered to the ankle before, during, and after participants submerged their hand in painful 10°C water (conditioning stimulus). Pain ratings and NFR were assessed in response to test stimuli. All groups demonstrated significant inhibition of pain during CPM. However, only the no-TE group demonstrated significant inhibition of NFR. The no-SA group showed no inhibition of NFR, whereas the SA group showed significant facilitation of NFR. These findings suggest that trauma exposure may impair inhibitory cerebrospinal circuits, but that SA may specifically promote facilitation of spinal nociception.
Perspective: This study suggests trauma exposure disrupts cerebrospinal inhibition of spinal nociception but that exposure to sexual assault further promotes chronic pain risk by facilitating spinal nociception. This help may help elucidate the pain risk mechanisms in trauma survivors.
Keywords: conditioned pain modulation, sexual assault, nociceptive flexion reflex, trauma, pain
Introduction
Sexual assault (SA), defined as exposure to any nonconsensual sexual act72, occurs in 1-in-4 women and 1-in-100 men, with a new incident occurring every 98 seconds in the U.S.16. SA has been linked to many negative outcomes, including chronic pain27. Indeed, many people with chronic pain report experiencing SA (7-91%)74, but only a minority of SA survivors report sustaining a physical injury during the SA40,73. Thus, injuries from SA are not likely responsible for chronic pain risk.
Laboratory studies suggest SA may promote chronic pain by disrupting pain processing. For example, SA survivors are hyperalgesic and have greater temporal summation of pain (a marker of central sensitization) compared to non-traumatized controls30,62. Moreover, our recent study found that SA survivors displayed general hyperalgesia and failed to inhibit spinal nociception (assessed by the nociceptive flexion reflex, NFR) in response to pleasant stimuli during an emotional modulation task33. Interestingly, inhibition of pain perception by pleasant stimuli (relative to neutral stimuli) was intact during the task, but not enough to offset the observed hyperalgesia33. Given that studies indicate a cortico-cortical circuit (e.g., anterior insula, orbitofrontal cortex [OFC], subgenual cingulate cortex [sgCC]) mediates emotional modulation of pain whereas a cerebrospinal circuit (e.g., ventromedial [vmPFC] and medial prefrontal cortices [mPFC], sgCC, amygdala, pons) mediates emotional modulation of NFR15,53,56,59,69, these results suggest that SA survivors have deficits in the cerebrospinal circuit responsible for NFR inhibition (Fig 1). These deficits could allow ascending nociception from the spinal cord to go “unchecked” and possibly promote chronic pain3,25,50,82. However, it is not clear whether this deficit is specific to modulation via emotions.
Fig. 1.
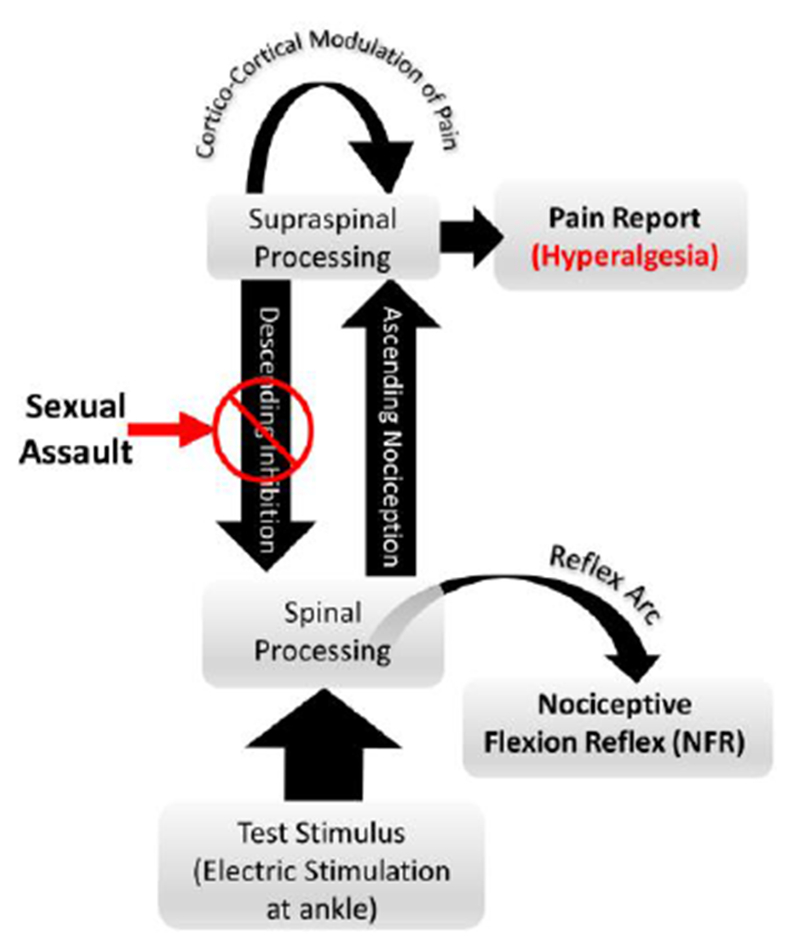
Putative effects of sexual assault (SA) on modulation of pain processing. Prior research has suggested that multiple circuits exist for modulating pain and spinal nociception59. Specifically, spinal nociception is modulated by a descending (cerebrospinal) circuit that can inhibit ascending pain signals early in pain transmission. However, there are additional cortico-cortical circuits that can further modulate pain at the suprapinal level, later in pain tranmission and perception. A study of emotional modulation of pain and the nociceptive flexion reflex (NFR, a measure of spinal nociception) found that SA survivors failed to inhibit spinal nociception and experienced hyperalgesia in response to electric stimulations33. Although pleasant emotions did inhibit pain perception in SA survivors (relative to a neutral emotional state), this effect was not strong enough to offset the observed hyperalgesia.
Descending inhibition is most often assessed experimentally by the conditioned pain modulation task (CPM; pain inhibiting pain)6,36,80. Clinically, responses to this task are important because studies have shown that a disruption of CPM-related pain inhibition is related to future chronic pain onset81,82. Moreover, CPM-related inhibitory processes also involve cortico-cortical and cerebrospinal circuits14,41,47,65. For example, Piche et al47 examined pain ratings and NFRs in response to painful electric test stimuli while participants placed their hand in painfully cold water (conditioning stimulus, CS). They found that pain inhibition was associated with a circuit involving the OFC, posterior cingulate cortex (PCC), anterior cingulate cortex (ACC), sgCC, anterior insula, amygdala, parahippocampal gyrus (PHG), and mPFC. By contrast, NFR inhibition was associated with a cerebrospinal circuit that included the primary somatosensory cortex (SI), paracentral lobule, supplementary motor area (SMA), pre-SMA, ACC, PCC, PHG, PFC, thalamus, and connections with the pons, periaqueductal grey (PAG), and rostral ventromedial medulla (RVM). Thus, CPM could be used to assess endogenous inhibitory circuits in SA survivors.
In a recent prospective study of 286 healthy, pain-free participants, we found that deficits in CPM-related NFR inhibition, but not deficits in CPM-related pain inhibition, predicted future onset of chronic pain34. This suggests a role of the CPM cerebrospinal circuit in chronic pain risk. Given this, the present study investigated whether currently pain-free participants with SA (n=32) had deficits in CPM of pain and NFR. To control for general exposure to trauma, this group was compared to a pain-free group with a history of non-SA trauma (no-SA, n=32), who were matched43 on age, sex, race, and mean number of non-SA traumas. These two groups were compared to a pain-free control group without a history of trauma exposure (no-TE, n=40). It was predicted that SA survivors would be hyperalgesic and fail to inhibit NFR. However, given evidence from a prior study of emotional modulation of pain33, it was unclear whether they would fail to inhibit pain.
Methods
Study Participants
Participants were recruited from the community as part of a larger study investigating risk factors for chronic pain in Native Americans and non-Hispanic Whites. Exclusion criteria included: 1) <18 years old, 2) any history of cardiovascular, neuroendocrine, musculoskeletal, neurological disorders, 3) history of or current chronic pain, 4) BMI>35, 5) recent use of anti-depressant, anxiolytic, analgesic, stimulant, and anti-hypertensive medication, 6) current psychotic symptoms (assessed by Psychosis Screening Questionnaire10) or substance use problems, and/or 7) an inability to read/speak English. Healthy, pain-free subjects were recruited to determine if disrupted pain modulation occurs prior to the onset of chronic pain and to rule out that disease status explains any group differences.
Participants completed laboratory testing over the course of 2 days, with each session lasting 4-6 hours. During each day of testing, participants completed a variety of tasks (painful and non-painful) with mandatory breaks in between each task to avoid/reduce any carry-over effects. The current study will focus on the results from the CPM task, which was administered towards the end of one of the testing days and lasted approximately 15 minutes.
251 participants enrolled in and attended the first testing session, however 27 withdrew prior to or during CPM. Multilevel modeling (MLM) does not exclude cases listwise28; therefore, participants who began CPM were still considered for the current study (N=224). Of those, 32 participants reported a history of SA (28 female) on the Life Events Checklist (a commonly used measure of potentially traumatic events described below). Of the 191 participants without a history of SA, a sample of 32 (28 female) trauma-exposed persons were selected as a control group matched on age, race, sex, and mean number of non-SA traumas (no-SA group). This matching procedure was intended to minimize the confounding influence of any group differences due to general trauma exposure, age, sex, and race. Finally, those participants who reported no trauma exposure (n=40, 13 female) were selected as no-trauma controls (no-TE). This design allows us to examine the effect that SA has on endogenous pain inhibition while controlling for the general effect of trauma exposure. However, due to demographic differences in base rates of trauma exposure in the population44, the no-TE group was not matched on all variables (similar to control groups used in other studies of trauma exposure43,49,62,68). Thus, analyses controlled for these variables. Table 1 presents participant characteristics by group. Note: some participants in the SA and no-SA groups also participated in our published study of emotional modulation of pain and NFR in SA survivors33.
Table 1.
Participant characteristics by group
| Categorical Variable | No-TE (n=40) |
No-SA (n=32) |
SA (n=32) |
Inferential Statistics |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | X2 | p-value | Comparisons | ||
| Sex (Female) | 13 | 32.5% | 28 | 87.5% | 28 | 87.5% | 33.349 | <.001 | no-TE < (SA = no-SA) | |
| Race | 9.742 | .045 | ||||||||
| NHW | 22 | 55.0% | 10 | 31.3% | 10 | 31.3% | no-TE > (SA = no-SA) | |||
| NA | 14 | 35.0% | 21 | 65.6% | 21 | 65.6% | no-TE < (SA = no-SA) | |||
| Other | 4 | 10.0% | 1 | 3.1% | 1 | 3.1% | ||||
| Employment Status | 8.204 | .084 | ||||||||
| >40 hrs/week | 8 | 21.1% | 14 | 43.8% | 7 | 21.9% | ||||
| <40 hrs/wk | 16 | 42.1% | 9 | 28.1% | 18 | 56.3% | ||||
| Unemployed | 14 | 36.8% | 9 | 28.1% | 7 | 21.9% | ||||
| Continuous Variable | M | SD | M | SD | M | SD | F | p-value | partial η2 | Comparisons |
| Age (years) | 25.650 | 10.888 | 31.000 | 12.342 | 30.406 | 12.357 | 2.275 | .108 | 0.043 | |
| Body mass index (kg/m2) | 24.679 | 4.278 | 26.523 | 4.745 | 25.560 | 4.117 | 1.534 | .221 | 0.030 | |
| non-SA Trauma # (LEC, 0-17)§ | - | - | 2.156 | 1.298 | 2.156 | 1.816 | <.001 | >.999 | 0.000 | |
| Global Psych Distress (SCL-90, 0-4) | 0.210 | 0.293 | 0.312 | 0.247 | 0.479 | 0.295 | 8.093 | .001 | 0.139 | SA > (no-SA = no-TE) |
| Pain Catastrophizing (PCS, 0-52) | 7.975 | 7.708 | 7.719 | 8.467 | 11.531 | 9.123 | 2.125 | .125 | 0.040 | |
| State Anxiety (STAI, 20-80) | 32.700 | 7.901 | 31.813 | 7.258 | 35.344 | 6.968 | 1.980 | .143 | 0.038 | |
| Perceived Stress (PSS, 0-40) | 12.250 | 5.420 | 12.594 | 6.440 | 15.613 | 5.264 | 3.454 | .035 | 0.065 | SA > (no-SA = no-TE) |
| NFR Threshold (0-50 mA) | 16.913 | 10.185 | 15.867 | 9.392 | 18.594 | 10.335 | 0.608 | .547 | 0.012 | |
| 3-Stimulus Threshold (0-50 mA) | 12.650 | 6.379 | 12.625 | 6.272 | 14.750 | 7.162 | 1.134 | .326 | 0.022 | |
| Stimulus Intensity (0-50 mA) | 26.233 | 12.779 | 23.922 | 11.297 | 25.636 | 12.292 | 0.333 | .717 | 0.007 | |
| Cold Water Pain Intensity (0-100)§§ | 51.200 | 27.151 | 56.750 | 24.870 | 66.438 | 27.630 | 2.935 | .058 | 0.055 | SA > no-TE |
Note: SA = participants with a history of sexual assault. No-SA = matched control group without a history of sexual assault. No-TE = control group without a history of trauma exposure. NHW = non-Hispanic white. NA = Native American. LEC = Life Events Checklist. SCL-90 = Symptom Checklist −90. PCS = Pain Catastrophizing Scale. STAI = State Trait Anxiety Inventory. PANAS = Positive and Negative Affect Schedule. PSS = Perceived Stress Scale.
This analysis was conducted with an independent samples t-test because the no-TE group reported no traumas (Cohen’s d reported for the effect size instead of partial eta squared). Comparisons report differences between groups at p<.05.
These comparison analyses were conducted post-hoc due to a marginal overall significant difference between groups.
This study was approved by the Institutional Review Boards of The University of Tulsa, Cherokee Nation, and the Oklahoma City Area Indian Health Service. During the informed consent process, participants were provided a detailed overview of all procedures and informed they could withdraw at any time. All participants provided verbal and written informed consent prior to enrollment and were provided $100 honorarium for the completion of each testing day (or $10/hour of every hour of testing completed).
A priori power analyses with 3 within-subject levels (CPM phases: baseline, cold water, post), 3 between-subject levels (no-TE, no-SA, SA), α=.05, intercorrelations among the repeated measures = .70, power=.80, and effect sizes from previous papers evaluating group differences in CPM (d=.37)29 suggested a total sample size of 39. Thus, our sample of 104 appears adequate.
Testing Apparatus
Study procedures were controlled by a computer with dual monitors, analog-to-digital converter (USB-6212 BNC; National Instruments, Austin, TX, USA) and LabVIEW software (National Instruments). Participants completed study procedures in an experimental room and used one monitor to complete electronic questionnaires and provide pain ratings, while a researcher located in an adjacent room monitored physiology via the second monitor. Study procedures were conducted in a sound attenuated and electrically shielded room. Throughout testing, participants wore sound-attenuating headphones to listen to pre-recorded instructions and communicate with the experimenter. In addition to monitoring physiology, the researcher monitored the participant via a video camera for study procedure compliance.
Electric stimuli were delivered to the left ankle over the retromalleolar pathway of the sural nerve by a stimulator (Digitimer DS7A; Hertfordshire, England) and a bipolar electrode (Nicolet, Model#019-40400, Madison, WI). Each electric stimulation was delivered as a train of five 1-ms rectangular wave pulses at 250-Hz and was perceived as a single stimulus. The timing of the delivery of electric stimuli was computer controlled. For safety purposes, the maximum intensity of each electric stimulation intensity was set to 50-mA.
To assess the NFR electromyography (EMG), two active Ag-AgCl electrodes were applied over the left biceps femoris muscle (located approximately 10-cm superior to the popliteal fossa). The EMG signal was filtered (10-Hz to 300-Hz) and amplified (×10,000) using a Grass Technologies (West Warwick, RI) Model 15LT amplifier (with AC Module 15A54). An electrode was placed over the lateral epicondyle of the femur to serve as a ground. The participant’s skin was cleaned with alcohol and exfoliated (NuPrep gel; Weaver and Company, Aurora, CO) to achieve impedances <5kΩ for EMG and stimulating electrode. Electrodes were filled with conductive gel (EC60, Grass Technologies), and EMG signals were sampled at 1000-Hz.
Questionnaires
Participants provided demographic information and health status via a custom-built questionnaire to assess background information and study inclusion/exclusion criteria. SA history was determined via the Life Events Checklist (LEC)32. A person was placed in the SA group if they endorsed “happened to me” for either of 2 items assessing SA32. The remaining 14 items on the LEC that were answered “happened to me” were summed to indicate the number of non-SA traumas (for matching purposes and determining the no-TE group).
Participants completed additional questionnaires to assess groups’ differences in psychological characteristics known to affect pain20,39 and to allow for these variables to be entered as covariates if needed. The Symptom Checklist-90-Revised (SCL-90-R) assesses various psychological symptoms17. The Global Severity Index (GSI) of the SCL-90-R was used to assess current overall psychological distress (higher scores indicate greater distress). The Pain Catastrophizing Scale assesses catastrophic cognitions (e.g., rumination, magnification, helplessness) associated with pain (higher scores indicate greater catastrophizing) and was administered via traditional instructions67. The State-Trait Anxiety Inventory (STAI) assesses the severity of state anxiety (higher scores indicate greater anxiety)64. The Perceived Stress Scale (PSS) assesses psychological stress within the past month (higher scores indicate more perceived stress)13.
Determination of Electric Stimulus Intensity used during CPM
Suprathreshold electric stimulus intensity (in mA) was individually calibrated to each participant prior to CPM testing. Because NFR magnitudes were a dependent variable in the current study, it was important to ensure that NFRs were reliably evoked throughout CPM testing. We have previously shown that setting the stimulus intensity above both NFR threshold and 3-stimulus threshold achieves this goal, whereas only setting it above NFR threshold does not.70 Interestingly, stimuli above NFR threshold and 3-stimulus threshold do not evoke pain in all individuals. Thus, a third calibration procedure was used (Pain30) if necessary to ensure that stimuli were experienced as at least mildly painful in all participants. As a result, stimulus intensity was set to the highest of: 1.2x the intensity of NFR threshold, 1.2x the intensity of 3-stimulus threshold, and 1x Pain30 (if necessary). During all 3 procedures, participants rated their pain intensity in response to each electric stimulus on a computer-presented visual analog scale (VAS) that ranged from 0 (“no pain sensation”) to 100 (“the most intense pain sensation imaginable”)55,71
Nociceptive Flexion Reflex (NFR) Threshold.
NFR is a spinally-mediated withdrawal reflex evoked by Aδ fiber activation, wherein the limb (e.g., leg) withdraws from a noxious stimulus54,55,57,60. Given that the reflex requires the activation of Aδ fibers but its reflex arc does not require supraspinal regions (it is observed in spinally transected individuals58,78), the NFR is used as a correlate of spinal nociception. Moreover, NFR can be modulated by descending input from supraspinal centers6,45 and human studies have shown that NFR is inhibited by CPM7,11,33,47.
NFR threshold was assessed using a well-validated paradigm that involved 3 ascending-descending staircases of stimulations54. During this procedure an NFR was said to occur if the mean rectified biceps femoris EMG in the 90 to 150-ms post-stimulus interval exceeded the mean rectified biceps femoris EMG activity during the 60-ms pre-stimulus baseline interval by at least 1.4 SD of the −60 to 0 ms prestimulation baseline EMG activity54. The first ascending staircase began at 0-mA and increased in 2-mA increments until a reflex was observed (peak). Following the first reflex, the stimulus intensity decreased in 1-mA steps until the reflex was no longer observed (trough). The subsequent 2 ascending-descending staircases implemented 1-mA step increments until all 3 peak and troughs were obtained. To minimize predictability and reflex habituation, the interval between electric stimulations varied randomly (8 to 12-s). NFR threshold was defined as the average stimulus intensity (mA) of the peaks and troughs of the last 2 ascending-descending staircases.
Pain30.
In the event that stimuli at NFR threshold did not elicit at least mild pain (determined by a rating ≥30 on the VAS), the Pain30 task was implemented. If assessed, the computer started the stimulus intensity (mA) at NFR threshold and increased the intensity in 2-mA increments until a VAS rating ≥30 was obtained. 14 participants (35%) in the no-TE group, 12 (38%) in the no-SA group, and 6 (19%) in the SA group underwent Pain30 assessment, χ2=3.19, p=.203.
3-Stimulus Threshold.
3-stimulus threshold assessed NFRs in response to a 3-stimulus series (i.e., each stimulation consisted of 3 trains of electric stimuli), with an interval of 0.5-s between each stimulation (stimulations=train of five 1-ms pulses at 250-Hz). The first series began at 0-mA and then the series increased by 2-mA until an NFR was evoked by the 3rd stimulation in the series. That intensity was designated as 3-stimulus threshold.
In addition to determining the suprathreshold stimulus intensity used during CPM, NFR threshold and 3-stimulus threshold were also used to assess group differences in general pain/nociceptive sensitivity. Pain30 was not used because it was not obtained for all participants.
Conditioned Pain Modulation (CPM)
CPM is a validated paradigm used to assess brain-to-spinal cord pain inhibitory circuitry38 (a human analog of the Diffuse Noxious Inhibitory Controls used with animals36). In brief, this task involves the assessment of pain in response to a test stimulus before, during, and after a tonic CS delivered at a distal body site from the test stimulus. In healthy humans, the conditioning stimulus should inhibit pain evoked by the test stimuli36. In the present study, the test stimuli were electrical stimulations delivered to the left ankle at a random interstimulus interval between 8-12-s. The CS was a circulating cold-water bath (Thermo Fisher Scientific, Pittsburgh, PA) maintained at a temperature of 10±0.1°C.
The CPM task consisted of three 2-min phases: baseline (test stimuli delivered prior to cold water), conditioning (test stimuli delivered while hand/arm is submerged in cold water, and post-test (test stimuli delivered after conditioning). A 2-min rest occurred between baseline and conditioning and a 5-min rest occurred between conditioning and post-test. During conditioning, participants were instructed to submerge their right hand up to their forearm in the water and to keep their hand palm down with fingers spread. During each 2-min phase, 5 electric test stimuli were delivered following a 20-s wait period. Participants provided pain ratings in response to the electric stimulations verbally and an experimenter, in an adjacent room, recorded the ratings. Following the conditioning phase, participants completed the VAS intensity scale for the pain elicited by the cold water. Pain from the cold water was also used to assess group differences in general pain sensitivity.
Pain ratings.
During each CPM phase, participants verbally provided pain ratings using a numerical rating scale (NRS). The NRS ranged from 0 “no pain” to 100 “worst possible pain.” Anchors in between 0 and 100 were 20 “mild pain”, 40 “moderate pain”, 60 “severe pain”, and 80 “very severe pain”.
Nociceptive flexion reflex (NFR) magnitudes.
NFR magnitudes were used to assess within-subjects changes in spinal nociception60. NFR magnitudes were calculated as a change from baseline in μV [NFR change = mean rectified EMG of 90 to 150-ms post-stimulation interval minus mean of rectified EMG from −60 to 0-ms-prestimulation interval]. Stimulation trials with NFR baselines higher than 3.0 μV were excluded from analyses due to excessive muscle tension and/or noise in the recording (5.6% of trials were excluded).
Testing Procedures
Figure 2 presents the tasks on the CPM testing day. The other testing day consisted of tasks assessing temporal summation of heat, pain thresholds/tolerances for electric, ischemic, cold, heat, and pressure stimuli. Testing day order was counterbalanced across participants but stratified by race and sex. Prior to pain tasks on the first testing day, participants completed a demographics questionnaire, the LEC, PCS, and STAI. Within the 10- and 20-minute breaks (Fig 2), participants completed several questionnaires presented in a randomized order including the SCL-90-R and the PSS.
Fig 2.
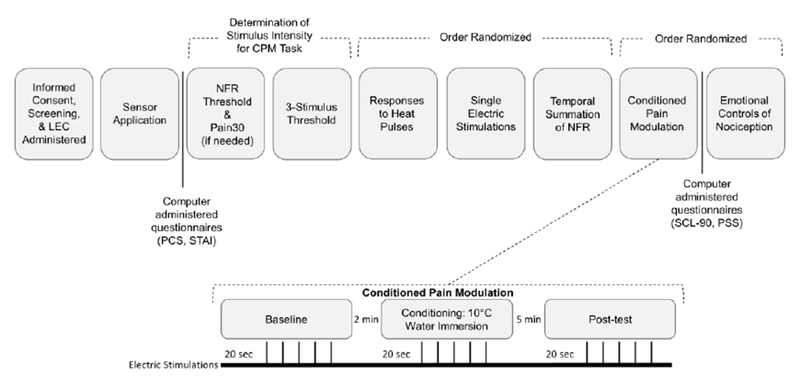
Testing day timeline and experimental procedures for the Conditioned Pain Modulation (CPM) testing day. The CPM task is expanded to show the 3 conditions and the administration of testing stimuli. 5 electrical stimulations were delivered during each phase. The interstimulus interval between each electric stimulation ranged from 8-12 seconds.
Statistical Analyses
Group differences in background variables were assessed via 1-way analysis of variance (ANOVA) for continuous variables (such as age) and chi-squared analyses for nominal variables (such as race), using group membership (no-TE, no-SA, SA) as the independent variable (IV).
Data were tested for normality and within-cell outliers were identified using Wilcox’s MAD-median approach77 and winsorized (if necessary). Primary outcomes (pain ratings, NFR magnitude) were analyzed via multilevel models (MLM) (MIXED procedure, SPSS. 20.0, IBM, Armonk, NY). Analyses of electric pain and NFR had 15 rows of data per participant, corresponding to the 5 electric stimuli delivered during each of the 3 phases (baseline, conditioning, and post-test). Level 1 units were responses to electric stimulations (pain/NFR). Level 2 units were participants. All models included a random intercept to model Level 2 variance in the dependent variable (DV). The MIXED procedure used in SPSS implements Satterthwaite estimation procedures which produce non-integer denominator degrees of freedom (which were rounded for ease of reporting). These degrees of freedom vary between analyses. Primary IVs within the MLMs were Group (no-TE, no-SA, SA) and CPM Phase (baseline, conditioning, post-test). Additionally, a continuous variable that coded for the sequence of the 5 stimulations delivered during each CPM phase was entered to model habituation/sensitization effects (i.e., Stimulus Number). DVs within the MLMs were electric pain ratings and NFR change. Fisher’s LSD was used to test significant F-tests during follow-up tests. Significance was set at α=.05 (2-tailed). In the event that significant group differences were found for background/psychological variables that might influence pain/NFR, these were entered into MLMs as covariates to control for these variables.
Results
Background Variables and Missing Data
Descriptive and inferential statistics for group differences in background characteristics are reported in Table 1. Due to an equipment issue, SCL-90 and PSS data for one participant from the SA group were lost.
SA and no-SA groups were successfully matched on sex, race, age, and mean number of non-SA traumas. However, the no-TE group was more likely to be male and NHW. The 3 groups did not differ on age, employment status, BMI, pain catastrophizing, or state anxiety. However, the SA group reported more psychological distress on the SCL-90 (ps <.03) and more stress on the PSS (ps < .04).
One person in the SA group had excessive muscle tension in the EMG during NFR recording (i.e., baseline EMG >3μV); thus, this person was excluded from NFR analysis.
General Pain Sensitivity
Groups did not differ on NFR threshold or 3-stimulus threshold. This suggests trauma exposure and SA history does not affect general (tonic) sensitivity of spinal neurons. This also explains why suprathreshold stimulus intensity used during CPM testing did not significantly differ between groups (Table 1).
Group differences in cold water pain (conditioning stimulus) were marginally significant (p=.055). Given this, exploratory post-hoc analyses were performed that found the SA group reported more pain in response to the cold water than the no-TE group (p = .018), but other comparisons were non-significant (ps > .14).
CPM of Pain and NFR
Given the group differences noted, global distress (GSI of SCL-90), perceived stress (PSS), sex, cold water pain, and minority status were entered as covariates in the MLM models. Also, stimulus intensity was entered as a covariate into the model predicting pain ratings to account for the individually calibrated intensities used during the CPM procedure. To control for sex, men were contrast coded as −1 and women were coded as 1. To control for race, NHWs were coded −1 and all others were coded 1. Continuous control variables were grand mean centered.
Results of multilevel models predicting pain ratings during CPM are reported in Table 2, whereas means and SEMs for pain ratings by group and CPM phase are reported in Table 3. A significant main effect of CPM phase for pain ratings indicated that pain in response to the electric stimuli was significantly reduced during the cold water (conditioning) relative to baseline and post phases (ps<.001; Fig 3). But, this effect did not differ by group as indicated by the nonsignificant Group x Phase interaction. The significant effect of Stimulus Number was associated with a positive regression slope (B=0.665) suggesting that pain sensitized across the 5 stimulations within each CPM phase. Further, cold water pain was significantly related to electric pain ratings (B=.317), as was the suprathreshold stimulus intensity (B=.424). Thus, higher values on both variables were associated with greater electric pain.
Table 2.
Results of multilevel models for conditioned modulation of pain.
| Predictors of Pain during CPM | dfnum | dfdenom | F | p |
|---|---|---|---|---|
| Intercept | 1 | 104.951 | 34.44 | <.001 |
| Race | 1 | 103.012 | 0.649 | 0.422 |
| Sex | 1 | 102.997 | 1.553 | 0.216 |
| Global Psych Distress | 1 | 102.999 | 2.76 | 0.100 |
| Perceived Stress | 1 | 102.993 | 2.125 | 0.148 |
| Cold Water Pain | 1 | 102.998 | 25.486 | <.001 |
| Stimulus Intensity | 1 | 103.001 | 9.543 | 0.003 |
| Stimulus Number | 1 | 1349.204 | 27.398 | <.001 |
| Group | 2 | 103.035 | 0.104 | 0.902 |
| CPM Phase | 2 | 457.585 | 72.525 | <.001 |
| Group × Phase | 4 | 612.615 | 0.995 | 0.409 |
Note. CPM = conditioned pain modulation.
Table 3.
Means and SEMs for pain ratings during the 3 phases of conditioned pain modulation (CPM)
| CPM Phase | ||||||
|---|---|---|---|---|---|---|
| Baseline |
Conditioning |
Post |
||||
| Group | M | SEM | M | SEM | M | SEM |
| no-TE | 39.853 | 2.910 | 34.092 | 2.900 | 39.340 | 2.910 |
| no-SA | 39.344 | 2.941 | 32.274 | 2.927 | 38.181 | 2.939 |
| SA | 36.885 | 3.099 | 31.429 | 3.088 | 38.775 | 3.098 |
Note. no-TE = participants with no trauma exposure. no-SA = trauma exposed participants without a history of sexual assault. SA = participants with a history of sexual assault. SEM = standard error of the mean.
Fig. 3.
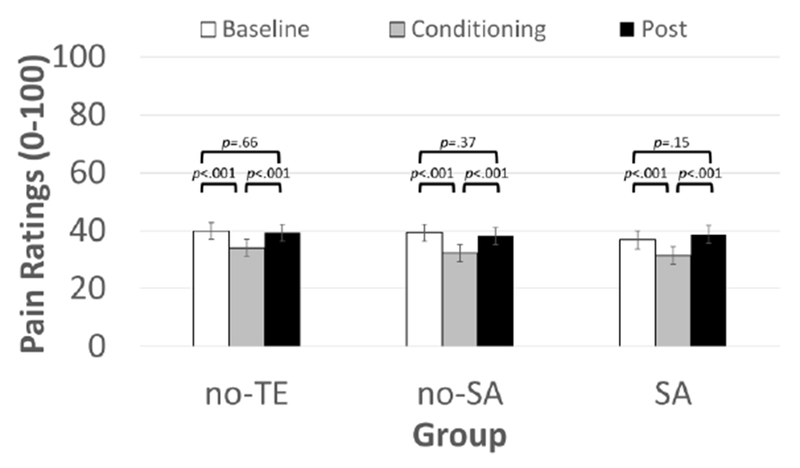
Conditoned modulation of pain intensity by CPM phase in sexual assault (SA) survivors, a matched control group of non-SA trauma-exposed persons (no-SA), and an unmatched control group of persons without trauma exposure (no-TE). All groups showed intact pain modulation, in which their pain intensity ratings were the lower during the conditoning phase relative to the baseline and post-test phases. Means±SEM.
Results of multilevel models predicting NFRs during CPM are reported in Table 4, whereas means and SEMs for NFRs by group and CPM phase are reported in Table 5. In contrast to pain, CPM of NFR did differ by group as indicated by the significant Group x CPM Phase interaction (Fig 4). The no-TE group demonstrated a significant reduction of NFR magnitudes during the post phase, relative to the baseline and conditioning phases (ps< .03). This suggests inhibition of NFR following the cold water. The no-SA group showed no differences in NFR across the 3 phases (ps > .20), suggesting a lack of CPM inhibition. The SA group demonstrated a significant increase in NFR magnitudes during the conditioning phase, relative to the baseline and post phases (ps < .001), suggesting facilitation of NFR. The significant effect of Stimulus Number was associated with a negative regression slope (B= −0.196) suggesting that NFRs habituated across the 5 stimulations within each CPM phase. Further, cold water pain was significantly related to NFRs (B= −.020); thus, greater cold water pain was associated with smaller NFRs.
Table 4.
Results of multilevel models for conditioned modulation of the nociceptive flexion reflex (NFR).
| Predictors of NFR during CPM | dfnum | dfdenom | F | p |
|---|---|---|---|---|
| Intercept | 1 | 143.692 | 269.887 | <.001 |
| Race | 1 | 101.768 | 0.177 | 0.675 |
| Sex | 1 | 100.886 | 0.32 | 0.573 |
| Global Psych Distress | 1 | 100.908 | 1.435 | 0.234 |
| Perceived Stress | 1 | 100.971 | 1.093 | 0.298 |
| Cold Water Pain | 1 | 101.481 | 12.432 | 0.001 |
| Stimulus Number | 1 | 1319.956 | 63.717 | <.001 |
| Group | 2 | 101.014 | 0.452 | 0.638 |
| CPM Phase | 2 | 473.075 | 10.015 | <.001 |
| Group × Phase | 4 | 489.621 | 4.651 | 0.001 |
Note. CPM = conditioned pain modulation.
Table 5.
Means and SEMs for nociceptive flexion reflex (NFR) magnitudes during the 3 phases of conditioned pain modulation (CPM)
| CPM Phase | ||||||
|---|---|---|---|---|---|---|
| Baseline |
Conditioning |
Post |
||||
| Group | M | SEM | M | SEM | M | SEM |
| no-TE | 2.267 | 0.289 | 2.444 | 0.290 | 1.896 | 0.290 |
| no-SA | 2.439 | 0.297 | 2.205 | 0.300 | 2.207 | 0.299 |
| SA | 2.336 | 0.316 | 3.118 | 0.317 | 2.301 | 0.316 |
Note. no-TE = participants with no trauma exposure. no-SA = trauma exposed participants without a history of sexual assault. SA = participants with a history of sexual assault. SEM = standard error of the mean.
Fig. 4.
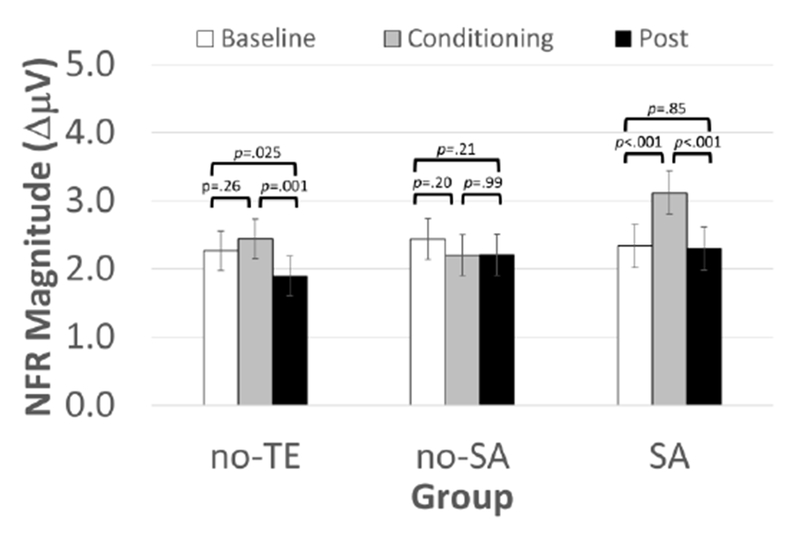
Conditioned modulation of the nociceptive flexion reflex (NFR) magnitude by CPM phase in sexual assault (SA) survivors, a matched control group of non-SA trauma-exposed persons (no-SA), and an unmatched control group of persons without trauma exposure (no-TE). The no-TE group demonstrated significant reduction in NFR during the post-test phase, relative to the other phases. However, the other two groups failed to show significant inhibition. In fact, the SA group showed significant NFR facilitation during the conditioning phase, relative to the other phases. Means±SEM.
Discussion
Contrary to our hypothesis, there was weak evidence for hyperalgesia in the SA group. Electric pain during CPM, cold water pain, NFR threshold, and 3-stimulus threshold were not significantly different across groups. Only exploratory mean comparisons found that the SA group reported greater cold water pain than the no-TE group. Moreover, all groups displayed intact CPM-related pain inhibition.
By contrast, CPM-related NFR inhibition was disrupted by trauma exposure. The no-SA group failed to show significant NFR inhibition and NFR was facilitated in the SA group. Only the no-TE group demonstrated significant NFR inhibition, but this was only observed during posttest (after offset of the CS). It is not clear why inhibition of the NFR was delayed relative to the inhibition of pain; however, it is noteworthy that other studies have reported difficulties observing NFR inhibition during the CS35,47, and delayed CPM inhibition of spinal reflexes have been noted elsewhere79. One possible explanation could be our use of 10°C water as the CS. When Goffaux et al26 used 6.5°C water, they found a large NFR inhibition during the conditioning phase. This is consistent with studies finding the magnitude of CPM-related inhibition is correlated with CS intensity31. Despite this, our CS temperature cannot explain why SA participants showed NFR facilitation. Together, findings suggest trauma exposure disrupts descending inhibition of spinal nociception, and that SA exposure may tip the balance towards facilitation. There are several potential implications of these findings.
First, Yarnitsky82 argues that a person’s pain modulation profile, which ranges along a continuum between anti-nociceptive to pro-nociceptive, determines the likelihood of chronic pain development. Following this logic, the SA group should be at a higher risk for pain than both the no-SA and no-TE groups because their cerebrospinal modulatory system is shifted to facilitation (i.e., extreme pro-nociception). Ostensibly, the no-SA group is still at a higher risk than the noTE group due to the inability to engage cerebrospinal inhibition.
Consistent with this argument, a recent study from our laboratory assessed CPM of pain and NFR in 286 healthy, pain-free men and women34. After testing, participants were assessed every 6-months to determine if they developed chronic pain (defined as persistent pain >3mos). Of the 208 responders to follow-up assessments, 16% developed chronic pain. Deficiencies in CPM of NFR, but not CPM of pain, predicted chronic pain development, even after controlling for age, sex, general health at testing, depression/anxiety, and somatization. Together, this suggests that a disruption of NFR inhibition may place SA survivors at risk for chronic pain. However, this conclusion should be taken with caution until a longitudinal study of SA and chronic pain can be conducted.
Second, our findings suggest a role of the CPM-related cerebrospinal circuit in trauma-related pain risk. To our knowledge, only one study has examined the differential circuits involved with CPM of pain vs. CPM of NFR. Piche et al47 found that CPM of pain involved the OFC, PCC, ACC, sgCC, anterior insula, amygdala, PHG, and mPFC, whereas CPM of NFR involved the SI, paracentral lobule, SMA, pre-SMA, ACC, PCC, PHG, PFC, thalamus, and connections with the brainstem descending modulation circuit (pons, PAG, RVM). Given their findings, our data suggests deficits in SI, SMA, pre-SMA, thalamus, and/or the brainstem modulatory circuit are responsible for chronic pain risk following trauma (Fig 1).
Third, SA and no-SA groups both experienced a disruption of NFR modulation, but only the SA group displayed facilitation. Currently it is not clear whether this reflects a qualitative or quantitative difference. We made efforts to match the SA and no-SA groups on important variables, including number of non-SA traumas. However, this means the SA group experienced more traumas overall. It is possible then that trauma exposure exerts a cumulative (quantitative) effect on spinal nociception, pushing it towards pro-nociception/facilitation. Consistent with this, a recent study from our lab found that trauma exposure has a dose-response relationship with temporal summation of the NFR (a marker of spinal cord hyperexcitability)66, such that persons with more trauma exposure demonstrated greater summation (i.e., spinal hyperexcitability).
By contrast, differences seen between SA and no-SA groups could reflect qualitative differences brought on uniquely by SA. For example, studies have noted that SA confers a risk for negative sequelae above and beyond other types of trauma (i.e., mugging, physical assault, etc.)46. At this time the mechanisms contributing to the unique risk from SA are unknown. However, the mechanisms may include: 1) epigenetic changes to the serotonin transporter promoter region9,63 which could affect serotonergic neurons of the brainstem modulation circuit, 2) structural changes in brain regions responsible for pain processing/modulation2,42, and/or 3) changes in the HPA axis and/or stress responsivity75,83. Additional research is needed to resolve this issue.
Fourth, our findings provide additional evidence that NFR modulation dysfunction may be a unique response to trauma exposure. Our lab developed a method to study emotional modulation of pain and NFR and have repeatedly shown that, in healthy participants, positive emotions inhibit pain/NFR and negative emotions enhance pain/NFR51–53,69. Recently we found that SA was associated with an inability to inhibit NFR during positive emotions, even though pain perception was inhibited 33. This is similar to the current findings indicating that CPM inhibited pain, but not NFR, in SA and no-SA. Interestingly, we have found that persons with major depressive disorder (MDD)69, sleep disturbance15, and fibromyalgia53 show a disruption of emotional modulation of pain, but not a disruption of emotional modulation of NFR. Given that MDD, sleep disturbance, fibromyalgia and trauma exposure are all risk factors for chronic pain1,4,12,22, then together findings suggest a disruption of NFR modulation (but not pain modulation) could be a unique pain risk factor for trauma survivors.
Finally, these findings may have treatment implications if SA and no-SA groups ultimately develop pain. As Yarnitsky notes82, a reduced capacity to engage CPM inhibition implies a problem with serotonin and/or norepinephrine neurons involved with the brainstem descending modulation circuit. Thus, drugs that block the reuptake of serotonin and/or norepinephrine may rectify the dysfunctional NFR modulation. Alternatively, recent studies have shown that brief, cognitive and behavioral interventions can be used to train individuals to inhibit spinal neurons8,18,19,37. These interventions alone or in combination could be helpful in stopping the transition to chronic pain or treating the pain once established in these individuals.
Strengths and Limitations
The present study assessed NFR so that inferences could be made about descending modulation of spinal nociception in SA survivors and trauma-exposed persons. Further, matching was used to control for possible confounding between the SA and no-SA groups, and statistical controls were used to account for potential confounds between traumatized groups and the no-TE group. Additionally, multilevel models were used to improve statistical power. Finally, our sample consisted of ethnically-diverse male and female participants, providing a better representation of SA and trauma survivors.
That said, our study is not without limitations. We assessed healthy, pain-free individuals to establish an association between SA and pain dysregulation prior to disease onset. Thus, it is unclear whether these findings will generalize to those with chronic pain. Further, due to our limited sample size, we are unable to examine within-group differences that may be of interest (e.g., sex/racial differences). Also, because of base rates in trauma exposure, we were unable to match the no-TE group to the other groups. In particular, the no-TE group was less likely to be female than the SA and no-SA group. Given evidence that females are more sensitive to experimental pain than men5,21,76, this could have affected our results. However, no sex differences have been found for CPM-related NFR inhibition23,24,48,61, despite evidence for sex differences in CPM-related pain inhibition48. Thus, our group differences in NFR modulation are not likely to have been confounded by sex distributions.
Additionally, we are not able to evaluate if SA characteristics impacted our findings, because the severity, frequency, and length of time passed since the SA are not measured by the LEC. We also did not assess for PTSD or other psychiatric diagnoses, so we are unable to determine if clinically significant distress impacted our results. Also, our procedure for matching SA and no-SA participants by age, sex, and race on a case-by-case method may have created dependencies between the samples that we were not able to account for within statistical models. Finally, participants were provided an overview of the study prior to their enrollment, thus it is possible that self-selection bias may limit the generalizability of our findings.
Highlight Points:
Trauma exposure disrupts cerebrospinal inhibition of spinal nociception
Sexual assault is related to facilitation of spinal nociception
This disruption may place sexual assault survivors at risk for chronic pain
Summary.
Findings suggest that SA survivors and persons exposed to non-SA traumas display intact CPM-related pain inhibition. However, NFR was facilitated in SA survivors by CPM, whereas no NFR modulation was observed in non-SA trauma-exposed participants. This implies that trauma exposure impairs cerebrospinal inhibitory circuits, whereas SA exposure shifts descending modulation towards facilitation. This may represent one pathway to chronic pain for trauma exposed persons, including those with SA histories.
Acknowledgments
Disclosure of Conflicts of Interest and Source of Funding: Research reported was supported by the National Institute on Minority Health and Health Disparities of the National Institute of Health under Award Number R01MD007807. Edward Lannon, Shreela Palit, and Yvette Güereca were supported by a National Science Foundation Graduate Research Fellowship Program. The content is solely the responsibility of the authors and does not necessarily reflect the views of the National Institutes of Health, National Science Foundation, Indian Health Service, or the Cherokee Nation. The authors report no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Afari N, Ahumada SM, Wright LJ, Mostoufi S, Golnari G, Reis V, Cuneo JG: Psychological trauma and functional somatic syndromes: a systematic review and metaanalysis. Psychosom Med 76:2, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH: Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. The Journal of neuropsychiatry and clinical neurosciences 20:292–301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K: Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain 9:463–484, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bair MJ, Robinson RL, Katon W, Kroenke K: Depression and pain comorbidity: a literature review. Arch Intern Med 163:2433–2445, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bartley EJ, Fillingim RB: Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 111:52–58, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartley EJ, Rhudy JL: Endogenous inhibition of the nociceptive flexion reflex (NFR) and pain ratings during the menstrual cycle in healthy women. Annals of Behavioral Medicine 43:343–351, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Bartley EJ, Rhudy JL: Endogenous inhibition of the nociceptive flexion reflex (NFR) and pain ratings during the menstrual cycle in healthy women. Annals Of Behavioral Medicine: A Publication Of The Society Of Behavioral Medicine 43:343–351,2012 [DOI] [PubMed] [Google Scholar]
- 8.Bäumler M, Feller M, Krafft S, Schiffer M, Sommer J, Straube A, Weinges F, Ruscheweyh R: Learned control over spinal nociception: Transfer and stability of training success in a long-term study. Clin Neurophysiol 128:2462–2469, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Beach SR, Brody GH, Todorov AA, Gunter TD, Philibert RA: Methylation at 5HTT mediates the impact of child sex abuse on women’s antisocial behavior: an examination of the Iowa adoptee sample. Psychosom Med 73:83, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bebbington P, Nayani T: The psychosis screening questionnaire. International Journal of Methods in Psychatric Research 5:11–19, 1995 [Google Scholar]
- 11.Biurrun Manresa JA, Fritsche R, Vuilleumier PH, Oehler C, Mørch CD, Arendt-Nielsen L, Andersen OK, Curatolo M: Is the Conditioned Pain Modulation Paradigm Reliable? A Test-Retest Assessment Using the Nociceptive Withdrawal Reflex. PLOS ONE 9:e100241,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clauw DJ: Fibromyalgia: a clinical review. JAMA 311:1547–1555, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Cohen S, Kamarck T, Mermelstein R: Perceived stress scale. Measuring stress: A guide for health and social scientists 1994 [Google Scholar]
- 14.De Broucker T, Cesaro P, Willer JC, Le Bars D: Diffuse noxious inhibitory controls in man. Involvement of the spinoreticular tract. Brain: A Journal Of Neurology 113 ( Pt 4):1223, 1990 [DOI] [PubMed] [Google Scholar]
- 15.DelVentura JL, Terry EL, Bartley EJ, Rhudy JL: Emotional modulation of pain and spinal nociception in persons with severe insomnia symptoms. Ann Behav Med 47:303–315, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Department of Justice: National Crime Victimization Survey 2010-2014. Office of Justice Programs, Bureau of Justice Statistics; 2015 [Google Scholar]
- 17.Derogatis LR: SCL-90-R : symptom checklist-90-R : administration, scoring & procedures manual. Vol. [Minneapolis, Minn.]: [National Computer Systems, Inc.]; 1994. [Google Scholar]
- 18.Emery CF, France CR, Harris J, Norman G, Vanarsdalen C: Effects of progressive muscle relaxation training on nociceptive flexion reflex threshold in healthy young adults: a randomized trial. Pain 138:375–379, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Emery CF, Keefe FJ, France CR, Affleck G, Waters S, Fondow MD, McKee DC, France JL, Hackshaw KV, Caldwell DS, Stainbrook D: Effects of a brief coping skills training intervention on nociceptive flexion reflex threshold in patients having osteoarthritic knee pain: a preliminary laboratory study of sex differences. J Pain Symptom Manage 31:262–269, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Ferreira VM, Sherman AM: The relationship of optimism, pain and social support to well-being in older adults with osteoarthritis. Aging & Mental Health 11:89–98, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL, III: Sex, gender, and pain: A review of recent clinical and experimental findings. The Journal of Pain 10:447–485, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finan PH, Goodin BR, Smith MT: The association of sleep and pain: an update and a path forward. The Journal of Pain 14:1539–1552, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.France CR, Suchowiecki S: A comparison of diffuse noxious inhibitory controls in men and women. Pain 81:77–84, 1999 [DOI] [PubMed] [Google Scholar]
- 24.France CR, Suchowiecki S: Assessing supraspinal modulation of pain perception in individuals at risk for hypertension. Psychophysiol 38:107–113, 2001 [PubMed] [Google Scholar]
- 25.Gebhart GF: Descending modulation of pain. Neurosci Biobehav Rev 27:729–737, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Goffaux P, Redmond WJ, Rainville P, Marchand S: Descending analgesia--when the spine echoes what the brain expects. Pain 130:137, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Golding JM: Sexual-assault history and long-term physical health problems: Evidence from clinical and population epidemiology. Current Directions in Psychological Science 8:191–194, 1999 [Google Scholar]
- 28.Goldstein H: Multilevel statistical models. Vol 922John Wiley & Sons; 2011. [Google Scholar]
- 29.Goodin BR, McGuire L, Allshouse M, Stapleton L, Haythornthwaite JA, Burns N, Mayes LA, Edwards RR: Associations between catastrophizing and endogenous pain-inhibitory processes: sex differences. The Journal of Pain 10:180–190, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Granot M, Somer E, Zisman-Ilani Y, Beny A, Sadger R, Mirkin R, Moont R, Yovell Y: Characteristics of response to experimental pain in sexually abused women. The Clinical Journal of Pain 27:616–622, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Granot M, Weissman-Fogel I, Crispel Y, Pud D, Granovsky Y, Sprecher E, Yarnitsky D: Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: Do conditioning stimulus painfulness, gender and personality variables matter? Pain 136:142–149, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Gray MJ, Litz BT, Hsu JL, Lombardo TW: Psychometric Properties of the Life Events Checklist. Assessment 11:330–341, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Hellman N, Kuhn BL, Lannon EW, Payne MF, Sturycz CA, Palit S, Shadlow JO, Rhudy JL: Emotional Modulation of Pain and Spinal Nociception in Sexual Assault Survivors. Psychosom Med 80:861–868, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber FA, Kuhn BL, Lannon EW, Sturycz CA, Payne MF, Hellman N, Toledo TA, Guereca YM, DeMuth M, Palit S, Shadlow JO, Rhudy JL: Less Efficient Endogenous Inhibition of Spinal Nociception Predicts Chronic Pain Onset: A Prospective Analysis from the Oklahoma Study of Native American Pain Risk (OK-SNAP) Paper presented at: American Pain Society, 2019; Milwaukee, WC [Google Scholar]
- 35.Jurth C, Rehberg B, von Dincklage F: Reliability of subjective pain ratings and nociceptive flexion reflex responses as measures of conditioned pain modulation. Pain Research and Management 19:93–96, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy DL, Kemp HI, Ridout D, Yarnitsky D, Rice ASC: Reliability of conditioned pain modulation: A systematic review. PAIN 157:2410–2419, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krafft S, Göhmann HD, Sommer J, Straube A, Ruscheweyh R: Learned control over spinal nociception in patients with chronic back pain. European Journal of Pain 21:1538–1549, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Lewis GN, Rice DA, McNair PJ: Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. The Journal of Pain 13:936–944, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Linton SJ, Shaw WS: Impact of psychological factors in the experience of pain. Physical Therapy 91:700–711, 2011 [DOI] [PubMed] [Google Scholar]
- 40.McLean SA, Soward AC, Ballina LE, Rossi C, Rotolo S, Wheeler R, Foley KA, Batts J, Casto T, Collette R, Holbrook D, Goodman E, Rauch S, Liberzon I: Acute Severe Pain Is a Common Consequence of Sexual Assault. The Journal of Pain 13:736–741,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moont R, Crispel Y, Lev R, Pud D, Yarnitsky D: Temporal changes in cortical activation during conditioned pain modulation (CPM), a LORETA study. PAIN® 152:1469–1477, 2011 [DOI] [PubMed] [Google Scholar]
- 42.New AS, Fan J, Murrough JW, Liu X, Liebman RE, Guise KG, Tang CY, Charney DS: A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry 66:656–664, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Noel M, Wilson AC, Holley AL, Durkin L, Patton M, Palermo TM: Posttraumatic stress disorder symptoms in youth with vs without chronic pain. PAIN 157:2277–2284, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olff M: Sex and gender differences in post-traumatic stress disorder: an update. European Journal of Psychotraumatology 8:1351204, 2017 [Google Scholar]
- 45.Palit S, Bartley EJ, Kuhn BL, Kerr KL, DelVentura JL, Terry EL, Rhudy JL: endogenous inhibition of pain and spinal nociception in women with premenstrual dysphoric disorder. Journal of pain research 9:57, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perrin M, Vandeleur CL, Castelao E, Rothen S, Glaus J, Vollenweider P, Preisig M: Determinants of the development of post-traumatic stress disorder, in the general population. Social psychiatry and psychiatric epidemiology 49:447–457, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Piché M, Arsenault M, Rainville P: Cerebral and cerebrospinal processes underlying counterirritation analgesia. J Neurosci 29:14236–14246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Popescu A, LeResche L, Truelove EL, Drangsholt MT: Gender differences in pain modulation by diffuse noxious inhibitory controls: a systematic review. Pain 150:309–318, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Pyne JM, Constans JI, Wiederhold MD, Gibson DP, Kimbrell T, Kramer TL, Pitcock JA, Han X, Williams DK, Chartrand D, Gevirtz RN, Spira J, Wiederhold BK, McCraty R, McCune TR: Heart rate variability: Pre-deployment predictor of post-deployment PTSD symptoms. Biological Psychology 121:91–98, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren K, Dubner R: Central nervous system plasticity and persistent pain. J Orofac Pain 13:155–163, 1999 [PubMed] [Google Scholar]
- 51.Rhudy JL, Bartley EJ, Palit S, Kuhn BL, Kerr KL, Martin SL, DelVentura JL, Terry EL: Affective disturbance associated with premenstrual dysphoric disorder (PMDD) does not disrupt emotional modulation of pain and spinal nociception. Pain 155:2144–2152, 2014 [DOI] [PubMed] [Google Scholar]
- 52.Rhudy JL, Bartley EJ, Williams AE, McCabe KM, Chandler MC, Russell JL, Kerr KL: Are there sex differences in affective modulation of spinal nociception and pain? The Journal of Pain 11:1429–1441, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Rhudy JL, DelVentura JL, Terry EL, Bartley EJ, Olech E, Palit S, Kerr KL: Emotional modulation of pain and spinal nociception in fibromyalgia. Pain 154:1045–1056, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rhudy JL, France CR: Defining the nociceptive flexion reflex (NFR) threshold in human participants: a comparison of different scoring criteria. PAIN 128:244–253, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rhudy JL, Williams AE, McCabe KM, Nguye~n MV, Rambo P: Affective modulation of nociception at spinal and supraspinal levels. Psychophysiol 42:579–587, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Rhudy JL, Williams AE, McCabe KM, Rambo PL, Russell JL: Emotional modulation of spinal nociception and pain: The impact of predictable noxious stimulation. Pain 126:221–233, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Rhudy JL, Williams AE, McCabe KM, Russell JL, Maynard LJ: Emotional control of nociceptive reactions (ECON): do affective valence and arousal play a role? PAIN 136:250–261, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Roby-Brami A, Bussel B, Willer J, LeBars D: An electrophysiological investigation into the pain-relieving effects of heterotopic nociceptive stimuli: probable involvement of a supraspinal loop. Brain 110:1497–1508, 1987 [DOI] [PubMed] [Google Scholar]
- 59.Roy M, Piche M, Chen J- I, Peretz I, Rainville P: Cerebral and spinal modulation of pain by emotions. PNAS Proceedings of the National Academy of Sciences of the United States of America 106:20900–20905, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Wilier JC: The lower limb flexion reflex in humans. Progress In Neurobiology 77:353–395, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Serrao M, Rossi P, Sandrini G, Parisi L, Amabile GA, Nappi G, Pierelli F: Effects of diffuse noxious inhibitory controls on temporal summation of the Rill reflex in humans. Pain 112:353–360, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Sherman AL, Morris MC, Bruehl S, Westbrook TD, Walker LS: Heightened Temporal Summation of Pain in Patients with Functional Gastrointestinal Disorders and History of Trauma. Ann Behav Med 49:785–792, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, Tang Y, Gillespie CF, Cubells JF, Ressler KJ: Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 156:700–708, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spielberger CD, Gorsuch RL, Lushene RE: Manual for the state-trait anxiety inventory. 1970 [Google Scholar]
- 65.Sprenger C, Bingel U, Buchel C: Treating pain with pain: supraspinal mechanisms of endogenous analgesia elicited by heterotopic noxious conditioning stimulation. PAIN® 152:428–439, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Sturycz C, Hellman N, Kuhn B, Lannon E, Palit S, Güereca Y, Payne M, Shadlow J, Rhudy J: (447) Does trauma exposure affect temporal summation of pain and the nociceptive flexion reflex? The Journal of Pain 18:S85–S86, 2017 [Google Scholar]
- 67.Sullivan MJL, Bishop SR, Pivik J: The pain catastrophizing scale: development and validation. Psychological Assessment 1995 [Google Scholar]
- 68.Sumner JA, Chen Q, Roberts AL, Winning A, Rimm EB, Gilsanz P, Glymour MM, Tworoger SS, Koenen KC, Kubzansky LD: Cross-Sectional and Longitudinal Associations of Chronic Posttraumatic Stress Disorder With Inflammatory and Endothelial Function Markers in Women. Biological Psychiatry 82:875–884, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Terry EL, DelVentura JL, Bartley EJ, Vincent A, Rhudy JL: Emotional modulation of pain and spinal nociception in persons with major depressive disorder (MDD). Pain 154:2759–2768, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Terry EL, France CR, Bartley EJ, DelVentura JL, Kerr KL, Vincent AL, Rhudy JL: Standardizing procedures to study sensitization of human spinal nociceptive processes: Comparing parameters for temporal summation of the nociceptive flexion reflex (TS-NFR). Int J Psychophysiol 81:263–274, 2011 [DOI] [PubMed] [Google Scholar]
- 71.Terry EL, France CR, Bartley EJ, DelVentura JL, Kerr KL, Vincent AL, Rhudy JL: Standardizing procedures to study sensitization of human spinal nociceptive processes: comparing parameters for temporal summation of the nociceptive flexion reflex (TS-NFR). International Journal of Psychophysiology 81:263–274, 2011 [DOI] [PubMed] [Google Scholar]
- 72.The United States Department of Justice: Sexual assault. The Office on Vioolence Against Women 2016
- 73.Ulirsch J, Ballina L, Soward A, Rossi C, Hauda W, Holbrook D, Wheeler R, Foley KA, Batts J, Collette R: Pain and somatic symptoms are sequelae of sexual assault: results of a prospective longitudinal study. European Journal of Pain 18:559–566, 2014 [DOI] [PubMed] [Google Scholar]
- 74.Walker E, Keegan D, Gardner G, Sullivan M, Bernstein D, Katon WJ: Psychosocial Factors in Fibromyalgia Compared With Rheumatoid Arthritis: II. Sexual, Physical, and Emotional Abuse and Neglect. Psychosom Med 59:572, 1997 [DOI] [PubMed] [Google Scholar]
- 75.Walsh K, Galea S, Koenen KC: Mechanisms underlying sexual violence exposure and psychosocial sequelae: A theoretical and empirical review. Clinical Psychology: Science and Practice 19:260–275, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wiesenfeld-Hallin Z: Sex differences in pain perception. Gender Med 2:137–145, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Wilcox RR, Keselman HJ: Modern Robust Data Analysis Methods: Measures of Central Tendency. Psychological Methods 8:254–274, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Wilier JC, Bussel B: Possible explanation for analgesia mediated by direct spinal effect of morphine. Lancet 1:158, 1980 [DOI] [PubMed] [Google Scholar]
- 79.Williams AE, Rhudy JL: Supraspinal modulation of trigeminal nociception and pain. Headache 49:704–720, 2009 [DOI] [PubMed] [Google Scholar]
- 80.Yarnitsky D: Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain 156:S24–S31, 2015 [DOI] [PubMed] [Google Scholar]
- 81.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best L- A, Granot M: Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain 138:22–28, 2008 [DOI] [PubMed] [Google Scholar]
- 82.Yarnitsky D, Granot M, Granovsky Y: Pain modulation profile and pain therapy: between pro-and antinociception. Pain 155:663–665, 2014 [DOI] [PubMed] [Google Scholar]
- 83.Yehuda R, Resnick HS, Schmeidler J, Yang R- K, Pitman RK: Predictors of cortisol and 3-methoxy-4-hydroxyphenylglycol responses in the acute aftermath of rape. Biol Psychiatry 43:855–859, 1998 [DOI] [PubMed] [Google Scholar]


