Abstract
Background
Viral infections contribute to morbidity in cystic fibrosis (CF), but the impact of respiratory viruses on the development of airway disease is poorly understood.
Methods
Infants with CF identified by newborn screening were enrolled prior to 4 months of age to participate in a prospective observational study at 4 centers. Clinical data were collected at clinic visits and weekly phone calls. Multiplex PCR assays were performed on nasopharyngeal swabs to detect respiratory viruses during routine visits and when symptomatic. Participants underwent bronchoscopy with bronchoalveolar lavage (BAL) and a subset underwent pulmonary function testing. We present findings through 8.5 months of life.
Results
Seventy infants were enrolled, mean age 3.1 ± 0.8 months. Rhinovirus was the most prevalent virus (66%), followed by parainfluenza (19%), and coronavirus (16%). Participants had a median of 1.5 viral positive swabs (range 0–10). Past viral infection was associated with elevated neutrophil concentrations and bacterial isolates in BAL fluid, including recovery of classic CF bacterial pathogens. When antibiotics were prescribed for respiratory-related indications, viruses were identified in 52% of those instances.
Conclusions
Early viral infections were associated with greater neutrophilic inflammation and bacterial pathogens. Early viral infections appear to contribute to initiation of lower airway inflammation in infants with CF. Antibiotics were commonly prescribed in the setting of a viral infection. Future investigations examining longitudinal relationships between viral infections, airway microbiome, and antibiotic use will allow us to elucidate the interplay between these factors in young children with CF.
Keywords: Cystic fibrosis, Viruses, Inflammation, Bacteria, Pathogens
Highlights
-
•
In this infant CF population, rhinovirus was the most commonly detected virus.
-
•
Respiratory viruses were associated with detection of classic CF pathogens on BAL.
-
•
Those with classic CF pathogens had elevation of inflammatory markers (IL-8, NE).
-
•
Antibiotics were commonly prescribed for respiratory indications in these infants.
-
•
Fifty-two percent of CF infants prescribed antibiotics for symptoms had a virus.
1. Introduction
Cystic fibrosis (CF) lung disease begins in the first months of life with lower airway inflammation, airflow limitation and structural abnormalities on chest CT [[1], [2], [3], [4]]. Historically, airway inflammation has been attributed to bacterial infection, though viral infections also play an important role in CF pulmonary disease [5,6]. Furthermore, evidence of structural airway disease including bronchiectasis has been reported in infants with CF, even in the absence of bacterial pathogens [4]. These findings suggest other factors, such as viral infections, may initiate the inflammation, chronic infection and progressive lung disease characteristic of CF lung disease.
Infection with respiratory viruses is linked to acute clinical deterioration in children with CF [5,7]. Compared to healthy counterparts, infants with CF acquire viral infections with a similar frequency, but are at greater risk for more prolonged, severe lower respiratory tract symptoms [5,8] and persistent lower airway obstruction [8]. Infants with CF hospitalized for viral infections have greater pulmonary inflammation, based on higher neutrophil, interleukin-8 (IL-8) and neutrophil elastase (NE) concentrations in bronchoalveolar lavage (BAL) fluid compared to controls [9]. Viral infection in school-aged children with CF leads to declining forced expiratory volume in one second (FEV1) values [10], and rhinovirus infection specifically is a risk factor for failure to recover FEV1 following pulmonary exacerbation [11]. Viral infections in children with CF have been associated with new acquisition of common bacterial pathogens including Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis [6], and severe viral infections requiring hospitalization have been linked to early infection with Pseudomonas aeruginosa [9]. Antiviral interventions, such as Palivizumab, reduce the rate and length of hospitalization due to RSV infection in CF infants [12]; however, have shown no difference in age at first acquisition of P. aeruginosa or S. aureus [13].
Healthy infants are typically treated conservatively with observation and supportive care until viral-induced symptoms resolve; however, management decisions for an infant with CF may be more complex. Understanding how respiratory viruses contribute to the early acquisition or evolution of bacterial flora in the CF airway could lead to modifications in immunization strategies and treatment decisions.
We hypothesized that viral infections alter the bacterial flora and inflammatory profile in the airway and contribute to the development of pulmonary disease in infants with CF. We describe the frequency of respiratory viral infections in infants with CF and explore potential sequelae, including their association with respiratory symptoms, airway inflammation, bacterial colonization, and pulmonary function measures. We also examine antibiotic prescribing patterns in the setting of viral illnesses.
2. Methods
Participants were enrolled at four sites, Riley Hospital for Children (Indianapolis, USA); St. Louis Children's Hospital (St. Louis, USA); Princess Margaret Hospital for Children (Perth, Australia); and Royal Children's Hospital (Melbourne, Australia). Study approval was obtained from the regulatory boards at each institution. Written informed consent was obtained from parents prior to enrollment.
All participants were identified through newborn screening and enrolled by 4 months of age. Inclusion criteria included a diagnosis of CF based on sweat chloride levels >60 mEq/L or two pathogenic cystic fibrosis transmembrane conductance regulator (CFTR) variants. Exclusion criteria included the inability to successfully or safely perform study visits.
Study visits were scheduled at 1 to 3-month intervals, coordinating with routine clinic appointments. A history and physical examination were performed at visits. Parents were contacted weekly via telephone to assess for new respiratory symptoms using a standardized questionnaire (Online supplement). Nasopharyngeal (NP) swabs were obtained at research clinic visits, and when participants were symptomatic, the parents collected a nasopharyngeal swab at home (FLOQSwabs™ and UTM™ Viral Transport Media, COPAN Diagnostics, Murrieta, CA). Parents were trained in collection techniques by research personnel in clinic (Online supplement). Viral analysis was performed at the Special Projects Laboratory at Washington University. Respiratory virus polymerase chain reaction (PCR) was performed using the GenMark eSensor Respiratory Viral Panel (GenMark Diagnostics Inc. Carlsbad, CA).
Infant pulmonary function testing (PFT) was performed in a subset of infants using the raised volume technique coupled with plethysmography [14,15]. Bronchoscopy with lavage was performed on a different day, <4 weeks apart as per protocol. If bronchoscopy was performed for pulmonary symptoms during that time frame, the research protocol for BAL fluid collection was followed. Inflammatory mediators, including tumor necrosis factor alpha (TNF)-α, IL-1β, and IL-8, were analyzed using commercially available sandwich enzyme immunoassays according to the manufacturer's recommended protocols (R&D Systems, Minneapolis, MN). Functional activity of NE was measured using a specific peptide chromogenic substrate (Elastin Products, Owensville, MO). The urea dilution method was used to determine cell counts and cytokine concentrations in the epithelial lining fluid (ELF) [16]. For our analyses, classical CF pathogens were defined as: Haemophilus influenzae, methicillin-resistant (MRSA) and -sensitive (MSSA) Staphylococcus aureus, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia. Clinical treatment decisions were left to the discretion of the treating physician. Study data were collected and managed using REDCap electronic data capture tools [17] hosted at the Indiana Clinical and Translational Sciences Institute.
2.1. Statistical analysis
Analyses were performed evaluating the infants enrolled in the prospective, longitudinal study following these children during the first year of life. For this report, we established a cut-off of 260 days of age to include available physiologic measures and a standard time period for tracking viral and symptom data. The data were analyzed cross-sectionally, using the longitudinal data. For analyses, participants were divided into two groups, “never virus” and “any virus” for each of the respective endpoints (infant PFT, bronchoscopy, or 260 days of age). Infant height and weight z-scores were generated using the WHO age adjusted algorithm [18].
Comparisons were performed using Student's t-tests for continuous variables, with log-transformations or Wilcoxon-Kruskal-Wallis tests when the data were skewed, and Chi-Square tests for categorical variables, with Fisher's Exact tests when cell counts were small. Generalized Linear Models (GLMs) were run when including covariates, to control for possible confounding effects, including age and site. Generalized Estimating Equations (GEEs) were performed with observation level data, to account for the intra-participant correlation present with repeated measures. GEEs allowed for the use of covariates, and models were run unadjusted and adjusted for age and site. Age and site were weakly associated, and so models were run with only one at a time and then together to ensure collinearity was not an issue. GEE models were also utilized as they allow non-normally distributed data to be used and perform for both continuous and binary endpoints. Analyses were done using SAS v9.4 (SAS Institute, Cary, NC) with p-values <.05 being considered statistically significant.
3. Results
3.1. Participant characteristics
Participants were screened from May 2013 to December 2016. At the time of this analysis, 109 participants were screened, 75 were enrolled and 70 included for analysis (20 from the US and 50 from Australia) (Fig. 1 online supplement). A total of 467 NP swabs were tested, median 6 NP swabs per participant (range 1–21). Total swabs tested did not differ by country (p = .12). Segregation of participants based on viral infection, “any virus” (n = 53) versus “never virus” (n = 17) through 260 days of age is outlined in Table 1 . Groups did not differ in age, race, height, weight, CFTR genotype or environmental exposures. However, at enrollment the any virus group had significantly more males (p = .02).
Table 1.
Baseline demographic and clinical characteristics of the viral infection cohorts.
| Entire cohort (n = 70) | Never virus (n = 17) | Any virus (n = 53) | p-Value | |
|---|---|---|---|---|
| Age at enrollment (months) | 3.0 (0.8) | 3.0 (0.5) | 3.0 (0.9) | .92 |
| Sex (Male) | 29 (41.4) | 3 (17.7) | 26 (49.1) | .02 |
| Race (Caucasian) | 68 (97.1) | 17 (100) | 51 (96.2) | .72 |
| Weight at enrollment (kg) | 5.52 (0.98) | 5.44 (0.74) | 5.54 (1.05) | .70 |
| Weight at enrollment (z-score) | −0.82 (1.18) | −0.75 (1.08) | −0.84 (1.22) | .78 |
| Height at enrollment (cm) | 59.18 (4.47) | 59.59 (2.29) | 59.04 (5.02) | .55 |
| Height at enrollment (z-score) | −0.63 (1.68) | −0.23 (0.94) | −0.76 (1.86) | .14 |
| CF genotype | ||||
| -ΔF508 heterozygous | 28 (40.0) | 5 (29.4) | 23 (43.4) | .46 |
| -ΔF508 homozygous | 40 (57.1) | 11 (64.7) | 29 (54.7) | |
| -Other | 2 (2.9) | 1 (5.9) | 1 (1.9) | |
| Smoke exposure (n = 67) | 14 (20.9) | 3 (17.7) | 11 (22.0) | .70 |
| Day Care (n = 67) | 1 (1.5) | 0 (0) | 1 (2.0) | .56 |
| Feeding (n = 62) | ||||
| Any breast feeding | 36 (58.1) | 11 (73.3) | 25 (53.2) | .17 |
Values are means (standard deviation) for continuous variables and frequency (percent) for categorical variables.
3.2. Viral detection and respiratory symptoms
Fifty-three (76%) participants had at least one positive swab (median 1.5, range 0–10). Viral detection was similar across study sites. Human rhinovirus (HRV) was the most frequently detected virus, found at least once in 66% of participants. Parainfluenza virus, coronavirus, RSV and human metapneumovirus (HMPV) were detected in 19%, 16%, 9% and 7% of participants, respectively (Table 2 ). Viral co-infection was detected in 11% of participants (n = 8). Viral positivity was not significantly affected by who collected the NP swab, research personnel versus parents.
Table 2.
Respiratory viruses detected from nasopharyngeal samples collected from infants through 260 days of age.
| NP swabs (n = 467) | Overall prevalence (n = 70) | |
|---|---|---|
| Never virus | 310 (66%) | 17 (24%) |
| Any virus | 157 (37%) | 53 (76%) |
| Rhinovirus | 110 (24%) | 46 (66%) |
| Parainfluenza virus | 21 (4%) | 13 (19%) |
| Coronavirus | 17 (4%) | 11 (16%) |
| Respiratory syncytial virus | 12 (3%) | 6 (9%) |
| Human metapneumovirus | 9 (2%) | 5 (7%) |
| Influenza virus | 1 (<1%) | 1 (1%) |
| Adenovirus | 2 (<1%) | 1 (1%) |
The median (IQR) rate of any recorded respiratory symptom for our participants was 26% (0–90%). The most commonly reported symptoms were cough and nasal congestion, reported at 15% (0–59%) and 17% (0–86%) of encounters. Cough and nasal congestion were associated with a greater likelihood of virus detection (OR 1.15, 95% CI 1.06–1.26 and OR 1.15, 95% CI 1.04–1.27, respectively) (Table 3 ). Wheezing, fever, altered breathing and decreased activity level were also reported, but not significantly related to viral positivity. Overall, 21% of the swabs collected in asymptomatic participants were positive for a virus; this occurred at least once in 34% (n = 24) of participants. In the absence of symptoms, the proportion of swabs positive for HRV (21%) or coronavirus (31%) was higher than for the other viruses tested (7%), p < .01. Overall, 11 infants received palivizumab for RSV prophylaxis before the age of one, all from the US. However, there were no differences in the rates of RSV between continents (US 2/20 vs AUS 4/50, p = .29).
Table 3.
Respiratory symptoms associated with viral infections in infants with cystic fibrosis.
| Overalla | Never virus (n = 17) | Any virus (n = 53) | p-Value⁎ | Odds ratio of detecting a Virus⁎⁎ | p-Value⁎⁎ | |
|---|---|---|---|---|---|---|
| No symptoms | 74% (10−100) | 83% (10–100) | 70% (24–96) | .06 | Reference | N/A |
| Any symptom | 26% (0–90) | 17% (0–90) | 30% (4–76) | .06 | 1.26 (1.16–1.37) | <.01 |
| Cough | 15% (0–59) | 8% (0–41) | 16% (25–59) | .06 | 1.15 (1.06, 1.26) | <.01 |
| Nasal congestion | 17% (0–86) | 8% (0–86) | 19% (33–59) | .04 | 1.15 (1.04, 1.27) | <.01 |
| Wheeze | 4% (0–65) | 0% (0–14) | 4% (0–65) | .09 | 0.99 (0.89, 1.10) | .84 |
| Fever | 0% (0–9) | 0% (0–5) | 0% (0–9) | .15 | 1.30 (0.99, 1.69) | .06 |
| Altered breathing | 0% (0–15) | 0% (0–15) | 0% (0–14) | .11 | 1.16 (0.98, 1.37) | .08 |
| Decreased activity | 0% (0–28) | 0% (0–15) | 0% (0–28) | .19 | 0.93 (0.80, 1.07) | .30 |
| For each additional Symptom Reported | 1.10 (1.06–1.15) | <.01 | ||||
Median (25th–75th) rate of symptom reporting over all encounters through 8 months of age.
p-Values from Wilcoxon rank-sum tests.
Analyses were performed using GEE models with repeated measures, adjusted for the presence of other symptoms.
There were 21 hospitalizations for respiratory indications in 18 participants. The median age at first hospitalization was 3.3 months (range 0–8 month). Of the 21 hospitalizations, a NP swab was collected within 7 days of hospitalization in 13. Eleven (85%) were positive for a respiratory virus. Viruses included rhinovirus (n = 5), RSV (n = 2), influenza (n = 1), parainfluenza (n = 1), human metapneumovirus (n = 1), and co-infection with adenovirus and human metapneumovirus (n = 1). Hospitalization rates did not differ between US and Australian sites nor between the “never virus” and “any virus” groups (data not shown).
3.3. Respiratory viruses, pulmonary inflammation, and bacterial isolates
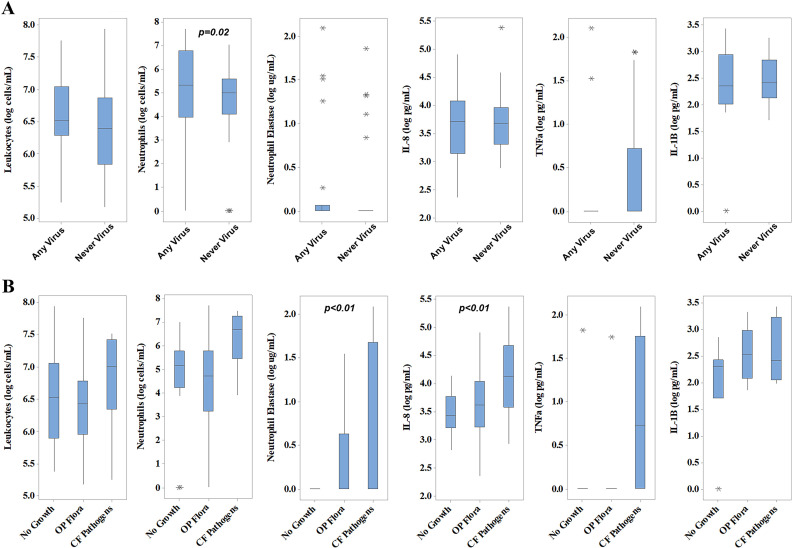
Bronchoscopy with BAL was performed in 68 infants; 66 had sufficient data for analyses (mean age 4.2 ± 1.6 months). Participants in the “any virus” group (n = 22) were significantly older than the “never virus” group (n = 44), 5.1 ± 1.9 month and 3.8 ± 1.2 month, respectively (p < .01). There was no significant difference in sex between the groups at time of bronchoscopy. Three of the 66 infants had pulmonary symptoms reported at time of BAL. For the any virus group, the median time between last detected virus and bronchoscopy was 24 days (range 0–89). The median time between last NP swab and bronchoscopy was 3 days (IQR 1–12 days). Lavage absolute neutrophil counts were higher in participants with previous viral infection (2.3 × 105 vs. 1.1 × 105 cells/mL ELF) in both unadjusted analyses (p = .01) and adjusted for age at time of BAL (p = .02) (Fig. 1 , panel A). There was no relationship between prior virus detection and current inflammatory markers in BAL (TNF-α, IL-1β, IL-8, NE).
Fig. 1.
Inflammatory cells and soluble inflammatory markers in lavage fluid (BALF) collected from infants during the study period. Raw data is plotted and p-values <.05 are shown. Any virus (n = 22); Never virus (n = 44).
Detection of a virus before BAL was associated with bacterial growth (45% in the “never virus group” vs 77% in the “any virus” group, p = .02) and the isolation of classic CF bacterial pathogens from BAL fluid (5% in the “never virus” group vs 32% in the “any virus” group, p = .01) (Table 4 ). P. aeruginosa was isolated from BAL fluid in only one participant (Table 4). Adjusting analyses to account for a viral infection in close proximity to the time of bronchoscopy (within 7 days) did not alter these results (data not shown).
Table 4.
Microbial isolates and their association with viral infections in infants with cystic fibrosis.
| Never virus (n = 44) | Any virus (n = 22) | p-Value | p-Value† | |
|---|---|---|---|---|
| No growth | 23 (52) | 5 (23) | .03 | .04 |
| Oropharyngeal flora | 18 (41) | 15 (68) | .07 | .05 |
| Any bacterial growth | 20 (45) | 17 (77) | .02 | .02 |
| Any classic CF pathogen | 2 (5) | 7 (32) | .01 | .01 |
| -Haemophilus influenzae | 1 (2) | 1 (5) | 1.0 | .56 |
| -Methicillin susceptibleStaphylococcus aureus | 0 (0) | 4 (18) | .01 | UTC |
| -Methicillin resistantStaphylococcus aureus | 0 (0) | 1 (5) | .33 | UTC |
| -Pseudomonas aeruginosa, non-mucoid | 0 (0) | 1 (5) | .33 | UTC |
| -Stenotrophomonas maltophilia | 1 (2) | 0 (0) | 1.0 | UTC |
| Moraxella catarrhalis | 0 (0) | 2 (9) | .11 | UTC |
| Escherichia coli | 2 (5) | 1 (5) | 1.0 | .96 |
| Klebsiella pneumoniae | 0 (0) | 1 (5) | .33 | UTC |
| Pseudomonas putida | 1 (2) | 0 (0) | 1.0 | UTC |
| Morganella morganii | 0 (0) | 1 (5) | .33 | UTC |
| Enterobacter cloacae | 1 (2) | 1 (5) | 1.0 | .64 |
| Any fungal growth | 2 (5) | 3 (14) | .32 | .20 |
| Candida albicans | 2 (5) | 3 (14) | .32 | .20 |
Values are frequencies (percentages) with p-values from Fisher's Exact Test and from generalized linear models (GLM) † adjusting for age at time of BAL (continent was highly correlated with age). There was no difference in sex at time of bronchoscopy. UTC = unable to calculate.
The presence of classic pathogens in BAL fluid was in turn associated with greater pulmonary inflammation, with higher concentrations of NE (p < .01) and IL-8 (p < .01) when compared to those with “no growth” or “oropharyngeal flora only” (Fig. 2, panel B online supplement). Oropharyngeal swab data available in online supplement (Fig. 3, Table 1 online supplement).
3.4. Antibiotic prescriptions and viral infections
Administration of prophylactic anti-staphylococcal antibiotics in early infancy is standard practice at the Australian centers. All Australian participants were treated with amoxicillin-clavulanate during the study time period. All but three were started on prophylaxis prior to BAL and the remaining three were started shortly after BAL. New antibiotic courses were defined as new prescriptions for respiratory related indications, including respiratory symptoms, otitis media, and/or bacterial eradication. During the first 260 days of life, infants in the US were prescribed more courses of systemic antibiotics (median 2, 0–7) for any indication versus Australian infants (median 0, 0–3) (p < .01). Moreover, American infants received more antibiotic courses related specifically to new respiratory symptoms (median 1, 0–5) than Australian infants (median 0, 0–3) (p < .01) and for all respiratory-related indications (US median 1.5, 0–7 and Australia median 0, 0–3) (p < .01). When viral testing was performed within 7 days of starting new antibiotics for respiratory indications, a virus was detected in 56% (n = 15) and 47% (n = 7) of cases in the US and Australia, respectively (p = .75) (Fig. 4 online supplement). Twenty-four participants (66%) were prescribed a new antibiotic any time before BAL. After adjusting for continents, infants who had ever received systemic antibiotics, irrespective of anti-staphylococcal prophylaxis, had higher neutrophil elastase (p < .01) and TNF-α (p < .01) concentrations in BAL fluid than those who had not. Of the nine infants who had classic CF pathogens detected on BAL, one was started on antibiotics in response to the lavage findings. Six infants were already on antibiotics at the time of BAL, one for pneumonia, three for a cough, one for otitis media and one for results from a prior surveillance oropharyngeal culture.
3.5. Respiratory virus detection and infant pulmonary function measures
Twenty-eight participants successfully completed infant pulmonary function testing (mean age 4.8 ± 1.3 month), 10 (36%) “never virus” and 18 (64%) “any virus.” There was no difference in age (p = .11), sex, or any pulmonary function measure between participants in the “never virus” vs “any virus” groups (Table 2 online supplement).
4. Discussion
While respiratory viral infections are common in infancy, their impact on CF lung disease is largely unknown. In this study, viral infections were associated with increased airway inflammation and more bacterial isolates, including classic CF bacterial pathogens in the lower airway. In turn, the presence of those classic CF pathogens was also associated with increased NE and IL-8 in the lower airways. Human rhinovirus was the most common virus detected, isolated more frequently in those with symptoms including cough and nasal congestion. In 52% of cases where antibiotics were prescribed for respiratory-related indications, a virus was detected.
Our finding of human rhinovirus being the most commonly detected virus agrees with recent publications showing a high prevalence of HRV in early life, both in healthy children and those with CF [[19], [20], [21]]. Older studies commonly report RSV, influenza and adenovirus in the youngest populations, detected using immunofluorescence staining or virus isolated from tissue or respiratory secretions [8,9]. Indeed, these differences in viral prevalence between studies can be explained by the advent and use of PCR assays as a sensitive diagnostic tool for respiratory viruses. Furthermore, in our study, samples were collected in outpatient settings, either in clinic or at home. Eighteen participants (26%) were hospitalized for respiratory illnesses during our study. This is similar to previous studies in which CF infants hospitalized with viral infections ranged from 23 to 39% [8,9].
We show 1–2 respiratory viruses identified during the first 8 months of life (median 1.5), consistent with community-based birth cohorts with 2–5 acute respiratory illnesses during infancy [19]. The percentage of viral positive swabs in our study was 37%. Prior studies in healthy infants show a wide range of viral positivity, anywhere from 23% [23] to 69% [19]. Comparing to studies examining viruses in the first year of life [19,20,22,24], we found comparable findings as in those studies viral positivity ranged from 27% [24] to 69% [19] in healthy infants. Our rates of detection in home versus clinic collected samples were not significantly different. This method of parental collection has been successfully used in prior studies. One study performed in the emergency room showed parental collected samples were equivalent to physician collected samples [25]. Others have demonstrated success with parents collecting samples at home and then mailing swabs to study coordinators [26,27]. One study did report mold in mailed samples [28]; however, these samples, unlike ours, were not maintained as frozen during storage and shipping. Home sampling by parents provides feasibility for frequent and/or rapid sampling at the onset of symptoms, without the barriers of participants coming into clinic or research coordinators going to participants' homes.
Unsurprisingly, viral detection was higher in our participants who were symptomatic, most often cough and nasal congestion. This is consistent with commonly reported viral symptoms in other CF cohorts [5,7]. Increased lower respiratory tract symptoms have been reported with HRV infection, especially in infants who have underlying lung diseases such as CF [5,7]. In our cohort, lower respiratory involvement, evidenced by wheezing, was reported exclusively in the any virus group. However it was infrequently reported and no significant difference was detected between groups. Our findings highlight the important role of viruses as a cause of early CF respiratory symptoms.
Bacterial pathogens are an important factor in the evolution of CF pulmonary disease. Through early surveillance, pathogens in the lower airway in infants with CF have been linked to inflammation and pulmonary function decline [1,2,29]. We found viral infection was associated with higher concentrations of lavage neutrophils as well as the presence of bacterial isolates, including classic CF pathogens. These pathogens were associated with higher NE and IL-8 concentrations in BAL, consistent with previous studies [1,3,4]. Based on our protocol, bronchoscopy was typically performed during a time of clinical stability and not during an acute viral infection. This may explain why there was less of a significant inflammatory response detected in the any virus group. However bacteria present in the lower airways clearly had more of an inflammatory effect, as would be expected.
In our participants, we found early viral infection was linked to lower airway bacterial isolates, with an increased incidence of classic CF bacterial pathogens. This contrasts with previous reports showing no changes in colonization or infection with typical CF pathogens following viral infection [6,30]. Of note, these studies primarily involved school-aged children who underwent BAL to detect viruses [6,9]. We obtained nasopharyngeal swabs to detect viruses and BAL to detect bacterial pathogens which may contribute to the difference in findings. There are several lines of evidence to support a mechanistic link between viral infections and lower airway bacterial colonization. Viral infections can damage the epithelial barrier, thus facilitating binding and translocation of bacteria into the airway [31,32]. In addition, viruses can decrease bacterial clearance contributing to the persistence of bacteria within the airway [33]. In COPD, rhinovirus specifically has been shown to impair bacterial phagocytosis by macrophages [34]. Others, using in vitro experiments, have shown alveolar macrophages are less able to mount an immune response to bacteria following HRV infection [35,36]. Viruses clearly play a role in the airway, the most significant of which may be their interaction with immune defense and bacteria.
Lastly, we examined antibiotic use in this young cohort of infants with CF. In contrast to the standard practice of watchful waiting and supportive care in non-CF infants with viral infections, the choice to use antibiotics in young children with CF with respiratory viral infections is more complicated. On one hand, we have to consider antibiotic stewardship [37,38], along with the potential effects antibiotics have on commensal organisms and the developing microbiome [[39], [40], [41]], and the growing sensitivity among clinicians regarding the use of antibiotics in CF care for symptoms that do not meet the clinical criteria of a pulmonary exacerbation [42]. These issues are juxtaposed by the potential benefits antibiotic treatment may have for the individual. For example the treatment or prevention of concurrent or subsequent bacterial infection, bacteria that may be linked to a poorer long-term prognosis [1,2,29]. Our data show a virus was present in roughly half of all instances in which a physician decided to start a new course of antibiotics; however bacterial cultures were not routinely collected at or after these events. Therefore, we are limited in our ability to address the potential benefits or detriments of these treatment decisions. These questions, particularly in infants and young children with CF, would benefit greatly from further investigation.
This study has several limitations. First, very few individuals had detected early episodes of RSV, HMPV or influenza, limiting our ability to specifically investigate the effects of each individual virus. A relatively small number of viral infections occurred in participants during the first 8 months of life (median 1.5). Therefore, we divided the groups into “never virus” and “any virus.” We assumed participants did not have viruses before enrollment in the “never virus” group, unless medical history indicated otherwise. It is possible that individuals with prior or undetected viral infections were included in the “never virus” group(s). As we did not have physiologic measures prior to any viral infection, we were unable to assess whether children with more severe lung disease were more likely to be symptomatic with a virus or possibly the reverse. Also, per the study design, the majority of the NP swabs were collected when infants were symptomatic. Therefore, we could not accurately estimate asymptomatic carrier rates. Some infants completed their bronchoscopy and infant PFT visits within 1 month of enrollment, limiting the viral data available prior to and between these visits. This short observation period may have influenced our results. Only a subset of participants was able to complete pulmonary function testing, yielding a low number of participants for that analysis. This small sample size may have contributed to the lack of statistically significant differences between groups. Lastly, we did not have a control group due to the nature of the study protocol which included bronchoscopy and infant lung function testing, sedated procedures that would not be electively performed on healthy infants. However, there are multiple epidemiologic studies of viral infections in healthy infants to which our prevalence results are comparable [19,20,22,24].
5. Conclusions
Viral infections in CF infants do not appear to be more frequent than those reported in healthy controls, however are associated with significant changes in the lower airway, including neutrophilic inflammation and greater frequency of classic CF bacterial isolates. Systemic antibiotics were prescribed in symptomatic participants, more than half (52%) in the setting of a viral infection. Future studies examining the relationship between viral infection, antibiotic use, and the airway microbiome over time will allow us to better understand these interactions and identify appropriate interventions.
Acknowledgments
Acknowledgments
The authors would like to thank the families who participated and the research coordinators who collected data and cared for the infants including Miriam Davis, Jane Quante, Nadeene Clarke, Rachel Foong, and Alana Harper. They would like to thank the personnel from the Washington University Virology Laboratory at St. Louis Children's Hospital for their expertise and contributions.
Financial support and conflicts of interest
The authors were supported by the National Institutes of Health (NIH) grants, HL116211 (SDD, TWF, SR), National Health and Medical Research Council award, NHMRC1043768, NHMRC1000896 (SMS), NHMRCAPP1025550 (GLH), the Cystic Fibrosis Foundation Fellowship Training Grant (ARD), the Indiana Physician Scientist Award (Eli Lilly) (SDD), and the Royal Children's Hospital Cystic Fibrosis Research Trust (SR). The views expressed do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S government.
There are no previous publications with any overlapping information presented in this report. Portions of this work have been presented in abstract form at the North American Cystic Fibrosis Conference and American Thoracic Society conference in 2015. This work is not and will not be submitted to any other journal while under consideration. Neither author has an actual or perceived conflict of interest concerning the information presented in the paper. None of the authors received honoraria or grant to write the manuscript. All authors listed on the manuscript have reviewed and approved the content of the submission, and take full responsibility for the information provided.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcf.2019.02.004.
Appendix A. Supplementary data
Supplementary material
References
- 1.Rosenfeld M., Gibson R.L., McNamara S., Emerson J., Burns J.L., Castile R. Early pulmonary infection, inflammation and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol. 2001;32:356–366. doi: 10.1002/ppul.1144. [DOI] [PubMed] [Google Scholar]
- 2.Dakin C.J., Numa A.H., Wang H., Morton J.R., Vertzyas C.C., Henry R.L. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 2002;165(7):904–910. doi: 10.1164/ajrccm.165.7.2010139. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong D.S., Hook S.M., Jamsen K.M., Nixon G.M., Carzino R., Carlin J.B. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol. 2005;40(6):500–510. doi: 10.1002/ppul.20294. [DOI] [PubMed] [Google Scholar]
- 4.Sly P.D., Brennan S., Gangell C., de Klerk N., Murray C., Mott L. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180(2):146–152. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 5.van Ewijk B.E., van der Zalm M.M., Wolfs T.F., Fleer A., Kimpen J.L., Wilbrink B. Prevalence and impact of respiratory viral infections in young children with cystic fibrosis: prospective cohort study. Pediatrics. 2008;122(6):1171–1176. doi: 10.1542/peds.2007-3139. [DOI] [PubMed] [Google Scholar]
- 6.Esther C.R., Jr., Lin F.C., Kerr A., Miller M.B., Gilligan P.H. Respiratory viruses are associated with common respiratory pathogens in cystic fibrosis. Pediatr Pulmonol. 2014;49(9):926–931. doi: 10.1002/ppul.22917. [DOI] [PubMed] [Google Scholar]
- 7.Burns J.L., Emerson J., Kuypers J., Campbell A.P., Gibson R.L., McNamara S. Respiratory viruses in children with cystic fibrosis: viral detection and clinical findings. Influenza Other Respi Viruses. 2012;6(3):218–223. doi: 10.1111/j.1750-2659.2011.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiatt P.W., Grace S.C., Kozinetz C.A., Raboudi S.H., Treece D.G., Taber L.H. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics. 1999;103(3):619–626. doi: 10.1542/peds.103.3.619. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong D., Grimwood K., Carlin J.B., Carzino R., Hull J., Olinsky A. Severe viral respiratory infections in infants with cystic fibrosis. Pediatr Pulmonol. 1998;26(6):371–379. doi: 10.1002/(sici)1099-0496(199812)26:6<371::aid-ppul1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Smyth A.R., Smyth R.L., Tong C.Y., Hart C.A., Heaf D.P. Effect of respiratory virus infections including rhinovirus on clinical status in cystic fibrosis. Arch Dis Child. 1995;73(2):117–120. doi: 10.1136/adc.73.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cousin M., Molinari N., Foulongne V., Caimmi D., Vachier I., Abely M. Rhinovirus-associated pulmonary exacerbations show a lack of FEV1 improvement in children with cystic fibrosis. Influenza Other Respi Viruses. 2016;10(2):109–112. doi: 10.1111/irv.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kua K.P., Lee S.W.H. Systematic review of the safety and efficacy of Palivizumab among infants and young children with cystic fibrosis. Pharmacotherapy. 2017;37(6):755–769. doi: 10.1002/phar.1936. [DOI] [PubMed] [Google Scholar]
- 13.Buchs C., Dalphin M.L., Sanchez S., Perceval M., Coutier L., Mainguy C. Palivizumab prophylaxis in infants with cystic fibrosis does not delay first isolation of Pseudomonas aeruginosa or Staphylococcus aureus. Eur J Pediatr. 2017;176(7):891–897. doi: 10.1007/s00431-017-2926-8. [DOI] [PubMed] [Google Scholar]
- 14.Stocks J., Godfrey S., Beardsmore C., Bar-Yishay E., Castile R. Plethysmographic measurements of lung volume and airway resistance. Eur Respir J. 2001;17(2):302–312. doi: 10.1183/09031936.01.17203020. [DOI] [PubMed] [Google Scholar]
- 15.ATS/ERS statement: raised volume forced expirations in infants: guidelines for current practiceAm J Respir Crit Care Med. 2005;172(11):1463–1471. doi: 10.1164/rccm.200408-1141ST. [DOI] [PubMed] [Google Scholar]
- 16.Rennard S.I., Basset G., Lecossier D., O'Donnell K.M., Pinkston P., Martin P.G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol. 1986;60(2):532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 17.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Child Growth Standards, Anthro version 3.2.2 2011. Available from: http://www.who.int/childgrowth/software/en/.
- 19.Kusel M.M., de Klerk N.H., Holt P.G., Kebadze T., Johnston S.L., Sly P.D. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25(8):680–686. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 20.Muller L., Mack I., Tapparel C., Kaiser L., Alves M.P., Kieninger E. Human rhinovirus types and association with respiratory symptoms during the first year of life. Pediatr Infect Dis J. 2015;34(8):907–909. doi: 10.1097/INF.0000000000000758. [DOI] [PubMed] [Google Scholar]
- 21.Stelzer-Braid S., Liu N., Doumit M., D'Cunha R., Belessis Y., Jaffe A. Association of rhinovirus with exacerbations in young children affected by cystic fibrosis: preliminary data. J Med Virol. 2017;89(8):1494–1497. doi: 10.1002/jmv.24794. [DOI] [PubMed] [Google Scholar]
- 22.Korten I., Kieninger E., Klenja S., Mack I., Schlapfer N., Barbani M.T. Respiratory viruses in healthy infants and infants with cystic fibrosis: a prospective cohort study. Thorax. 2018;73(1):13–20. doi: 10.1136/thoraxjnl-2016-209553. [DOI] [PubMed] [Google Scholar]
- 23.Principi N., Zampiero A., Gambino M., Scala A., Senatore L., Lelii M. Prospective evaluation of rhinovirus infection in healthy young children. J Clin Virol. 2015;66:83–89. doi: 10.1016/j.jcv.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Mack I., Kieninger E., Cangiano G., Tapparel C., Kuehni C., Spycher B. Rhinovirus infections and associated respiratory morbidity in infants: a prospective cohort study. Pediatr Infect Dis J. 2016;35(10):1069–1074. doi: 10.1097/INF.0000000000001240. [DOI] [PubMed] [Google Scholar]
- 25.Esposito S., Molteni C.G., Daleno C., Valzano A., Tagliabue C., Galeone C. Collection by trained pediatricians or parents of mid-turbinate nasal flocked swabs for the detection of influenza viruses in childhood. Virol J. 2010;7:85. doi: 10.1186/1743-422X-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emerson J., Cochrane E., McNamara S., Kuypers J., Gibson R.L., Campbell A.P. Home self-collection of nasal swabs for diagnosis of acute respiratory virus infections in children with cystic fibrosis. J Pediatr Infect Dis Soc. 2013;2(4):345–351. doi: 10.1093/jpids/pit039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarna M., Lambert S.B., Sloots T.P., Whiley D.M., Alsaleh A., Mhango L. Viruses causing lower respiratory symptoms in young children: findings from the ORChID birth cohort. Thorax. 2018;73(10):969–979. doi: 10.1136/thoraxjnl-2017-210233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gangell C.L., Shackleton C., Poreddy S., Kappers J., Gaydon J.E., Sloots T.P. Feasibility of parental collected nasal swabs for virus detection in young children with cystic fibrosis. J Cyst Fibros. 2014 doi: 10.1016/j.jcf/2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pillarisetti N., Williamson E., Linnane B., Skoric B., Robertson C.F., Robinson P. Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med. 2011;184(1):75–81. doi: 10.1164/rccm.201011-1892OC. [DOI] [PubMed] [Google Scholar]
- 30.Olesen H.V., Nielsen L.P., Schiotz P.O. Viral and atypical bacterial infections in the outpatient pediatric cystic fibrosis clinic. Pediatr Pulmonol. 2006;41(12):1197–1204. doi: 10.1002/ppul.20517. [DOI] [PubMed] [Google Scholar]
- 31.Sajjan U., Wang Q., Zhao Y., Gruenert D.C., Hershenson M.B. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am J Respir Crit Care Med. 2008;178(12):1271–1281. doi: 10.1164/rccm.200801-136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Ewijk B.E., Wolfs T.F., Aerts P.C., Van Kessel K.P., Fleer A., Kimpen J.L. RSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cells. Pediatr Res. 2007;61(4):398–403. doi: 10.1203/pdr.0b013e3180332d1c. [DOI] [PubMed] [Google Scholar]
- 33.Stark J.M., Stark M.A., Colasurdo G.N., LeVine A.M. Decreased bacterial clearance from the lungs of mice following primary respiratory syncytial virus infection. J Med Virol. 2006;78(6):829–838. doi: 10.1002/jmv.20631. [DOI] [PubMed] [Google Scholar]
- 34.Finney L.J., Belchamber K.B.R., Fenwick P.S., Kemp S.V., Edwards M.R., Mallia P. Human rhinovirus impairs the innate immune response to Bacteria in alveolar macrophages in COPD. Am J Respir Crit Care Med. 2018 doi: 10.1164/rccm.201806-1095OC. [DOI] [PubMed] [Google Scholar]
- 35.Oliver B.G., Lim S., Wark P., Laza-Stanca V., King N., Black J.L. Rhinovirus exposure impairs immune responses to bacterial products in human alveolar macrophages. Thorax. 2008;63(6):519–525. doi: 10.1136/thx.2007.081752. [DOI] [PubMed] [Google Scholar]
- 36.Jubrail J., Africano-Gomez K., Herit F., Baturcam E., Mayer G., Cunoosamy D.M. HRV16 impairs macrophages cytokine response to a secondary bacterial trigger. Front Immunol. 2018;9:2908. doi: 10.3389/fimmu.2018.02908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben-David D., Rubinstein E. Appropriate use of antibiotics for respiratory infections: review of recent statements and position papers. Curr Opin Infect Dis. 2002;15(2):151–156. doi: 10.1097/00001432-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 38.File T.M., Jr. Judicious use of antibiotics to treat respiratory tract infections. Curr Opin Infect Dis. 2002;15(2):149–150. doi: 10.1097/00001432-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Prevaes S.M., de Winter-de Groot K.M., Janssens H.M., de Steenhuijsen Piters W.A., Tramper-Stranders G.A., Wyllie A.L. Development of the nasopharyngeal microbiota in infants with cystic fibrosis. Am J Respir Crit Care Med. 2016;193(5):504–515. doi: 10.1164/rccm.201509-1759OC. [DOI] [PubMed] [Google Scholar]
- 40.Mika M., Korten I., Qi W., Regamey N., Frey U., Casaulta C. The nasal microbiota in infants with cystic fibrosis in the first year of life: a prospective cohort study. Lancet Respir Med. 2016;4:627–635. doi: 10.1016/S2213-2600(16)30081-9. [DOI] [PubMed] [Google Scholar]
- 41.Pittman J.E., Wylie K.M., Akers K., Storch G.A., Hatch J., Quante J. Association of antibiotics, airway microbiome, and inflammation in infants with cystic fibrosis. Ann Am Thorac Soc. 2017;14(10):1548–1555. doi: 10.1513/AnnalsATS.201702-121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waters V., Ratjen F. Pulmonary exacerbations in children with cystic fibrosis. Ann Am Thorac Soc. 2015;12(Suppl. 2):S200–S206. doi: 10.1513/AnnalsATS.201502-098AW. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material