Abstract
Aim: The present study aimed to investigate the association between shape and location of atherosclerotic plaques and intraplaque hemorrhage (IPH) in carotid arteries using magnetic resonance (MR) imaging.
Methods: Overall, 114 symptomatic patients (mean age: 64.9±10.9 years; 81 males) who underwent MR imaging and had advanced carotid plaques were included in analysis. IPH presence and carotid plaque shape and location (below and above bifurcation) were evaluated. The plaque shape was defined as follows: type-I: the arc-length of plaque is greater in the upstream; type-II: the arc-length of plaque in downstream and upstream is equal; and type-III: the arc-length of plaque is greater in downstream. The plaque shape and location were compared between plaques with and without IPH and their associations with IPH were determined.
Results: Of 181detectedplaques, 57 (31.5%) had IPH. Compared with plaques without IPH, those with IPH had higher incidence of the plaque shape of type-I (66.7% vs. 32.2%, P<0.001), lower incidence of plaque shape of type-III (24.6% vs. 50.0%, P=0.001), and were more likely located above carotid bifurcation (71.9% vs. 48.4%, P=0.003). The plaque shape of type-I (OR, 4.01; 95%CI, 1.36–11.83; P=0.012) and location above bifurcation (OR, 3.21; 95%CI, 1.07–9.61; P=0.037) of carotid plaques were significantly associated with IPH after adjusting for confounder factors.
Conclusions: Carotid plaque shape and location are significantly associated with the occurrence of IPH. Our findings could provide new insights for the pathogenesis of IPH and vulnerably plaques.
Keywords: Carotid artery, Atherosclerosis, Intraplaque hemorrhage, Risk factor, Magnetic resonance imaging
Introduction
Vulnerable atherosclerotic plaques in carotid arteries have been demonstrated to have a significant correlation with cerebrovascular ischemic events, such as ischemic stroke and transient ischemic attack1-3). Intraplaque hemorrhage (IPH), one of compositional characteristics of vulnerable plaques, plays a critical role in stratifying the risk of future events for patients with carotid atherosclerosis4-6). The mechanism of IPH in carotid plaques remains unclear. It is important to investigate the risk factors for IPH to stabilize atherosclerotic plaque and thereby prevent future cerebrovascular events.
Previous evidences have shown that local factors of atherosclerotic plaques play an important role in the occurrence of carotid IPH. Investigators have demonstrated that the likelihood of IPH increased with an increase of plaque burden as measured by intima-media thickness or plaque volume7, 8). In addition, carotid plaque calcification9, 10) and ulceration11) have been found to be associated with the presence of IPH. It has been proved that higher maximum shear stresses that act on plaques were independently correlated with occurrence of IPH12). The shear stresses may vary with different carotid plaque shape and location because of the change in hemodynamics caused by the turbulent flow in carotid arteries. However, the relationship between IPH and the carotid plaque shape and location remains unclear.
High-resolution multicontrast magnetic resonance imaging (MRI) has been demonstrated to be capable of evaluating location and morphological features of carotid plaques13, 14). Furthermore, MRI exhibits excellent capability in characterizing carotid plaque IPH validated by histology15, 16)ss.
Aim
The present study aimed to investigate the association between IPH and carotid plaque shape and location of carotid plaques using the multicontrast MR imaging techniques.
Methods
Study Population
The study enrolled patients who suffered from recent cerebrovascular ischemic symptoms (within two weeks), including stroke or transient ischemic attack and had atherosclerotic plaque in at least one carotid artery as determined by ultrasound. All patients underwent MR vessel wall imaging examination for bilateral carotid arteries. The exclusion criteria were as follows: 1) contraindication to MR examination; 2) previous history of carotid endarterectomy (CEA); 3) previous history of radiotherapy at neck; and 4) claustrophobia. Demographic and clinical information including age, sex, body mass index (BMI), hypertension, diabetes, hyperlipidemia, lipid levels, smoking history, coronary heart disease, and statin utilization was collected from clinical record. The study protocol conforms to the ethical guidelines of the Declaration of Helsinki and was approved by the Institution's ethics committee for research on humans. All participants provided written consent forms.
Carotid Artery MR Imaging
Carotid MR imaging was performed on a 3.0T MR scanner (Achieva TX, Philips Healthcare, Best, The Netherlands) using a dedicated 8-channel phase array carotid coil. MR imaging parameters for the three-dimensional time-of-flight (TOF) imaging were as follows: repetition time (TR)/echo time (TE) of 29 ms/4.9 ms, field of view(FOV) of 140 mm×140 mm, flip angle of 20°, matrix size of 256×256, and slice thickness of 2 mm. Parameters for two-dimensional T1-weighted (T1W) quadruple inversion recovery imaging were as follows: TR/TE of 800 ms/10 ms, FOV of 140 mm×140 mm, flip angle of 90°, matrix size of 256×256, and slice thickness of 2 mm and those for axial T2-weighted (T2W) multislice double inversion recovery imaging were as follows: TR/TE of 4800 ms/50 ms, FOV of 140 mmx140 mm, flip angle of 90°, matrix size of 256×256, and slice thickness of 2 mm. The MR scan was centered to the bifurcation of the symptomatic side of carotid artery.
MR Image Analysis
The MR vessel wall images of symptomatic carotid arteries were reviewed by two radiologists with >2 years' experience in cerebrovascular imaging using custom-designed software CASCADE (Vascular Imaging Lab, University of Washington)17) with consensus. A 4-point scale (1 = poor, 2 = marginal, 3 = good, and 4 = excellent) was utilized to assess the image quality per slice18), and images with quality score <2 were excluded from the study. The lumen and wall boundaries were manually outlined and the lumen area, wall area, and maximum wall thickness (WTmax) were measured. The presence or absence of ulceration and plaque compositions, such as lipid-rich necrotic core (LRNC), IPH, and calcification, were determined16). Only advanced carotid plaques with compositions, such as LRNC, IPH, calcification, or ulceration, were included for further analysis. Carotid plaques were classified as type-I, type-II, and type-III according to the symmetric features of the plaque shape in the longitudinal direction (Fig. 1): instances where in the greater arc-length of carotid plaques was located in the downstream arterial wall above the location of WTmax were classified as type-I; instances where in the arc-length of carotid plaques in downstream and upstream arterial wall from the location of WTmax was equal were classified as type-II; instances where in the greater arc-length of carotid plaques was located in upstream arterial wall below the location of WTmax were classified as type-III. When the surface of carotid plaques was irregular, the arc-length was estimated using smoothing algorithm. The location of carotid plaques was divided into two categories—above bifurcation and below bifurcation—that were defined according to the location of WTmax of carotid plaque (Fig. 2).
Fig. 1.
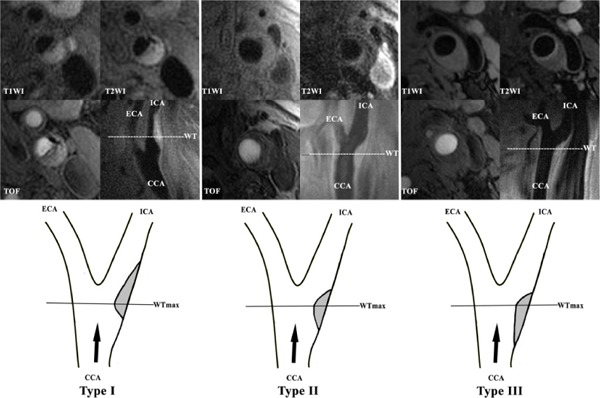
Examples of different types of plaque shape
T1W images (the top row) and their corresponding diagrams (the bottom row) of the three shape types are shown. Type-I was defined when the greater arc-length of carotid plaques was located in the downstream arterial wall above the location of WTmax; MR images show that the carotid plaque has IPH (high-intensity on all the sequences), LRNC (iso-intensity on T1WI and low-intensity on T2WI), and calcification (low-intensity on all the sequences). Type-II was defined if the arc-length of carotid plaques in downstream and upstream arterial wall from the location of WTmax was equal;. Furthermore, MR images show that the carotid plaque had LRNC (iso-intenstiy on T1WI and low-intensity on T2WI). Type-III was defined if the greater arc-length of carotid plaques was located in upstream arterial wall below the location of WTmax.MR images show that the carotid plaque had LRNC (iso-intenstiy on T1WI and low-intensity on T2WI). (WTmax: maximum wall thickness; ICA: internal carotid artery; ECA: external carotid artery; CCA: common carotid artery; BIF: bifurcation of carotid artery; Black arrow: direction of blood flow; IPH: intraplaque hemorrhage; LRNC: lipid-rich necrotic core).
Fig. 2.
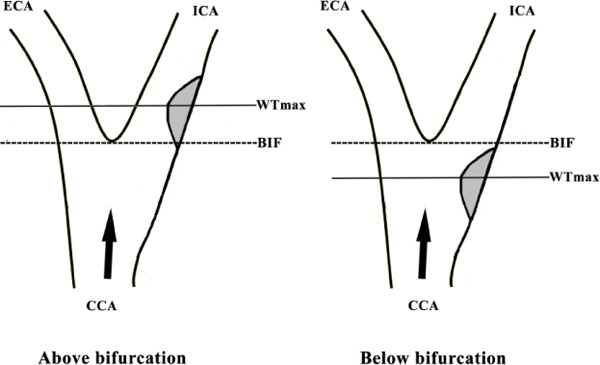
Diagram of location of carotid plaques
Plaques located above bifurcation and below bifurcation are defined according to the location of the WTmax of carotid plaque (WTmax: maximum wall thickness; ICA: internal carotid artery; ECA: external carotid artery; CCA: common carotid artery; BIF: bifurcation of carotid artery; Black arrow: direction of blood flow)
Statistical Analysis
The continuous variables are presented as mean±standard deviation (SD) and categorical variables are expressed as percentage. The clinical characteristics and carotid plaque measurements were compared between plaques with and without IPH using independent Student t-test or chi-square test. Logistic regression analysis with generalized estimating equation correction was used to calculate the odds ratio (OR) and corresponding 95% confidence interval (CI) of carotid plaque shape and location in discriminating presence of IPH. Two-sided p<0.05 was considered significant; statistical analyses were performed using the software of SPSS 22.0 (IBM, Chicago, IL).
Results
Overall, 131 patients with complex carotid plaque compositions were enrolled in the study from May 2010 to August 2015. Of these, 17patients were excluded because of poor image quality (n=5) and insufficient longitudinal coverage (n=12). Among the remaining114 patients, mean age was 64.9±10.9 years old, 81 (71.1%) were males, 44 (38.6%) had diabetes mellitus, 87 (76.3%) had hypertension, 65 (57.0%) had history of smoking, 64 (56.1%) had hyperlipidemia, and 48 (42.1%) used statins. Table 1 shows the clinical characteristics of the population.
Table 1. Comparison of patients'clinical characteristics between plaques with and without IPH.
| Mean±SD or n (%) | |||
|---|---|---|---|
| With IPH | Without IPH | P | |
| (n = 57) | (n = 124) | ||
| Gender, male | 46 (80.7) | 85 (68.5) | 0.089 |
| Age, y | 68.7±11.1 | 63.3±10.2 | 0.298 |
| BMI kg/m2 | 24.3±3.3 | 24.1±3.0 | 0.461 |
| Hypertension | 44 (77.2) | 92 (74.2) | 0.665 |
| Hyperlipidemia | 29 (50.9) | 71 (57.3) | 0.423 |
| Diabetes | 18 (31.6) | 50 (40.3) | 0.259 |
| CHD | 9 (15.9) | 28 (22.6) | 0.720 |
| Low density protein, mmol/L | 2.5±0.89 | 3.0±1.0 | 0.720 |
| High density protein, mmol/L | 1.0±0.2 | 1.0±0.3 | 0.793 |
| Total density protein, mmol/L | 4.0±0.9 | 4.5±1.0 | 0.977 |
| Triglyceride, mmol/L | 1.3±0.4 | 1.5±0.5 | 0.360 |
| Statin use | 15 (26.3) | 60 (48.4) | 0.005 |
| History of smoke | 39 (68.4) | 60 (48.4) | 0.012 |
IPH: intraplaque hemorrhage; SD: standard deviation; BMI: body mass index; CHD: coronary heart disease.
Imaging Characteristics of Carotid Atherosclerotic Plaque
Of 114 patients, 67 (58.8%) had bilateral carotid plaques and 47 (41.2%) had unilateral carotid plaques. Among all 181detected plaques, 174 (96.1%) had LRNC, 57 (31.5%) had IPH, 121 (66.9%) had calcification, and 16 (8.8%) had ulceration. The maximum wall thickness, stenosis, and mean wall area of carotid arteries were 3.7±1.7 mm, 28.2%±35.5%, and 35.9±11.1 mm2, respectively.
Compared with plaques without IPH, those with IPH had greater maximum wall thickness (5.1±1.9 mm vs. 3.1±1.1 mm, P<0.001), mean wall area (44.9±12.5 mm2 vs. 31.7±7.4 mm2, P=0.001), and stenosis (50.4%±39.1% vs.18.0%±28.5%, P<0.001), had higher incidence of plaque shape of type-I (66.7% vs. 32.2%, P<0.001) and lower incidence of plaque shape of type-III (24.6% vs. 50.0%, P=0.001),and were more likely to be located above the bifurcation of carotid arteries (71.9% vs. 48.4%, P=0.003) (Table 2).
Table 2. Characteristics of burden, compositions, shape and location of carotid plaques between plaques with IPH and without IPH.
| Mean±SD or n (%) | |||
|---|---|---|---|
| With IPH | Without IPH | P | |
| (n = 57) | (n = 124) | ||
| Plaque burden and compositions | |||
| Mean total vessel area | 82.4±16.4 | 73.0±20.7 | 0.114 |
| Stenosis | 50.4±39.1 | 18.0±28.5 | <0.001 |
| Mean wall area | 44.9±12.5 | 31.7±7.4 | 0.001 |
| Maximum wall thickness | 5.1±1.9 | 3.1±1.1 | <0.001 |
| Lipid-rich necrotic core | 57 (100.0) | 117 (94.3) | 0.067 |
| Calcification | 44 (77.2) | 79 (63.7) | 0.071 |
| Ulceration | 12 (21.1) | 4 (3.2) | <0.001 |
| Shape of carotid plaque | |||
| Type-I | 38 (66.7) | 40 (32.2) | <0.001 |
| Type-II | 5 (8.8) | 22 (17.7) | 0.116 |
| Type-III | 14 (24.6) | 62 (50.0) | 0.001 |
| Location of carotid plaque | |||
| Above bifurcation | 41 (71.9) | 60 (48.4) | 0.003 |
| Below bifurcation | 16 (28.1) | 64 (51.6) | |
IPH: intraplaque hemorrhage; SD: standard deviation
Association between Carotid Plaque Shape and Location and IPH
Univariate logistic regression analysis showed that the plaque shape of type-I (OR, 4.11; 95%CI, 1.95–8.63; P<0.001) and type-III (OR, 0.33; 95%CI, 0.17–0.65; P=0.001) and location above bifurcation (OR, 3.28; 95%CI, 1.40–7.67; P=0.006) of carotid plaques were significantly associated with the presence of IPH. Adjusting for clinical confounding factors, including age, sex, smoking history, and statin utilization, revealed a significant association of plaque shape of type-I (OR, 4.26; 95%CI, 1.41–11.25; P<0.001) and type-III (OR, 0.29; 95%CI, 0.13–0.65; P=0.003)and location above bifurcation(OR, 4.02; 95%CI, 1.48–10.95; P=0.006) with IPH. After further adjustment for maximum wall thickness, stenosis of carotid artery, and ulceration, the association of plaque shape of type-I (OR, 4.01; 95%CI, 1.36–11.83; P=0.012) and location above bifurcation (OR, 3.21; 95%CI, 1.07–9.61; P=0.037) with IPH remained significant (Table 3).
Table 3. Logistic regression hazard models of risk factors for the presence of IPH.
| Presence of IPH | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate regression | Model 1* | Model 2† | ||||||||
| OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | ||
| Shape | ||||||||||
| Type-I | 4.11 | 1.95–8.63 | <0.001 | 4.26 | 1.41–11.25 | <0.001 | 4.01 | 1.36–11.83 | 0.012 | |
| Type-II | 0.50 | 0.21–1.18 | 0.114 | 0.45 | 0.15–1.31 | 0.141 | 0.39 | 0.11–2.34 | 0.515 | |
| Type-III | 0.33 | 0.17–0.65 | 0.001 | 0.29 | 0.13–0.65 | 0.003 | 0.46 | 0.18–1.11 | 0.119 | |
| Location | ||||||||||
| Above BIF | 3.28 | 1.40–7.67 | 0.006 | 4.02 | 1.48–10.95 | 0.006 | 3.21 | 1.07–9.61 | 0.037 | |
IPH: intraplaque hemorrhage; OR: odds ratio; BIF: bifurcation of carotid artery. Model 1 is adjusted for age, sex, smoking history, and statin use; Model 2 is adjusted for age, sex, smoking history, statin use, maximum wall thickness, stenosis, and ulceration.
Discussion
The present study investigated the relationship between the shape and location of carotid atherosclerotic plaque and the presence of IPH. We found that compared with plaques without IPH, those with IPH had higher incidence of plaque shape of type-I, lower incidence of shape of type-III and were more likely to be located above the bifurcation of carotid arteries than those without IPH. In addition, we found that the plaque shape of type-I and location above bifurcation of carotid plaque were significantly associated with the presence of IPH after adjusting for con founding factors. Our findings indicate that carotid plaque shape and location might be potential indicators for IPH in symptomatic patients.
In the present study, we found that compared with patients without IPH in carotid plaques, those with IPH were significantly less likely to use statins but more likely to smoke. Our findings are consistent with previous studies19, 20). Derksen et al.20) have demonstrated that IPH in carotid plaques was less frequently detected in patients who used statins (P= 0.002) and statins usage was independently associated with carotid IPH (OR, 0.52; CI, 0.32–0.85; P=0.009). It is known that statins could inhabit inflammatory status and biological activity related with inflammation in plaques. Furthermore, neovasculature formation within plaques could be reduced by statins usage. The reduction of inflammation and angiogenesis might play a key role in a decrease of IPH in carotid plaques19, 21). In the present study, we observed a higher incidence of smoking in patients with carotid IPH, supporting earlier results of a Rotterdam study by Van et al.22). They found that current smokers were more likely to have IPH in plaques (OR, 1.6; CI, 1.2–2.3), suggesting that smoking might be a dependent indicator of carotid IPH in patients with carotid atherosclerosis. The nicotine in cigarettes could accelerate the heart rate and elevate the blood pressure of patients. A previous study has reported that elevated blood pressure would increase the risk of IPH in carotid arteries (OR, 1.4; CI, 1.1–1.8)22).
Our data have shown that the carotid plaque shape of type-I was independently associated with the occurrence of IPH. The mechanism of the relationship between the plaque shape and IPH presence remains unclear. However, plaques with different shapes had different flow biomechanical features acting on their surface that could contribute to the different incidence rates of IPH. In a previous study, Tuenter et al have found that higher maximum shear stresses on plaque surface were independently associated with the presence of carotid IPH (OR, 12.14; 95%CI, 3.21–45.94; P=0.001)12). Moreover, another study by Huang et al has shown that the value of mean plaque wall stress from hemorrhage parts of plaque was higher than that from non-hemorrhage parts (75.6 vs. 68.1 kPa, P=0.003)23). The possible mechanism could be that higher maximum shear stress accelerates the expression of vascular endothelial growth factor, which will induce angiogenesis, disrupt the vascular barrier function in plaques, and subsequently lead to the occurrence of IPH in plaques24). In the present study, compared with plaques with shape of type-II and type-III, those with shape of type-I had the higher slope toward the upstream and might suffer a higher shear stress which would stimulate the occurrence of carotid IPH25).The mechanism of the relationship between the plaque shape and IPH needs further investigation.
Furthermore, in the present study, we observed that carotid plaques located above the bifurcation of carotid artery were more likely to have IPH. However, the underlying mechanism of the location of plaques affecting the occurrence of IPH remains unclear. It has been demonstrated that individual segments of extracranial carotid artery, including common carotid artery (CCA), BIF, and internal carotid artery (ICA), have unique histological26) and anatomic features27) and were differently exposed to turbulent flow28). The different types of turbulent flow and hemodynamic characteristics between segments above and below carotid bifurcation might result in the different incidence of carotid IPH. In addition, plaques located at different segments of carotid arteries have been demonstrated to have different progression rate. A previous study by Mackinnon et al.29) has shown that the progression rate of carotid plaques at the ICA was significantly greater compared with the CCA. Previous studies have demonstrated that IPH could accelerate the progression of carotid plaques30, 31). This suggests that the higher incidence of IPH above BIF in the present study is a contributor of higher progression rate of carotid plaques located at the ICA.
Several limitations in our study should be noted. First, only qualitative evaluation was performed to characterize the carotid plaque shape because of the limitation of two-dimensional imaging techniques utilized in the present study. In recent years, three-dimensional MR vessel wall imaging techniques have been proposed for characterization of carotid plaques with isotropic high spatial resolution and large longitudinal coverage32, 33) that allow accurate quantification of plaque morphology. These three-dimensional vessel wall imaging techniques could potentially be used to quantitatively evaluate plaque shape, such as the value of the slope of plaque surface. Second, in the present study, because of limited longitudinal coverage, the location of carotid plaques was classified into only two categories according to their location to carotid bifurcation. Future studies are warranted to investigate the relationship between IPH and carotid plaque located in the more proximal segments of CCA or more distal segments of ICA as long as large coverage imaging data available. Third, the hemodynamic characteristics of carotid arteries may play important role in occurrence of carotid IPH. To determine the hemodynamic features in carotid plaques with different shape and location patterns could be helpful for better understanding their relationship with carotid IPH.
Conclusion
Carotid plaque shape and location are independently associated with the occurrence of IPH. Our findings may provide new insights for the pathogenesis of IPH and vulnerably plaques.
Acknowledgments
This study was supported by the grants of National Natural Science Foundation of China (81771825), Beijing Science and Technology Project (D171100003017003), and Ministry of Science and Technology of China (2017YFC1307904).
Abbreviations
- IPH
intraplaque hemorrhage
- MR
magnetic resonance
- OR
odds ratio
- CI
confidence interval
- CEA
carotid endarterectomy
- BMI
body mass index
- TOF
time-of-flight
- FOV
field of view
- LRNC
lipid-rich necrotic core
- WTmax
maximum wall thickness
- CCA
common carotid artery
- BIF
bifurcation of carotid artery
- ICA
internal carotid artery
Conflicts of Interest
None.
References
- 1). Kume S, Hama S, Yamane K, Wada S, Nishida T, Kurisu K: Vulnerable carotid arterial plaque causing repeated ischemic stroke can be detected with b-mode ultrasonography as a mobile component: Jellyfish sign. Neurosurg Revi, 2010; 33: 419-430 [DOI] [PubMed] [Google Scholar]
- 2). Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, Tran N, Polissar NL, Isaac C, Ferguson MS, Garden GA, Cramer SC, Maravilla KR, Hashimoto B, Hatsukami TS: Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: A prospective assessment with mri--initial results. Stroke, 2006; 37: 818-823 [DOI] [PubMed] [Google Scholar]
- 3). Liu XS, Zhao HL, Cao Y, Lu Q, Levison Xu JR: Comparison of carotid atherosclerotic plaque characteristics by high-resolution black-blood MR imaging between patients with first-time and recurrent acute ischemic stroke. AJNR Am J Neuroradiol, 2012; 33: 1257-1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Hellings WE, Peeters W, Moll FL, Piers SR, van Setten J, Van der Spek PJ, de Vries JP, Seldenrijk KA, De Bruin PC, Vink A, Velema E, de Kleijn DP, Pasterkamp G: Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: A prognostic study. Circulation, 2010; 121: 1941-1950 [DOI] [PubMed] [Google Scholar]
- 5). Kurosaki Y, Yoshida K, Endo H, Chin M, Yamagata S: Association between carotid atherosclerosis plaque with high signal intensity on t1-weighted imaging and subsequent ipsilateral ischemic events. Neurosurgery, 2011; 68: 62-67 [DOI] [PubMed] [Google Scholar]
- 6). Altaf N, Daniels L, Morgan PS, Auer D, MacSweeney ST, Moody AR, Gladman JR: Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg, 2008; 47: 337-342 [DOI] [PubMed] [Google Scholar]
- 7). Sun J, Song Y, Chen H, Kerwin WS, Hippe DS, Dong L, Chen M, Zhou C, Hatsukami TS, Yuan C: Adventitial perfusion and intraplaque hemorrhage: A dynamic contrast-enhanced mri study in the carotid artery. Stroke, 2013; 44: 1031-1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Zhao X, Underhill HR, Zhao Q, Cai J, Li F, Oikawa M, Dong L, Ota H, Hatsukami TS, Chu B, Yuan C: Discriminating carotid atherosclerotic lesion severity by luminal stenosis and plaque burden: A comparison utilizing high-resolution magnetic resonance imaging at 3.0 tesla. Stroke, 2011; 42: 347-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Xu X, Ju H, Cai J, Cai Y, Wang X, Wang Q: High-resolution mr study of the relationship between superficial calcification and the stability of carotid atherosclerotic plaque. Int J Cardiovasc imaging, 2010; 26 Suppl 1: 143-150 [DOI] [PubMed] [Google Scholar]
- 10). Lin R, Chen S, Liu G, Xue Y, Zhao X: Association between carotid atherosclerotic plaque calcification and intraplaque hemorrhage: A magnetic resonance imaging study. Arterioscler Thromb Vasc Biol, 2017; 37: 1228-1233 [DOI] [PubMed] [Google Scholar]
- 11). Cui Y, Qiao H, Ma L, Lu M, Yang J, Yao G, Cai J, Zhao X: Association of age and size of carotid artery intraplaque hemorrhage and minor fibrous cap disruption: A high resolution magnetic resonance imaging study. J Atheroscler Thromb, 2018; 25: 1222-1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Tuenter A, Selwaness M, Arias Lorza A, Schuurbiers JCH, Speelman L, Cibis M, van der Lugt A, de Bruijne M, van der Steen AFW, Franco OH, Vernooij MW, Wentzel JJ, High shear stress relates to intraplaque haemorrhage in asymptomatic carotid plaques. Atherosclerosis, 2016; 251: 348-354 [DOI] [PubMed] [Google Scholar]
- 13). Gao S, van ‘t Klooster R, van Wijk DF, Nederveen AJ, Lelieveldt BP, van der Geest RJ: Repeatability of in vivo quantification of atherosclerotic carotid artery plaque components by supervised multispectral classification. MAGMA, 2015; 28: 535-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Watase H, Sun J, Hippe DS, Balu N, Li F, Zhao X, Mani V, Fayad ZA, Fuster V, Hatsukami TS, Yuan C: Carotid artery remodeling is segment specific: An in vivo study by vessel wall magnetic resonance imaging. Arterioscler Thromb Vasc Biol, 2018; 38: 927-934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Chu B, Kampschulte A, Ferguson MS, Kerwin WS, Yarnykh VL, O'Brien KD, Polissar NL, Hatsukami TS, Yuan C: Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke, 2004; 35: 1079-1084 [DOI] [PubMed] [Google Scholar]
- 16). Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C: Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation, 2002; 106: 1368-1373 [DOI] [PubMed] [Google Scholar]
- 17). Kerwin W, Xu D, Liu F, Saam T, Underhill H, Takaya N, Chu B, Hatsukami T, Yuan C. Magnetic resonance imaging of carotid atherosclerosis: Plaque analysis. Topics in magnetic resonance imaging: Top Magn Reson Imaging, 2007; 18: 371-378 [DOI] [PubMed] [Google Scholar]
- 18). Li D, Zhao H, Chen X, Chen S, Qiao H, He L, Li R, Xu J, Yuan C, Zhao X: Identification of intraplaque haemorrhage in carotid artery by simultaneous non-contrast angiography and intraplaque haemorrhage (snap) imaging: A magnetic resonance vessel wall imaging study. Eur Radiol, 2018; 28: 1681-1686 [DOI] [PubMed] [Google Scholar]
- 19). Pucci A, Sheiban I, Formato L, Celeste A, Brscic E, Moretti C, De Bernardi A, Alberti A, Bergamasco L, Trevi G, Fuster V: In vivo coronary plaque histology in patients with stable and acute coronary syndromes: Relationships with hyperlipidemic status and statin treatment. Atherosclerosis, 2007; 194: 189-195 [DOI] [PubMed] [Google Scholar]
- 20). Derksen WJ, Peeters W, Tersteeg C, de Vries JP, de Kleijn DP, Moll FL, van der Wal AC, Pasterkamp G, Vink A: Age and coumarin-type anticoagulation are associated with the occurrence of intraplaque hemorrhage, while statins are associated less with intraplaque hemorrhage: A large histopathological study in carotid and femoral plaques. Atherosclerosis, 2011; 214: 139-143 [DOI] [PubMed] [Google Scholar]
- 21). Verhoeven BA, Moll FL, Koekkoek JA, van der Wal AC, de Kleijn DP, de Vries JP, Verheijen JH, Velema E, Busser E, Schoneveld A, Virmani R, Pasterkamp Pasterkamp: Statin treatment is not associated with consistent alterations in inflammatory status of carotid atherosclerotic plaques: A retrospective study in 378 patients undergoing carotid endarterectomy. Stroke, 2006; 37: 2054-2060 [DOI] [PubMed] [Google Scholar]
- 22). van den Bouwhuijsen QJ, Vernooij MW, Hofman A, Krestin GP, van der Lugt A, Witteman JC: Determinants of magnetic resonance imaging detected carotid plaque components: The rotterdam study. Eur Heart J, 2012; 33: 221-229 [DOI] [PubMed] [Google Scholar]
- 23). Huang X, Teng Z, Canton G, Ferguson M, Yuan C, Tang D: Intraplaque hemorrhage is associated with higher structural stresses in human atherosclerotic plaques: An in vivo mri-based 3d fluid-structure interaction study. Biomed Eng Online, 2010; 9: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Wang Y, Qiu J, Luo S, Xie X, Zheng Y, Zhang K, Ye Z, Liu W, Gregersen H, Wang G: High shear stress induces atherosclerotic vulnerable plaque formation through angiogenesis. Regen Biomater, 2016; 3: 257-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Jing LN, Gao PY, Lin Y, Sui BB, Qin HQ, Ma L, Xue J: Distribution of wall shear stress in carotid plaques using magnetic resonouce imaging and computational fluid dynamics analysis: a preliminary study. Chin Med J(Enql), 2011; 124: 1465-1469 [PubMed] [Google Scholar]
- 26). Liao YC, Lin HF, Rundek T, Cheng R, Guo YC, Sacco RL, Juo SH: Segment-specific genetic effects on carotid intima-media thickness: The northern manhattan study. Stroke, 2008; 39: 3159-3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Carvalho JL, Nielsen JF, Nayak KS: Feasibility of in vivo measurement of carotid wall shear rate using spiral fourier velocity encoded mri. Magn Reson Med, 2010; 63: 1537-1547 [DOI] [PubMed] [Google Scholar]
- 28). Espeland MA, Tang R, Terry JG, Davis DH, Mercuri M, Crouse JR: Associations of risk factors with segment-specific intimal-medial thickness of the extracranial carotid artery. Stroke, 1999; 30: 1047-1055 [DOI] [PubMed] [Google Scholar]
- 29). Mackinnon AD, Jerrard-Dunne P, Sitzer M, Buehler A, von Kegler S, Markus HS: Rates and determinants of sitespecific progression of carotid artery intima-media thickness: The carotid atherosclerosis progression study. Stroke, 2004; 35: 2150-2154 [DOI] [PubMed] [Google Scholar]
- 30). Takaya N, Yuan C, Chu B, Saam T, Polissar NL, Jarvik GP, Isaac C, McDonough J, Natiello C, Small R, Ferguson MS, Hatsukami TS: Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation, 2005; 111: 2768-2775 [DOI] [PubMed] [Google Scholar]
- 31). Sun J, Underhill HR, Hippe DS, Xue Y, Yuan C, Hatsukami TS: Sustained acceleration in carotid atherosclerotic plaque progression with intraplaque hemorrhage: a long-term time course study. JACC Cardiovasc Imaging, 2012; 5: 798-804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Zhou Z, Li R, Zhao X, He L, Wang X, Wang J, Balu N, Yuan C: Evaluation of 3d multi-contrast joint intra- and extracranial vessel wall cardiovascular magnetic resonance. J Cardiovasc Magn Reson, 2015; 17: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Narumi S, Sasaki M, Natori T, Yamaguchi Oura M, Ogasawara K, Kobayashi M, Sato Y, Ogasawara Y, Hitomi J, Terayama Y: Carotid plaque characterization using 3d t1-weighted mr imaging with histopathologic validation: A comparison with 2d technique. AJNR. Am J Neuroradiol, 2015; 36: 751-756 [DOI] [PMC free article] [PubMed] [Google Scholar]


