Abstract
Lipoprotein apheresis has been developed as the treatment for refractory familial hypercholesterolemia (FH) to remove low-density lipoprotein (LDL), which is the main pathogenic factor. Currently, three procedures are available in Japan, including the plasma exchange, double-membrane filtration, and selective LDL adsorption. Selective LDL adsorption, which was developed in Japan, has been one of the most common treatment methods in the world. Lipoprotein apheresis enabled the prevention of atherosclerosis progression even in homozygous FH (HoFH) patients. However, in our observational study, HoFH patients who started lipoprotein apheresis in adulthood had a poorer prognosis than those who started in childhood. Therefore, HoFH patients need to start lipoprotein apheresis as early as possible. Although the indication for lipoprotein apheresis in heterozygous FH (HeFH) patients has been decreasing with the advent of strong statins, our observational study showed that HeFH patients who discontinued lipoprotein apheresis had a poorer prognosis than patients who continued apheresis therapy. These results suggest that it is beneficial for very-high-risk HeFH patients to be treated by lipoprotein apheresis even if their LDL cholesterol is controlled well by lipid-lowering agents. Since launching a new class of lipid-lowering agents, proprotein convertase subtilisin/kexin type 9 (PCSK9) antibody and microsome triglyceride transfer protein inhibitors, the indication for lipoprotein apheresis in FH has been changing. However, despite the development of these drugs, lipoprotein apheresis is still an option with a high therapeutic effect for FH patients with severe atherosclerotic cardiovascular disease.
Keywords: Lipoprotein apheresis, Familial hypercholesterolemia, Atherosclerotic cardiovascular disease, PCSK9 inhibitor, MTP inhibitor
Introduction
Familial hypercholesterolemia (FH) is characterized by hypercholesterolemia, cutaneous and tendon xanthomas, and premature atherosclerotic cardiovascular disease1). FH is a genetic disorder caused by a mutation in the gene related to the low-density lipoprotein (LDL) receptor pathway, such as LDL receptor, proprotein convertase subtilisin/kexin type 9 (PCSK9), apolipoprotein B, and LDLRAP1 gene mutations2). Homozygous FH (HoFH) patients harbor homozygous mutations, compound heterozygous mutations, and double-heterozygous mutations of these genes, showing severe symptoms, such as severe hypercholesterolemia (600–1,000 mg/dL), cutaneous and tendon xanthomas, aortic valvular stenosis, and supra-aortic valvular stenosis from childhood1). Heterozygous FH (HeFH) patients show milder symptoms than HoFH patients.
The development of hydroxymethylglutaryl-CoA reductase inhibitors (statins) has enabled the effective treatment of HeFH3). Indeed, the average age at coronary artery disease (CAD) onset significantly increased after the widespread use of statins compared with that before October 1989, when statins were approved in Japan4). PCSK9 inhibitors were developed and have already been on the market, which made it possible to control low-density lipoprotein cholesterol (LDL-C) levels more intensively even in patients with severe forms of HeFH. However, HoFH patients are usually resistant to most lipid-lowering drugs, including statins, ezetimibe, resins, and PCSK9 inhibitors, conwhich require LDL receptor activity to reduce LDL-C levels5). Recently, an inhibitor of microsome triglyceride transfer protein (MTP) was developed and shown to reduce LDL-C levels in HoFH through an LDL receptor-independent pathway6, 7). However, lipoprotein apheresis is still one of the major strategies to control LDL-C levels in HoFH and in several other types of severe hypercholesterolemia, such as autosomal recessive hypercholesterolemia.
History of Lipoprotein Apheresis
In Japan, plasma exchange, selective LDL adsorption, and double-membrane filtration plasmapheresis (DFPP) are available, and selective LDL adsorption and DFPP are now widely used.
The first trial of lipoprotein apheresis in HoFH patients was performed as plasma exchange by de Gennes et al.8). This therapeutic approach led to the improvement of coronary artery stenosis and regression of xanthoma accompanied by LDL-C reduction. However, plasma exchange has the disadvantage that various important substances, such as immunoglobulins, are removed along with LDL. Therefore, at present, plasma exchange may be used for only HoFH patients aged <10 years who cannot be treated with selective LDL adsorption or DFPP due to their small capacity for extracorporeal circulation.
In the 1980s, columns with dextran sulfatecoated cellulose beads were proven to be a potent specific sorbent of apolipoprotein B-containing lipoproteins9). The early system of selective LDL adsorption (LA-40 system) had a large column (400 mL) with this sorbent, which had limited capacity to remove LDL. Currently, a selective LDL adsorption system (LA-15 system, Kaneka, Osaka, Japan) has two small columns (150 mL each) that are alternately reused during the procedure by eluting adsorbed LDL on a saturated column with 5% NaCl. This current system has made it possible to treat a larger volume of plasma and reduce cardiovascular burden during apheresis therapy. Therefore, this system can be available for patients with cardiac dysfunction or small body mass, and currently, the LA-15 system is widely used all over the world. On the other hand, the negative charges of the columns used in the LDL adsorption system were found to promote the production of bradykinin via activation of the coagulation system10). Because angiotensin-converting enzyme inhibitors (ACEIs) have inhibitory activity against the degradation of bradykinin, patients who are taking ACEIs should not be treated with this system.
The mechanism of DFPP is the selective removal of macromolecules based on molecular weight and fil ter pore size11). Initially, treatable plasma volume was limited in this procedure because of the increase in transmembrane pressure. However, improvement of the sieving properties has enabled us to treat larger plasma volumes. The disadvantage of this method was that in addition to LDL, the plasma concentrations of other proteins such as albumin and immunoglobulin, as well as the antiatherogenic high-density lipoprotein, were significantly decreased11). However, bradykinin levels are not elevated by DFPP, and this method can be used in patients taking ACEIs.
Mechanism of the Preventive Effect on Atherosclerosis
Selective LDL adsorption therapy can remove not only LDL, but also cell adhesion molecules such as intracellular adhesion molecule-1 and vascular cell adhesion molecule12, 13), coagulation factors such as fibrinogen and thrombosis factor, and plasminogen activator inhibitor-1 13). Selective LDL adsorption can also remove inflammatory cytokines, such as tumor necrosis factor- and interleukin-1 14), and reduce reactive oxygen species production via suppression of NADPH oxidase15). These factors play significant roles in the progression of atherosclerosis. Thus, LDL adsorption therapy can prevent atherosclerosis as a result of and independent of LDL removal. LDL adsorption therapy was also reported to improve vascular endothelial function via the induction of bradykinin and nitric oxide production16).
Lipoprotein apheresis also improves atherogenic lipoprotein metabolism in addition to direct removal of LDL. In patients with peripheral artery disease, oxidized LDL is removed by LDL adsorption, and the removal rate of oxidized LDL is associated with the improvement in walking distance17). We also found that small, dense LDL, which has strong atherogenic action, was efficiently removed by lipoprotein apheresis in FH patients18). Furthermore, we reported that lipoprotein apheresis could remove Apolipoprotein C III19), which is an endogenous lipoprotein lipase inhibitor that promotes atherosclerosis progression20). LDL adsorption therapy could also remove PCSK9 21, 22), which plays a pivotal role in lipid metabolism by enhancing endosomal and lysosomal degradation of the LDL receptor in the liver22, 23). In addition, PCSK9 is known to be involved in inflammatory processes by upregulating proinflammatory gene expression24). An elevated plasma concentration of lipoprotein (a) (Lp(a)) is an independent risk factor for cardiovascular disease. Lipoprotein apheresis is very efficient in decreasing Lp(a) concentrations, and when performed weekly or biweekly, the mean interval conwhich centrations are markedly decreased (approximately 25%–40% reduction)25). Thus, LDL adsorption has various antiatherosclerotic effects in addition to LDL removal (Fig. 1).
Fig. 1.
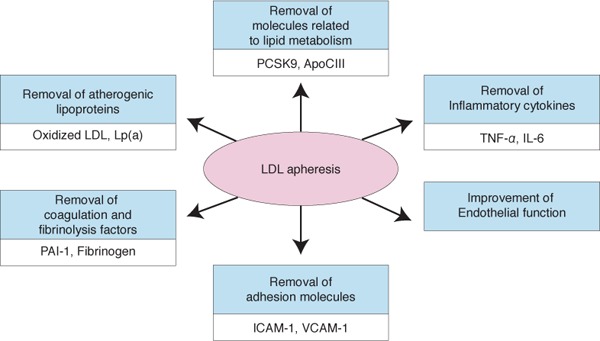
Schema of the pleiotropic effects of lipoprotein apheresis.
Clinical Effect of Lipoprotein Apheresis and Therapeutic Target LDL-C Levels in FH
Lipoprotein Apheresis in HoFH
In HoFH patients, a very high level of LDL-C from birth leads to atheromatous lesions of the arterial wall. As a result, HoFH patients have severe CAD and valvular disease, which may determine the prognosis of the disease26, 27). For the last several decades, lipoprotein apheresis has been the only practical method to control LDL-C levels in HoFH patients because statins have no or very limited LDL-C lowering effect in HoFH patients. Indeed, lipoprotein apheresis can prevent the progression of atherosclerosis even in HoFH patients. For example, a Lipoprotein Apheresis Atherosclerosis Regression Study (LAARS) showed that the regression of coronary artery stenosis was observed in four out of seven HoFH patients28). HoFH patients usually begin lipoprotein apheresis at approximately 10 years old. Before LDL adsorption therapy, these patients are treated with plasma exchange therapy. We investigated the long-term effect of lipoprotein apheresis in 18 patients with HoFH (observation period was 3–20 years.) (Table 1). Six patients started treatment with lipoprotein apheresis in childhood. Among these six patients, only one was free from atherosclerotic disease, and another was free from apparent atherosclerotic disease, although the atherosclerotic change in the carotid artery worsened after pregnancy. Three patients underwent aortic valve replacement after 18–35 years of lipoprotein apheresis. On the other hand, all 11 patients who started lipoprotein apheresis in adulthood developed a severe atherosclerotic disease. Two of these patients died from myocardial infarction (MI), one patient died from heart failure, and four patients underwent coronary artery bypass surgery before or at the initiation of lipoprotein apheresis. Græsdal A et al. also reported that patients who underwent lipoprotein apheresis before 10 years old revealed mild atherosclerotic changes, whereas two patients who started lipoprotein apheresis in adulthood already had CAD before initiation of lipoprotein apheresis29). Therefore, it is important for HoFH patients to be accurately diagnosed and to begin lipoprotein apheresis as early as possible.
Table 1. Clinical course of HoFH patients undergoing liporprotein apheresis treatment.
| Age at diagnosis (y.o.) | Age at the introduction of LDL apheresis (y.o.) | Cardiovascular finding | |
|---|---|---|---|
| 39F | 4 | 4 | Atherosclerosis in carotid artery progressed after pregnancy |
| 30F | 1 | 5 | No atherosclerotic finding |
| 43F | 1 | 6 | Angina pectoris occurred at 2 years, total occlusion was apparent in the left main trunk at 5 years, CABG was performed at 10 and 15 years, PCI was performed at 24 years, and aortic valve replacement and CABG were performed at 41 years |
| 33M | 0 | 6 | Deformation of the aortic valve at 5 years and aortic valve stenosis was slightly improved at 15 years, but aortic valve replacement was performed at 30 years |
| 44F | 3 | 9 | RCA total occlusion was apparent at 25 years |
| 47M | 8 | 12 | Deformation of the aortic valve at 9 years, aortic valve regurgitation was apparent at 19 years, and aortic valve replacement was performed at 30 years |
| 52M | 5 | 19 | CABG was performed at 40 years |
| 35F | 3 | 22 | CABG was performed for triple-vessel disease at 18 years |
| 49F | unknown | 22 | CABG was performed at 18 years. At 49 years, the patient died from heart failure |
| 31F | unknown | 25 | Aortic valve stenosis at 29 years and death from MI at 31 years |
| 29M | unknown | 27 | Patient died from MI at 29 years |
| 44M | 34 | 34 | Multiple coronary artery regions with 50% stenosis |
| 66M | 30 | 30 | Patient underwent 7 PCIs over 30 years |
| 45F | 36 | 36 | Patient died from peritonitis at 45 years |
| 54F | 3 | 47 | Patient had 50% stenosis in coronary arteries |
| 68F | 52 | 52 | CABG was performed at 57 years |
| 24M | 22 | 22 | CABG was performed at 22 years |
| 72F | 37 | 65 | CABG and aortic valve replacement were performed at 64 years |
CABG: coronary artery bypass graft surgery, PCI: percutaneous coronary intervention, RCA: right coronary artery, MI: myocardial infarction
However, many HoFH patients fail to be diagnosed accurately in their childhood before atherosclerosis has progressed. A-Hit 1 registry showed that only 33% of HoFH in Turkey were diagnosed before 7 years old30). There is no international guideline for targeting LDL-C levels in lipoprotein apheresis. The novel guideline of the Japanese Atherosclerosis Society recommends that the target LDL-C level is below 100 mg/dL in HoFH patients with primary prevention and 70 mg/dL in those with secondary prevention31) since this target has become achievable following the development of new lipid-lowering drugs such as PCSK9 inhibitors and MTP inhibitors. For pediatric FH patients, the target LDL-C level is below 140 mg/dL32). In Japan, lipoprotein apheresis in HoFH patients is covered by health insurance systems. HoFH has also been designated as a specified disease in the Specified Disease Treatment Research Program.
The recommendation of the HEART-UK Lipoprotein apheresis Working Group is as follows33).
-
1)
An acute reduction in total cholesterol of ≥ 65% or in LDL cholesterol of ≥ 70% on average during each procedure.
-
2)
An interval mean total cholesterol of <270 mg/dl or LDL cholesterol <252 mg/dl (or decreases of >60% or >65%, respectively, from baseline values).
-
3)
A baseline level of total cholesterol of <349 mg/dl or LDL cholesterol <329 mg/dl (or decreases of >50% or >55%, respectively, from baseline values).
April of 2019, The LIPOSORBER LA-15 System was approved by US Food and Drug Administration (FDA) and indicated for use in performing low density lipoprotein cholesterol (LDL-C) apheresis to acutely remove LDL-C from the plasma of the following high risk patient populations for whom diet has been ineffective and maximum drug therapy has either been ineffective or not tolerated:
Group A - Functional Hypercholesterolemic Homozygotes with LDL-C >500 mg/dL;
Group B - Functional Hypercholesterolemic Heterozygotes with LDL-C ≥ 300 mg/dL; and
Group C - Functional Hypercholesterolemic Heterozygotes with LDL-C ≥ 100 mg/dL and either documented coronary artery disease or documented peripheral artery disease.
Documented coronary artery disease (CAD) includes CAD diagnosed by invasive or computed tomography (CT) coronary angiography, or by electron beam (ultrafast) CT (EBCT), or documented by a history of myocardial infarction (MI), percutaneous coronary intervention (PCI), or coronary artery bypass graft (CABG) surgery.
Lipoprotein Apheresis in HeFH
The introduction of strong statins has improved the prognosis of HeFH patients worldwide. Indeed, we reported that the average age at CAD onset was significantly higher after the widespread use of statins than before October 1989, when statins were approved in Japan4). On the other hand, LDL-C levels in many of the HeFH patients cannot be decreased to the target level by a combination of ezetimibe and high-intensity statins. Lipoprotein apheresis used to be the only strategy to further reduce LDL-C levels in these patients. There is evidence of the usefulness of lipoprotein apheresis for the prevention of atherosclerosis progression in HeFH patients. The LAARS demonstrated that lipoprotein apheresis significantly prevented CAD progression compared with only medication34). The progression of CAD was well prevented by lipoprotein apheresis, especially in cases where LDL-C levels were reduced to <100 mg/dL after apheresis, although the mean LDL-C level before apheresis was 249 mg/dL and the mean LDL-C level after apheresis was 105 mg/dL in the LAARS34). Mabuchi et al. also reported that the incidence of CAD in HeFH patients who were undergoing lipoprotein apheresis was lower than that of those receiving only medication therapy in the Hokuriku-FHLipoprotein Apheresis Study35). Furthermore, lipoprotein apheresis could induce the regression of atherosclerotic plaques in coronary arteries of HeFH patients (LDL Apheresis Coronary Morphology and Reserve Trial)36). The Japan LDL Apheresis Coronary Atherosclerosis Prospective Study showed that the frequency of regression or attenuation of change in coronary artery stenosis was significantly higher in the apheresis group than in the control group among HeFH patients37).
As shown in Fig.2, in our observational study before the PCSK9 antibody was launched, HeFH patients who discontinued lipoprotein apheresis had a poorer prognosis than those who continued apheresis, although patients who discontinued lipoprotein apheresis had almost the same levels of LDL-C as patients who continued lipoprotein apheresis38). These reports indicate that HeFH patients with severe atherosclerotic disease experience a beneficial effect from lipoprotein apheresis for the prevention of atherosclerotic disease. Thompson GR et al. recommended that patients with HeFH for whom there is objective evidence of continuing the significant progression of coronary disease and whose LDL cholesterol remains > 193 mg/dL or decreases by <40% despite combination drug therapy should be considered for lipoprotein apheresis33). The International Panel on Management of FH (2004) indicated that suggested efficacy targets for apheresis in HeFH patients are an interval mean LDL-C of ≤ 100 mg/dL or a reduction of ≥ 60% from baseline values39). The Japanese Atherosclerosis Society recommends a target LDL-C level in HeFH patients of below 100 mg/dL or a reduction of ≥ 50% in patients with primary prevention and of 70 mg/dL in patients with secondary prevention31).
Fig. 2.
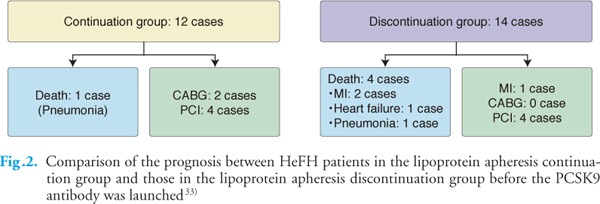
Comparison of the prognosis between HeFH patients in the lipoprotein apheresis continuation group and those in the lipoprotein apheresis discontinuation group before the PCSK9 antibody was launched33)
Pregnancy and Lipoprotein Apheresis in FH
There are increased stresses on the cardiovascular system throughout pregnancy and delivery. Both blood volume and cardiac output increase by 25% to 80%40). The main point of concern has been the aggravation of coronary insufficiency in the mother mainly due to these hemodynamic changes in pregnancy41). High lipid levels may also affect placental vasculature and cause the retardation of fetal growth42). Furthermore, statin therapy is contraindicated during pregnancy. Therefore, during pregnancy, HoFH and HeFH patients with CAD should be treated by lipoprotein apheresis to prevent further progression of coronary atherosclerosis and to better tolerate the increased stress of delivery. In fact, our recent report of seven pregnant HoFH patients showed that one patient who refused lipoprotein apheresis during pregnancy and another patient whose adherence to lipoprotein apheresis was poor died from acute MI43). The other five patients who underwent lipoprotein apheresis delivered successfully. Therefore, the Japanese Atherosclerosis Society recommends lipoprotein apheresis for HoFH and HeFH patients with CAD during the course of pregnancy to control their LDL-C level not only for their own sake but also for normal fetal growth. In any case, pregnancy and delivery in women with CAD are hazardous and should be monitored very closely. Furthermore, we should pay attention to the symptoms of bradykinin overproduction, such as nausea, bradycardia, and hypotension43).
Novel Lipid-Lowering Drugs and Lipoprotein Apheresis
Recently, PCSK9 antibodies (evolocumab and alirocumab) have become commercially available. A FOURIER trial demonstrated that LDL-C reduction by evolocumab significantly decreased the incidence of cardiovascular events44). Another clinical trial also demonstrated that an approximately 60% reduction of LDL-C was achieved by evolocumab in HeFH patients45). The ODDESEY ESCAPE study showed that 63.4% of HeFH patients undergoing lipoprotein apheresis could discontinue lipoprotein apheresis following alirocumab treatment46). Therefore, the number of FH patients treated by lipoprotein apheresis is decreasing since the LDL-C levels in most HeFH patients can be controlled well by the application of the PCSK9 antibody. Kawashiri et al. also reported that biweekly evolocumab treatment was superior to biweekly lipoprotein apheresis in terms of LDL-Clowering effects, even in HeFH patients47). However, it is still unclear whether lipoprotein apheresis withdrawal due to sufficient LDL reduction by PCSK9 antibody increases the risk of cardiovascular events in HeFH patients with severe atherosclerosis. The PCSK9 antibody can partly decrease LDL-C levels even in HoFH patients. The TESLA part B trial showed that evolocumab achieved an approximately 30% reduction of LDL-C in 33 HoFH patients48). The TAUSSIG study, which had 106 HoFH patients, including 34 patients undergoing lipoprotein apheresis, showed that evolocumab decreased LDL-C levels by 20.6%5). However, the PCSK9 antibody had no LDL-C-lowering effect in LDL receptor-negative HoFH patients, although the treatment has a partial effect in HoFH patients with LDL receptor defects49). On the other hand, as shown in Fig.3, in patients with double-heterozygous LDLR/PCSK9 gain-offunction mutations, LDL-C can be controlled well by the application of PCSK9 antibody, and lipoprotein apheresis could be discontinued.
Fig. 3.
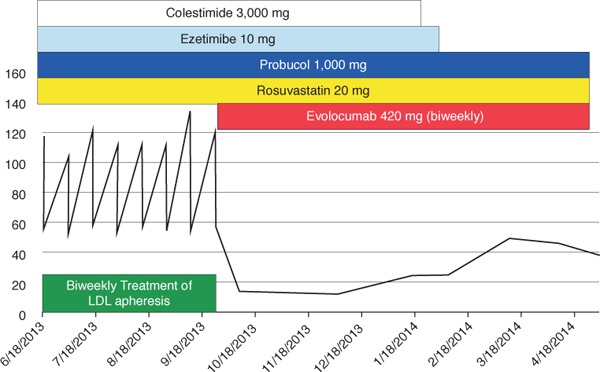
Changes in LDL-C levels in a double-heterozygous FH patient after application of the PCSK9 antibody
She was diagnosed with FH at 37 years. CABG and aortic valve replacement were performed at 64 years.
Recently, MTP inhibitor, which inhibits very low density lipoprotein (VLDL) synthesis in the liver and chylomicron in the intestine50), has also become commercially available. MTP inhibitor can reduce LDL-C even in HoFH patients6, 7). In a Japanese study, three of six patients (50%) who underwent apheresis were able to increase the interval between apheresis treatments7). However, because HoFH patients usually have severe atherosclerosis, they need to continue lipoprotein apheresis to not only control LDL-C levels but also reduce molecules responsible for the development of atherosclerosis. MTP inhibitor may cause diarrhea and/or liver dysfunction, the frequency of which is increased by higher doses. It is important to advise patients to reduce lipid intake to avoid side effects of MTP inhibitor, such as diarrhea.
Thus, lower target LDL-C levels can be achieved in HeFH and HoFH patients than before because of the launch of the new classes of lipid-lowering drugs. Further development of new drugs, including siRNA targeting PCSK951) and antibodies against AGPTL3 52), may help further prevent the development of atherosclerosis in HeFH and HoFH patients in the future.
Conclusion
Lipoprotein apheresis has various preventive effects on atherosclerosis progression in addition to strong LDL-C reduction. In the past, lipoprotein apheresis was the only way to prevent cardiovascular events in patients with severe FH. Now, with the development of new lipid-lowering drugs, LDL-C levels can be controlled much better than before. We need to decide whether to discontinue or reduce the frequency of Lipoprotein apheresis carefully, particularly in severe FH patients.
Acknowledgments and Notice of Grant Support
This research was supported by Health, Labor and Welfare Sciences Research Grant for Research on Rare and Intractable Diseases.
Conflict of Interest
Mariko Harada-Shiba received honoraria from Sanofi, Astellas, Astellas Amgen, research grant from Aegerion and Kaneka.
Hisashi Makino received honoraria from Sanofi.
Masatsune Ogura received honoraria from Sanofi, Astellas and Astellas Amgen.
References
- 1). Goldstein JL, Brown MS: The metabolic basis of inherited disease. MacGraw-Hill, New York, NY, 1982 [Google Scholar]
- 2). Santos RD, Gidding SS, Hegele RA, Cuchel MA, Barter PJ, Watts GF, Baum SJ, Catapano AL, Chapman MJ, Defesche JC, Folco E, Freiberger T, Genest J, Hovingh GK, Harada-Shiba M, Humphries SE, Jackson AS, Mata P, Moriarty PM, Raal FJ, Al-Rasadi K, Ray KK, Reiner Z, Sijbrands EJ, Yamashita S: Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International atherosclerosis society severe familial hypercholesterolemia panel. Lancet Diabetes Endocrinol, 2016; 4: 850-861 [DOI] [PubMed] [Google Scholar]
- 3). Illingworth DR: How effective is drug therapy in heterozygous familial hypercholesterolemia?. Am J Cardiol, 1993; 72: 54d-58d [DOI] [PubMed] [Google Scholar]
- 4). Harada-Shiba M, Sugisawa T, Makino H, Abe M, Tsushima M, Yoshimasa Y, Yamashita T, Miyamoto Y, Yamamoto A, Tomoike H, Yokoyama S: Impact of statin treatment on the clinical fate of heterozygous familial hypercholesterolemia. J Atheroscler Thromb, 2010; 17: 667-674 [DOI] [PubMed] [Google Scholar]
- 5). Raal FJ, Hovingh GK, Blom D, Santos RD, Harada-Shiba M, Bruckert E, Couture P, Soran H, Watts GF, Kurtz C, Honarpour N, Tang L, Kasichayanula S, Wasserman SM, Stein EA: Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolaemia: an interim subset analysis of the open-label TAUSSIG study. Lancet Diabetes Endocrinol, 2017; 5: 280-290 [DOI] [PubMed] [Google Scholar]
- 6). Cuchel M, Meagher EA, Theron HD, Blom DJ, Marais AD, Hegele RA, Averna MR, Sirtori CR, Shah PK, Gaudet D, Stefanutti C, Vigna GB, Du Plessis AM, Propert KJ, Sasiela WJ, Bloedon LT, Rader DJ: Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet, 2013; 381: 40-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Harada-Shiba M, Ikewaki K, Nohara A, Otsubo Y, Yanagi K, Yoshida M, Chang Q, Foulds P: Efficacy and safety of lomitapide in Japanese patients with homozygous familial hypercholesterolemia. J Atheroscler Thromb, 2017; 24: 402-411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). de Gennes JL, Touraine R, Maunand B, Truffert J, Laudat P: Homozygous cutaneo-tendinous forms of hypercholesteremic xanthomatosis in an exemplary familial case. Trial of plasmapheresis ans heroic treatment. Bull Mem Soc Med Hop Paris, 1967; 118: 1377-1402 [PubMed] [Google Scholar]
- 9). Yokoyama S, Hayashi R, Kikkawa T, Tani N, Takada S, Hatanaka K, Yamamoto A: Specific sorbent of apolipoprotein B-containing lipoproteins for plasmapheresis. Characterization and experimental use in hypercholesterolemic rabbits. Arteriosclerosis, 1984; 4: 276-282 [DOI] [PubMed] [Google Scholar]
- 10). Krieter DH, Steinke J, Kerkhoff M, Fink E, Lemke HD, Zingler C, Muller GA, Schuff-Werner P: Contact activation in low-density lipoprotein apheresis systems. Artif Organs, 2005; 29: 47-52 [DOI] [PubMed] [Google Scholar]
- 11). Geiss HC, Parhofer KG, Donner MG, Schwandt P: Low density lipoprotein apheresis by membrane differential filtration (cascade filtration). Ther Apher, 1999; 3: 199-202 [DOI] [PubMed] [Google Scholar]
- 12). Sampietro T, Tuoni M, Ferdeghini M, Ciardi A, Marraccini P, Prontera C, Sassi G, Taddei M, Bionda A, Plasma cholesterol regulates soluble cell adhesion molecule expression in familial hypercholesterolemia. Circulation, 1997; 96: 1381-1385 [DOI] [PubMed] [Google Scholar]
- 13). Utsumi K, Kawabe M, Hirama A, Ueda K, Kamada Y, Arii K, Komaba Y, Katsura K, Iino Y, Katayama Y: Effects of selective LDL apheresis on plasma concentrations of ICAM-1, VCAM-1 and P-selectin in diabetic patients with arteriosclerosis obliterans and receiving maintenance hemodialysis. Clin Chim Acta, 2007; 377: 198-200 [DOI] [PubMed] [Google Scholar]
- 14). Hovland A, Hardersen R, Nielsen EW, Mollnes TE, Lappegard KT: Hematologic and hemostatic changes induced by different columns during LDL apheresis. J Clin Apher, 2010; 25: 294-300 [DOI] [PubMed] [Google Scholar]
- 15). Hara T, Kiyomoto H, Hitomi H, Moriwaki K, Ihara G, Kaifu K, Fujita Y, Higashiyama C, Nishiyama A, Kohno M: Low-density lipoprotein apheresis for haemodialysis patients with peripheral arterial disease reduces reactive oxygen species production via suppression of NADPH oxidase gene expression in leucocytes. Nephrol Dial Transplant, 2009; 24: 3818-3825 [DOI] [PubMed] [Google Scholar]
- 16). Tamai O, Matsuoka H, Itabe H, Wada Y, Kohno K, Imaizumi T: Single LDL apheresis improves endotheliumdependent vasodilatation in hypercholesterolemic humans. Circulation, 1997; 95: 76-82 [DOI] [PubMed] [Google Scholar]
- 17). Tamura K, Tsurumi-Ikeya Y, Wakui H, Maeda A, Ohsawa M, Azushima K, Kanaoka T, Uneda K, Haku S, Azuma K, Mitsuhashi H, Tamura N, Toya Y, Tokita Y, Kokuho T, Umemura S: Therapeutic potential of low-density lipoprotein apheresis in the management of peripheral artery disease in patients with chronic kidney disease. Ther Apher Dial, 2013; 17: 185-192 [DOI] [PubMed] [Google Scholar]
- 18). Makino H, Tamanaha T, Harada-Shiba M: LDL apheresis in Japan. Transfus Apher Sci, 2017; 56: 677-681 [DOI] [PubMed] [Google Scholar]
- 19). Yuasa Y, Osaki T, Makino H, Iwamoto N, Kishimoto I, Usami M, Minamino N, Harada-Shiba M: Proteomic analysis of proteins eliminated by low-density lipoprotein apheresis. Ther Apher Dial, 2014; 18: 93-102 [DOI] [PubMed] [Google Scholar]
- 20). Sathiyakumar V, Kapoor K, Jones SR, Banach M, Martin SS, Toth PP: Novel therapeutic targets for managing dyslipidemia. Trends Pharmacol Sci, 2018; 39: 733-747 [DOI] [PubMed] [Google Scholar]
- 21). Tavori H, Giunzioni I, Linton MF, Fazio S: Loss of plasma proprotein convertase subtilisin/kexin 9 (PCSK9) after lipoprotein apheresis. Circ Res, 2013; 113: 1290-1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Hori M, Ishihara M, Yuasa Y, Makino H, Yanagi K, Tamanaha T, Kishimoto I, Kujiraoka T, Hattori H, Harada-Shiba M: Removal of plasma mature and furincleaved proprotein convertase subtilisin/kexin 9 by low-density lipoprotein-apheresis in familial hypercholesterolemia: development and application of a new assay for PCSK9. J Clin Endocrinol Metab, 2015; 100: E41-E49 [DOI] [PubMed] [Google Scholar]
- 23). Lambert G, Charlton F, Rye KA, Piper DE: Molecular basis of PCSK9 function. Atherosclerosis, 2009; 203: 1-7 [DOI] [PubMed] [Google Scholar]
- 24). Tang Z, Jiang L, Peng J, Ren Z, Wei D, Wu C, Pan L, Jiang Z, Liu L: PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-kappaB activation in THP-1-derived macrophages. Int J Mol Med, 2012; 30: 931-938 [DOI] [PubMed] [Google Scholar]
- 25). Waldmann E, Parhofer KG: Lipoprotein apheresis to treat elevated lipoprotein (a). J Lipid Res, 2016; 57: 1751-1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Yamamoto A, Kamiya T, Yamamura T, Yokoyama S, Horiguchi Y, Funahashi T, Kawaguchi A, Miyake Y, Beppu S, Ishikawa K, Matsuzawa Y, Takaichi S: Clinical features of familial hypercholesterolemia. Arteriosclerosis, 1989; 9: I66-I74 [PubMed] [Google Scholar]
- 27). Beppu S, Minura Y, Sakakibara H, Nagata S, Park YD, Nambu S, Yamamoto A: Supravalvular aortic stenosis and coronary ostial stenosis in familial hypercholesterolemia: two-dimensional echocardiographic assessment. Circulation, 1983; 67: 878-884 [DOI] [PubMed] [Google Scholar]
- 28). Tatami R, Inoue N, Itoh H, Kishino B, Koga N, Nakashima Y, Nishide T, Okamura K, Saito Y, Teramoto T, Yasugi T, Yamamoto A, Goto Y, LARS Investigators : Regression of coronary atherosclerosis by combined LDL-apheresis and lipid-lowering drug therapy in patients with familial hypercholesterolemia: a multicenter study. The LARS Investigators. Atherosclerosis, 1992; 95: 1-13 [DOI] [PubMed] [Google Scholar]
- 29). Graesdal A, Bogsrud MP, Holven KB, Nenseter MS, Narverud I, Langslet G, Brekke M, Retterstol K, Arnesen KE, Ose L: Apheresis in homozygous familial hypercholesterolemia: the results of a follow-up of all Norwegian patients with homozygous familial hypercholesterolemia. J Clin Lipidol, 2012; 6: 331-339 [DOI] [PubMed] [Google Scholar]
- 30). Kayikcioglu M, Tokgozoglu L, Yilmaz M, Kaynar L, Aktan M, Durmus RB, Gokce C, Temizhan A, Ozcebe OI, Akyol TK, Okutan H, Sag S, Gul OO, Salcioglu Z, Yenercag M, Altunkeser BB, Kuku I, Yasar HY, Kurtoglu E, Kose MD, Demircioglu S, Pekkolay Z, Ilhan O: A nation-wide survey of patients with homozygous familial hypercholesterolemia phenotype undergoing LDL-apheresis in Turkey (A-HIT 1 registry). Atherosclerosis, 2018; 270: 42-48 [DOI] [PubMed] [Google Scholar]
- 31). Harada-Shiba M, Arai H, Ishigaki Y, Ishibashi S, Okamura T, Ogura M, Dobashi K, Nohara A, Bujo H, Miyauchi K, Yamashita S, Yokote K: Guidelines for diagnosis and treatment of familial hypercholesterolemia 2017. J Atheroscler Thromb, 2018; 25: 751-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Harada-Shiba M, Ohta T, Ohtake A, Ogura M, Dobashi K, Nohara A, Yamashita S, Yokote K, Joint Working Group by Japan Pediatric Society and Japan Atherosclerosis Society for Making Guidance of Pediatric Familial Hypercholesterolemia : Guidance for pediatric familial hypercholesterolemia 2017. J Atheroscler Thromb, 2018; 25: 539-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Thompson GR, Barbir M, Davies D, Dobral P, Gesinde M, Livingston M, Mandry P, Marais AD, Matthews S, Neuwirth C, Pottle A, le Roux C, Scullard D, Tyler C, Watkins S: Efficacy criteria and cholesterol targets for LDL apheresis. Atherosclerosis, 2010; 208: 317-321 [DOI] [PubMed] [Google Scholar]
- 34). Kroon AA, Aengevaeren WR, van der Werf T, Uijen GJ, Reiber JH, Bruschke AV, Stalenhoef AF: LDL-apheresis atherosclerosis regression study (LAARS). Effect of aggressive versus conventional lipid lowering treatment on coronary atherosclerosis. Circulation, 1996; 93: 1826-1835 [DOI] [PubMed] [Google Scholar]
- 35). Mabuchi H, Koizumi J, Shimizu M, Kajinami K, Miyamoto S, Ueda K, Takegoshi T: Long-term efficacy of low-density lipoprotein apheresis on coronary heart disease in familial hypercholesterolemia. Hokuriku-FHLDL-apheresis study group. Am J Cardiol, 1998; 82: 1489-1495 [DOI] [PubMed] [Google Scholar]
- 36). Matsuzaki M, Hiramori K, Imaizumi T, Kitabatake A, Hishida H, Nomura M, Fujii T, Sakuma I, Fukami K, Honda T, Ogawa H, Yamagishi M: Intravascular ultrasound evaluation of coronary plaque regression by low density lipoprotein-apheresis in familial hypercholesterolemia: the low density lipoprotein-apheresis coronary morphology and reserve trial (LACMART). J Am Coll Cardiol, 2002; 40: 220-227 [DOI] [PubMed] [Google Scholar]
- 37). Nishimura S, Sekiguchi M, Kano T, Ishiwata S, Nagasaki F, Nishide T, Okimoto T, Kutsumi Y, Kuwabara Y, Takatsu F, Nishikawa H, Daida H, Yamaguchi H: Effects of intensive lipid lowering by low-density lipoprotein apheresis on regression of coronary atherosclerosis in patients with familial hypercholesterolemia: Japan low-density lipoprotein apheresis coronary atherosclerosis prospective study (L-CAPS). Atherosclerosis, 1999; 144: 409-417 [DOI] [PubMed] [Google Scholar]
- 38). Makino H, Tamanaha T, Harada-Shiba M: LDL adsorption. Hemoperfusion, plasmaperfusion and other clinical uses of general, biospecific, immuno and leucocyte adsorbents, pp385-397, World Scientific, Singapore, 2015 [Google Scholar]
- 39). Civeira F, International Panel on Management of Familial Hypercholesterolemia : Guidelines for the diagnosis and management of heterozygous familial hypercholesterolemia. Atherosclerosis, 2004; 173: 55-68 [DOI] [PubMed] [Google Scholar]
- 40). Hankins G, Wendel JG, Leveno KJ, Stoneham J: Myocardial infarction during pregnancy: a review. Obstet Gynecol, 1985; 65: 139-146 [PubMed] [Google Scholar]
- 41). Barss V, Phillippe M, Greene MF, Covell L: Pregnancy complicated by homozygous hypercholesterolemia. Obstet Gynecol, 1985; 65: 756-757 [PubMed] [Google Scholar]
- 42). Beigel Y, Hod M, Fuchs J, Lurie J, Friedman S, Green P, Merlob P, Melamed R, Ovadia J: Pregnancy in a homozygous familial hypercholesterolemic patient treated with long-term plasma exchange. Am J Obstet Gynecol, 1990; 162: 77-78 [DOI] [PubMed] [Google Scholar]
- 43). Ogura M, Makino H, Kamiya C, Yoshimatsu J, Soran H, Eatough R, Perrone G, Harada-Shiba M, Stefanutti C: Lipoprotein apheresis is essential for managing pregnancies in patients with homozygous familial hypercholesterolemia: seven case series and discussion. Atherosclerosis, 2016; 254: 179-183 [DOI] [PubMed] [Google Scholar]
- 44). Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR: Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med, 2017; 376: 1713-1722 [DOI] [PubMed] [Google Scholar]
- 45). Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, Hovingh GK, Cariou B, Gouni-Berthold I, Somaratne R, Bridges I, Scott R, Wasserman SM, Gaudet D, RUTHERFORD-2 Investigators : PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, doubleblind, placebo-controlled trial. Lancet, 2015; 385: 331-340 [DOI] [PubMed] [Google Scholar]
- 46). Moriarty PM, Parhofer KG, Babirak SP, Cornier MA, Duell PB, Hohenstein B, Leebmann J, Ramlow W, Schettler V, Simha V, Steinhagen-Thiessen E, Thompson PD, Vogt A, von Stritzky B, Du Y, Manvelian G: Alirocumab in patients with heterozygous familial hypercholesterolaemia undergoing lipoprotein apheresis: the ODYSSEY ESCAPE trial. Eur Heart J, 2016; 37: 3588-3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Kawashiri MA, Nohara A, Higashikata T, Tada H, Nakanishi C, Okada H, Konno T, Sakata K, Hayashi K, Inazu A, Mabuchi H, Yamagishi M: Impact of evolocumab treatment on low-density lipoprotein cholesterol levels in heterozygous familial hypercholesterolemic patients withdrawing from regular apheresis. Atherosclerosis, 2017; 265: 225-230 [DOI] [PubMed] [Google Scholar]
- 48). Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, Wasserman SM, Stein EA, TESLA Investigators : Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet, 2015; 385: 341-350 [DOI] [PubMed] [Google Scholar]
- 49). Stein EA, Honarpour N, Wasserman SM, Xu F, Scott R, Raal FJ: Effect of the proprotein convertase subtilisin/ kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation, 2013; 128: 2113-2120 [DOI] [PubMed] [Google Scholar]
- 50). Berriot-Varoqueaux N, Aggerbeck LP, Samson-Bouma M, Wetterau JR: The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu Rev Nutr, 2000; 20: 663-697 [DOI] [PubMed] [Google Scholar]
- 51). Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, Hall T, Troquay RP, Turner T, Visseren FL, Wijngaard P, Wright RS, Kastelein JJ: Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med, 2017; 376: 1430-1440 [DOI] [PubMed] [Google Scholar]
- 52). Gaudet D, Gipe DA, Pordy R, Ahmad Z, Cuchel M, Shah PK, Chyu KY, Sasiela WJ, Chan KC, Brisson D, Khoury E, Banerjee P, Gusarova V, Gromada J, Stahl N, Yancopoulos GD, Hovingh GK: ANGPTL3 inhibition in homozygous familial hypercholesterolemia. N Engl J Med, 2017; 377: 296-297 [DOI] [PubMed] [Google Scholar]


