Abstract
Background
Pain due to pancreatic cancer/PCa or chronic pancreatitis/CP, is notoriously resistant to the strongest pain medications. Here, we aimed at deciphering the specific molecular mediators of pain at surgical-stage pancreatic disease and to discover novel translational targets.
Methods
We performed a systematic, quantitative analysis of the neurotransmitter/neuroenzmye profile within intrapancreatic nerves of CP and PCa patients. Ex vivo neuronal cultures treated with human pancreatic extracts, conditional genetically engineered knockout mouse models of PCa and CP, and the cerulein-induced CP model were employed to explore the therapeutic potential of the identified targets.
Findings
We identified a unique enrichment of neuronal nitric-oxide-synthase (nNOS) in the pancreatic nerves of CP patients with increasing pain severity. Employment of ex vivo neuronal cultures treated with pancreatic tissue extracts of CP patients, and brain-derived-neurotrophic-factor-deficient (BDNF+/−) mice revealed neuronal enrichment of nNOS to be a consequence of BDNF loss in the progressively destroyed pancreatic tissue. Mechanistically, nNOS upregulation in sensory neurons was induced by tryptase secreted from perineural mast cells. In a head-to-head comparison of several genetically induced, painless mouse models of PCa (KPC, KC mice) or CP (Ptf1a-Cre;Atg5fl/fl) against the hypersecretion/cerulein-induced, painful CP mouse model, we show that a similar nNOS enrichment is present in the painful cerulein-CP model, but absent in painless genetic models. Consequently, mice afflicted with painful cerulein-induced CP could be significantly relieved upon treatment with the specific nNOS inhibitor NPLA.
Interpretation
We propose nNOS inhibition as a novel strategy to treat the unbearable pain in CP.
Fund
Deutsche Forschungsgemeinschaft/DFG (DE2428/3-1 and 3-2).
Keywords: Pain, Neurology of the digestive tract, nNOS, Nitrergic, Chronic pancreatitis, Pancreatic cancer, Genetically engineered mice, Atg5
Research in context.
Evidence before this study
Treating pain due to pancreatic cancer (PCa) chronic pancreatitis (CP) is one of the leading challenges in clinical gastroenterology. The majority of these patients develop pain recurrence or opiate-dependence despite endoscopic or surgical interventions. As such, novel analgesic regimens for treating pancreatic pain are urgently needed.
Added value of this study
Here, we identified an enrichment of neuronal-nitric-oxide-synthase (nNOS) in the nerves of patients with CP, which was in contrast with PCa patients. Mechanistically, nNOS enrichment in CP tissues was found to be linked to loss of BDNF and to mast cell tryptase activity in the pancreas. By employing several genetically induced, painless mouse models of PCa against genetic or hypersecretion-induced, painful CP mouse models, we show that a similar nNOS enrichment is present specifically in the painful cerulein-CP model, but absent in painless genetic models. Accordingly, mice afflicted with painful, cerulein-induced CP could be significantly relieved upon treatment with the specific nNOS inhibitor NPLA.
Implications of all the available evidence
As there are no molecularly targeted therapies against the severe pain in pancreatic diseases, we demonstrate that nNOS inhibition represents a potential novel strategy for treating the unbearable pain due to human CP.
Alt-text: Unlabelled Box
1. Introduction
Pain is currently the biggest health-related socio-economic burden in the western world. In fact, the annual cost of pain treatment in the United States was reported to be approximately 600 billion US$, surpassing any medical condition [1]. In the United Kingdom, pain alone due to pancreatic diseases causes costs around 638 million US$, which is probably an underestimation [2]. Pain can turn into torture for patients, if it becomes chronic, and even more, as the most potent painkillers, such as opiates, gradually and totally lose their efficacy [3]. Pancreatic pain, typically encountered in chronic pancreatitis (CP) and pancreatic cancer (PCa), is not only chronic and opiate-resistant, but also ruins lives by causing severe loss of appetite, weight loss, disability, mental decline, and unemployment [4]. Resistance of pancreatic pain to medical and surgical treatments is the cause of a huge physical, psychological, and socio-economic problem.
The neuropathic nature of pain in CP and PCa, which both inflict major damage on pancreatic nerves, is a novel discovery [5]. This neural damage in the pancreas induces an uncontrolled neuro-excitatory program, chronic central and peripheral neuro-sensitization, and pain memory [[5], [6], [7]]. In parallel, over-sensitized damaged neurons and their fibers undergo remodeling and uncontrolled regeneration, and this hyper-innervation, in a vicious cycle, in turn augments inflammation and cancer progression [[8], [9], [10]]. For the development of novel and effective analgesic therapies, it is mandatory to decipher the signaling events between the diseased pancreas and the nervous system. So far, however, the neuropeptides and neurotransmitters that are specifically involved in pain transmission between the diseased pancreas and the nervous system have not been systematically analyzed.
In the present study, we quantified the amounts of the four major nociceptive neuropeptides/neuroenzymes in mammalians [8] including substance P (SP), calcitonin-gene-related-peptide (CGRP), vasoactive intestinal peptide (VIP), and neuronal nitric oxide synthase (nNOS), in all nerves of human surgical pancreas specimens derived from resected CP and PCa patients. We revealed a specific increase in the amount of nNOS in intra-pancreatic nerves of CP patients correlating with pain severity. In ex vivo neuronal cultures incubated with human pancreatic tissue extracts, and in genetically modified mice, we show that nNOS levels in pancreatic nerves are controlled by the microenvironmental levels of brain-derived-neurotrophic factor (BDNF). Accordingly, in mouse models of CP with neuropathy similar to human disease, administration of a specific inhibitor of nNOS ameliorated the abdominal, pain-associated mechano-sensitivity. Mechanistically, nNOS upregulation in the sensory neurons was induced by a protease, i.e. the mast cell tryptase that is secreted from perineural mast cells in CP tissues. Thus, we propose nNOS inhibition as a novel strategy for treating pancreatic pain.
2. Materials & methods
2.1. Patients, tissues, and pain degree
Human chronic pancreatitis (CP) and pancreatic cancer (PCa) surgical tissue specimens were collected from the pancreatic head of patients after informed consent and following tumor resection. Patient characteristics are depicted in Table 1a. Specifically, the main indication for surgery among the CP patients was intractable pain that was not amenable to medical or endoscopic therapy, or suspected malignancy (the final histology confirmed CP). All patients had an inflammatory mass in the pancreas that necessitated a resection (rather than draining) type of surgery. For all PCa patients, the indication for surgery was high suspicion for malignancy, even in the absence of a positive preoperative biopsy. All patients underwent primary resection and none received neoadjuvant therapy. The indication for surgery was decided after interdisciplinary discussion of the cases.
Table 1a.
Characteristics of the included patients.
| Number of patients (n) | Gender |
Etiology of CP | Median age (yrs) | Differentiation |
UICC grade |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Moderate | Poor | IIa | II b | III | ||||
| NP | 10 | 6 | 4 | 40 | ||||||
| CP | 23 | 18 | 5 | Alcoholic: 10 Biliary: 2 Idiopathic: 7 Autoimmune: 2 Other:2 |
46 | |||||
| PCa | 19 | 12 | 7 | 62 | 14 | 5 | 3 | 15 | 1 | |
NP: normal pancreas, CP: chronic pancreatitis, PDAC: pancreatic ductal adenocarcinoma, UICC: union internationale contre cancer, yrs: years.
The resected pancreatic tissue samples were divided into parts, which were immediately fixed in 4% paraformaldehyde followed by paraffin-embedding, as described previously [11]. The pain scoring was performed as reported and standardized previously [[11], [12], [13], [14]]. Briefly, the pain score (pain intensity and frequency) was prospectively registered prior to surgery. Pain intensity was scored by using the following scale: 0/none, 1/mild, 2/moderate and 3/strong pain. Pain frequency was scored as 3/daily, 2/weekly and 1/monthly. To calculate the degree of pain, points of pain intensity were multiplied with pain frequency of each individual, resulting in three groups: Pain 0 (0 point) patients without pain, Pain I (1–3 points) patients with mild pain, and Pain II (4–9 points) patients with moderate to severe pain [12,13].
2.2. Immunohistochemistry (IHC) & quantitative assessment of neuro-immunoreactivity in human and mouse specimens
Consecutive 3 μm sections from paraffin-embedded normal pancreas/NP, CP and PCa samples or mouse tissues were analyzed for the immunoreactivity of each nerve for SP, CGRP, VIP, and nNOS. One additional section was stained against the pan-neural marker protein-gene-product 9.5 (PGP9.5) to detect all nerves and to facilitate their re-identification on the consecutive sections.
The degree of pancreatic neuritis and neural invasion on each nerve was classified as no neuritis/score 0, peri-neuritis/I and endo-neuritis/II, or as no invasion/0, perineural invasion/I or endoneural invasion/II, as described previously [11]. In particular, score I was given if inflammatory or cancer cells were detected solely in contact with epineurium, and score II, if inflammatory or cancer cells were also present in the endoneurium of nerves [11]. Each nerve on every immunostained section was photomicrographed, and measured for the per cent proportion of the stained nerve area by using the threshold function of the ImageJ software (Wayne Rasband, NIH, version 1.44), as described previously [15]. Two different sections that were at least 30 μm apart were analyzed from each patient. A total of ca. 10,000 nerves were analyzed in the present study.
2.2.1. Mouse models of chronic pancreatitis and pancreatic cancer
-
•
Secretagogue/hypersecretion-induced CP: Here, the cholecystokinin/CCK analogue cerulein (50 μg/kg/injection in saline; Sigma-Aldirch, St. Louis, USA) or saline (control) was administered intraperitoneally 6 times at hourly intervals, three times per week for 8 weeks, to age- and gender-matched C57BL/6 J mice (n = 8 each).
-
•
Genetically-induced CP: To include a genetically induced CP model representing hereditary CP, Ptf1a-creex1 mice were crossed to mice bearing loxP-flanked knockin inserts at the autophagy-related protein Atg5 locus, generating Ptf1a-Cre;Atg5fl/fl, termed herein Atg5Δ/Δpan (n = 4). This model is an atrophic CP, which at the age of 18 weeks, exhibits histological atrophy of the pancreas, without any overt signs of pain [16].
-
•
Conditional, genetically induced PCa models: Mutant Kras together with mutated or absent trp53 induces metastatic pancreatic ductal adenocarcinoma in mice, reflecting all major features of human PCa [17,18]. Here, the LSL-KrasG12D knock-in [19], p48-creex1 [20] and Trp53fl/fl [21] strains were interbred to obtain LSL-KrasG12D;Trp53fl/fl;p48-cre (termed herein KPC, n = 2) or LSL-KrasG12D; p48- creex1 (termed KC) mice.
-
•
BDNF+/− mice: The B6.129S4-Bdnftm1Jae/J mouse strain was obtained from the Jackson Laboratory, Bar Harbor, ME USA. The mice were sacrificed at the age of 18–24 weeks for histological analysis of nNOS immunoreactivity in the pancreas.
Detailed information on the remaining methods, i.e. ex vivo neuroplasticity assays, antibodies (Table 1b), pain/mechanosensitivity testing, study approval, and statistics can be found in the “supplementary methods” section.
Table 1b.
Primary and secondary antibodies, and recombinant proteins.
| Antibody | Species | Type | Dilution | Source |
|---|---|---|---|---|
| Alexa Fluor® Goat anti-mouse IgG 488/594 | Goat | Polyclonal | 1:200 (IF) | Invitrogen, Karlsruhe, Germany |
| Alexa Fluor® Goat anti-rabbit IgG 488/594 | Goat | Polyclonal | 1:200 (IF) | Invitrogen, Karlsruhe, Germany |
| Alexa Fluor® Donkey anti-goat IgG 594 | Donkey | Polyclonal | 1:200 (IF) | Invitrogen, Karlsruhe, Germany |
| beta-III-tubulin | Mouse | Monoclonal | 1:100 (IF) | Merck Millipore, Darmstadt, Germany |
| PGP9.5 | Mouse | Monoclonal | 1: 2000 (IHC), 1:200 (IF) | DAKO, Hamburg, Germany |
| CGRP | Rabbit | Polyclonal | 1:150 (IF), 1:200 (IHC) | Merck Millipore, Darmstadt, Germany |
| Substance P | Mouse | Monoclonal | 1:30 (IF), 1:150 (IHC) | Santa Cruz, Dallas, TX, USA |
| VIP | Rabbit | Polyclonal | 1:80 (IF), 1:500 (IHC) | Sigma-Aldrich, St. Louis, MO, USA |
| nNOS | Rabbit | Polyclonal | 1:500 (IHC) | Cell Signaling, Cambridge, UK |
| nNOS | Goat | Polyclonal | 1:500 (IF) | CalBiochem (San Diego, CA, USA) |
| BDNF | Mouse | Monoclonal | 10 μg/ml (N) | CalBiochem (San Diego, CA, USA) |
| NT-3 | Mouse | Monoclonal | 1 μg/ml (N) | R&D systems, Germany |
| NGF | Rabbit | Polyclonal | 3 μg/ml (N) | Acris Antibodies, Herford, Germany |
| S100 | Mouse | Monoclonal | 1:150 (IF) | Merck Millipore, Darmstadt, Germany |
| Recombinant protein | ||||
| rhBDNF | Human | 10 ng/ml | Sigma-Aldrich, St. Louis, MO, USA |
IHC: immunohistochemistry, IF: immunofluorescence, N: neutralization, rh: recombinant human.
2.3. Study approval
The study was approved by the ethics committee of the Technical University of Munich, Germany (Approval-Nr.: 550/16 s). All animal experiments were carried out in accordance with the regulations of the Government of Upper Bavaria (Approval Nrs. 55.2–1-54-2532-223–2015 and 55.2–1-54-2532-20-2015).
3. Results
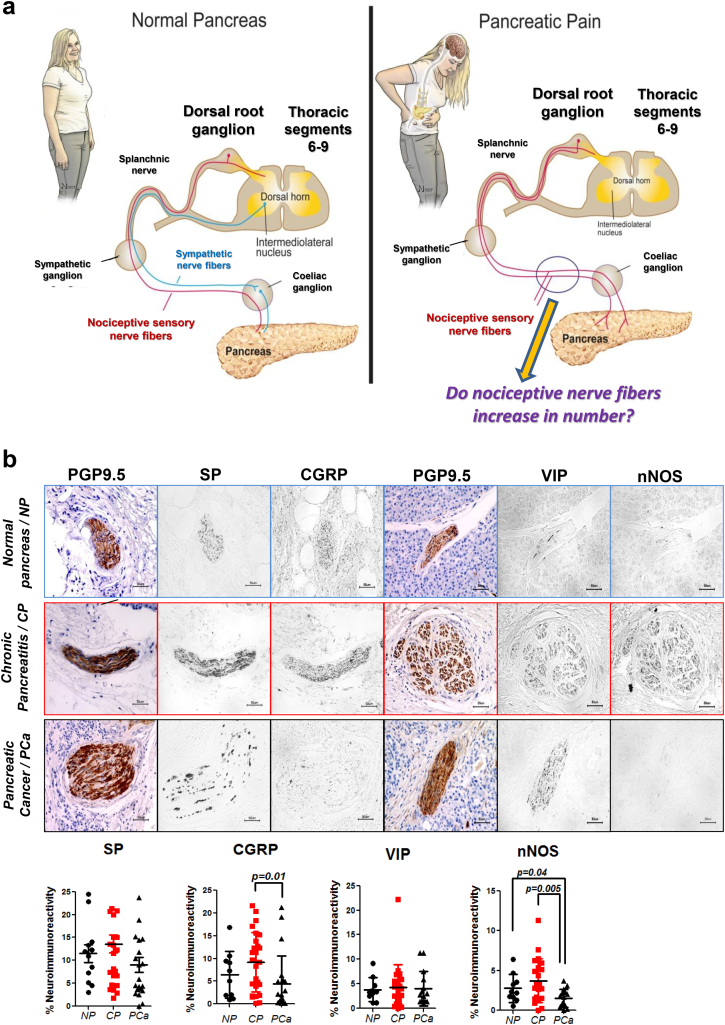
3.1. Pain in CP promotes the emergence of specifically nNOS-containing, i.e. nitrergic, nerve fibers
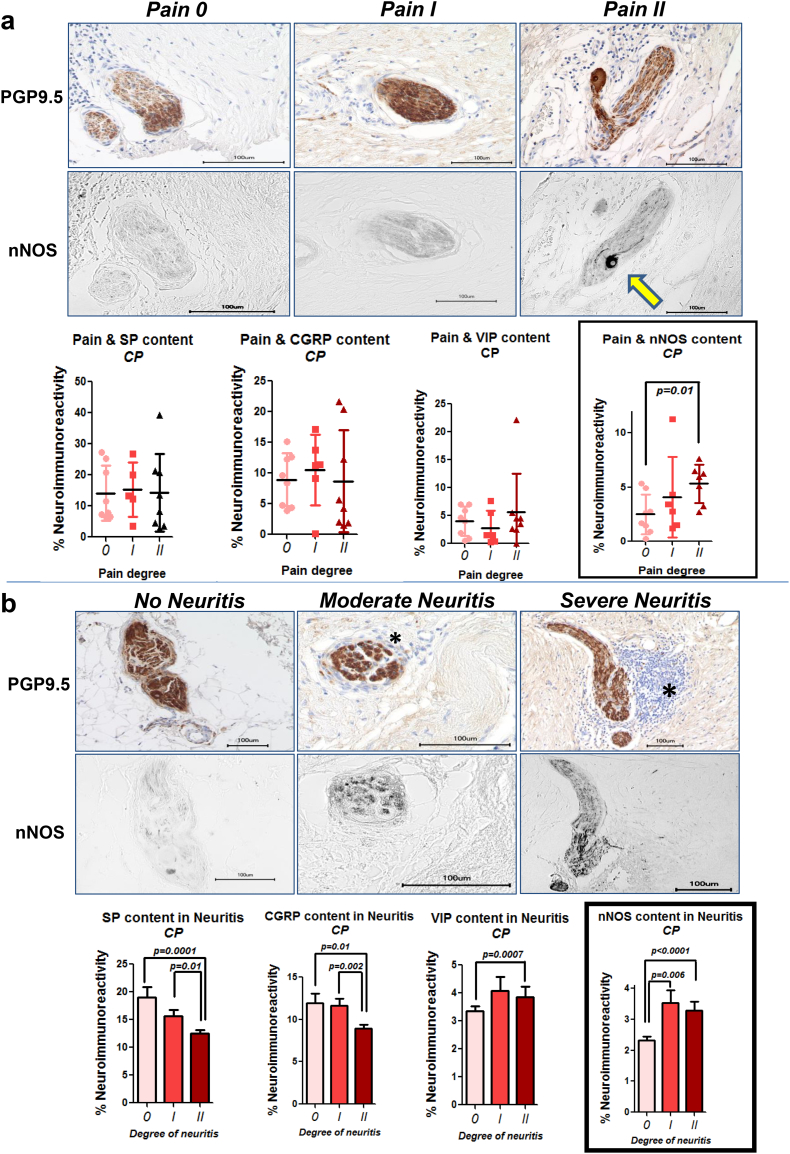
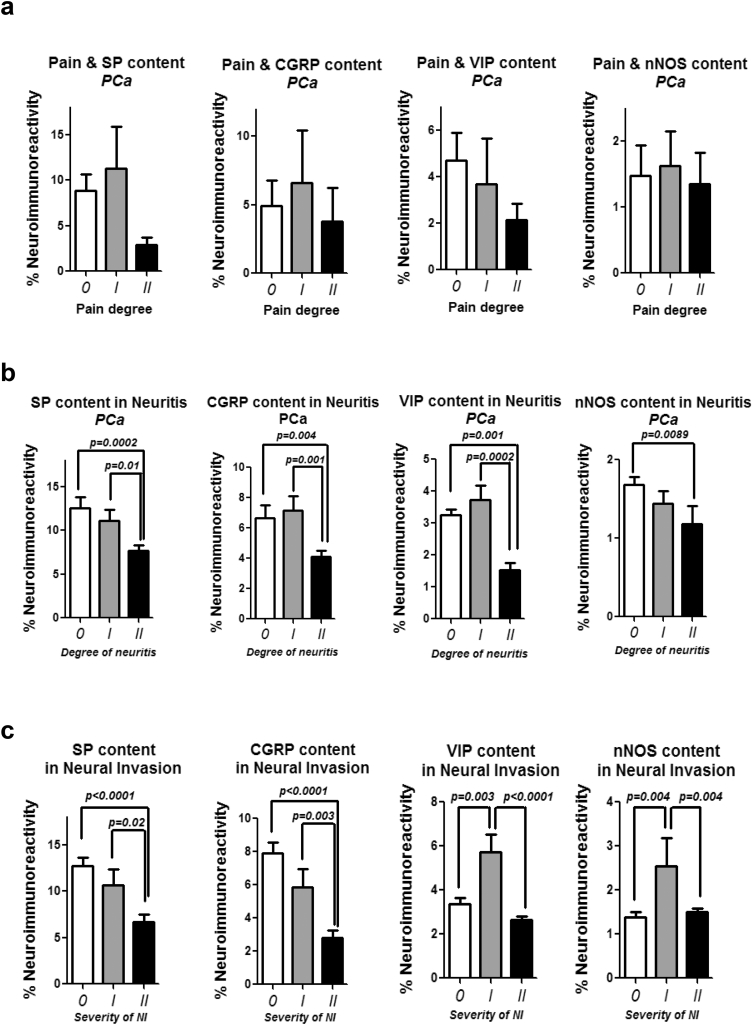
The previously described loss of sympathetic nerve fibers in painful CP and PCa [15] prompted us to investigate the amount of pain-related (“nociceptive”) neuropeptides in the pancreatic nerves of these patients (Fig. 1a). Here, we focused on SP, CGRP, VIP, and nNOS, since these represent the most prevalent, nociception-related neuropeptides in GI organs [8]. We detected no changes in SP and VIP content of nerves in CP and PCa when compared to normal human pancreas/NP (Fig. 1b). Furthermore, nerves of PCa patients showed loss of CGRP and nNOS expression. Since CP and PCa patients do not always have a painful course of disease, and since loss of sympathetic nerves is rather prominent in painful disease [15], we analyzed the intra-neural content of these neuropeptides in correlation with the severity of pain sensation. Among these neuropeptides, we observed an exclusive upregulation of nNOS in pancreatic nerves of CP patients, whereas the other three neuropeptides were unaltered between increasing degrees of pain in either CP or PCa (Pain degree 0: 2.5 ± 1.8%, Pain degree I: 4.1 ± 3.7%, Pain degree II: 5.3 ± 1.8%, p = .0092, Fig. 2a, Fig. 3a). Neuro-inflammation is considered as a major mediator of visceral, neuropathic pain in the GI tract [22]. In CP and PCa, neuro-inflammation is frequent, is termed “neuritis”, and correlates to the severity of pain [11]. Therefore, we classified pancreatic nerves in CP based on their degree of neuritis, and correlated this to their neuropeptide content. In analogy with the pain-dependent increase of nNOS in pancreatic nerves in CP, we detected greater amounts of nNOS with both degrees, i.e. moderate and severe degree of neuritis (3.5 ± 5.0%, 3.3 ± 3.7%, respectively, vs. 2.3 ± 3.6% without neuritis, p < .0001, Fig. 2b). There was a similar enrichment of VIP in nerves with severe neuro-inflammation in CP, yet the other, more classical nociceptive neuropeptides SP and CGRP did not correlate to pain or to neuritis in CP (Fig. 2b). In PCa, increasing degree of neuritis correlated with decreasing amounts of all four neuropeptides (Fig. 3b). Furthermore, perineural invasion, but not endoneural invasion, was present around nerves with higher VIP and nNOS content (Fig. 3c). Overall, we could hereby identify nNOS to be specifically up-regulated in pancreatic nerves of CP patients with severe pain.
Fig. 1.
The rationale behind the study: neural remodeling & distribution of pain-related neuropeptides in chronic pancreatitis (CP) and pancreatic cancer (PCa). a. Left: The pancreas is innervated by afferent (sensory) nerve fibers (red) originating from the dorsal root ganglia (thoracic segments 8–11), and by efferent sympathetic fibers (blue) derived from neurons of the intermediolateral nucleus. These fibers converge in the splanchnic nerves before they join in the celiac ganglion, and then enter the pancreas. Right: In pain-associated diseases of the pancreas such as CP and PCa, sympathetic fibers are decreased in number. This observation raises the question as to whether pain−/nociception related sensory fibers (red) increase in number instead. b. In the present study, intrapancreatic nerves in resection specimens from patients with CP, PCa or normal human pancreas (NP) were immunolabeled against the pan-neural marker protein-gene-product 9.5 (PGP9.5), and against four nociception-related neuropeptides (i.e., substance P/SP, calcitonin-gene-related-peptide/CGRP, vasoactive intestinal peptide/VIP, and neuronal nitric oxide synthase/nNOS). The area immunostained by each antigen was proportioned to the area of each nerve and expressed as “%neuroimmunoreactivity”. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Increasing pain severity and more severe neuro-inflammation correlate with increased nNOS-content of intrapancreatic nerves. a. Pain severity and frequency of CP patients [12] was correlated with neuro-immunoreactivity of the neuropeptides SP, CGRP, VIP, and nNOS. Patients with more severe pain (degree II) exhibited more nNOS in their pancreatic nerves (arrow) than patients with no pain (degree 0). b. Intrapancreatic neuro-inflammation, which is a defining feature of pancreatic neuropathy in CP [11], was classified as moderate if inflammatory cells were solely around the nerve, and as severe if they were also present within the interior of the nerve, as shown previously [15]. Increasing severity of neuritis was observed around nerves with greater nNOS content. Thus, intrapancreatic nerves of patients with more pain and neuritis showed upregulated nNOS. The asterisk indicates inflammatory cells.
Fig. 3.
Impact of pain, neuro-inflammation and neural invasion on the neuropeptide content of nerves in human PCa. a. Preoperative pain status of PCa patients was classified as degree 0, I or II on an ordinary scale and correlated to the mean immunoreactivity of pancreatic nerves for the pain-related neuropeptides SP, CGRP, VIP and nNOS (Ceyhan et al., Gastroenterology 2009). Here, there was no correlation between the pain status and the neuropeptide content of nerves in PCa. Unpaired t-test. b. The degree of neuritis was classified histologically around each pancreatic nerve as no neuritis (degree 0), peri-neuritis (degree I, i.e. inflammation solely around the nerve), or endo-neuritis (degree II, i.e. inflammation reaching the interior of the nerve), and correlated to the neuropeptide content of nerves in PCa. Increasing severity of neuritis was each time accompanied by diminishing amounts of SP CGRP, VIP and nNOS in PCa nerves, suggesting the presence of inflammatory cells primarily around non-nociception related nerve fibers in PCa. Unpaired t-test. c. Similar to the grading of neuritis, neural invasion (NI) by cancer cells was also classified as no invasion (severity 0), perineural invasion (severity I), and endoneural invasion (severity II). Here, increasing severity of NI was associated with lower SP and CGRP content, whereas in perineural invasion, the content of pancreatic nerves for VIP and nNOS was markedly higher. Unpaired t-test.
3.2. nNOS enrichment in CP nerves is dependent on the loss of pancreatic BDNF expression and on mast cell-derived tryptase activity
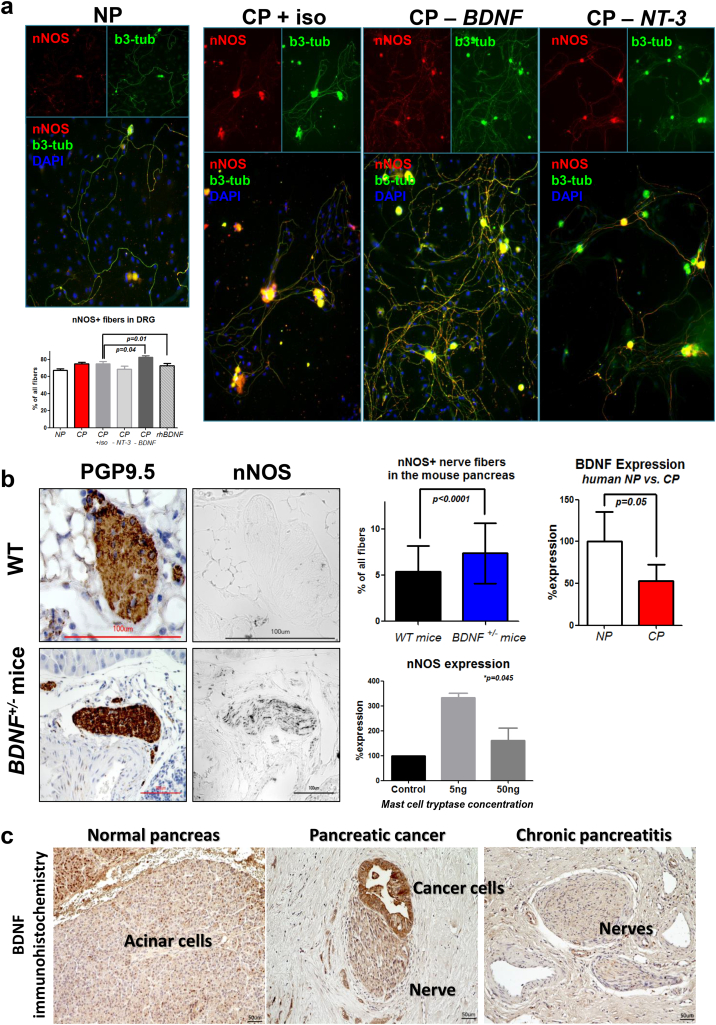
To understand the underlying mechanisms of nNOS enrichment in painful CP, we performed an ex vivo/in vitro neuroplasticity assay in which we stimulated murine dorsal root ganglia (DRG) neurons with tissue extracts of human NP, CP, or PCa patients and quantified the density of neurites (Fig. 4, Supplementary Fig. 1). This assay allows identification of molecular mediators of neuroplasticity by selective depletion in these extracts via neutralizing antibodies. We analyzed the contribution of neurotrophic factors that are known to selectively induce the sprouting of nociceptive, i.e. pain-mediating, nerve fibers that contain nociceptive neuropeptides, such as SP and CGRP. For this purpose, we not only determined the overall neurite density of DRG cultures treated with human CP extracts, but also selectively determined the density of neurites that contained the neuropeptides of interest, i.e. SP, CGRP, VIP, or nNOS (Fig. 4, Supplementary Figs. 2–4). We found that sprouting of SP-containing nerve fibers from DRG was diminished upon blockade of neurotrophin-3/NT-3, and not of BDNF from human CP extracts (Supplementary Fig. 2). Interestingly numbers of nNOS-containing nitrergic fibers of DRG were even increased upon neutralization of BNDF (CP extract+control isotype antibody: 75.2 ± 5.3 vs. CP + anti-BDNF: 82.6 ± 4.0%, p = .04, Fig. 4a). Accordingly, the density of nNOS-containing fibers of DRG upon treatment with recombinant human BDNF (rhBDNF) was lower than that induced by CP extracts (rhBDNF: 72.6 ± 6.9%, p = .01, Fig. 4a). To validate the potential antagonistic relationship between BDNF and nNOS in nerves, we analyzed the density of nNOS+ fibers in the pancreatic nerves of heterozygous BDNF+/− mice, since homozygous BDNF−/− mice do normally not survive until the adult age [23]. Here, similarly, we detected more intra-neural nNOS in the pancreas of BDNF+/− mice (7.4 ± 3.3%) as compared to wildtype (WT) mice (5.4 ± 2.8%, p = .0001, Fig. 4b). In accordance, BDNF in the pancreatic tissue of CP patients, who underwent resection due to severe pain, was downregulated at mRNA level when compared to NP tissues (CP: 52.7% of NP, p = .05, Fig. 4b-c, Table 1c). Thus, overall, these findings suggested that downregulation of BDNF in the course of CP promotes the emergence of pain-promoting nitrergic nerve fibers in the pancreas.
Fig. 4.
Depletion of BDNF, or mast cell tryptase, in the CP microenvironment induces sprouting of nNOS-containing nitrergic nerve fibers, ex vivo and in vivo. a. In the ex vivo neuroplasticity assays, blocking antibodies against brain-derived-neurotrophic factor (CP-BDNF) or against neurotrophin-3 (CP-NT-3) were supplied into the human CP tissue extracts before they were applied into the growth medium of DRG neurons. Depletion of BDNF augmented the density of nitrergic fibers in DRG cultures. b. Similar to the ex vivo observations, this antagonism of BDNF against nNOS in nerves was also observed in BDNF+/− mice (n = 4), which exhibited a markedly greater neuro-immunoreactivity for nNOS than wildtype/WT mice, n = 5). In accordance, at mRNA level, BDNF was downregulated in human CP tissues (n = 21) when compared to nomal human pancreas (NP) tissues (n = 21), accounting for the enhanced nNOS activity in CP. Painful CP is coupled with perineural mast cell enrichment [24]. nNOS mRNA expression was also enhanced in DRG neurons treated with mast cell tryptase in their growth media in a dose-dependent manner. p-value of the one-way ANOVA. Protein-gene-product 9.5 (PGP9.5): pan-neural marker used for the identification of nerves in the pancreas. c. The expression of brain derived neurotrophic factor (BDNF) is diminished in human chronic pancreatitis tissues. In normal human pancreas, we observed a BDNF expression in acinar cells, frequently within nuclei. In pancreatic cancer, BDNF was mostly present in cancer cells and nerves. In chronic pancreatitis, though, BDNF was hardly detectable in the pancreas.
Table 1c.
Primer sequences.
| Sequence | |
|---|---|
| Human | |
| BDNF | For: 5′-ATTGGCTGGCGATTCATAAG-3′ |
| Rev: 5′-GTTTCCCTTCTGGTCATGGA-3′ | |
| Cyclophilin B | For: 5′-TGTGGTGTTTGGCAAAGTTC-3′ |
| Rev: 5′-GTTTATCCCGGCTGTCTGTC-3′ | |
| Mouse | |
| BDNF | For: 5′-TGCAGGGGCATAGACAAAAGG-3′ |
| Rev: 5′-CTTATGAATCGCCAGCCAATTCTC-3′ | |
| GAPDH | For: 5′-AATGTGTCCGTCGTGGATCTG-3′ |
| Rev: 5′-CAACCTGGTCCTCAGTGTAGC-3′ | |
| nNOS | For: 5′-CTGGTGAAGGAACGGGTCAG3′ |
| Rev: CCGATCATTGACGGCGAGAAT-3′ |
Beyond the loss of BDNF as a factor contributing to nNOS enrichment, we also analyzed the possibility that mast cells may be linked to nNOS upregulation in nerves in CP. In fact, we recently reported a specific accumulation of mast cells around nerves in CP patients with pain [24]. Thus, we postulated that mast cell-derived mediators may cause the nNOS upreguation in the DRG neurons that supply the pancreas. For this analysis, we treated murine DRG neurons from the thoraco-lumbar area with increasing concentrations of mast cell tryptase in their growth medium. Indeed, nNOS expression was upregulated in DRG neurons upon different concentrations of mast cell tryptase treatment (Fig. 4b), suggesting mast cells as another potential inducer of nNOS expression in sensory neurons supplying the pancreas.
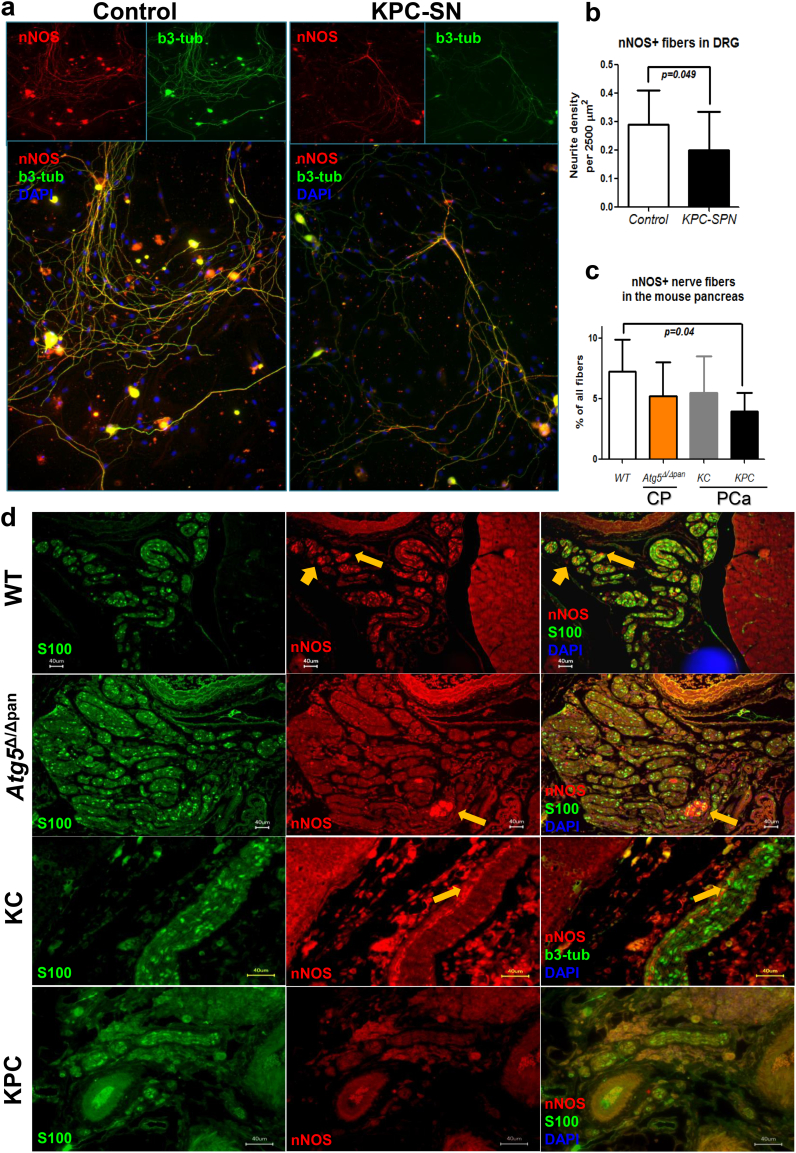
To demonstrate the reproducibility of the loss of nitrergic innervation in PCa, we next analyzed the density of nNOS+ fibers in murine DRG cultures treated with culture supernatants of cancer cells derived from the genetically engineered, KPC (Ptf1a-Cre;LSL-KrasG12D;p53fl/fl) mouse model of PCa (Fig. 5a). Here, DRG neurons treated with the KPC supernatants exhibited a lower nNOS+ fiber density than DRG neurons cultured in their normal medium (Control-DRG: 0.3 ± 0.1%, vs. KPC: 0.2 ± 0.1%, p = .049, Fig. 5a). In analogy, when we histologically quantified the proportion of nNOS+ fibers in the pancreatic nerves of KPC mice, we indeed found that KPC mice also harboured less nitrergic fibers (4.0 ± 1.6%) in their pancreas than WT mice (7.3 ± 2.6%, p = .04, Fig. 5b-c). This decrease was gradual during pancreatic carcinogenesis, since the KC mice (Ptf1a-Cre;LSL-KrasG12D) at the age of 16–24 weeks, which solely harbor the precursor lesions of PCa, had an intermediate nitrergic fiber content (5.5 ± 3.0%, Fig. 5c-d).
Fig. 5.
Genetically induced mouse models of CP and PCa differ in their capacity to simulate the alterations in nitrergic signaling in human disease. a-b. Cancer cells derived from the genetically induced KPC (Ptf1a-Cre;LSL-KrasG12D;p53fl/fl) murine model of PCa were cultivated until 80% confluence for the collection of their supernatants. Murine DRG neurons were then treated with the KPC cancer cell supernatants or with the murine cancer cell medium as control and compared for their neurite density. In analogy with the human disease, KPC supernatants suppressed the density of nNOS-containing fibers. Unpaired t-test. b3-tub: beta-III-tubulin. c-d. The neuroimmunoreactivity for nNOS was compared in pancreatic nerves of either wildtype/WT mice (18 week-old, n = 7), or mice with genetically induced CP (atrophic CP upon pancreas-specific ablation of the autophagy-related protein Atg5, i.e. Ptf1a-Cre;Atg5fl/fl, termed herein Atg5Δ/Δpan, 18 week-old, n = 4), in KC (Ptf1a-Cre;LSL-KrasG12D, n = 4) mice that give rise to the precursor (PanIN) lesions of PCa, and in KPC mice (Ptf1a-Cre;LSL-KrasG12D;p53fl/fl, n = 4) at the age of 4–6 weeks that have overt PCa. In accordance with human PCa and with the ex vivo assays (a-b), mice with PCa displayed fewer nitrergic fibers. The genetically induced Atg5Δ/Δpan model of CP did not exhibit any increase in nitrergic signaling when compared to WT mice.
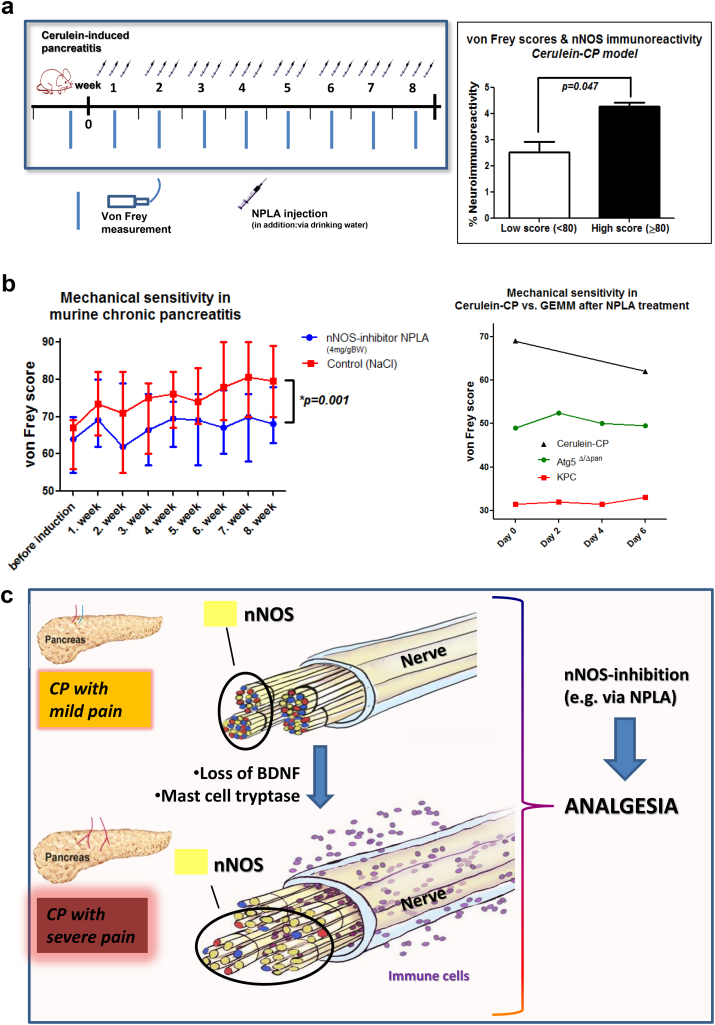
In the next step, we analyzed mouse models of CP for their nNOS content (Fig. 5c-d). For this purpose, we first used a rather novel, genetically-induced model of CP, which is based on pancreas-specific ablation of the autophagy-related protein Atg5 (Ptf1a-Cre;Atg5fl/fl, termed herein Atg5Δ/Δpan). This model is an atrophic CP, which, at the age of 18 weeks, exhibits histological atrophy of the pancreas, without any overt signs of pain [16]. In the second model, we made use of a well-characterized CP model that is based on repetitive intraperitoneal injections of the secretagogue cerulein, resulting in recurrent bouts of acute pancreatitis, and over the course of eight weeks, leads to painful CP [25]. Comparison of these models with WT mice did not reveal any change in the amount of nNOS-containing fibers in any of the genetic models of CP (Fig. 5c-d). In contrast, though, the amount of nNOS-containing nerve fibers in the pancreas correlated to higher abdominal pain/mechanosensitivity (von Frey) scores in the painful cerulein-CP model (Fig. 6a). Thus, these models allowed subsequent comparisons of the effect of nNOS inhibitors on pain sensation of these mice. Furthermore, the histological observations that we made in human specimens were simulatable in the ex vivo neuroplasticity assays with murine neurons, and were largely reproducible in a mouse-model-dependent manner (Supplementary Figs. 5–7).
Fig. 6.
nNOS inhibitors ameliorate pain in human-like models of CP. a. Since the genetic Atg5Δ/Δpan model of CP did not show any increase in nNOS activity in nerves as the human disease, we tested a more classical secretagogue (cerulein)-induced model of CP, which is based on three times per week injections of cerulein over eight weeks. The mechanosensitivity of these mice in the abdominal area was determined by means of von Frey filaments as an indicator of their pain. The amount of nNOS-containing nerve fibers in the pancreas correlated to higher abdominal pain/von Frey scores in the cerulein-CP model. Unpaired t-test. b. The Cerulein-CP mice were treated either with solvent (sodium chloride/NaCl, n = 8) or with a highly specific inhibitor of nNOS, i.e. „Nω-Propyl-L-arginine hydrochloride“/ NPLA (4 mg/kg body weight, n = 8) via drinking water and three times per week subcutaneous/s.c. injections. Mice treated with NPLA constantly exhibited lower mechanosensitivity/von Frey scores and thus less pain than the control solvent-treated mice (unpaired t-test). In a head-to-head comparison of the responsiveness of different mouse models to NPLA treatment, a one-week treatment with combined oral and s.c. NPLA did not change the pain/von Frey scores of KPC (n = 2) or Atg5Δ/Δpan (n = 3) mice. In contrast, even a one-week combined oral and s.c. NPLA treatment was sufficient to lower the pain score of the mice with cerulein-induced CP, which, in contrast to other models, exhibited human-like nNOS upregulation in their pancreatic nerves. c. In pain-associated CP, loss of BDNF expression during disease progression tends to specifically increase the number of nitrergic/ nNOS-containing nerve fibers in the pancreas, rendering nNOS inhibition as an innovative form of pain therapy in CP.
3.3. nNOS inhibition ameliorates pain specifically in CP, but not in PCa
In contrast to its two isoenzymes (inducible NOS/iNOS and endothelial NOS/eNOS), nNOS has not yet been reported to be relevant for pain sensation in pancreatic diseases. Based on the pain-specific upregulation of nNOS in human CP and the cerulein-CP model, we evaluated the potential of nNOS as a novel analgesic/therapeutic target in CP (Fig. 6a-b). For this purpose, we treated different models of CP with the specific nNOS inhibitor N(ω)-propyl-L-arginine (NPLA, 2 mg/kg body weight, Tocris, Bristol, UK) over varying periods and tracked the abdominal mechanosensitivity (assessed via von Frey filaments) of the respective mice in comparison with mice treated with the solvent agent (sodium chloride/NaCl/solvent). In the pain-associated cerulein-induced CP model, we found prominently higher von Frey scores in the solvent-treated animals (74.7 ± 4.7, Fig. 6b, “Control/NaCl” treatment). However, mice treated with NPLA during the eight weeks of CP induction exhibited lower mechanosensitivity/von Frey scores than the solvent-treated mice (67.9 ± 2.3, p = .001, Fig. 6b).
To validate our hypothesis on the specific, nNOS-mediated pain sensation in painful CP, we also treated Atg5Δ/Δpan and KPC mice with NPLA, since we showed that these mice rather exhibit a loss, rather than an increase, in their neural nNOS content. While a one-week treatment with NPLA in developed CP led to reduced von Frey scores of cerulein-CP mice, the von Frey scores of Atg5Δ/Δpan and KPC mice treated with NPLA were not affected, suggesting no analgesic effect of NPLA in these mice with genetically induced CP and PCa, Fig. 6b).
Thus, application of a single, specific nNOS inhibitor led to reduction in pain sensation, specifically in mice with painful CP, suggesting nNOS inhibition as a potential, novel analgesic target for CP.
4. Discussion
The present study aimed at identifying novel therapeutic targets for treating the cardinal symptom of pancreatic diseases, i.e. abdominal pain, since current regimens do not yield satisfying long-term results and cannot prevent opiate dependence [26]. Through a detailed analysis of the neuropeptide content of human pancreatic nerves in CP and PCa, by means of ex vivo neuronal assays, and via genetically-induced and established animal models, we demonstrate a previously unknown, key role for nitrergic signaling in the mediation of CP-associated pain. In particular, we provide evidence for nNOS inhibition as a potential, novel analgesic therapy option for CP.
Pain dictates the unpleasant course of life in CP patients, since pain results in depression, anxiety, loss of mental performance, loss of job, and loss of social contacts [27]. Thus, treating pain is the first priority in CP, since CP cannot be sufficiently treated in a causal manner and cannot be reverted [5]. In addition to medical measures such as administration of non-opiate analagesics, opiate analgesics, and psychotherapy, patients frequently undergo endoscopic treatments (stent-placement) due to strictures in the pancreatic or bile duct, or in the duodenum. However, these measures often fail, leaving surgery, i.e. drainage or resection of the pancreas in part or in total, as the last resort [28]. Neural remodeling involving the switch of nerve fiber subtypes within the pancreas and loss of sympathetic innervation [15], together with central nervous alterations observed in functional MRI [29], suggested that “neuropathic” pain mechanisms may be the factor that renders pain management in CP so difficult [5]. Based on this premise, recent studies made use of medications such as pregabalin, that are normally used to treat neuropathic pain conditions like diabetic neuropathy, post-herpetic or trigeminal neuralgia [30]. In fact, pregabalin was effective in relieving neuropathic pain and in improving the overall health status in CP patients in a randomized controlled trial [30]. These studies underline that understanding “pancreatic neuropathy” will lead to the development of more effective and targeted therapy regimens.
For this reason, we hypothesized that deciphering the neurotransmitter−/neuropeptide code of the altered, enlarged nerves in pancreatic diseases may provide clues for such novel strategies. In addition to the classical pain-mediating neuropeptides such as SP and CGRP, we also analyzed two additional neuropeptides/neuroenzymes, i.e. VIP and nNOS which, although not always linked to pain, were shown to be upregulated in other gastrointestinal neuropathies such as chronic gastritis, reflux esophagitis, or motility disorders [8]. Unexpectedly, SP and CGRP content of intrapancreatic nerves did not correlate to the pain severity of CP or PCa patients. As these neuropeptides are mediators of neurogenic inflammation [31], we postulate that they might primarily contribute to the maintanence of tissue inflammation, but less to neuro-inflammation or to the specific stimulation of nociceptors. By analyzing the nerves from resection specimens of CP patients, we could for the first time identify nNOS as a neuronal enzyme that was enriched in pancreatic nerves of CP patients in a pain-severity-dependent manner. Importantly, we identified among different in vivo models of CP and PCa the ones that exhibit a similar nNOS enrichment, and verified that only those painful models with a similar neural nNOS upregulation also responded to nNOS inhibition as an analgesic therapy.
Inhibition of nNOS has recently been considered as a novel option for pain therapy in other neuropathic pain conditions [32,33]. nNOS is one of the three producers of nitric oxide (NO) and is localized at the neuronal postsynaptic terminals, near N-methyl-d-aspartate (NMDA) receptors that play a role in chronic pain and central sensitization [34,35]. Following stimulation of Ca2+-permeable NMDA receptors, nNOS is activated over a Ca2+ influx and calmodulin activation [36]. The resulting NO increase in the neuron activates cGMP and protein kinase G (PKG) signaling and affects multiple cellular processes including neurotransmission, cell metabolism, long-term potentiation and –depression, and neuropathic pain [37]. Thus, pathological NMDA receptor activation through excessive nNOS activity at the spinal or peripheral sensory neuronal level may be one of the key reasons for the recently discovered link between nNOS activity and neuropathic pain. Accordingly, small molecule inhibitors of nNOS have recently been shown to alleviate neuropathic pain after spinal nerve injury or chemotherapy-induced neuropathic pain [38,39]. Development of novel and specific nNOS inhibitors is a hot topic in current pharmacological research on analgesics [38]. In the present study, we used one of the most specific, available nNOS inhibitors that, in contrast with other arginin-based analogue inhibitors of nNOS, has the least affinity for eNOS or iNOS.
The present study also once again underlined that pain mechanisms in CP and in PCa seem to differ, since nNOS enrichment was specific for pain in CP and not PCa. Although both diseases exhibit loss of sympathetic nerves, the extent of peripheral glial activation is much more pronounced in PCa [40]. Furthermore, surgical transection of pancreatic afferents in the splanchnic nerves is of clinical benefit for PCa patients, but not for CP patients [5]. Thus, despite structural similarities such as nerve hypertrophy or nerve sprouting, there are obvious mechanistic differences in the pain caused by CP versus by PCa. Nonetheless, by identifying neuropeptides or neurotransmitters enriched in human pancreatic nerves and by the choice of suitable animal models harboring comparable neuropeptide alterations, we believe that it is possible to identify further, promising analgesic targets. Thus, our findings indicate that due to its human-like neural nNOS enrichment, cerulein-induced CP may be a more suitable animal model for in vivo mechanistic pain studies than the genetically induced, Atg5-depletion model of CP.
In summary, the present study elucidated nNOS inhibition as a novel form of pain therapy in CP due to the nNOS enrichment in pancreatic nerves inherent to this disease. Molecular profiling of intra-organ nerve endings in GI diseases seems to bear the potential of unraveling further analgesic targets. Based on our findings, we believe that nNOS inhibitors should find access into early phase clinical trials on pain in CP for alleviation of the huge burden that pain inflicts on these patients.
Acknowledgments
Acknowledgments
IED was supported by grant of the Deutsche Forschungsgemeinschaft/DFG (DE2428 3-1 and 3-2). The funders had no role in study design, data collection, data analysis, interpretation, or writing of the report.
Beyond this, the authors declare no conflicts of interest and have no financial disclosures.
Author contributions
IED, HF, and GOC designed the study. IED, TH, DGC, ÖCS, SK ST, TK, CMR, ET, ML, DHB, BG, BEU, KND, ML performed the experiments. ML, MS, MM, HA, and AK contributed novel ideas and modified the design. All authors contributed to the draft of the manuscript and approved the final version.
Financial disclosures
IED was supported by a grant of the Deutsche Forschungsgemeinschaf/DFG (DE2428/3-1 and 3-2).
Conflicts of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.07.055.
Appendix A. Supplementary data
Supplementary material
References
- 1.Holmes D. The pain drain. Nature. 2016;535:S2–S3. doi: 10.1038/535S2a. [DOI] [PubMed] [Google Scholar]
- 2.Hall T.C., Garcea G., Webb M.A., Al-Leswas D., Metcalfe M.S., Dennison A.R. The socio-economic impact of chronic pancreatitis: a systematic review. J Eval Clin Pract. 2014;20:203–207. doi: 10.1111/jep.12117. [DOI] [PubMed] [Google Scholar]
- 3.Grayson M. Pain. Nature. 2016;535:S1. doi: 10.1038/535S1a. [DOI] [PubMed] [Google Scholar]
- 4.Machicado J.D., Amann S.T., Anderson M.A., Abberbock J., Sherman S., Conwell D.L. Quality of life in chronic pancreatitis is determined by constant pain, disability/unemployment, current smoking, and associated co-morbidities. Am J Gastroenterol. 2017;112:633–642. doi: 10.1038/ajg.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demir I.E., Friess H., Ceyhan G.O. Neural plasticity in pancreatitis and pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2015;12:649–659. doi: 10.1038/nrgastro.2015.166. [DOI] [PubMed] [Google Scholar]
- 6.Demir I.E., Tieftrunk E., Maak M., Friess H., Ceyhan G.O. Pain mechanisms in chronic pancreatitis: of a master and his fire. Langenbecks Arch Surg. 2011;396:151–160. doi: 10.1007/s00423-010-0731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenstein M. Neuropathy: a name for their pain. Nature. 2016;535:S10–S11. doi: 10.1038/535S10a. [DOI] [PubMed] [Google Scholar]
- 8.Demir I.E., Schafer K.H., Tieftrunk E., Friess H., Ceyhan G.O. Neural plasticity in the gastrointestinal tract: chronic inflammation, neurotrophic signals, and hypersensitivity. Acta Neuropathol. 2013;125:491–509. doi: 10.1007/s00401-013-1099-4. [DOI] [PubMed] [Google Scholar]
- 9.Saloman J.L., Albers K.M., Li D., Hartman D.J., Crawford H.C., Muha E.A. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci U S A. 2016;113:3078–3083. doi: 10.1073/pnas.1512603113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boilly B., Faulkner S., Jobling P., Hondermarck H. Nerve dependence: from regeneration to cancer. Cancer Cell. 2017;31:342–354. doi: 10.1016/j.ccell.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Ceyhan G.O., Bergmann F., Kadihasanoglu M., Altintas B., Demir I.E., Hinz U. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136:177–186. doi: 10.1053/j.gastro.2008.09.029. (e1) [DOI] [PubMed] [Google Scholar]
- 12.Ceyhan G.O., Bergmann F., Kadihasanoglu M., Erkan M., Park W., Hinz U. The neurotrophic factor artemin influences the extent of neural damage and growth in chronic pancreatitis. Gut. 2007;56:534–544. doi: 10.1136/gut.2006.105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demir I.E., Wang K., Tieftrunk E., Giese N.A., Xing B., Friess H. Neuronal plasticity in chronic pancreatitis is mediated via the neurturin/GFRalpha2 axis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1017–G1028. doi: 10.1152/ajpgi.00517.2011. [DOI] [PubMed] [Google Scholar]
- 14.Wang K., Demir I.E., D'Haese J.G., Tieftrunk E., Kujundzic K., Schorn S. The neurotrophic factor neurturin contributes toward an aggressive cancer cell phenotype, neuropathic pain and neuronal plasticity in pancreatic cancer. Carcinogenesis. 2014;35:103–113. doi: 10.1093/carcin/bgt312. [DOI] [PubMed] [Google Scholar]
- 15.Ceyhan G.O., Demir I.E., Rauch U., Bergmann F., Muller M.W., Buchler M.W. Pancreatic neuropathy results in "neural remodeling" and altered pancreatic innervation in chronic pancreatitis and pancreatic cancer. Am J Gastroenterol. 2009;104:2555–2565. doi: 10.1038/ajg.2009.380. [DOI] [PubMed] [Google Scholar]
- 16.Diakopoulos K.N., Lesina M., Wormann S., Song L., Aichler M., Schild L. Impaired autophagy induces chronic atrophic pancreatitis in mice via sex- and nutrition-dependent processes. Gastroenterology. 2015;148:626–638. doi: 10.1053/j.gastro.2014.12.003. (e17) [DOI] [PubMed] [Google Scholar]
- 17.Hingorani S.R., Wang L., Multani A.S., Combs C., Deramaudt T.B., Hruban R.H. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Lesina M., Kurkowski M.U., Ludes K., Rose-John S., Treiber M., Kloppel G. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Jackson E.L., Willis N., Mercer K., Bronson R.T., Crowley D., Montoya R. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakhai H., Sel S., Favor J., Mendoza-Torres L., Paulsen F., Duncker G.I. Ptf1a is essential for the differentiation of GABAergic and glycinergic amacrine cells and horizontal cells in the mouse retina. Development. 2007;134:1151–1160. doi: 10.1242/dev.02781. [DOI] [PubMed] [Google Scholar]
- 21.Jonkers J., Meuwissen R., van der Gulden H., Peterse H., van der Valk M., Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 22.Scholz J., Woolf C.J. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 23.Ward N.L., Hagg T. BDNF is needed for postnatal maturation of basal forebrain and neostriatum cholinergic neurons in vivo. Exp Neurol. 2000;162:297–310. doi: 10.1006/exnr.1999.7346. [DOI] [PubMed] [Google Scholar]
- 24.Demir I.E., Schorn S., Schremmer-Danninger E., Wang K., Kehl T., Giese N.A. Perineural mast cells are specifically enriched in pancreatic neuritis and neuropathic pain in pancreatic cancer and chronic pancreatitis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michalski C.W., Laukert T., Sauliunaite D., Pacher P., Bergmann F., Agarwal N. Cannabinoids ameliorate pain and reduce disease pathology in cerulein-induced acute pancreatitis. Gastroenterology. 2007;132:1968–1978. doi: 10.1053/j.gastro.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drewes A.M., Bouwense S.A.W., Campbell C.M., Ceyhan G.O., Delhaye M., Demir I.E. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology. 2017;17:720–731. doi: 10.1016/j.pan.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Ammann R.W., Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology. 1999;116:1132–1140. doi: 10.1016/s0016-5085(99)70016-8. [DOI] [PubMed] [Google Scholar]
- 28.da Silveira A.B., Freitas M.A., de Oliveira E.C., Neto S.G., Luquetti A.O., Furness J.B. Neuronal plasticity of the enteric nervous system is correlated with chagasic megacolon development. Parasitology. 2008;135:1337–1342. doi: 10.1017/S0031182008004770. [DOI] [PubMed] [Google Scholar]
- 29.Olesen S.S., Brock C., Krarup A.L., Funch-Jensen P., Arendt-Nielsen L., Wilder-Smith O.H. Descending inhibitory pain modulation is impaired in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2010;8:724–730. doi: 10.1016/j.cgh.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Olesen S.S., Bouwense S.A., Wilder-Smith O.H., van Goor H., Drewes A.M. Pregabalin reduces pain in patients with chronic pancreatitis in a randomized, controlled trial. Gastroenterology. 2011;141:536–543. doi: 10.1053/j.gastro.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Nathan J.D., Peng R.Y., Wang Y., McVey D.C., Vigna S.R., Liddle R.A. Primary sensory neurons: a common final pathway for inflammation in experimental pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G938–G946. doi: 10.1152/ajpgi.00105.2002. [DOI] [PubMed] [Google Scholar]
- 32.Levy D., Zochodne D.W. NO pain: potential roles of nitric oxide in neuropathic pain. Pain Pract. 2004;4:11–18. doi: 10.1111/j.1533-2500.2004.04002.x. [DOI] [PubMed] [Google Scholar]
- 33.Ahlawat A., Rana A., Goyal N., Sharma S. Potential role of nitric oxide synthase isoforms in pathophysiology of neuropathic pain. Inflammopharmacology. 2014;22:269–278. doi: 10.1007/s10787-014-0213-0. [DOI] [PubMed] [Google Scholar]
- 34.Brenman J.E., Chao D.S., Gee S.H., McGee A.W., Craven S.E., Santillano D.R. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 35.Petrenko A.B., Yamakura T., Baba H., Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97:1108–1116. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- 36.Steinert J.R., Chernova T., Forsythe I.D. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist. 2010;16:435–452. doi: 10.1177/1073858410366481. [DOI] [PubMed] [Google Scholar]
- 37.Kawano T., Zoga V., Kimura M., Liang M.Y., Wu H.E., Gemes G. Nitric oxide activates ATP-sensitive potassium channels in mammalian sensory neurons: action by direct S-nitrosylation. Mol Pain. 2009;5:12. doi: 10.1186/1744-8069-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee P., Cinelli M.A., Kang S., Silverman R.B. Development of nitric oxide synthase inhibitors for neurodegeneration and neuropathic pain. Chem Soc Rev. 2015;43:6814–6838. doi: 10.1039/c3cs60467e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guan Y., Yaster M., Raja S.N., Tao Y.X. Genetic knockout and pharmacologic inhibition of neuronal nitric oxide synthase attenuate nerve injury-induced mechanical hypersensitivity in mice. Mol Pain. 2007;3:29. doi: 10.1186/1744-8069-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demir I.E., Tieftrunk E., Schorn S., Saricaoglu O.C., Pfitzinger P.L., Teller S. Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut. 2016;65:1001–1014. doi: 10.1136/gutjnl-2015-309784. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material