Abstract
Background
Cigarette smoke is the main risk factor of pulmonary emphysema development, which is characterized by alveolar wall destruction. Mitochondria are important for alveolar type II (ATII) cell metabolism due to ATP generation.
Methods
We isolated ATII cells from control non-smoker and smoker organ donors, and after lung transplant of patients with emphysema to determine mitochondrial function, dynamics and mitochondrial (mt) DNA damage.
Findings
We found high mitochondrial superoxide generation and mtDNA damage in ATII cells in emphysema. This correlated with decreased mtDNA amount. We also detected high TOP1-cc and low TDP1 levels in mitochondria in ATII cells in emphysema. This contributed to the decreased resolution of TOP1-cc leading to accumulation of mtDNA damage and mitochondrial dysfunction. Moreover, we used lung tissue obtained from areas with mild and severe emphysema from the same patients. We found a correlation between the impaired fusion and fission as indicated by low MFN1, OPA1, FIS1, and p-DRP1 levels and this disease severity. We detected lower TDP1 expression in severe compared to mild emphysema.
Interpretation
We found high DNA damage and impairment of DNA damage repair in mitochondria in ATII cells isolated from emphysema patients, which contribute to abnormal mitochondrial dynamics. Our findings provide molecular mechanisms of mitochondrial dysfunction in this disease.
Fund
This work was supported by National Institutes of Health (NIH) grant R01 HL118171 (B.K.) and the Catalyst Award from the American Lung Association (K.B.).
Keywords: COPD, Emphysema, Lung, DNA damage, Alveolar type II cells, Mitochondria
Research in context.
Evidence before this study
Alveolar type II (ATII) cells have a stem cell potential, they proliferate and restore the epithelium after damage. Mitochondria are important for ATII cell function due to ATP generation. Emphysema is characterized by alveolar wall destruction, however, its pathophysiology is not well known. Studies using isolated primary ATII cells from patients with this disease are very limited.
Added value of this study
In this study, we isolated ATII cells from emphysema patients and control organ donors. We observed impaired ATII cell function in this disease pathogenesis and progression as determined by the low mitochondrial amount and impaired mitochondrial dynamics. Our results also indicated decreased TDP1 levels, which is involved in DNA damage repair, leading to increased mitochondrial TOP1-cc levels, DNA damage and dysfunction.
Implications of all the available evidence
Our results reveal a novel mechanism underlying the impaired human primary ATII cell function in emphysema. Mitochondrial dysfunction caused by decreased mitochondrial DNA damage repair contributes to ATII cell death. Strategies restoring mitochondrial function in ATII cells may slow down emphysema development.
Alt-text: Unlabelled Box
1. Introduction
Pulmonary emphysema is characterized by a unique pattern of alveolar wall destruction associated with high reactive oxygen species (ROS) generation induced mainly by cigarette smoking [1]. Increased ROS levels can lead to an imbalance between oxidant and antioxidant systems [1,2]. High oxidative stress causes DNA damage and further impairs the efficient repair of damaged DNA and the maintenance of DNA integrity [3].
Alveolar type II (ATII) cells self-renew, proliferate, and differentiate to alveolar type I cells to restore the epithelium after damage [4]. Mitochondria convert energy from nutrients into ATP through oxidative phosphorylation (OXPHOS). These organelles are important for ATII cell metabolism [5]. ROS are eliminated through activation of the antioxidant defense system. However, its impairment can cause ROS accumulation leading to mitochondrial DNA (mtDNA) damage, mutations, and deletions. MtDNA maintenance is controlled by nuclear gene expression [6]. MtDNA contains genes encoding for essential subunits of the OXPHOS system. Moreover, mtDNA is more prone to oxidative damage than nuclear DNA due to the lack of protective histones [7]. Damage to mtDNA and alterations of copy number disrupts mitochondrial function. Mitochondria constantly fuse and divide via fusion and fission machinery, respectively to eliminate damaged organelles and protect the integrity of mtDNA [8]. Mitochondrial dynamics is regulated by mitofusin 1 (MFN1), mitofusin 2 (MFN2), optic atrophy-1 (OPA1), mitochondrial fission 1 (FIS1) and dynamin family member dynamin-related protein 1 (DRP1). Degradation of defective mitochondria occurs through mitophagy. Mitochondrial dysfunction was reported in airway epithelial cells in patients with chronic obstructive pulmonary disease (COPD) and a murine model of COPD [[9], [10], [11]].
Non-homologous end joining (NHEJ) recognizes and ligates DNA double-strand breaks (DSBs) [12]. NHEJ is considered as an error-prone DSBs repair pathway. Inaccurate repair of DSBs can generate mutations and deletions leading to cell death. Moreover, mtDNA damage is mostly repaired by nuclear DNA repair proteins. Topoisomerases are required for transiently cutting and re-ligating DNA backbone to maintain proper DNA supercoiling density [13]. MtDNA damage can cause stalling of topoisomerase 1 DNA covalent complex in mtDNA (TOP1mt-cc) leading to the impairment of transcription and/or replication. We have recently shown the cytoprotective function of DJ-1 in human primary ATII cells [14]. DJ-1 is localized in mitochondria, cytoplasm, and nucleus. Recent studies have shown the importance of DJ-1 in maintaining mitochondrial function and activating the antioxidant defense system under oxidative stress conditions [15].
In this report, we analyzed, for the first time, mitochondrial function in isolated ATII cells from emphysema patients. We hypothesize that high levels of ROS in ATII cells in this disease contributes to mtDNA damage and stalling of TOP1-cc in mitochondria. Low tyrosyl-DNA-phosphodiesterase (TDP1) decreases the resolution of TOP1mt-cc, which contribute to mitochondrial dysfunction, ATII cell injury, and death.
2. Materials and methods
2.1. Human lungs and ATII cell isolation
Lungs were obtained from non-smoker or smoker organ donors through the Gift of Life Donor Program (Philadelphia, PA, USA). We selected donors without a history of chronic lung disease with reasonable lung function with a PaO2/FIO2 ratio of >225, X-ray and clinical history that did not indicate infection and limited time on a ventilator. Non-smokers never smoked and smokers smoked 5–20 cigarettes per day for at least 3 years. Lung tissue from patients with emphysema was obtained from lung transplantation through Temple Lung Center Biobank (Temple University, Philadelphia, PA, USA; N = 3–8 per group, 49–62 years old, females and males). ATII cells were isolated as we previously described [14,16,17] and freshly isolated ATII cells were used in this study. Briefly, we instilled 12.9 U/ml elastase (Worthington, Lakewood, NJ, USA) and the lung was minced followed by centrifugation to collect cell suspension. The cells were filtrated and purified using a density gradient made of Optiprep (Accurate Chemical Scientific Corp., Westbury, NY, USA). We used EpCAM microbeads (Miltenyi Biotec, Germany) for a positive selection as we reported [18].
Lung tissue cores were obtained from areas with mild and severe emphysema as we previously described [19]. Briefly, lungs were removed from the thorax, inflated with air and frozen by liquid nitrogen vapor followed by being cut into 2-cm thick slices in the same plane as the CT scan. Tissue cores were collected from areas with mild and severe emphysema using a sharpened steel cylinder diameter of 1 cm. Emphysema was quantified by the percent of the lung voxels on inspiratory CT scan with attenuation < −950 HU (Insp−950) [20]. CT scans were subjected to a standard quality control procedure. Computerized image analysis was performed with 3D SLICER software [21]. Emphysema was considered absent in subjects with values for Insp−950 < 4% in smokers, to account for the fact that the increased lung density in smokers results in a decrease in emphysema index [22]. Severe emphysema was defined by Insp−950 > 14% in smokers. Subjects (N = 8) provided written informed consent before surgery for the use of these specimens and the relevant clinical and radiological data required for research. The study was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Boards at Partners Healthcare and the Committee for the Protection of Human Subjects at Temple University.
2.2. Mitochondrial parameters
Mitochondrial function was analyzed in freshly isolated ATII cells from non-smokers, smokers and emphysema patients. Mitochondrial membrane potential (ΔΨm) was measured using the cationic potentiometric fluorescent dye, tetramethylrhodamine, methyl ester (TMRM; Thermo-Fisher, Waltham, MA, USA). ATII cells were incubated with 100 nM TMRM for 15 min at 37 °C and washed with PBS. The fluorescence intensity was monitored using a live cell confocal microscope (Zeiss). Mitochondrial superoxide generation was measured using MitoSOX Red (Thermo-Fisher). ATII cells were loaded with 10 μM MitoSOX Red for 10 min at 37 °C as we described previously [23]. MitoSOX Red fluorescence was quantified by using ImageJ software (NIH). Quantification of mitochondrial swelling was performed in ATII cells using SP-C (Millipore, Burlington, MA, USA) and TOM20 (Santa Cruz Biotechnology, TX, USA) staining and NIH ImageJ software [24]. The mitochondrial swelling index was calculated as the average of mitochondrial area/perimeter, normalized to mitochondrial circularity.
2.3. Analysis of mitochondrial DNA
MtDNA amount, mtDNA damage and common deletions were evaluated as previously described [25,26]. We used a DNA isolation kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. DNA concentration in all samples was quantitated by Nanodrop, and 15 ng was used for qPCR. We amplified 85 bp and 162 bp segments of nuclear and mtDNA, respectively. Primers used for qPCR are shown in Table E1A. MtDNA copy number was determined by the ratio of the mitochondrial to nuclear DNA fragments. ∆Ct method was applied to calculate the relative mtDNA copy number. For analysis of mtDNA damage, we used primers shown in Table E1B to amplify long (1.8 kb) and short mtDNA fragments (162 bp) by qPCR [27]. The fluorescence intensity from amplified long mtDNA fragments versus short mtDNA fragments reflects mtDNA damage. Common deletions indicate the most frequent inaccurately repaired mtDNA damage [28] and were analyzed by amplification of 15 ng of DNA by qPCR and normalized to short mtDNA fragment. Primers used for common deletions are shown in Table E1C.
2.4. RT-PCR
Total RNA was extracted from ATII cells and lung tissue obtained from control non-smokers, smokers, and emphysema patients using Trizol (Thermo-Fisher) according to the manufacturer's instructions. The cDNA synthesis was carried out using SuperScript IV Reverse Transcript kit (Thermo-Fisher). SYBR Green Master Mix (Thermo-Fisher) was applied for PCR amplification using a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Primers used for TDP1, MFN1, and DRP1 mRNA expression are listed in Table E2. Obtained values were normalized to 1 for control non-smokers. Data were analyzed using the ΔΔCt method.
2.5. Chromatin immunoprecipitation (CHIP) assay
Complexes of DJ-1 or TDP1 with DNA were obtained through pulldown using DJ-1 or TDP-1 antibodies (Santa Cruz Biotechnology), respectively followed by applying G Mag Sepharose beads (GE Healthcare, Bensalem, PA, USA) as previously published [29]. The qPCR was used to detect the presence of mtDNA among the isolated complexes and a short mtDNA fragment (162 bp) was amplified. We used ∆Ct method to calculate the relative mtDNA copy number. Housekeeping GAPDH was used as a control. Primers used for qPCR are listed in Table E3.
2.6. Western blotting
Western blotting was performed as we described previously [17,19,30]. Briefly, cells were lysed and lung tissue was homogenized. Mitochondrial fraction was prepared using subcellular fractionation kit (G-Biosciences, St. Louis, MO, USA) per manufacturer's instructions. Protein samples were separated by SDS-PAGE electrophoresis (Thermo-Fisher) and transferred to nitrocellulose membranes. We used the following antibodies: DRP1, TOM20, mtTFA, MFN1, MFN2, POLγ, TDP1, DJ-1 (all from Santa Cruz Biotechnology), TOP1-cc (Millipore), p-DRP1 (Ser616) (Cell Signaling Technology, Danvers, MA, USA), GAPDH (Abcam, Cambridge, MA, USA) and β-actin (Sigma, St. Louis, MO, USA). We used horseradish peroxidase (HRP)-conjugated AffiniPure donkey anti-rabbit immunoglobulin (Ig) G or anti-mouse IgG purchased from Jackson ImmunoResearch (West Grove, PA, USA). The blots were developed using an enhanced chemiluminesence kit for Western blotting (Millipore) according to the manufacturer's instructions. Images were quantitated using NIH Image J software.
2.7. Immunostaining
ATII cells or paraffin-embedded human lung tissue sections were incubated with SP-C, TOP1-cc (both from Millipore), SP-A (Novus Biologicals, Littleton, CO, USA), pro-SP-C, p-DRP1, TOM20, MFN1, OPA1, FIS1, p63, CD68, TDP1 or DJ-1 (all from Santa Cruz Biotechnology). Secondary antibodies Alexa Fluor 594, Alexa Fluor 488 or Alexa 647 (Invitrogen Corp., Carlsbad, CA, USA) were applied for 1 h. Mitochondrial nucleoids were identified by DNA-intercalating dye Picogreen (Lumiprobe, Hunt Valley, MD, USA) as previously described [29,31]. Sections were mounted with Vectashield medium containing DAPI (Abcam) to detect nuclei. Images were obtained using a confocal laser-scanning microscope (Zeiss). Pearson's correlation coefficient was used to analyze the colocalization of proteins of interest and TOM20 in SP-A-positive ATII cells in non-smokers, smokers, and patients with emphysema. Protein fluorescence intensity and colocalization were quantified by Image J (NIH) and normalized to control non-smokers as 1.
2.8. Statistical analysis
Data are expressed as the means ± s.e.m. Statistically significant differences among experimental groups were determined by one-way ANOVA. A value of p < .05 was considered significant.
3. Results
3.1. Mitochondrial dysfunction in primary ATII cells in emphysema patients
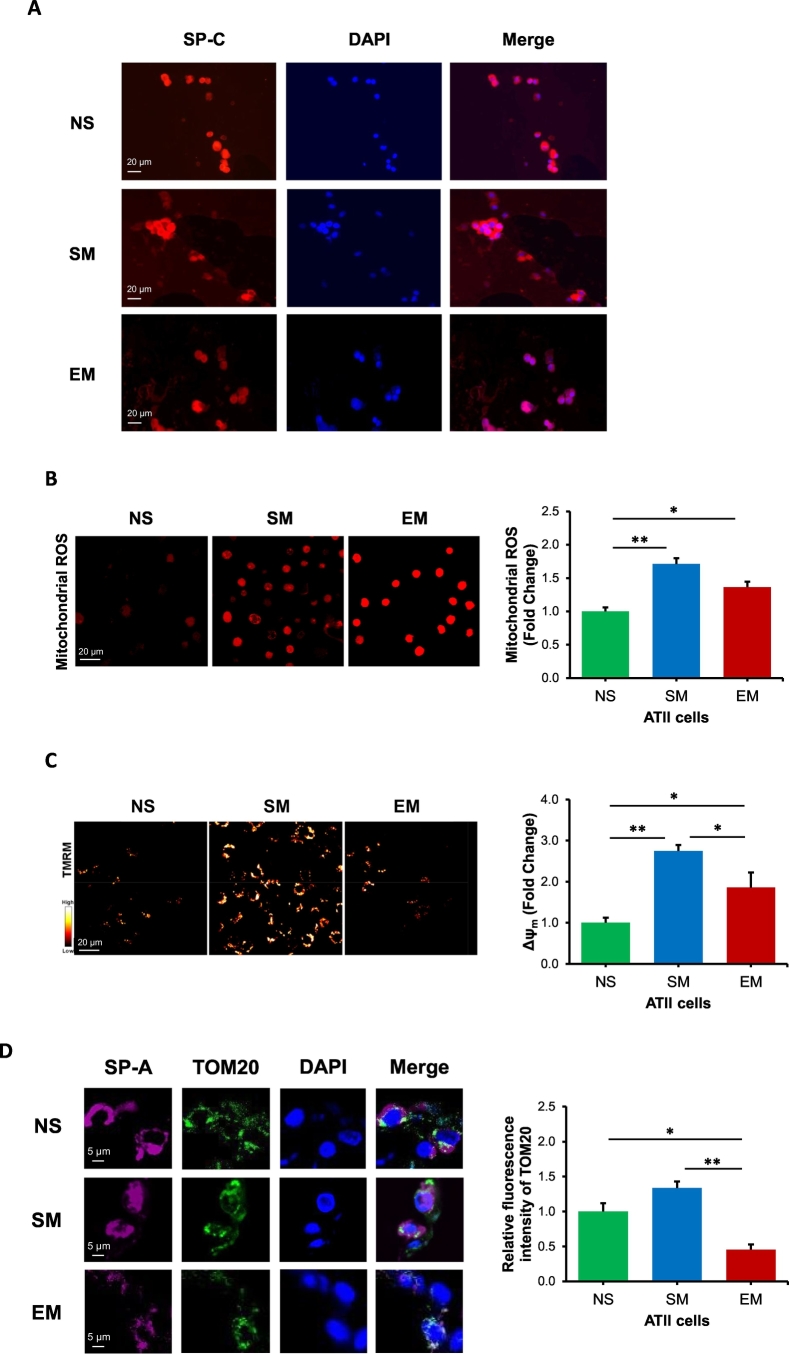
ATII cells were isolated as we previously described [32]. We recovered 200–300 × 106 ATII cells with no major difference in the number of cells among the non-smoker or smoker donor lungs. We isolated 50–100 × 106 ATII cells from lung transplants of emphysema patients. ATII cell viability was up to 93%. The purity of freshly isolated ATII cells was confirmed using SP-C, p63, and CD68 by immunocytofluorescence (Figs. 1A, E1). Mitochondria generate ATP and are important for ATII cell metabolism [5]. We further analyzed mitochondrial superoxide production, mitochondrial function and mtDNA amount in freshly isolated ATII cells. We found a significant increase in mitochondrial ROS fluorescence intensity in ATII cells obtained from smokers and emphysema patients in comparison with non-smokers (Fig. 1B). Interestingly, we detected significantly higher mitochondrial membrane potential (ΔΨm) in ATII cells isolated from smokers and individuals with emphysema in comparison with control non-smokers (Fig. 1C).
Fig. 1.
Mitochondrial dysfunction in human primary ATII cells in emphysema. Freshly isolated ATII cells from non-smokers (NS), smokers (SM) and emphysema patients (EM) were used for all experiments. A – Purity of freshly isolated ATII cells was determined by SP-C expression by immunocytofluorescence. B – Mitochondrial ROS generation was analyzed by MitoSOX using confocal fluorescence microscopy. C – Mitochondrial membrane potential was determined by TMRM and confocal fluorescence microscopy. D - TOM20 expression in ATII cells identified using SP-A antibody in lung tissue sections by immunohistofluorescence. E – MtTFA expression in ATII cells by Western blotting. F - MtDNA amount was analyzed by qPCR. G - Mitochondrial swelling in ATII cells. Data is expressed as means ± s.e.m., N = 3–6 lungs per group, *P < .05; **P < .001.
ROS is a trigger of mitochondrial fragmentation [33], and oxidative stress generated by cigarette smoke contributes to emphysema development [34]. We compared the mitochondrial levels in ATII cells. We found that ATII cells obtained from emphysema patients have decreased TOM20 expression in comparison with control smokers and non-smokers as detected by immunohistofluorescence using confocal microscopy (Figs. 1D, E2). This suggests a lower mitochondrial population in this disease compared to controls. We did not detect significant differences in mitochondrial abundance in ATII cells isolated from smokers and non-smokers. Next, we analyzed mitochondrial transcription factor A (mtTFA) expression, which has a critical role in mitochondrial biogenesis. Decreased mtTFA expression was observed in ATII cells in emphysema (Fig. 1E). We also determined mtDNA amount in ATII cells by qPCR analysis. Our results indicate decreased mtDNA in emphysema patients in comparison with non-smokers and smokers (Fig. 1F), which correlated with decreased TOM20 expression in ATII cells in individuals with this disease (Figs. 1D, E2). Of note, we did not detect differences in mtDNA amounts between smokers and non-smokers. Mitochondrial swelling was detected in ATII cells from emphysema compared to controls (Figs. 1G, E3). Together, our results indicate mitochondrial dysfunction in ATII cells in this disease.
3.2. The impairment of mitochondrial dynamics in ATII cells in emphysema
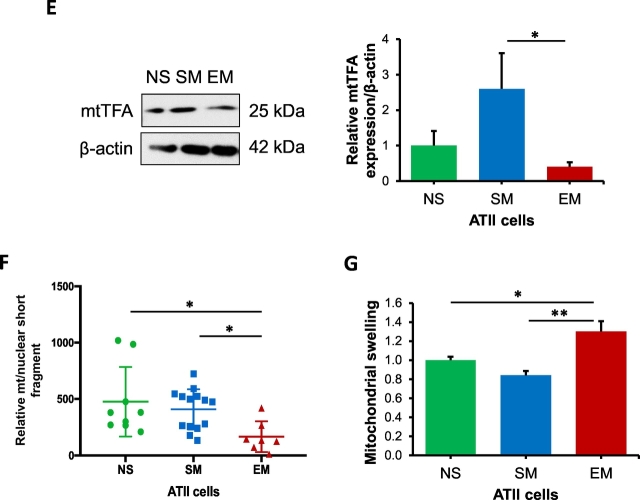
Mitochondrial fission removes damaged mitochondrial components through mitophagy [35]. We found higher p-DRP1 levels in freshly isolated ATII cells from smokers in comparison with non-smokers and emphysema by Western blotting (Fig. 2A). However, we did not detect significant differences in DRP1 mRNA expression between all groups by RT-PCR (Fig. 2B). Our results also indicate decreased p-DRP1 and TOM20 colocalization in smokers and individuals with emphysema compared to non-smokers by immunohistofluorescence (Fig. 2C). P-DRP1 activation leads to its translocation to mitochondria, which initiates mitochondrial fission [36,37]. Therefore, we also analyzed the levels of p-DRP1 in mitochondrial fractions obtained from lung tissue by Western blotting (Fig. 2D). Our results show a significant decrease in p-DRP1 expression in emphysema compared to non-smokers. In addition, lower FIS1 expression was observed in ATII cells in emphysema compared to smokers by immunocytofluorescence (Fig. 2E). The discrepancy between our results obtained using lung tissue and ATII cells can be caused by the presence of different cell types in former samples.
Fig. 2.
Decreased mitochondrial fission in ATII cells in emphysema. Freshly isolated ATII cells and lung tissue were obtained from non-smokers (NS), smokers (SM) and emphysema patients (EM). A - Western blot images of p-DRP1 expression in ATII cells. B – DRP1 mRNA expression in ATII cells. C – p-DRP1 expression (green) in mitochondria (red) in ATII cells (violet) identified using TOM20 and SP-A antibodies, respectively in lung tissue sections by immunohistofluorescence. Pearson's correlation coefficient was used to determine p-DRP1 and TOM20 co-localization in ATII cells. D – p-DRP1 levels and quantification in mitochondrial fractions obtained from lung tissue by Western blotting. E – FIS1 expression (green) in ATII cells (red) identified using pro-SP-C antibody in lung tissue sections by immunohistofluorescence. Data is expressed as means ± s.e.m., N = 3–4 lungs per group, *P < .05; **P < .001.
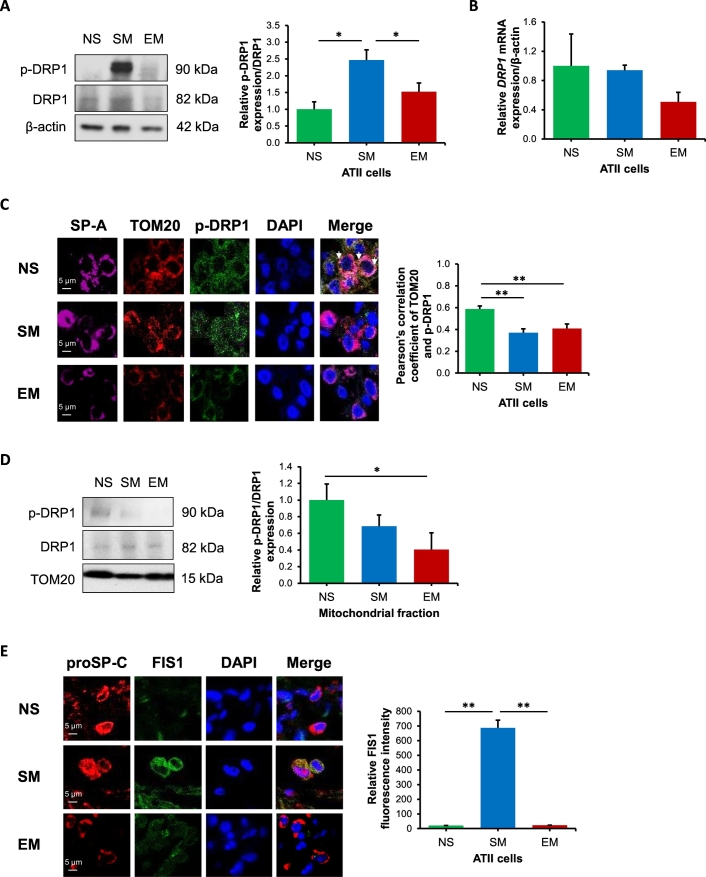
The fusion of mitochondria is considered as a rescue mechanism for damaged organelles [35]. We found lower MFN1 expression in ATII cells obtained from emphysema compared to smokers by Western blotting (Fig. 3A). Moreover, we detected increased MFN1 levels in ATII cells in smokers in comparison with non-smokers. The same correlation was found for MFN1 mRNA expression by RT-PCR (Fig. 3B). We also analyzed mitochondrial MFN1 levels in ATII cells using TOM20 and SP-A staining by immunohistofluorescence (Fig. 3C). We found decreased MFN1 and TOM20 colocalization in mitochondria in ATII cells in smokers and emphysema patients compared to non-smokers. In addition, we found lower MFN2 expression in ATII cells obtained from emphysema patients compared to smokers (Fig. E4). We also determined MFN1 expression in mitochondrial fractions obtained from lung tissue and observed its higher levels in emphysema patients compared to controls (Fig. 3D). The discrepancy between results obtained from ATII cells and lung tissue can be explained by different cell types present in the latter samples. Moreover, we observed decreased OPA1 expression in ATII cells obtained from emphysema compared to smokers by immunocytofluorescence (Fig. 3E). Our data suggest increased fission and fusion in ATII cells in smokers. On the other hand, we observed the impairment of these processes in ATII cells in emphysema, which suggests decreased mitochondrial dynamics.
Fig. 3.
Reduced mitochondrial fusion in ATII cells in emphysema. Freshly isolated ATII cells and lung tissue were obtained from non-smokers (NS), smokers (SM) and emphysema patients (EM). A – MFN1 expression was determined in ATII cells by Western blotting. MFN1 levels were normalized to β-actin and control non-smokers. B – MFN1 mRNA expression in ATII cells. C – MFN1 expression (green) in mitochondria (red) in ATII cells (violet) identified using TOM20 and SP-A antibodies, respectively in lung tissue sections by immunohistofluorescence. MFN1 and TOM20 co-localization in ATII cells is shown using Pearson's correlation coefficient. D – MFN1 levels and quantification in mitochondrial fractions obtained from lung tissue by Western blotting. MFN1 levels were normalized to TOM20 and control non-smokers. E – OPA1 expression in ATII cells identified by proSP-C marker in lung tissue sections. Data is expressed as means ± s.e.m., N = 3–7 lungs per group, *P < .05; **P < .001.
3.3. TOP1-cc formation in mitochondria in ATII cells in emphysema
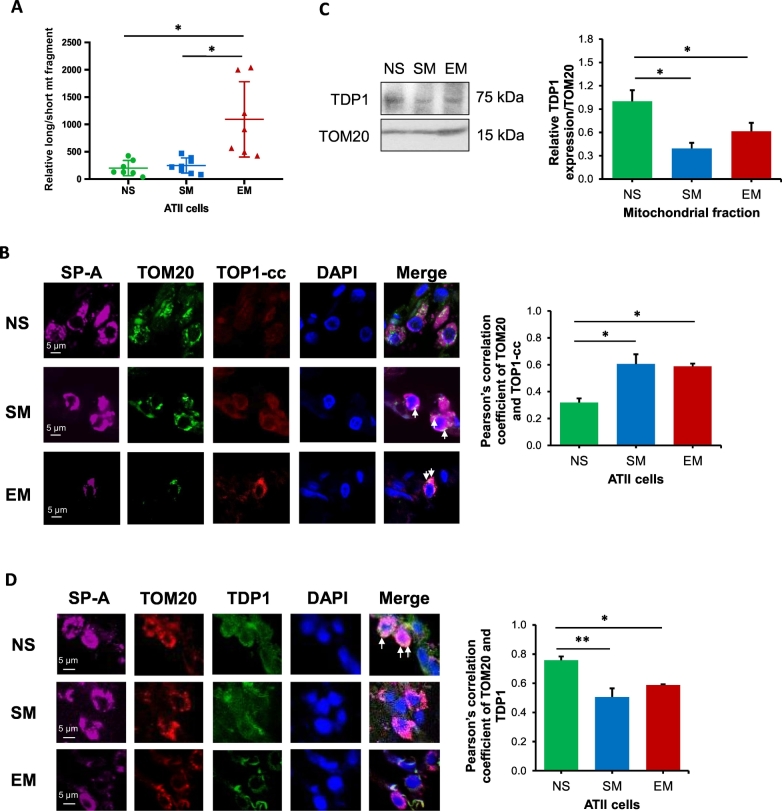
Lack of protective histones in mtDNA and its localization in the proximity to the mitochondrial respiratory chain increases mtDNA vulnerability to ROS-induced damage [38]. We found a significant increase in mtDNA damage in ATII cells in emphysema compared to control non-smokers and smokers (Fig. 4A). Interestingly, we did not see higher mtDNA damage in smokers in comparison with non-smokers. Next, we wanted to determine further the molecular mechanism involved in mtDNA damage observed in ATII cells in emphysema. We detected higher TOP1-cc and TOM20 co-localization in mitochondria in ATII cells in smokers and individuals with this disease by immunohistofluorescence, which suggests higher susceptibility to DNA damage (Fig. 4B). We also analyzed DNA polymerase γ (POLγ) expression, which is involved in replication-dependent repair pathway [39]. However, its levels were unaltered in ATII cells isolated from emphysema patients in comparison with controls by Western blotting (Fig. E5). Next, we checked whether observed high TOP1mt-cc levels were caused by the impairment of resolution of this covalent complex by TDP1. There was no significant difference in TDP1 expression in ATII cells isolated from all groups by Western blotting (Fig. E6A) and RT-PCR (Fig. E6B). We analyzed TDP1 levels in mitochondrial fractions obtained from lung tissue by Western blotting and detected lower TDP1 expression in smokers and emphysema compared to non-smokers (Fig. 4C). Decreased TDP1 co-localization in mitochondria was also observed in ATII cells in individuals with this disease and smokers using TOM20 and SP-A staining by immunohistofluorescence (Fig. 4D). However, increased mtDNA damage and decreased mtDNA amount was observed only in ATII cells in emphysema, which can be caused by the impairment of the resolution of TOP1mt-cc by TDP1. We further analyzed TDP1 localization in nucleoids in mitochondria in ATII cells as described below.
Fig. 4.
High mtDNA damage in ATII cells in emphysema. Freshly isolated ATII cells from non-smokers (NS), smokers (SM) and emphysema patients (EM) were used for all experiments. A – MtDNA damage was determined by qPCR and is shown as relative long/short mtDNA fragment. B – TOP1-cc levels (red) were analyzed in lung tissue sections by immunohistofluorescence. SP-A (violet) and TOM20 (green) were used to identify ATII cells and mitochondria, respectively. Co-localization of TOP1-cc in mitochondria using Pearson's correlation coefficient is also shown. C – TDP1 expression and quantification in mitochondrial fractions obtained from lung tissue by Western blotting. TDP1 levels were normalized to TOM20 and control non-smokers. D – TDP1 expression (green) was analyzed in mitochondria (red) in ATII cells (violet) in lung tissue sections. Pearson's correlation coefficient was used to determine TDP1 and TOM20 co-localization in ATII cells. Data is expressed as means ± s.e.m., N = 3–4 lungs per group, *P < .05; **P < .001.
3.4. The impairment of TOP1-cc resolution in mitochondria in emphysema progression
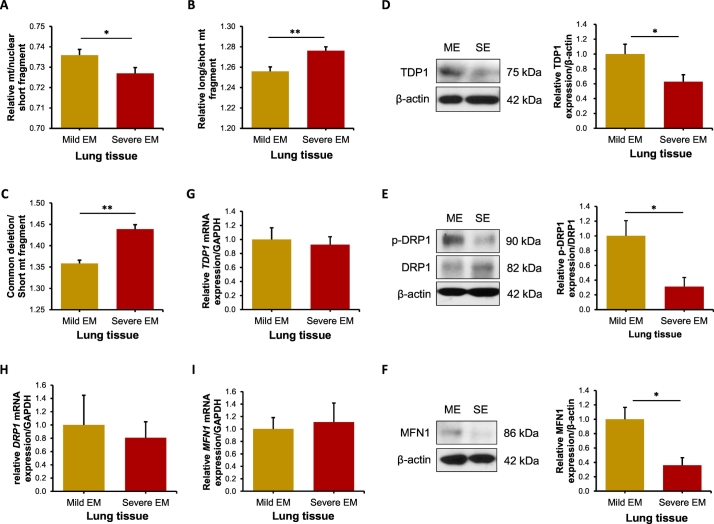
The pathophysiology of emphysema is not well understood [40]. We used lung tissue obtained from areas with mild and severe emphysema of the same patient as we previously described [19]. Our results indicate a significant decrease in mtDNA amount in severe compared to mild emphysema (Fig. 5A). We also found higher mtDNA damage in areas with severe than mild disease (Fig. 5B). Next, we analyzed the levels of common deletions, which reflect the most frequent inaccurately repaired mtDNA damage [28]. We detected greater mtDNA common deletion levels in severe in comparison with mild emphysema (Fig. 5C).
Fig. 5.
Increased mtDNA damage and decreased mtDNA amount and mitochondrial dynamics in emphysema progression. Lung tissue was obtained from areas with mild and severe emphysema (EM) of the same patient. MtDNA amount (mt/nuclear short fragment) (A), mtDNA damage (long/short mt fragment) (B) and common deletion (C) were determined by qPCR. TDP1 (D), p-DRP1 (E) and MFN1 (F) levels were determined in lung tissue by Western blotting. Protein expression was normalized to β-actin. TDP1 (G), DRP1 (H) and MFN1 (I) mRNA levels were determined by RT-PCR. Data is expressed as means ± s.e.m., N = 3–8 lungs per group, *P < .0, **P < .001.
Since we observed high TOP1-cc levels in mitochondria in emphysema patients compared to control non-smokers and smokers (Fig. 4B), we also determined TDP1 levels in emphysema progression. Interestingly, we found decreased TDP1 levels in severe emphysema (Fig. 5D).
Impaired mitochondrial fission and fusion was observed in ATII cells in emphysema compared to controls (Fig. 2, Fig. 3); therefore, we also analyzed mitochondrial dynamics in emphysema progression. Our data indicate lower p-DRP1 (Fig. 5E) and MFN1 (Fig. 5F) levels in areas with severe compared to mild disease. We determined TDP1 (Fig. 5G), DRP1 (Fig. 5H) and MFN1 (Fig. 5I) mRNA expression; however, we did not detect significant differences in emphysema progression. We also analyzed variability and did not observe significant differences between two mild or two severe regions obtained from individuals with this disease (Fig. E7). P-DRP1, MFN1 and TDP1 expression was determined in ATII cells in mild and severe emphysema by immunohistofluorescence (Fig. E8). Our results indicate their decreased levels compared to control smokers. Together, our data suggest high mtDNA damage and mitochondrial dysfunction in this disease progression.
3.5. Decreased TDP1 localization in nucleoids in mitochondria in ATII cells in emphysema
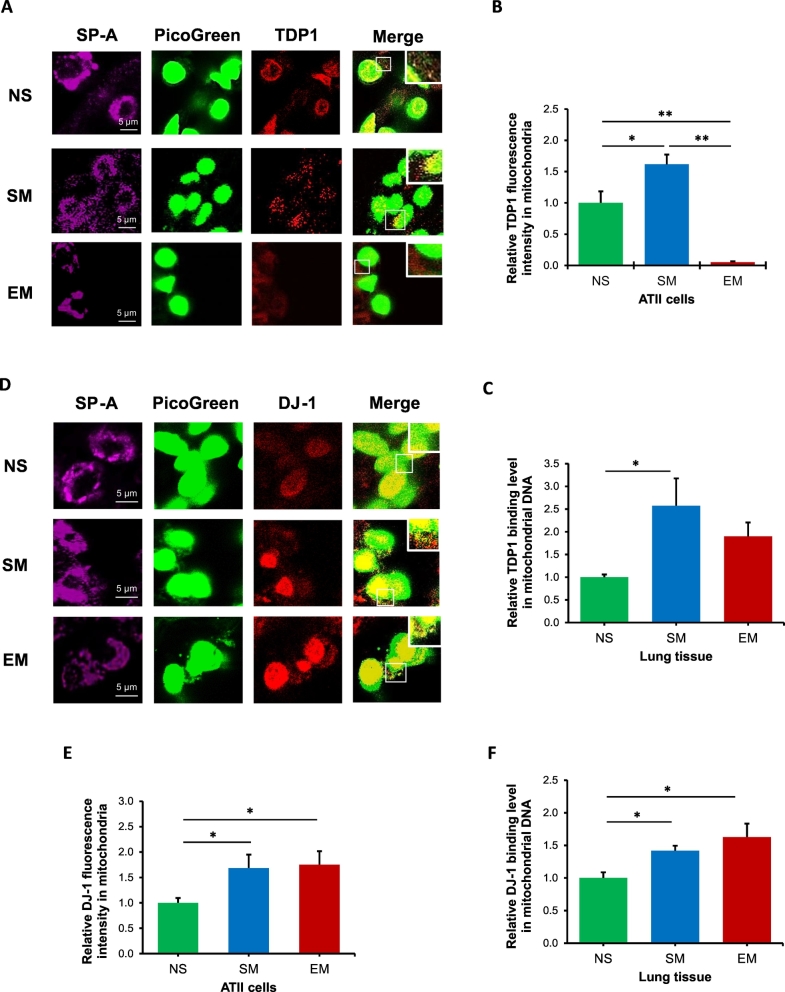
We found decreased TDP1 expression and increased TOP1mt-cc accumulation in mitochondria in ATII cells in smokers and emphysema (Fig. 4). We further analyzed TDP1 localization in mitochondrial nucleoids, which are mitochondrial DNA–protein structures containing a variety of mtDNA repair proteins [31]. First, we found TDP1 localization in nucleoids in ATII cells in smokers, providing further evidence of its mitochondrial function in these cells (Fig. 6A). Second, we detected lower mitochondrial TDP1 localization in nucleoids in ATII cells in emphysema compared to non-smokers and smokers (Fig. 6B), which suggests inefficient mtDNA damage repair. Higher TDP1 association with mtDNA in lung tissue by CHIP assay in smokers was observed, which indicates functional repair of potential mtDNA damage (Fig. 6C). Together, our data suggest an important role of TDP1 in nucleoids in mtDNA damage repair and mitochondrial function.
Fig. 6.
TDP1 and DJ-1 localization in nucleoids in mitochondria in ATII cells. Lung tissue was obtained from non-smokers (NS), smokers (SM) and emphysema patients (EM). A - TDP1 expression (red) was analyzed in ATII cells identified using SP-A antibody (violet) in lung tissue sections by immunohistofluorescence. MtDNA was detected by Picogreen (green). B – Relative TDP1 fluorescence intensity was determined in mitochondrial nucleoids in ATII cells. C - TDP1 binding to mtDNA was determined in lung tissue by CHIP assay. D - DJ-1 expression (red) was analyzed in mtDNA (green) in ATII cells (violet) by immunohistofluorescence. E - Relative DJ-1 fluorescence intensity in mitochondrial nucleoids in ATII cells is also shown. F – DJ-1 binding to mtDNA was analyzed in lung tissue by CHIP assay. Data is expressed as means ± s.e.m., N = 3 per group, *P < .05, **P < .001.
DJ-1 is a chaperone-like protein localized in mitochondria, cytoplasm, and nucleus [41]. We found DJ-1 localization in mitochondrial nucleoids in ATII cells (Fig. 6D) and higher DJ-1 levels in smokers and emphysema (Fig. 6E). To further analyze the role of DJ-1, we assessed its association with mtDNA in lung tissue by CHIP assay (Fig. 6F). Our results suggest that DJ-1 binds to mtDNA and higher binding activity was detected in smokers and emphysema patients compared to non-smokers. Further studies are required to determine whether this binding is direct or indirect and define DJ-1 oxidation status in mitochondria, which may correlate with this protein activity.
4. Discussion
Cigarette smoke causes a progressive disruption of alveolar maintenance and variable degrees of inflammation [42]. Susceptible individuals would have activated molecular and cellular processes involved in alveolar destruction, protease/antiprotease imbalance, oxidative stress, mitochondrial dysfunction, and apoptosis. It has been reported that mitochondrial function and transfer play an important role in the cellular bioenergetics, homeostasis of alveolar epithelium and tissue regeneration after lung injury [43]. Mitochondria are highly dynamic organelles and their fusion and fission are necessary to maintain their population [44]. The impairment of mitochondrial motility causes fragmentation of these organelles, alteration of mitochondrial membrane potential and decreased mtDNA amount leading to cell injury and death [45]. Fission provides quality control by segregating and targeting damaged mitochondria for elimination. This process is mediated by a cytosolic p-DRP1 and FIS1 [36,37,46]. DRP1 is recruited from the cytosol to form spirals around mitochondria that constrict to serve both inner and outer membranes. We found that ATII cells isolated from emphysema patients have decreased mitochondrial fission as detected by lower p-DRP1 and FIS1 levels compared to controls. Mitochondrial fusion serves to mix and unify the mitochondrial compartment and is thought to constitute a defense mechanism [47]. It is regulated by mitochondrial fusion proteins MFN1, MFN2 and OPA1. We found their lower expression in ATII cells isolated from emphysema patients in comparison with control smokers. This suggests impaired mitochondrial dynamics in this disease pathophysiology and progression, which may negatively affect mitochondrial function.
It was reported that ROS contributes to emphysema pathophysiology [48]. We found high mitochondrial superoxide production and mitochondrial membrane potential in ATII cells obtained from smokers and emphysema patients compared to non-smokers. Our results are in agreement with Ballweg et al. [49], who observed increased mitochondrial potential after MLE12 cell treatment with cigarette smoke extract. This was accompanied by functional mitochondrial changes, such as mitochondrial fragmentation and mitophagy, which depended on the degree of cellular stress. An increase in mitochondrial membrane potential may indicate disruption of mitochondrial functions on the inner or outer membrane and the early and necessary events for the initiation of apoptosis [50,51]. It has been well established that cells pretreated with F1F0-ATPase inhibitor oligomycin exhibits hyperpolarization of mitochondrial potential followed by cell death [52]. In this context, the high mitochondrial membrane potential promotes proton leak rather than ATP production. Our results suggest that cigarette smoke may cause mitochondrial hyperpolarization, which could participate in ATII cell death with low ATP levels.
MtTFA plays essential roles in the transcription, replication, and packaging of circular mtDNA into nucleoids and has a critical function in mitochondrial biogenesis [53]. Our data indicate decreased mtTFA expression in ATII cells in emphysema. We also analyzed mtDNA amount and mtDNA damage as well as activation of DNA damage repair to determine their contribution to mitochondrial function. ATII cells isolated from emphysema patients had higher mtDNA damage and impaired mtDNA damage repair. We detected mitochondrial dysfunction with this disease progression. Interestingly, we observed lower mtDNA damage and higher mtDNA amount in control smokers compared to emphysema patients. Of note, both groups had high mitochondrial superoxide levels. This suggests an efficient system of ROS-induced mtDNA damage repair below a cellular critical threshold in smokers. It may also indicate an adaptation to cellular stress induced by cigarette smoke [54]. On the other hand, low mitochondrial amount and mitochondrial swelling observed in ATII cells in emphysema may contribute to these organelles dysfunction. Mitochondrial swelling has been reported in primary bronchial epithelial cells obtained from COPD patients [55]. Together, our data suggest that mitochondrial dysfunction correlates with high mtDNA damage and low mtDNA damage repair in this disease.
We further investigated the mechanism of mitochondrial dysfunction in ATII cells in emphysema. MtDNA is susceptible to oxidative stress-induced damage [56]. Although it is predicted that mtDNA damage is repaired by nuclear double-strand DNA breaks (DSBs) repair proteins, the mechanisms underlying these processes are poorly understood. Topoisomerases have been extensively studied in the cancer field [13], but their contribution to lung diseases is only beginning to emerge. To further study the molecular mechanism, we analyzed TOP1mt-cc levels, which are formed by stalling mitochondrial TOP1 at mtDNA lesions [57]. TOP1 can also be trapped on oxidized damaged DNA [58]. The most frequent mtDNA aberrancy is the “common deletion”, which is located at the 8483–13,459 bp of the mitochondria genome [59]. We found a higher formation of common deletion in emphysema progression. This may be caused by an excessive TOP1mt-cc formation by oxidized DNA induced by high ROS levels. Furthermore, several studies demonstrated the involvement of TDP1 in the repair of a wide variety of oxidized DNA fragments [60]. TDP1 has an enzymatic activity that resolves TOP1mt-cc [57] and is also involved in DNA breaks repair including non-homologous end joining (NHEJ), a pathway that repairs nuclear DSBs [61]. However, TDP1 function in mitochondria is not well known. It has been shown that TOP1 depletion led to marked protection from cell death induced by oxidative stress, suggesting that TOP1-mediated DNA breaks are major contributors to cell injury, especially in situations where TDP1 is limiting [62]. We detected high mitochondrial ROS levels in both smokers and emphysema patients. However, high mtDNA damage in ATII cells in this disease suggests the impairment of mtDNA damage repair. This may cause accumulation of aberration leading to dysregulated mitochondrial dynamics and function. Recently, in human COPD, lung epithelial cells displayed increased expression of the mitophagy proteins. These findings implicate mitochondrial dysfunction in this disease [63,64]. Together, our results suggest an important role of TDP1 in nucleoids in mtDNA damage repair and mitochondrial function.
DJ-1 is a multifunctional protein localized in mitochondria, cytoplasm, and nucleus [15]. It has a redox sensitive cysteine at position 106, which can be easily oxidized [65]. We have shown the cytoplasmic and nuclear role of DJ-1 in human primary ATII cells obtained from smokers [14]. Of note, high ROS levels can cause DJ-1 overoxidation leading to loss of protein structure and biological function [66]. We performed a CHIP assay to analyze the DJ-1 role in mitochondria. We found increased DJ-1 binding to mtDNA in smokers and emphysema. Although, there are potential DJ-1 binding sites with different sequences in the human genome, is not clear whether DJ-1 directly binds to DNA or via other DNA-binding factors [67]. Further studies are required to assess DJ-1 oxidation on binding activity, function and localization in mitochondrial nucleoids in ATII cells in emphysema.
In summary, we observed high mtDNA damage, low mitochondrial amount and impaired mitochondrial dynamics in ATII cells in emphysema and this disease progression (Fig. E9). This suggests mitochondrial dysfunction and decreased mtDNA damage repair in this disease.
Author contributions
All authors provided intellectual input and critical feedback. B.K. and K.B. designed the study and wrote the manuscript. B.K., C.R.L., L.K., D.T., L.V., and K.B. performed experiments and analyzed results. D.T. and M.M. analyzed the mitochondrial function and provided critical reagents. N.M., S.B. and G.J.C. contributed to the design and interpretation of the study and provided human lungs.
Declaration of Competing Interest
None.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grant R01 HL118171 (B.K.) and the Catalyst Award from the American Lung Association (K.B.). The authors thank the Lung Center Tissue Bank at Temple University for providing the human lung specimens.
Footnotes
This article has an online supplementary material, which is accessible from this issue's table of contents at www.ebiomedicine.com. Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.07.063.
Contributor Information
Beata Kosmider, Email: beata.kosmider@temple.edu.
Karim Bahmed, Email: karim.bahmed@temple.edu.
Appendix A. Supplementary data
Supplementary material
References
- 1.Boukhenouna S., Wilson M.A., Bahmed K., Kosmider B. Reactive oxygen species in chronic obstructive pulmonary disease. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/5730395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman I., MacNee W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic Biol Med. 1996;21(5):669–681. doi: 10.1016/0891-5849(96)00155-4. [DOI] [PubMed] [Google Scholar]
- 3.Meek K., Dang V., Lees-Miller S.P. DNA-PK: the means to justify the ends? Adv Immunol. 2008;99:33–58. doi: 10.1016/S0065-2776(08)00602-0. [DOI] [PubMed] [Google Scholar]
- 4.Mason R.J. Biology of alveolar type II cells. Respirology. 2006;11 Suppl doi: 10.1111/j.1440-1843.2006.00800.x. S12-5. [DOI] [PubMed] [Google Scholar]
- 5.Guillot L., Nathan N., Tabary O., Thouvenin G., Le Rouzic P., Corvol H. Alveolar epithelial cells: master regulators of lung homeostasis. Int J Biochem Cell Biol. 2013;45(11):2568–2573. doi: 10.1016/j.biocel.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Scarpulla R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88(2):611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee A., Dasgupta S., Sidransky D. Mitochondrial subversion in cancer. Cancer Prev Res (Phila) 2011;4(5):638–654. doi: 10.1158/1940-6207.CAPR-10-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra P., Chan D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol. 2014;15(10):634–646. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cloonan S.M., Choi A.M. Mitochondria in lung disease. J Clin Invest. 2016;126(3):809–820. doi: 10.1172/JCI81113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X., Yang H., Sun H., Lu R., Zhang C., Gao N. Taurine ameliorates particulate matter-induced emphysema by switching on mitochondrial NADH dehydrogenase genes. Proc Natl Acad Sci U S A. 2017;114(45) doi: 10.1073/pnas.1712465114. E9655-E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal A., Mabalirajan U. Rejuvenating cellular respiration for optimizing respiratory function: targeting mitochondria. Am J Physiol Lung Cell Mol Physiol. 2016;310(2):L103–L113. doi: 10.1152/ajplung.00320.2015. [DOI] [PubMed] [Google Scholar]
- 12.Chiruvella K.K., Liang Z., Wilson T.E. Repair of double-strand breaks by end joining. Cold Spring Harb Perspect Biol. 2013;5(5) doi: 10.1101/cshperspect.a012757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nitiss J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9(5):338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bahmed K., Messier E.M., Zhou W., Tuder R.M., Freed C.R., Chu H.W. DJ-1 modulates Nrf2-mediated protection in human primary alveolar type II cells in smokers. Am J Respir Cell Mol Biol. 2016;55(3):439–449. doi: 10.1165/rcmb.2015-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCoy M.K., Cookson M.R. DJ-1 regulation of mitochondrial function and autophagy through oxidative stress. Autophagy. 2011;7(5):531–532. doi: 10.4161/auto.7.5.14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin CR B., Bahmed K R.J., Tomar D K., Kosmider B., Marchetti N R.J., Criner GJ K., Bolla S R.J., Wilson MA K., Madesh M R.J., Kosmider B K. The relationship between DJ-1 and S100A8 in human primary alveolar type II cells in emphysema. Am J Physiol Lung Cell Mol Physiol. 2019 doi: 10.1152/ajplung.00494.2018. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messier E.M., Day B.J., Bahmed K., Kleeberger S.R., Tuder R.M., Bowler R.P. N-acetylcysteine protects murine alveolar type II cells from cigarette smoke injury in a nuclear erythroid 2-related factor-2-independent manner. Am J Respir Cell Mol Biol. 2013;48(5):559–567. doi: 10.1165/rcmb.2012-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosmider B., Mason R.J., Bahmed K. Isolation and characterization of human alveolar type II cells. Methods Mol Biol. 2018;1809:83–90. doi: 10.1007/978-1-4939-8570-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin C.R., Bahmed K., Criner G.J., Marchetti N., Tuder R.M., Kelsen S. S100A8 protects human primary alveolar type II cells against injury and emphysema. Am J Respir Cell Mol Biol. 2019;60(3):299–307. doi: 10.1165/rcmb.2018-0144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gevenois P.A., De Vuyst P., de Maertelaer V., Zanen J., Jacobovitz D., Cosio M.G. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154(1):187–192. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- 21.Hersh C.P., Washko G.R., Jacobson F.L., Gill R., Estepar R.S., Reilly J.J. Interobserver variability in the determination of upper lobe-predominant emphysema. Chest. 2007;131(2):424–431. doi: 10.1378/chest.06-1040. [DOI] [PubMed] [Google Scholar]
- 22.Shaker S.B., Stavngaard T., Laursen L.C., Stoel B.C., Dirksen A. Rapid fall in lung density following smoking cessation in COPD. COPD. 2011;8(1):2–7. doi: 10.3109/15412555.2010.541306. [DOI] [PubMed] [Google Scholar]
- 23.Mukhopadhyay P., Rajesh M., Hasko G., Hawkins B.J., Madesh M., Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc. 2007;2(9):2295–2301. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dagda R.K., Zhu J., Kulich S.M., Chu C.T. Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: implications for Parkinson's disease. Autophagy. 2008;4(6):770–782. doi: 10.4161/auto.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furda A., Santos J.H., Meyer J.N., Van Houten B. Quantitative PCR-based measurement of nuclear and mitochondrial DNA damage and repair in mammalian cells. Methods Mol Biol. 2014;1105:419–437. doi: 10.1007/978-1-62703-739-6_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santos J.H., Meyer J.N., Mandavilli B.S., Van Houten B. Quantitative PCR-based measurement of nuclear and mitochondrial DNA damage and repair in mammalian cells. Methods Mol Biol. 2006;314:183–199. doi: 10.1385/1-59259-973-7:183. [DOI] [PubMed] [Google Scholar]
- 27.Bakkour S., Chafets D.M., Wen L., van der Meer P.F., Mundt J.M., Marschner S. Development of a mitochondrial DNA real-time polymerase chain reaction assay for quality control of pathogen reduction with riboflavin and ultraviolet light. Vox Sang. 2014;107(4):351–359. doi: 10.1111/vox.12173. [DOI] [PubMed] [Google Scholar]
- 28.Chen T., He J., Shen L., Fang H., Nie H., Jin T. The mitochondrial DNA 4,977-bp deletion and its implication in copy number alteration in colorectal cancer. BMC Med Genet. 2011;12(8):1–9. doi: 10.1186/1471-2350-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wisnovsky S., Jean S.R., Kelley S.O. Mitochondrial DNA repair and replication proteins revealed by targeted chemical probes. Nat Chem Biol. 2016;12(7):567–573. doi: 10.1038/nchembio.2102. [DOI] [PubMed] [Google Scholar]
- 30.Tan L.H., Bahmed K., Lin C.R., Marchetti N., Bolla S., Criner G.J. The cytoprotective role of DJ-1 and p45 NFE2 against human primary alveolar type II cell injury and emphysema. Sci Rep. 2018;8(1):3555. doi: 10.1038/s41598-018-21790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashley N., Harris D., Poulton J. Detection of mitochondrial DNA depletion in living human cells using PicoGreen staining. Exp Cell Res. 2005;303(2):432–446. doi: 10.1016/j.yexcr.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Kosmider B., Lin C.R., Vlasenko L., Marchetti N., Bolla S., Criner G.J. Impaired non-homologous end joining in human primary alveolar type II cells in emphysema. Sci Rep. 2019;9:920. doi: 10.1038/s41598-018-37000-z. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman J.R., Nunnari J. Mitochondrial form and function. Nature. 2014;505(7483):335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldkorn T., Filosto S., Chung S. Lung injury and lung cancer caused by cigarette smoke-induced oxidative stress: molecular mechanisms and therapeutic opportunities involving the ceramide-generating machinery and epidermal growth factor receptor. Antioxid Redox Signal. 2014;21(15):2149–2174. doi: 10.1089/ars.2013.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni H.M., Williams J.A., Ding W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6–13. doi: 10.1016/j.redox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zemirli N., Morel E., Molino D. Mitochondrial dynamics in basal and stressful conditions. Int J Mol Sci. 2018;19(2):1–19. doi: 10.3390/ijms19020564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu C., Huang Y., Li L. Drp1-dependent mitochondrial fission plays critical roles in physiological and pathological progresses in mammals. Int J Mol Sci. 2017;18(1):1–19. doi: 10.3390/ijms18010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nita M., Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev. 2016;2016:3164734. doi: 10.1155/2016/3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishra A., Saxena S., Kaushal A., Nagaraju G. RAD51C/XRCC3 facilitates mitochondrial DNA replication and maintains integrity of the mitochondrial genome. Mol Cell Biol. 2018;38(3) doi: 10.1128/MCB.00489-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taraseviciene-Stewart L., Burns N., Kraskauskas D., Nicolls M.R., Tuder R.M., Voelkel N.F. Mechanisms of autoimmune emphysema. Proc Am Thorac Soc. 2006;3(6):486–487. doi: 10.1513/pats.200603-063MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Lazzari F., Bisaglia M. DJ-1 as a deglycating enzyme: a unique function to explain a multifaceted protein? Neural Regen Res. 2017;12(11):1797–1798. doi: 10.4103/1673-5374.219035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuder R.M., Yoshida T., Arap W., Pasqualini R., Petrache I. State of the art. Cellular and molecular mechanisms of alveolar destruction in emphysema: an evolutionary perspective. Proc Am Thorac Soc. 2006;3(6):503–510. doi: 10.1513/pats.200603-054MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Islam M.N., Das S.R., Emin M.T., Wei M., Sun L., Westphalen K. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suen D.F., Norris K.L., Youle R.J. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22(12):1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knott A.B., Perkins G., Schwarzenbacher R., Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9(7):505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pagliuso A., Cossart P., Stavru F. The ever-growing complexity of the mitochondrial fission machinery. Cell Mol Life Sci. 2018;75(3):355–374. doi: 10.1007/s00018-017-2603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westermann B. Molecular machinery of mitochondrial fusion and fission. J Biol Chem. 2008;283(20):13501–13505. doi: 10.1074/jbc.R800011200. [DOI] [PubMed] [Google Scholar]
- 48.Trocme C., Deffert C., Cachat J., Donati Y., Tissot C., Papacatzis S. Macrophage-specific NOX2 contributes to the development of lung emphysema through modulation of SIRT1/MMP-9 pathways. J Pathol. 2015;235(1):65–78. doi: 10.1002/path.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ballweg K., Mutze K., Konigshoff M., Eickelberg O., Meiners S. Cigarette smoke extract affects mitochondrial function in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2014;307(11):L895–L907. doi: 10.1152/ajplung.00180.2014. [DOI] [PubMed] [Google Scholar]
- 50.Ling Y.H., Liebes L., Zou Y., Perez-Soler R. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to Bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J Biol Chem. 2003;278(36):33714–33723. doi: 10.1074/jbc.M302559200. [DOI] [PubMed] [Google Scholar]
- 51.Gottlob K., Majewski N., Kennedy S., Kandel E., Robey R.B., Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15(11):1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perry S.W., Norman J.P., Barbieri J., Brown E.B., Gelbard H.A. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50(2):98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee W.R., Na H., Lee S.W., Lim W.J., Kim N., Lee J.E. Transcriptomic analysis of mitochondrial TFAM depletion changing cell morphology and proliferation. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-18064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrusca D.N., Van Demark M., Gu Y., Justice M.J., Rogozea A., Hubbard W.C. Smoking exposure induces human lung endothelial cell adaptation to apoptotic stress. Am J Respir Cell Mol Biol. 2014;50(3):513–525. doi: 10.1165/rcmb.2013-0023OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoffmann R.F., Zarrintan S., Brandenburg S.M., Kol A., de Bruin H.G., Jafari S. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir Res. 2013;14:97. doi: 10.1186/1465-9921-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Madsen-Bouterse S.A., Zhong Q., Mohammad G., Ho Y.S., Kowluru R.A. Oxidative damage of mitochondrial DNA in diabetes and its protection by manganese superoxide dismutase. Free Radic Res. 2010;44(3):313–321. doi: 10.3109/10715760903494168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiang S.C., Meagher M., Kassouf N., Hafezparast M., McKinnon P.J., Haywood R. Mitochondrial protein-linked DNA breaks perturb mitochondrial gene transcription and trigger free radical-induced DNA damage. Sci Adv. 2017;3(4) doi: 10.1126/sciadv.1602506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sordet O., Khan Q.A., Pommier Y. Apoptotic topoisomerase I-DNA complexes induced by oxygen radicals and mitochondrial dysfunction. Cell Cycle. 2004;3(9):1095–1097. [PubMed] [Google Scholar]
- 59.Phillips A.F., Millet A.R., Tigano M., Dubois S.M., Crimmins H., Babin L. Single-molecule analysis of mtDNA replication uncovers the basis of the common deletion. Mol Cell. 2017;65(3):527–538. doi: 10.1016/j.molcel.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 60.El-Khamisy S.F., Saifi G.M., Weinfeld M., Johansson F., Helleday T., Lupski J.R. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature. 2005;434(7029):108–113. doi: 10.1038/nature03314. [DOI] [PubMed] [Google Scholar]
- 61.Bahmed K., Nitiss K.C., Nitiss J.L. UnTTrapping the ends: a new player in overcoming protein linked DNA damage. Cell Res. 2010;20(2):122–123. doi: 10.1038/cr.2010.17. [DOI] [PubMed] [Google Scholar]
- 62.Liao C., Beveridge R., Hudson J.J.R., Parker J.D., Chiang S.C., Ray S. UCHL3 regulates topoisomerase-induced chromosomal break repair by controlling TDP1 proteostasis. Cell Rep. 2018;23(11):3352–3365. doi: 10.1016/j.celrep.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryter S.W., Choi A.M. Autophagy in lung disease pathogenesis and therapeutics. Redox Biol. 2015;4:215–225. doi: 10.1016/j.redox.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mizumura K., Cloonan S.M., Nakahira K., Bhashyam A.R., Cervo M., Kitada T. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest. 2014;124(9):3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waak J., Weber S.S., Gorner K., Schall C., Ichijo H., Stehle T. Oxidizable residues mediating protein stability and cytoprotective interaction of DJ-1 with apoptosis signal-regulating kinase 1. J Biol Chem. 2009;284(21):14245–14257. doi: 10.1074/jbc.M806902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ariga H., Takahashi-Niki K., Kato I., Maita H., Niki T., Iguchi-Ariga S.M. Neuroprotective function of DJ-1 in Parkinson's disease. Oxid Med Cell Longev. 2013;2013 doi: 10.1155/2013/683920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamane T., Suzui S., Kitaura H., Takahashi-Niki K., Iguchi-Ariga S.M., Ariga H. Transcriptional activation of the cholecystokinin gene by DJ-1 through interaction of DJ-1 with RREB1 and the effect of DJ-1 on the cholecystokinin level in mice. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0078374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material