Abstract
Background
Severe energy deficits during military operations, produced by significant increases in exercise and limited dietary intake, result in conditions that degrade lean body mass and lower-body muscle function, which may be mediated by concomitant reductions in circulating testosterone.
Methods
We conducted a three-phase, proof-of-concept, single centre, randomised, double-blind, placebo-controlled trial (CinicalTrials.gov, NCT02734238) of non-obese men: 14-d run-in, free-living, eucaloric diet phase; 28-d live-in, 55% exercise- and diet-induced energy deficit phase with (200 mg testosterone enanthate per week, Testosterone, n = 24) or without (Placebo, n = 26) exogenous testosterone; and 14-d recovery, free-living, ad libitum diet phase. Body composition was the primary end point; secondary endpoints included lower-body muscle function and health-related biomarkers.
Findings
Following energy deficit, lean body mass increased in Testosterone and remained stable in Placebo, such that lean body mass significantly differed between groups [mean difference between groups (95% CI), 2.5 kg (3.3, 1.6); P < .0001]. Fat mass decreased similarly in both treatment groups [0.2 (−0.4, 0.7), P = 1]. Change in lean body mass was associated with change in total testosterone (r = 0.71, P < .0001). Supplemental testosterone had no effect on lower-body muscle function or health-related biomarkers.
Interpretation
Findings suggest that supplemental testosterone may increase lean body mass during short-term severe energy deficit in non-obese, young men, but it does not appear to attenuate lower-body functional decline.
Funding
Collaborative Research to Optimize Warfighter Nutrition projects I and II, Joint Program Committee-5, funded by the US Department of Defence.
Keywords: Hypothalamic-pituitary-gonadal axis, Hypogonadism, Military operational stress, Semi-starvation, Anabolism, Muscle mass, Lower-body muscle function
Research in context.
Evidence before this study
PubMed was searched for clinical trials published in English up to August 1, 2018, using search terms that included “weight loss”, “starvation”, “testosterone”, “hypogonadism”, “young men”, “non-obese”, “energy deficit”, “military”, and “muscle mass” alone or in combination. There have been no studies that have assessed whether restoring testosterone minimizes the catabolic effects of severe exercise- and diet-induced energy deficit in non-obese, young men reflective of military personnel participating in strenuous military operations.
Added value of this study
This proof-of-concept, single centre, randomised, double-blind, placebo controlled trial is the first, to our knowledge, assessing the efficacy of supplemental testosterone on body composition, muscle function, and clinical health biomarkers in healthy, non-obese, young men exposed to 28 d of severe exercise- and diet-induced energy deficit representative of strenuous military training and combat operations. Our study was rigorously controlled, particularly during the 28 d intervention phase, as diet and physical activity were individualized and continuously supervised on the inpatient unit at the Pennington Biomedical Research Centre. Despite the 28 d severe exercise- and diet-induced energy deficit, individuals receiving weekly injections of supplemental testosterone experienced an increase in lean mass and an attenuation in total weight loss compared to controls, with no difference in lower-body muscle functional decline or clinical health biomarkers.
Implications of all available evidence
Short-term (4 wk) use of supplemental testosterone minimizes weight loss and promotes lean mass gain in non-obese, young men exposed to military-relevant exercise- and diet-induced energy deficit, although the lean mass gain did not translate to improvements in muscle function. These data support further exploration of various pharmacologic interventions to mitigate the loss of muscle experienced by military personnel during periods of unavoidable, severe energy deficit, particularly those that may also safely enhance performance.
Alt-text: Unlabelled Box
1. Introduction
US military personnel conducting strenuous training and combat operations commonly experience sustained periods of severe, unavoidable energy deficit, the effects of which resemble the pathophysiology of semi-starvation [[1], [2], [3]]. Energy deficits produced during strenuous operations are due, in large part, to increases in aerobic-type physical activity and restricted dietary intake, which often result in conditions that degrade both lean body mass and lower-body muscle function [4]. Further, many military personnel experience these severe and unavoidable periods of exercise- and diet-induced energy deficit and associated loss of lean body mass repeatedly over their military career, raising concerns about the potential accumulated health effects of those energy deficits. The US military has sponsored considerable research to develop nutritional countermeasures to mitigate lean body mass loss under these conditions; however, dietary interventions, including those attempting to leverage the anabolic potential of increased protein intake, have been ineffective during severe energy deficit [[5], [6], [7]].
The extent to which lean body mass is lost in men during severe energy deficit may, in part, be attributable to reductions in testosterone. Energy deficits inhibit the hypothalamic-pituitary-gonadal axis by preventing luteinising hormone release and testosterone synthesis [8]. Reductions in testosterone are accompanied by muscle atrophy and decreased muscle strength [9]. Testosterone status is markedly degraded during sustained military operations that involve prolonged, strenuous physical work, reduced energy intake, altered sleep, and psychological stress. Most notably, Friedl et al. [1] demonstrated a 50–65% decline in testosterone in male Soldiers during the first 28 d of US Army Ranger School. Therefore, exogenous testosterone administration may be an effective biomedical strategy to counter declines in testosterone, lean body mass, and physical function that result from severe energy deficit.
In a recent study, supplementing obese men who had low testosterone concentrations (≤12.15 nmol/L) with exogenous testosterone during 56-wk of underfeeding resulted in significantly more fat mass and less lean body mass loss than those receiving placebo injections [10]. However, no study, to our knowledge, has attempted to induce testosterone decrements by replicating the stressors responsible for energy deficits observed during military operations or test the subsequent efficacy and safety of supplemental testosterone for maintaining lean body mass and lower-body muscle function in non-obese males exposed to those military-relevant stressors. The purpose of this study was to evaluate the effects of supplemental testosterone on body composition and lower-body muscle function in non-obese, young adult males, representative of US military personnel, exposed to severe exercise- and diet-induced energy deficit for 28 d. We hypothesized that the group receiving supplemental testosterone during energy deficit would lose more fat mass, less lean body mass, and an equivalent amount of total body mass and have attenuated lower-body muscle functional declines compared to placebo. In addition, we hypothesized that testosterone supplementation during the energy deficit, compared to placebo, would result in more lean body mass accretion and less fat mass gain during recovery from exercise- and diet-induced energy deficit [2,11].
2. Materials and methods
2.1. Participants
Participants were recruited from the Baton Rouge, LA community. Procedures regarding recruitment, randomisation, and follow-up of this study are provided in Fig. 1. Participants were healthy (no cardiometabolic disorders), physically active (≥2-d/wk aerobic and/or resistance exercise) men aged 18–39 y, with total testosterone concentrations within normal physiological range [10.4–34.7 nmol/L, based on clinical practice guidelines at the time the study was conceived [12]]. Participants met age-specific US Army body composition standards [13]. Participants with prostate-specific antigen concentrations > 3 μg/L, haematocrit > 50%, a positive urine drug screen, or reporting anabolic steroid, human growth hormone, or nutritional testosterone precursor-like supplement use within the past 6-mo were excluded. Additional participant inclusion and exclusion criteria as well as details of the recruitment process are published elsewhere [14]. This study was approved by the Pennington Biomedical Research Centre Institutional Review Board and the Human Research Protection Office of the US Army Medical Research and Materiel Command. A data safety monitoring board, consisting of a biostatistician, an exercise physiologist, a clinician, and a layperson, oversaw the safety of participants, monitored recruitment and adherence, and reviewed adverse events, proposed modifications, and reports of related studies as appropriate. The data safety monitoring board was blinded to the study treatments, received quarterly reports, met annually, and provided written documentation of their assessments and recommendations for study continuation. Extended details of the roles and responsibilities of the data and safety monitoring board are provided as Supplementary Material. Participants provided written informed consent. Participants were studied between April 12, 2016 and September 15, 2017. This study is registered at www.clinicaltrials.gov as NCT02734238.
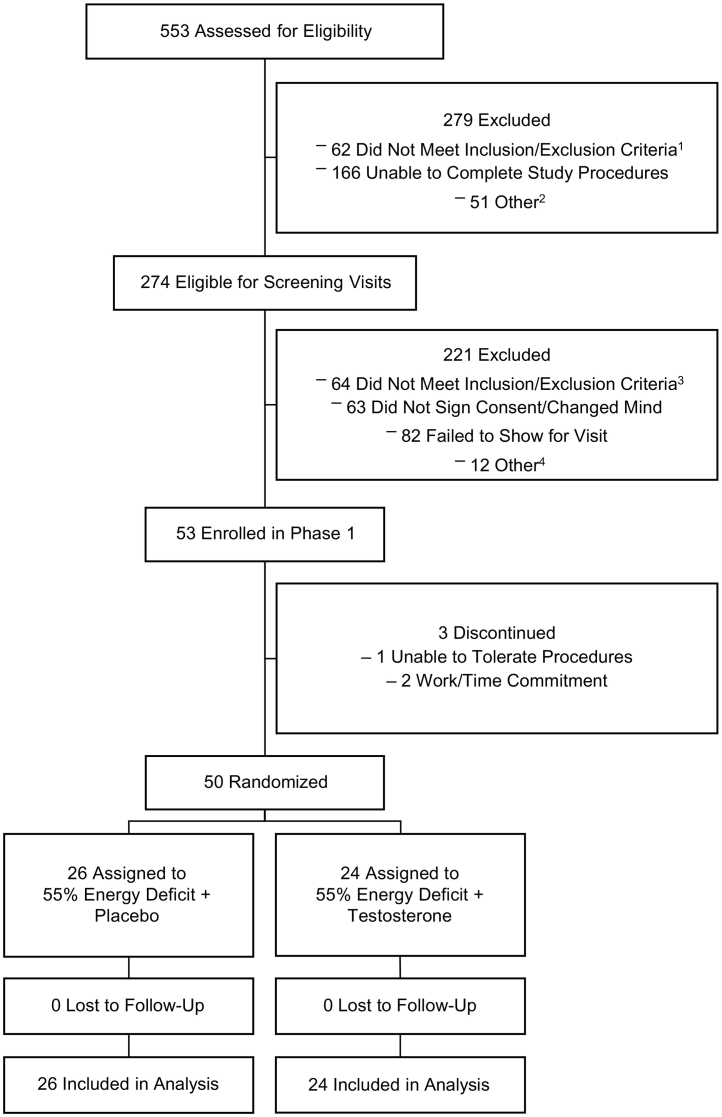
Fig. 1.
Participant flow chart. 553 individuals were assessed for eligibility, 279 were excluded, 274 were found eligible for screening, 221 were excluded following screening, 53 were enrolled, 3 discontinued participation prior to phase 2 (i.e., were not randomised), 50 were randomised and completed the intervention (24 into Testosterone and 26 into Placebo). 1Taking medications or supplements (n = 11), irremovable metal (n = 16), allergies or food intolerance (n = 5), not physically active (n = 2), age (n = 5), body mass index (n = 9), did not meet >1 inclusion/exclusion criteria (n = 14); 2Recruitment complete/full (n = 28), could not contact (n = 17), not a citizen (n = 5), unknown (n = 1); 3Medical history or lab results (n = 18), smoking/drug use (n = 12), body mass index (n = 20), dietary limitations (n = 3), non-compliant with screening procedures (n = 9), not willing to receive testosterone injections (n = 1), head circumference too large for MRI machine (n = 1); 4Recruitment complete/full (n = 4), schedule conflict (n = 8).
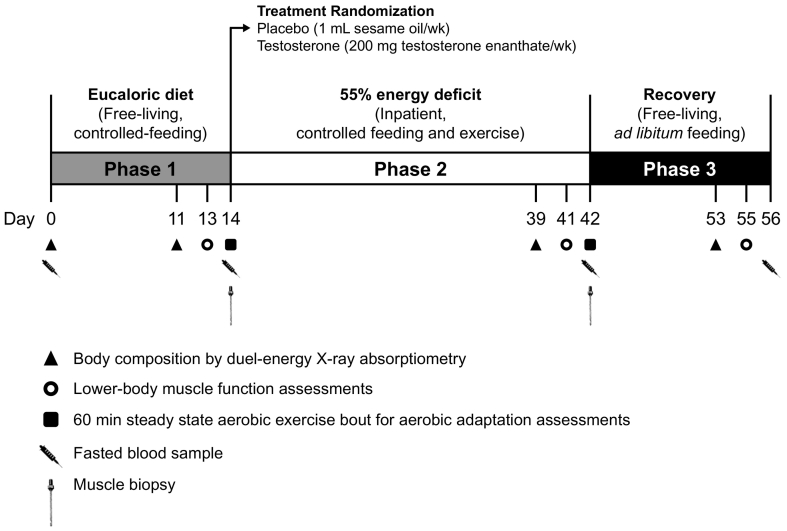
2.2. Study design and intervention
The 3-phase, proof-of-concept, single centre, randomised, double-blind, placebo-controlled, Optimizing Performance for Soldiers trial was conducted at Pennington Biomedical Research Centre to assess the effects of exogenous testosterone administration on body composition changes during severe energy deficit and recovery (Fig. 2). Phase 1 was a 14-d (days 1–14), free-living, eucaloric diet period. Total daily energy expenditure for diet prescriptions during Phase 1 was determined using the Mifflin St. Jeor Equation with an activity factor of 1.3 to account for activities of daily living, and in combination with results from 7-d accelerometer data and 3-d activity logs collected during screening visits [14]. Diets provided 1.6 ± 0.2 g protein/kg/d distributed equally across meals, 30% of total daily energy requirements from fat, with the remaining energy derived from carbohydrate (Supplementary Table 1). Participants maintained their habitual (documented by pre-study, survey questionnaire) physical activity levels during Phase 1. Diet and physical activity adherence were verified by research dietitians, accelerometry, and by ensuring body mass was maintained within ±2%.
Fig. 2.
Experimental design. Adapted from Pasiakos et al. [14].
Participants were admitted to the inpatient unit after completing Phase 1 and underwent a 28-d (Phase 2; days 15–42), highly controlled exercise- and diet-induced energy deficit equal to 55% of Phase 2 total daily energy expenditure. This 55% energy deficit during Phase 2 involved increasing exercise-induced energy expenditure to elevate total daily energy expenditure by 50% above Phase 1 total daily energy expenditure, and restricting energy intake to 45% of this elevated total daily energy expenditure (Supplementary Table 2). The macronutrient composition of the Phase 2 diet was consistent with Phase 1. All meals were provided and consumed under supervision.
To increase Phase 2 total daily energy expenditure, exercise consisted of varied, low-, moderate-, and high-intensity (40–85% of predetermined peak rate of oxygen uptake) aerobic-type exercise, including treadmill and/or outdoor walking and/or running, elliptical, stationary bike and weighted backpack (carrying 30% of body mass) walking (Supplementary Table 3). Steady-state, aerobic-type exercise was chosen as the primary means by which total daily energy expenditure was elevated during Phase 2 to reflect the aerobic-type physical work that occurs during sustained, strenuous military operations. High energy flux and limited dietary intake were the principal military-relevant stressors imposed in our study. Participants were not psychologically stressed, nor was sleep restricted in anyway during Phase 2. On average, participants performed 3.5 exercise sessions per day, all directly supervised by research staff. The physical activity prescription was verified biweekly and adjusted as needed to achieve the desired energy expenditure using open circuit indirect calorimetry (ParvoMedics TruOne 2400, East Sandy, UT). Light calisthenics were incorporated into the exercise routine every 3–4 d [14]. Adherence to the prescribed exercise was ≥89% and not different between groups (Supplementary Table 3).
After completing Phase 2, participants were released from the inpatient unit and instructed to return to their pre-study, ad libitum, habitual diet and physical activity routines; body mass and composition were assessed during this recovery period (Phase 3, days 43–56). Participants completed 3-d food records (1 weekend day, 2 weekdays) the week prior to day 56 that were reviewed by research dietitians.
2.3. Treatment randomisation and masking
At the start of Phase 2, participants were randomised according to a 1:1 ratio to receive either intramuscular injections of testosterone enanthate (200 mg/wk, Testosterone) or placebo (1 mL sesame oil, Placebo) on days 15, 21, 28, and 35 by a biostatistician unaffiliated with the study. The testosterone dosage was the same for each participant and chosen to maintain normal testosterone concentrations during severe energy deficit [14]. A computer-generated randomisation plan (SAS Institute, Cary, NC, USA, version 9.4) using the permuted-block method (n = 4/block) and age stratification (<29 or ≥29 y) was developed to allocate participants. The randomisation plan was delivered via a secured network to the study pharmacist who prepared the testosterone or placebo injections. The pharmacist was the only individual affiliated with the study that had access to the randomisation plan. The plan was kept in a password-protected file and accessed only when needed to allocate new participants to a group. Participants and all remaining study staff were blinded to group allocation until study completion.
2.4. Outcome measures
Body composition (total body mass, lean body mass, fat mass, and bone mineral content) measured on days 0, 11, 39, and 53 after an overnight fast and morning void by dual-energy X-ray absorptiometry (DXA; Lunar iDXA, GE Healthcare, Madison, WI, extended details on scanner positioning standards are provided as Supplementary Materials), was the primary study end point [14]. In this study, lean body mass is defined as total body mass minus fat mass and bone mineral content. All other outcomes described are considered secondary end points.
Semi-nude body weight was measured after an overnight fast and morning void by calibrated digital scale (GSE Inc. Model 450, GSE Scale Systems, Novi, MI) during each screening visit and daily throughout Phase 1 and Phase 2. During Phase 3, participants weighed themselves daily (semi-nude, after an overnight fast) using a calibrated scale provided by staff (Body Trace, Inc. Model BT003, New York, NY).
Percutaneous muscle biopsies of the vastus lateralis were collected in the rested, fasted state under local anaesthesia (1% lidocaine) using a 5 mm Bergstrom needle with manual suction on days 14 and 42 [15]. Muscle biopsy samples were used to characterize cross-sectional area and percent distribution of myosin heavy chain myofibres delineated as type II fast-twitch and type I slow-twitch at the end of Phase 1 and Phase 2. Muscle biopsy samples were also used to assess nuclear peroxisomal proliferator–activated receptor γ coactivator 1α percentage. Extended methodological details for these assessments are provided as Supplementary Material.
Lower-body muscular strength and endurance were assessed using isometric and isokinetic knee extension tests (Biodex Medical Systems, Shirley, NY) on days 13, 41, and 55. Knee extensor dynamometry was chosen to assess muscle function due to its reliability in assessing muscle strength in response to changes in lean mass [16]. Isometric peak torque was measured at 75° knee flexion (3 repetitions, separated by 30 s rest). Isokinetic peak torque was determined from six maximal knee extensions at 60° per second, and isokinetic total work was determined from 20 repeated maximal knee extensions at 180° per second. Muscle strength and endurance data are presented as absolute values and relative to lean body mass of the tested leg.
Participants also performed 60 min steady-state aerobic exercise bouts on days 14 and 42 to characterize submaximal aerobic adaptations (e.g., heart rate, ratings of perceived exertion, power output, energy expenditure, and substrate oxidation) to testosterone and severe energy deficit. Exercise intensity was matched within participants based on power output and total work performed to limit the confounding effects of weight loss on relative exercise intensity [14].
2.5. Sample analyses
Fasted blood samples were collected on days 0, 14, 42, and 56 between 06:00 h and 09:00 h to limit the potential confounding effects of circadian rhythm on total testosterone in young men [17]. Blood samples were analysed for total testosterone, follicle-stimulating hormone, estradiol, sex hormone-binding globulin, luteinising hormone, insulin, cortisol, and prostate-specific antigen (Siemens Immulite 2000, Llanberis, UK). Free testosterone was determined by calculation [18]. Insulin-like growth factor-1 was analysed using an enzyme-linked immunoassay (ALPCO, Salem, NH). Glucose, total cholesterol, HDL-cholesterol, triglycerides, and alanine aminotransferase were analysed on a Beckman DXC 600 Pro (Brea, CA). LDL-cholesterol was determined by calculation [19]. Complete blood counts were analysed on a Beckman DxH (Brea, CA). Systolic and diastolic blood pressure were measured manually on days 0, 15 (prior to the first treatment injection), and 42.
2.6. Sample size determination
Relevant data (means ± standard deviations) demonstrating the effects of moderate-to-severe energy deficit on lean body mass were used to determine statistical power and sample size [20,21]. Based on these estimates, with 90% statistical power, 5% type I error rate, and according to a 2-sided test, 22 males per group were needed (n = 44) [14]. Based on the variability in lean body mass loss in response to moderate-to-severe energy deficit, the sample size was increased to n = 50.
2.7. Data collection and management
Participants were assigned unique identification numbers that were used on all data collection forms, questionnaires, biological specimen tubes, and computer records. The master list linking participant names and identification numbers were kept in a password-protected computer file with access only granted to the principal investigator and study manager. Biological samples moved off-site for analysis (i.e., MyoSyntax performed the muscle cross-sectional area and nuclear peroxisomal proliferator–activated receptor γ coactivator 1α analyses) did not contain any personal identifiable information, nor did they have access to the master list at any time.
Data collection forms were kept under lock and key, or in password-protected files, and under the control of the principal investigator and project coordinator. The Pennington Biomedical Research Centre has a fully integrated, campus wide, automated Central Database management system that is fully validated and continually vetted for quality assurance. Data from self-report or questionnaires were also collected and managed using the REDCap electronic data capture tools hosted at the Pennington Biomedical Research Centre [22]. Data are backed up daily, and the Research Computing Core at the Pennington Biomedical Research Centre oversees all data management.
2.8. Statistical analysis
All analyses are considered 2-tailed, with α = .05 considered statistically significant. All primary analyses were intention-to-treat. When luteinising hormone (n = 15), follicle stimulating hormone (n = 27), estradiol (n = 3), and prostate-specific antigen (n = 2) values were below the level of detection, values for data analyses were imputed at one quarter of the detection value. A single testosterone observation was above 55.5 nmol/L and was imputed as 55.5 nmol/L. SAS (SAS Institute, Cary, NC, USA), version 9.4, was used for analyses.
Between group comparisons for Phase 1, Phase 2, and Phase 3 dietary intake and exercise-induced energy expenditure data were assessed using two sample Student's t-tests. Baseline and day 14 body weights were compared using a paired t-test to determine whether weight was maintained during Phase 1. Primary analysis of body composition, clinical, muscular function, and muscle fibre cross-sectional area, nuclear peroxisomal proliferator–activated receptor γ coactivator 1α, and aerobic adaptation parameters were performed using a mixed effect linear model. Models include the final measurement day of each phase as the outcome. Treatment (Testosterone and Placebo), phase (Phases 1–3), phase-by-treatment interaction, age, and pre-study values (only for body composition and clinical parameters, Table 1) were considered fixed effects covariates in the model. The random effect included an unstructured covariance matrix to account for the correlation within-subjects over time. Least squares means from the model estimated interaction effects. For outcomes with significant phase-by-treatment interactions, within group comparisons across phases (n = 6) and between group comparisons within each phase (n = 3) were made using two sample Student's t-tests based on the least squares means and adjusted using the Bonferroni correction (maximum of 9 comparisons). Unadjusted data are reported in Supplementary Table 4.
Table 1.
Pre-study participant characteristics in the intention-to-treat population.
| Testosterone (n = 24) | Placebo (n = 26) | |
|---|---|---|
| Race and ethnicity, n (%) | ||
| Non-Hispanic Black | 6 (25) | 7 (27) |
| Non-Hispanic White | 14 (58) | 16 (61) |
| Hispanic | 3 (13) | 0 (0) |
| Other | 1 (4) | 3 (12) |
| Age, y | 25 (5) | 25 (5) |
| Height, cm | 178 (9) | 178 (6) |
| Weight, kg | 81 (14) | 77 (10) |
| Body mass index, kg/m2 | 25 (3) | 24 (3) |
| Systolic blood pressure, mmHg | 119 (8) | 117 (10) |
| Diastolic blood pressure, mmHg | 73 (8) | 74 (6) |
| Total testosterone, nmol/L | 17 (5) | 15 (3)† |
| Free testosterone, nmol/L | 0.34 (0.10) | 0.31 (0.10) |
| Follicle stimulating hormone, IU/L | 3.3 (1.9) | 3.8 (2.2) |
| Estradiol, pmol/L | 157 (52) | 155 (53) |
| Sex-hormone binding globulin, nmol/L | 37 (18) | 32 (11) |
| Luteinising hormone, IU/L | 3.7 (1.3) | 4.4 (2.0) |
| Insulin-like growth factor-1, nmol/L | 37 (9) | 36 (12) |
| Glucose, mmol/L | 4.9 (0.4) | 4.9 (0.3) |
| Insulin, pmol/L | 68 (106) | 55 (51) |
| Cortisol, nmol/L | 371 (107) | 411 (141) |
| Total cholesterol, mmol/L | 4.2 (0.7) | 4.3 (0.9) |
| High-density lipoprotein cholesterol, mmol/L | 1.5 (0.3) | 1.4 (0.3) |
| Low-density lipoprotein cholesterol, mmol/L | 2.4 (0.6) | 2.6 (0.7) |
| Triglycerides, mmol/L | 0.81 (0.41) | 0.87 (0.50) |
| Haemoglobin, g/L | 150 (10) | 150 (11) |
| Haematocrit, % | 45 (2) | 44 (3) |
| Prostate-specific antigen, μg/L | 0.78 (0.61) | 0.67 (0.34) |
| Alanine aminotransferase, μkat/L | 0.39 (0.14) | 0.35 (0.13) |
| Body mass composition, kg | ||
| Total | 81.0 (13.7) | 76.5 (10.3) |
| Lean | 60.7 (10.1) | 56.0 (5.4)* |
| Fat | 17.0 (6.8) | 17.4 (6.6) |
| Peak oxygen uptake, L/min | 3.6 (0.8) | 3.4 (0.6) |
| Absolute intake, kcal/d or g/d | ||
| Energy | 2432 (777) | 2478 (950) |
| Carbohydrate | 258 (99) | 281 (114) |
| Protein | 118 (39) | 113 (40) |
| Fat | 106 (38) | 103 (49) |
| Relative intake, kcal/kg body mass/d or g/kg body mass/d | ||
| Energy | 32 (14) | 33 (13) |
| Carbohydrate | 3.4 (1.7) | 3.8 (1.6) |
| Protein | 1.5 (0.6) | 1.5 (0.6) |
| Fat | 1.4 (0.6) | 1.4 (0.7) |
Values are mean (standard deviation). Testosterone = 55% energy deficit + 200 mg testosterone enanthate per week during Phase 2, Placebo = 55% energy deficit + 1 mL sesame seed oil placebo per week during Phase 2.
3. Results
3.1. Inclusion
Non-obese, young men were enrolled (n = 53), randomised (n = 50), and completed (n = 50) this double-blinded, placebo-controlled trial (Fig. 1, Table 1). Throughout the study, adverse events (Table 2) were not different between Testosterone and Placebo.
Table 2.
Incidence of adverse events in the intention-to-treat population.
| Testosterone (n = 24) |
Placebo (n = 26) |
|||||
|---|---|---|---|---|---|---|
| Phase 1 | Phase 2 | Phase 3 | Phase 1 | Phase 2 | Phase 3 | |
| Death | 0 | 0 | 0 | 0 | 0 | 0 |
| Serious adverse event | ||||||
| Low absolute neutrophil count | 0 | 0 | 0 | 1 | 0 | 0 |
| Elevated alanine aminotransferase | 0 | 0 | 3 | 0 | 0 | 0 |
| Biopsy infection, cellulitis | 0 | 1 | 0 | 0 | 0 | 0 |
| Adverse event-related withdrawals | 0 | 0 | 0 | 0 | 0 | 0 |
| Adverse event | ||||||
| Biopsy pain | 20 | 20 | 0 | 17 | 23 | 2 |
| Blister/chafing | 0 | 8 | 1 | 0 | 7 | 1 |
| Foot pain | 0 | 7 | 0 | 0 | 7 | 0 |
| Joint/muscle soreness | 3 | 28 | 0 | 2 | 26 | 0 |
| Gastrointestinal | 1 | 5 | 1 | 0 | 5 | 1 |
| Dermatological | 1 | 5 | 1 | 3 | 10 | 0 |
| Insomnia | 0 | 9 | 0 | 0 | 7 | 0 |
| Headache | 2 | 4 | 3 | 0 | 2 | 3 |
| Allergy/eye irritation/ear pain | 3 | 2 | 3 | 4 | 5 | 0 |
| Low haemoglobin, haematocrit, and mean cell volume | 0 | 1 | 0 | 0 | 0 | 0 |
| Iron deficiency anaemia | 0 | 0 | 0 | 0 | 1 | 0 |
| Other1 | 1 | 4 | 0 | 2 | 2 | 1 |
Chi square tests were used to compare adverse event incidence in Testosterone (n = 24) and Placebo (n = 26) within each phase. There were no between group differences during any phase (P > .05). Percutaneous muscle biopsies of the vastus lateralis were obtained during P1 and P2. P1 = phase 1, P2 = phase 2, P3 = phase 3, Testosterone = 55% energy deficit + 200 mg testosterone enanthate per week during Phase 2, Placebo = 55% energy deficit + 1 mL sesame seed oil placebo per week during Phase 2. 1Other: Testosterone; P1, vasovagal/syncope (n = 1); P2, vasovagal/syncope (n = 1), fatigue (n = 1), blurred vision (n = 1), herpes simplex (n = 1); Placebo; P1, chest pain (n = 1), vertigo (n = 1); P2, vertigo (n = 1), dysuria (n = 1); P3, visual disturbance (n = 1).
3.2. Characteristics and effects during phase 1
During Phase 1, exercise-induced energy expenditure (mean ± standard deviation, Testosterone, 331 ± 387 kcal/d and Placebo, 360 ± 345 kcal/d) and dietary intake did not differ between groups (P > .05, Supplementary Table 1). Participants in both groups remained weight stable [Testosterone, d 0: 81 kg (77, 85) and d 14: 80 kg (76, 85), P = .38; Placebo, d 0: 77 kg (72, 81) and d 14: 76 kg (72, 81), P = .62].
3.3. Characteristics of phase 2
During Phase 2, total daily energy expenditure, exercise-induced energy expenditure, percent and absolute energy deficit, and modality-specific exercise did not differ (P > .05) between groups (Supplementary Tables 2–3). Except for relative protein intake, energy and macronutrient intake did not differ between treatments (Supplementary Table 1).
3.4. Effects of testosterone supplementation on circulating biomarkers during phase 2
Following energy deficit in Phase 2, concentrations of free testosterone decreased, total testosterone remained unchanged, and sex-hormone binding globulin increased in Placebo; whereas, total testosterone and free testosterone increased and sex-hormone binding globulin was unchanged in Testosterone, such that, total testosterone [all bracketed data are mean difference between groups (95% CI), 24.4 nmol/L (21.8, 27.0), P < .0001] and free testosterone [0.69 nmol/L (0.61, 0.77), P < .0001] were greater and sex-hormone binding globulin concentrations [15.9 (11.4, 20.4), P < .0001] were lower in Testosterone than Placebo (Table 3). Estradiol [116 pmol/L (91, 140), P < .0001], follicle stimulating hormone [2.6 IU/L (2.0, 3.2), P < .0001], and luteinising hormone [2.5 IU/L 1.7, 3.4, P < .0001] concentrations were different for Testosterone compared to Placebo (Table 3). Furthermore, Testosterone had higher haemoglobin [6.7 g/L (3.0, 10.3), P < .0048] and haematocrit [2.1% (0.9, 3.2), P < .0053] than Placebo (Table 3).
Table 3.
Adjusted least squares means for body composition, clinical biomarkers, and lower-body muscular strength and endurance in the intention-to-treat population.
| Testosterone (n = 24) |
Placebo (n = 26) |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Phase 1 | Phase 2 | Phase 3 | Phase 1 | Phase 2 | Phase 3 | Phase | Treat | Phase × Treat | |
| Body composition | |||||||||
| Body mass, kg | |||||||||
| Total | 78.0 (77.4, 78.7)a | 75.8 (75.1, 76.5)b,⁎ | 79.3 (78.6, 79.9)a,⁎ | 78.3 (77.7, 78.9)a | 73.3 (72.7, 74.0)b | 76.5 (75.8, 77.1)c | <0.0001 | <0.0001 | <0.0001 |
| Lean | 57.9 (57.3, 58.5)a | 60.4 (59.8, 61.0)b,⁎ | 63.1 (62.5, 63.7)c,⁎ | 58.3 (57.7, 58.9)a | 58.0 (57.4, 58.6)a | 60.5 (60.0, 61.1)b | <0.0001 | <0.0001 | <0.0001 |
| Fat1 | 16.8 (16.3, 17.2) | 12.0 (11.6, 12.4) | 12.8 (12.4, 13.2) | 16.8 (16.4, 17.2) | 12.2 (11.8, 12.6) | 12.8 (12.4, 13.2) | <0.0001 | 0.78 | 0.79 |
| Bone mineral content, kg | 3.27 (3.26, 3.28)a | 3.27 (3.26, 3.28)a | 3.26 (3.25,3.27)a | 3.27 (3.26, 3.28)a | 3.25 (3.24, 3.27)a | 3.24 (3.23, 3.25)b,⁎ | <0.0001 | 0.016 | 0.035 |
| Leg mass, kg | |||||||||
| Total | 26.7 (26.4, 27.0)a | 25.8 (25.5, 26.1)b | 26.9 (26.6, 27.2)a,⁎ | 26.8 (26.5, 27.1)a | 25.2 (24.9, 25.5)b | 26.1 (25.8, 26.4)c | <0.0001 | 0.0089 | 0.012 |
| Lean | 19.5 (19.3, 19.8)a | 20.1 (19.8, 20.4)b,⁎ | 21.2 (20.9, 21.4)c,⁎ | 19.6 (19.3, 19.8)a | 19.3 (19.0, 19.6)a | 20.2 (19.9, 20.4)b | <0.0001 | 0.0001 | <0.0001 |
| Fat | 6.0 (5.8, 6.1)a | 4.4 (4.3, 4.6)b | 4.5 (4.4, 4.6)b | 6.0 (5.8, 6.1)a | 4.7 (4.6, 4.8)b | 4.7 (4.6, 4.9)b | <0.0001 | 0.039 | 0.015 |
| Trunk mass, kg | |||||||||
| Total | 35.7 (35.3, 36.1)a | 34.8 (34.3, 35.2)b,⁎ | 36.7 (36.3, 37.1)c⁎ | 35.9 (35.5, 36.2)a | 33.4 (33.0, 33.8)b | 35.1 (34.7, 35.5)a | <0.0001 | <0.0001 | <0.0001 |
| Lean | 26.7 (26.3, 27.1)a | 28.5 (28.1, 28.8)b,⁎ | 29.8 (29.5, 30.2)c,⁎ | 27.0 (26.6, 27.3)a | 27.4 (27.1, 27.8)a | 28.7 (28.4, 29.1)b | <0.0001 | 0.0018 | <0.0001 |
| Fat1 | 8.0 (7.7, 8.3) | 5.3 (5.0, 5.6) | 5.9 (5.6, 6.2) | 8.0 (7.7, 8.3) | 5.1 (4.8, 5.4) | 5.6 (5.3, 5.8) | <0.0001 | 0.28 | 0.39 |
| Clinical health-biomarkers | |||||||||
| Total testosterone, nmol/L | 15.5 (13.7, 17.4)a | 36.2 (34.3, 38.0)b,⁎ | 7.8 (5.9, 9.8)c,⁎ | 14.6 (12.9, 16.4)a | 11.8 (10.0, 13.5)a | 14.6 (12.8, 16.3)a | <0.0001 | <0.0001 | <0.0001 |
| Free testosterone, nmol/L | 0.33 (0.27, 0.38)a | 0.87 (0.81, 0.92)b,⁎ | 0.15 (0.10, 0.21)c,⁎ | 0.31 (0.26, 0.36)a | 0.18 (0.13, 0.23)b | 0.29 (0.23, 0.34)a,b | <0.0001 | <0.0001 | <0.0001 |
| Follicle stimulating hormone, IU/L | 3.1 (2.7, 3.6)a | 0.3 (−0.2, 0.7)b,⁎ | 2.2 (1.7, 2.7)c,⁎ | 3.5 (3.0, 3.9)a | 2.8 (2.4, 3.3)a | 3.5 (3.0, 3.9)a | <0.0001 | <0.0001 | <0.0001 |
| Estradiol, pmol/L | 148 (130, 166)a | 247 (229, 265)b,⁎ | 144 (125, 162)a | 142 (124, 159)a | 131 (114, 148)a | 158 (141, 175)a | <0.0001 | <0.0001 | <0.0001 |
| Sex-hormone binding globulin, nmol/L | 31.6 (28.3, 34.8)a,b | 36.5 (33.2, 39.7)a,⁎ | 30.6 (27.2, 34.0)b | 31.1 (27.9, 34.2)a | 52.4 (49.2, 55.5)b | 35.7 (32.6, 38.8)a | <0.0001 | 0.0001 | <0.0001 |
| Luteinizing hormone, IU/L | 3.3 (2.6, 3.9)a | 0.3 (−0.4, 0.9)b,⁎ | 2.2 (1.6, 2.9)a,⁎ | 3.8 (3.2, 4.3)a,b | 2.8 (2.2, 3.4)a | 4.1 (3.5, 4.7)b | <0.0001 | <0.0001 | 0.0006 |
| Insulin-like growth factor-1, nmol/L2 | 38.5 (35.6, 41.5) | 25.0 (22.0, 27.9) | 36.6 (33.5, 39.7) | 38.2 (35.3, 41.0) | 25.8 (22.9, 28.6) | 37.5 (34.7, 40.4) | <0.0001 | 0.73 | 0.89 |
| Glucose, mmol/L1 | 4.71 (4.53, 4.88) | 4.14 (3.96, 4.32) | 4.93 (4.74, 5.11) | 4.63 (4.46, 4.81) | 4.19 (4.02, 4.36) | 4.91 (4.74, 5.08) | <0.0001 | 0.88 | 0.76 |
| Insulin, pmol/L2 | 59 (35, 83) | 15 (−9, 39) | 80 (55, 105) | 54 (31, 77) | 11 (−12, 34) | 87 (64, 110) | <0.0001 | 0.93 | 0.86 |
| Cortisol, nmol/L2 | 397 (356, 438) | 512 (472, 553) | 388 (345, 430) | 434 (395, 473) | 551 (512, 590) | 378 (339, 417) | <0.0001 | 0.73 | 0.89 |
| Total cholesterol, mmol/L1 | 3.85 (3.59, 4.11) | 4.39 (4.13, 4.65) | 4.97 (4.70, 5.24) | 3.86 (3.61, 4.11) | 4.12 (3.87, 4.37) | 4.98 (4.73, 5.23) | <0.0001 | 0.54 | 0.22 |
| High-density lipoprotein cholesterol, mmol/L | 1.22 (1.11, 1.32)a | 1.64 (1.54, 1.75)b | 1.85 (1.74, 1.96)c | 1.22 (1.12, 1.32)a | 1.75 (1.65, 1.85)b | 1.74 (1.64, 1.84)b | <0.0001 | 0.98 | 0.008 |
| Low-density lipoprotein cholesterol, mmol/L | 2.23 (2.03, 2.43) | 2.44 (2.24, 2.63) | 2.74 (2.53, 2.95) | 2.29 (2.10, 2.48) | 2.14 (1.95, 2.34) | 2.78 (2.59, 2.98) | <0.0001 | 0.53 | 0.054 |
| Triglyceride, mmol/L | 0.84 (0.69, 0.99)a | 0.65 (0.49, 0.80)a | 0.78 (0.62, 0.94)a | 0.80 (0.66, 0.95)a,b | 0.52 (0.37, 0.67)a | 1.03 (0.88, 1.17)b | 0.0001 | 0.63 | 0.038 |
| Haemoglobin, g/L | 145 (143, 148) a | 145 (142, 148)a,⁎ | 145 (142, 147)a | 144 (142, 147)a | 138 (136, 141)b | 139 (137, 142)b | 0.012 | 0.002 | 0.035 |
| Haematocrit, % | 42.9 (42.1, 43.7)a | 43.6 (42.7, 44.4)a,⁎ | 43.2 (42.3, 44.0)a | 42.8 (42.0, 43.6)a | 41.5 (40.7, 42.3)a | 41.5 (40.7, 42.3)a | 0.45 | 0.003 | 0.020 |
| Alanine aminotransferase, μkat/L3 | 0.32 (−0.20, 0.83) | 0.42 (−0.09, 0.94) | 2.65 (2.11, 3.18) | 0.38 (−0.12, 0.87) | 0.49 (0.00, 0.98) | 1.68 (1.19, 2.18) | <0.0001 | 0.19 | 0.074 |
| Prostate-specific antigen, μg/L1 | 0.77 (0.56, 0.98) | 0.68 (0.47, 0.89) | − | 0.59 (0.39, 0.79) | 0.43 (0.23, 0.63) | − | <0.0001 | 0.14 | 0.15 |
| Systolic blood pressure, mm Hg1 | 104 (99, 108) | 99 (94, 103) | − | 103 (98, 107) | 98 (94, 102) | − | 0.0002 | 0.80 | 0.75 |
| Diastolic blood pressure, mm Hg1 | 63 (60, 66) | 58 (55, 60) | − | 64 (61, 67) | 58 (55, 60) | − | <0.0001 | 0.73 | 0.66 |
| Lower-body muscular strength and endurance | |||||||||
| Isometric torque | |||||||||
| Peak, Nm4 | 245 (221, 269) | 219 (195, 243) | 214 (190, 238) | 243 (220, 266) | 219 (196, 243) | 229 (205, 252) | <0.0001 | 0.78 | 0.37 |
| Peak, Nm/kg lean body mass4 | 24 (22, 26) | 21 (19, 23) | 20 (18, 22) | 26 (24, 28) | 24 (22, 26) | 24 (22, 26) | <0.0001 | 0.014 | 0.31 |
| Isokinetic torque | |||||||||
| Peak, Nm4 | 200 (181, 219) | 164 (145, 183) | 174 (155, 193) | 195 (177, 213) | 163 (145, 181) | 168 (150, 186) | <0.0001 | 0.72 | 0.82 |
| Peak, Nm/kg lean body mass4 | 20 (18, 21) | 16 (14, 17) | 16 (14, 17) | 21 (19, 22) | 17 (16, 19) | 17 (16, 19) | <0.0001 | 0.12 | 0.72 |
| Total work, J1 | 3339 (3024, 3653) | 2800 (2485, 3115) | 3180 (2865, 3494) | 3423 (3121, 3726) | 2843 (2541, 3146) | 2985 (2683, 3288) | <0.0001 | 0.91 | 0.26 |
| Total work, J/kg lean body mass4 | 327 (303, 352) | 266 (242, 290) | 291 (267, 316) | 362 (339, 385) | 306 (282, 329) | 307 (284, 331) | <0.0001 | 0.033 | 0.33 |
Values are least squares mean (95% confidence interval). Data were analysed using linear mixed models, adjusted for age (all parameters) and pre-study value (only for body composition and clinical parameters). Bonferroni corrections were used for post hoc comparisons. Data not sharing the same letter superscript within a treatment are different by phase and *indicates a between group difference at a particular phase (phase-by-treat interaction). Main effect of phase, 1all phases are different, 2Phase 1 and Phase 3 are different than Phase 2, 3Phase 1 and Phase 2 are different than Phase 3, 4Phase 1 is different than Phase 2 and Phase 3. P < .05 considered statistically significant. Testosterone = 55% energy deficit + 200 mg testosterone enanthate per week during Phase 2, Placebo = 55% energy deficit + 1 mL sesame seed oil placebo per week during Phase 2.
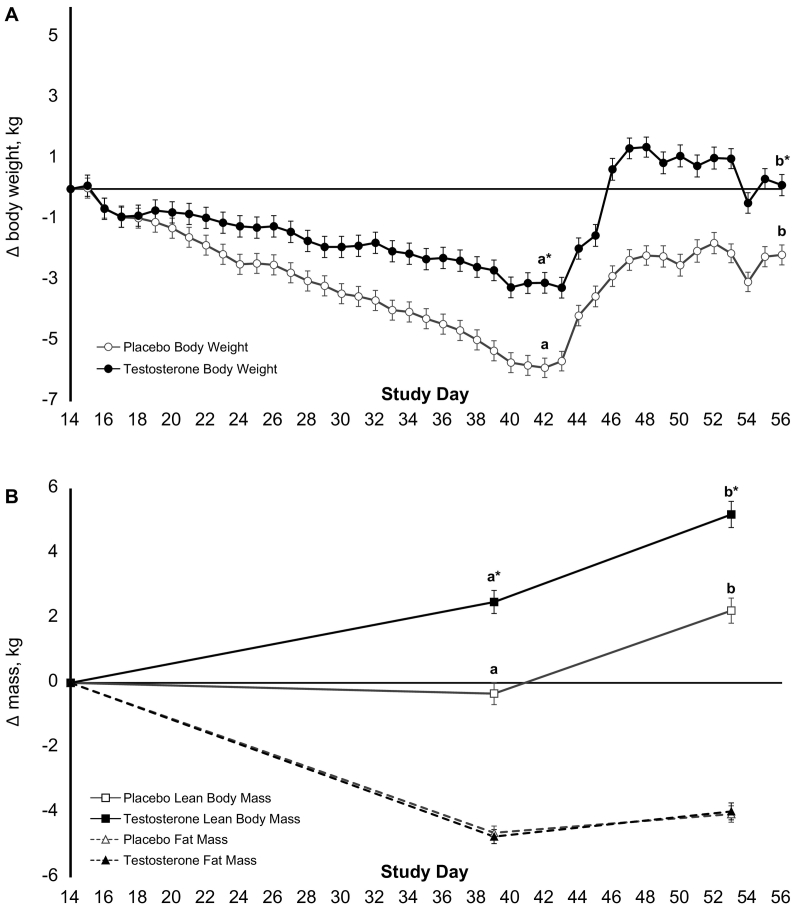
3.5. Effects of testosterone supplementation on body composition during phase 2
During Phase 2, Placebo lost more body weight than Testosterone (P < .0001, Fig. 3A). Fat mass decreased similarly in both treatment groups (Fig. 3B, Table 3). Bone mineral content did not differ between treatment groups (Table 3). Lean body mass increased in Testosterone, whereas lean body mass remained stable in Placebo, such that lean body mass significantly differed between groups at the end of Phase 2 [2.5 kg (3.3, 1.6); P < .0001]. The change in total testosterone was positively associated with change in lean body mass (r = 0.71, r2 = 0.50, P < .0001; Supplementary Fig. 1). Similarly, free testosterone was associated with the change in lean body mass (r = 0.69, r2 = 0.48, P < .001).
Fig. 3.
Change in body weight and composition during energy deficit and recovery. Least squares mean ± standard error change in body weight (A) and composition (B) during Phase 2 (55% energy deficit) and Phase 3 (recovery/weight regain) relative to the final body weight and composition measured during Phase 1 (eucaloric diet) for Testosterone (n = 24), 55% energy deficit + 200 mg testosterone enanthate per week during Phase 2 (n = 24) and Placebo, 55% energy deficit + 1 mL sesame seed oil placebo per week during Phase 2 (n = 26). Data were analysed using linear mixed models, adjusting for age and pre-study value. Bonferroni corrections were used for post hoc comparisons. Data not sharing the same letter superscript within a treatment are different; and *indicates a between group difference (phase-by-treatment interaction).
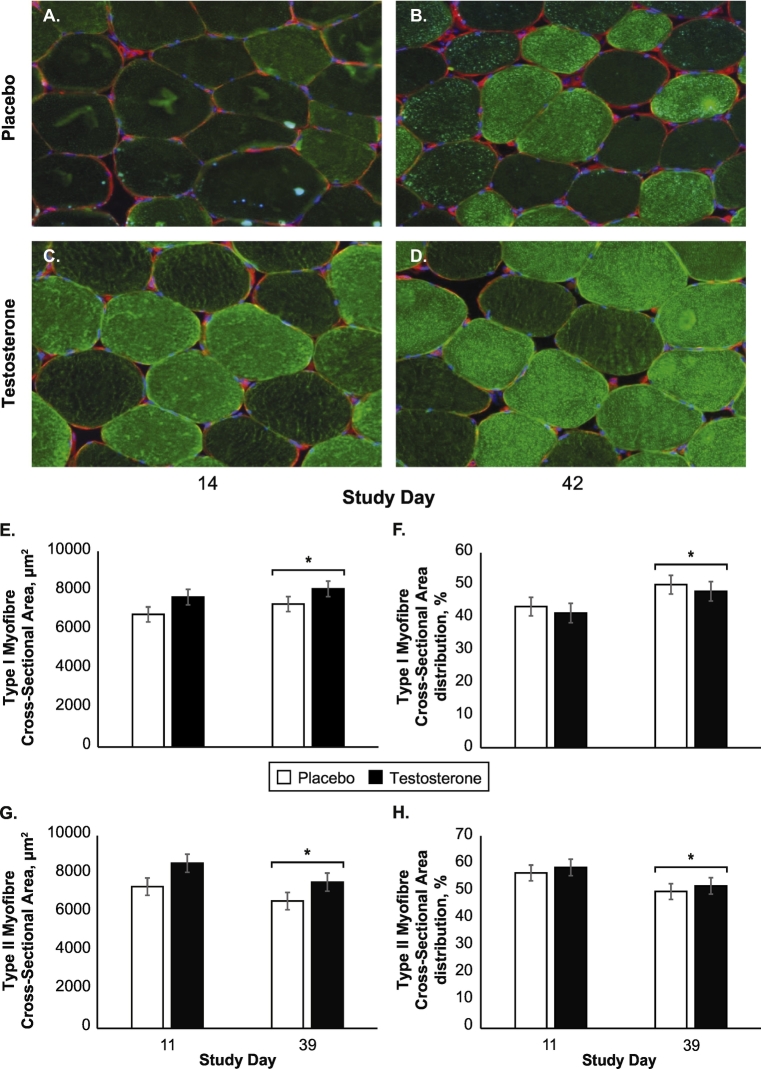
3.6. Effects of testosterone supplementation on muscle function, myofibre cross-sectional area, and aerobic adaptations during phase 2
Lower-body muscle function declined similarly for both groups during Phase 2, despite no loss of lean body mass in either group (Table 3). Type I slow-twitch myofibre cross-sectional area and percent myofibre distribution increased, whereas type II fast-twitch myofibre cross-sectional area and percent distribution decreased during Phase 2 as compared to Phase 1, independent of treatment (Fig. 4A–H, all P < .05). Independent of treatment, submaximal aerobic exercise economy was markedly improved for a given matched workload (i.e., greater reliance on fat oxidation and lower perceived effort, heart rate, energy expenditure, and oxygen uptake, all P < .05, Supplementary Table 5). There were no effects of energy deficit or treatment on basal (fasted, rested) nuclear percentage of peroxisomal proliferator–activated receptor γ coactivator 1α [Testosterone, d 14: 42.6% (33.4, 51.8) and d 42: 48.2% (38.1, 58.2); Placebo, d 14: 50.7% (41.9, 59.6) and d 42: 52.4% (42.8, 62.1), P-interaction = 0.42].
Fig. 4.
Muscle fibre type and percent distribution during energy balance and energy deficit. Representative images of serial cross-sections from vastus lateralis samples collected at rest, under fasted conditions during phase 1 (eucaloric phase, day 14) and phase 2 (55% energy deficit, day 42) for Placebo (A–B), 55% energy deficit + 1 mL sesame seed oil placebo per week during Phase 2 (n = 26) and Testosterone (C–D), 55% energy deficit + 200 mg testosterone enanthate per week during Phase 2 (n = 24). Images were taken with the 20× objective lens in a 15 × 10 grid. Laminin signals were stitched into one image to cover the entire specimen and analysed using Cell Profiler. Myosin heavy chain myofibres with a median intensity < 0.3 and those ≥0.3 were delineated as type II fast-twitch myofibres and type I slow-twitch myofibres, respectively. Least squares mean ± standard error in type 1 slow-twitch myofibre cross-sectional area (E), type 1 slow-twitch myofibre percent distribution (F), type II fast-twitch myofibre cross-sectional area (G), and type II fast-twitch myofibre percent distribution (H). Data were analysed using linear mixed models, adjusting for age. *Indicates a between phase difference (phase main effect).
3.7. Characteristics and effects during phase 3
During Phase 3, ad-libitum dietary intake was not different between groups (Supplementary Table 1). Total testosterone [6.7 nmol/L (4.1, 9.4), P < .0001], free testosterone [0.13 nmol/L (0.05, 0.21), P < .013], and follicle stimulating hormone [1.3 IU/L (0.6, 1.9), P < .0011] concentrations were lower for Testosterone than Placebo (Table 3), but no other health-related biomarkers differed by group. Both groups regained body weight; however, body weight remained greater in Testosterone than Placebo (P < .0001; Fig. 3A). Both groups also gained lean body mass, but lean body mass remained greater in Testosterone than Placebo [2.6 kg (1.8, 3.4), P < .0001; Fig. 3B, Table 3]. Bone mineral content decreased in Placebo and remained stable in Testosterone, such that bone mineral content significantly differed between groups at the end of Phase 3 [21 g [7,36]; P < .043]. Fasting insulin concentrations were greater following recovery compared to the energy deficit, with no difference between groups. Muscle function measures remained lower during Phase 3 than Phase 1, regardless of treatment allocation (P < .0001).
4. Discussion
In the current proof-of-concept study, we report that during 28-d of severe exercise- and diet-induced energy deficit, non-obese, young-adult males receiving weekly intramuscular injections of testosterone (200 mg testosterone enanthate/wk) gained lean body mass and lost less total mass compared to those receiving placebo injections. Testosterone fully recovered their body weight and were 2.8 kg heavier at the end of the 14-d recovery period than Placebo, due almost entirely to lean body mass. Testosterone did not differ from Placebo with respect to lower-body muscular strength and endurance, incidence of adverse events, systolic or diastolic blood pressure, or glucose, insulin, cortisol, lipid, lipoprotein, insulin-like growth factor 1, alanine aminotransferase, or prostate-specific antigen concentrations during energy deficit or recovery.
Suppression of the hypothalamic-pituitary-gonadal axis in energy deficient, non-obese men is well-documented, particularly during sustained, strenuous military operations [1,23,24]. However, no study, to our knowledge, has attempted to replicate these testosterone decrements in a tightly controlled clinical trial using military-relevant exercise and diet stressors. The inability of our severe exercise- and diet-induced energy deficit to reduce total testosterone concentrations to levels measured during US Army Ranger School [1], the basis for the design of the current study, provides 1) controlled scientific evidence to counter several of the points made in the paper by Friedl and colleagues [1], and 2) valuable information regarding the design of future trials attempting to induce non-pharmacologic decrements in endogenous testosterone status in a clinical setting.
In the current study, the 28-d exercise- and diet-induced energy deficit intervention was modelled to reflect the exercise- and diet-induced stress experienced by US Army Soldiers participating in US Army Ranger School [1], an example of severe military operational stress. Friedl and colleagues [1] suggested that the energy deficit alone, caused by increased energy expenditure and restricted dietary intake, was the primary determinant for the decline in total testosterone. This reasoning was based on the observed restoration of eugonadal status in Soldiers fed adequate energy following the first 28 d of Army Ranger School, but still exposed to the remaining stressors (i.e., prolonged and strenuous physical work, sleep deprivation, and psychological stress) [1]. The authors concluded that the energy deficit (~1100 kcal/d) alone, not the means by which the deficit was produced, was the primary determinant of the 50–65% decline in total testosterone observed during the first 28-d of US Army Ranger School. Therefore, the current study, which induced a greater daily energy deficit (~1950 kcal), was expected to elicit a similar decline in total testosterone and lean body mass.
This discrepancy between studies may suggest that, in addition to energy restriction and strenuous physical work, other factors, such as sleep deprivation, psychological stress, and the type of physical work employed meaningfully contribute to declines in testosterone status observed during strenuous military training. During real-world military operations, recovery is minimal and military personnel are often sleep deprived. In the current study, psychological stress was not imposed, and sleep was not restricted. Sleep restriction (5 h/night) has been shown to suppress total testosterone by as much as 15% [25]. In a study that involved strenuous military training and produced a substantial energy deficit, continuous sleep (3 h/d), compared to non-continuous sleep, attenuated reductions in total testosterone [24]. Furthermore, discrete aerobic-type exercise bouts were used to increase energy expenditure (3.5 sessions per day, averaging 2.5 h/d) during the 28-d energy deficit, followed by periods of rest. Energy deficits during real-world military operations often result from low-to-moderate increases in metabolic demand and are sustained for long periods, as physical/aerobic exercise-type activity can exceed 10 h/d. As such, the stressors applied in the current study may not have replicated the multi-faceted stress experienced during real-world military operations, which generally produce marked suppression of the hypothalamic-pituitary-gonadal axis and catabolism of lean body mass [6,7,26]. These realizations are critical for the design of future interventional studies of military operational stress and add important evidence to the literature regarding endogenous testosterone decrements that occur during physiologically and psychologically stressful events.
Finally, while the magnitude of the total testosterone decline (19%, NS) in the current study was less than that reported by others [1,27], the significance of a 42% decline in free testosterone – i.e., the bioactive form of testosterone – should be highlighted. Recent guidance from the US Endocrine Society recommends that free testosterone be relied upon for diagnosis of low testosterone in conditions where sex-hormone binding globulin is altered and total testosterone concentrations are borderline low [28,29]. For Placebo, sex-hormone binding globulin increased by 68% during Phase 2 compared to Phase 1 and total testosterone concentrations were borderline low [<13.9 nmol/L, [28]] at the end of Phase 2. Importantly, free testosterone concentrations [0.18 (0.13, 0.23) nmol/L] were below the lower-end of the normal range estimated using the Vermeulen equation [0.25–0.785 nmol/L, [30]] following Phase 2. Therefore, a more holistic examination of the data (i.e., total and free testosterone and sex-hormone binding globulin) suggests testosterone status was degraded by the severe exercise- and diet-induced energy deficit, albeit not to the same degree as during US Army Ranger School.
The hypertrophic effects of supplemental testosterone are well-described [31]. Bhasin et al. [32] reported a 5% (2.5 kg) and 9% (5.5 kg) increase in lean mass in non-obese, young men receiving weekly injections of 125 mg and 300 mg, respectively, of testosterone enanthate while consuming a eucaloric diet for 20-wk. We report a similar increase in lean body mass (~4%, 2.5 kg) after 4-wk in young men receiving 200 mg/wk. of testosterone enanthate while being underfed. The hypertrophic effects of testosterone, despite energy deficit, may be due to enhanced commitment of satellite cells to myogenesis [33] or androgen receptor-mediated cell signalling [34]. Given the magnitude of energy deficit in the current study, preservation of lean body mass in Placebo was unexpected. However, the combination of higher-protein feeding and high-volume exercise may, in part, explain this finding, due to the synergistic restorative effects of dietary protein and mechanical stress on muscle protein synthesis during energy deficit [20,[35], [36], [37]], providing the conditions by which lean body mass may be maintained [38]. The addition of supplemental testosterone may have provided an anabolic stimulus that was additive to the stimulus provided by protein and exercise, explaining, in part, the gain in lean body mass in Testosterone. This hypothesis is supported by studies showing that testosterone administration enhances muscle protein synthetic efficiency, meaning, for a given amount of intracellular essential amino acids, a greater proportion is routed towards protein synthesis than towards oxidative metabolism, thus conserving lean body mass [39,40].
Lower-body muscle function deteriorated similarly between treatments during the 28-d exercise- and diet-induced energy deficit. The decline in muscle function may be attributable to neuromuscular fatigue, secondary to multiple daily submaximal aerobic exercise sessions for 28 d [41]. Reduced motivation over time may also be a factor contributing to the observed decline in muscular strength and endurance [42,43]. However, the incongruence between the lean body mass change and muscle function in our study is not a unique observation. A similar inability to detect differences in muscle function with testosterone supplementation, despite greater lean mass, has also been demonstrated in young men receiving exogenous testosterone during 28 d of bed rest [44], in middle-aged, obese males receiving exogenous testosterone for 56 wk [10], and in older, overweight males receiving testosterone supplementation for 6 mo [45]. However, the lack of a functional benefit should not discount the potential advantage that may be gained by increasing or maintaining lean body mass in response to severe energy deficit. Accrual or preservation of muscle protein mass may afford greater physiological resiliency during severe military operational stress, particularly when repeated bouts of stress are unavoidable, and recovery is limited. For example, stress-mediated lean body mass losses approaching 10% have been associated with impaired immune function [46]. Lean body mass can be viewed as an amino acid reservoir, and increased protein mass, even to a limited extent, may prolong physiological homeostasis during periods of severe energy deficit.
The lack of a quantitative functional benefit for Testosterone despite gains in lean body mass may also raise methodological concerns about the use of DXA for body composition measurements. DXA is the most commonly employed body composition measurement tool in biomedical research [47], yet it provides estimates for only three body compartments (i.e., bone, fat, and lean soft tissue), and does not discern skeletal muscle mass from body water. If gains in lean body mass in Testosterone were due, at least in part, to an increase in extracellular water [48] and muscle glycogen content [49], and not solely to an increase in protein, those changes would not be delineated by DXA. Assessing total body water in combination with DXA (i.e., four compartment model) may have mitigated those concerns. If lean mass hydration is conservatively estimated to be 75%, total body protein accretion in TEST would be ~625 g. Gains in protein mass, without gains in muscle function, may be a reflection of the specific myofibres (i.e., protein fraction) affected by the intervention. More specifically, the reduction in type II fast-twitch myofibre cross-sectional area would support the functional declines observed with the isokinetic and isometric functional measures. The increase in type I slow-twitch myofibre cross-sectional area is consistent with the observed improvement in submaximal aerobic exercise economy. These findings, in addition to the possibility that gains in lean mass were due to fluid or non-contractile protein, may help explain why lean body mass accrual in Testosterone had no functional benefit.
5. Limitations
While the findings from the current study are strengthened by 100% retention and ≥89% adherence with diet and exercise prescriptions during the energy deficit phase, there are limitations to acknowledge, in addition to those already discussed with regards to DXA methodology and muscle function measures. This study was designed to provide testosterone supplementation at a dose that maintained total and free testosterone concentrations during a severe energy deficit, in order to attenuate expected lean body mass loss. However, following testosterone supplementation in Testosterone, total and free testosterone concentrations were 134% and 164% higher, respectively, than concentrations prior to supplementation. In addition, following Phase 2, total testosterone concentrations in Testosterone were approximately 14% above the upper-end of the harmonized reference range for testosterone (9.2–31.8 nmol/L) recently established by the Endocrine Society in 2018 [28]. Free testosterone concentrations were 11% above the upper-end of the reference range for free testosterone (0.25–0.785 nmol/L) established by Vermeulen and colleagues [18,30]. The increases in total and free testosterone above basal concentrations and the upper-end of the normal reference range were likely due to the administration of a greater dosage than the recommended initial dose for testosterone replacement therapy [28] and, possibly, the discrepancy between actual endogenous testosterone suppression produced by the severe energy deficit and the anticipated suppression, on which the chosen dosage was based [32]. As discussed previously, the anticipated suppression was based on military studies demonstrating marked reductions in total testosterone during demanding military operations that produced severe energy deficits and lean body mass loss [1,27,50]. Instead, we observed lean body mass gains and maintenance in Testosterone and Placebo, respectively; whereas, both groups lost the same amount of body fat. Administering a lower testosterone dose, implementing more demanding stressors that better mimic the multi-stressor environment of real-world military operations, or a combination of both may have facilitated greater changes in testosterone status and lean body mass.
The dose and duration of exogenous supplementation dictates the recovery of endogenous testosterone production [28]. In the current study, total testosterone concentrations in Testosterone were ~78% and ~50% lower following recovery compared to the energy deficit and run-in phases, respectively. Similarly, free testosterone concentrations in Testosterone were ~83% and ~55% lower following Phase 3 compared to Phases 1 and 2, respectively. Despite lower circulating total and free testosterone concentrations in Testosterone compared to Placebo, lean body mass remained greater in Testosterone than Placebo. However, muscle function measures remained lower in recovery than during the run-in phase and similar to the measures taken at the end of Phase 2, regardless of treatment group. Total and free testosterone concentrations were; however, restored to normal levels within 28 d (n = 13), 90 d (n = 8), and 11 mo (n = 1) after recovery (2 participants did not return for follow-up) [14]. The potential physiological and psychological consequences of supplemental testosterone-induced hypogonadism and subsequent endogenous and functional recovery require consideration. Finally, the benefits and risks of using supplemental testosterone to aid in total body mass and lean body mass retention and muscle function during and in recovery from severe energy deficit in non-obese, young-adult males beyond the 4 wk energy deficit and 2 wk recovery period studied in this trial are unknown.
6. Conclusions
Exogenous testosterone, to our knowledge, has not been administered as an intervention to counter anticipated reductions in testosterone status, lean body mass, and muscle function due to military operational stress. Findings from this proof-of-concept study show testosterone supplementation increases total and free testosterone, and lean body mass but has no effect on lower-body muscle function, type I slow-twitch and type II fast-twitch myofibre cross-sectional area, whole-body and muscle aerobic adaptations, circulating health biomarkers, or blood pressure during a tightly controlled clinical study resembling military operational stress that is severe, but less so than US Army Ranger School. This indicates testosterone supplementation is likely not warranted for operational scenarios that do not markedly decrease testosterone status and, when designing future studies, more sensitive outcomes to assess muscle function and physical performance (e.g., anaerobic/aerobic capacity, occupational and tactical military tasks, including timed load carriage marches, casualty drag, litter carries, and marksmanship) should be included. The primary findings from this study provide rationale for further exploration of practical pharmacologic interventions to mitigate losses of lean body mass experienced by military personnel during periods of unavoidable, severe energy deficit, particularly those that may enhance muscle function.
Contributors
Conceptualization, S.M.P., C.E.B., J.P.K., H.R.L., J.S.O., L.M.M., J.A.C., A.J.Y., M.A.M., W.J.E., O.V., O.T.C., K.M.G., and J.C.R.; Formal Analysis, S.M.P., C.E.B., R.A.B., and J.C.R.; Investigation, S.M.P., C.E.B., N.M.J., M.N.H., and J.C.R.; Writing – Original Draft, S.M.P., C.E.B., and J.C.R.; Writing – Reviewing & Editing, J.P.K., H.R.L., J.S.O., L.M.M., J.A.C., A.J.Y., M.A.M., W.J.E., O.V., O.T.C., K.M.G., N.M.J., R.A.B., and M.N.H.; Supervision,; Project Administration, S.M.P., C.E.B., K.M.G., M.N.H., and J.C.R.; Funding Acquisition, S.M.P. and J.C.R.
Data safety monitoring board
Timothy Church, (Chair, ACAP Health, Dallas, TX, USA); Michael Switzer, (Behavior Technology Lab, Pennington Biomedical Research Centre, Baton Rouge, LA, USA); William Johnson, (Biostatistics, Pennington Biomedical Research Centre, Baton Rouge, LA, USA); Brian Irvine, (School of Kinesiology, Louisiana State University, Baton Rouge, LA, USA).
Declaration of Competing Interest
J.C.W., O.T.C., and K.M.G. reported that their institution received funding from the US Department of Defence for work associated with this publication. H.R.L. reported receiving personal fees from Pfizer, Inc., for work outside this publication. All remaining authors declare no competing interests. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Army or the Department of Defence. Any citations of commercial organizations and trade names in this report do not constitute an official Department of the Army endorsement of approval of the products or services of these organizations.
Acknowledgements
The research reported in this publication is supported by the Collaborative Research to Optimize Warfighter Nutrition II and III projects and the Joint Program Committee-5, funded by the US Department of Defence. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. The full trial protocol can be accessed as online Supplementary material. The authors wish to thank the participants who consented and participated in this research experiment. The authors would like to thank Dr. Shalender Bhasin for sharing his expertise and assisting the research team on the design of the testosterone intervention. We would also like to thank the study team at Pennington Biomedical Research Centre for their significant contributions to study management and conduct, data collection, and analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.07.059.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
References
- 1.Friedl K.E., Moore R.J., Hoyt R.W., Marchitelli L.J., Martinez-Lopez L.E., Askew E.W. Endocrine markers of semistarvation in healthy lean men in a multistressor environment. J Appl Physiol (1985) 2000;88(5):1820–1830. doi: 10.1152/jappl.2000.88.5.1820. [DOI] [PubMed] [Google Scholar]
- 2.Nindl B.C., Friedl K.E., Frykman P.N., Marchitelli L.J., Shippee R.L., Patton J.F. Physical performance and metabolic recovery among lean, healthy men following a prolonged energy deficit. Int J Sports Med. 1997;18(5):317–324. doi: 10.1055/s-2007-972640. [DOI] [PubMed] [Google Scholar]
- 3.Murphy N.E., Carrigan C.T., Karl J.P., Pasiakos S.M., Margolis L.M. Threshold of energy deficit and lower-body performance declines in military personnel: a meta-regression. Sports Med. Sep 2018;48(9):2169–2178. doi: 10.1007/s40279-018-0945-x. (Review) [DOI] [PubMed] [Google Scholar]
- 4.Henning P.C., Park B.S., Kim J.S. Physiological decrements during sustained military operational stress. Mil Med. 2011;176(9):991–997. doi: 10.7205/milmed-d-11-00053. [DOI] [PubMed] [Google Scholar]
- 5.Berryman C.E., Young A.J., Karl J.P., Kenefick R.W., Margolis L.M., Cole R.E. Severe negative energy balance during 21 d at high altitude decreases fat-free mass regardless of dietary protein intake: a randomized controlled trial. FASEB J. 2018;32(2):894–905. doi: 10.1096/fj.201700915R. [DOI] [PubMed] [Google Scholar]
- 6.Margolis L.M., Murphy N.E., Martini S., Gundersen Y., Castellani J.W., Karl J.P. Effects of supplemental energy on protein balance during 4-d Arctic military training. Med Sci Sports Exerc. 2016;48(8):1604–1612. doi: 10.1249/MSS.0000000000000944. [DOI] [PubMed] [Google Scholar]
- 7.Margolis L.M., Murphy N.E., Martini S., Spitz M.G., Thrane I., McGraw S.M. Effects of winter military training on energy balance, whole-body protein balance, muscle damage, soreness, and physical performance. Appl Physiol Nutr Metab. 2014;39(12):1395–1401. doi: 10.1139/apnm-2014-0212. [DOI] [PubMed] [Google Scholar]
- 8.Trumble B.C., Brindle E., Kupsik M., O'Connor K.A. Responsiveness of the reproductive axis to a single missed evening meal in young adult males. Am J Hum Biol. 2010;22(6):775–781. doi: 10.1002/ajhb.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkelstein J.S., Yu E.W., Burnett-Bowie S.A. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(25):2457. doi: 10.1056/NEJMc1313169. [DOI] [PubMed] [Google Scholar]
- 10.Ng Tang Fui M., Prendergast L.A., Dupuis P., Raval M., Strauss B.J., Zajac J.D. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Med. 2016;14(1):153. doi: 10.1186/s12916-016-0700-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dulloo A.G., Jacquet J., Girardier L. Poststarvation hyperphagia and body fat overshooting in humans: a role for feedback signals from lean and fat tissues. Am J Clin Nutr. 1997;65(3):717–723. doi: 10.1093/ajcn/65.3.717. [DOI] [PubMed] [Google Scholar]
- 12.Bhasin S., Cunningham G.R., Hayes F.J., Matsumoto A.M., Snyder P.J., Swerdloff R.S. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 13.The Army Body Composition Program . Headquarters DotA, Navy, and Air Force; Washington: 2013. Army Regulation 600-9. [Google Scholar]
- 14.Pasiakos S.M., Berryman C.E., Karl J.P., Lieberman H.R., Orr J.S., Margolis L.M. Physiological and psychological effects of testosterone during severe energy deficit and recovery: a study protocol for a randomized, placebo-controlled trial for Optimizing Performance for Soldiers (OPS) Contemp Clin Trials. 2017;58:47–57. doi: 10.1016/j.cct.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Evans W.J., Phinney S.D., Young V.R. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc. 1982;14(1):101–102. [PubMed] [Google Scholar]
- 16.Frontera W.R., Hughes V.A., Dallal G.E., Evans W.J. Reliability of isokinetic muscle strength testing in 45- to 78-year-old men and women. Arch Phys Med Rehabil. 1993;74(11):1181–1185. [PubMed] [Google Scholar]
- 17.Welliver R.C., Jr., Wiser H.J., Brannigan R.E., Feia K., Monga M., Kohler T.S. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192(1):165–169. doi: 10.1016/j.juro.2014.01.085. [DOI] [PubMed] [Google Scholar]
- 18.Vermeulen A., Verdonck L., Kaufman J.M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 19.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 20.Pasiakos S.M., Cao J.J., Margolis L.M., Sauter E.R., Whigham L.D., McClung J.P. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: a randomized controlled trial. FASEB J. 2013;27(9):3837–3847. doi: 10.1096/fj.13-230227. [DOI] [PubMed] [Google Scholar]
- 21.Berryman C.E., Sepowitz J.J., McClung H.L., Lieberman H.R., Farina E.K., McClung J.P. Supplementing an energy adequate, higher-protein diet with protein does not enhance fat-free mass restoration after short-term severe negative energy balance. J Appl Physiol. 1985;2017 doi: 10.1152/japplphysiol.01039.2016. (jap 01039 2016) [DOI] [PubMed] [Google Scholar]
- 22.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opstad P.K. Androgenic hormones during prolonged physical stress, sleep, and energy deficiency. J Clin Endocrinol Metab. 1992;74(5):1176–1183. doi: 10.1210/jcem.74.5.1314847. [DOI] [PubMed] [Google Scholar]
- 24.Opstad P.K., Aakvaag A. The effect of sleep deprivation on the plasma levels of hormones during prolonged physical strain and calorie deficiency. Eur J Appl Physiol Occup Physiol. 1983;51(1):97–107. doi: 10.1007/BF00952542. [DOI] [PubMed] [Google Scholar]
- 25.Leproult R., Van Cauter E. Effect of 1 week of sleep restriction on testosterone levels in young healthy men. JAMA. 2011;305(21):2173–2174. doi: 10.1001/jama.2011.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nindl B.C., Barnes B.R., Alemany J.A., Frykman P.N., Shippee R.L., Friedl K.E. Physiological consequences of U.S. Army Ranger training. Med Sci Sports Exerc. 2007;39(8):1380–1387. doi: 10.1249/MSS.0b013e318067e2f7. [DOI] [PubMed] [Google Scholar]
- 27.Henning P.C., Scofield D.E., Spiering B.A., Staab J.S., Matheny R.W., Jr., Smith M.A. Recovery of endocrine and inflammatory mediators following an extended energy deficit. J Clin Endocrinol Metab. 2014;99(3):956–964. doi: 10.1210/jc.2013-3046. [DOI] [PubMed] [Google Scholar]
- 28.Bhasin S., Brito J.P., Cunningham G.R., Hayes F.J., Hodis H.N., Matsumoto A.M. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. May 1 2018;103(5):1715–1744. doi: 10.1210/jc.2018-00229. [DOI] [PubMed] [Google Scholar]
- 29.Yeap B.B., Wu F.C.W. Clinical practice update on testosterone therapy for male hypogonadism: contrasting perspectives to optimize care. Clin Endocrinol (Oxf) 2019;90(1):56–65. doi: 10.1111/cen.13888. [DOI] [PubMed] [Google Scholar]
- 30.Ho C.K., Stoddart M., Walton M., Anderson R.A., Beckett G.J. Calculated free testosterone in men: comparison of four equations and with free androgen index. Ann Clin Biochem. 2006;43(Pt 5):389–397. doi: 10.1258/000456306778520115. [DOI] [PubMed] [Google Scholar]
- 31.Corona G., Giagulli V.A., Maseroli E., Vignozzi L., Aversa A., Zitzmann M. Testosterone supplementation and body composition: results from a meta-analysis of observational studies. J Endocrinol Invest. 2016;39(9):967–981. doi: 10.1007/s40618-016-0480-2. [DOI] [PubMed] [Google Scholar]
- 32.Bhasin S., Woodhouse L., Casaburi R., Singh A.B., Bhasin D., Berman N. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281(6):E1172–E1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 33.Herbst K.L., Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7(3):271–277. doi: 10.1097/00075197-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 34.White J.P., Gao S., Puppa M.J., Sato S., Welle S.L., Carson J.A. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol. 2013;365(2):174–186. doi: 10.1016/j.mce.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasiakos S.M., Vislocky L.M., Carbone J.W., Altieri N., Konopelski K., Freake H.C. Acute energy deprivation affects skeletal muscle protein synthesis and associated intracellular signaling proteins in physically active adults. J Nutr. 2010;140(4):745–751. doi: 10.3945/jn.109.118372. [DOI] [PubMed] [Google Scholar]
- 36.Areta J.L., Burke L.M., Camera D.M., West D.W., Crawshay S., Moore D.R. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am J Physiol Endocrinol Metab. 2014;306(8):E989–E997. doi: 10.1152/ajpendo.00590.2013. [DOI] [PubMed] [Google Scholar]
- 37.Hector A.J., McGlory C., Damas F., Mazara N., Baker S.K., Phillips S.M. Pronounced energy restriction with elevated protein intake results in no change in proteolysis and reductions in skeletal muscle protein synthesis that are mitigated by resistance exercise. FASEB J. 2018;32(1):265–275. doi: 10.1096/fj.201700158RR. [DOI] [PubMed] [Google Scholar]
- 38.Longland T.M., Oikawa S.Y., Mitchell C.J., Devries M.C., Phillips S.M. Higher compared with lower dietary protein during an energy deficit combined with intense exercise promotes greater lean mass gain and fat mass loss: a randomized trial. Am J Clin Nutr. 2016;103(3):738–746. doi: 10.3945/ajcn.115.119339. [DOI] [PubMed] [Google Scholar]
- 39.Ferrando A.A., Tipton K.D., Doyle D., Phillips S.M., Cortiella J., Wolfe R.R. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol. 1998;275(5):E864–E871. doi: 10.1152/ajpendo.1998.275.5.E864. Pt 1. [DOI] [PubMed] [Google Scholar]
- 40.Sheffield-Moore M., Urban R.J., Wolf S.E., Jiang J., Catlin D.H., Herndon D.N. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrinol Metab. 1999;84(8):2705–2711. doi: 10.1210/jcem.84.8.5923. [DOI] [PubMed] [Google Scholar]
- 41.Millet G.Y., Lepers R. Alterations of neuromuscular function after prolonged running, cycling and skiing exercises. Sports Med. 2004;34(2):105–116. doi: 10.2165/00007256-200434020-00004. [DOI] [PubMed] [Google Scholar]
- 42.Carter J.M., Jeukendrup A.E., Jones D.A. The effect of carbohydrate mouth rinse on 1-h cycle time trial performance. Med Sci Sports Exerc. 2004;36(12):2107–2111. doi: 10.1249/01.mss.0000147585.65709.6f. [DOI] [PubMed] [Google Scholar]
- 43.Irwin B.C., Scorniaenchi J., Kerr N.L., Eisenmann J.C., Feltz D.L. Aerobic exercise is promoted when individual performance affects the group: a test of the Kohler motivation gain effect. Ann Behav Med. 2012;44(2):151–159. doi: 10.1007/s12160-012-9367-4. [DOI] [PubMed] [Google Scholar]
- 44.Zachwieja J.J., Smith S.R., Lovejoy J.C., Rood J.C., Windhauser M.M., Bray G.A. Testosterone administration preserves protein balance but not muscle strength during 28 days of bed rest. J Clin Endocrinol Metab. 1999;84(1):207–212. doi: 10.1210/jcem.84.1.5420. [DOI] [PubMed] [Google Scholar]
- 45.Emmelot-Vonk M.H., Verhaar H.J., Nakhai Pour H.R., Aleman A., Lock T.M., Bosch J.L. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299(1):39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 46.Demling R.H., DeSanti L. Involuntary weight loss and the nonhealing wound: the role of anabolic agents. Adv Wound Care. 1999;12(1 Suppl):1–14. [quiz 5-6] [PubMed] [Google Scholar]
- 47.Heymsfield S.B., Gonzalez M.C., Lu J., Jia G., Zheng J. Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc. 2015;74(4):355–366. doi: 10.1017/S0029665115000129. [DOI] [PubMed] [Google Scholar]
- 48.Johannsson G., Gibney J., Wolthers T., Leung K.C., Ho K.K. Independent and combined effects of testosterone and growth hormone on extracellular water in hypopituitary men. J Clin Endocrinol Metab. 2005;90(7):3989–3994. doi: 10.1210/jc.2005-0553. [DOI] [PubMed] [Google Scholar]
- 49.van Breda E., Keizer H.A., Geurten P., van Kranenburg G., Menheere P.P., Kuipers H. Modulation of glycogen metabolism of rat skeletal muscles by endurance training and testosterone treatment. Pflugers Arch. 1993;424(3–4):294–300. doi: 10.1007/BF00384355. [DOI] [PubMed] [Google Scholar]
- 50.Friedl K.E., Moore R.J., Martinez-Lopez L.E., Vogel J.A., Askew E.W., Marchitelli L.J. Lower limit of body fat in healthy active men. J Appl Physiol (1985) 1994;77(2):933–940. doi: 10.1152/jappl.1994.77.2.933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2