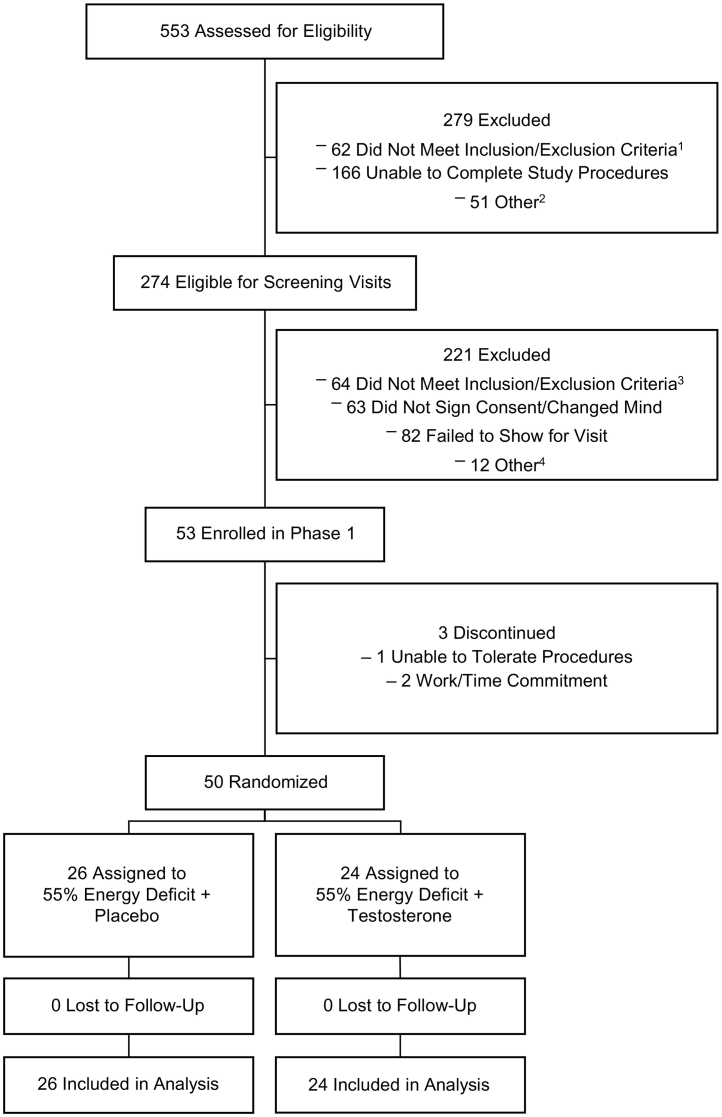
Fig. 1.
Participant flow chart. 553 individuals were assessed for eligibility, 279 were excluded, 274 were found eligible for screening, 221 were excluded following screening, 53 were enrolled, 3 discontinued participation prior to phase 2 (i.e., were not randomised), 50 were randomised and completed the intervention (24 into Testosterone and 26 into Placebo). 1Taking medications or supplements (n = 11), irremovable metal (n = 16), allergies or food intolerance (n = 5), not physically active (n = 2), age (n = 5), body mass index (n = 9), did not meet >1 inclusion/exclusion criteria (n = 14); 2Recruitment complete/full (n = 28), could not contact (n = 17), not a citizen (n = 5), unknown (n = 1); 3Medical history or lab results (n = 18), smoking/drug use (n = 12), body mass index (n = 20), dietary limitations (n = 3), non-compliant with screening procedures (n = 9), not willing to receive testosterone injections (n = 1), head circumference too large for MRI machine (n = 1); 4Recruitment complete/full (n = 4), schedule conflict (n = 8).