Abstract
Background
The imprinted gene Delta like non-canonical Notch ligand 1 (Dlk1) is considered an inhibitor of adipogenesis, but its in vivo impact on fat mass indeed remains elusive and controversial.
Methods
Fat deposits were assessed by MRI and DXA scanning in two cohorts of non-diabetic men, whereas glucose disposal rate (GDR) was determined during euglycemic hyperinsulinemic clamp. Blood analyte measurements were used for correlation and mediation analysis to investigate how age, BMI, and fat percentage affect the relation between DLK1 and GDR. Confirmatory animal studies performed in normal (NC) and high fat diet (HFD) fed Dlk1+/+ and Dlk1−/− mice included DXA scanning, glucose tolerance tests (GTTs), blood measurements, and skeletal muscle glucose uptake studies by positron emission tomography (PET), histology, qRT-PCR, and in vitro cell studies.
Findings
Overall, DLK1 is positively correlated with fat amounts, which is consistent with a negative linear relationship between DLK1 and GDR. This relationship is not mediated by age, BMI, or fat percentage. In support, DLK1 also correlates positively with HOMA-IR and ADIPO-IR in these humans, but has no linear relationship with the early diabetic inflammation marker MCP-1. In Dlk1−/− mice, the increase in fat percentage and adipocyte size induced by HFD is attenuated, and these animals are protected against insulin resistance. These Dlk1 effects seem independent of gluconeogenesis, but at least partly relies on increased in vivo glucose uptake in skeletal muscles by Dlk1 regulating the major glucose transporter Glut4 in vivo as well as in two independent cell lines.
Interpretation
Thus, instead of an adipogenic inhibitor, Dlk1 should be regarded as a factor causally linked to obesity and insulin resistance, and may be used to predict development of type 2 diabetes.
Fund
The Danish Diabetes Academy supported by the Novo Nordisk Foundation, The Danish National Research Council (#09-073648), The Lundbeck Foundation, University of Southern Denmark, and Dep. Of Clinical Biochemistry and Pharmacology/Odense University Hospital, the Swedish Research Council, the Swedish Diabetes Foundation, the Strategic Research Program in Diabetes at Karolinska Institute and an EFSD/Lilly grant.
Keywords: Dlk1, Type 2 diabetes, Obesity, Insulin resistance, Glucose uptake
Research in context.
Evidence before this study
Before undertaking this study, we searched PubMed, MEDLINE and references from relevant articles on Dlk1 in metabolism and critically evaluated the quality and originality of the methods and results of animal studies and clinical trials. With the exception of a few studies, the overall view has been that Dlk1 acts as an inhibitor of adipogenesis and fat accumulation. Additionally, a few studies have addressed Dlk1's role in glucose homeostasis with diverging results.
Added value of this study
Our data contradict prior knowledge and suggest that in non-diabetic and otherwise healthy humans, DLK1 associates with increased fat amounts, and independent hereof directly affects the glucose disposal rate. Animal and cell studies confirm this and further suggest that Dlk1 affects glucose homeostasis through an effect on skeletal muscle glucose uptake.
Implications of all the available evidence
Together with prior knowledge, our data underscore a role of Dlk1 in obesity and implications hereof. Our results in specific hold promise for using DLK1 as an early prognostic marker of individuals that may develop type 2 diabetes (T2D), but currently are diagnosed as non-diabetics. Moreover, together with existing Dlk1 knowledge, results herein may be further used to unravel the complex biology underlying T2D and eventually lead to new treatment targets.
Alt-text: Unlabelled Box
1. Introduction
Obesity has become one of the major burdens of modern societies and causes numerous complications, including type 2 diabetes (T2D). More efficient anti-obesity strategies seem to require a combination of approaches [1], and identification of new drug targets is therefore warranted. Dysregulated expression of imprinted genes is known to exert a marked impact on embryonic growth and development, but exciting new evidence also suggests a postnatal effect where imprinted genes are implicated in human metabolic disorders [2]. The Dlk1-Dio3 imprinted gene cluster is localized to mouse chromosome 12 and human chromosome 14. Defects in this cluster are associated with obesity, leanness, insulin resistance, and impaired glucose tolerance and metabolic disorders [2]. Delta like non-canonical Notch ligand 1 (Dlk1) is one out of four protein-encoding paternally expressed genes in the Dlk1-Dio3 domain. The DLK1 protein exists in both transmembrane and shed forms, and is highly homologous to the NOTCH-DELTA protein family [3,4]. However, although others and we have recently shown that DLK1 interacts with NOTCH1, also Notch-independent mechanisms have been described [[4], [5], [6], [7], [8], [9]]. Indeed, the underlying mechanisms are still unclear, and the proposed functions of DLK1 are diverse and debated. Inhibition of adipogenesis by Dlk1 was already described twenty-five years ago, and Dlk1 has for long been considered as “the preadipocyte marker” [[10], [11], [12], [13], [14]]. However, it should be emphasized that the majority of evidence for this relies on in vitro work obtained in preadipocyte cell lines [11,12] and only a few rodent studies exist [10,[13], [14], [15], [16]]. Remarkably, more recent studies oppose this idea and note that DLK1 may enhance adipocyte differentiation in some cells (mesenchymal C3H10T1/2 cell line) [17], or has no effect on adipose-tissue expansion [18]. In addition, several clinical studies relating serum DLK1 levels to obesity/fat mass/weight gain show different relationships in humans affected by various diseases [[19], [20], [21], [22]]. Thus, the impact from Dlk1 on in vivo obesity remains controversial and even lacks for healthy individuals.
Thus, to clarify whether novel strategies exploiting Dlk1 may be relevant for anti-obesity drug development or metabolic profiling, we set out to delineate by state-of-art scanning techniques whether Dlk1 has a substantial impact on obesity in healthy human individuals, while performing complementary studies in Dlk1 transgenic animals.
2. Research design and methods
2.1. Approvals
All human studies were approved by relevant ethical committees and a written informed consent was obtained from every subject at each site [[23], [24], [25], [26]]. The animal study was approved by the Danish Council for Supervision with Experimental Animals (#2012-15-2934-00313 and # 2016–15–0201 − 00941).
2.2. Study populations
The clinical details on the study subjects from the Swedish cohort have been published previously [23]. Briefly, 56 women with a median age of 41.5 (IQR, 12), were subdivided into obese (median BMI: 41.3; IQR, 11.8; n = 30) and non-obese (median BMI: 24.1; IQR, 2.78; n = 26).
Subjects from one Italian cohort [24,26] included 35 healthy non-diabetic men with a median age of 47 years (IQR, 15.1) and median BMI of 27.4 (IQR, 4.3), all of whom underwent Magnetic Resonance Imaging (MRI) scans. An extended Italian cohort, examined by Computer Tomography (CT) and with available data on their epicardial fat, consisted of 158 non-diabetic men, with a median age of 62 years (IQR, 18) and a median BMI of 26.5 (IQR, 3.4).
A Danish cohort encompassed 48 healthy non-diabetic men [25] with a median age of 68 years (IQR, 9) and a median BMI of 30.0 (IQR, 5.6). Six patients from the initial population of 54 subjects were excluded based on a diabetic MCP-1 value (n = 2), DLK1 levels >100 (ng/mL) (n = 1), missing measures (n = 2), and hemolysis in blood sample (n = 1). Dual X-ray absorptiometry (DXA) was used to measure percentage of body fat (n = 48) and quantification of visceral and subcutaneous fat was performed by MRI (n = 43) [27]. Euglycemic hyperinsulinemic clamps (n = 48) were performed as follows [25]: Briefly, after a 120-min. Basal tracer equilibration period, insulin (Actrapid; Novo Nordisk, DK) was infused at a rate of 40 mU/m2/min. For 180 min leading to physiological hyperinsulinemia at approximately 400 pmol/L in the insulin-stimulated period. Plasma glucose levels were clamped at approximately 5 mmol/L, using a variable infusion rate of 20% glucose based on bedside plasma glucose measurements (ABL800 Flex, Radiometer, DK) every 10–20 min. Mean glucose disposal rate (mg/min/m2) at steady state (the last 20 min. of clamp) was taken as an estimate of whole-body insulin sensitivity.
2.3. Animals and diets
To account for Dlk1's paternal inheritance, we generated and maintained Dlk1−/− and Dlk1+/+ colonies by homozygous breeding and backcrossed as previously described [28,29]. Breeding and housing was done under standard conditions with a 12-h light/12-h dark cycle. Animals were fed ad libitum with a normal chow (NC; 10% fat, 20% protein, 70% carbohydrate) or with a high-fat diet (HFD; 45% fat, 35% carbohydrate, 20% protein) (Brogaarden, Denmark) and had free access to water. For all animal experiments, age- and sex-matched animals were used as indicated and where relevant (GTT and PET/CT), animal groups were measured in a mixed order.
2.4. Mouse and human plasma measurements
Blood from animals was collected from the tail vein, and DLK1 levels were measured using a mouse DLK1 ELISA essentially as previously described [30], except that two monoclonal antibodies (CC-5 and CC-11, generated in-house) against mouse DLK1 were used as capture/detection antibodies. Human serum DLK1 was measured as formerly described [31]. Plasma levels of Monocyte Chemoattractant Protein-1 (MCP-1) were measured by ELISA as previously reported [32]. Glucagon was measured by ELISA as recommended by the manufacturer (Mercodia).
2.5. Glucose tolerance test
A Glucose Tolerance Test (GTT) was performed in Dlk1−/− and Dlk1+/+ males/females fed HFD or NC. Briefly, animals were fasted for 6 h [33], and following an intraperitoneal (i.p.) injection of d-Glucose (2 g/kg body weight), blood glucose levels were monitored at indicated time points using the One Touch Ultra® system (LifeScan). In an immunoneutralization experiment, Dlk1+/+ animals (n = 15) fed HFD for 13 weeks were injected i.p. with a mouse monoclonal antibody (IgG1, k, in house) recognizing an epitope on the extracellular part of the mouse DLK1 protein, or with an isotype-matched control antibody (10 μg MAb/g body weight). GTT was performed one and three days after injection.
2.6. Homeostasis model assessment for insulin resistance
The homeostasis model assessment method was used to calculate insulin resistance (HOMA-IR), as described by others [34] from fasting plasma glucose and insulin levels: HOMA-IR = (FPI × FPG)/22.5, where FPI is the fasting plasma insulin concentration (mU/L) and FPG is the fasting plasma glucose level (mmol/L). Adipo-IR, a measure of adipose tissue insulin resistance [35] = Fasting FFAs (mM) × Insulin (pM).
2.7. Body composition (DXA)
Body composition, including total fat mass, was determined by dual energy X-ray absorptiometry (DXA) using the Lunar PIXImus Mouse Densitometer (Wipro GE Healthcare, WI) with the mice under inhalation anesthesia using 2% Isoflurane.
2.8. Small animal positron emission-/computer-tomography (PET/CT) imaging
Small-animal PET/CT was performed on a Siemens INVEON multimodality preclinical scanner. Mice (Dlk1+/+ n = 15, Dlk1−/− n = 13) were fasted overnight prior to PET/CT scanning at baseline (day 0) and one day later (day 1). Mice were subcutaneously injected with 75 μl saline or 0.75 U/(Kg body weight) short acting insulin (Humulin, NOVO Nordisk, DK) respectively, 15 min. Before injection of 18F-Fluoro-deoxy-glucose (FDG). Mice were anesthetized (2% isoflurane), and a bolus injection of FDG (day 0 95.5 ± 9.1 MBq; day 1: 91.6 ± 13.4 MBq) was administered via a tail vein catheter. Prior to the PET scan a two-bed CT scan was performed for attenuation correction of the PET data and anatomic orientation. CT and PET images were co-registered using a transformation matrix and CT-based attenuation correction was applied to the PET data. The PET data were reconstructed using the Siemens INVEON pre-clinical software and data analysis of the PET/CT fused images was performed with INVEON software version 4.2 (IRW, Siemens). FDG uptake in each volume of interest (VOI) was reported as standardized uptake values (SUV). VOIs of the skeletal limb hind muscles were normalized using brain uptake as a reference tissue that metabolizes glucose independent of insulin.
2.9. In vitro cell transfections
Mouse C2C12 myoblast as well as 3 T3-L1 preadipocytes were cultured as previously described [28,36]. For plasmid transfection we used vectors expressing the entire DLK1 protein (pLHDLK1-HA), protease site-lacking DLK1 (pLHDLK1ΔP-HA), soluble DLK1 (pLHDLK1E-HA) or empty vector (pLHCX-HA) [37]. Plasmids were purified using a QIAGEN Plasmid Miniprep Kit (Qiagen) and the concentration measuered by Nanodrop (Saveen Werner, DK). Purity was evaluated by visualization on a 1% agarose gel. Transfections were performed using electroporation (Amaxa Nucleofector™ Technology, Lonza) at optimal confluence according to the manufacturer's instructions. Myoblasts (2.7–3.7 × 106) were suspended in 100 μL of Cell line Mirus Nucleofector solution (Lonza) with two different concentrations of plasmids. Transfected cells reached a 10-fold increase in the expression of DLK1. Cells were analyzed by immunostaining or qPCR. For preadipocytes, siRNA transfections were performed as previously described [36].
2.10. qPCR and mRNA arrays
Total RNA extraction and qPCR analysis were performed as previously described [28]. Relative expression levels were obtained after normalizing the raw data against multiple endogenous control genes as determined by the qBase+ platform (Biogazelle). In the Swedish cohort, total RNA was isolated from subcutaneous abdominal adipose tissue biopsies. Biotinylated complementary RNA was subsequently analyzed using the GeneChip Human Gene 1.0 ST Array (Affymetrix Inc., Santa Clara, CA) and standardized protocols as described previously [23]. Data are deposited at the National Center for Biotechnology Information Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE25402.
2.11. Adipocyte size quantification and Immuno(cyto/histo)chemistry
Average adipocyte volume (pL) was determined using HE-stained sections of formaldehyde fixed (FFPE) gonadal adipose tissue obtained from six week-old Dlk1+/+ (n = 12), Dlk1−/− (n = 12) mice fed NC or HFD for 28 weeks before being sacrificed. Two sections (each comprising five fields) from each animal were used for quantification. Images were acquired using a Leica DMI4000B Cool Fluo Package instrument equipped with a Leica DFC340 FX Digital Camera and analyzed using ImageJ 1.48 software with MRI adipocyte size tool. Average adipocyte volume was calculated from the Feret's diameter values. Immunohistochemistry of FFPE tissues was performed as previously described [28] with rabbit anti-mouse DLK1 (1:2000; in-house) [30] or, rabbit anti-Glut4 (1:1000; ab33780, Abcam) antibodies. Secondary antibodies used were Alexa 555- or 488-conjugated donkey anti-IgG (1:200, Molecular Probes), and mounting medium contained DAPI (Vectashield, Vector Labs). Images were captured using a Leica DMI4000B instrument equipped with a Leica DFC340 FX Digital Camera. Exposure and picture processing were applied equally to m.gastrocnemius sample sections and controls. GLUT 4 protein expression differed within all specimens with some areas showing high membrane appearance, whereas others showing much less membrane GLUT4 localization. To minimize subjectivity in GLUT4 quantification, a picture was acquired for all cross-sectional areas (high and low) in a given specimen. Analysis was performed using the ImageJ software, where ROIs were used to remove areas such as large vessels and mesenchyme. For each specimen, two averages of the mean intensity were calculated for high- (n = 6) and low (n = 4–6, 3 specimens did not include low intensity areas of cross section) intensity areas, and a final average (high and low (n = 4–6)) of the entire section was calculated.
2.12. Statistical analyses
The statistical significance of the difference between means was determined by either two-tailed t-tests, or by one- or two-way ANOVA followed by appropriate post-hoc tests as indicated. Clinical values were presented using the median and interquartile range (IQR). Associations between DLK1 and clinical parameters of interest were evaluated by correlation analysis using Spearman's rank correlation coefficient or the Pearson correlation coefficient as appropriate. Six observations were removed from the data set for various reasons (diabetic MCP, lacking measures, hemolysis, or DLK1 outlier). A mediation analysis was performed to investigate how age, BMI, and fat percentage affect the relation between DLK1 and GDR. The mediating process includes the dependent variable GDR, the independent variable DLK1, and the mediators age, BMI, and fat percentage. The mediators BMI and fat percentage are relatively strongly correlated (r = 0.7) but removing each of these mediators in turn from the model does not affect the estimation and significance tests substantially. The maximum variance inflation factor among DLK1, age, BMI, and fat percentage is 2.93, so there is no evidence of severe multicollinearity. Finally, in order to improve homogeneity of variances, a logarithmic transformation was applied to GDR (Supplemental Table S1). However, since the two mediation analyses are in substantial agreement, we interpret the results from the analysis based on untransformed data. For statistical analysis, we used GraphPad Prism and the R software environment (version 3.5.1).
3. Results
3.1. DLK1 is associated with increased body fat in humans
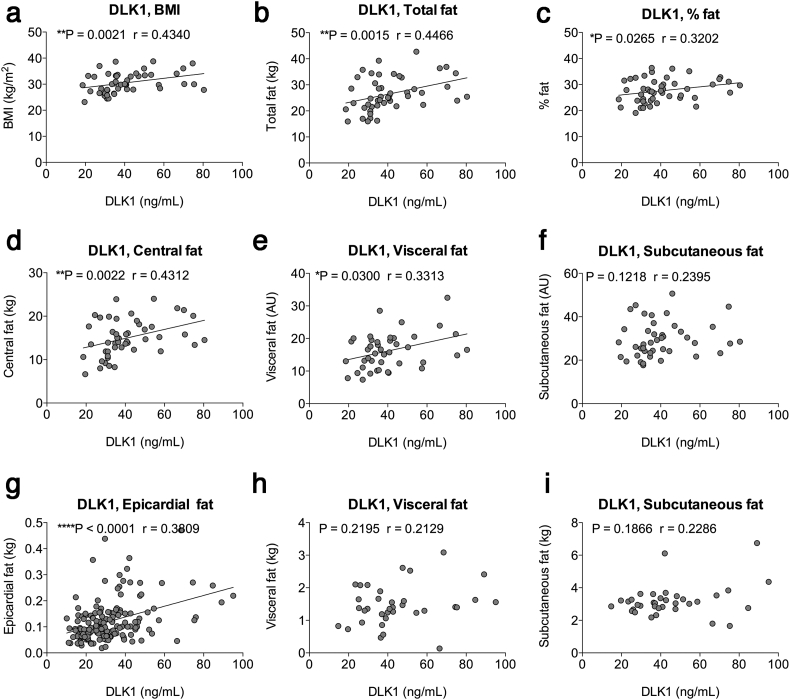
Circulating DLK1 levels were measured in blood samples from healthy to slightly overweight, non-diabetic Danish men (68 years (9) (median; IQR); n = 52) with a median BMI of 29.8 (5.4)) [27]. The median concentration of serum DLK1 was 36.15 ng/ml (17.2) and correlated positively with BMI (Fig. 1a) as also previously shown [19]. As determined by Magnetic Resonance Imaging (MRI) (Fig. 1b-f), the absolute amount of fat as well as the percentage of fat increased significantly with increasing levels of circulating DLK1 in these non-diabetic individuals (Fig. 1b-c). The relative amount of abdominal fat is in general associated with an increased risk of developing metabolic syndrome. We found that increased levels of circulating DLK1 were associated to increased amounts of central fat mass (Fig. 1d), and this seemed to be a consequence of increased amounts of visceral-, but not subcutaneous fat (Fig. 1e-f). Increased epicardial fat is also a marker of the metabolic syndrome [38]. In a population of 158 healthy, non-diabetic middle-aged Italian men (median age: 58 years (21 (IQR) and median BMI: 26.8 (3.7)) undergoing CT scans [24,26], the median concentration of serum DLK1 was 28.9 ng/ml (17.1) and correlated positively with BMI (p = .0068; Spearman's r = 0.2143). In this population, the amount of epicardial fat was significantly associated with DLK1 levels (Fig. 1g) further supporting a possible link between DLK1 and increased fat in humans, as well as with the development of the metabolic syndrome. No significant correlations were observed between DLK1 and visceral or subcutaneous fat amounts (Fig. 1h-i) in this population, but these latter data may be undermined by a low power (N = 35; see methods) of the subpopulation undergoing whole body scan.
Fig. 1.
DLK1 levels correlate with fat accumulation in humans. (a-f) Correlation analysis of circulating DLK1 in a Danish male cohort (n = 48, median age 68 years, IQR 9; median BMI 29.8, IQR 5.5), with (a) BMI, (b) Total fat (kg), (c) Percentage of fat, (d) Central fat mass (kg), (e) Visceral fat mass (arbitrary unit (AU) = pixels*10−3), (f) subcutaneous fat mass (AU). (g-i) Correlation analysis of circulating DLK1 in an Italian cohort (n = 158) consisting of non-diabetic men median age: 58 years IQR 21 and median BMI: 26.8, IQR 3.7, with (g) Epicardial fat amount, and for a subpopulation of n = 35 (median age 47 years, IQR 15.1; median BMI 27.4, IQR, 4.3) (h) Visceral fat mass (kg) and (i) subcutaneous fat mass (kg). Data were analyzed using Spearman and Pearson correlation analysis, as appropriate. Trend lines imply visual aid and not causality.
Together, these data suggest that in normal to overweight non-diabetic human subjects, DLK1 levels are associated with increased body fat, thus contradicting that DLK1 acts as an adipogenic inhibitor exclusively.
3.2. Dlk1 deficiency protects against diet-induced obesity in mice
To directly investigate our positive correlations between increased DLK1 and body fat content we used a Dlk1−/− mouse strain, which has been used by several research groups in growth-related studies [28]. Adult Dlk1−/− mice were completely negative for Dlk1 in all examined tissues, whereas Dlk1+/+ mice expressed Dlk1 in specific tissues, mainly of neuro-endocrine function (Supplemental Fig. S1).
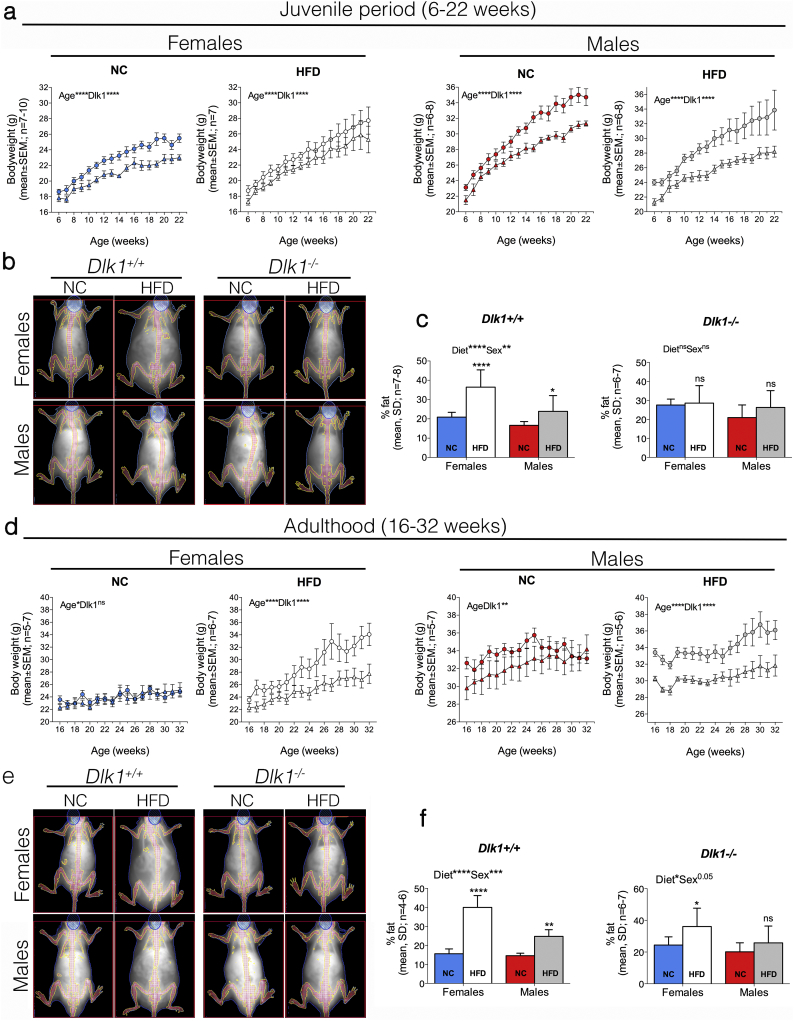
As an accepted model for the induction of obesity, we compared Dlk1+/+ and Dlk1−/− mice fed a normal chow (NC) or a high-fat diet (HFD) starting at the age of 6 weeks (= puberty in mice) (Fig. 2a-c). In both females and males, and irrespective of diet, we found that the weight of Dlk1−/− mice was significantly reduced as compared to that of Dlk1+/+ control mice (Fig. 2a). Yet, the percentage of weight gain on NC and HFD was similar in both genotypes (Supplemental Fig. S2), which is in agreement with previous observations that food intake is unaffected by Dlk1 [15,18,39]. To obtain accurate fat content measures in these mice, we used DXA scanning (Fig. 2b). On NC diet, the fat percentage was slightly increased in Dlk1−/− as compared to Dlk1+/+ at NC in both females and males (Fig. 2b-c), a result which was consistent with previous data suggesting that Dlk1 negatively affects adipogenesis [3,13,40]. However, whereas Dlk1+/+ animals substantially increased their fat percentage upon HFD diet, Dlk1−/− animals were resistant upon HFD and exhibited a fat percentage similar to their NC fed controls (Fig. 2b-c).
Fig. 2.
Dlk1−/− mice weigh less than Dlk1+/+ mice regardless of the type of diet, and are resistant to HFD induced obesity. Six-week-old (a-c) and 16-week-old (d-f) Dlk1+/+ and Dlk1−/− mice were fed normal chow (NC) or high-fat diet (HFD, Altromin, 42% fat). Body weight was determined every week (a, d) and DXA scanning (diet age: 23 weeks; b-c and e-f) was performed to quantify percentage of body fat. Statistical difference was tested by a two-way ANOVA followed by Fisher's LSD test; *P < .05, **P < .01, ***P < .001, ****P < .0001, ns (not significant).
Previous studies performed in mice have shown that Dlk1 inhibits adipocyte enlargement during obesity [[13], [14], [15]]. We found that Dlk1+/+ animals independently of sex, exhibited an expected increase in fat cell volume upon HFD whereas Dlk1−/− animals showed no change, despite having slightly larger adipocytes on NC diet as compared to Dlk1+/+ animals (Supplemental Fig. S3). Furthermore, we did not find a negative association between DLK1 mRNA and fat cell size in WAT subcutaneous biopsies obtained from normal to overweight human subjects (Supplemental Fig. S3).
Since adipose tissue develops during the postnatal and juvenile life [41], the results of an early postnatal HFD feeding protocol as tested above may have influenced fat tissue development by itself. Thus, we next tested whether the absence of Dlk1 affected fat expansion in adult animals by starting the diet intervention at the age of 16 weeks. In contrast to the findings in juvenile animals (Fig. 2a), the difference in body weight between genotypes in NC fed mice, particularly in females, were no longer evident (Fig. 2d). For Dlk1−/− females on HFD, this resulted in a substantially lower weight gain (Supplemental Fig. S2). As for the juvenile animals, DXA scanning showed a slightly increased fat percentage at basal levels in Dlk1−/− versus Dlk1+/+ animals (Fig. 2e-f). Yet, an increase in % fat was still highly substantial for Dlk1+/+ animals on HFD induction, with only a modest effect in Dlk1−/− female animals and none in males (Fig. 2e-f). Together these animal studies demonstrate that irrespective of obesity onset, mice lacking Dlk1 are protected from HFD-induced obesity, although they may have slightly more adipose tissue under isocaloric conditions. Thus, these data supports our human data showing a positive association between DLK1 and fat masses.
3.3. DLK1 has a direct negative impact on glucose homeostasis
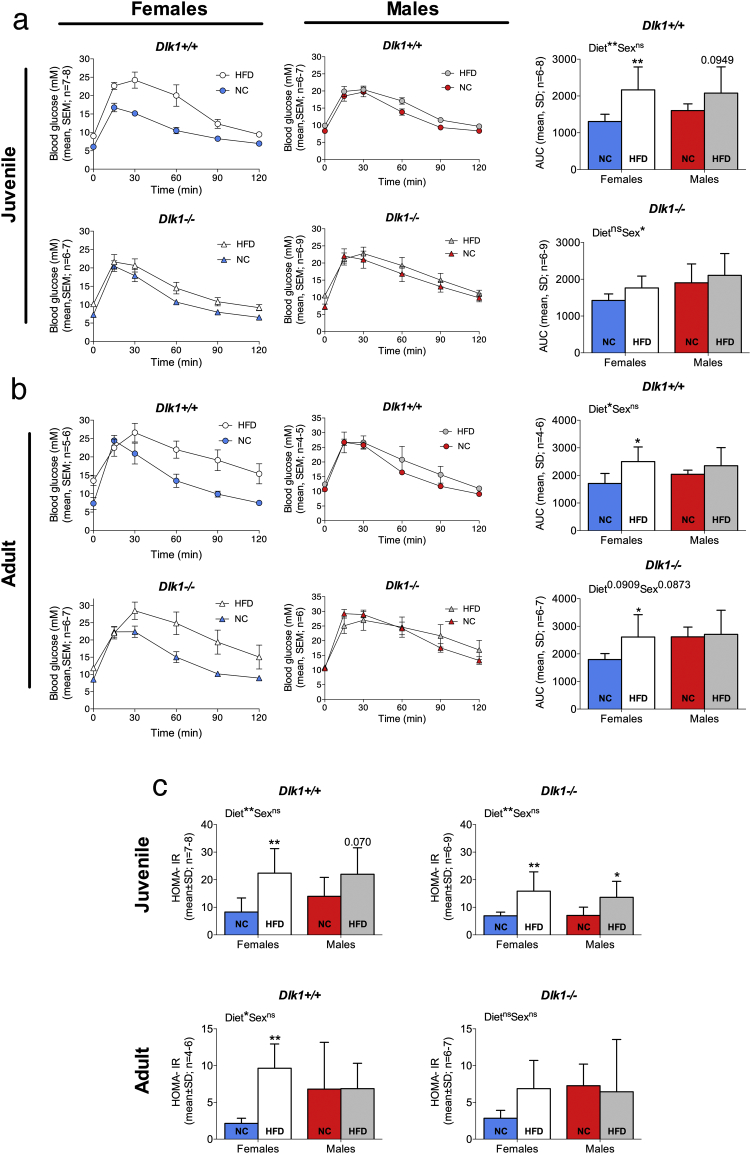
Since our data revealed that a lack of Dlk1 protects against diet-induced obesity, we next hypothesized that it would also protect against impaired glucose tolerance as induced by HFD. Except for adult males, all animals on HFD displayed higher fasting blood glucose levels than their NC-fed controls (Supplemental Fig. S4). This was independent of genotype, sex or age at onset (6- or 16-weeks of age) of diet-induced obesity (Supplemental Fig. S4) and verified that HFD resulted in insulin resistance. As expected, blood glucose was significantly higher in the adult animal group compared to the juvenile (Supplemental Fig. S4), reflecting that increasing age lead to decreased insulin sensitivity. However, neither genotype nor sex influenced these results, suggesting that Dlk1 does not have an impact on the normal age-related negative changes in glucose homeostasis as such.
We next subjected our animals to glucose tolerance tests (GTT) (Fig. 3a-b). Overall, GTT profiles (Fig. 3a-b) reflected that females responded to HFD by a greater reduction in glucose tolerance than males. This is in agreement with the fat percentage profiles described above (Fig. 2c, f) and as also noticed by others it underscores the importance of sex, at least in mouse [42,43]. However, while impairment of glucose clearance was obvious in juvenile Dlk1+/+ females the Dlk1−/− animals were protected against glucose intolerance (Fig. 3a). This phenotype was, however, attenuated in adult animals where the ability to clear glucose was similar in both genotypes independent of sex (Fig. 3b). Suppressing the bioavailable DLK1 levels in vivo by 73% (Supplemental Fig. S5) with an antibody in juvenile Dlk1+/+ females on HFD did not result in any acute changes in blood glucose clearance (Supplemental Fig. S5). Notably, while fasting glucose levels were unaffected by genotype (Supplemental Fig. S4) fasting insulin levels were significantly lower in Dlk1−/− animals as compared to Dlk1+/+ controls, leading to reduced HOMA-IR levels (Fig. 3c), an estimate of insulin resistance based on fasting levels of glucose and insulin.
Fig. 3.
Lack of Dlk1 improves glucose homeostasis in female mice. (a, c (Top)) Six-week-old or (b, c (Bottom)) 16-week-old Dlk1+/+ or Dlk1−/− mice were fed normal chow (NC) or high-fat diet (HFD) for 25 weeks. Glucose tolerance tests (GTT) were performed at the end of the experiment and fasting insulin and glucose levels (Supplemental Fig. S4) were measured to calculate the HOMA-IR index. For A-B, the area under the curve (AUC) was calculated for each mouse and used for statistical testing. a-c statistical difference was tested by a two-way ANOVA followed by Fisher's LSD test; *P < .05, **P < .01, ns (not significant).
Thus, in agreement with the above data, where Dlk1 enhanced obesity, it similarly has a negative impact on insulin sensitivity.
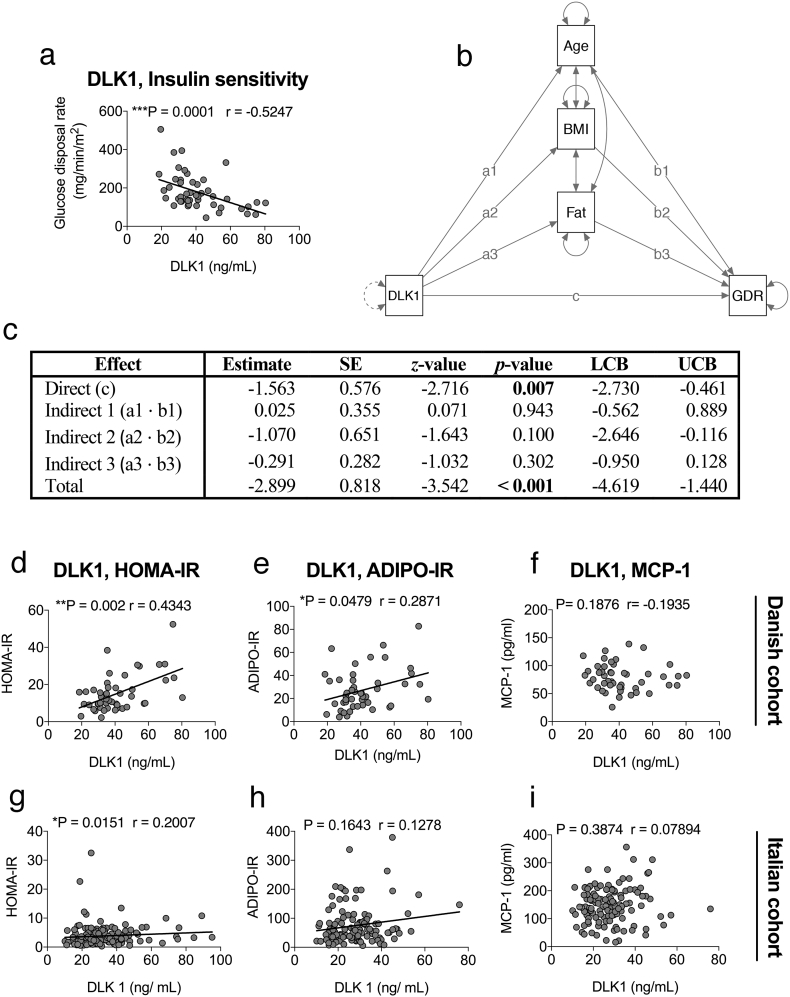
To extend these data into humans, we analyzed whole body insulin sensitivity by the gold standard technique, the euglycaemic hyperinsulinaemic clamp, in relation to serum DLK1 levels. Indeed, serum DLK1 is negatively correlated with glucose disposal rates (GDR) measured in 48 (68 year-old (9); median BMI: 30.0 (5.6)) of the 58 previously described Danish men (Fig. 4a). Importantly, there is no evidence of mediation of the effect of DLK1 by age, BMI, or fat percentage (Fig. 4b-c and Supplemental Table S1). The direct effect from DLK1 to GDR is statistically significant (p = .007). None of the three indirect effects explaining the extent to which the effect of DLK1 is mediated by age, BMI, and fat percentage, respectively, are statistically significant at the 0.05 significance level. The total effect is highly statistically significant (p < .001).
Fig. 4.
Dlk1 is related to an adverse metabolic profile connected with insulin resistance in humans. Spearman correlation analysis of circulating DLK1 in 48 men (68-year-old, IQR 9; median BMI 30, IQR 5.6) from a Danish cohort with (a) glucose disposal rate. (b) Path diagram of the multiple-mediator model. The direct effect from DLK1 to GDR is labeled c. The effects from DLK1 to age, BMI, and fat percentage are labeled a1, a2, and a3, respectively. The effects from age, BMI, and fat percentage to GDR are labeled b1, b2, and b3, respectively. The three indirect effects explaining the extent to which the effect of DLK1 is mediated by age, BMI, and fat percentage are given by the products a1 · b1, a2 · b2, and a3 · b3, respectively. The total effect is given by the sum of direct and indirect effects: c + (a1 · b1) + (a2 · b2) + (a3 · b3). Dependent variable: GDR. Independent variable: DLK1. Mediators: Age, BMI, and fat percentage. (c) Summary of the mediation analysis. Estimates, standard errors, z-values, p-values, and 95% lower and upper confidence bounds (LCB and UCB, respectively). Bootstrapping with 5000 draws was used to estimate the standard errors. (d-f) Spearman correlation analysis in the Danish cohort of circulating DLK1 and (d) HOMA-IR, (e) Adipo-IR, and (f) MCP-1. Spearman correlation analysis of circulating DLK1 in an Italian cohort of men (n = 157; median age: 58 years, IQR 21; median BMI 26.8, IQR 3.7) with (g) HOMA-IR, (h) Adipo-IR, and (i) MCP-1. Trend lines imply visual aid and not causality.
Moreover, serum DLK1 was positively correlated with HOMA-IR and Adipo-IR [35] (Fig. 4d-e), the latter indicating that insulin resistance in adipose tissue may also be affected by Dlk1 and contribute to the reduction of whole-body insulin sensitivity. As noted above, the examined cohorts herein were not established type 2 diabetics and had relatively low levels of the inflammation marker MCP-1 which did not correlate with DLK1 levels (Fig. 4f). Similar results were observed in the case of the Italian cohort (Fig. 4g-i). These data thus extend our animal results into healthy, non-diabetic humans and indicate that Dlk1 has a direct effect on glucose disposal, and that plasma DLK1 may be an early predictor of insulin resistance and TD2 in healthy humans (at least in men) well before the onset of an MCP-1 increase.
3.4. Dlk1 impacts glucose homeostasis upon diet-induced obesity, through regulation of glucose uptake in skeletal muscle
We finally assessed whether our results could be explained by DLK1 mediating inhibition of gluconeogenesis in the liver or by inhibition of skeletal muscle glucose uptake. Measuring the level of glucagon in the NC and HFD treated juvenile female animals showed an increase upon HFD, but it did not reveal any apparent difference between genotypes (Supplemental Fig. S6). This supports that fasting glucose levels are similar between genotypes (Supplemental Fig. S4), and indicates that gluconeogenesis is unaffected in Dlk1−/− animals, and therefore not responsible for the observed resistance to obesity-induced insulin resistance (Fig. 2). In agreement, hepatic glucose production in the population of Danish men did not correlate to DLK1 levels at neither basal nor clamp conditions (Supplemental Fig. S6).
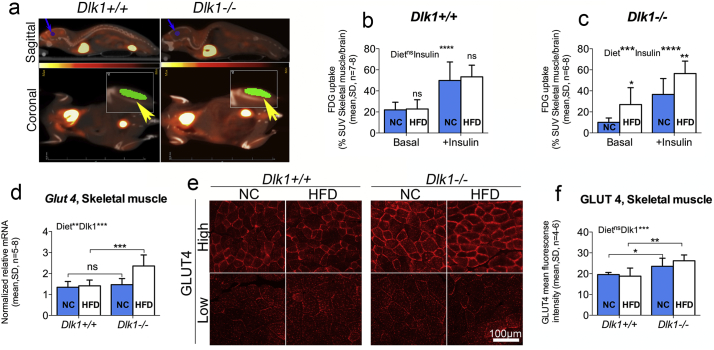
We then tested glucose uptake in skeletal muscle directly by using 18F-FDG PET/CT-scanning (Fig. 5a). For this study, female Dlk1+/+ and Dlk1−/− mice were fed short-term (6 weeks versus 25 and 28 weeks used above, respectively) with NC or HFD, starting at 3 weeks, which has been shown applicable to induce glucose intolerance by others [44]. The standardized 18F-FDG uptake values (SUVs) for each animal (skeletal hind limb muscle vs. brain) were similar to those described by others [45], and all animals responded well to insulin treatment with an enhanced FDG uptake (Fig. 5b-c). Most interestingly, Dlk1−/− mice responded to the short duration of high calorie intake by increasing their skeletal muscle glucose uptake significantly (Fig. 5b), supporting a higher ability for body glucose clearance as observed above (Fig. 3a). In contrast, Dlk1+/+ animals were unaffected in their glucose uptake ability by this short-term HFD (Fig. 5b). At the molecular level, we found that expression of the major glucose transporter Glut4 was increased in Dlk1−/− animals as compared with Dlk1+/+ controls (Fig. 5d), which was verified at the protein level (Fig. 5e-f) using GLUT4 immunohistochemical quantification as exploited by others [[46], [47], [48], [49], [50]].
Fig. 5.
Lack of Dlk1 improves skeletal muscle glucose uptake. (a-c) Three-week-old Dlk1+/+ and Dlk1−/− female mice were fed NC or HFD short-term for 6 weeks and subjected twice (± insulin) to PET/CT scanning for determination of skeletal muscle glucose (18F-FDG) uptake. (a) Fields used for quantification are marked by arrows (Yellow: Skeletal muscle; Blue: Brain). (b-c) For each mouse, 18F-FDG standardized uptake value (SUV) for skeletal muscle was normalized to the brain SUV, and statistical difference was tested by a two-way ANOVA followed by Fisher's LSD test. (d-f) Six-week-old female Dlk1+/+ and Dlk1−/− mice were fed normal chow (NC) or high-fat diet (HFD) long-term (28 weeks), and (d) Glut4 (Slc2a4) mRNA levels were determined in hind limb (m. Gastrocnemius) skeletal muscles using qPCR and (e-f) verified by GLUT4 quantification in immunohistochemical stainings. For qPCR, raw data were normalized against Gapdh and beta-actin (qBASE- M:0.390; CV:0.137). For GLUT 4 IHC staining, mean intensity GLUT4 fluorescence was quantified in areas of both low and high GLUT4 to minimize subjectivity (see materials and methods for details), and an overall average was calculated for each animal in (F). Statistical difference was tested by a two-way ANOVA followed by Fisher's LSD test. *P < .05, **P < .01, ***P < .001, ****P < .0001, ns (not significant). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Dlk1 negatively regulates expression of Glut4-the major glucose transporter
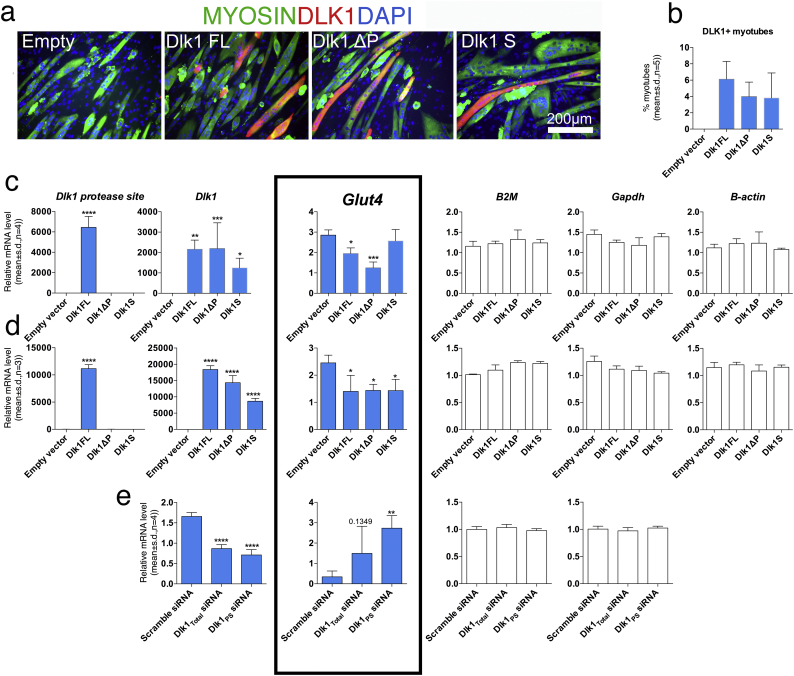
To confirm Dlk1s impact on Glut4 expression, we transfected skeletal myoblasts with different Dlk1 expression constructs and differentiated the cells into multinucleated myotubes (Fig. 6a). However, only a minor fraction of myotubes expressed DLK1 (Fig. 6b), and thus disabled further Glut4 evaluation. As an alternative, non-differentiated myoblasts showed a transfection efficiency of 40% (data not shown), and although they express Glut 4 at lower levels than myotubes, we attempted to evaluate Glut4 expression in these cells. Despite the limitations, indeed we did find Glut4 to be decreased in Dlk1-transfected cultures as compared to cultures transfected with empty vector (Fig. 6c), and this was further aggravated when DLK1 overexpression was increased up to 10-fold (Fig. 6d). Furthermore, we found that differentiated preadipocytes that are easily transfected and express high amounts of endogenous DLK1, substantially increased their Glut4 expression upon siRNA mediated knock-down of Dlk1 (Fig. 6e). Together, this points to Dlk1 as a regulator of GLUT4 mediated glucose uptake and may at least partially explain why HFD-fed Dlk1−/− animals are less obese and insulin resistant as compared with Dlk1+/+ mice.
Fig. 6.
In vitro Dlk1 overexpression and knock-down inhibits and enhances Glut4 expression, respectively. (a) Myoblasts were differentiated into multinucleated myofibers and transfected with empty vector and different Dlk1 constructs (full-length (FL), lacking the protease recognition site (ΔP) and the extracellular soluble domain (S) before DLK1/MYOSIN/DAPI staining and (b) quantification of percentage DLK1 positive myofibers. (c-d) Myoblasts were transfected with empty vector and Dlk1 constructs as above, but at two different concentrations before qPCR. (e) Dlk1 expression 3 T3-L1 preadipocytes that were transfected with siRNA-Scramble, or siRNAs against all Dlk1 isoforms (Dlk1Total) or Dlk1 isoforms containing the protease site (Dlk1PS) before differentiation and qPCR. For c-e, qPCR raw data were normalized against multiple stably expressed control genes as determined by the Qbase-plus platform. Statistical difference was tested by a one-way ANOVA followed by Fisher's LSD test. *P < .05, **P < .01, ***P < .001, ****P < .0001.
4. Discussion
Our data clearly demonstrate that Dlk1 is not an inhibitor of in vivo fat tissue mass in healthy to overweight humans or mice, although it may exert a slightly inhibiting role on adipogenesis during development leading to a modest increase in fat mass accumulation as previously considered by others and us hereto [3,10,11,13,15,19,21,36]. On the contrary, Dlk1 is associated with increased obesity. Moreover, we found that Dlk1 has a negative and direct impact on whole body glucose homeostasis upon obesity, by regulating GLUT4 mediated skeletal muscle glucose uptake. This supports the idea that imprinted genes play an important role in metabolism after birth [2], and may serve as key targets for new interventions.
Specifically, in this study we demonstrate that absence of Dlk1 is not permissive for fat accumulation in young or adult mice, and that Dlk1−/− mice after an HFD diet indeed are resistant to obesity, as compared to Dlk1+/+ animals. Thus, in times of excess dietary lipids, Dlk1 somehow mediates lipid storage. This could be due to a direct effect from Dlk1 on fat tissue or an indirect effect e.g. on insulin secretion in the pancreas.
Our data are in agreement with a recent developmental study conducted by the Sul's group, in which ablation of Dlk1 at E10.5 or P1-P21 reduced WAT development dramatically [10], thus contradicting their earlier papers where Dlk1-null mice have been reported to be more obese [13] and Dlk1-overexpressing mice to be less obese [14,15]. Also a recent study by Ferguson's group briefly noted that overexpression of Dlk1 in the adult (24-weeks) hyperphagic ob/ob mouse did not negatively alter body fat percentage [18]. Herein, we furthermore evaluate in humans, which reconciles that Dlk1 is positively associated with obesity, and the perception of Dlk1 being an adipogenic inhibitor should be changed. Technical issues may explain some of the reported diversity. For instance, weighing the individual fat pads as done previously [14,15,18] may be a questionable measurement of changes in body fat if not normalized to body weight; a parameter that is always found to be affected by Dlk1. Indeed, Schmidt and co-workers pointed out that the fat pad weight when expressed as percentage of body weight, was decreased in Dlk1-null female, but not male animals [51], which is in agreement with our data. In the present study we overcome the issue of weighing fat pads by using DXA and MRI scanning to measure body fat in animals and humans. Moreover, the previously published studies overexpressing DLK1 in transgenic mice were based on a fusion protein consisting of two DLK1 extracellular domains fused with the Fc-part of a human immunoglobulin. Such a structure is dissimilar from physiological DLK1 and may explain differences [14,15]. Likewise, the two existing Dlk1−/− mouse strains generated by the Sul [13] and the Laborda [29] (used herein) groups differ with respect to Dlk1 gene disruption strategy, which may potentially explain the differences in phenotype. Nevertheless, the animal data herein is strikingly supportive of the human data where Dlk1 associates positively with obesity and not vice versa. Likewise, in clinical studies, the discrepancies between Dlk1 being a positive mediator of obesity in our healthy individuals and other studies considering it as an obesity guard [[20], [21], [22]] may be explained by age, gender, and especially the health status of included subjects. In regard to the latter, we alone use healthy non-diabetic individuals whereas others either compare groups of diseased and healthy individuals, solely diseased individuals or use a mixture of healthy and diseased individuals for their correlations. Moreover, the choice of assay technology is also likely to influence the outcome and herein we have used a DLK1 ELISA that is well characterized and based on high affinity DLK1 antibodies [31] whereas most other studies have used commercially available kits. Yet, the underlying mechanism of DLK1 associating with increased amounts of adipose tissue is still unclear. Our animal study suggests that when dietary fats are in excess, Dlk1 mediates lipid storage as visualized by increased fat deposits and fat cell hypertrophy. From previous studies by us and others, we know that the number of DLK1+ cells in fat is discrete, and mainly refers to cells of the vascular lineage [52,53]. A lack of correlation between DLK1 mRNA and fat cell size in humans indicates that any direct effect of such DLK1+ residing cells would be regulated at the post-transcriptional level. However, herein it seems more likely that either the soluble circulating DLK1 affects lipid storage directly or alternatively, that DLK1 present in the pancreatic beta cells somehow mediates insulin secretion thereby indirectly stimulating fat accumulation in adipose tissue.
In humans, we observe by the gold-standard euglycaemic hyperinsulinaemic clamp a negative impact of DLK1 on insulin sensitivity, with glucose disposal rates being negatively correlated with circulating DLK1 levels. Our data thus firmly establish DLK1´s role in glucose metabolism in healthy subjects where it is intriguing to speculate that circulating DLK1 may predict a pre-diabetic phenotype as discussed below. Our results with the healthy non-diabetic cohorts supports previous studies correlating DLK1 positively to insulin resistance in non-healthy or mixed (healthy/non-healthy) cohorts [14,19,20,54,55]. Interestingly, using similar or alternative approaches other groups have shown the opposite [18,22,40,56] or no association [57]. Of these, Flehmig and co-workers [56] used a distance-based hierarchical cluster analysis approach to find patterns between serum adipokine levels and clinical parameters related to obesity, inflammation and glucose metabolism. In their moderate to severely obese cohort, DLK1 segregated into an insulin resistance/hyperglycemia cluster rather than an obesity cluster but DLK1 levels were found to be significantly lower in individuals with T2D than without. So, while several studies reach the same general conclusion that DLK1 has an impact on glucose metabolism, the underlying data are surprisingly very different. This can possibly be ascribed to differences in study populations (and species) and/or the use of different assays for DLK1 quantification as stated above. Independent of DLK1 assay technology however our data herein support previous studies on Dlk1 overexpressing mice where Villena et al. reported attenuation of insulin sensitivity by DLK1 during an euglycaemic hyperinsulinaemic clamp although they observed Dlk1 to negatively affect obesity [15]. Likewise, Abdallah et al. showed that insulin sensitivity is improved by the absence of Dlk1 55. In our human cohort, a mediation analysis revealed that the effect of Dlk1 on glucose disposal in healthy individuals is not mediated by age, BMI, or fat percentage. Although we did not observe any effect from Dlk1 on glucagon levels and fasting glucose levels in animals nor any correlations between DLK1 and hepatic glucose production in humans, we cannot exclude that in another context, such as prolonged fasting, Dlk1 could be implicated in gluconeogenesis as reported in mice [40]. Yet, our data more likely suggest that Dlk1 impacts glucose homeostasis by inhibiting in vivo glucose uptake in skeletal muscle through regulation of the glucose transport machinery. Our GLUT 4 immunohistochemical staining profile agrees with that observed by others in the field [[46], [47], [48], [49], [50]] and reflect our Glut4 mRNA data, which underscore that Dlk1 affects Glut4 regulation. Moreover, we confirmed this independently in two different cell lines, with two distinct gene manipulation strategies. This suggests that the in vivo Dlk1 phenotype on glucose homeostasis cannot solely be explained by Dlk1 impacting muscle growth during development. This is further supported by our relative skeletal muscle/brain 18F-FDG PET/CT glucose uptake experiments, which are independent on muscle size. Yet, since DLK1 expression is very low in normal adult skeletal muscle, it will be interesting to see whether the observed Glut4 regulation is mediated by soluble DLK1 [14] working as a hormone and part of a novel feedback axis within glucose homeostasis. Alternatively, Dlk1's effect could also be mediated by insulin and its regulation of GLUT4 translocation. Our short-term in vivo anti-DLK1 strategy did not result in any acute changes in glucose levels, which may suggest that the phenotype is due to more chronic changes or that the organ responsible for the DLK1 fraction mediating insulin resistance is not reached by the anti-DLK1 strategy. We have previously noted that antibody-based in vivo neutralization of DLK1 triggers an unknown compensatory feedback loop [58] and our unpublished data show that long-term anti-DLK1 targeting through the circulation indeed increases circulating DLK1 when treatment stops. Currently, we can only speculate on the underlying mechanism, yet in a treatment perspective that seeks minimal side effects, the major source of circulating DLK1 in this feedback loop needs to be pinpointed and may help further clarify the potential of targeting DLK1 in vivo in relation to preventing/treating T2D. Moreover, recent work by others has demonstrated that the NOTCH pathway is implicated in metabolism and it is likely that the observed effect of Dlk1 on the glucose machinery is mediated by a NOTCH-dependent mechanism.
In perspective, it will also be of interest to see, in a longitudinal human study, whether increased Dlk1 levels indeed predispose for developing the metabolic syndrome later in life. In this regard, it is noteworthy that increased levels of circulating DLK1 in our study were associated with decreased glucose disposal rates in subjects with no diagnosed insulin resistance. The pro-inflammatory chemokine MCP-1 (CCL2) has been linked to obesity and insulin resistance for many years and the general notion hitherto is that elevated MCP-1 levels precede the inflammatory processes that eventually lead to insulin resistance [59]. Whereas others report 195 and 224 pg/mL MCP-1 levels for individuals with impaired glucose tolerance or Type 2 diabetes [60], respectively, our non-diabetic cohorts exhibited significantly lower levels (79 and 148 pg/mL) further confirming the non-diabetic status of our used cohorts. Moreover, we noted that MCP-1 levels in our healthy subjects did not correlate with GDR, nor DLK1 and interestingly, recent data suggest that MCP-1 (and inflammation) is triggered by insulin resistance and not vice versa [61]. Our findings are in support of the latter, thus regardless of the order of events DLK1 definitely relates to insulin sensitivity at an earlier timepoint than MCP-1. Certainly, more studies are required to establish if Dlk1 may be used together with other parameters as a true early marker/predictor of insulin resistance. Moreover, demonstrating that Dlk1 is positively associated with obesity and insulin resistance might suggest it to be useful in the development of drugs for preventing or treating metabolic syndrome and/or type 2 diabetes.
Disclosure statement
The Authors declare competing interests. The University of Southern Denmark/Region of South Denmark has filed patent applications related to this work with CHJ and DCA listed as inventors.
Acknowledgments
Acknowledgements
We would like to thank Charlotte Nielsen, Tonja L. Jørgensen, and Anette Kliem (LMCC, Odense University Hospital) for excellent technical assistance on this study. Moreover, we thank Senior Scientist Jakob L. Hansen (Novo Nordisk, DK) for help with mouse insulin measurements. Contributions: CHJ: Conception and design, Collection of data, Data analysis and interpretation, Manuscript writing and final approval. RK: Collection of data, Data analysis and interpretation, Manuscript writing. CB, SH, MA, LLC, AG, PM, PA, MR, CDJ, SH, CB, JL, JJH: Collection of data, Data analysis, Manuscript editing, and Final approval of manuscript. DCA: Conception and design, Collection of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript, and Financial support. Furthermore, DCA is the guarantor of the study. Funding: The work was supported by research grants from The Danish Diabetes Academy supported by the Novo Nordisk Foundation, The Danish National Research Council (#09-073648), The Lundbeck Foundation, University of Southern Denmark, and Dep. Of Clinical Biochemistry and Pharmacology/Odense University Hospital, the Swedish Research Council, the Swedish Diabetes Foundation, the Strategic Research Program in Diabetes at Karolinska Institute and an EFSD/Lilly grant.
Data and resource availability
All data generated or analyzed during this study are included in the published article (and its online supplementary files).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.07.070.
Appendix A. Supplementary data
Supplementary material
References
- 1.Maksimov M.L., Svistunov A.A., Tarasov V.V. Approaches for the development of drugs for treatment of obesity and metabolic syndrome. Curr. Pharm. Des. 2016;22(7):895–903. doi: 10.2174/1381612822666151209153047. [DOI] [PubMed] [Google Scholar]
- 2.Peters J. The role of genomic imprinting in biology and disease: an expanding view. Nat. Rev. Genet. 2014;15(8):517–530. doi: 10.1038/nrg3766. [DOI] [PubMed] [Google Scholar]
- 3.Smas C.M., Sul H.S. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73(4):725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- 4.Baladron V., Ruiz-Hidalgo M.J., Nueda M.L. Dlk acts as a negative regulator of Notch1 activation through interactions with specific EGF-like repeats. Exp. Cell Res. 2005;303(2):343–359. doi: 10.1016/j.yexcr.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Traustadottir G.A., Jensen C.H., Thomassen M. Evidence of non-canonical NOTCH signaling: Delta-like 1 homolog (DLK1) directly interacts with the NOTCH1 receptor in mammals. Cell. Signal. 2016;28(4):246–254. doi: 10.1016/j.cellsig.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Begum A., Lin Q., Yu C., Kim Y., Yun Z. Interaction of delta-like 1 homolog (drosophila) with prohibitins and its impact on tumor cell clonogenicity. Mol. Cancer Res. 2014;12(1):155–164. doi: 10.1158/1541-7786.MCR-13-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray S.J., Takada S., Harrison E., Shen S.C., Ferguson-Smith A.C. The atypical mammalian ligand Delta-like homologue 1 (Dlk1) can regulate notch signalling in drosophila. BMC Dev. Biol. 2008;8:11. doi: 10.1186/1471-213X-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferron S.R., Charalambous M., Radford E. Postnatal loss of Dlk1 imprinting in stem cells and niche astrocytes regulates neurogenesis. Nature. 2011;475(7356):381–385. doi: 10.1038/nature10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Zhao L., Smas C., Sul H.S. Pref-1 interacts with fibronectin to inhibit adipocyte differentiation. Mol. Cell. Biol. 2010;30(14):3480–3492. doi: 10.1128/MCB.00057-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudak C.S., Gulyaeva O., Wang Y. Pref-1 marks very early mesenchymal precursors required for adipose tissue development and expansion. Cell Rep. 2014;8(3):678–687. doi: 10.1016/j.celrep.2014.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sul H.S. Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Mol. Endocrinol. 2009;23(11):1717–1725. doi: 10.1210/me.2009-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garces C., Ruiz-Hidalgo M.J., Bonvini E., Goldstein J., Laborda J. Adipocyte differentiation is modulated by secreted delta-like (dlk) variants and requires the expression of membrane-associated dlk. Diff. Res. Bio. Div. 1999;64(2):103–114. doi: 10.1046/j.1432-0436.1999.6420103.x. [DOI] [PubMed] [Google Scholar]
- 13.Moon Y.S., Smas C.M., Lee K. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol. Cell. Biol. 2002;22(15):5585–5592. doi: 10.1128/MCB.22.15.5585-5592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee K., Villena J.A., Moon Y.S. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1) J. Clin. Invest. 2003;111(4):453–461. doi: 10.1172/JCI15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villena J.A., Choi C.S., Wang Y. Resistance to high-fat diet-induced obesity but exacerbated insulin resistance in mice overexpressing preadipocyte factor-1 (Pref-1): a new model of partial lipodystrophy. Diabetes. 2008;57(12):3258–3266. doi: 10.2337/db07-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdallah B.M., Ding M., Jensen C.H. Dlk1/FA1 is a novel endocrine regulator of bone and fat mass and its serum level is modulated by growth hormone. Endocrinology. 2007;148(7):3111–3121. doi: 10.1210/en.2007-0171. [DOI] [PubMed] [Google Scholar]
- 17.Nueda M.L., Baladron V., Sanchez-Solana B., Ballesteros M.A., Laborda J. The EGF-like protein dlk1 inhibits notch signaling and potentiates adipogenesis of mesenchymal cells. J. Mol. Biol. 2007;367(5):1281–1293. doi: 10.1016/j.jmb.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 18.Charalambous M., Da Rocha S.T., Radford E.J. DLK1/PREF1 regulates nutrient metabolism and protects from steatosis. Proc. Natl. Acad. Sci. U. S. A. 2014;111(45):16088–16093. doi: 10.1073/pnas.1406119111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chacon M.R., Miranda M., Jensen C.H. Human serum levels of fetal antigen 1 (FA1/Dlk1) increase with obesity, are negatively associated with insulin sensitivity and modulate inflammation in vitro. Int. J. Obes. 2008;32(7):1122–1129. doi: 10.1038/ijo.2008.40. [DOI] [PubMed] [Google Scholar]
- 20.Kavalkova P., Touskova V., Roubicek T. Serum preadipocyte factor-1 concentrations in females with obesity and type 2 diabetes mellitus: the influence of very low calorie diet, acute hyperinsulinemia, and fenofibrate treatment. Horm. Metab. Res. 2013;45(11):820–826. doi: 10.1055/s-0033-1353210. [DOI] [PubMed] [Google Scholar]
- 21.Liangpunsakul S., Bennett R., Westerhold C. Increasing serum pre-adipocyte factor-1 (Pref-1) correlates with decreased body fat, increased free fatty acids, and level of recent alcohol consumption in excessive alcohol drinkers. Alcohol. 2014;48(8):795–800. doi: 10.1016/j.alcohol.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S.H., Rhee M., Yang H.K. Serum preadipocyte factor 1 concentrations and risk of developing diabetes: a nested case-control study. Diabet. Med. 2016;33(5):631–638. doi: 10.1111/dme.12871. [DOI] [PubMed] [Google Scholar]
- 23.Arner E., Mejhert N., Kulyte A. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes. 2012;61(8):1986–1993. doi: 10.2337/db11-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basta G., Del Turco S., Navarra T. Inverse association between circulating levels of soluble receptor for advanced glycation end-products and coronary plaque burden. J. Atheroscler. Thromb. 2012;19(10):941–948. doi: 10.5551/jat.10561. [DOI] [PubMed] [Google Scholar]
- 25.Frederiksen L., Hojlund K., Hougaard D.M., Brixen K., Andersen M. Testosterone therapy increased muscle mass and lipid oxidation in aging men. Age. 2012;34(1):145–156. doi: 10.1007/s11357-011-9213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sironi A.M., Petz R., De Marchi D. Impact of increased visceral and cardiac fat on cardiometabolic risk and disease. Diabet. Med. 2012;29(5):622–627. doi: 10.1111/j.1464-5491.2011.03503.x. [DOI] [PubMed] [Google Scholar]
- 27.Frederiksen L., Hojlund K., Hougaard D.M. Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. Eur. J. Endocrinol. 2012;166(3):469–476. doi: 10.1530/EJE-11-0565. European Federation of Endocrine Societies. [DOI] [PubMed] [Google Scholar]
- 28.Andersen D.C., Laborda J., Baladron V., Kassem M., Sheikh S.P., Jensen C.H. Dual role of delta-like 1 homolog (DLK1) in skeletal muscle development and adult muscle regeneration. Development. 2013;140(18):3743–3753. doi: 10.1242/dev.095810. [DOI] [PubMed] [Google Scholar]
- 29.Raghunandan R., Ruiz-Hidalgo M., Jia Y. Dlk1 influences differentiation and function of B lymphocytes. Stem Cells Dev. 2008;17(3):495–507. doi: 10.1089/scd.2007.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachmann E., Krogh T.N., Hojrup P., Skjodt K., Teisner B. Mouse fetal antigen 1 (mFA1), the circulating gene product of mdlk, pref-1 and SCP-1: isolation, characterization and biology. J. Reprod. Fertil. 1996;107(2):279–285. doi: 10.1530/jrf.0.1070279. [DOI] [PubMed] [Google Scholar]
- 31.Jensen C.H., Krogh T.N., Stoving R.K., Holmskov U., Teisner B. Fetal antigen 1 (FA1), a circulating member of the epidermal growth factor (EGF) superfamily: ELISA development, physiology and metabolism in relation to renal function. Clinica Chimica Acta; Int. J. Clin. Chem. 1997;268(1–2):1–20. doi: 10.1016/s0009-8981(97)00152-6. [DOI] [PubMed] [Google Scholar]
- 32.Glintborg D., Christensen L.L., Kvorning T. Strength training and testosterone treatment have opposing effects on migration inhibitor factor levels in ageing men. Mediat. Inflamm. 2013;2013:539156. doi: 10.1155/2013/539156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrikopoulos S., Blair A.R., Deluca N., Fam B.C., Proietto J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008;295(6):E1323–E1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 34.Wallace T.M., Levy J.C., Matthews D.R. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 35.Gastaldelli A., Harrison S.A., Belfort-Aguilar R. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology. 2009;50(4):1087–1093. doi: 10.1002/hep.23116. [DOI] [PubMed] [Google Scholar]
- 36.Mortensen S.B., Jensen C.H., Schneider M. Membrane-tethered delta-like 1 homolog (DLK1) restricts adipose tissue size by inhibiting preadipocyte proliferation. Diabetes. 2012;61(11):2814–2822. doi: 10.2337/db12-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez-Solana B., Nueda M.L., Ruvira M.D. The EGF-like proteins DLK1 and DLK2 function as inhibitory non-canonical ligands of NOTCH1 receptor that modulate each other's activities. Biochim. Biophys. Acta. 2011;1813(6):1153–1164. doi: 10.1016/j.bbamcr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Lim C., Ahn M.I., Jung J.I., Beck K.S. Simple quantification of paracardial and epicardial fat dimensions at low-dose chest CT: correlation with metabolic risk factors and usefulness in predicting metabolic syndrome. Jpn. J. Radiol. 2018;36(9):528–536. doi: 10.1007/s11604-018-0752-1. [DOI] [PubMed] [Google Scholar]
- 39.Charalambous M., Ferron S.R., da Rocha S.T. Imprinted gene dosage is critical for the transition to independent life. Cell Metab. 2012;15(2):209–221. doi: 10.1016/j.cmet.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee Y.H., Yun M.R., Kim H.M. Exogenous administration of DLK1 ameliorates hepatic steatosis and regulates gluconeogenesis via activation of AMPK. Int. J. Obes. 2005:2015. doi: 10.1038/ijo.2015.173. [DOI] [PubMed] [Google Scholar]
- 41.Spalding K.L., Arner E., Westermark P.O. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 42.Le N.H., Kim C.S., Park T. Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediat. Inflamm. 2014;2014 doi: 10.1155/2014/834294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S.R., Khamoui A.V., Jo E. Effects of chronic high-fat feeding on skeletal muscle mass and function in middle-aged mice. Aging Clin. Exp. Res. 2015;27(4):403–411. doi: 10.1007/s40520-015-0316-5. [DOI] [PubMed] [Google Scholar]
- 44.Winzell M.S., Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl. 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 45.Kreissl M.C., Stout D.B., Wong K.P. Influence of dietary state and insulin on myocardial, skeletal muscle and brain [F]-fluorodeoxyglucose kinetics in mice. EJNMMI Res. 2011;1:8. doi: 10.1186/2191-219X-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bradley H., Shaw C.S., Bendtsen C. Visualization and quantitation of GLUT4 translocation in human skeletal muscle following glucose ingestion and exercise. Phys. Rep. 2015;3(5) doi: 10.14814/phy2.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradley H., Shaw C.S., Worthington P.L., Shepherd S.O., Cocks M., Wagenmakers A.J. Quantitative immunofluorescence microscopy of subcellular GLUT4 distribution in human skeletal muscle: effects of endurance and sprint interval training. Phys. Rep. 2014;2(7) doi: 10.14814/phy2.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lauritzen H.P., Galbo H., Brandauer J., Goodyear L.J., Ploug T. Large GLUT4 vesicles are stationary while locally and reversibly depleted during transient insulin stimulation of skeletal muscle of living mice: imaging analysis of GLUT4-enhanced green fluorescent protein vesicle dynamics. Diabetes. 2008;57(2):315–324. doi: 10.2337/db06-1578. [DOI] [PubMed] [Google Scholar]
- 49.Ploug T., van Deurs B., Ai H., Cushman S.W., Ralston E. Analysis of GLUT4 distribution in whole skeletal muscle fibers: identification of distinct storage compartments that are recruited by insulin and muscle contractions. J. Cell Biol. 1998;142(6):1429–1446. doi: 10.1083/jcb.142.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodnick K.J., Slot J.W., Studelska D.R. Immunocytochemical and biochemical studies of GLUT4 in rat skeletal muscle. J. Biol. Chem. 1992;267(9):6278–6285. [PubMed] [Google Scholar]
- 51.Appelbe O.K., Yevtodiyenko A., Muniz-Talavera H., Schmidt J.V. Conditional deletions refine the embryonic requirement for Dlk1. Mech. Dev. 2013;130(2–3):143–159. doi: 10.1016/j.mod.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andersen D.C., Jensen L., Schroder H.D., Jensen C.H. "the preadipocyte factor" DLK1 marks adult mouse adipose tissue residing vascular cells that lack in vitro adipogenic differentiation potential. FEBS Lett. 2009;583(17):2947–2953. doi: 10.1016/j.febslet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Zwierzina M.E., Ejaz A., Bitsche M. Characterization of DLK1(PREF1)(+)/CD34(+) cells in vascular stroma of human white adipose tissue. Stem Cell Res. 2015;15(2):403–418. doi: 10.1016/j.scr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Hermida C., Garces C., de Oya M. The serum levels of the EGF-like homeotic protein dlk1 correlate with different metabolic parameters in two hormonally different children populations in Spain. Clin. Endocrinol. 2008;69(2):216–224. doi: 10.1111/j.1365-2265.2008.03170.x. [DOI] [PubMed] [Google Scholar]
- 55.Abdallah B.M., Ditzel N., Laborda J., Karsenty G., Kassem M. DLK1 regulates whole body glucose metabolism: a negative feedback regulation of the osteocalcin-insulin loop. Diabetes. 2015;64(9):3069–3080. doi: 10.2337/db14-1642. [DOI] [PubMed] [Google Scholar]
- 56.Flehmig G., Scholz M., Kloting N. Identification of adipokine clusters related to parameters of fat mass, insulin sensitivity and inflammation. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0099785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wurst U., Ebert T., Kralisch S., Stumvoll M., Fasshauer M. Serum levels of the adipokine Pref-1 in gestational diabetes mellitus. Cytokine. 2015;71(2):161–164. doi: 10.1016/j.cyto.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 58.Figeac F., Andersen D.C., Nielsen C.A.N. Antibody-based inhibition of circulating DLK1 protects from estrogen deficiency-induced bone loss in mice. Bone. 2018;110:312–320. doi: 10.1016/j.bone.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 59.de Luca C., Olefsky J.M. Inflammation and insulin resistance. FEBS Lett. 2008;582(1):97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piemonti L., Calori G., Lattuada G. Association between plasma monocyte chemoattractant protein-1 concentration and cardiovascular disease mortality in middle-aged diabetic and nondiabetic individuals. Diabetes Care. 2009;32(11):2105–2110. doi: 10.2337/dc09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimobayashi M., Albert V., Woelnerhanssen B. Insulin resistance causes inflammation in adipose tissue. J. Clin. Invest. 2018;128(4):1538–1550. doi: 10.1172/JCI96139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material