Mycoplasma genitalium is a common sexually transmitted infection with a propensity to acquire resistance to commonly used antimicrobial therapies. Bacterial load has been linked to patient symptoms and the success of treatment. In this study, we demonstrate methodology to estimate load from routine diagnostic assays using the ResistancePlus MG test (SpeeDx Pty Ltd., Australia).
KEYWORDS: Mycoplasma genitalium, diagnostic test, infection load, sample type, urine
ABSTRACT
Mycoplasma genitalium is a common sexually transmitted infection with a propensity to acquire resistance to commonly used antimicrobial therapies. Bacterial load has been linked to patient symptoms and the success of treatment. In this study, we demonstrate methodology to estimate load from routine diagnostic assays using the ResistancePlus MG test (SpeeDx Pty Ltd., Australia). The method gave comparable quantitation to an M. genitalium-specific 16S rRNA quantitative PCR (qPCR; Spearman r = 0.94) for the samples analyzed (n = 499, including urine and swab types as detailed below) and was, therefore, employed to analyze typical load levels for samples in a diagnostic laboratory (total of 1,012 tests). When stratified by sample type, female urine (median, 826 genomes/ml) had the lowest load. This was significantly lower than median loads for all other sample types (male urine [6.91 × 103 genomes/ml], anal swabs [5.50 × 103], cervical swabs [8.15 × 103], endocervical swabs [3.97 × 103], and vaginal swabs [6.95 × 103]) (P < 0.0001). There were no significant differences in load estimates between the other sample types. Reproducibility of load estimates conducted on the same samples was high (r > 0.85). In conclusion, this methodology to provide load estimates for M. genitalium can be easily integrated into routine diagnostic laboratory workflow. Given the association between organism load, symptoms, and treatment success, load assessment has future diagnostic potential.
INTRODUCTION
Mycoplasma genitalium is a sexually transmitted bacterium associated with urethritis in men and adverse reproductive outcomes in women (1). Over the last decade, there has been a dramatic increase in the level of antibiotic resistance to first-line treatment azithromycin, with recent macrolide resistance mutation (MRM) levels as high as 68% in some populations (2). Resistance to fluoroquinolones, the second-line treatment, is also increasing (3).
The load of M. genitalium may have important clinical implications. High infection load has been associated with macrolide treatment failure in several studies (4–6) and has also been correlated with symptoms. In a recent study, men-who-have-sex-with-men with proctitis had significantly higher loads of M. genitalium than those that were asymptomatically colonized (7). In a separate study, urine and urethral swab specimens from symptomatic men had higher M. genitalium loads than infected asymptomatic men (8). The quantitation of load, therefore, has the potential to aid the clinical management of the patient, especially with respect to antibiotic treatment; however, there are no commercial assays at present that provide this information.
The ResistancePlus MG assay (SpeeDx Pty Ltd., Australia) offers a means for detecting M. genitalium and MRM simultaneously (9). In this study, we present a method to integrate M. genitalium load estimates into the routine diagnostic workflow using the ResistancePlus MG assay. The method was validated against an existing noncommercial quantitation method and then applied to over 1,000 diagnostic samples to explore the distribution of bacterial load in various sample types. Finally, the reproducibility of the method was assessed by reviewing results among samples that underwent repeat testing.
MATERIALS AND METHODS
Establishment of a standard curve.
The ResistancePlus MG test targets the mgpB gene of M. genitalium encoding the MGPA protein. A quantitated 500-bp synthetic double-stranded DNA “gBlock” of mgpB (GenBank accession number L43967; positions 221639 to 222138; Integrated DNA Technologies) was 10-fold serially diluted (from 105 molecules/μl extinction). This was analyzed in triplicate using the ResistancePlus MG assay, which was set up according to the manufacturer’s instructions. Run data were used to generate a reference standard curve, as an “External Standard Curve” as specified in the Lightcycler 480 II instrument operator manual (Roche Diagnostics, Mannheim, Germany).
Samples analyzed in this study.
Samples collected at Melbourne Sexual Health Centre, The Royal Women’s Hospital, and external referrals were tested as they were received by the diagnostic laboratory. Urine samples were processed by centrifugation (1 ml, 16,000 relative centrifugal force [rcf], 15 min, room temperature), the supernatant discarded, and the pellet resuspended in 200 μl of phosphate-buffered saline (PBS). Swabs were processed by swirling in PBS (500 μl). Samples were extracted (200 μl) by a MagNA Pure 96 instrument (Roche) with the DNA and Viral NA Small Volume Kit on the universal pathogen protocol with an elution volume of 100 μl. The ResistancePlus MG assay was performed as part of routine diagnostic testing according to the manufacturer’s instructions using 5 μl of sample extract (published limit of detection for this assay, 10 copies of mgpB) (10) with the manufacturer’s internal control. Each assay included a midrange-concentration quantitated standard from a dilution series in addition to the recommended controls (positive control and internal control).
A summary of sample and data collection is presented in Fig. 1. As depicted in Fig. 1A, data, including sex, age, sample type, and sample load (determined by retrospective analysis, as described in the following section) were extracted for samples (n = 531; 319 urine samples, 63 anal/rectal swabs, 89 cervical/endocervical swabs, 54 vaginal swabs, and 6 urethral swabs) testing positive for M. genitalium between 16 June 2016 to 30 January 2017. Estimates of load were previously determined for a subset of 499 samples using a validated M. genitalium-specific 16S rRNA quantitative PCR method (not used for routine clinical care; published limit of detection, 6 copies of 16S rRNA gene) (2, 11). These data were used for a comparison of quantitation methods and also for analysis of load by sample type. As depicted in Fig. 1B, data were also extracted for M. genitalium-positive samples tested between 11 August 2017 and 19 May 2018 (n = 481; 293 urine samples, 59 anal swab, 65 cervical/endocervical swabs, 63 vaginal swabs, and 1 urethral swab). These data were used for an analysis of load stratified by sample type. Records were excluded if any data were missing; results corresponded to a repeat test from the same sample or if the amplification curve was flat upon visual inspection of the raw results.
FIG 1.
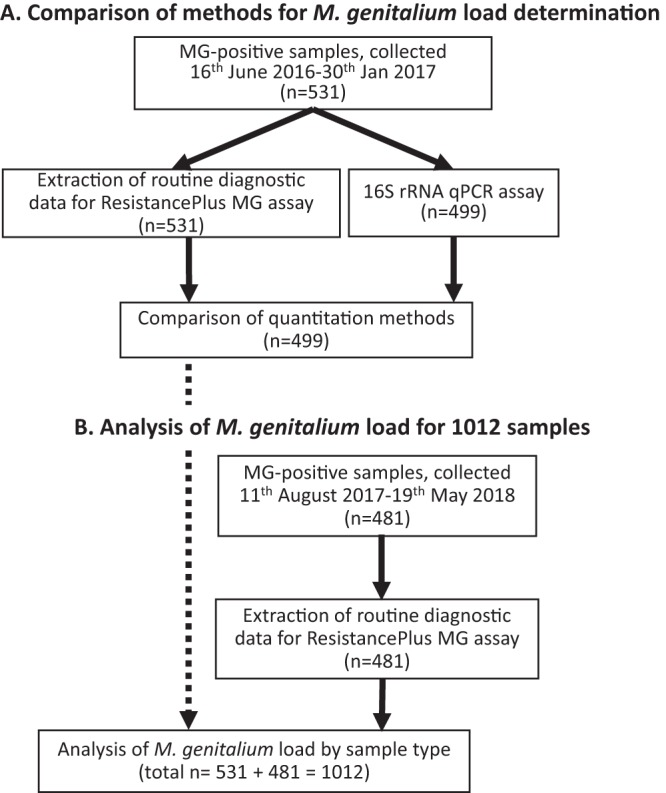
Overview of sample collection, data extraction, and analysis. (A) For 531 diagnostic tests, data extraction was performed from the routine diagnostic ResistancePlus MG assay. A 16S rRNA qPCR was performed on a subset of 499 samples for a comparison of load estimation methods. (B) Data were extracted for routine diagnostic ResistancePlus MG assay for an additional 481 samples and combined with the results for the 531 samples (from above), making a total of 1,012 samples.
Retrospective analysis of sample load by ResistancePlus MG assay.
A retrospective analysis was performed on stored ResistancePlus MG diagnostic data for all 1,012 samples. For setup on the Lightcycler 480 II instrument, when specifying names under “Sample Editor,” the “Abs Quant” workflow was selected. Next to the name for the standard, in “Channel A (465 to 510 nm),” the option “Standard” was selected under “Quantitation sample type” and concentration entered. After assay completion, prior to calculating quantification cycle (Cq) and concentration values, the reference standard curve was selected under “Use Efficiency” and then “STD curve (External).” Data analysis was performed using “Abs Quant/2nd Derivative Max for All Samples.” Results were only included for assay runs where the quantitated standard had not drifted outside ±1 cycles.
Data analysis.
For a pairwise comparison of quantitation methods, calculated load was log10 transformed and then analyzed by linear regression and Spearman correlation (GraphPad Prism v7.04). For other analyses, the load per 5-μl sample was converted to number of M. genitalium genomes per swab or 1-ml urine and then log10 transformed. Differences in loads between groups were analyzed with the Kruskal-Wallis one-way analysis of variance (ANOVA) with Dunn’s multiple-comparison test (GraphPad Prism v7.04). Data for the collected samples (531 samples; median log10 load, 3.71) and data extraction (481 samples; median log10 load, 3.66) were not significantly different (P = 0.185) and so were combined for the analysis of infection load stratified by sample type.
It is standard practice for the diagnostic laboratory to repeat the analysis of samples (in duplicate) where the result is unclear (e.g., if amplification curves show low efficiency). The data set was analyzed to determine the reproducibility of load analysis.
Ethics approval to perform an audit of M. genitalium load was obtained from the Royal Women’s Hospital research and ethics committees.
RESULTS AND DISCUSSION
Comparison of load determination by M. genitalium-specific 16S rRNA quantitative PCR and the ResistancePlus MG method.
Diagnostic samples (n = 499) were analyzed for sample load by both an M. genitalium-specific 16S rRNA quantitative PCR and the ResistancePlus MG method. The log10-transformed loads from both methods were compared by scatter plot with linear regression. There was a strong correlation (Spearman r = 0.94, regression gradient, 0.815) (Fig. 2), indicating the ResistancePlus MG method has a good performance compared with an established quantitation method. The median load per 5 μl of extract for 16S rRNA qPCR was higher than for the ResistancePlus MG method (2.775 [range, −0.260 to 6.75] versus 2.276 [range, −0.148 to 5.784]; P < 0.001); this finding, combined with the gradient of the regression line, indicates that the 16S rRNA qPCR method provides a marginally higher estimate of sample load.
FIG 2.
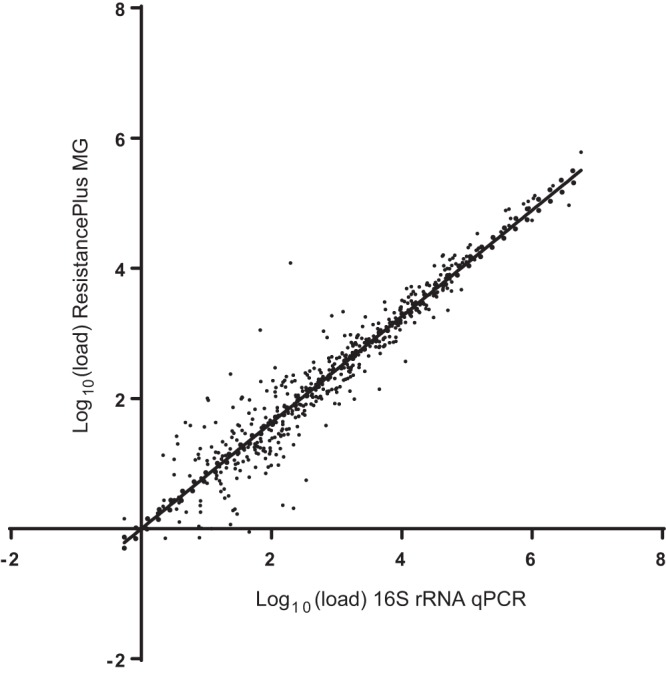
Comparison of load estimation by 16S rRNA qPCR and ResistancePlus MG assay. Sample loads (genome equivalents per 5-μl sample extract) were graphed in a scatter plot with linear regression.
Analysis of sample load in typical diagnostic specimens.
In retrospective analysis of 1,012 diagnostic samples, load estimates of genomes per milliliter of urine ranged from 1.12 (log10 load, 0.049) to 1.22 × 107 (log10 load, 7.09), with a median of 3.74 × 103 (log10 load, 3.57). For swabs, the load of genomes per swab ranged from 27.9 (log10 load, 1.45) to 9.3 × 106 (log10 load, 6.97), with a median of 6.8 × 103 (log10 load, 3.83). This large variation of load is similar to reports examining male first-void urine and urethral specimens (8) and urine specimens in men and women undergoing treatment (range, 49.6 to 7.28 × 105 bacteria per milliliter) (12).
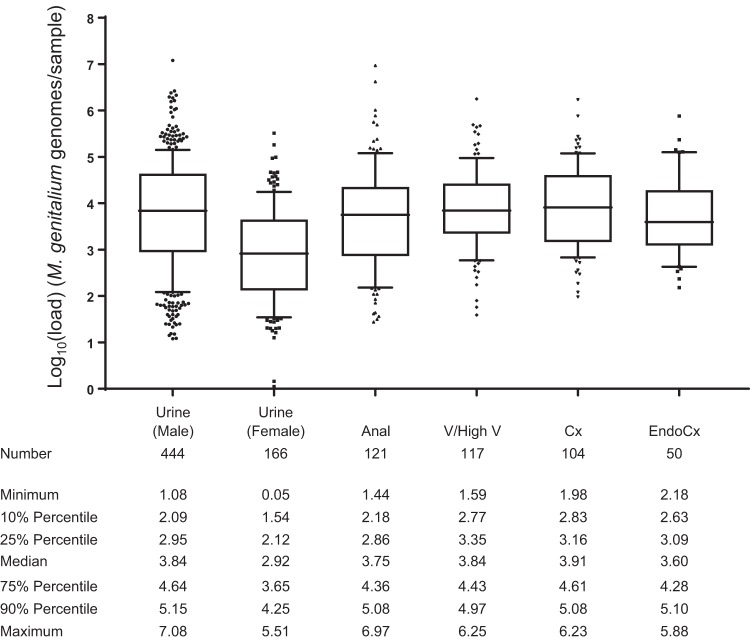
Load estimates were stratified by sample type for comparison (Fig. 3). There was no significant difference in load estimates between most sample types, possibly a result of the wide distribution of values. Notably, the infection load for urine was significantly higher for males (median log10 load, 3.84, or 6.91 × 103 genomes/ml) than females (median log10 load, 2.92, or 826 genomes/ml; P < 0.0001). This could reflect differences in the collection of urine, tissue tropisms, or site of infection (e.g., urethra versus other sites in women). This result demonstrates that it is acceptable to aggregate or directly compare certain sample types (e.g., different types of swabs and male urine) when interpreting sample load, with the exception of female urine.
FIG 3.
Diagnostic specimen sample loads stratified by sample type. Sample loads estimated from the ResistancePlus MG assay (expressed as genome equivalents per swab or per 1 ml for urine) were log10 transformed and plotted. Analysis of the range and distribution of loads is presented in tabular form. Whiskers represent the 10th/90th percentiles, and outliers are indicated. Samples types not represented in the figure include urethral swabs (n = 7), rectal swab (n = 1), and urine samples where sex was indicated as “other” (n = 2). Only the median for female urine samples was significantly different to other sample types (P < 0.0001).
Importantly, female urine load also differed significantly from each of the other female sample types (P < 0.0001). A previous study utilizing a smaller number of primary samples from women also identified that vaginal swab specimens had higher median load levels than urine samples (13). These observations may help explain why some studies have reported that urine has inferior diagnostic potential compared with cervical or vaginal swabs (14, 15).
Load estimates are reproducible upon repeated analysis.
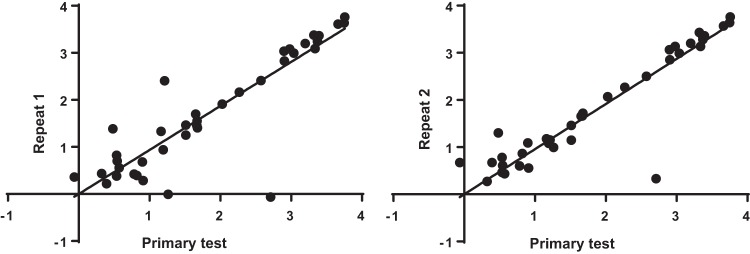
There were 39 samples that were repeated twice on a separate diagnostic assay run (making a total of 3 tests for each individual sample). There was strong correlation between the first test and second test (r = 0.858; linear regression gradient, 0.935) and first test and third test (r = 0.892; linear regression gradient, 0.957) (Fig. 4). This finding demonstrates robust reproducibility of sample load estimates. Notably, the linear regression had a gradient marginally less than 1, suggesting a low level of sample degradation between the first and repeat tests (of note, samples and extracts were stored in at 4°C between tests, typically for 24 h).
FIG 4.
Analysis of the reproducibility of load estimates. Sample load estimates for the primary test (log transformed, n = 39 samples) were separately compared with two repeat analysis tests by scatter plot with linear regression.
Study limitations.
This study has a number of limitations. Factors extrinsic to the quantity of bacteria at an infection site can influence the load reported by an assay, and these factors were not accounted for in this study, e.g., type of swab, the swabbing technique, efficiency of swab resuspension, and volume collected (dilution factor). Additionally, transport and storage conditions (duration and temperature) may affect the amount of detectable analyte. Few urethral swabs were available for analysis, as this is no longer a common sample type in Australia. Clinical symptoms and treatment outcomes for this data set are unknown, so infection load could not be correlated with symptoms. Additionally, a sample taken from a patient may not be representative of the ongoing M. genitalium infection levels. Also, for some samples with very high or low sample load, the quantification was extrapolated beyond the range of standards in the standard curve. Finally, the reproducibility of repeat analysis may have been over estimated, as data were extracted for positive detection of M. genitalium, and if a repeat test was negative it, would not have been extracted for the analysis.
Conclusions.
This study presents a method to determine M. genitalium load that is a convenient addition to the routine commercial diagnostic assays. This is highly relevant to research studies that wish to correlate load with symptom status, clinical syndrome, and treatment outcomes. Load may be useful in clinical care in the future given that high-load infections have been shown to be more likely to fail azithromycin and develop macrolide resistance. These data also suggest that urine in females is less optimal than vaginal and cervical swabs for M. genitalium testing.
ACKNOWLEDGMENTS
Jenny Su is acknowledged for performing some of the laboratory analysis.
This work was supported by an Innovations Connections grant from the Department of Industry, Innovation and Science, The Australian Federal Government (number ICG000220), and the Victorian Medical Research Acceleration Fund, the State Government of Victoria, Australia.
REFERENCES
- 1.Lis R, Rowhani-Rahbar A, Manhart LE. 2015. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 61:418–426. doi: 10.1093/cid/civ312. [DOI] [PubMed] [Google Scholar]
- 2.Read TRH, Fairley CK, Murray GL, Jensen JS, Danielewski J, Worthington K, Doyle M, Mokany E, Tan L, Chow EPF, Garland SM, Bradshaw CS. 2019. Outcomes of resistance-guided sequential treatment of Mycoplasma genitalium infections: a prospective evaluation. Clin Infect Dis 68:554–560. doi: 10.1093/cid/ciy477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray GL, Bradshaw CS, Bissessor M, Danielewski J, Garland SM, Jensen JS, Fairley CK, Tabrizi SN. 2017. Increasing macrolide and fluoroquinolone resistance in Mycoplasma genitalium. Emerg Infect Dis 23:809–812. doi: 10.3201/eid2305.161745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bissessor M, Tabrizi SN, Twin J, Abdo H, Fairley CK, Chen MY, Vodstrcil LA, Jensen JS, Hocking JS, Garland SM, Bradshaw CS. 2015. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis 60:1228–1236. doi: 10.1093/cid/ciu1162. [DOI] [PubMed] [Google Scholar]
- 5.Walker J, Fairley CK, Bradshaw CS, Tabrizi SN, Twin J, Chen MY, Taylor N, Donovan B, Kaldor JM, McNamee K, Urban E, Walker S, Currie M, Birden H, Bowden FJ, Gunn J, Pirotta M, Gurrin L, Harindra V, Garland SM, Hocking JS. 2013. Mycoplasma genitalium incidence, organism load, and treatment failure in a cohort of young Australian women. Clin Infect Dis 56:1094–1100. doi: 10.1093/cid/cis1210. [DOI] [PubMed] [Google Scholar]
- 6.Guschin A, Ryzhikh P, Rumyantseva T, Gomberg M, Unemo M. 2015. Treatment efficacy, treatment failures and selection of macrolide resistance in patients with high load of Mycoplasma genitalium during treatment of male urethritis with josamycin. BMC Infect Dis 15:40. doi: 10.1186/s12879-015-0781-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bissessor M, Tabrizi SN, Bradshaw CS, Fairley CK, Hocking JS, Garland SM, Twin J, Poljak M, Peel J, Chen MY. 2016. The contribution of Mycoplasma genitalium to the aetiology of sexually acquired infectious proctitis in men who have sex with men. Clin Microbiol Infect 22:260–265. doi: 10.1016/j.cmi.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Jensen JS, Bjornelius E, Dohn B, Lidbrink P. 2004. Use of TaqMan 5′ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J Clin Microbiol 42:683–692. doi: 10.1128/JCM.42.2.683-692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabrizi SN, Su J, Bradshaw CS, Fairley CK, Walker S, Tan LY, Mokany E, Garland SM. 2017. Prospective evaluation of ResistancePlus MG, a new multiplex qPCR assay for the detection of Mycoplasma genitalium and macrolide resistance. J Clin Microbiol 55:1915–1919. doi: 10.1128/JCM.02312-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabrizi SN, Tan LY, Walker S, Twin J, Poljak M, Bradshaw CS, Fairley CK, Bissessor M, Mokany E, Todd AV, Garland SM. 2016. Multiplex assay for simultaneous detection of Mycoplasma genitalium and macrolide resistance using PlexZyme and PlexPrime technology. PLoS One 11:e0156740. doi: 10.1371/journal.pone.0156740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Twin J, Taylor N, Garland SM, Hocking JS, Walker J, Bradshaw CS, Fairley CK, Tabrizi SN. 2011. Comparison of two Mycoplasma genitalium real-time PCR detection methodologies. J Clin Microbiol 49:1140–1142. doi: 10.1128/JCM.02328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosse M, Nordbo SA, Pukstad B. 2016. Bacterial load in daily urine samples of patients infected with Mycoplasma genitalium, mutation analysis, and response to treatment. Infect Dis Obstet Gynecol 2016:8382469. doi: 10.1155/2016/8382469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlsen KH, Jensen JS. 2010. Mycoplasma genitalium PCR: does freezing of specimens affect sensitivity? J Clin Microbiol 48:3624–3627. doi: 10.1128/JCM.00232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lillis RA, Nsuami MJ, Myers L, Martin DH. 2011. Utility of urine, vaginal, cervical, and rectal specimens for detection of Mycoplasma genitalium in women. J Clin Microbiol 49:1990–1992. doi: 10.1128/JCM.00129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wroblewski JK, Manhart LE, Dickey KA, Hudspeth MK, Totten PA. 2006. Comparison of transcription-mediated amplification and PCR assay results for various genital specimen types for detection of Mycoplasma genitalium. J Clin Microbiol 44:3306–3312. doi: 10.1128/JCM.00553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]