Abstract
Non-diagnostic results can affect the diagnostic performance of percutaneous transthoracic needle biopsy (PTNB) but have not been critically meta-analyzed yet. To meta-analyze the incidence and malignancy rate of non-diagnostic results, 3-by-2 table approaches rather than the conventional 2-by-2 approaches are needed to know its impact on the diagnostic performance of PTNB. A systematic literature search identified studies evaluating the diagnostic performance of PTNB with extractable outcomes. A total of 143 studies with 35,059 biopsies were included. The pooled incidence of non-diagnostic results was 6.8% (95% CI, 6.0–7.6%; I2 = 0.91). The pooled malignancy rate of non-diagnostic results was 59.3% (95% CI, 51.7–66.8%; I2 = 0.80), and was correlated with the prevalence of malignancy (correlation coefficient, 0.66; 95% CI, 0.42–0.91). Pooled percentage decrease of sensitivity and specificity due to non-diagnostic results were 4.5% (95% CI, 3.2–5.7%; I2 = 0.64) and 10.7% (95% CI, 7.7–13.7%; I2 = 0.70), respectively, and the pooled incidence of non-diagnostic results was 4.4% (95% CI, 3.2–5.8%; I2 = 0.83) in lesions ultimately diagnosed as malignancies and 10.4% (95% CI, 7.5–13.8%; I2 = 0.74) in benign disease. In conclusion, non-diagnostic results averagely occurred in 6.8% of PTNB and more than half of the results were malignancies. The non-diagnostic results decreased specificity and sensitivity by 10.7% and 4.5%, respectively, demanding efforts to minimize the non-diagnostic results in PTNB.
Subject terms: Cancer imaging, Oncology
Introduction
Percutaneous transthoracic needle biopsy (PTNB) is a safe, accurate diagnostic procedure for evaluating pulmonary lesions, with an average sensitivity of 90% and specificity of 97%1,2. With the introduction of advanced imaging modalities for needle guidance, the diagnostic accuracy of computed tomography (CT) fluoroscopy- and cone-beam CT-guided biopsies increased to 95.2%3 and 97.0%4, respectively.
The diagnostic accuracy of PTNB is currently assessed using a 2-by-2 table, in which the PTNB results are clearly separated into positive and negative results, and then are compared with reference standards to create the following 4 cells: true positivity, false positivity, false negativity, and true negativity5. However, non-contributory results can be designated as neither positive nor negative PTNB results; these occur when the PTNB specimen is non-diagnostic, meaning that it does not provide any information for differentiating malignancy from benign disease3. The non-contributory results are expected to affect the diagnostic accuracy but are often omitted in a 2-by-2 table approach. The simple exclusion of non-diagnostic results from the 2-by-2 table leads to an overestimation of diagnostic accuracy6–9.
Non-diagnostic results has not yet been critically analyzed and it can be incorporated by using a 3-by-2 table to assess diagnostic accuracy, which enables a more realistic evaluation10. Thus, we meta-analyzed the incidence, malignancy rate of non-diagnostic biopsy results, and its impact on the diagnostic performance of PTNB for focal lung lesions using conventional 2-by-2 and 3-by-2 table approaches to handle non-diagnostic results.
Results
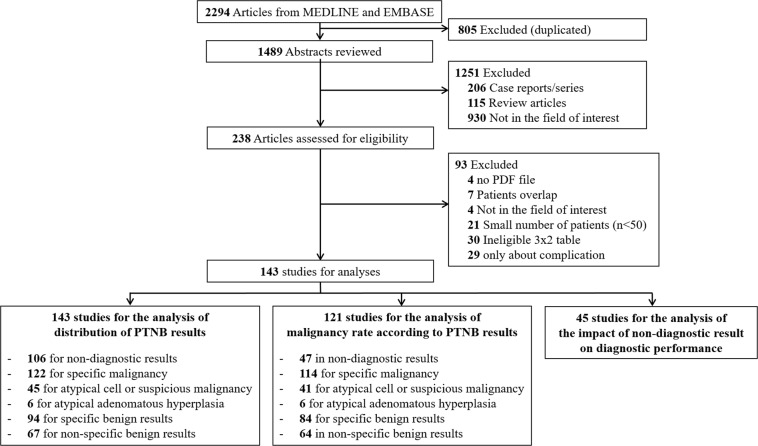
Of the 2294 references identified in the initial database search, 1433,4,6–9,11–147 with 35,059 biopsies were finally included in our analysis (Fig. 1).
Figure 1.
Flow diagram of the literature search.
The baseline characteristics and results of the 143 included studies are summarized in Supplementary Table A1. The number of total attempted biopsies ranged from 50 to 994 (median, 160; interquartile range [IQR], 100–316) and the median of the median or mean size of the pulmonary lesions was 31 mm (IQR, 24–37 mm). In 84 studies, fine needle aspiration (FNA) was mainly performed, and core biopsy was primarily analyzed in 59 studies. CT was the most frequently used modality for needle guidance (n = 77), followed by fluoroscopy (n = 37), cone-beam CT (n = 12), and CT fluoroscopy (n = 5); 12 other studies used 2 or more modalities for needle guidance. The median prevalence of malignancy was 78.6% (IQR, 69.1–84.3%; range, 54.2–96.5%). When assessed by the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 tool, the included studies appeared to have a relatively low risk of bias in index test domain. However, the risk of bias was unclear in approximately two-fifths of included studies in patient selection, reference standard, flow and timing domains (Supplementary Fig. A1).
Incidence of non-diagnostic and other PTNB results
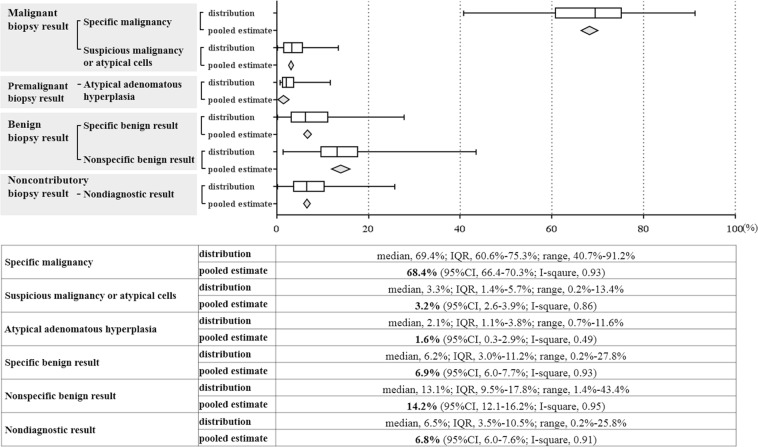
The pooled incidence of non-diagnostic results was 6.8% (95% CI, 6.0–7.6%; I2 = 0.91; 24668 biopsies in 106 studies)4,6–8,11,12,19,22–24,26–35,38,40–42,44–49,52,54,55,59–62,65–67,69–86,89,91–97,99–103,106–113,115–118,120,122–126,128–133,135–143,146,147.
The pooled incidence rates of other pathology findings of PTNB specimens are shown in Fig. 2: specific malignancy, 68.4% (95% CI, 66.4–70.3%, I2 = 0.93; 29739 biopsies in 122 studies)3,4,6–9,11–23,25–70,72–76,78,79,81–86,88–93,96,98–105,108,110,113–115,118–121,124,126–130,132–134,137–147; atypical cells, 3.2% (95% CI, 2.6–3.9%, I2 = 0.86; 11032 biopsies in 45 studies)4,9,13,14,17–20,26,27,29,30,34,37,41,42,46,47,53,64,66,69,70,74–76,78,79,85,86,89–91,98,101,104,108,115,120,121,126,129,133,144,147; AAH, 1.6% (95% CI, 0.3–2.9%, I2 = 0.49; 849 biopsies in 6 studies)8,76,84,126,128,138; non-specific benign results, 14.2% (95% CI, 12.1–16.2%; I2 = 0.95; 16455 biopsies in 67 studies)4,6,8,11,12,19,22,23,26–28,30,31,33,34,38,40,42,44,45,47–49,52,59–62,65,67,69,70,72,74–76,78,79,81–86,91,101,108,110,115,118–120,124,126,128–130,132,133,137–140,143,146–148; specific benign results, 6.9% (95% CI, 6.0–7.7%, I2 = 0.93; 22390 biopsies in 94 studies)4,6,9,12–16,18,21–23,26–28,30,31,33–40,42,43,45,46,48,50–53,56–65,67–70,72–76,79,81–83,85–88,90–93,98–101,103,104,108,110,114,115,118–121,124,126–130,132,134,139–141,143–145,147.
Figure 2.
Distribution of pathology reports of biopsy specimen in percutaneous thoracic needle biopsy.
On univariate meta-regression analyses, the incidence of non-diagnostic results were significantly lower with core biopsy (versus FNA; p = 0.010), CT or CTF guidance (versus fluoroscopy; p = 0.041), usage of 18 gauge needle or larger (versus 20 gauge or smaller; p = 0.137) (Supplementary Table A2). The core biopsy was significantly associated with the lower frequencies of non-specific benign results (P = 0.035), and higher frequencies of specific benign results (P = 0.001) than FNA. Lesion size significantly affected the incidence of specific malignancy, but did not affect the incidence of non-diagnostic results (Supplementary Table A2).
Final malignancy rate of non-diagnostic and other PTNB results
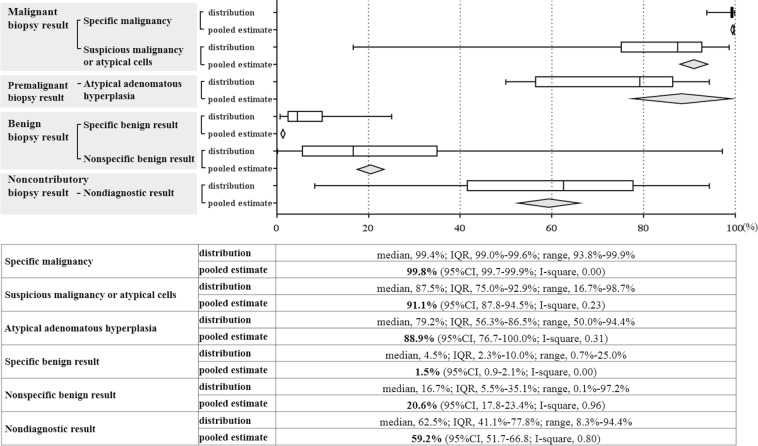
The final malignancy rate differed according to the PTNB pathology findings (Fig. 3). The pooled final malignancy rate of non-diagnostic results was 59.3% (95% CI, 51.7–66.8%; I2 = 0.80, 709 biopsies in 47 studies)4,6–8,11,12,19,22,23,26–28,33,34,38,40,42,45,48,52,54,55,59–61,65,70,72,76,78,79,81–83,85,91,92,96,101,108,115,129,137–140,147. The malignancy rate was moderately positively correlated with the overall prevalence of malignancy in the study (correlation coefficient, 0.66; 95% CI, 0.42–0.91).
Figure 3.
Malignancy rate according to pathology reports of biopsy specimen in percutaneous thoracic needle biopsy.
With regard to other pathology findings, the malignancy rate was the highest for specific malignancy (99.8%, 95% CI, 99.7–99.9%; I2 = 0.00; 11,884 biopsies in 114 studies)3,4,6–9,11–23,25–68,70,72–76,78,79,81–86,88,90–93,96,99–101,103–105,108,113–115,118–120,124,126–130,132,134,138–147, followed by atypical cells (91.1%, 95% CI, 87.8–94.5%; I2 = 0.23; 312 biopsies in 41 studies)4,9,13,14,17–20,26,27,29,30,34,37,41,42,46,47,53,64,66,70,74–76,79,85,86,89–91,98,101,104,108,115,120,126,129,144,147, AAH (88.9%, 95% CI, 76.7–100.0%; I2 = 0.31; 18 biopsies in 6 studies)8,76,84,126,128,138, non-specific benign results (20.6%, 95% CI, 17.8–23.4%; I2 = 0.96; 2574 biopsies in 64 studies)4,6,8,11,12,19,22,23,26–28,30,33,34,38,40,42,44,45,47–49,52,59–62,65,67,69,70,72,74–76,78,79,81–86,91,96,101,108,110,115,119,120,124,126,129,130,132,133,137–140,143,146,147, and specific benign results (1.5%, 95% CI, 0.9–2.1%; I2 = 0.00; 1601 biopsies in 84 studies)4,6,9,12–16,18,21–23,26–28,30,31,33–40,42,43,45,46,48,50–53,56–65,67–70,72–76,79,81–83,85–88,90–93,98–101,103,104,108,110,114,115,118–121,124,126–130,132,134,139–141,143–145,147.
Impact of non-diagnostic results on the diagnostic performance of PTNB
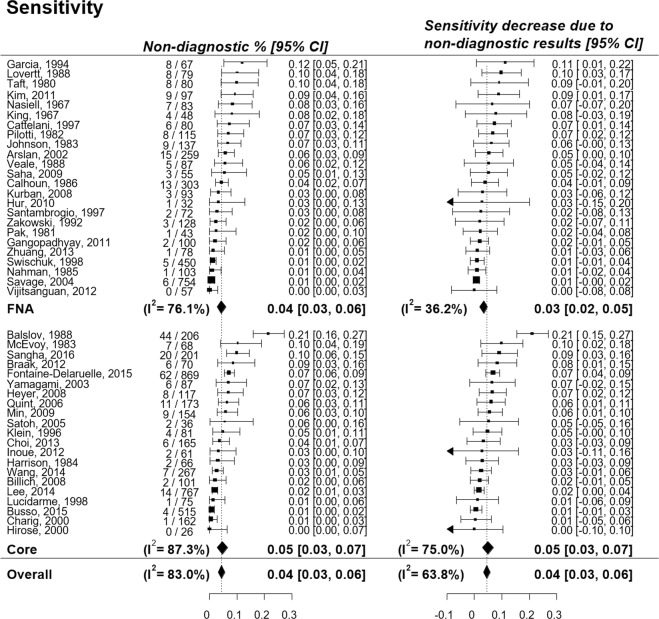
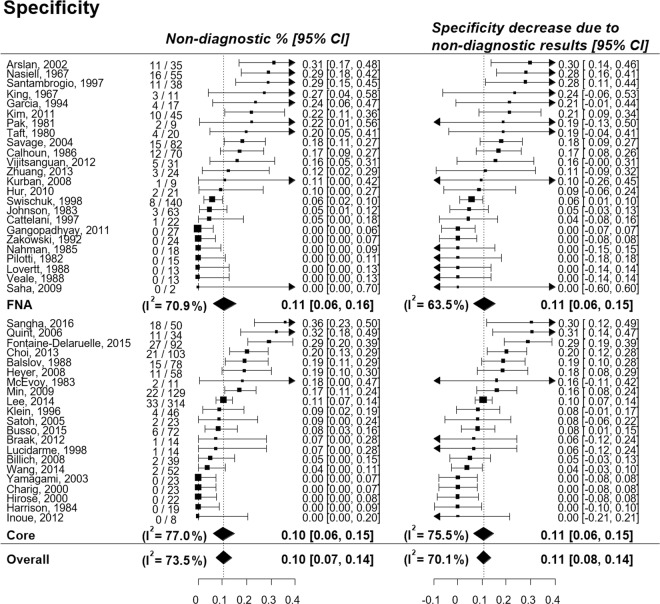
The pooled percentage decrease of sensitivity and specificity due to non-diagnostic results was 4.5% (95% CI, 3.2–5.7%; I2 = 0.64) and 10.7% (95% CI, 7.7–13.7%; I2 = 0.70), respectively (Figs 4, 5). The pooled incidence of non-diagnostic results was 4.4% (95% CI, 3.2–5.8%; I2 = 0.83) in lesions with a final diagnosis of malignancy and 10.4% (95% CI, 7.5–13.8%; I2 = 0.74) in lesions with a final diagnosis of benign disease, resulting in a larger reduction of specificity than of sensitivity. The subgroup analysis according to the biopsy method showed that changes in the pooled sensitivities and specificities were similar between the biopsy methods (Supplementary Fig. A2).
Figure 4.
Percentage decrease of sensitivity of percutaneous transthoracic needle biopsy due to non-diagnostic results.
Figure 5.
Percentage decrease of specificity of percutaneous transthoracic needle biopsy due to non-diagnostic results.
Funnel plot asymmetry was assessed for the incidence of non-diagnostic PTNB results presented in 106 studies. The double arcsine-transformed incidence was used to stabilize variance149. The funnel plots were not asymmetrical and the P value for the Egger test was 0.291, indicating no obvious publication bias. (Supplementary Fig. A3).
Discussion
Our meta-analysis revealed that 6.8% of successful PTNB procedures averagely did not offer any information for differentiating malignancies from benign disease. The pooled malignancy rate of non-diagnostic PTNB results was 59.3%, which was much higher than that of non-specific and specific benign results (20.6% and 1.5%, respectively). Although we pooled the incidence and malignancy rates to suggest a summary of those estimates, there was substantial heterogeneity across studies. Non-diagnostic results decreased the sensitivity and specificity of PTNB by 4.5% and 10.7%, respectively, which followed the pooled incidence of non-diagnostic results of 4.4% in lesions ultimately diagnosed as malignancies and those of 10.4% in lesions with a final diagnosis of benign disease. Additionally, multivariate meta-regression analysis revealed that the core biopsy showed significantly less frequent non-diagnostic results than FNA (P = 0.015).
Non-diagnostic PTNB results included specimens with blood, necrosis, normal lung parenchyma, or insufficient tissue to make any diagnosis, and accounted for 6.8% of PTNB specimens. Although the incidence of non-diagnostic results was significantly lower in core biopsy than in FNA, substantial heterogeneity still existed when studies were separately pooled according to the biopsy method. Presumably, lesion characteristics, such as location, distance from the pleura, and necrotic proportions of a lesion affect the likelihood of obtaining a non-diagnostic result3,91, although such characteristics could not be considered in this meta-regression analysis.
In non-diagnostic results, the median and pooled estimates of the malignancy rate were 62.5% and 59.3%, respectively, meaning that subsequent diagnostic procedures such as repeated biopsy, surgical exploration, or other imaging investigations such as positron emission tomography were clinically necessary. However, the malignancy rate was substantially heterogeneous across the studies. The heterogeneity seems to have mainly originated from the heterogeneous prevalence of malignancy across studies, given the moderate correlation between the malignancy rate and the overall prevalence of malignancy in the study (correlation coefficient, 0.66). This could result from differences in the study population and the institutional practice of PTNB across the studies.
Non-specific benign results had a relatively high proportion of final malignancy diagnoses (20.6%; 95% CI, 17.8–23.4%) when compared to specific benign results (1.5%; 95% CI, 0.9–2.1%). Similarly to non-diagnostic results, non-specific benign results often required a repeated PTNB or a more invasive procedure such as surgical exploration, especially when there was a discrepancy between a high clinical suspicion of malignancy and non-specific benign PTNB results150. Furthermore, lesions with a final diagnosis of malignancy were frequently accompanied by AAH, for which the proportion of final malignancy diagnoses (88.9%; 95% CI, 76.7–100.0%) was close to that of atypical cells or suspicious for malignancy (91.1%; 95% CI, 87.8–94.5%). Although AAH was defined as a peripheral focal proliferation of atypical cuboidal or columnar epithelial cells along the alveoli and respiratory bronchioles151, it is a premalignant lesion that forms a continuous spectrum with adenocarcinoma, and a clear distinction between AAH and pre- or minimally-invasive adenocarcinoma cannot be conclusive in PTNB specimens.
By using both the conventional 2-by-2 table approach and the intention-to-diagnose approach, we found that non-diagnostic results decreased the sensitivity and specificity of PTNB by 4.5% and 10.7%, respectively. Interestingly, the degree of reduction in sensitivity and specificity were almost the same as the incidence of non-diagnostic results in lesions with a final diagnosis of malignancy and benign disease, respectively. This is because the occurrence of non-diagnostic results directly led to the decrease of diagnostic accuracy as the accuracy of PTNB in a 2-by-2 table analysis was close to 100% (Figs 4, 5 and Supplementary Table A3).
Our study has several limitations. First, the degree of suspicion (pretest probability) for malignancy could not be considered in our analysis. Second, we regarded repeat PTNBs as separate initial PTNBs, and inter-exam correlations between repeat PTNBs could not be considered due to difficulties in accessing the raw data. Third, the reasons for statistical heterogeneity were not fully identified despite the meta-regression analysis. A detailed examination of the lesion characteristics could have helped identify the causes of heterogeneity, but this information was not extractable from the included studies. Additionally, meta-regression analysis regarding the experience and subspecialty of operators was not included due to the limited number of included studies. Fourth, some inconsistencies existed across the studies in terms of the reference standard for the final diagnosis of malignancy, such as different durations of post-PTNB observation or different rates of surgical confirmation, even though most studies included in our analysis were similar. Fifth, we included studies consisting of 50 or more PTNBs of focal parenchymal lung lesions by referring to the American College of Chest Physicians (ACCP) guideline for the diagnosis of lung cancer in 20132. Although this might potentially cause a biased inclusion of relevant studies, most of the studies (15/21) applied a particular inclusion criteria including pulmonary lesions difficult to be biopsied (9/21, 42.9%), cancer or benign lesion only (4/21, 19.0%), or a new biopsy technique (2/21, 9.5%). Other 6 (28.6%) studies were ineligible to construct a 2 × 3 table. Lastly, we did not include ultrasonography-guided biopsy, because we tried to investigate the diagnostic performances of intrapulmonary lesion, and it was not able to separate the diagnostic performance for subpleural lesions from that for chest wall lesion in the ultrasonography-guided biopsy studies.
In conclusion, the pooled incidence of non-diagnostic results was 6.8% in PTNB procedures and more than half of the non-diagnostic results were from malignancies. In in the 3-by-2 table approach, non-diagnostic results averagely decreased specificity and sensitivity by 10.7% and 4.5%, respectively, which followed incidences of non-diagnostic results in benign disease and malignancies. Because the previous 2 × 2 table analysis could result in reporting a biased diagnostic accuracy, accurate information of non-diagnostic rate can be transmitted to the patients more accurately with the approach of the 3 × 2 table analysis. Additionally, as true malignancy might be masked averagely in 60% of non-diagnostic biopsy results, thoracic interventionists should continue their efforts to minimize non-diagnostic results to maintain the diagnostic accuracy of PTNB, along with discreetly making an effort to identify the actual pathology of pulmonary lesions with the non-diagnostic results.
Methods
Search strategy
Two authors (K.J.C and S.H.Y) independently performed literature searches of the Ovid-MEDLINE and Embase databases to identify relevant publications using keywords related to ‘lung,’ ‘biopsy,’ and ‘accuracy’ (Supplementary Table A4), and the searches of two authors were harmonized by consensus. Searches were limited to English-language publications and human studies published through March 2016.
Inclusion criteria
The following inclusion criteria were applied to determine eligibility: (i) study population consisting of 50 or more PTNBs of focal parenchymal lung lesions2; (ii) study fully or partly addressing the diagnostic performance of PTNB; (iii) radiological guidance of fluoroscopy, CT, cone-beam CT, or CT fluoroscopy; (iv) a sufficient description of the data for outcomes to be extracted. In cases of partially or completely overlapping study populations, the study with the most biopsies was included. Case reports, review articles, editorials, letters, comments, and conference proceedings were excluded.
Definition of outcomes
We assessed 3 outcomes related to non-diagnostic results in this meta-analysis: (1) the incidence of non-diagnostic results, along with that of other pathology findings of PTNB specimens; (2) the final malignancy rate of non-diagnostic results, along with that of PTNB specimens with other pathology findings; (3) the impact of non-diagnostic results on the diagnostic performance of PTNB.
The pathology findings of PTNB specimens were divided into 6 categories which consisted of malignant, premalignant, benign, non-diagnostic findings: specific malignancy, atypical cells or suspicious for malignancy, atypical adenomatous hyperplasia (AAH), non-diagnostic results, non-specific benign disease, and specific benign diseases84,91. Specific benign diseases included benign lung tumors, infectious pneumonia, pulmonary tuberculosis, silicosis, vasculitis, or others. Non-specific benign disease referred to acute or chronic non-specific inflammation, granuloma, focal fibrosis, or a specimen without evidence of malignancy150. Non-diagnostic results were defined as a pathologic report of PTNB specimen only having blood, necrosis, normal lung parenchyma, or insufficient tissue to make any diagnosis. The final diagnosis was confirmed by the pathologic evaluation of a surgical specimen or clinico-radiologic follow-up for 1 year or longer2. Repeated biopsies for the same lesion were regarded as separate initial PTNBs.
To evaluate the impact of non-diagnostic results on the diagnostic performance of PTNB, we constructed a 3-by-2 table where a non-diagnostic PTNB results were added to the middle of the rows between positive and negative PTNB results on a per-biopsy basis. Positive PTNB results included specific malignancy and atypical cells or suspicious for malignancy, whereas negative PTNB results included AAH, non-specific benign disease, and specific benign diseases.
Data extraction and quality assessment
Data extraction and quality assessment were performed independently by 2 authors (K.J.C and S.H.Y). The quality of the included studies was assessed based on the QUADAS-2 criteria152. In case of disagreement between the two authors, a consensus was reached through further discussion with rechecking the text of the study.
Statistical analysis
A random-effects model was used to estimate the pooled incidences of pathology findings in PTNB specimens and the pooled proportion of final malignancy diagnoses according to the pathology findings of PTNB. The pooled estimates were shown with the distribution of individual study results instead of funnel plot as the funnel plot could not be presented in the text due to large numbers of studies included in the meta-analyses. Statistical heterogeneity across the included studies was assessed using forest plots and the I-squared statistic. To explore reasons for between-study heterogeneity, meta-regression was performed for the incidences of pathology finding in PTNB specimens. The correlation between the final malignancy rate of the non-diagnostic results and the prevalence of malignancy was estimated using a bivariate generalized linear model153.
To evaluate the diagnostic accuracy of PTNB, 2 approaches of handling non-diagnostic results were applied: the conventional approach of excluding non-diagnostic results and a conservative intention-to-diagnose approach10 (Tables 1 and 2). In the intention-to-diagnose approach, sensitivity and specificity were calculated as the proportion of positive PTNB results among technically successful procedures with a final malignancy diagnosis and the proportion of negative PTNB results among technically successful procedures with a final diagnosis of benign disease, respectively.
Table 1.
Definition of sensitivity and specificity in the conventional approach.
| Biopsy result | |||
|---|---|---|---|
| Malignancy | Benign | ||
| Final result | Malignancy | a (true positive) | c (false negative) |
| Benign | b (false positive) | d (true negative) | |
Table 2.
Definition of sensitivity and specificity in the intention-to-diagnose approach.
| Biopsy result | ||||
|---|---|---|---|---|
| Malignancy | Non-diagnostic result | Benign | ||
| Final result | Malignancy | a (true positive) | e | c (false negative) |
| Benign | b (false positive) | f | d (true negative) | |
A trivariate generalized linear model153 was used to explore correlations of the 2 diagnostic measures of sensitivity and specificity with prevalence, as the diagnostic measures may vary with prevalence due to different definitions of the reference standard or different distributions of disease severity154. Since negligible correlations between prevalence and the 2 diagnostic measures were observed in the trivariate generalized linear model, a bivariate generalized linear model155 was employed to estimate the pooled percentage decrease of sensitivity and specificity due to non-diagnostic results. The incidence of non-diagnostic results was examined by final disease status in order to explore the reason for different degrees of decrease between sensitivity and specificity in the intention-to-diagnose analysis. Subgroup analysis was conducted for the biopsy method (core biopsy versus FNA), as the biopsy method may affect the diagnostic accuracy.
The potential for publication bias was visually evaluated using funnel plots and the Egger test for asymmetry156. Analyses were performed using MetaAnalyst version 3.1 (Tufts Medical Center, Boston, MA, USA)157, the NLMIXED procedure in SAS 9.3 (SAS Corp., Cary, NC, USA), and the metafor package158 in R 3.4.0.
Supplementary information
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HC15C3390).
Author Contributions
K.C., S.Y. and C.P. designed the study. K.C. and S.Y. performed the literature search. K.C., H.H., S.Y. and S.H. performed statistical analysis. S.H., G.J., C.P. and J.G. supervised the findings of this work. All authors discussed the results and contributed to the final manuscript.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. Non-financial competing interests: The authors declare no non-financial competing Interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kum Ju Chae and Hyunsook Hong contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-48805-x.
References
- 1.Manhire A, et al. Guidelines for radiologically guided lung biopsy. Thorax. 2003;58:920. doi: 10.1136/thorax.58.11.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e142S–e165S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 3.Hiraki T, et al. CT fluoroscopy-guided biopsy of 1,000 pulmonary lesions performed with 20-gauge coaxial cutting needles: Diagnostic yield and risk factors for diagnostic failure. Chest. 2009;136:1612–1617. doi: 10.1378/chest.09-0370. [DOI] [PubMed] [Google Scholar]
- 4.Lee SM, et al. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of lung nodules: Clinical experience in 1108 patients. Radiology. 2014;271:291–300. doi: 10.1148/radiol.13131265. [DOI] [PubMed] [Google Scholar]
- 5.Šimundić A-M. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC. 2009;19:203–211. [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SH, et al. Percutaneous CT-guided aspiration and core biopsy of pulmonary nodules smaller than 1 cm: Analysis of outcomes of 305 procedures from a tertiary referral center. Am. J. Roentgenol. 2013;201:964–970. doi: 10.2214/AJR.12.10156. [DOI] [PubMed] [Google Scholar]
- 7.Kim GR, et al. CT fluoroscopy-guided lung biopsy versus conventional CT-guided lung biopsy: A prospective controlled study to assess radiation doses and diagnostic performance. Eur. Radiol. 2011;21:232–239. doi: 10.1007/s00330-010-1936-y. [DOI] [PubMed] [Google Scholar]
- 8.Inoue D, et al. CT fluoroscopy-guided cutting needle biopsy of focal pure ground-glass opacity lung lesions: Diagnostic yield in 83 lesions. Eur. J. Radiol. 2012;81:354–359. doi: 10.1016/j.ejrad.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Flower CD, Verney GI. Percutaneous needle biopsy of thoracic lesions–an evaluation of 300 biopsies. Clin. Radiol. 1979;30:215–218. doi: 10.1016/S0009-9260(79)80166-X. [DOI] [PubMed] [Google Scholar]
- 10.Schuetz GM, Schlattmann P, Dewey M. Use of 3x2 tables with an intention to diagnose approach to assess clinical performance of diagnostic tests: meta-analytical evaluation of coronary CT angiography studies. BMJ. 2012;345:e6717. doi: 10.1136/bmj.e6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King EB, Russell WM. Needle aspiration biopsy of the lung–technique and cytologic morphology. Acta Cytol. 1967;11:319–324. [PubMed] [Google Scholar]
- 12.Nasiell M. Diagnosis of lung cancer by aspiration biopsy and a comparison between this method and exfoliative cytology. Acta Cytol. 1967;11:114–119. [PubMed] [Google Scholar]
- 13.Stevens GM, Weigen JF, Lillington GA. Needle aspiration biopsy of localized pulmonary lesions with amplified fluoroscopic guidance. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1968;103:561–571. doi: 10.2214/ajr.103.3.561. [DOI] [PubMed] [Google Scholar]
- 14.Pavy RD, Antic R, Begley M. Percutaneous aspiration biopsy of discrete lung lesions. Cancer. 1974;34:2109–2117. doi: 10.1002/1097-0142(197412)34:6<2109::AID-CNCR2820340635>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 15.Francis D. Aspiration biopsies from diagnostically difficult pulmonary lesions. A consecutive case material. Acta Pathol. Microbiol. Scand. A. 1977;85a:235–239. doi: 10.1111/j.1699-0463.1977.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 16.House AJ, Thomson KR. Evaluation of a new transthoracic needle for biopsy of benign and malignant lung lesions. Am. J. Roentgenol. 1977;129:215–220. doi: 10.2214/ajr.129.2.215. [DOI] [PubMed] [Google Scholar]
- 17.Lalli AF, McCormack LJ, Zelch M, Reich NE, Belovich D. Aspiration biopsies of chest lesions. Radiology. 1978;127:35–40. doi: 10.1148/127.1.35. [DOI] [PubMed] [Google Scholar]
- 18.Poe RH, Tobin RE. Sensitivity and specificity of needle biopsy in lung malignancy. Am. Rev. Respir. Dis. 1980;122:725–729. doi: 10.1164/arrd.1980.122.5.725. [DOI] [PubMed] [Google Scholar]
- 19.Taft PD, Szyfelbein WM, Greene R. A study of variability in cytologic diagnoses based on pulmonary aspiration specimens. Am. J. Clin. Pathol. 1980;73:36–40. doi: 10.1093/ajcp/73.1.36. [DOI] [PubMed] [Google Scholar]
- 20.Westcott JL. Direct percutaneous needle aspiration of localized pulmonary lesions: result in 422 patients. Radiology. 1980;137:31–35. doi: 10.1148/radiology.137.1.7422857. [DOI] [PubMed] [Google Scholar]
- 21.Allison DJ, Hemingway AP. Percutaneous needle biopsy of the lung. Br. Med. J. 1981;282:875–878. doi: 10.1136/bmj.282.6267.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pak HY, Yokota S, Teplitz RL, Shaw SL, Werner JL. Rapid staining techniques employed in fine needle aspirations of the lung. Acta Cytol. 1981;25:178–184. [PubMed] [Google Scholar]
- 23.Pilotti S, Rilke F, Gribaudi G, Damascelli B. Fine needle aspiration biopsy cytology of primary and metastatic pulmonary tumors. Acta Cytol. 1982;26:661–666. [PubMed] [Google Scholar]
- 24.Samuelsson L, Albrechtsson U, Tylen U. Fine-needle biopsy of chest lesions. Radiologe. 1982;22:493–496. [PubMed] [Google Scholar]
- 25.Vine HS, Kasdon EJ, Simon M. Percutaneous lung biopsy using the Lee needle and a track-obliterating technique. Radiology. 1982;144:921–922. doi: 10.1148/radiology.144.4.7111747. [DOI] [PubMed] [Google Scholar]
- 26.Johnson RD, Gobien RP, Valicenti JF., Jr. Current status of radiologically directed pulmonary thin needle aspiration biopsy. An analysis of 200 consecutive biopsies and review of the literature. Ann. Clin. Lab. Sci. 1983;13:225–239. [PubMed] [Google Scholar]
- 27.McEvoy RD, Begley MD, Antic R. Percutaneous biopsy of intrapulmonary mass lesions. Experience with a disposable cutting needle. Cancer. 1983;51:2321–2326. doi: 10.1002/1097-0142(19830615)51:12<2321::aid-cncr2820511226>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Harrison BD, Thorpe RS, Kitchener PG, McCann BG, Pilling JR. Percutaneous Trucut lung biopsy in the diagnosis of localised pulmonary lesions. Thorax. 1984;39:493–499. doi: 10.1136/thx.39.7.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens GM, Jackman RJ. Outpatient needle biopsy of the lung: its safety and utility. Radiology. 1984;151:301–304. doi: 10.1148/radiology.151.2.6709896. [DOI] [PubMed] [Google Scholar]
- 30.Crosby JH, Hager B, Hoeg K. Transthoracic fine-needle aspiration. Experience in a cancer center. Cancer. 1985;56:2504–2507. doi: 10.1002/1097-0142(19851115)56:10<2504::aid-cncr2820561030>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 31.Greene R, Szyfelbein WM, Isler RJ, Stark P, Janstsch H. Supplementary tissue-core histology from fine-needle transthoracic aspiration biopsy. Am. J. Roentgenol. 1985;144:787–792. doi: 10.2214/ajr.144.4.787. [DOI] [PubMed] [Google Scholar]
- 32.Lees WR, Hall-Craggs MA, Manhire A. Five years’ experience of fine-needle aspiration biopsy: 454 consecutive cases. Clin. Radiol. 1985;36:517–520. doi: 10.1016/S0009-9260(85)80204-X. [DOI] [PubMed] [Google Scholar]
- 33.Nahman BJ, Van Aman ME, McLemore WE, O’Toole RV. Use of the Rotex needle in percutaneous biopsy of pulmonary malignancy. Am. J. Roentgenol. 1985;145:97–99. doi: 10.2214/ajr.145.1.97. [DOI] [PubMed] [Google Scholar]
- 34.Calhoun P, et al. The clinical outcome of needle aspirations of the lung when cancer is not diagnosed. Ann. Thorac. Surg. 1986;41:592–596. doi: 10.1016/S0003-4975(10)63066-4. [DOI] [PubMed] [Google Scholar]
- 35.Winning AJ, McIvor J, Seed WA, Husain OA, Metaxas N. Interpretation of negative results in fine needle aspiration of discrete pulmonary lesions. Thorax. 1986;41:875–879. doi: 10.1136/thx.41.11.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanley JH, et al. Lung lesions: cytologic diagnosis by fine-needle biopsy. Radiology. 1987;162:389–391. doi: 10.1148/radiology.162.2.3797651. [DOI] [PubMed] [Google Scholar]
- 37.Weisbrod GL, Herman SJ, Tao LC. Preliminary experience with a dual cutting edge needle in thoracic percutaneous fine-needle aspiration biopsy. Radiology. 1987;163:75–78. doi: 10.1148/radiology.163.1.3823460. [DOI] [PubMed] [Google Scholar]
- 38.Balslov S, Vestbo J, Viskum KA. Value of Tru-cut lung biopsy in focal and diffuse lung disease. Thorax. 1988;43:147–150. doi: 10.1136/thx.43.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine MS, Weiss JM, Harrell JH, Cameron TJ, Moser KM. Transthoracic needle aspiration biopsy following negative fiberoptic bronchoscopy in solitary pulmonary nodules. Chest. 1988;93:1152–1155. doi: 10.1378/chest.93.6.1152. [DOI] [PubMed] [Google Scholar]
- 40.Lovett JV, Manalo PB, Barcia TC, Bomberger RA, McGregor DB. Diagnosis of pulmonary masses by fine-needle aspiration. Am. J. Surg. 1988;156:441–445. doi: 10.1016/S0002-9610(88)80523-3. [DOI] [PubMed] [Google Scholar]
- 41.Simpson RW, Johnson DA, Wold LE, Goellner JR. Transthoracic needle aspiration biopsy. Review of 233 cases. Acta Cytol. 1988;32:101–104. [PubMed] [Google Scholar]
- 42.Veale D, Gilmartin JJ, Sumerling MD, Wadehra V, Gibson GJ. Prospective evaluation of fine needle aspiration in the diagnosis of lung cancer. Thorax. 1988;43:540–544. doi: 10.1136/thx.43.7.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins CD, Breatnach E, Nath PH. Percutaneous needle biopsy of lung nodules following failed bronchoscopic biopsy. Eur. J. Radiol. 1992;15:49–53. doi: 10.1016/0720-048X(92)90203-L. [DOI] [PubMed] [Google Scholar]
- 44.Cristallini EG, et al. Fine needle aspiration biopsy in the diagnosis of intrathoracic masses. Acta Cytol. 1992;36:416–422. [PubMed] [Google Scholar]
- 45.Zakowski MF, Gatscha RM, Zaman MB. Negative predictive value of pulmonary fine needle aspiration cytology. Acta Cytol. 1992;36:283–286. [PubMed] [Google Scholar]
- 46.Grode G, Faurschou P, Milman N. Percutaneous transthoracic fine-needle lung biopsy with 3 different needles. A retrospective study of results and complications in 224 patients. Respiration. 1993;60:284–288. doi: 10.1159/000196217. [DOI] [PubMed] [Google Scholar]
- 47.Burbank F, Kaye K, Belville J, Ekuan J, Blumenfeld M. Image-guided automated core biopsies of the breast, chest, abdomen, and pelvis. Radiology. 1994;191:165–171. doi: 10.1148/radiology.191.1.8134564. [DOI] [PubMed] [Google Scholar]
- 48.Garcia Rio F, et al. Value of CT-guided fine needle aspiration in solitary pulmonary nodules with negative fiberoptic bronchoscopy. Acta Radiol. 1994;35:478–480. doi: 10.1177/028418519403500517. [DOI] [PubMed] [Google Scholar]
- 49.Bocking A, Klose KC, Kyll HJ, Hauptmann S. Cytologic versus histologic evaluation of needle biopsy of the lung, hilum and mediastinum. Sensitivity, specificity and typing accuracy. Acta Cytol. 1995;39:463–471. [PubMed] [Google Scholar]
- 50.Gasparini S, et al. Integration of transbronchial and percutaneous approach in the diagnosis of peripheral pulmonary nodules or masses: Experience with 1,027 consecutive cases. Chest. 1995;108:131–137. doi: 10.1378/chest.108.1.131. [DOI] [PubMed] [Google Scholar]
- 51.Milman N, Faurschou P, Grode G. Diagnostic yield of transthoracic needle aspiration biopsy following negative fiberoptic bronchoscopy in 103 patients with peripheral circumscribed pulmonary lesions. Respiration. 1995;62:1–3. doi: 10.1159/000196380. [DOI] [PubMed] [Google Scholar]
- 52.Klein JS, Salomon G, Stewart EA. Transthoracic needle biopsy with a coaxially placed 20-gauge automated cutting needle: results in 122 patients. Radiology. 1996;198:715–720. doi: 10.1148/radiology.198.3.8628859. [DOI] [PubMed] [Google Scholar]
- 53.Li H, Boiselle PM, Shepard JO, Trotman-Dickenson B, McLoud TC. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: comparison of small and large pulmonary nodules. Am. J. Roentgenol. 1996;167:105–109. doi: 10.2214/ajr.167.1.8659351. [DOI] [PubMed] [Google Scholar]
- 54.Cattelani L, et al. CT-guided transthoracic needle biopsy in the diagnosis of chest tumours. J. Cardiovasc. Surg. (Torino) 1997;38:539–542. [PubMed] [Google Scholar]
- 55.Santambrogio L, et al. CT-guided fine-needle aspiration cytology of solitary pulmonary nodules: a prospective, randomized study of immediate cytologic evaluation. Chest. 1997;112:423–425. doi: 10.1378/chest.112.2.423. [DOI] [PubMed] [Google Scholar]
- 56.Westcott JL, Rao N, Colley DP. Transthoracic needle biopsy of small pulmonary nodules. Radiology. 1997;202:97–103. doi: 10.1148/radiology.202.1.8988197. [DOI] [PubMed] [Google Scholar]
- 57.Yankelevitz DF, Henschke CI, Koizumi JH, Altorki NK, Libby D. CT-guided transthoracic needle biopsy of small solitary pulmonary nodules. Clin. Imaging. 1997;21:107–110. doi: 10.1016/S0899-7071(96)00011-3. [DOI] [PubMed] [Google Scholar]
- 58.Larscheid RC, Thorpe PE, Scott WJ. Percutaneous transthoracic needle aspiration biopsy: a comprehensive review of its current role in the diagnosis and treatment of lung tumors. Chest. 1998;114:704–709. doi: 10.1378/chest.114.3.704. [DOI] [PubMed] [Google Scholar]
- 59.Lucidarme O, Howarth N, Finet JF, Grenier PA. Intrapulmonary lesions: percutaneous automated biopsy with a detachable, 18-gauge, coaxial cutting needle. Radiology. 1998;207:759–765. doi: 10.1148/radiology.207.3.9609901. [DOI] [PubMed] [Google Scholar]
- 60.Swischuk JL, et al. Percutaneous transthoracic needle biopsy of the lung: review of 612 lesions. J. Vasc. Interv. Radiol. 1998;9:347–352. doi: 10.1016/S1051-0443(98)70279-9. [DOI] [PubMed] [Google Scholar]
- 61.Charig MJ, Phillips AJ. CT-guided cutting needle biopsy of lung lesions–safety and efficacy of an out-patient service. Clin. Radiol. 2000;55:964–969. doi: 10.1053/crad.2000.0964. [DOI] [PubMed] [Google Scholar]
- 62.Hirose T, et al. Computed tomographic fluoroscopy-guided transthoracic needle biopsy for diagnosis of pulmonary nodules. Jpn. J. Clin. Oncol. 2000;30:259–262. doi: 10.1093/jjco/hyd070. [DOI] [PubMed] [Google Scholar]
- 63.Laurent F, et al. CT-guided transthoracic needle biopsy of pulmonary nodules smaller than 20 mm: results with an automated 20-gauge coaxial cutting needle. Clin. Radiol. 2000;55:281–287. doi: 10.1053/crad.1999.0368. [DOI] [PubMed] [Google Scholar]
- 64.Lopez Hanninen E, Vogl TJ, Ricke J, Felix R. CT-guided percutaneous core biopsies of pulmonary lesions. Diagnostic accuracy, complications and therapeutic impact. Acta Radiol. 2001;42:151–155. doi: 10.1034/j.1600-0455.2001.042002151.x. [DOI] [PubMed] [Google Scholar]
- 65.Arslan S, et al. CT- guided transthoracic fine needle aspiration of pulmonary lesions: accuracy and complications in 294 patients. Med. Sci. Monit. 2002;8:Cr493–497. [PubMed] [Google Scholar]
- 66.Wallace MJ, et al. CT-guided percutaneous fine-needle aspiration biopsy of small (<or =1-cm) pulmonary lesions. Radiology. 2002;225:823–828. doi: 10.1148/radiol.2253011465. [DOI] [PubMed] [Google Scholar]
- 67.Yu LS, Deheinzelin D, Younes RN, Chojniak R. Computed tomography-guided cutting needle biopsy of pulmonary lesions. Rev. Hosp. Clin. Fac. Med. Sao Paulo. 2002;57:15–18. doi: 10.1590/S0041-87812002000100003. [DOI] [PubMed] [Google Scholar]
- 68.Anderson JM, Murchison J, Patel D. CT-guided lung biopsy: Factors influencing diagnostic yield and complication rate. Clin. Radiol. 2003;58:791–797. doi: 10.1016/S0009-9260(03)00221-6. [DOI] [PubMed] [Google Scholar]
- 69.Geraghty PR, et al. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology. 2003;229:475–481. doi: 10.1148/radiol.2291020499. [DOI] [PubMed] [Google Scholar]
- 70.Yamagami T, et al. Usefulness of new automated cutting needle for tissue-core biopsy of lung nodules under CT fluoroscopic guidance. Chest. 2003;124:147–154. doi: 10.1378/chest.124.1.147. [DOI] [PubMed] [Google Scholar]
- 71.Mullan CP, et al. CT-guided fine-needle aspiration of lung nodules: Effect on outcome of using coaxial technique and immediate cytological evaluation. Ulster Med. J. 2004;73:32–36. [PMC free article] [PubMed] [Google Scholar]
- 72.Savage C, et al. Transthoracic Image-guided Biopsy of Lung Nodules: When Is Benign Really Benign? J. Vasc. Interv. Radiol. 2004;15:161–164. doi: 10.1097/01.RVI.0000109397.74740.8D. [DOI] [PubMed] [Google Scholar]
- 73.Gupta S, et al. Small (</=2-cm) subpleural pulmonary lesions: short- versus long-needle-path CT-guided Biopsy–comparison of diagnostic yields and complications. Radiology. 2005;234:631–637. doi: 10.1148/radiol.2342031423. [DOI] [PubMed] [Google Scholar]
- 74.Loubeyre P, Copercini M, Dietrich PY. Percutaneous CT-guided multisampling core needle biopsy of thoracic lesions. Am. J. Roentgenol. 2005;185:1294–1298. doi: 10.2214/ajr.04.1344. [DOI] [PubMed] [Google Scholar]
- 75.Mazza E, et al. On-site evaluation of percutaneous CT-guided fine needle aspiration of pulmonary lesions. A study of 321 cases. Radiol. Med. 2005;110:141–148. [PubMed] [Google Scholar]
- 76.Satoh S, et al. CT-guided automated cutting needle biopsy by a combined method for accurate specific diagnosis of focal lung lesions. Radiat. Med. 2005;23:30–36. [PubMed] [Google Scholar]
- 77.Bakhshayesh Karam M, et al. CT-guided percutaneous fine-needle aspiration biopsy of pulmonary lesions. Tanaffos. 2006;5:37–44. [Google Scholar]
- 78.Lourenco R, et al. CT-guided percutaneous transthoracic biopsy in the evaluation of undetermined pulmonary lesions. Rev. Port. Pneumol. 2006;12:503–524. doi: 10.1016/S0873-2159(15)30448-7. [DOI] [PubMed] [Google Scholar]
- 79.Quint LE, Kretschmer M, Chang A, Nan B. CT-guided thoracic core biopsies: value of a negative result. Cancer Imaging. 2006;6:163–167. doi: 10.1102/1470-7330.2006.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Halloush RA, et al. Fine needle aspiration cytology of lung lesions: A clinicopathological and cytopathological review of 150 cases with emphasis on the relation between the number of passes and the incidence of pneumothorax. Cytopathology. 2007;18:44–51. doi: 10.1111/j.1365-2303.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- 81.Priola AM, et al. Accuracy of CT-guided transthoracic needle biopsy of lung lesions: Factors affecting diagnostic yield. Radiologia Medica. 2007;112:1142–1159. doi: 10.1007/s11547-007-0212-y. [DOI] [PubMed] [Google Scholar]
- 82.Billich C, et al. CT-guided lung biopsy: incidence of pneumothorax after instillation of NaCl into the biopsy track. Eur. Radiol. 2008;18:1146–1152. doi: 10.1007/s00330-008-0872-6. [DOI] [PubMed] [Google Scholar]
- 83.Heyer CM, et al. Computed tomography-navigated transthoracic core biopsy of pulmonary lesions: which factors affect diagnostic yield and complication rates? Acad. Radiol. 2008;15:1017–1026. doi: 10.1016/j.acra.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 84.Kim TJ, et al. Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. Am. J. Roentgenol. 2008;190:234–239. doi: 10.2214/ajr.07.2441. [DOI] [PubMed] [Google Scholar]
- 85.Kurban LA, Gomersall L, Weir J, Wade P. Fluoroscopy-guided percutaneous lung biopsy: a valuable alternative to computed tomography. Acta Radiol. 2008;49:876–882. doi: 10.1080/02841850802225893. [DOI] [PubMed] [Google Scholar]
- 86.Laspas F, et al. Percutaneous CT-guided fine-needle aspiration of pulmonary lesions: Results and complications in 409 patients. J. Med. Imaging Radiat. Oncol. 2008;52:458–462. doi: 10.1111/j.1440-1673.2008.01990.x. [DOI] [PubMed] [Google Scholar]
- 87.Ng YL, et al. CT-guided percutaneous fine-needle aspiration biopsy of pulmonary nodules measuring 10 mm or less. Clin. Radiol. 2008;63:272–277. doi: 10.1016/j.crad.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 88.Chakrabarti B, et al. Risk assessment of pneumothorax and pulmonary haemorrhage complicating percutaneous co-axial cutting needle lung biopsy. Respir. Med. 2009;103:449–455. doi: 10.1016/j.rmed.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 89.Guimaraes MD, Chojniak R, Gross JL, Bitencourt AG. Predictive success factors for CT-guided fine needle aspiration biopsy of pulmonary lesions. Clinics (Sao Paulo, Brazil) 2009;64:1139–1144. doi: 10.1590/s1807-59322009001200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kothary N, Lock L, Sze DY, Hofmann LV. Computed tomography-guided percutaneous needle biopsy of pulmonary nodules: Impact of nodule size on diagnostic accuracy. Clin. Lung Cancer. 2009;10:360–363. doi: 10.3816/CLC.2009.n.049. [DOI] [PubMed] [Google Scholar]
- 91.Min JW, et al. Clinical significance of non-diagnostic pathology results from percutaneous transthoracic needle lung biopsy: experience of a tertiary hospital without an on-site cytopathologist. Respirology. 2009;14:1042–1050. doi: 10.1111/j.1440-1843.2009.01610.x. [DOI] [PubMed] [Google Scholar]
- 92.Saha A, Kumar K, Choudhuri MK. Computed tomography-guided fine needle aspiration cytology of thoracic mass lesions: A study of 57 cases. J. Cytol. 2009;26:55–59. doi: 10.4103/0970-9371.55222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Uskul BT, et al. CT-guided transthoracic fine needle aspiration of pulmonary lesions: accuracy and complications in 134 cases. Tuberk Toraks. 2009;57:177–185. [PubMed] [Google Scholar]
- 94.Yildirim E, et al. CT-guided cutting needle lung biopsy using modified coaxial technique: Factors effecting risk of complications. Eur. J. Radiol. 2009;70:57–60. doi: 10.1016/j.ejrad.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 95.Davoudi M, Shanfi MSZ, Rahim F. Study the complications of Ct-guidance fine needle biopsy in intra-thoracic masses. Int J Cancer Research. 2010;6:243–250. doi: 10.3923/ijcr.2010.243.250. [DOI] [Google Scholar]
- 96.Hur J, et al. Computed tomographic fluoroscopy-guided needle aspiration biopsy as a second biopsy technique after indeterminate transbronchial biopsy results for pulmonary lesions: Comparison with second transbronchial biopsy. J. Comput. Assist. Tomogr. 2010;34:290–295. doi: 10.1097/RCT.0b013e3181bc93ef. [DOI] [PubMed] [Google Scholar]
- 97.Kakizawa H, et al. Risk factors for severity of pneumothorax after CT-guided percutaneous lung biopsy using the single-needle method. Hiroshima J. Med. Sci. 2010;59:43–50. [PubMed] [Google Scholar]
- 98.Lee IJ, et al. Percutaneous needle aspiration biopsy (PCNAB) of lung lesions: 5 years results with focusing on repeat PCNAB. Eur. J. Radiol. 2010;73:551–554. doi: 10.1016/j.ejrad.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 99.Priola AM, et al. Diagnostic accuracy and complication rate of CT-guided fine needle aspiration biopsy of lung lesions: a study based on the experience of the cytopathologist. Acta Radiol. 2010;51:527–533. doi: 10.3109/02841851003691979. [DOI] [PubMed] [Google Scholar]
- 100.Schoellnast H, et al. CT-guided biopsy of lesions of the lung, liver, pancreas or of enlarged lymph nodes. Value of additional fine needle aspiration (FNA) to core needle biopsy (CNB) in an offsite pathologist setting. Acad. Radiol. 2010;17:1275–1281. doi: 10.1016/j.acra.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 101.Gangopadhyay M, Chakrabarti I, Ghosh N, Giri A. Computed tomography guided fine needle aspiration cytology of mass lesions of lung: Our experience. Indian J. Med. Paediatr. Oncol. 2011;32:192–196. doi: 10.4103/0971-5851.95139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guimaraes MD, De Andrade MQ, Da Fonte AC, Chojniak R, Gross JL. CT-guided cutting needle biopsy of lung lesions - An effective procedure for adequate material and specific diagnose. Eur. J. Radiol. 2011;80:e488–e490. doi: 10.1016/j.ejrad.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 103.Lee YJ, et al. Inconclusive Result from CT Guided Transthoracic Needle Aspiration and Biopsy: Affecting Factors and Final Outcome. J Lung Cancer. 2011;10:94–101. doi: 10.6058/jlc.2011.10.2.94. [DOI] [Google Scholar]
- 104.Lima CD, et al. Results and complications of CT-guided transthoracic fine-needle aspiration biopsy of pulmonary lesions. J. Bras. Pneumol. 2011;37:209–216. doi: 10.1590/S1806-37132011000200011. [DOI] [PubMed] [Google Scholar]
- 105.Matsui Y, et al. Role of computed tomography fluoroscopy-guided cutting needle biopsy of lung lesions after transbronchial examination resulting in negative diagnosis. Clin. Lung Cancer. 2011;12:51–55. doi: 10.3816/CLC.2011.n.007. [DOI] [PubMed] [Google Scholar]
- 106.Yamauchi Y, et al. Diagnostic performance of percutaneous core-needle lung biopsy under CT scan fluoroscopic guidance for pulmonary lesions measuring </=10 mm. Chest. 2011;140:1669–1670. doi: 10.1378/chest.11-1821. [DOI] [PubMed] [Google Scholar]
- 107.Beslic S, Zukic F, Milisic S. Percutaneous transthoracic CT guided biopsies of lung lesions; Fine needle aspiration biopsy versus core biopsy. Radiology and Oncology. 2012;46:19–22. doi: 10.2478/v10019-012-0004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Braak SJ, Herder GJ, van Heesewijk JP, van Strijen MJ. Pulmonary masses: initial results of cone-beam CT guidance with needle planning software for percutaneous lung biopsy. Cardiovasc. Intervent. Radiol. 2012;35:1414–1421. doi: 10.1007/s00270-011-0302-z. [DOI] [PubMed] [Google Scholar]
- 109.Maataoui A, Vogl TJ, Jacobi V, Khan MF. Diagnostic accuracy of CT readings on coin lesions in the lung as compared with transthoracic CT-guided needle biopsy results. Pneumologie. 2012;66:432–436. doi: 10.1055/s-0032-1309978. [DOI] [PubMed] [Google Scholar]
- 110.McSweeney SE, O’Regan KN, Mc Laughlin PD, Crush L, Maher MM. Evaluation of the efficacy and safety of percutaneous biopsy of lung. Open Respir. Med. J. 2012;6:82–88. doi: 10.2174/1874306401206010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nakatani M, et al. Analysis of factors influencing accuracy and complications in CT-guided lung biopsy. Minim. Invasive Ther. Allied Technol. 2012;21:415–422. doi: 10.3109/13645706.2012.662155. [DOI] [PubMed] [Google Scholar]
- 112.O’Neill AC, et al. Rapid needle-out patient-rollover time after percutaneous CT-guided transthoracic biopsy of lung nodules: effect on pneumothorax rate. Radiology. 2012;262:314–319. doi: 10.1148/radiol.11103506. [DOI] [PubMed] [Google Scholar]
- 113.Prosch H, et al. CT fluoroscopy-guided vs. multislice CT biopsy mode-guided lung biopsies: accuracy, complications and radiation dose. Eur. J. Radiol. 2012;81:1029–1033. doi: 10.1016/j.ejrad.2011.01.064. [DOI] [PubMed] [Google Scholar]
- 114.Uruga H, et al. Diagnostic efficacy of CT-guided transthoracic needle biopsy and fine needle aspiration in cases of pulmonary infectious disease. Jpn J Radiol. 2012;30:589–593. doi: 10.1007/s11604-012-0094-3. [DOI] [PubMed] [Google Scholar]
- 115.Vijitsanguan C, Subhunnachart P, Nikomprasart S. Efficacy of computed tomography-guided fine needle aspiration in diagnosis of lung mass by trained internists. J. Med. Assoc. Thai. 2012;95(Suppl 8):S31–36. [PubMed] [Google Scholar]
- 116.Yoshimatsu R, et al. Comparison of fully automated and semi-automated biopsy needles for lung biopsy under CT fluoroscopic guidance. Br. J. Radiol. 2012;85:208–213. doi: 10.1259/bjr/15132704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Asai N, et al. Is emphysema a risk factor for pneumothorax in CT-guided lung biopsy? Springerplus. 2013;2:196. doi: 10.1186/2193-1801-2-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Filippo M, et al. Predictive factors of diagnostic accuracy of CT-guided transthoracic fine-needle aspiration for solid noncalcified, subsolid and mixed pulmonary nodules. Radiol. Med. 2013;118:1071–1081. doi: 10.1007/s11547-013-0965-4. [DOI] [PubMed] [Google Scholar]
- 119.Li Y, Du Y, Yang HF, Yu JH, Xu XX. CT-guided percutaneous core needle biopsy for small (≤20 mm) pulmonary lesions. Clin. Radiol. 2013;68:e43–e48. doi: 10.1016/j.crad.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 120.Loh SE, et al. CT-guided thoracic biopsy:Evaluating diagnostic yield and complications. Ann. Acad. Med. Singapore. 2013;42:285–290. [PubMed] [Google Scholar]
- 121.Malone LJ, Stanfill RM, Wang H, Fahey KM, Bertino RE. Effect of intraparenchymal blood patch on rates of pneumothorax and pneumothorax requiring chest tube placement after percutaneous lung biopsy. Am. J. Roentgenol. 2013;200:1238–1243. doi: 10.2214/ajr.12.8980. [DOI] [PubMed] [Google Scholar]
- 122.Min L, et al. Breath-hold after forced expiration before removal of the biopsy needle decreased the rate of pneumothorax in CT-guided transthoracic lung biopsy. Eur. J. Radiol. 2013;82:187–190. doi: 10.1016/j.ejrad.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 123.Mondal SK, et al. Computed tomogram guided fine-needle aspiration cytology of lung mass with histological correlation: A study in Eastern India. South Asian J Cancer. 2013;2:14–18. doi: 10.4103/2278-330x.105881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Poulou LS, et al. Computed tomography-guided needle aspiration and biopsy of pulmonary lesions: a single-center experience in 1000 patients. Acta Radiol. 2013;54:640–645. doi: 10.1177/0284185113481595. [DOI] [PubMed] [Google Scholar]
- 125.Sconfienza LM, et al. Pleural and peripheral lung lesions: Comparison of US- and CT-guided biopsy. Radiology. 2013;266:930–935. doi: 10.1148/radiol.12112077. [DOI] [PubMed] [Google Scholar]
- 126.Tachibana K, et al. Immediate cytology improves accuracy and decreases complication rate in real-time computed tomography-guided needle lung biopsy. Diagn. Cytopathol. 2013;41:1063–1068. doi: 10.1002/dc.22940. [DOI] [PubMed] [Google Scholar]
- 127.Tuna T, et al. Diagnostic efficacy of computed tomography-guided transthoracic needle aspiration and biopsy in patients with pulmonary disease. Onco Targets Ther. 2013;6:1553–1557. doi: 10.2147/ott.s45013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yamagami T, Yoshimatsu R, Miura H, Yamada K, Takahata A, Matsumoto T, Hasebe T. Diagnostic performance of percutaneous lung biopsy using automated biopsy needles under CT-fluoroscopic guidance for ground-glass opacity lesions. The British Journal of Radiology. 2013;86(1022):20120447. doi: 10.1259/bjr.20120447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhuang YP, Wang HY, Zhang J, Feng Y, Zhang L. Diagnostic accuracy and safety of CT-guided fine needle aspiration biopsy in cavitary pulmonary lesions. Eur. J. Radiol. 2013;82:182–186. doi: 10.1016/j.ejrad.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 130.Floridi C, et al. C-arm cone-beam computed tomography needle path overlay for percutaneous biopsy of pulmonary nodules. Radiol. Med. 2014;119:820–827. doi: 10.1007/s11547-014-0406-z. [DOI] [PubMed] [Google Scholar]
- 131.Guimarães MD, Marchiori E, Hochhegger B, Chojniak R, Gross JL. CT-guided biopsy of lung lesions: defining the best needle option for a specific diagnosis. Clinics (Sao Paulo, Brazil) 2014;69:335–340. doi: 10.6061/clinics/2014(05)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jiao DC, et al. Clinical applications of the C-arm cone-beam CT-based 3D needle guidance system in performing percutaneous transthoracic needle biopsy of pulmonary lesions. Diagn Interv Radiol. 2014;20:470–474. doi: 10.5152/dir.2014.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Konjengbam R, Singh NB, Gatphoh SG. Computed tomography guided percutaneous transthoracic fine needle aspiration cytology of pulmonary mass lesions: Two years cross sectional study of 61 cases. J Med Soc. 2014;28:112–116. doi: 10.4103/0972-4958.141098. [DOI] [Google Scholar]
- 134.Kravtsov V, et al. Diagnostic aspects of fine needle aspiration for lung lesions: series of 245 cases. Asian Pac. J. Cancer Prev. 2014;15:9865–9869. doi: 10.7314/APJCP.2014.15.22.9865. [DOI] [PubMed] [Google Scholar]
- 135.Mendiratta-Lala M, et al. CT-guided core biopsy and percutaneous fiducial seed placement in the lung: Can these procedures be combined without an increase in complication rate or decrease in technical success? Eur. J. Radiol. 2014;83:720–725. doi: 10.1016/j.ejrad.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 136.Patel MV, Ahmed O, Jilani D, Zangan S. Computed tomography-guided percutaneous lung biopsy: impact of lesion proximity to diaphragm on biopsy yield and pneumothorax rate. J. Thorac. Imaging. 2014;29:344–349. doi: 10.1097/rti.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 137.Shrestha MK, Ghartimagar D, Ghosh A. Computed tomogram guided fine-needle aspiration cytology of lung and mediastinal masses with cytological correlation: a study of 257 cases in Western region of Nepal. Nepal Med. Coll. J. 2014;16:80–83. [PubMed] [Google Scholar]
- 138.Wang Y, Li W, He X, Li G, Xu L. Computed tomography-guided core needle biopsy of lung lesions: Diagnostic yield and correlation between factors and complications. Oncol. Lett. 2014;7:288–294. doi: 10.3892/ol.2013.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Busso M, et al. Safety and diagnostic performance of image-guided lung biopsy in the targeted therapy era. Radiol. Med. 2015;120:1024–1030. doi: 10.1007/s11547-015-0538-9. [DOI] [PubMed] [Google Scholar]
- 140.Fontaine-Delaruelle C, et al. Negative Predictive Value of Transthoracic Core-Needle Biopsy: A Multicenter Study. Chest. 2015;148:472–480. doi: 10.1378/chest.14-1907. [DOI] [PubMed] [Google Scholar]
- 141.Jaconi M, et al. C-arm cone-beam CT-guided transthoracic lung core needle biopsy as a standard diagnostic tool. Medicine. 2015;94:e698. doi: 10.1097/MD.0000000000000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schulze R, et al. Complications in CT-Guided, Semi-Automatic Coaxial Core Biopsy of Potentially Malignant Pulmonary Lesions. Rofo. 2015;187:697–702. doi: 10.1055/s-0034-1399648. [DOI] [PubMed] [Google Scholar]
- 143.Takeshita J, et al. CT-guided fine-needle aspiration and core needle biopsies of pulmonary lesions: a single-center experience with 750 biopsies in Japan. Am. J. Roentgenol. 2015;204:29–34. doi: 10.2214/ajr.14.13151. [DOI] [PubMed] [Google Scholar]
- 144.Yaffe D, Koslow M, Haskiya H, Shitrit D. A novel technique for CT-guided transthoracic biopsy of lung lesions: improved biopsy accuracy and safety. Eur. Radiol. 2015;25:3354–3360. doi: 10.1007/s00330-015-3750-z. [DOI] [PubMed] [Google Scholar]
- 145.Haas BM, et al. Nondiagnostic Computed Tomography-guided Percutaneous Lung Biopsies Are More Likely When Infection Is Suspected. J. Thorac. Imaging. 2016;31:151–155. doi: 10.1097/rti.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 146.Rotolo N, et al. Comparison of cone-beam CT-guided and CT fluoroscopy-guided transthoracic needle biopsy of lung nodules. Eur. Radiol. 2016;26:381–389. doi: 10.1007/s00330-015-3861-6. [DOI] [PubMed] [Google Scholar]
- 147.Sangha Bippan S., Hague Cameron J., Jessup Jennifer, O'Connor Robert, Mayo John R. Transthoracic Computed Tomography–Guided Lung Nodule Biopsy: Comparison of Core Needle and Fine Needle Aspiration Techniques. Canadian Association of Radiologists Journal. 2016;67(3):284–289. doi: 10.1016/j.carj.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 148.Hur J, et al. Diagnostic accuracy of CT fluoroscopy-guided needle aspiration biopsy of ground-glass opacity pulmonary lesions. Am. J. Roentgenol. 2009;192:629–634. doi: 10.2214/AJR.08.1366. [DOI] [PubMed] [Google Scholar]
- 149.Freeman, M. F. & Tukey, J. W. Transformations related to the angular and the square root. The Annals of Mathematical Statistics, 607–611 (1950).
- 150.Kim JI, Park CM, Kim H, Lee JH, Goo JM. Non-specific benign pathological results on transthoracic core-needle biopsy: how to differentiate false-negatives? Eur. Radiol. 2017;27:3888–3895. doi: 10.1007/s00330-017-4766-3. [DOI] [PubMed] [Google Scholar]
- 151.Weng S, et al. Multiple atypical adenomatous hyperplasia of type II pneumonocytes and bronchiolo-alveolar carcinoma. Histopathology. 1990;16:101–103. doi: 10.1111/j.1365-2559.1990.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 152.Whiting PF, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 153.Chu H, Nie L, Cole SR, Poole C. Meta‐analysis of diagnostic accuracy studies accounting for disease prevalence: Alternative parameterizations and model selection. Stat. Med. 2009;28:2384–2399. doi: 10.1002/sim.3627. [DOI] [PubMed] [Google Scholar]
- 154.Leeflang MM, Bossuyt PM, Irwig L. Diagnostic test accuracy may vary with prevalence: implications for evidence-based diagnosis. J. Clin. Epidemiol. 2009;62:5–12. doi: 10.1016/j.jclinepi.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 155.Chu Haitao, Cole Stephen R. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiology. 2006;59(12):1331–1332. doi: 10.1016/j.jclinepi.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 156.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med. Res. Methodol. 2009;9:80. doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.