Heart failure with preserved ejection fraction (HFpEF) and obesity are 2 common and often coexisting conditions that reduce cardiorespiratory fitness (CRF) 1, 2.
Intake of unsaturated fatty acids (UFAs), which consist of monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), has recently been associated with favorable CRF, body composition, and cardiac diastolic function in patients with obesity and HFpEF (3). A high UFA diet also prevented weight gain and cardiac diastolic dysfunction (3) in a mouse model of Western diet-induced cardiac dysfunction (4), despite similar total caloric and total fats intake. However, the feasibility of a dietary intervention aimed at increasing daily UFA intake in a prospective trial is required in this population to test its potential efficacy in a large randomized controlled trial.
We hypothesized that a 12-week dietary intervention aimed at increasing UFA consumption was feasible in patients with obesity and HFpEF, and would result in increased consumption of UFAs at 12 weeks (primary endpoint), as assessed by both dietary recall and biomarkers. To test this hypothesis, we performed a proof-of-concept trial and enrolled 9 patients with obesity, symptomatic HFpEF (i.e., dyspnea, fatigue), and reduced CRF (<80% of predicted) measured with maximal cardiopulmonary exercise testing, in a single-arm dietary intervention. All study participants provided written consent (NCT03310099).
Dietary intervention consisted of an individualized, in-person meeting with a dietitian at baseline and every 4 weeks thereafter, with a weekly telephone call to support dietary adherence. Dietary intervention was aimed at consuming a recommended daily amount (or more, without upper limit for consumption) of UFA-rich foods: extra-virgin olive oil (54 g), canola oil (54 g), unsalted or lightly salted mixed dry tree nuts (walnuts, hazelnuts, almonds, pecans), and peanuts (28 g), without providing recommendations on caloric intake. In patients who could not consume the recommended foods for personal and/or cultural preferences, the following foods were recommended: unsalted mixed seeds (28 g), Hass avocado (50 g), and fatty fish (salmon, tuna, trout, mackerel, sardines) (170 g). We provided $100 of financial support to purchase the recommended UFA-rich foods at baseline and 12-week visits, as well as $50 at 4- and 8-week visits. At each visit, a standardized 5-pass, 24-h dietary recall (3) was administered by a dietitian and nonfasting plasma UFA (oleic acid [MUFA], α-linolenic acid, and linoleic acid [PUFA]) levels were measured (Salveo Diagnostics Inc., Henrico, Virginia). We also measured exercise time, peak oxygen consumption (VO2) and peak oxygen (O2) pulse at baseline and at 12 weeks using a metabolic cart interfaced with a treadmill using a conservative ramping protocol. Physical activity was assessed using the International Physical Activity Questionnaire-short version (IPAQ) to calculate the cumulative metabolic equivalent of task-minutes per week (MET-min/week). The Shapiro-Wilk test was used to assess deviation from a Gaussian distribution with paired Student’s t-tests and Pearson’s rank test with mean ± SD used to examine changes between baseline and 12 weeks for normally distributed variables. Wilcoxon’s test and Spearman’s rank test with median and interquartile ranges were used to compare abnormally distributed variables, respectively. Statistical analysis was performed using SPSS version 24.0 (IBM, Armonk, New York).
Of 9 patients (6 African American) enrolled, 5 were women; mean age was 54 ± 5 years. All patients had arterial hypertension, and 5 had type 2 diabetes mellitus. Baseline left ventricular EF, N-terminal pro–brain natriuretic peptide, E/e′ ratio, and left atrial volume were 58 ± 4%, 29 pg/ml (range 17.5 to 54 pg/ml), 8.7 ± 2.9, and 57.9 ± 29.7 ml/m2, respectively. Baseline peak VO2 was 16.3 ± 5.9 ml/kg1/min1 or 53 ± 12% of predicted according to age, sex, height, and ideal body weight. Baseline exercise time and O2 pulse were 10.7 ± 3.2 min and 12.8 ± 4.8 ml/beat, respectively.
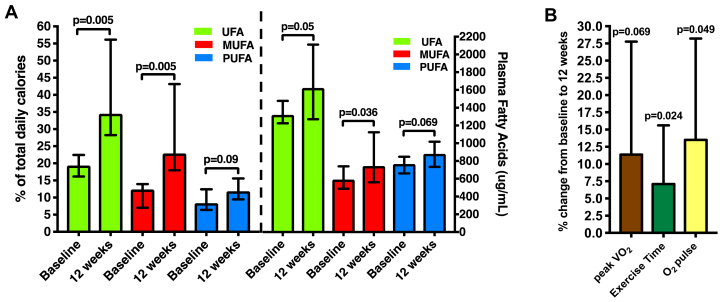
After 12 weeks, patients reported a significant increase in the proportion of daily calories derived from dietary UFAs and demonstrated an increase in plasma UFA biomarkers (Figure 1A). In addition to increased UFA consumption, we observed a significant increase in proportion of calories from total fat consumption (p = 0.007), but not a proportion of calories from saturated fatty acids, protein, total calories and milligrams of sodium intake (all p > 0.05). We found a significant reduction in the proportion of calories from total carbohydrates (p = 0.011) and fructose (p = 0.02).
Figure 1.
Dietary and Plasma Level of UFAs, MUFAs, and PUFAs
(A) Dietary and plasma level of unsaturated fatty acids (UFAs) (monounsaturated fatty acids [MUFAs], polyunsaturated fatty acids [PUFA]) and (B) changes in peak oxygen consumption (VO2), exercise time, and O2 pulse.
Baseline plasma UFAs, MUFAs, and PUFAs were positively associated with peak VO2 (R = +0.79; p = 0.036; R = +0.75; p = 0.052; R = +0.79; p = 0.036, respectively) and O2 pulse (R = +0.86; p = 0.014; R = +0.89; p = 0.007; R = +0.86; p = 0.014, respectively). After 12 weeks of UFA supplementation, we observed a significant improvement in exercise time and O2 pulse (Figure 1B) with a trend toward a significant increase in peak VO2 (p = 0.069), without significant changes in the respiratory exchange ratio (1.09 ± 0.09 to 1.07 ± 0.11; p = 0.44), body mass index (40.0 to 40.2 kg/m2; p = 0.21) or IPAQ-estimated physical activity (1,155 to 1,030 MET-min/wk; p = 0.67). Changes in peak VO2 tended to associate with changes in plasma UFAs (R = +0.71; p = 0.071), MUFAs (R = +0.75; p = 0.052), and PUFAs (R = +0.71; p = 0.071), although the associations did not reach statistical significance (p < 0.05).
Although limited by the small sample size and single-arm intervention, for the first time, we showed that a dietary intervention aimed at increasing UFA consumption was feasible and had the potential to improve CRF in patients with severe obesity and HFpEF. Larger randomized controlled trials to test the efficacy of UFA supplementation on CRF and clinical outcomes, as well as understanding the mechanisms through which UFAs may exert these beneficial effects are clearly warranted. Finally, understanding whether the improvements in CRF induced by increased UFA consumption result from improvement in cardiac function or noncardiac factors, such as favorable changes in body composition (5), remains a critical question to investigate in future studies.
Footnotes
Please note: The study was supported by the VCU Department of Internal Medicine Pilot Project Grant Program 2017 and by the VCU Pauley Heart Center Pilot Project Grant Program 2017. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Carbone S., Canada J.M., Buckley L.F. Obesity contributes to exercise intolerance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68:2487–2488. doi: 10.1016/j.jacc.2016.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obokata M., Reddy Y.N.V., Pislaru S.V., Melenovsky V., Borlaug B.A. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. doi: 10.1161/CIRCULATIONAHA.116.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carbone S., Canada J.M., Buckley L.F. Dietary fat, sugar consumption, and cardiorespiratory fitness in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol Basic Transl Sci. 2017;2:513–525. doi: 10.1016/j.jacbts.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbone S., Mauro A.G., Mezzaroma E. A high-sugar and high-fat diet impairs cardiac systolic and diastolic function in mice. Int J Cardiol. 2015;198:66–69. doi: 10.1016/j.ijcard.2015.06.136. [DOI] [PubMed] [Google Scholar]
- 5.Del Buono M.G., Arena R., Borlaug B.A. Exercise intolerance in patients with heart failure. JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:2209–2225. doi: 10.1016/j.jacc.2019.01.072. [DOI] [PubMed] [Google Scholar]