Abstract
Olive (Olea europaea L.) is a very important edible oil crop and has been cultivated for about 4,000 years in the Mediterranean area. Due to its nutritional and economic importance, researches on germplasm characterization received extensive attention. In this study, using the genotyping-by-sequencing (GBS) technology, we carried out genetic diversity analysis on 57 olive cultivars with different geographical origins. In total, 73,482 high-quality single-nucleotide polymorphisms (SNPs) with minor allele frequency (MAF) > 5%, call rate > 50%, and heterozygosity rate < 10% were obtained at the whole genome level. Genetic structure and phylogenetic analysis showed that the 57 olive cultivars could be classified into two groups (Group I and Group II). No clear geographical distributions of cultivars were observed generally between the two groups. The average nucleotide diversities (π) specific for Group I and Group II were 0.317 and 0.305. The fixation index (F ST) between Group I and Group II was 0.033. In Group II, cultivars could be further divided into two subgroups (Group IIa and Group IIb), which seem to be associated with their fruit sizes. The five Chinese-bred cultivars were all clustered in Group II, showing a closer genetic relationship with those from the central Mediterranean region and limited genetic background. It is therefore necessary for Chinese olive breeding programs to incorporate other genetic basis by utilizing germplasm from the other regions particularly from the east Mediterranean region as breeding parents. The results showed that GBS is an effective marker choice for cultivar characterization and genetic diversity analysis in olive and will help us better understand the genetic backgrounds of the crop.
Keywords: olive, genotyping-by-sequencing technology, single-nucleotide polymorphism exploration, phylogenetic analysis, genetic diversity
Introduction
Olive (Olea europaea L.) is one of the valuable fruit trees and the second largest woody oil plant in the world. Olive tree is native to Asia Minor and has been domesticated and cultivated around 4,000 years in Mediterranean countries (Zohary and Spiegel-Roy, 1975; Zohary and Hopf, 1994). One of the main purposes to grow olive trees is to produce fresh virgin olive oil. It is rich in monounsaturated fatty acids and has high nutritional quality, which is considered as “liquid gold” and benefits our health (Sebastiani and Busconi, 2017). Because of the high nutritional and great economic value, the olive industry has developed rapidly in recent years (Pérez-Jiménez et al., 2007; Zhan et al., 2015). At present, olives have been introduced and planted in more than 40 countries including America, Australia, and China (Kaniewski et al., 2012). Through long-term natural selection, artificial selection, cultivation, and domestication, numerous genetic resources have been formed (Wright, 1978). However, the genetic relationship among the cultivars is not yet clear, although the olive germplasms are extremely abundant. Therefore, researches on identification, classification, and genetic diversity analysis of olive cultivars are imperative, which not only helps to utilize the existing olive cultivars more effectively but also benefits genetic improvement and breeding of olive cultivars.
For these purposes, scientists have done lots of works on the germplasm resources and genetics analysis of olives. Using morphological characters, agronomic traits, biochemical markers, and molecular markers, Pontikis et al. (1980) and Ouazzani et al. (1993) elucidated 27 and 133 olive cultivars by analyzing isozyme banding patterns and applied biochemical markers to distinguish olive cultivars, respectively. Molecular markers such as random amplified polymorphism DNA (RAPD), amplified fragment length polymorphism (AFLP), sequence-related amplified polymorphism (SRAP), simple sequence repeats (SSR), inter-simple sequence repeat (ISSR), and single-nucleotide polymorphism (SNP) have been used to evaluate the genetic variation of olive germplasms (Hess et al., 2000; Rallo et al., 2000; Besnard et al., 2001; Grati-Kamoun et al., 2006; Reale et al., 2006; Isk et al., 2011; Kaya et al., 2013; Diez et al., 2015; Zhan et al., 2015; Khaleghi et al., 2017; Mousavi et al., 2017; Rienzo et al., 2018). Through the integration of molecular markers SSR, SNP and diversity array technology (DArT), and agronomical traits, Belaj et al. (2012) studied the pattern of genetic diversity among 361 olive cultivars and found that a certain association would exist between the geographical origin and genetic structure of olive, especially for the differentiated cultivars from eastern and western Mediterranean. Bartolini et al. (2005) established a public OLEA database (http://www.oleadb.it/) by integrating information of morphological, agronomical, and biochemical traits and molecular markers of more than 1,000 cultivars, which greatly benefits cultivar consultancy and further researches as well. The high efficiency and reliability of molecular markers make it an effective tool for the study of genetic diversity, molecular marker-assisted breeding, species identification, genetic map construction, and excellent gene mapping for olives.
Genotyping-by-sequencing (GBS) technology is a new method based on next-generation sequencing (Poland and Rife, 2012; Torkamaneh et al., 2016). The protocol enables high-throughput sequencing of multiplexed samples and combines genome-wide molecular marker discovery and genotyping (Torkamaneh et al., 2016). This greatly reduces the cost of gene sequencing and enables multi-sample high-throughput parallel sequencing as well. Besides, GBS technology was also available for no-reference species (Elshire et al., 2011; He et al., 2014; Torkamaneh et al., 2016). The method has been used for genetic diversity analysis in both animals and plants such as cattle (Donato et al., 2013), watermelon (Nimmakayala et al., 2014), wheat (Lin et al., 2015), spinach (Shi et al., 2017), and tetraploid ryegrass (Guo et al., 2018). İpek et al., 2016 identified 10,941 SNPs from a cross between the olive cultivars “Gemlik” and “Edincik Su” using GBS and constructed a high-density genetic map. Using the GBS data of 94 Italian olive cultivars, D’Agostino et al. (2018) obtained 22,088 and 8,088 SNPs by reference-based and reference-independent SNP calling pipeline and found the varied genetic diversity of Italian cultivars.
China is a newly emerging olive-oil-producing region in the world. It has been only 50 years since the large-scale introduction and cultivation of olive trees. It is generally estimated that the current area of olive trees in the country is about 80,000 hectares, and the annual output of olive oil is about 6,000 tons, which is simply unable to meet the demand for high-quality edible oil in the Chinese market. Most of the Chinese olive gardens have been newly built since the beginning of this century and have not yet entered fructifications or full production. Because of the huge climate and soil differences with the Mediterranean region, olive trees of most cultivars generally show a poor adaptability to local environmental conditions when grown in China, resulting in lower yield compared to their traditional cultivation regions. It is therefore important to make genetic assessments on olive germplasms so as to screen elite cultivar suitable for growing in China. Until now, the sequencing of two cultivated olive trees O. europaea cv. Leccino (Barghini et al., 2014) and O. europaea cv. Farga (Cruz et al., 2016) and one wild olive tree O. europaea var. sylvestris (Unver et al., 2017) has been completed. The publication and availability of genomic data provide us a quick and effective way to characterize olive germplasm resources. In this study, we analyzed the genetic background of 57 olive cultivars of different geographic origins at the whole genome level with the available database and GBS technology, aiming at carrying out the identification and evaluation of olive germplasm and providing core reference for further introduction of olive germplasm in China.
Materials and Methods
Plant Materials
A total of 57 olive cultivars were collected and analyzed in this study ( Table 1 ), which were originally collected from eight different countries. The majority were from Italy and Spain with 27 and 19 cultivars each, while the remaining were from China (n = 5), France (n = 2), Greece (n = 1), Azerbaijan (n = 1), Portugal (n = 1), and Algeria (n = 1). The different olive fruit sizes (weight) were also downloaded from OLEA database (http://www.oleadb.it/) and shown in Table 1 , including five levels: L (low: less than 2.0 g), M (medium: 2.0 to 4.0 g), MH (medium-high: 4.0 to 6.0 g), H (high: 6.0 to 8.0 g), and VH (very high: greater than 8.0 g). The Student’s t test was conducted to establish whether the statistics of fruit sizes were significant within different groups. Among all cultivars, 37 were used for olive oil purposes, 5 as table olive, and 15 for double purposes. All cultivars were grown in the experimental field with an average space 2 × 3 m in Zhejiang Academy of Agricultural Sciences (30◦18′28′′ N–120◦11′44′′ E), Hangzhou, Zhejiang Province, China. Young olive leaves were sampled, quickly frozen in liquid nitrogen, and stored at −70°C freezer for further analysis.
Table 1.
Information of the 57 olive cultivars analyzed in the study.
| Material no. | Olive germplasm | Origin | Fruit weighta | Usageb | Cluster/sub-cluster |
|---|---|---|---|---|---|
| 1 | Alfafara | Spain | H | O | Group I |
| 2 | Arbequina | Spain | L | O | Group IIa |
| 3 | Arbosana | Spain | L | O | Group IIa |
| 4 | Arroniz | Spain | M | O | Group I |
| 5 | Ascolana tenera | Italy | H | T | Group IIb |
| 6 | Bianchera | Italy | M | O | Group IIb |
| 7 | Bouteillan | France | H | T/O | Group I |
| 8 | Canino | Italy | L | O | Group IIa |
| 9 | Carrasqueno | Spain | M | T/O | Group I |
| 10 | Castellana | Spain | M | O | Group I |
| 11 | Changlot real | Spain | M | O | Group IIa |
| 12 | Chemlal de Kabylie | Algeria | M | O | Group IIa |
| 13 | Chenggu 32 | China | M | T/O | Group IIa |
| 14 | Cipressino | Italy | M | O | Group IIb |
| 15 | Cobrancosa | Portugal | H | O | Group I |
| 16 | Coratina | Italy | M | O | Group IIb |
| 17 | Cornezuelo de Jaen | Spain | M | T/O | Group I |
| 18 | Cornicabra | Spain | M | O | Group I |
| 19 | Dolce agogia | Italy | M | O | Group IIa |
| 20 | Empeltre | Spain | M | O | Group IIa |
| 21 | Ezhi 8 | China | M | T/O | Group IIa |
| 22 | Fecciaro | Italy | MH | O | Group IIa |
| 23 | Frantoio | Italy | M | O | Group IIb |
| 24 | Frantoio selezione quarrata | Italy | M | O | Group IIa |
| 25 | Gentile di chieti | Italy | M | O | Group IIb |
| 26 | Grignan | Italy | H | O | Group I |
| 27 | Grossa di spagna | Italy | VH | T | Group I |
| 28 | Hojiblanca | Spain | H | T/O | Group I |
| 29 | Huaou 5 | China | MH | O | Group IIa |
| 30 | I-77 | Italy | M | O | Group IIa |
| 31 | I-79 | Italy | M | O | Group IIb |
| 32 | Koroneiki | Greece | L | O | Group IIa |
| 33 | Leccino | Italy | M | O | Group IIa |
| 34 | Limona | Italy | M | T | Group IIa |
| 35 | Manzanilla | Spain | H | T/O | Group I |
| 36 | Manzanilla cacerena | Spain | H | T/O | Group I |
| 37 | Manzanilla sevillana | Spain | H | T | Group I |
| 38 | Maurino | Italy | L | O | Group IIa |
| 39 | Moraiolo | Italy | L | O | Group IIa |
| 40 | Morcona | Italy | M | O | Group IIb |
| 41 | Nevadillo fino | Spain | M | O | Group I |
| 42 | Nikitskii I | Azerbaijan | MH | T/O | Group IIa |
| 43 | Nociara | Italy | M | T/O | Group IIb |
| 44 | Nostrale di rigali | Italy | M | O | Group IIb |
| 45 | Olivo de caniles | Spain | – | UN | Group I |
| 46 | Pendolino | Italy | L | O | Group IIa |
| 47 | Peranzana | Italy | M | T/O | Group I |
| 48 | Picholine | France | M | T/O | Group IIb |
| 49 | Picual | Spain | M | O | Group I |
| 50 | Redondilla | Spain | MH | T/O | Group I |
| 51 | Rosciola | Italy | L | O | Group IIa |
| 52 | Santa caterina | Italy | H | T | Group I |
| 53 | Taggiasca | Italy | M | O | Group IIb |
| 54 | Verdial de badajoz | Spain | VH | O | Group I |
| 55 | Yuntai | China | M | T/O | Group IIa |
| 56 | Zen | Italy | L | O | Group IIa |
| 57 | Zhonglan | China | L | O | Group IIa |
Fruit weight: low, L (less than 2.0 g; medium, M (2.0 to 4.0 g); medium-high, MH (4.0 to 6.0 g); high, H (6.0 to 8.0 g); very high, VH (greater than 8.0 g)
T, Table olive; O, Olive oil; T/O, Double purpose.
DNA Extraction and GBS Library Construction
Genomic DNA of the 57 olive cultivars was extracted with the cetyl-trimethyl-ammonium-bromide (CTAB) method as described by Murray and Thompson (1980). Qualified DNA samples, after checking on agarose gel, were digested with ApeKI (New England Biolabs, USA) and then ligated to either barcoded adaptors or common adaptors. Only short samples featuring both barcode and common adaptor were enriched by PCR amplification and then purified by magnetic beads with a range of 250–300 bp. Finally, paired-end sequencing was performed on an Illumina HiSeq 2000 platform at Beijing Genomics Institute (BGI) in Hong Kong.
GBS-SNP Procedure
The bioinformatics pipeline for GBS-SNP is summarized in Supplementary Figure S1 . In detail, raw reads were filtered and split into clean reads by the following steps: 1) remove reads with adaptors; 2) remove low-quality reads, of which more than 50% had quality value ≤ 12; 3) remove reads whose unknown bases were ≥ 3%; 4) remove reads that do not contain barcode (4–8 bp) at 5-most of reads used to be identified by different samples (one barcode corresponds to one sample); and 5) trim the barcode after step 4 and then remove reads lacking key sequence of the enzymes at 5-most.
Clean reads were then aligned to the olive reference sequences O. europaea cv. Farga (Cruz et al., 2016) using SOAP2 software (Hurgobin, 2016). Subsequently, SOAPsnp was used to call SNP (Li et al., 2008; https://sourceforge.net/projects/soapsnp/). The main parameters are shown in Supplementary Table S1 .
The Bayesian model was applied to calculate the probability of genotypes. The genotype with the highest probability was selected as the genotype of the sequencing individual at the specific locus. Using the consensus sequence, polymorphic loci against the reference sequence were selected and then filtered under certain requirements. The call frequency, minor allele frequency (MAF), heterozygosity rate, and polymorphism information content (PIC) (Botstein et al., 1980) were calculated and analyzed using EXCEL 2013 software based on the SNP genotyping.
Population Characteristics and Linkage Disequilibrium Analysis
To reflect the genetic relationship of olive cultivars, the SNPs with missing data > 0.5 were excluded and the remaining data with MAF > 5% and heterozygosity rate < 10% were selected for further analysis. Genetic structure analysis was conducted using admixture 1.3 (Alexander et al., 2009) and the number of populations (K) was calculated from K = 1 to 10. Meanwhile, a phylogenetic tree was constructed using MEGA X software with the neighbor-joining method (www.megasoftware.net; Kumar et al., 2018) and further edited by Figtree software (https://sourceforge.net/projects/figtree/). The parameters were as follows: Test of phylogeny, bootstrap method; no. of bootstrap replications, 1,000; Model/method, maximum composite likelihood; Substitutions to include, d: Transitions + Transversions; Gaps/missing data treatment, pairwise deletion. Principal component analysis (PCA) was performed using TASSEL 5.0 software (https://tassel.bitbucket.io/) with an identity-by-state (IBS) matrix data. Pairwise IBS allele-sharing was calculated using PLINK V1.90 presented by multidimensional scaling (MDS) plot (Purcell et al., 2007). The correlation coefficient (r 2) of alleles was calculated to measure linkage disequilibrium (LD) in each group level using PLINK V1.90 (Purcell et al., 2007).
Population Diversity Analysis
VCFtools (https://vcftools.github.io/) was employed to calculate the parameters of population genetic diversity. The degree of polymorphism within a population was measured by the average number of nucleotide differences per site (π; Nei and Li, 1979), and the genetic differentiation between groups was measured by fixation index (F ST; Holsinger and Weir, 2009).
Results
General Characteristics of GBS in Olive
To understand the genetic relationship of olive germplasm, 57 olive cultivars mainly from Italy and Spain were sequenced using GBS technology ( Table 1 ). The data were presented in Supplementary Table S2. After filtering, raw reads were split into clean reads and finally generated 352.93 million (M) clean reads with average 6.19 M reads per sample (ranging from 3.66 M to 12.01 M). Statistics on sequence data further showed that the quality value 20 (Q20) ≥ 97.3%, quality value 30 (Q30) ≥ 92.8%, and the GC contents distributed in a range of 46.4–56.4%, indicating that GBS was a valuable molecular method qualified for germplasm characterization in olive.
GBS-SNP Analysis
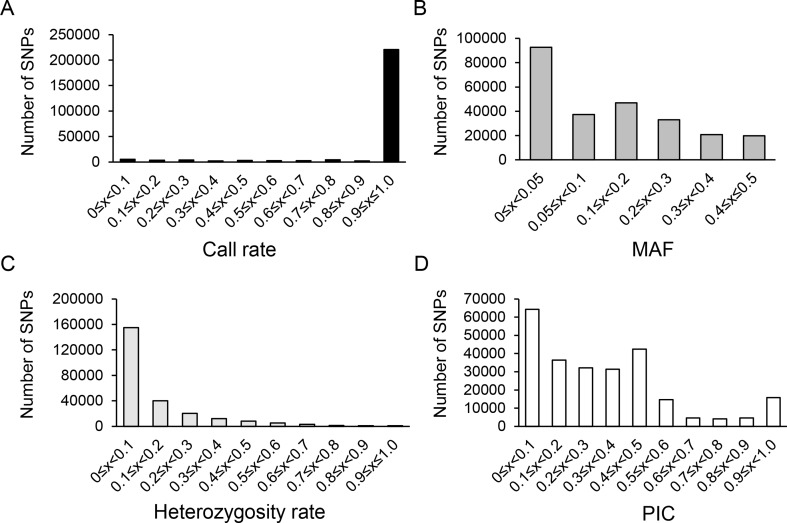
Clean reads were mapped to olive reference genome O. europaea cv. Farga using SOAP2 (Cruz et al., 2016; Hurgobin, 2016) and SNP call (Li et al., 2008; https://sourceforge.net/projects/soapsnp/). A total of 250,583 SNPs was generated with an average mapping rate of 44.2%. As shown in Figure 1 , 88.0% of all the SNPs had call rate in the range of 90–100%, and 67.1% had MAF > 5%. Besides, the heterozygosity rate was mainly in the range of 0–10%, which accounted for 61.8% of all SNPs. PIC was mainly in the range of 0–50%, with 3.8% of all SNPs having a PIC = 50%.
Figure 1.
Characteristic statistics of SNPs using 250,583 SNPs. (A) SNPs distribution on the olive scaffolds. (B) Distribution of genic and inter-genic regions of selected SNPs. The X-axis represented the statistical SNP characteristic parameters including loci call frequency (A), minor allele frequency (MAF) (B), heterozygosity rate (C) and polymorphism information content, PIC (D). The Y-axis represented the number of SNPs.
The 250,583 SNPs were further filtered under the condition of MAF > 5%, call rate > 50%, and heterozygosity rate < 10% and generated 73,482 SNPs used for genetic diversity analysis with a mean depth of 49.5 reads/SNP. The missing calls of filtered SNPs were in the range of 0.2–3.3% with an average of 1.4%, while the heterozygous calls were in the range of 1.3–6.4% with an average of 2.9%. Various SNP types were determined as follows: [A/G] SNP type had 20,456 SNPs (27.84%); [C/T], 20,418 (27.79%); [A/C], 8,194 (11.15%); [A/T], 8,108 (11.03%); [G/T], 7,700 (10.48%); [C/G], 5,700 (7.76%). The remaining SNPs (2,900; 3.95%) displayed three or four polymorphic types. Meanwhile, among all the cultivars investigated, 13 cultivars (Bouteillan, Coratina, Ezhi 8, Hojiblanca, Huaou 5, Manzanilla, Nevadillofino, Nikitskii I, Olivo de caniles, Pendolino, Picual, Santa caterina, and Zhonglan) showed heterozygous calls of less than 2.0%, whereas 5 cultivars (Chenggu 32, Cipressino, Nociara, Nostrale di rigali, and Taggiasca) displayed higher heterozygous calls of more than 5.0%. The filtered SNPs among single cultivar are listed in Supplementary Table S3 .
Genetic Structure and Phylogenetic Analysis
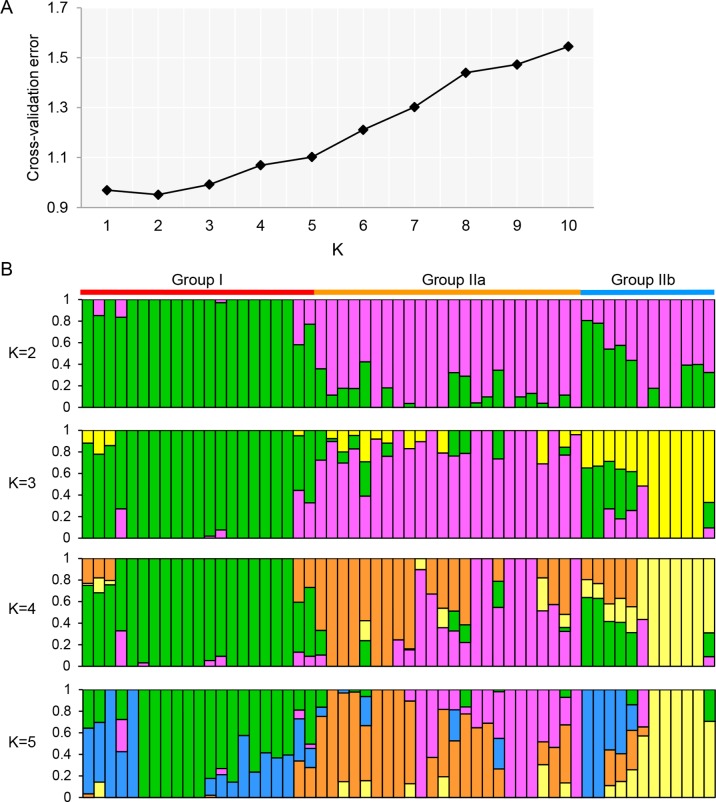
Genetic structure and phylogenetic analysis were further performed to gain an insight into the genetic diversity of olive cultivars. The 73,482 SNPs of high-quality data were used to investigate the population structure among 57 olive cultivars. Using admixture 1.3, the cross-validation errors were examined under the models with K = 1–10. As suggested, a good value of K will exhibit the lowest cross-validation error compared to other K values (Alexander et al., 2009). Here, the minimum value of the cross-validation errors was 0.95 when K = 2 and the values continuously increased with K from 3 to 10 ( Figure 2A ). To classify groups, we considered a genotype unequivocally assigned to a group when its admixture coefficient was >80% (Q > 0.8) as previously described (Diez et al., 2015). The cultivars were classified into two groups at K = 2, except for 14 cultivars that could not be unequivocally assigned to any of the two groups ( Figure 2B ). The first group contained 20 cultivars from six countries (Italy, Spain, Greece, China, Azerbaijan, and Algeria), and the second group contained 23 cultivars from four countries (Italy, Spain, France, and Portugal). To further investigate the population structure, the analyses at K = 3–5 were also performed ( Figure 2B ). When K = 3, three groups were identified with 36 cultivars including a new group that consisted of 5 cultivars (Nostrale di rigali, Taggiasca, Frantoio, I-79, and Ascolana tenera from Italy). The new groups were also identified at K = 4 and 5. However, just 33 and 28 cultivars could be unequivocally assigned to groups, respectively.
Figure 2.
Population structure analyses of 57 olive cultivars based on the GBS-SNP genotyping. (A) Cross-validation plot for the number of population (K) values. The X-axis and Y-axis represented the different K values (K = 1–10) and cross-validation error. (B) Stacked bar plot for the K value = 2, 3, 4, and 5. The population structure analysis was performed by admixture 1.3 (Alexander et al., 2009). The X-axis represented the individual cultivar with K colored segments and the Y-axis represented the ancestry qi proportion, correspondingly.
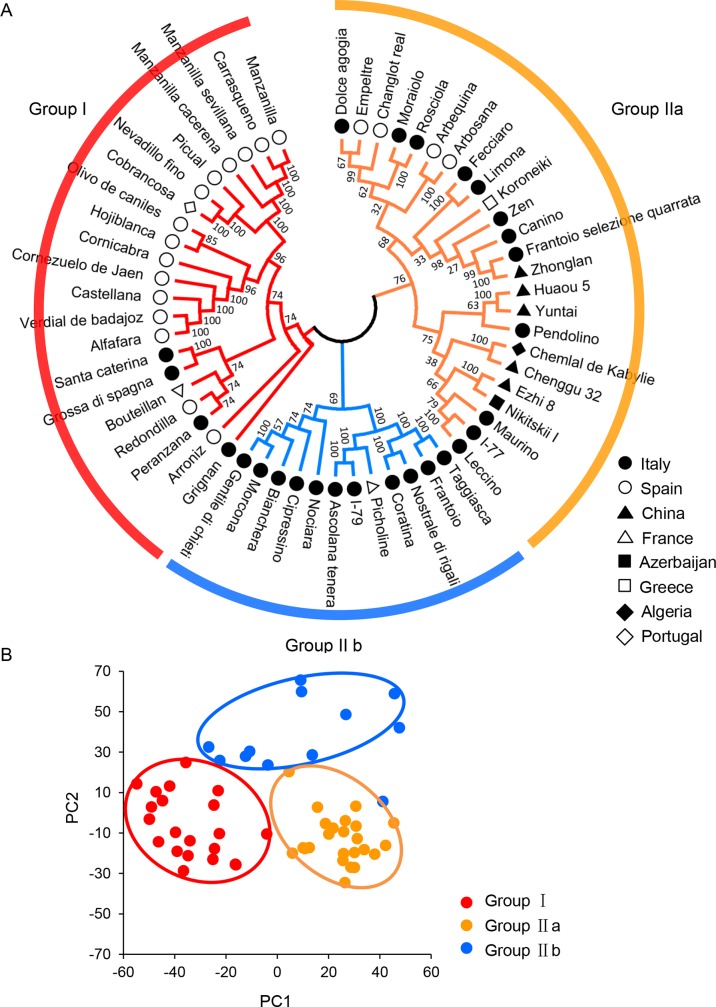
With phylogenetic analysis, neighbor-joining tree using MEGA X software also clearly clustered the 57 cultivars into two main groups (Group I and Group II), which was consistent with the model-based population structure at K = 2 ( Figure 2B and Figure 3A ). Group I consisted of 21 cultivars (36.8%) from four countries, i.e., Spain (n = 15), Italy (n = 4), France (n = 1), and Portugal (n = 1), while Group II included the remaining 36 cultivars (63.2%). Group II could be further classified into two subgroups named Group IIa and Group IIb. In Group IIa, there were 24 cultivars (42.1%) from six countries, including 12 cultivars from Italy, 4 from Spain, 5 from China, 1 from Azerbaijan, 1 from Greece, and 1 from Algeria. In Group IIb, there were totally 12 cultivars (21.1%) from only two countries including 11 cultivars from Italy and 1 from France. Moreover, the distribution of the two dimensions generated by PCA of all 57 cultivars agreed well with the classification of all the cultivars into two clusters ( Figure 3B ), which was also consistent with the model-based population structure and phylogenetic analysis. The relationships among the 57 olive cultivars were further analyzed with the IBS allele-sharing values. The bin for all the cultivars filled between 0.59 and 0.88, with the majority (1,515, 94.7%) distributed in 0.65–0.75 ( Supplementary Figure S2A ). The 10 pairs with allele-sharing values > 0.85 could be seen in Supplementary Table S4 . Besides, the multidimensional scaling (MDS) plot of genome-wide IBS pairwise distances also displayed a clear separation of two groups (Group I and Group II), while the cultivars in Group IIa and Group IIb were interlaced partially ( Supplementary Figure S2B ).
Figure 3.
Phylogenetic analyses of olive cultivars. (A) Phylogenetic analysis of olive cultivars. Phylogenetic tree was constructed using MEGA X software (www.megasoftware.net) with the neighbor-joining method (Kumar et al., 2018). The parameters were as follows: Test of phylogeny, bootstrap method; no. of bootstrap replications, 1,000; Model/method, maximum composite likelihood; Substitutions to include, d: Transitions + Transversions; Gaps/missing data treatment, pairwise deletion. (B) Principal component analysis (PCA) of olive cultivars using TASSEL 5.0 software (https://tassel.bitbucket.io/).
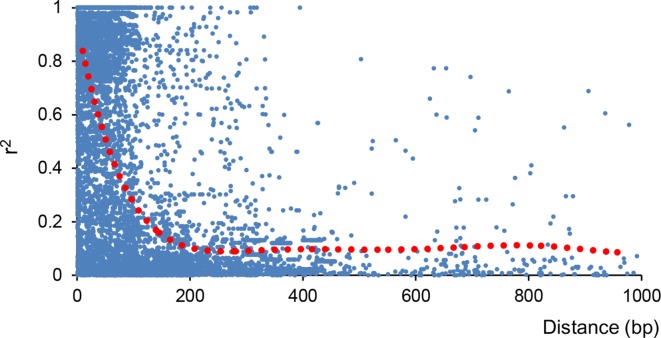
Moreover, linkage disequilibrium (LD) decreased with physical distance among SNPs in all 57 olive cultivars. For more than 5,000 scaffolds that differ in size, LD decay was estimated considering only those SNP markers identified in the 30 longest scaffolds as the method described by D’Agostino et al. (2018). The extent of LD was measured as the scaffold distance when LD decreased to half of its maximum value. We also found a rapid decay of LD ( Figure 4 ), with average r 2 dropping from 0.74 to 0.41 (80 bp) and 0.33 (90 bp), which was slightly higher than that in a previous report (D’Agostino et al., 2018).
Figure 4.
Decay of linkage disequilibrium (LD) in all 57 olive cultivars. Decay of LD indicated by correlation coefficient (r2) was calculated using PLINK V1.90 (Purcell et al., 2007).
Genetic Diversity Analysis
To explore the genetic differentiation among populations, we used VCFtools (https://vcftools.github.io/) to conduct genetic diversity analysis ( Figure 5 ). The average nucleotide diversity (π) of the total sites was 0.318 for the whole set of olive cultivars, which was bigger than the π values specific for Group I (0.26) and Group II (0.32). Moreover, both of the cultivars in Group IIa and Group IIb shared the π values 0.30 ( Figure 5A ). The fixation index (F ST) for Group I–Group II, Group I–Group IIa, Group I–Group IIb, and Group IIa–Group IIb were 0.08, 0.09, 0.10, and 0.06, respectively ( Figure 5B ), indicating that the olive cultivars here displayed moderate genetic differentiation. While the π values of cultivars from Italy and Spain were 0.32 and 0.28, the F ST of cultivars between Italy and Spain was 0.046 ( Figure 5 ), which suggests that the cultivars between Italy and Spain showed a slight genetic differentiation and the cultivars from Italy had more variability.
Figure 5.
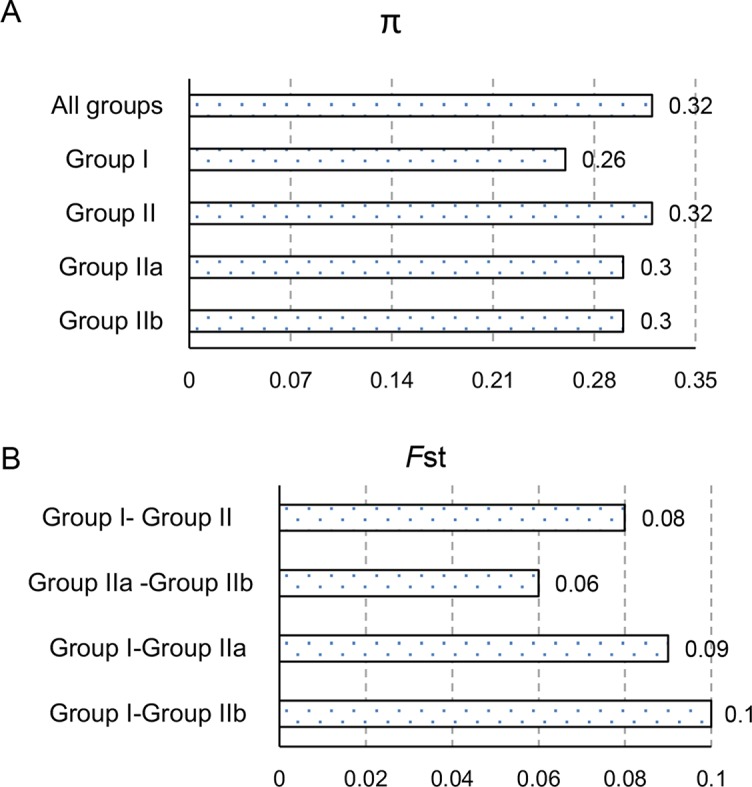
Genetic diversity analyses of different olive groups. The levels of nucleotide diversity, π (A) and fixation index, F ST (B) between groups were calculated using VCFtools (https://vcftools.github.io/).
Discussion
GBS-SNP Exploration in Olive
Molecular markers such as RAPD, AFLP, and SSR have been widely used in germplasm characterizations and genetic diversity analysis in plants including olive in the past two decades (Sebastiani and Busconi, 2017). In recent years, the next-generation sequencing and transcriptomic analysis become the main approaches to study the genetic characteristics of plants (Metzker, 2010; Bolger et al., 2014), due to their high efficiency of genome-wide sequencing. GBS technology, as one of the easily handled and powerful new methods, has been widely used in modern molecular breeding. To our knowledge, only a few publications related to sequencing on several olive genotypes such as O. europaea cv. Leccino, O. europaea cv. Farga, and O. europaea var. sylvestris are available (Barghini et al., 2014; Cruz et al., 2016; Unver et al., 2017), and the excavation of polymorphic loci at the whole genome only were done by İpek et al. (2016) and D’Agostino et al. (2018) with the GBS technology. In the present study, we analyzed the genetic variability of 57 olive cultivars by GBS-SNPs. A total of 352.93 million clean reads with an average data size of 588.63 Mb were generated, and as many as 73,482 high-quality SNPs were obtained subsequently after mapping to olive reference genome O. europaea cv. Farga and filtering. Compared with the GBS-SNP results in previous studies (İpek et al., 2016; D’Agostino et al., 2018), this study collected various cultivars with different genetic backgrounds and captured more SNPs, with the average density being higher. The genetic diversity analysis in olive germplasm is usually performed using SSR/AFLP markers and the identification of SNPs at the whole genome level is also lacking. The results will enrich the availability of genome information of olive and could be further used for genetic diversity study and modern molecular breeding.
Genetic Diversity Among Olive Cultivars
In the previous studies, olive cultivars were classified as three gene pools including east, central, and west Mediterranean regions based on their geographic origins (Sarri et al., 2006; Belaj et al., 2012). The geographic origins had a certain correlation with the genetic differentiation (Belaj et al., 2012). The present study using model-based population analysis classified 57 cultivars into two groups (Group I and Group II), and Group II was further divided into two subgroups (Group IIa and Group IIb). Both neighbor-joining tree ( Figure 3A ) and PCA ( Figure 3B ) showed consistent results and support each other, but did not support the two groups related to geographic origins. Interestingly, based on the standard of olive fruit sizes (weight) conducted by the OLEA database (http://www.oleadb.it/), the different olive fruit sizes (weight) with five levels were observed among different groups ( Table 1 ) as L (low: less than 2.0 g), M (medium: 2.0 to 4.0 g), MH (medium-high: 4.0 to 6.0 g), H (high: 6.0 to 8.0 g), and VH (very high: greater than 8.0 g). In Group I, all cultivars had medium to very high fruits, which were significantly higher (heavier) in Group I than in Group II (IIa and IIb) (P = 2.6 × 10−5; Student’s t test). In Group IIa, all cultivars had low to medium–large fruits, while in Group IIb, all cultivars except Ascolana tenera from Italy had medium fruits. The fruit size in Group IIb was bigger than that in Group IIa, with P = 0.045 (Student’s t test). The three groups had a significant association with fruit size, which was similar to the results reported by Biton et al. (2015). A set of 145,974 SNPs were developed using next-generation sequencing technology and subsequently used a subset of 138 SNPs to analyze 119 cultivars maintained in the Israeli germplasm collection (Biton et al., 2015). Comprehensive analysis showed that olive cultivars were grouped more in terms of their functions (oil, table or double purpose) than in terms of their geographic origin (Biton et al., 2015).
LD analysis in all 57 olive cultivars indicated that olive genomes had short LD distance and rapid LD decays ( Figure 4 ). The LD decay distance (∼85 bp) was much shorter than that reported in pear (211 bp; Wu et al., 2018) and apple (161 bp; Duan et al., 2017). The F ST between each group pairs ( Figure 5 ) had a similar result with previous reports by D’Agostino et al. (2018) and Rienzo et al. (2018), but it was relatively lower than that in other tree plants, such as pear and apple (Duan et al., 2017; Wu et al., 2018). The above results implied a relatively weak selection and a moderate differentiation during the genetic domestication of olive, which might be due to the vegetative propagation approach and the low self-fruitful rate (Xu, 2001). Furthermore, previous studies showed that there was relative differentiation among Spanish and Italian cultivars and a clear distinction between Spanish cultivars and those from Greece and Turkey (Besnard et al., 2001; Owen et al., 2005). The cultivars in this study from Italy and Spain were distributed in both Group I and II. However, there was a clear distinction between the cultivars from the two countries within both groups; for example, none of 12 cultivars in Group IIb was from Spain. Combined with cluster analysis ( Figure 3 ) and nucleotide diversity analysis, it could be inferred that compared to Spain cultivars, the Italian cultivars may have more genetic variability, which was consistent with the previous results obtained by D’Agostino et al. (2018).
As a new olive production area, most of the cultivars widely cultivated currently in China were introduced from Mediterranean countries, and some were selected and bred by Chinese olive breeding programs from cultivars such as Coligno, Ascolano Tenera, Nikitskii I, Nikitskii II, Leccino, and Kalinio (Xu, 2001; Li, 2010). Among the five cultivars developed in China in this study, Chenggu 32, Zhonglan, Yuntai, Ezhi 8, and Huaou 5, except Huaou 5 with unknown parents, the female parents of the other four cultivars were all from the central Mediterranean countries (Xu, 2001). The results of cluster analysis indicated that the five cultivars were all in Group IIa with a close genetic relationship with the cultivars from the central Mediterranean region. Previous studies showed that there was a relatively narrow genetic basis of the Chinese-bred cultivars (Xu, 2001; Li, 2010; Zhan et al., 2015). Most olive cultivars introduced in China came from the central and western regions of the Mediterranean during the 1960s to 1970s, and the germplasm from the eastern region was less (Xu, 2001). Therefore, it is necessary to introduce olive germplasm from the eastern regions of the Mediterranean in the future in order to broaden the genetic basis of the Chinese olive germplasm.
Effectiveness of GBS for Characterizing Genetic Relationships Among Olive Cultivars
Among the cultivars analyzed in this study, Frantoio and Taggiasca, Picual and Nevadillo fino, and three Manzanilla cultivars (Manzanilla, Manzanilla cacerena, and Manzanilla sevillana) and Carrasquena were generally considered to be synonymous, which were very similar in morphological and genetic characteristics (Bartolini et al., 2005; Belaj et al., 2012). Cluster analysis showed that these cultivars did have high genetic homogeneity and clustered pairwisely or together, respectively ( Figure 3 ), with higher IBS values > 0.85 ( Supplementary Table S4 ). Interestingly, the cultivar Manzanilla cacerena with the other three cultivars shared IBS values of about 0.76–0.77, which were relatively low than those found in other pairs. Ezhi8 was an excellent cultivar selected from a hybrid population of free pollination. We do not know exactly its parents, but it is commonly believed that it was derived from Nikitskii I, a cultivar originated in Azerbaijan, according to their similarities in morphological traits. In this study, the two cultivars Ezhi8 and Nikitskii I were clustered together to show their close kinship with the IBS value = 0.84, confirming the general knowledge about their genetic relationships. A similar result was also found in cultivars Huaou 5 and Yuntai, which shared the highest IBS value in this study (0.88) ( Supplementary Table S4 ). The two cultivars with similar morphological traits such as tree shape, leaf shape, leaf size, fruit shape, and fruit size were clustered closely as well. In summary, GBS-SNP loci here will correct effectively the relationship among different cultivars and further benefit the development of core germplasm loci.
Author Contributions
SZ and AS conceived the study and EN carried out the analysis and wrote the manuscript. SZ, AS, and BM revised the manuscript. All authors approved the final manuscript.
Funding
This study was financially supported by the International Science & Technology Cooperation Program of China (No. 2013DFG32780).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00755/full#supplementary-material
Bioinformatics analysis pipeline for GBS-SNP. The raw reads were subjected to quality control and split into clean reads. Using SOAP2 (Hurgobin, 2016) and SOAPsnp (Li et al., 2008), the clean reads were aligned to the olive reference sequences O. europaea cv. Farga (Cruz et al., 2016) and further to call SNP respectively. The main parameters were shown in Supplementary Table S2 .
Multi-dimensional scaling (MDS) plot of identity-by-state (IBS) distance matrix. Pair-wise IBS allele-sharing using the 73,482 SNPs among 57 olive cultivars were calculated using PLINK96 V1.90 and visualized with the MDS plot (Purcell et al., 2007).
The main parameters for sequences alignment and SNP calling.
Summary of sequencing data of 57 olive cultivars.
Statistics of GBS SNP genotyping.
Pairs of cultivars with identity-by-state (IBS) allele-sharing values > 0.85.
References
- Alexander D. H., Novembre J., Lange K. (2009). Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664. 10.1101/gr.094052.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barghini E., Natali L., Cossu R. M., Giordani T., Pindo M., Cattonaro F., et al. (2014). The peculiar landscape of repetitive sequences in the olive (Olea europaea L.) genome. Genome Biol. Evol. 6, 776–791. 10.1093/gbe/evu058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini G., Prevost G., Messeri C., Carignani C. (2005). Olive germplasm: cultivars and world-wide collections. FAO/Plant Production and Protection. Available at: www.oleadb.it.
- Belaj A., Dominguez-García M. C., Atienza S. G., Urdíroz N. M., De la Rosa R., Satovic Z., et al. (2012). Developing a core collection of olive (Olea europaea L.)based on molecular markers (DARTs, SSRs, SNPs) and agronomic traits. Tree Genet. Genomes 8, 365–378. 10.1007/s11295-011-0447-6 [DOI] [Google Scholar]
- Besnard G., Breton C., Baradat P., Khadari B., Bervillé A. (2001). Cultivar identification in olive based on RAPD markers. J. Am. Soc. Hort. Sci. 126, 668–675. 10.21273/JASHS.126.6.668 [DOI] [Google Scholar]
- Biton I., Doron-Faigenboim A., Jamwal M., Mani Y., Eshed R., Rosen A., et al. (2015). Development of a large set of SNP markers for assessing phylogenetic relationships between the olive cultivars composing the Israeli olive germplasm collection. Mol. Breeding 35, 107. 10.1007/s11032-015-0304-7 [DOI] [Google Scholar]
- Bolger M. E., Weisshaar B., Scholz U., Stein N., Usadel B., Mayer K. F. (2014). Plant genome sequencing—Applications for crop improvement. Curr. Opin. Biotechnol. 26, 31–37. 10.1016/j.copbio.2013.08.019 [DOI] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. (1980). Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 32, 314–331. 10.1016/0165-1161(81)90274-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz F., Julca I., Gómez-Garrido J., Loska D., Marcet-Houben M., Cano E., et al. (2016). Genome sequence of the olive tree, Olea europaea . Gigascience 5, 29. 10.1186/s13742-016-0134-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino N., Taranto F., Camposeo S., Mangini G., Fanelli V., Gadaleta S., et al. (2018). GBS-derived SNP catalogue unveiled wide genetic variability and geographical relationships of Italian olive cultivars. Sci. Rep. 8, 15877. 10.1038/s41598-018-34207-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez C. M., Trujillo I., Martinez-Urdiroz N., Barranco D., Rallo L., Marfil P., et al. (2015). Olive domestication and diversification in the Mediterranean Basin. New Phytol. 206, 436–447. 10.1111/nph.13181 [DOI] [PubMed] [Google Scholar]
- Donato M. D., Peters S. O., Mitchell S. E., Hussain T., Imumorin I. G. (2013). Genotyping-by-sequencing (GBS): a novel, efficient and cost-effective genotyping method for cattle using next-generation sequencing. PLoS One 8, e62137. 10.1371/journal.pone.0062137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan N., Bai Y., Sun H., Wang N., Ma Y., Li M., et al. (2017). Genome re-sequencing reveals the history of apple and supports a two-stage model for fruit enlargement. Nat. Commun. 8, 249. 10.1038/s41467-017-00336-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshire R. J., Glaubitz J. C., Sun Q., Poland J. A., Kawamoto K., Buckler E. S., et al. (2011). A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS One 6, e19379. 10.1371/journal.pone.0019379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grati-Kamoun N., Lamy-Mahmoud F., Rebaï A., Gargouri A., Panaud O., Saar A. (2006). Genetic diversity of Tunisian olive tree (Olea europaea L.) cultivars assessed by AFLP markers. Genet. Resour. Crop. Evol. 53, 265–275. 10.1007/s10722-004-6130-0 [DOI] [Google Scholar]
- Guo X., Cericola F., Fè D., Pedersen M. G., Lenk I., Jensen C. S., et al. (2018). Genomic prediction in tetraploid ryegrass using allele frequencies based on genotyping by sequencing. Front. Plant Sci. 9, 1165. 10.3389/fpls.2018.01165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Zhao X., Laroche A., Lu Z. X., Liu H., Li Z. (2014). Genotyping-by-sequencing (GBS), an ultimate marker-assisted selection (MAS) tool to accelerate plant breeding. Front. Plant Sci. 5, 484. 10.3389/fpls.2014.00484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J., Kadereit J. W., Vargas P. (2000). The colonization history of Olea europaea L. in Macaronesia based on internal transcribed spacer 1 (ITS-1) sequences, randomly amplified polymorphic DNAs (RAPD), and intersimple sequence repeats (ISSR). Mol. Ecol. 9, 857–868. 10.1046/j.1365-294x.2000.00942.x [DOI] [PubMed] [Google Scholar]
- Holsinger K. E., Weir B. S. (2009). Genetics in geographically structured populations: defining, estimating and interpreting F ST. Nat. Rev. Genet. 10, 639–650. 10.1038/nrg2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurgobin B. (2016). Short read alignment using SOAP2. Methods Mol. Biol. 1374, 241–252. 10.1007/978-1-4939-3167-5_13 [DOI] [PubMed] [Google Scholar]
- İpek A., Yılmaz K., Sıkıcı P., Tangu N. A., Öz A. T., Bayraktar M., et al. (2016). SNP discovery by GBS in olive and the construction of a high-density genetic linkage map. Biochem. Genet. 54, 313–325. 10.1007/s10528-016-9721-5 [DOI] [PubMed] [Google Scholar]
- Isk N., Doğanlar S., Frary A. (2011). Genetic diversity of Turkish olive varieties assessed by simple sequence repeat and sequence-related amplified polymorphism markers. Crop Sci. 51, 1646–1654. 10.2135/cropsci2010.11.0625 [DOI] [Google Scholar]
- Kaya H. B., Cetin O., Kaya H., Sahin M., Sefer F., Kahraman A., et al. (2013). SNP discovery by illumina-based transcriptome sequencing of the olive and the genetic characterization of Turkish olive genotypes revealed by AFLP, SSR and SNP markers. PLoS One 8, e73674. 10.1371/journal.pone.0073674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniewski D., Van Campo E., Boiy T., Terral J. F., Khadari B., Besnard G. (2012). Primary domestication and early uses of the emblematic olive tree: palaeobotanical, historical and molecular evidences from the middle east. Biol. Rev. Camb. Philos. Soc. 87, 885–899. 10.1111/j.1469-185X.2012.00229.x [DOI] [PubMed] [Google Scholar]
- Khaleghi E., Sorkheh K., Chaleshtori M. H., Ercisli S. (2017). Elucidate genetic diversity and population structure of Olea europaea L. germplasm in Iran using AFLP and IRAP molecular markers. 3 Biotech. 7, 71. 10.1007/s13205-017-0669-x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. (2010). Retrospect and prospect to introduce and develop olive production in China. Beijing: China Forestry Press. [Google Scholar]
- Li R., Li Y., Kristiansen K., Wang J. (2008). SOAP: Short oligonucleotide alignment program. Bioinformatics 24, 713–714. 10.1093/bioinformatics/btn025 [DOI] [PubMed] [Google Scholar]
- Lin M., Cai S., Wang S., Liu S., Zhang G., Bai G. (2015). Genotyping-by-sequencing (GBS) identified SNP tightly linked to QTL for pre-harvest sprouting resistance. Theor. Appl. Genet. 128, 1385–1395. 10.1007/s00122-015-2513-1 [DOI] [PubMed] [Google Scholar]
- Metzker M. L. (2010). Sequencing technologies—The next generation. Nat. Rev. Genet. 11, 31–46. 10.1038/nrg2626 [DOI] [PubMed] [Google Scholar]
- Mousavi S., Mariotti R., Regni L., Nasini L., Bufacchi M., Pandolfi S., et al. (2017). The first molecular identification of an olive collection applying standard simple sequence repeats and novel expressed sequence tag markers. Front. Plant Sci. 8, 1283. 10.3389/fpls.2017.01283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. (1980). Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8, 4321–4325. 10.1093/nar/8.19.4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Li W. H. (1979). Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 76, 5269–5273. 10.1073/pnas.76.10.5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmakayala P., Levi A., Abburi L., Abburi V. L., Tomason Y. R., Saminathan T., et al. (2014). Single nucleotide polymorphisms generated by genotyping by sequencing to characterize genome-wide diversity, linkage disequilibrium, and selective sweeps in cultivated watermelon. BMC Genomics 15, 767. 10.1186/1471-2164-15-767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouazzani N., Lumaret R., Villemur P., Giusto F. D. (1993). Leaf allozyme variation in cultivated and wild olive trees (Olea europaea L.) J. Hered. 84, 34–42. 10.1093/oxfordjournals.jhered.a111274 [DOI] [PubMed] [Google Scholar]
- Owen C. A., Bita E. C., Banilas G., Hajjar S. E., Sellianakis V., Aksoy U., et al. (2005). AFLP reveals structural details of genetic diversity within cultivated olive germplasm from the Eastern Mediterranean. Theor. Appl. Genet. 110, 1169–1176. 10.1007/s00122-004-1861-z [DOI] [PubMed] [Google Scholar]
- Pérez-Jiménez F., Ruano J., Perez-Martinez P., Lopez-Segura F., Lopez-Miranda J. (2007). The influence of olive oil on human health: not a question of fat alone. Mol. Nutr. Food Res. 51, 1199–1208. 10.1002/mnfr.200600273 [DOI] [PubMed] [Google Scholar]
- Poland J. A., Rife T. W. (2012). Genotyping-by-Sequencing for plant breeding and genetics. Plant Genome 5, 92–102. 10.3835/plantgenome2012.05.0005 [DOI] [Google Scholar]
- Pontikis C. A., Loukas M., Kousounis G. (1980). The use of biochemical markers to distinguish olive cultivars. J. Hortic. Sci. Biotech. 55, 333–343. 10.1080/00221589.1980.11514942 [DOI] [Google Scholar]
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M. A., Bender D., et al. (2007). PLINK: a toolset for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallo P., Dorado G., Martin A. (2000). Development of simple sequence repeats (SSRs) in olive tree (Olea europaea L.) Theor. Appl. Genet. 101, 984–989. 10.1007/s00122-003-1332-y [DOI] [Google Scholar]
- Reale S., Doveri S., Díaz A., Angiolillo A., Lucentini L., Pilla F., et al. (2006). SNP-based markers or discriminating olive (Olea europaea L.) cultivars. Genome 49, 1193–1205. 10.1139/g06-068 [DOI] [PubMed] [Google Scholar]
- Rienzo V. D., Sion S., Taranto F., D’Agostino N., Montemurro C., Fanelli V., et al. (2018). Genetic flow among olive populations within the Mediterranean basin. Peer J. 6, e5260. 10.7717/peerj.5260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarri V., Baldoni L., Porceddu A., Cultrera N. G., Contento A., Frediani M., et al. (2006) Microsatellite markers are powerful tools for discriminating among olive cultivars and assigning them to geographically defined populations. Genome 49, 1606–1615. 10.1139/g06-126 [DOI] [PubMed] [Google Scholar]
- Sebastiani L., Busconi M. (2017). Recent developments in olive (Olea europaea L.) genetics and genomics: applications in taxonomy, varietal identification, traceability and breeding. Plant Cell Rep. 36, 1345–1360. 10.1007/s00299-017-2145-9 [DOI] [PubMed] [Google Scholar]
- Shi A., Qin J., Mou B., Correll J., Weng Y., Brenner D., et al. (2017). Genetic diversity and population structure analysis of spinach by single-nucleotide polymorphisms identified through genotyping-by-sequencing. PLoS One 12, e0188745. 10.1371/journal.pone.0188745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkamaneh D., Laroche J., Belzile F. (2016). Genome-Wide SNP calling from genotyping by sequencing (GBS) data: a comparison of seven pipelines and two sequencing technologies. PLoS One 11, e0161333. 10.1371/journal.pone.0161333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unver T., Wu Z., Sterck L., Turktas M., Lohaus R., Li Z., et al. (2017). Genome of wild olive and the evolution of oil biosynthesis. Proc. Natl. Acad. Sci. U.S.A 114, E9413–E9422. 10.1073/pnas.1708621114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. (1978). Evolution and the genetics of populations. Chicago: University of Chicago Press. [Google Scholar]
- Wu J., Wang Y., Xu J., Korban S. S., Fei Z., Tao S., et al. (2018). Diversification and independent domestication of Asian and European pears. Genome Biol. 19, 77. 10.1186/s13059-018-1452-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W. (2001). Utilization of olive germplasm in China. Changchun: Changchun Press. [Google Scholar]
- Zhan M. M., Cheng Z. Z., Su G. C., Wang A. Y., Chen H. P., Shan Z., et al. (2015). Genetic relationships analysis of olive cultivars grown in China. Genet. Mol. Res. 14, 5958–5969. 10.4238/2015.June.1.13 [DOI] [PubMed] [Google Scholar]
- Zohary D., Hopf M. (1994). Domestication of plants in the Old World. 2nd edn. Oxford: Clarendon Press. [Google Scholar]
- Zohary D., Spiegel-Roy P. (1975). Beginnings of fruit growing in the Old World. Science 187, 319–327. 10.1126/science.187.4174.319 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bioinformatics analysis pipeline for GBS-SNP. The raw reads were subjected to quality control and split into clean reads. Using SOAP2 (Hurgobin, 2016) and SOAPsnp (Li et al., 2008), the clean reads were aligned to the olive reference sequences O. europaea cv. Farga (Cruz et al., 2016) and further to call SNP respectively. The main parameters were shown in Supplementary Table S2 .
Multi-dimensional scaling (MDS) plot of identity-by-state (IBS) distance matrix. Pair-wise IBS allele-sharing using the 73,482 SNPs among 57 olive cultivars were calculated using PLINK96 V1.90 and visualized with the MDS plot (Purcell et al., 2007).
The main parameters for sequences alignment and SNP calling.
Summary of sequencing data of 57 olive cultivars.
Statistics of GBS SNP genotyping.
Pairs of cultivars with identity-by-state (IBS) allele-sharing values > 0.85.