Abstract
Nonbacterial thrombotic endocarditis (NBTE) associated with malignancy is rare; its infrequency and similarity to other diagnoses make it a significant diagnostic challenge. A 63-year-old woman on rivaroxaban for prior deep vein thrombosis presented with left upper extremity weakness and left facial droop with imaging demonstrating multiple strokes. Echocardiograms revealed mitral and aortic valve vegetations. The patient was switched to apixaban and started on vancomycin and ceftriaxone for presumed culture-negative endocarditis. Despite continuing apixaban, her hospital course was complicated by new acute embolic infarcts. Workup confirmed non-mucinous metastatic biliary adenocarcinoma. The patient was placed on a heparin drip then switched to low molecular weight heparin without further embolic events and was discharged to a rehabilitation facility in stable condition with plans for chemotherapy as an outpatient. These clinical, imaging, and histologic findings were consistent with a rare case of NBTE associated with primary non-mucinous gallbladder malignancy complicated by recurrent strokes in which direct oral anticoagulants did not provide adequate anticoagulation.
KEY WORDS: cancer, stroke, thromboembolism, clinical vignette
CASE
A 63-year-old woman presented to the emergency department with 2 hours of left arm weakness. Her past medical history was notable for hypertension, hyperlipidemia, and sigmoid colon adenocarcinoma treated with resection and adjuvant chemotherapy 3 years prior to her current presentation. She was recently diagnosed with a left lower extremity deep vein thrombosis and pulmonary embolism 4 months prior to this presentation for which she was treated with rivaroxaban. In addition to left arm weakness, physical exam revealed flattening of the left nasolabial fold and left arm pronator drift. Brain magnetic resonance imaging showed multiple acute infarcts in the right frontal lobe, parietal lobe, and basal ganglia and left frontal and parietal lobe acute punctate infarcts. Computed tomography angiography (CTA) of the head and neck demonstrated a filling defect at the right internal carotid artery and anterior and middle cerebral arteries. In combination, these clinical and imaging findings were suspicious for a thromboembolic cerebrovascular accident. Tissue plasminogen activator (tPA) was not administered due to the patient’s anticoagulation with rivaroxaban, and the patient was admitted to the hospital.
During her hospitalization, the patient continued to undergo workup for her cerebrovascular accident. Beyond her notable neurological findings, her exam was otherwise unremarkable; specifically, the patient was afebrile, had no heart murmur, and had no skin lesions. Initial labs were notable for mild leukocytosis. Transthoracic and transesophageal echocardiogram revealed small echodensities on the mitral and aortic valves, concerning for endocarditis. Three blood cultures were obtained, and the patient was started on vancomycin and ceftriaxone for empiric management of infective endocarditis. Valvular surgery was deferred in the setting of the patient’s acute stroke. Blood cultures revealed no growth. The patient was discharged to a rehabilitation facility with a peripherally inserted central catheter for continuation of empiric vancomycin and ceftriaxone for an extended course, and her rivaroxaban was switched to apixaban without a documented reason.
Two weeks later, the patient presented to the hospital from the rehabilitation facility with fever and rash. She was found to have a widespread morbilliform desquamating rash without mucosal involvement. She demonstrated persistent left hemiparesis without any stigmata of infective endocarditis. The patient was transferred to another hospital, where she was determined to have a drug reaction with eosinophilia and systemic symptoms (DRESS) from vancomycin and ceftriaxone. She was started on systemic steroids.
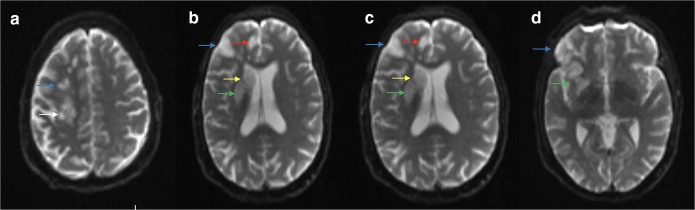
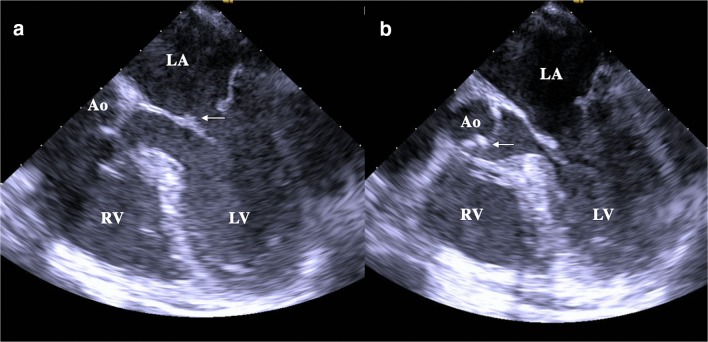
One day after admission, the patient developed lethargy and confusion and was found to have left-sided neglect and dysarthria in addition to her preexisting left hemiparesis. Magnetic resonance imaging (MRI) of her brain demonstrated acute infarcts involving the right frontal lobe, right parietal lobe, insular cortex, basal ganglia, corona radiata, and centrum semiovale (Fig. 1a-d). A transesophageal echocardiogram showed vegetations on the atrial side of the mitral valve and ventricular side of the aortic valve (Fig. 2a, b). Her anticoagulation was switched from apixaban to a heparin infusion.
Figure 1.
Repeat brain magnetic resonance imaging (MRI) after the patient’s recurrent stroke event. Diffusion weighted imaging, axial view. 1a: Blue arrow indicates area of restricted diffusion in frontal lobe. White arrow indicates area of restricted diffusion in parietal lobe. Other smaller areas of restricted diffusion are also present in frontal lobe. 1b: White arrow indicates area of restricted diffusion in parietal lobe. 1c: Blue arrow indicates area of restricted diffusion in frontal cortex. Red arrow indicates area of restricted diffusion in insular cortex. Green arrow indicates area of restricted diffusion in corona radiata. Yellow arrow indicates area of restricted diffusion near basal ganglia. 1d: Blue arrow indicates area of restricted diffusion in frontal cortex. Green arrow indicates area of restricted diffusion in corona radiata.
Figure 2.
Transesophageal echocardiogram. 2a: White arrow indicates small vegetation attached to the atrial aspect of the posterior mitral valve leaflet. 2b: White arrow indicates small vegetation on left ventricular side of the aortic valve. LA, left atrium; LV, left ventricle; RV, right ventricle; Ao, aorta.
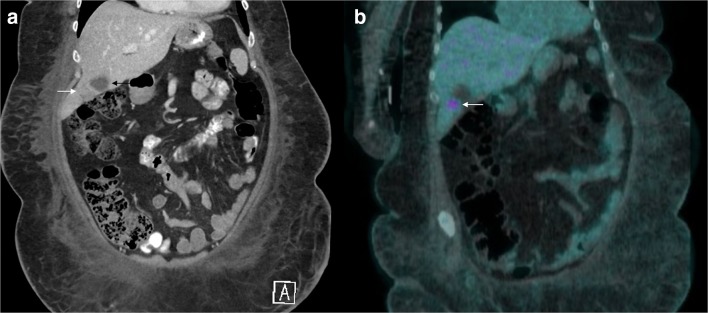
A broad workup was initiated for causes of culture-negative and nonbacterial thrombotic endocarditis (NBTE). All autoimmune labs were negative, including antinuclear antibody, antiphospholipid antibody screen, anti-neutrophilic cytoplasmic antibody, and rheumatoid factor. Infectious workup was also negative, including serum galactomannan, serum beta-d-glucan, and serologies for Bartonella, Brucella, Coxiella, Legionella, and Strongyloides. Two additional sets of blood cultures were drawn, which included extended culture incubation, and remained without evidence of bacterial growth. Labs to assess disseminated intravascular coagulation were unremarkable. CA 19-9 was elevated at 164 (normal < 35 units/mL), indicating potential pancreas, liver, or gallbladder involvement. Computed tomography (CT) of the abdomen and pelvis revealed an enlarged porta hepatis lymph node and abnormal focal circumferential gallbladder wall thickening at the fundus (Fig. 3a) and a positron emission tomography (PET)/computed tomography scan showed 18 2-fluoro-2-deoxy-D-glucose (FDG) uptake near the liver (Fig. 3b), indicating metabolically active tissue concerning for malignancy. An endoscopic ultrasound with biopsy of the porta hepatis lymph node was performed. Histopathology of the biopsy was consistent with a non-mucinous metastatic biliary adenocarcinoma.
Figure 3.
Abdominal Imaging. 3a: Computed tomography scan of the abdomen and pelvis with oral and intravenous Omnipaque contrast, coronal view. Black arrow indicates gallbladder with focal circumferential wall thickening at the fundus (white arrow) measuring up to 2.2 cm on the coronal plane. 3b: Positron emission tomography/computed tomography scan with 18 2-fluoro-2-deoxy-D-glucose (FDG), coronal view. White arrow indicates notable FDG uptake near the liver.
The patient was diagnosed with nonbacterial thrombotic endocarditis (NBTE) from a new primary gallbladder adenocarcinoma with locally advanced involvement of the porta hepatis node. She was transitioned to subcutaneous injections of enoxaparin without further embolic events and was discharged to a rehabilitation facility in stable condition with plans for outpatient chemotherapy for her cancer.
DISCUSSION
NBTE refers to the development of sterile vegetations of fibrin and platelets on cardiac valves in the absence of bloodstream bacterial infection. The pathogenesis remains incompletely understood but may involve endothelial damage with subsequent exposure of subendothelial tissue to circulating platelets, immune complex deposition, hypoxia with resulting plasma tissue factor abnormalities, and hypercoagulability possibly due to hypo- and hyper-fibrogenemia, thrombocytopenia, decreased factors V, VIII, and XII and antithrombin III, and increased fibrinolysis.1 Unlike infective endocarditis, which may present with cardiac and systemic findings on physical exam as well as microorganism growth on cultures, NBTE presents with embolic stroke as its first manifestation in up to 50% of patients2 (Table 1). NBTE predominantly affects left-sided heart valves; mitral valves are affected more frequently than aortic valves, which individually are found more frequently than combined mitral and aortic valvular involvement.3, 4 Transesophageal echocardiography (TEE) is the preferred imaging modality to detect the vegetations associated with NBTE, as it has a higher sensitivity in detecting valvular lesions compared to transthoracic echocardiography.5 However, definitive diagnosis can only be made through histological examination of the cardiac valve, which reveals friable, white, and tan vegetations consisting of platelets and fibrin without microorganisms.1
Table 1.
Comparison of Nonbacterial Thrombotic Endocarditis (NBTE) and Infective Endocarditis
| NBTE | Infective endocarditis | |
|---|---|---|
| Pathophysiology | Mechanism: deposition of thrombi on previously undamaged heart valves in the absence of a bloodstream bacterial infection, likely with contribution of immune complex deposition, hypoxia resulting in increased tissue factor, pro-thrombotic states, and carcinomatosis |
Two main mechanisms: 1) predisposing valvular structural abnormalities resulting in adhesion of circulating microorganisms to the valvular surface with propagation as vegetation or systemic emboli 2) initially sterile vegetation composed of fibrin and platelets colonized by circulating microorganisms resulting in further inflammation affecting vegetations |
| Clinical symptoms | ||
| Fever | – | + |
| Cardiac murmur | − (rarely +) | +* |
| Systemic vasculitic phenomena (splinter hemorrhages, Roth spots, glomerulonephritis) | – | + |
| Embolization to major organs | + | + |
| Recurrent embolization | +* | ± |
| Labs | ||
| Leukocytosis | – | + |
| CRP | +/− | + |
| Positive blood cultures | – | +* |
| Valvular involvement | Mitral valve involvement most common followed by aortic valve and then both aortic and mitral valve involvement; rarely associated with valvular abscess and rupture | Auriculo-ventricular valves or aortic valve most common depending on etiology; commonly associated with valvular abscess and rupture |
| Vegetation findings | Often small but variable in size, minimal inflammatory response, composed primarily of platelet and fibrin, no microorganisms present | Variable size; pattern degree of inflammation depending on infecting organism but inflammatory response present, microorganisms present |
| Brain MRI findings | Multiple disseminated strokes with variable lesion size | Single lesion, territorial infarction, or multiple disseminated punctuate lesions |
| Therapeutic management | ||
| Anticoagulation | + | Only if other anticoagulation indications including mechanical valve or atrial fibrillation |
| Antibiotics | – | + |
+ = yes; − = no
CRP C-reactive protein, MRI magnetic resonance imaging, NBTE nonbacterial thrombotic endocarditis
*Indicates hallmark feature
NBTE is often associated with hypercoagulable states, including solid malignancies, hematologic malignancies such as lymphoma, disseminated intravascular coagulation, and autoimmune conditions such as primary antiphospholipid syndrome and systemic lupus erythematosus.1 Although less than 5% of patients with cancer are found to have NBTE on autopsy, undetected cancer is a major contributor to NBTE, with approximately 60% of NBTE patients found to have malignancy on autopsy.6 The incidence of NBTE as the initial sign of malignancy is unknown due to NBTE’s infrequency; similarly, the incidence of clinically apparent NBTE in patients with known cancer is difficult to determine. However, 20–30% of incident venous thromboembolic events (VTE) have been associated with underlying cancer7 and up to 10% of patients with cancer develop clinically apparent VTE,8 which may provide an upper bound to the estimate. Solid tumors commonly associated with NBTE include primary lung, pancreas, and ovarian adenocarcinoma.1, 3, 4, 9 Staging of cancer in malignancy-related NBTE is rarely performed, but NBTE may occur in early-stage cancer10 and advanced disease.11
This patient’s NBTE was a consequence of a primary biliary malignancy, a rare cause of NBTE, accounting for only 4–14% of malignancies found on autopsy of patients with NBTE.2 Among the biliary cancers, NBTE associated with primary gallbladder malignancy is even rarer, with only three case reports prior to our patient reporting an association between primary gallbladder cancer and NBTE11–13 (Table 2). In comparing the previous three cases and our patient, the clinical presentation for all four patients was similar; three patients presented with acute hemiparesis, and one patient presented with acute angina with subsequent development of fevers, partial blindness, lower extremity pitting edema, and ataxia. Brain imaging demonstrated evidence of stroke in all patients, with artery occlusion found in three cases. Our patient and previously reported patients had left-sided cardiac valvular involvement, two with both mitral and aortic valvular vegetations, one with vegetations only on the mitral valve, and one with vegetations only on the aortic valve. The associated malignancy was attributed to gallbladder cancer in all cases, although only one case reported a mucin-producing tumor on histopathology. IV heparin was started in three cases, including our patient. Three patients underwent therapy to address their primary malignancy, although gallbladder resection was not performed in one patient with extensive omental seeding found on laparoscopic surgery.
Table 2.
Comparison of Previous Case Reports of Gallbladder-Associated Nonbacterial Thrombotic Endocarditis and This Clinical Vignette
| Case 112 | Case 211 | Case 313 | Case 4 (this clinical vignette) | |
|---|---|---|---|---|
| Patient age and gender | 41-year-old woman | 68-year-old man | 62-year-old woman | 63-year-old woman |
| Presenting clinical symptoms | Acute left hemiparesis | Acute chest pain with development of fevers, bilateral pitting edema, visual field loss, confusion, ataxia | Acute left hemiparesis | Acute left hemiparesis |
| Initial exam findings | Left facial and upper extremity motor weakness | One nailbed splinter hemorrhage, leg pitting edema, bilateral inferior hemianopia, diastolic murmur | Left upper and lower limb motor weakness | Left nasolabial flattening and upper extremity motor weakness |
| Subsequent clinical findings | Seizures on hospital day 2 | Deterioration with obtundation, arterial emboli to the right lower limb, and cardiovascular collapse during hospitalization | Left hemispatial neglect and sensory change found on transfer to new hospital 1 week after presentation | Lethargy, confusion, left-sided neglect, dysarthria 2 weeks after initial presentation during hospitalization for DRESS |
| Notable lab findings | Not reported | Elevated CRP and ESR, normal lumbar puncture labs | Elevated CEA, elevated d-dimer; normal PT, PTT, fibrinogen, ATIII |
Initial: normal PT and INR After acute new onset stroke*: PT 24.3, INR 2.3, PTT 47.4, normal fibrinogen; elevated CA 19-9 |
| Brain imaging findings |
CT (initial): negative CTA (1 day after presentation): occlusion of the cortical branches of the right MCA CT (with clinical deterioration): new ischemic lesions in both hemispheres |
MRI (at initial presentation): restricted diffusion in white matter and cortical sulci with edema in right temporal region |
MRI (initial): negative MRI (1 week following initial presentation): multiple cerebral and cerebellar infarctions MRA (1 week after presentation): right ICA occlusion |
MRI (initial): multiple acute infarcts in the right frontal lobe, parietal lobe, and basal ganglia, and left frontal and parietal lobe CTA (initial): right ICA, ACA, and MCA filling defects MRI (after new acute symptoms): acute infarcts of right frontal lobe, right parietal lobe, insular cortex, basal ganglia, corona radiata, centrum semiovale |
| Vegetation location | Aortic and mitral valves | Aortic valve | Mitral valve | Aortic and mitral valves |
| Management | IV heparin and low molecular dextran |
Broad-spectrum antibiotics Urgent aortic valve replacement |
IV heparin Surgical resection of gallbladder |
Initially started on apixaban and broad-spectrum antibiotics After acute recurrent stroke: IV heparin then LMWH Outpatient chemotherapy |
| Prognosis | Hospitalization complicated by seizures due to recurrent ischemic strokes leading to cerebral edema and brain death; died on hospital day 6 | Hospitalization complicated by hemodynamic instability and new onset ascites leading to urgent valve replacement and abdominal laparoscopic exploration; transitioned to comfort care and died shortly after | Disappearance of vegetation on transesophageal echocardiogram 2 weeks after initiation of heparin; no known recurrence of stroke | No known recurrence of stroke |
| Valve histopathology findings | Loosely adherent aortic and mitral valve vegetations with fibrin; valvular leaflet without destruction or bacteria | Fibrinous aggregations with mixed inflammatory cell infiltrate and scanty neutrophils and degenerate histiocytes without identification or later growth of microorganisms | N/A | N/A |
| Malignancy/histopathology findings |
Mass filling gallbladder cavity Histopathology: adenocarcinoma |
Large intra-hepatic solid lesion, a thickened gallbladder, extensive omental seeding Histopathology: disseminated adenocarcinoma |
24 mm mass on gallbladder Histopathology: poorly differentiated tumor cells and the production of mucin |
Abnormally focal circumferential thickness of gallbladder fundus, enlarged porta hepatis lymph node Histopathology: biliary adenocarcinoma on nodal biopsy |
| Associated malignancy | Gallbladder adenocarcinoma (no reported mucin) | Probable primary gallbladder adenocarcinoma with omental seeding (no reported mucin) | Mucin-producing gallbladder carcinoma | Probable primary gallbladder malignancy without mucin production with involvement of the porta hepatis node |
ACA anterior cerebral artery, ATIII antithrombin III, CEA carcinoembryonic antigen, CRP C-reactive protein, CT computerized tomography, CTA computerized tomography angiography, DRESS drug reaction with eosinophilia and systemic symptoms, ESR erythrocyte sedimentation rate, ICA internal carotid artery, INR international normalized ratio, LMWH low molecular weight heparin, MCA middle cerebral artery, MRI magnetic resonance imaging, MRA magnetic resonance angiography, PT prothrombin time, PTT partial thromboplastin time
*Patient on a direct oral anticoagulant at time of labs
Treatment of NBTE has three components. First, systemic anticoagulation is needed to limit recurrent embolization. Second, correction of the underlying cause should be performed if possible, including treatment of malignancy. Third, surgical intervention on the affected cardiac valve should be considered. Although general consensus for indications for valve surgery in NBTE are similar to those in infective endocarditis, including heart failure and large vegetations, case reports have suggested that the most common indication for valve replacement in NBTE is recurrent thromboembolism despite therapeutic anticoagulation.14
It is well-established that NBTE poses a higher risk for systemic embolization compared to infective endocarditis. One review article cites the incidence of stroke as 33% in NBTE patients compared to 19% in infective endocarditis patients.5 This increased embolization is hypothesized to relate to the nature of the platelet and fibrin microthrombi, which are less adherent to valves and more easily dislodged than thrombi from infective endocarditis.15 Consequently, patients with NBTE are often anticoagulated indefinitely, including those without evidence of embolic events.16 In contrast, anticoagulation in infective endocarditis is only indicated for mechanical valves or atrial fibrillation due to the lack of demonstrated benefit on embolization and potential to harm infective endocarditis patients due to increased risk of hemorrhagic conversion.16
Despite the risk of embolization, no formal randomized control trials established the ideal anticoagulation therapy for NBTE. Current guidelines16 recommend heparin as first-line therapy for NBTE, although evidence remains weak (grade 2C). Additionally, there is evidence from in vitro, animal, and retrospective studies suggesting that heparin is more effective at reducing embolization compared to alternatives such as warfarin or direct thrombin inhibitors.17 The proposed mechanism of this efficacy relates to the unique mechanism of heparin inhibiting selectins and the role of mucin in interacting with selectins. The selectin family, which includes P-selectins stored in platelets and expressed on the platelet surface after platelet activation, L-selectins present on leukocytes, and E-selectins present on endothelial cells, are carbohydrate-binding adhesion molecules involved in inflammation and thrombosis. Selectins, specifically P-selectin, have many pro-thrombotic roles, including platelet aggregation through adhesive properties,18 platelet activation through signaling properties,18 and inducing expression of tissue factor, the main initiator of the coagulation cascade leading to thrombus stabilization.19 P-selectin induces tissue factor expression on monocytes. It has been proposed that tumor mucins interact with P-selectin and L-selectin, resulting in initial activation and aggregation of platelets and consequent microthrombi formation.20 Additional studies have shown that heparin can inhibit P-selectin and L-selectin recognition of ligands.20, 21 Therefore, the inhibition of selectins by heparin may prevent mucin interaction with selectins, which in turn decreases platelet aggregation and activation. This mechanism may contribute to the clinical superiority of heparin over warfarin in secondary prevention of thromboembolism in patients with malignancy,22 as warfarin has not been shown to affect selectins. Direct oral anticoagulants also appear to lack the ability to disrupt mucin-selectin binding; hirudin, a direct thrombin inhibitor, did not affect selectin-mucin binding,20 and interactions between selectins and direct factor Xa inhibitors have not yet been reported.
Interestingly, the patient in this clinical vignette did not have a mucin-producing tumor, as histopathology was negative for mucin. This may suggest mucin is not a requirement for the development of NBTE; rather, NBTE may be a consequence of additional pro-thrombotic mechanisms of malignancy, including the production of tissue factor and factor X-activating proteases, as well as other known mediators of platelet activation such as ADP.15 These molecules, which contribute to a pro-inflammatory state, may contribute to selectin involvement even without mucin-selectin interaction and therefore still suggest a role for heparin use in non-mucinous malignancy-related NBTE. The increased efficacy of heparin in our patient may support this, as direct Xa inhibitors were not effective in preventing embolic events, and it is important to note that she did not have any additional embolic events after initiation of heparin therapy.
CONCLUSION
In summary, this clinical vignette describes an unusual case of NBTE due to a non-mucinous primary gallbladder malignancy with local involvement of the portal hepatis lymph node with recurrent thromboembolic events despite anticoagulation with direct factor Xa inhibitors. Thus, we provide new evidence suggesting that direct factor Xa inhibitors, in addition to previously studied direct oral anticoagulants such as direct thrombin inhibitors, may not be effective in preventing thromboembolic consequences of NBTE. This substantiates previous findings that heparin, including LMWH, is a superior anticoagulation agent for NBTE, which may be partially due to its interactions with selectins in both mucinous and non-mucinous tumors.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Asopa S, Patel A, Khan OA, Sharma R, Ohri SK. Non-bacterial thrombotic endocarditis. Eur J Cardiothorac Surg. 2007;32(5):696–701. doi: 10.1016/j.ejcts.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 2.Lopez J, Ross RS, Fishbein MC, Siegel RJ. Nonbacterial thrombotic endocarditis: A review. Am Heart J. 1987;113(3):773–784. doi: 10.1016/0002-8703(87)90719-8. [DOI] [PubMed] [Google Scholar]
- 3.Edoute Y, Haim N, Rinkevich D, Brenner B, Reisner SA. Cardiac valvular vegetations in cancer patients: A prospective echocardiographic study of 200 patients. Am J Med. 1997;102(3):252–258. doi: 10.1016/S0002-9343(96)00457-3. [DOI] [PubMed] [Google Scholar]
- 4.Bedikian A, Valdivieso M, Luna M, Bodey GP. Nonbacterial thrombotic endocarditis in cancer patients: Comparison of characteristics of patients with and without concomitant disseminated intravascular coagulation. Med Pediatr Oncol. 1978;4(2):149–157. doi: 10.1002/mpo.2950040211. [DOI] [PubMed] [Google Scholar]
- 5.Biswas A, Yassin M. Comparison between transthoracic and transesophageal echocardiogram in the diagnosis of endocarditis: A retrospective analysis. International Journal of Critical Illness and Injury Science. 2015;5(2):130. doi: 10.4103/2229-5151.158429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen T, Deangelis LM. Stroke in cancer patients. Curr Neurol Neurosci Rep. 2006;6(3):187–192. doi: 10.1007/s11910-006-0004-0. [DOI] [PubMed] [Google Scholar]
- 7.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712–1723. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 8.El-Shami K, Griffiths E, Streiff M. Nonbacterial Thrombotic Endocarditis in Cancer Patients: Pathogenesis, Diagnosis, and Treatment. Oncologist. 2007;12(5):518–523. doi: 10.1634/theoncologist.12-5-518. [DOI] [PubMed] [Google Scholar]
- 9.Kooiker JC, Maclean JM, Sumi SM. Cerebral Embolism, Marantic Endocarditis, and Cancer. Arch Neurol. 1976;33(4):260–264. doi: 10.1001/archneur.1976.00500040044006. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka H, Ito M, Yoshida K, Asakura T, Taniguchi H. Nonbacterial thrombotic endocarditis complicated with stage Ia ovarian cancer. Int J Clin Oncol. 2009;14(4):369–371. doi: 10.1007/s10147-008-0852-5. [DOI] [PubMed] [Google Scholar]
- 11.Scalia GM, Tandon AK, Robertson JA. Stroke, Aortic Vegetations and Disseminated Adenocarcinoma – A Case of Marantic Endocarditis. Heart Lung Circ. 2012;21(4):234–236. doi: 10.1016/j.hlc.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Okuchi K, Fujioka M, Iwanaga H, Koshimae N, Sakaki T. Fulminant cerebral infarctions caused by nonbacterial thrombotic endocarditis due to gallbladder cancer. Acta Neurochir. 1997;139(10):995–996. doi: 10.1007/BF01411313. [DOI] [PubMed] [Google Scholar]
- 13.Yamane A, Sadahiro H, Goto H, et al. Multiple Ischemic Strokes Caused by Nonbacterial Thrombotic Endocarditis Because of Gallbladder Cancer: A Case Report. J Stroke Cerebrovasc Dis. 2014;23(6):1727–1729. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Kaneyuki D, Matsuura K, Ueda H, Kohno H, Kanbe M, Matsumiya G. Surgical management of nonbacterial thrombotic endocarditis in malignancy. Surg Case Rep 2017;3(1). [DOI] [PMC free article] [PubMed]
- 15.Eiken PW, Edwards WD, Tazelaar HD, Mcbane RD, Zehr KJ. Surgical Pathology of Nonbacterial Thrombotic Endocarditis in 30 Patients, 1985–2000. Mayo Clin Proc. 2001;76(12):1204–1212. doi: 10.4065/76.12.1204. [DOI] [PubMed] [Google Scholar]
- 16.Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and Thrombolytic Therapy for Valvular Disease. Chest. 2012;141(2). [DOI] [PMC free article] [PubMed]
- 17.Varki A. Trousseaus syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110(6):1723–1729. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Théorêt J-F, Yacoub D, Hachem A, Gillis M-A, Merhi Y. P-selectin ligation induces platelet activation and enhances microaggregate and thrombus formation. Thromb Res. 2011;128(3):243–250. doi: 10.1016/j.thromres.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Celi A, Pellegrini G, Lorenzet R, et al. P-selectin induces the expression of tissue factor on monocytes. Proc Natl Acad Sci. 1994;91(19):8767–8771. doi: 10.1073/pnas.91.19.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahrenbrock M, Borsig L, Le D, Varki N, Varki A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Investig. 2003;112(6):853–862. doi: 10.1172/JCI200318882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson R, Cecconi O, Roberts W, Aruffo A, Linhard R, Bevilacqua M. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood. 1993;82(11):3253–3258. doi: 10.1182/blood.V82.11.3253.3253. [DOI] [PubMed] [Google Scholar]
- 22.Meyer G, Marjanovic Z, Valcke J. Comparison of low-molecular-weight heparin and warfarin for secondary prevention of venous thromboembolism in patients with cancer. A randomized controlled study. ACC Curr J Rev. 2003;12(1):15. doi: 10.1016/S1062-1458(02)00998-4. [DOI] [PubMed] [Google Scholar]