Abstract
Background
Fetuin-A is a hepatokine that involved in the pathogenesis of insulin resistance. Previous epidemiological studies have found a positive association between blood fetuin-A and type 2 diabetes mellitus (T2DM) risk among Caucasians and African Americans. We aimed to investigate the prospective relationship between fetuin-A and T2DM in an Asian population for the first time.
Methods
A nested case-control study was established within a prospective cohort of Chinese living in Singapore. At blood collection (1999 to 2004), all participants were free of diagnosed T2DM and aged 50 to 79 years. At subsequent follow-up (2006 to 2010), 558 people reported to have T2DM and were classified as incident cases, and 558 controls were randomly chosen from the participants who did not develop T2DM to match with cases on age, sex, dialect group, and date of blood collection. Plasma fetuin-A levels were measured retrospectively in cases and controls using samples collected at baseline. Conditional logistic regression models were used to compute the odds ratio (OR) and 95% confidence interval (CI). Restricted cubic spline analysis was used to examine a potential non-linear association between fetuin-A levels and T2DM risk.
Results
Compared with those in the lowest fetuin-A quintile, participants in the highest quintile had a two-fold increased risk of developing T2DM (OR, 2.06; 95% CI, 1.21 to 3.51). A non-linear association was observed (P nonlinearity=0.005), where the association between fetuin-A levels and T2DM risk plateaued at plasma concentrations around 830 µg/mL.
Conclusion
There is a positive association between plasma fetuin-A levels and risk of developing T2DM in this Chinese population.
Keywords: Alpha-2-HS-glycoprotein; Association; Case-control studies; Diabetes mellitus, type 2; Epidemiology
INTRODUCTION
Fetuin-A is predominately synthesized by the liver, a vital organ in maintaining glucose homeostasis [1]. Fetuin-A binds to the insulin receptor tyrosine kinase and reduces downstream signaling in fat and muscle, thus enhancing peripheral insulin resistance and leading to the onset of type 2 diabetes mellitus (T2DM) [2,3,4]. Consistently, animal studies have found that fetuin-A knockout mice are more insulin-sensitive compared with the wild-type controls [3,4], and treating wild-type mice with exogenous fetuin-A severely deteriorated insulin resistance [5]. Moreover, epidemiological studies have reported a positive association between fetuin-A levels and T2DM, and a recent meta-analysis pooled results from seven prospective studies and reported a 23% increased risk of T2DM per standard deviation (SD) increment in fetuin-A levels [6]. Thus, the overall evidence suggested that fetuin-A could be an important biomarker involved in the pathogenesis of T2DM development.
Previous epidemiological studies have been mainly conducted in Caucasians and African American populations [7,8,9,10,11,12] with a small study among 120 Indians with pre-diabetes [13]. In addition, the mean body mass index (BMI) of study participants ranged from 25.4 to 28.9 kg/m2 in the previous studies [6], whereas the evidence among lean and general Asian populations is scarce. Asians develop T2DM at lower BMIs compared to Western counterparts [14]. Two prior studies among lean Japanese [15] and Chinese populations [16] (both mean BMIs approximately 23.0 kg/m2) have observed much lower cutoff values of the liver enzyme alanine aminotransferase (ALT) in predicting T2DM risk compared with a United States population [17]. In addition, the risk estimate associated with ALT for T2DM risk was slightly higher among Asians than Caucasians (1.34 vs. 1.22) in a meta-analysis on ALT and T2DM risk [18]. Thus, these lines of evidence suggested that the liver may play an important role in T2DM onset in relatively lean Asian population. In addition, compared with non-Asian populations, Asians are more likely to develop insulin resistant and have lower β-cell function [19]. Considering this potential biological difference between Asians and Caucasians, it is important to examine whether fetuin-A, released predominately by the liver, could predict the onset of T2DM in a lean and healthy Chinese population. In addition, it is not clear whether the association between fetuin-A and T2DM risk could be modified by sex, age, or glucose levels [7,8,9,10,11,12].
To address the abovementioned issues, we conducted a nested case-control study within the prospective Singapore Chinese Health Study to quantify the association between plasma fetuin-A levels and T2DM risk among a general Chinese population with mean BMI of 23.0 kg/m2, and the associations among different subgroups.
METHODS
Study population
The Singapore Chinese Health Study was established between 1993 and 1998 [20]. At baseline, we recruited 63,257 Chinese adults between 45 to 74 years old, and we conducted a face-to-face interview to collect information on medical history, diet and lifestyle habits using a structured questionnaire. Subsequently, we conducted follow-up I interviews (1999 to 2004), and re-contacted 52,322 participants. Among them, we collected bio-specimens from 32,535 participants. From 2006 to 2010, we conducted follow-up II interviews via telephone and re-interviewed 39,528 participants. Among them, 25,477 (78.3%) donated bio-specimens at follow-up I. Informed consent was completed at the baseline interview and all procedures were complied with the 1964 Helsinki declaration. The protocol was approved by the Institutional Review Boards at University of Pittsburgh and National University of Singapore (NUS-IRB Ref Code: 06-027).
Ascertainment of T2DM
Self-reported physician-diagnosed T2DM information was collected by asking whether the subject has been told by a doctor that he/she had diabetes at baseline and two follow-up interviews. If the participant reported “yes” to the question, the age at first diagnosis was then asked. The accuracy of the self-reported T2DM data was validated in a separate study, where 98.9% cases were confirmed by either medical records or complementary information on diagnostic tests and therapy [21].
Establishment of the nested case-control study
Within this cohort, a nested case-control study comprising 558 case-control pairs was established. All cases and controls reported no history of T2DM, cardiovascular disease and cancer at baseline recruitment (1993 to 1998) and blood collection at follow-up I (1999 to 2004). At follow-up II visit (2006 to 2010), 571 participants reported to have physician-diagnosed T2DM, and were considered as incident cases. Among participants who reported no T2DM at follow-up II and had glycosylated hemoglobin (HbA1c) <6.0%, we randomly chose 571 controls to match with cases on age (±3 years), sex, dialect group (Hokkien, Cantonese), and date of blood collection (±6 months). A total of 13 participants with extreme fetuin-A levels (>3 SD over mean fetuin-A levels) were excluded, leaving 558 case-control pairs for the current analysis (Supplementary Fig. 1).
Laboratory measurements
We collected 20-mL peripheral blood from each consenting participant in the morning, and then immediately transported the sample on ice to the laboratory. We separated all specimens into various components (i.e., serum, plasma, buffy coat, red blood cells), and put in freezers (−80℃) for long-term storage. The measurements of the biomarkers were conducted retrospectively after the selection of cases and controls at the National University Hospital Reference Laboratory, Singapore. Specifically, one aliquot of serum and plasma from the same case-control pair was analyzed in the same batch. Plasma levels of fetuin-A and adiponectin were measured via Sandwich ELISA (Bio-Rad Laboratories, Hercules, CA, USA). The measurement range of fetuin-A ranged from 2.5 to 2,000 µg/mL, and the inter- and intra-assay variation was 3.9% to 4.9% and 7.3% to 8.4%, respectively. Plasma levels of lipids (total cholesterol [TC], triglyceride [TG], high density lipoprotein cholesterol [HDL-C]), liver enzyme (ALT) and inflammatory marker (high-sensitivity C-reactive protein [hs-CRP]) were measured by colorimetric method (AU5800 Analyzer; Beckman Coulter, Brea, CA, USA). HbA1c levels were measured in red blood cells via HPLC method (Bio-Rad Laboratories).
Statistical analysis
Pearson correlation coefficients between fetuin-A and age, BMI, TC, HDL-C, TG ALT, adiponectin, hs-CRP, random insulin, random glucose, and HbA1c levels were calculated among control participants after natural-log transformation of skewed variables (fetuin-A, ALT, TG, and hs-CRP). We divided subjects into sex-specific quintiles using the fetuin-A distribution among controls. We used conditional logistic regression models to compute the odds ratio (OR) of fetuin-A for T2DM risk. Model 1 included age (continuous), education levels (no, primary school, secondary or above), smoking habits (never smokers, past/current smokers), alcohol drinking (<weekly, ≥weekly), moderate-to-vigorous physical activity levels (<0.5, 0.5 to 3.9, and ≥4.0 hours/week), history of hypertension (yes, no), fasting status (yes, no), and BMI (continuous). Furthermore, we further adjusted for other T2DM biomarkers (hs-CRP, TG, HDL-C, adiponectin, ALT and random glucose). In addition, we used restricted cubic spline analysis to assess the linearity of the association between fetuin-A and T2DM risk with 3 knots at 25, 50, and 75 percentiles of fetuin-A concentrations. We chose 3 knots because it yielded better model fit compared to using 4 or 5 knots assessed by Akaike information criteria. Furthermore, we tested potential interaction with some baseline characteristics including age, sex, BMI, alcohol consumption, physical activity, fasting status, plasma levels of TG, HDL-C, ALT, adiponectin, hs-CRP, and HbA1c levels. Because of the detection of a non-linear relation between fetuin-A and T2DM, in the stratified analysis, we presented the OR and 95% confidence interval (CI) associated with fetuin-A concentrations in quintiles, and tested P interaction by adding an interaction term (binary factor×continuous fetuin-A) to the regression models using restricted cubic spline analysis.
We performed all analyses with Stata software version 14.0 (Stata Corp., College Station, TX, USA). We considered two-sided P values <0.05 to be statistically significant.
RESULTS
The mean age of T2DM diagnosis was 63.2±6.4 years and the mean time between bio-specimen collection and T2DM diagnosis was 4.0±1.7 years. The baseline description of demography, diet, lifestyle habits and medical history of cases and controls are presented in Table 1. The mean age of the participants at blood donation was 59.7±6.1 years and 58.2% were women. Cases had higher BMI and more likelihood to have hypertension compared to their age-and-sex matched controls. Cases and controls had similar education levels, fasting status, and dietary and lifestyle habits in smoking, alcohol consumption, and physical activity. For the blood biomarkers, cases had worse metabolic profile. Except for HDL-C and adiponectin that were higher in controls, cases had higher levels of lipids (TC, TG), liver enzyme (ALT), inflammation (hs-CRP), random insulin, and glucose levels (random glucose and HbA1c). The median (interquartile range [IQR]), concentrations of fetuin-A were 711 µg/mL (IQR, 542 to 916) in cases and 640 µg/mL (IQR, 490 to 844) in controls. Among total population, plasma fetuin-A levels were weakly correlated with BMI, ALT, TC, and hs-CRP (Pearson's coefficient ranged from 0.08 to 0.10; P<0.01) (Supplementary Table 1). However, fetuin-A was not significantly correlated with age, HDL-C, TG, adiponectin, random insulin, random glucose or HbA1c levels in controls (Pearson's coefficient r ranged from −0.05 to 0.05; P> 0.05) (Supplementary Table 1).
Table 1. Baseline characteristics and fetuin-A levels of diabetes cases and matched controls, the Singapore Chinese Health Studya.
| Characteristic | Cases (n=558) | Controls (n=558) | P valueb |
|---|---|---|---|
| Age at blood taken, yr | 59.6±6.11 | 59.7±6.18 | - |
| Female sex | 325 (58.2) | 325 (58.2) | - |
| Dialect | - | ||
| Cantonese | 280 (50.2) | 280 (50.2) | |
| Hokkien | 278 (49.8) | 278 (49.8) | |
| Body mass index, kg/m2 | 24.8±3.62 | 22.8±3.29 | <0.001 |
| Level of education | 0.226 | ||
| No formal education | 102 (18.3) | 96 (17.2) | |
| Primary school | 249 (44.6) | 227 (40.7) | |
| Secondary and above | 207 (37.1) | 235 (42.1) | |
| History of hypertension | 257 (46.1) | 145 (26.0) | <0.001 |
| Cigarette smoking | 0.162 | ||
| Never smokers | 400 (71.7) | 413 (74.0) | |
| Former smoker | 61 (10.9) | 70 (12.5) | |
| Current smokers | 97 (17.4) | 75 (13.4) | |
| Weekly moderate activity, hr/wk | 0.091 | ||
| <0.5 | 445 (79.8) | 444 (79.6) | |
| 0.5–3.9 | 82 (14.7) | 67 (12.0) | |
| ≥4 | 31 (5.6) | 47 (8.4) | |
| Alcohol intake | 0.838 | ||
| Abstainers | 486 (87.1) | 486 (87.1) | |
| Weekly drinkers | 54 (9.7) | 57 (10.2) | |
| Daily drinkers | 18 (3.2) | 15 (2.7) | |
| Fasting status (yes) | 171 (30.7) | 154 (27.6) | 0.263 |
| Fetuin-A, µg/mL | 711 (542–916) | 640 (490–844) | <0.001 |
| ALT, IU/L | 27 (20–37) | 20 (15–27) | <0.001 |
| TC, mmol/L | 5.30±0.95 | 5.20±0.84 | 0.067 |
| HDL-C, mmol/L | 1.08±0.24 | 1.23±0.32 | <0.001 |
| TG, mmol/L | 2.15 (1.45–2.94) | 1.54 (1.07–2.23) | <0.001 |
| Adiponectin, µg/mL | 7.02±2.70 | 9.08±3.78 | <0.001 |
| hs-CRP, mg/L | 1.80 (0.90–3.40) | 1.20 (0.60–2.30) | <0.001 |
| Random insulin, mIU/L | 14.6 (7.90–35.2) | 8.85 (4.40–21.6) | <0.001 |
| Random glucose, mmol/L | 6.00 (4.80–8.90) | 4.50 (4.10–5.30) | <0.001 |
| HbA1c, % | 6.40 (5.90–7.20) | 5.60 (5.40–5.80) | <0.001 |
| HbA1c, mmol/mol | 46.0 (41.0–55.0) | 38.0 (36.0–40.0) | <0.001 |
Values are presented as mean±standard deviation, number (%), or median (interquartile range).
ALT, alanine aminotransferase; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; TG, triglyceride; hs-CRP, high sensitivity C-reactive protein; HbA1c, glycosylated hemoglobin.
aCases and controls are matched on age at blood taken (±3 years), gender, dialect, and date of blood collection (±6 months), bP values based on the chi-square test for categorical variables, Student's t-test and Mann-Whitney test for continuous variable.
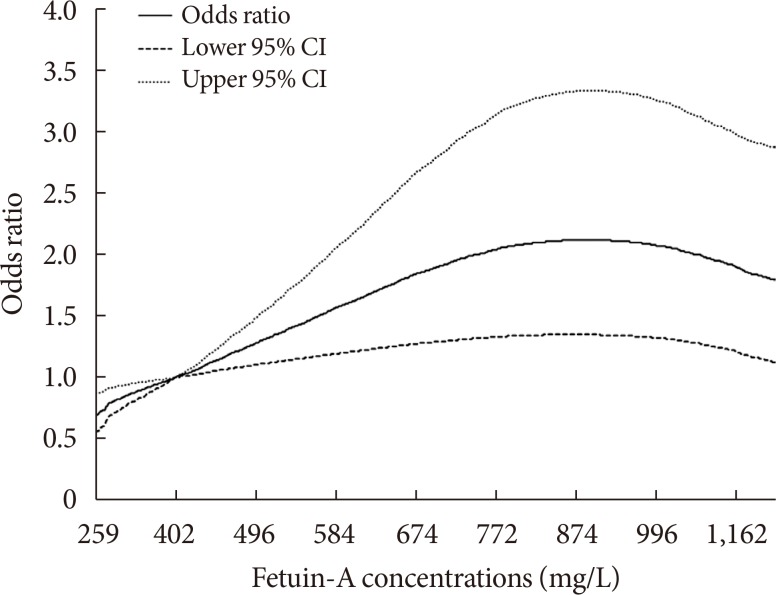
Inspection of cubic spline analysis revealed that fetuin-A levels were positively associated with T2DM risk up to circulating concentrations of approximately 830 µg/mL (approximately 70th percentile of total population), above which the association plateaued in model 1 with adjustment for potential confounding factors (P nonlinearity=0.005) (Fig. 1). Per 100 µg/mL increment in fetuin-A levels was associated with a 24% higher risk of developing T2DM (OR, 1.24; 95% CI, 1.01 to 1.53) before the inflection point (data not shown). Similarly, when analyzing the fetuin-A concentrations as quintiles, graded increments in risk up to quintile 3 was observed while the association plateaued in quintile 4 and 5 (Table 2).
Fig. 1. Spline analysis of the association between fetuin-A concentrations and incident type 2 diabetes mellitus risk. The solid line represents point estimates of relative risk for the association between fetuin-A levels and risk of incident type 2 diabetes mellitus, and the dotted lines represent the upper and lower bound of 95% confidence interval (CI). Study participants with the highest 5% of fetuin-A were excluded to minimize the potential impact of outliers. Cubic spline analysis was used to examine the association between fetuin-A concentrations and risk of developing type 2 diabetes mellitus using the model 1 from Table 2 (P nonlinearity=0.005).
Table 2. ORs (95% CIs) of type 2 diabetes mellitus associated with sex-specific quintiles of fetuin-A, the Singapore Chinese Health Study.
| Variable | Quintiles of fetuin-A | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| Male, µg/mL, median (range) | 396 (262–470) | 513 (470–569) | 619 (570–685) | 788 (685–899) | 1,057 (906–1,499) |
| Female, µg/mL, median (range) | 384 (259–454) | 522 (455–588) | 658 (589–721) | 825 (724–938) | 1,125 (938–1,682) |
| Cases/controls | 86/114 | 82/113 | 110/113 | 149/109 | 131/109 |
| Crude model, OR (95% CI) | 1.00 | 1.08 (0.70–1.67) | 1.68 (1.07–2.65) | 2.45 (1.56–3.85) | 2.26 (1.41–3.62) |
| Adjusted modela, OR (95% CI) | 1.00 | 1.21 (0.74–1.97) | 2.10 (1.25–3.51) | 2.09 (1.26–3.48) | 2.06 (1.21–3.51) |
OR, odds ratio; CI, confidence interval.
aAdjusted model: adjusted for age at blood taken (continuous), smoking status (never, ever smoker), alcohol intake (never, weekly or daily), weekly moderate-to-vigorous physical activity levels (<0.5, 0.5 to 3.9, and ≥4 hours/week), education levels (no, primary school, secondary or above), history of hypertension (yes, no), fasting status (yes, no), and body mass index (continuous).
In the stratified analyses, no significant interactions were observed (all P interaction >0.10) (Supplementary Table 2), although the association was slightly stronger in certain subgroups. When stratified by HbA1c levels, the association between fetuin-A and T2DM risk remained strong among those with baseline HbA1c ≥6.5% (OR comparing extreme quintiles of fetuin-A was 2.47; 95% CI, 1.15 to 5.29), whereas the association did not reach statistical significance among those with HbA1c levels <6.5% (OR comparing extreme quintiles of fetuin-A was 1.85; 95% CI, 0.84 to 4.08) (Supplementary Table 2).
Because of the non-linear association between fetuin-A and T2DM, we also categorized participants into four groups that comprise tertiles among participants with fetuin-A levels smaller than 830 µg/mL, and a fourth category of people with fetuin-A levels higher or equal to 830 µg/mL (Table 3). Compared with the lowest group of fetuin-A levels, there was a two-fold higher risk of developing T2DM in the highest group after adjustment for established T2DM risk factors in Model 1 (P trend=0.002) (Table 3). Among people with fetuin-A levels smaller than 830 µg/mL, further adjustment for other blood biomarkers (hs-CRP, adiponectin, TG/HDL-C, ALT, and random glucose) either individually or collectively had little impact on the association (ORs ranged from 1.76 to 2.11 comparing group 3 vs. group 1) (Table 3). Comparatively, among people with higher fetuin-A levels (≥830 µg/mL), the association was substantially attenuated and the OR became non-significant when adjusting for all the biomarkers together (OR comparing group 4 vs. group 1 was 1.60; 95% CI, 0.92 to 2.78) (Table 3).
Table 3. ORs (95% CIs) of type 2 diabetes mellitus associated with different levels of fetuin-A, the Singapore Chinese Health Study.
| Variable | Four groups fetuin-A | P value | |||
|---|---|---|---|---|---|
| Group 1a | Group 2a | Group 3a | Group 4 | ||
| Male, µg/mL, median (range) | 412 (262–487) | 564 (490–628) | 727 (632–829) | 998 (833–1,499) | |
| Female, µg/mL, median (range) | 405 (259–488) | 556 (489–631) | 714 (632–829) | 1,027 (830–1,682) | |
| Cases/controls | 105/137 | 107/137 | 151/137 | 195/147 | |
| Crude model, OR (95% CI) | 1.00 | 1.38 (0.87–2.21) | 2.02 (1.24–3.30) | 2.07 (1.27–3.38) | 0.001 |
| Model 1b, OR (95% CI) | 1.00 | 1.41 (0.89–2.23) | 2.02 (1.25–3.27) | 2.16 (1.34–3.47) | 0.002 |
| Model 1b+hs-CRP, OR (95% CI) | 1.00 | 1.38 (0.86–2.20) | 1.94 (1.19–3.16) | 2.03 (1.25–3.31) | 0.003 |
| Model 1b+adiponectin, OR (95% CI) | 1.00 | 1.32 (0.81–2.16) | 1.98 (1.19–3.31) | 1.83 (1.10–3.05) | 0.013 |
| Model 1b+TG/HDL-C, OR (95% CI) | 1.00 | 1.30 (0.79–2.16) | 2.11 (1.26–3.54) | 1.86 (1.11–3.13) | 0.009 |
| Model 1b+ALT, OR (95% CI) | 1.00 | 1.29 (0.79–2.11) | 1.92 (1.15–3.19) | 1.80 (1.08–3.00) | 0.015 |
| Model 1b+random glucose, OR (95% CI) | 1.00 | 1.22 (0.70–2.13) | 1.76 (0.99–3.13) | 1.98 (1.10–3.55) | 0.011 |
| Model 2c, OR (95% CI) | 1.00 | 1.28 (0.74–2.20) | 1.84 (1.05–3.23) | 1.60 (0.92–2.78) | 0.169 |
OR, odds ratio; CI, confidence interval; hs-CRP, high sensitivity C-reactive protein; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; ALT, alanine aminotransferase.
aGroup 1–3 were categorized into tertiles among controls with fetuin-A levels less than 830 mg/L, bModel 1: adjusted for age at blood taken (continuous), smoking status (never, ever smoker), alcohol intake (never, weekly or daily), moderate-to-vigorous weekly activity levels (<0.5, 0.5 to 3.9, and ≥ 4 hours/week), education levels (no, primary school, secondary or above), history of hypertension (yes, no), fasting status (yes, no), and body mass index (continuous), cModel 2: Model 1 plus hs-CRP (mg/L), adiponectin (µg/mL), TG-to-HDL-C ratio, ALT (IU/L) and random glucose (mmol/L; all in quartiles).
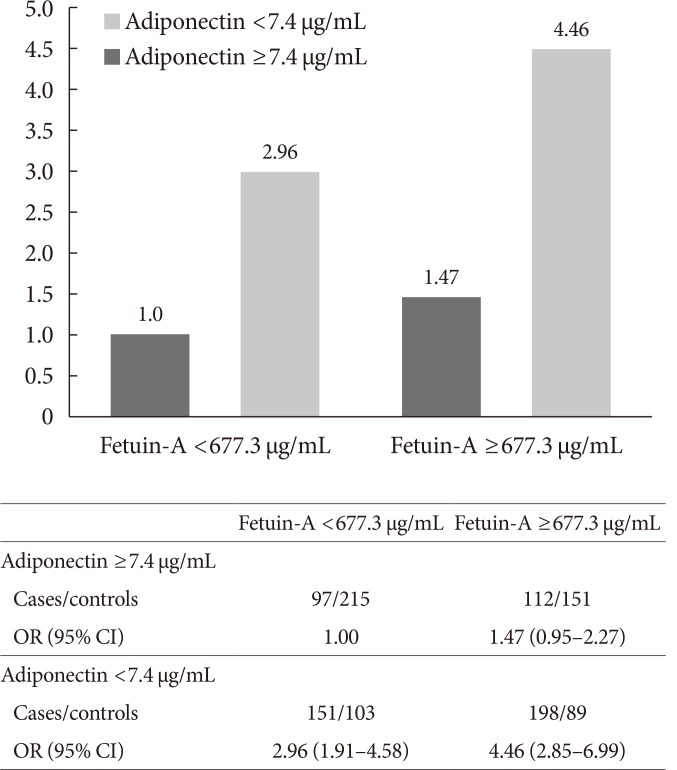
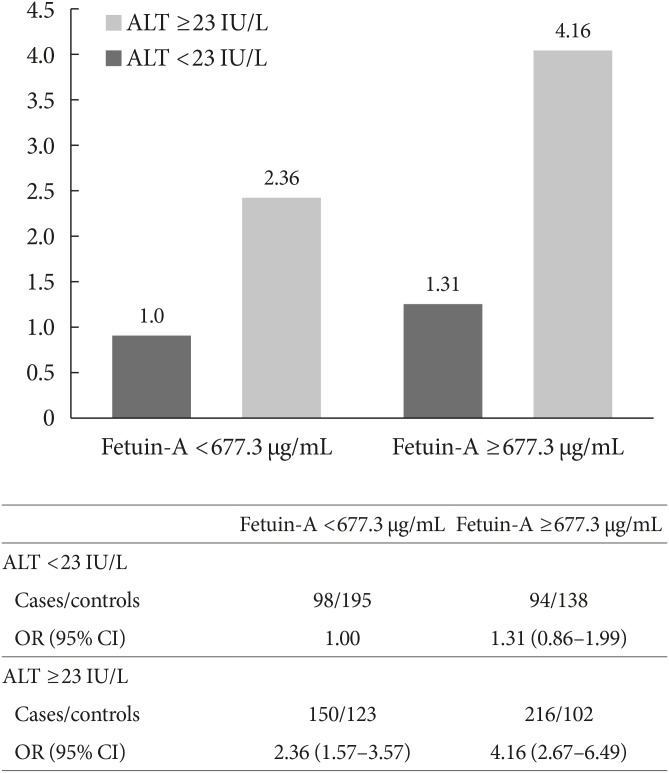
We subsequently examined the joint impact of fetuin-A and adiponectin, as well as fetuin-A and ALT on T2DM risks. Although the multiplicative interaction was not significant between fetuin-A and adiponectin levels on T2DM risk (P interaction =0.93), the combined group of high fetuin-A (≥median 677.3 µg/mL) and low adiponectin levels (<median 7.4 µg/mL) had a much higher risk of T2DM (OR, 4.46; 95% CI, 2.85 to 3.66) than the reference group of low fetuin-A and high adiponectin levels (Fig. 2). Similarly, the joint impact of high fetuin-A and high ALT levels (≥median 23 IU/L) was associated with a significantly higher risk of T2DM risk compared to the reference group of low fetuin-A and low ALT levels (OR, 4.16; 95% CI, 2.67 to 6.49), although the multiplicative interaction was not significant (P=0.90) (Fig. 3).
Fig. 2. Total type 2 diabetes mellitus risk according to joint distribution of fetuin-A and adiponectin. Multivariable model adjusted for age at blood taken (continuous), smoking status (never, ever smoker), alcohol intake (never, weekly or daily), weekly moderate-to-vigorous physical activity levels (<0.5, 0.5 to 3.9, and ≥4 hours/week), education levels (no, primary school, secondary or above), history of hypertension (yes, no), fasting status (yes, no), and body mass index (continuous). OR, odds ratio; CI, confidence interval.
Fig. 3. Total type 2 diabetes mellitus risk according to joint distribution of fetuin-A and alanine transaminase (ALT). Multivariable model adjusted for age at blood taken (continuous), smoking status (never, ever smoker), alcohol intake (never, weekly or daily), weekly moderate-to-vigorous physical activity levels (<0.5, 0.5 to 3.9, and ≥4 hours/week), education levels (no, primary school, secondary or above), history of hypertension (yes, no), fasting status (yes, no), and body mass index (continuous). OR, odds ratio; CI, confidence interval.
DISCUSSION
In this prospective nested case-control study among Chinese men and women in Singapore, we found a non-linear association between fetuin-A levels and T2DM risk: the association was gradually increased up to concentrations of approximately 830 µg/mL (70th percentile in the total population), and plateaued with further increment in fetuin-A levels. To the best of our knowledge, this is the largest and first prospective study in a healthy Asian population to evaluate the relationship between fetuin-A and T2DM risk, and reported a non-linear association.
Fetuin-A has increasingly attracted attention, so far a total of 27 case-control studies and seven prospective studies have evaluated the association between fetuin-A and T2DM risk [6,22]. In the meta-analysis of 27 case-control studies, significantly higher fetuin-A levels have been observed in T2DM cases compared to controls [22], which were consistent with the current study. Another meta-analysis of seven prospective studies has assumed a linear association between fetuin-A and T2DM risk: the pooled relative risk was 1.23 (95% CI, 1.16 to 1.31; P<0.001) per SD increment in fetuin-A levels for T2DM risk [6], which was similar to the risk estimate found in the current study before the inflection point (OR, 1.24; 95% CI, 1.01 to 1.53) per 100 µg/mL increment in fetuin-A levels. In six out of the seven studies included in the meta-analysis [6], fetuin-A levels ranged from 14 to 984 µg/mL [8,9,10,11,12,13]. In comparison, our participants had higher fetuin-A levels with wider range (294 to 1,627 µg/mL), which enabled us to test the association among higher fetuin-A categories. Notably, although the assay method used in the current study (Sandwich ELISA) was different from the majority of the previous studies (Epitope Diagnostics ELISA) [9,10,11,12], the influence of inter-laboratory variation on the methodology cannot fully account for the wider range of fetuin-A levels observed in the current study because one previous study used the same assay method (Epitope Diagnostics ELISA) has also observed a wide fetuin-A range (approximately 200 to 2,600 µg/mL) [7]. This study was conducted among older white and black adults from the Health, Aging and Body Composition (Health ABC) study, and it reported a marginally significant association for T2DM risk per 1-log increment of fetuin-A levels (OR, 1.57; 95% CI, 0.99 to 2.49) [7]. The Health ABC study had fewer people with T2DM (n=135) compared to the current study (n=558), and thus may have limited power to observe a non-linear association between fetuin-A levels and T2DM risk. Since there are no other studies with wide fetuin-A range that is comparable to the current study, it is unclear whether the plateau of the association at higher fetuin-A levels was a chance finding or a true relationship, and more studies are warranted. In addition, although the risk estimate of fetuin-A associated with T2DM risk was slightly stronger in men compared to women, the interaction was not statistically significant (P interaction =0.32) in the current study. This finding corroborates with several cohort studies [7,8,9,11], although a significant sex-interaction in the association between fetuin-A and T2DM risk has been reported in two other studies [10,12]. Furthermore, different from previous findings [6], we did not observe a significant correlation between fetuin-A and age, as well as fetuin-A and adiponectin even when sex was accounted for.
When stratified by baseline HbA1c levels, we observed a positive association between fetuin-A levels and increased risk of undiagnosed diabetes (HbA1c ≥6.5%) but not incident diabetes (HbA1c <6.5%), suggesting that fetuin-A might not be a causal factor for diabetes, but rather a secondary outcome of hyperglycemia. Stefan et al. [8] observed a significant effect modification between fetuin-A and glucose levels where a strong association among participants with elevated glucose levels (≥100 mg/dL), but no association among participants with normal glucose levels (P interaction =0.023). However, Laughlin et al. [10] and Sun et al. [11] both found a positive association between fetuin-A and incident diabetes (HbA1c <6.5%). Since no other studies evaluated the potential impact of high baseline HbA1c levels on the association, future studies in Asian population are warranted. Nevertheless, the sample size was much smaller in the stratified analyses and results should be interpreted cautiously.
Several lines of experimental evidence suggest that fetuin-A may involve in important etiological pathways of T2DM development, such as in regulation of insulin sensitivity, inflammation, development of non-alcoholic fatty liver disease (NAFLD) and dyslipidemia. Fetuin-A has been shown to inhibit insulin receptor tyrosine kinase and subsequently decrease glucose clearance and enhance insulin resistance [23,24]. In addition, fetuin-A could suppress adiponectin expression [5,25], an important adipokine that is released from adipose tissue, and work against T2DM development through regulating insulin sensitivity [26]. In addition, lipid-induced fetuin-A expression in hepatocytes and increased fetuin-A blood levels could also regulate lipolysis and lipid disturbances in the human body [27,28]. Moreover, fetuin-A is involved in the pathogenesis of subclinical inflammation [5], and higher fetuin-A levels have also been associated with hepatic insulin resistance and fat content in patients with NAFLD [12,29]. Furthermore, a recent study has found potential interaction between fetuin-A levels and NAFLD in association with insulin resistance [30].
Hence, in our analysis, we have attempted to study if adding markers of insulin sensitivity, inflammation, NAFLD and dyslipidemia could impact the association between fetuin-A and T2DM. As a result, the current study has shown that the association between fetuin-A and T2DM is independent of adiponectin, inflammatory marker (hs-CRP), estimate of NAFLD (ALT) and lipids (TG and HDL-C) among people with fetuin-A levels smaller than 830 µg/mL, which was consistent with previous findings [7,8,9,10,11,12,30]. In contrast, among people with higher fetuin-A levels (≥830 µg/mL), adjustment for those blood biomarkers attenuated the association, indicating that the association between fetuin-A and T2DM risk may be mediated by one or several of the abovementioned pathways. In addition, we have found that higher fetuin-A/low adiponectin and high fetuin-A/high ALT jointly were associated with in creased risk of T2DM, which were consistent with previous findings [11,31], and thus indicated that the pathway of fetuin-A may not be greatly overlapped with adiponectin or ALT in the development of T2DM risk.
The current study had several limitations merit consideration. First, we measured fetuin-A only once, which may not represent its long-term levels. However, fetuin-A has been shown to be stable over several years in a previous study [32]. In addition, some important information such as family history of T2DM was not available; thus, residual confounding factors may exist. Furthermore, the current study did not measure the levels of free fatty acids, and thus could not explore their potential interaction on the association between fetuin-A and T2DM risk, as suggested by recent findings [33]. Moreover, more than 70% of blood samples were non-fasting; however, we compared baseline fetuin-A levels by fasting status, and further performed stratified analysis and found neither fetuin-A concentrations nor their association with T2DM risk differed between two groups, suggesting that fasting status did not impact the associations in the current study. Consistently, the EPIC-Potsdam Study also used primarily non-fasting blood samples (2/3 non-fasting samples) and reported a positive association between fetuin-A and T2DM [8].
In conclusion, raised fetuin-A levels were positively associated with T2DM risk in this Chinese population even after adjustment for BMI and other lifestyle risk factors. The relationship plateaued at levels around 830 µg/mL fetuin-A level. Recent studies have shown that fetuin-A levels may be reduced by pharmacological (peroxisome proliferator-activated receptor gamma agonist pioglitazone) [34], surgical (gastric bypass surgery) [35], dietary and physical activity interventions [23]. Further research is needed to validate our findings and clinical studies are warranted to investigate the feasibility of targeting fetuin-A to reduce the risk of T2DM in high-risk populations.
ACKNOWLEDGMENTS
We thank Siew-Hong Low of the National University of Singapore for supervising the fieldwork of the Singapore Chinese Health Study, and Renwei Wang for the maintenance of the cohort study database. We also thank the founding principal investigator of the Singapore Chinese Health Study, Mimi C. Yu.
This work was supported by the National Medical Research Council, Singapore (NMRC/CIRG/1354/2013) and National Institutes of Health, USA (RO1 CA144034 and UM1 CA182876). Woon-Puay Koh is supported by the National Medical Research Council, Singapore (NMRC/CSA/0055/2013). An Pan is supported by the National Key Research and Development Program of China (2017YFC0907504).
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
- Conception or design: W.P.K., A.P.
- Acquisition, analysis, or interpretation of data: Y.W., W.P.K., M.K.J., J.M.Y., A.P.
- Drafting the work or revising: Y.W., W.P.K., M.K.J., J.M.Y., A.P.
- Final approval of the manuscript: Y.W., W.P.K., M.K.J., J.M.Y., A.P.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2018.0171.
The pair-wise Pearson correlation coefficients between fetuin-A, age, BMI, plasma measures of ALT, lipids, hs-CRP, adiponectin, random insulin, random glucose and HbA1c levels, the Singapore Chinese Health Study
Odds ratios (95% confidence intervals) of type 2 diabetes mellitus by stratified analysis, the Singapore Chinese Health Study
Participant flowchart of the type 2 diabetes mellitus nested case-control study in the Singapore Chinese Health Study. HbA1c, glycosylated hemoglobin.
References
- 1.Levinthal GN, Tavill AS. Liver disease and diabetes mellitus. Clin Diabetes. 1999;17:1–20. [Google Scholar]
- 2.Rauth G, Poschke O, Fink E, Eulitz M, Tippmer S, Kellerer M, Haring HU, Nawratil P, Haasemann M, Jahnen-Dechent W, Muller-Esterl W. The nucleotide and partial amino acid sequences of rat fetuin. Identity with the natural tyrosine kinase inhibitor of the rat insulin receptor. Eur J Biochem. 1992;204:523–529. doi: 10.1111/j.1432-1033.1992.tb16663.x. [DOI] [PubMed] [Google Scholar]
- 3.Auberger P, Falquerho L, Contreres JO, Pages G, Le Cam G, Rossi B, Le Cam A. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell. 1989;58:631–640. doi: 10.1016/0092-8674(89)90098-6. [DOI] [PubMed] [Google Scholar]
- 4.Mathews ST, Singh GP, Ranalletta M, Cintron VJ, Qiang X, Goustin AS, Jen KL, Charron MJ, Jahnen-Dechent W, Grunberger G. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002;51:2450–2458. doi: 10.2337/diabetes.51.8.2450. [DOI] [PubMed] [Google Scholar]
- 5.Hennige AM, Staiger H, Wicke C, Machicao F, Fritsche A, Haring HU, Stefan N. Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS One. 2008;3:e1765. doi: 10.1371/journal.pone.0001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo VY, Cao B, Cai C, Cheng KK, Cheung BMY. Fetuin-A levels and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Acta Diabetol. 2018;55:87–98. doi: 10.1007/s00592-017-1068-9. [DOI] [PubMed] [Google Scholar]
- 7.Ix JH, Wassel CL, Kanaya AM, Vittinghoff E, Johnson KC, Koster A, Cauley JA, Harris TB, Cummings SR, Shlipak MG; Fetuin-A and incident diabetes mellitus in older persons. JAMA. 2008;300:182–188. doi: 10.1001/jama.300.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefan N, Fritsche A, Weikert C, Boeing H, Joost HG, Haring HU, Schulze MB. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes. 2008;57:2762–2767. doi: 10.2337/db08-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ix JH, Biggs ML, Mukamal KJ, Kizer JR, Zieman SJ, Siscovick DS, Mozzaffarian D, Jensen MK, Nelson L, Ruderman N, Djousse L. Association of fetuin-A with incident diabetes mellitus in community-living older adults: the cardiovascular health study. Circulation. 2012;125:2316–2322. doi: 10.1161/CIRCULATIONAHA.111.072751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laughlin GA, Barrett-Connor E, Cummins KM, Daniels LB, Wassel CL, Ix JH. Sex-specific association of fetuin-A with type 2 diabetes in older community-dwelling adults: the Rancho Bernardo study. Diabetes Care. 2013;36:1994–2000. doi: 10.2337/dc12-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Q, Cornelis MC, Manson JE, Hu FB. Plasma levels of fetuin-A and hepatic enzymes and risk of type 2 diabetes in women in the U.S. Diabetes. 2013;62:49–55. doi: 10.2337/db12-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aroner SA, Mukamal KJ, St-Jules DE, Budoff MJ, Katz R, Criqui MH, Allison MA, de Boer IH, Siscovick DS, Ix JH, Jensen MK. Fetuin-a and risk of diabetes independent of liver fat content: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2017;185:54–64. doi: 10.1093/aje/kww095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta D, Mondal SA, Kumar M, Hasanoor Reza AH, Biswas D, Singh P, Chakrabarti S, Mukhopadhyay S. Serum fetuin-A concentration predicts glycaemic outcomes in people with prediabetes: a prospective study from eastern India. Diabet Med. 2014;31:1594–1599. doi: 10.1111/dme.12539. [DOI] [PubMed] [Google Scholar]
- 14.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doi Y, Kubo M, Yonemoto K, Ninomiya T, Iwase M, Tanizaki Y, Shikata K, Iida M, Kiyohara Y. Liver enzymes as a predictor for incident diabetes in a Japanese population: the Hisayama study. Obesity (Silver Spring) 2007;15:1841–1850. doi: 10.1038/oby.2007.218. [DOI] [PubMed] [Google Scholar]
- 16.Wang YL, Koh WP, Yuan JM, Pan A. Association between liver enzymes and incident type 2 diabetes in Singapore Chinese men and women. BMJ Open Diabetes Res Care. 2016;4:e000296. doi: 10.1136/bmjdrc-2016-000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenzo C, Hanley AJ, Rewers MJ, Haffner SM. Discriminatory value of alanine aminotransferase for diabetes prediction: the Insulin Resistance Atherosclerosis Study. Diabet Med. 2016;33:348–355. doi: 10.1111/dme.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunutsor SK, Apekey TA, Walley J. Liver aminotransferases and risk of incident type 2 diabetes: a systematic review and meta-analysis. Am J Epidemiol. 2013;178:159–171. doi: 10.1093/aje/kws469. [DOI] [PubMed] [Google Scholar]
- 19.Chan JC, Yeung R, Luk A. The Asian diabetes phenotypes: challenges and opportunities. Diabetes Res Clin Pract. 2014;105:135–139. doi: 10.1016/j.diabres.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, Yu MC. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer. 2001;39:187–195. doi: 10.1207/S15327914nc392_5. [DOI] [PubMed] [Google Scholar]
- 21.Odegaard AO, Pereira MA, Koh WP, Arakawa K, Lee HP, Yu MC. Coffee, tea, and incident type 2 diabetes: the Singapore Chinese Health Study. Am J Clin Nutr. 2008;88:979–985. doi: 10.1093/ajcn/88.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roshanzamir F, Miraghajani M, Rouhani MH, Mansourian M, Ghiasvand R, Safavi SM. The association between circulating fetuin-A levels and type 2 diabetes mellitus risk: systematic review and meta-analysis of observational studies. J Endocrinol Invest. 2018;41:33–47. doi: 10.1007/s40618-017-0697-8. [DOI] [PubMed] [Google Scholar]
- 23.Reinehr T, Roth CL. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. J Clin Endocrinol Metab. 2008;93:4479–4485. doi: 10.1210/jc.2008-1505. [DOI] [PubMed] [Google Scholar]
- 24.Siddiq A, Lepretre F, Hercberg S, Froguel P, Gibson F. A synonymous coding polymorphism in the alpha2-Heremans-schmid glycoprotein gene is associated with type 2 diabetes in French Caucasians. Diabetes. 2005;54:2477–2481. doi: 10.2337/diabetes.54.8.2477. [DOI] [PubMed] [Google Scholar]
- 25.Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol. 2010;21:406–412. doi: 10.1681/ASN.2009080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 27.Ix JH, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley MA. Association between human fetuin-A and the metabolic syndrome: data from the Heart and Soul Study. Circulation. 2006;113:1760–1767. doi: 10.1161/CIRCULATIONAHA.105.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dasgupta S, Bhattacharya S, Biswas A, Majumdar SS, Mukhopadhyay S, Ray S, Bhattacharya S. NF-kappaB mediates lipid-induced fetuin-A expression in hepatocytes that impairs adipocyte function effecting insulin resistance. Biochem J. 2010;429:451–462. doi: 10.1042/BJ20100330. [DOI] [PubMed] [Google Scholar]
- 29.Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Krober SM, Machicao F, Fritsche A, Haring HU. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 2006;29:853–857. doi: 10.2337/diacare.29.04.06.dc05-1938. [DOI] [PubMed] [Google Scholar]
- 30.Stefan N, Schick F, Haring HU. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:2236–2237. doi: 10.1056/NEJMc1412427. [DOI] [PubMed] [Google Scholar]
- 31.Stefan N, Sun Q, Fritsche A, Machann J, Schick F, Gerst F, Jeppesen C, Joost HG, Hu FB, Boeing H, Ullrich S, Haring HU, Schulze MB. Impact of the adipokine adiponectin and the hepatokine fetuin-A on the development of type 2 diabetes: prospective cohort- and cross-sectional phenotyping studies. PLoS One. 2014;9:e92238. doi: 10.1371/journal.pone.0092238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotsopoulos J, Tworoger SS, Campos H, Chung FL, Clevenger CV, Franke AA, Mantzoros CS, Ricchiuti V, Willett WC, Hankinson SE, Eliassen AH. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses' Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19:938–946. doi: 10.1158/1055-9965.EPI-09-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefan N, Haring HU. Circulating fetuin-A and free fatty acids interact to predict insulin resistance in humans. Nat Med. 2013;19:394–395. doi: 10.1038/nm.3116. [DOI] [PubMed] [Google Scholar]
- 34.Mori K, Emoto M, Araki T, Yokoyama H, Lee E, Teramura M, Koyama H, Shoji T, Inaba M, Nishizawa Y. Effects of pioglitazone on serum fetuin-A levels in patients with type 2 diabetes mellitus. Metabolism. 2008;57:1248–1252. doi: 10.1016/j.metabol.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 35.Brix JM, Stingl H, Hollerl F, Schernthaner GH, Kopp HP, Schernthaner G. Elevated fetuin-A concentrations in morbid obesity decrease after dramatic weight loss. J Clin Endocrinol Metab. 2010;95:4877–4881. doi: 10.1210/jc.2010-0148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The pair-wise Pearson correlation coefficients between fetuin-A, age, BMI, plasma measures of ALT, lipids, hs-CRP, adiponectin, random insulin, random glucose and HbA1c levels, the Singapore Chinese Health Study
Odds ratios (95% confidence intervals) of type 2 diabetes mellitus by stratified analysis, the Singapore Chinese Health Study
Participant flowchart of the type 2 diabetes mellitus nested case-control study in the Singapore Chinese Health Study. HbA1c, glycosylated hemoglobin.