Abstract
Hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA) represent the most common primary liver malignancies whose outcome is influenced by the immune response. In the present study, we evaluated the tumor-infiltrating leukocyte (TIL) populations in 21 HCC patients and 8 CCA patients by flow cytometry immediately after the surgical procedure. Moreover, CD4+ T cells, CD8+ T cells, monocytes, and macrophages were purified by cell sorting for further analysis of gene expression by quantitative reverse-transcription polymerase chain reaction. Regarding tumor-infiltrating macrophages, we observed a significantly higher expression of markers associated with M2 phenotype and a higher expression of PD-L1 in patients with HCC in comparison to CCA. In addition, for HCC patients, we found a significant increase in the expression of CD200R in macrophages from tumors that were in grade G3-G4 as compared to tumors in grade G1-G2. Besides, a significantly higher frequency of tumor-infiltrating lymphocytes, CD8+CD56+ T cells, and natural killer cells was detected in HCC biopsies in comparison to CCA. In summary, this study has revealed functional and phenotypic differences in TIL cell subpopulations between CCA and HCC, as well as among different histopathological grades and tumor aggressiveness degrees, and it has provided evidence to better understand the tumor immune microenvironment of CCA and HCC.
Introduction
Hepatocellular carcinoma (HCC) is the most frequent type of liver cancer and presents high morbidity and mortality rates [1], [2]. It has a poor prognosis, generally due to its late presentation and, thus, late diagnostic. Cholangiocarcinoma (CCA), a malignancy that originates from biliary epithelia, is an aggressive cancer with high mortality rates [3] and, along with HCC, represents a major primary liver cancer. CCA is difficult to diagnose due to its silent and nonspecific clinical features, and in most cases, the symptoms occur when the tumor has reached an advanced stage [4]. For patients with advanced disease, current systemic treatment options provide only limited therapeutic benefit for a subset of patients, and novel therapeutic options to treat these carcinomas are needed [5]. The tumor microenvironment plays a vital role in tumor epigenetics, tumor differentiation, immune escape, and metastasis [6]. Accordingly, an increasing body of evidence supports the utility of immunotherapy as antitumor treatment, including active vaccination, adoptive cell transfer therapy, and immune checkpoint blockade [7]. There are many clinical trials to assess these therapies, and the results have demonstrated a definite clinical application value [8]. Therefore, an evaluation of the tumor microenvironment may facilitate and prioritize development of new immunotherapy strategies for HCC and CCA patients. Previous clinical studies have found that the composition of tumor-infiltrating leukocytes (TILs) may correlate with prognosis in cancer patients [9], [10], [11]. In HCC, most studies indicate that high levels of CD8+ and CD3+ TILs have a better prognostic value on overall survival, while high levels of FOXP3+ TILs have been associated with a worse prognosis [7]. In addition, high presence of CD206+ tumor-associated macrophages (TAMs) was markedly correlated with aggressive tumor phenotypes, such as multiple tumor number and advanced TNM stage, as well as being associated with poor overall survival in HCC patients [12]. In the same way, low versus high CD206+ TAMs density has been associated with better overall survival in HCC, suggesting that the characterization of CD206+ tumor-infiltrating macrophages could be considered to improve the risk stratification system. In CCA, high tumor-associated neutrophils, low CD8+ T cell, and high Treg populations, as well as the enrichment of CD163+ TAMs, have been correlated with poor prognosis and have been associated with worse overall survival in human extrahepatic CCA patients with surgical resection [13]. Moreover, high levels of TAMs in the tumor invasive front or absence of histologic tumor necrosis has been associated with a significantly improved recurrence-free and overall survival of patients with intrahepatic CCA [14].
In this study, we have functional and phenotypically characterized, by flow cytometry and by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) expression analysis, the TIL populations of a group of patients with HCC and CCA immediately after surgical resection, determining its association with tumor grades and aggressiveness, in order to detect prognosis biomarkers as well as identify new molecular targets for the development of new immunotherapy strategies in neoadjuvant, adjuvant, or palliative setting.
Materials and Methods
Patients
Twenty-one patients with HCC (3 women and 18 men; average age: 62.2 ± 14.5 years) and 8 patients with CCA (5 women and 3 men; average age: 61.0 ± 14.7 years) were included in this study. The clinical background of the patients included in this study is summarized in Table 1. Patients were classified according to the eighth TNM classification, and tumors were categorized depending on their histopathological grading. Moreover, for purposes of classification, HCC patients were dichotomized into low risk and high risk, taking into consideration the pathological features described in the literature as predictors of worst outcome and poor prognosis. Tumors were considered of high risk if one or more of the following features were present: microvascular invasion [15]; stage III or superior in the American Joint Committee for Cancer Classification [16]; tumor grade equal or superior than G3, as defined by Edmonson [17], [18]; and cytokeratin 19 expression [19].
Table 1.
Clinical Data from HCC and CCA Patients Enrolled in This Study
| CCA (n = 8) |
HCC (n = 21) |
||
|---|---|---|---|
| Age | 61 ± 15 | 62 ± 15 | |
| Sex | Female | 5 (63%) | 3 (14%) |
| Male | 3 (38%) | 18 (86%) | |
| TNM | Stage I | 1 (13%) | 3 (14%) |
| Stage II | 4 (50%) | 16 (76%) | |
| Stage IIIA | 0 (0%) | 1 (5%) | |
| Stage IV | 3 (38%) | 1 (5%) | |
| Histologic grade (G) | G1 | 2 (25%) | 2 (9%) |
| G2 | 3 (38%) | 12 (57%) | |
| G3 | 3 (38%) | 6 (29%) | |
| G4 | 0 (0%) | 1 (5%) | |
| Risk stratification | Low | 2 (25%) | 10 (48%) |
| High | 6 (75%) | 11 (52%) | |
| HBsAg | Positive | 0 (0%) | 1 (5%) |
| HCV | Positive | 0 (0%) | 6 (29%) |
| Cirrhosis | 0 (0%) | 16 (76%) | |
| Relapse | 3 (38%) | 1 (5%) | |
| Death | 3 (38%) | 2 (9%) | |
| Liver transplant | 0 (0%) | 7 (33%) | |
Number (N) and percentage (%) of cases are indicated.
Samples were collected immediately after surgical procedure, and no patients received any antitumor therapy or medication prior to surgery. Cells from biopsies were mechanically extracted from tumors using syringes and needles in phosphate-buffered saline (PBS; Gibco, Life Technologies, Carlsbad, CA) and immediately processed.
Study Approval
The experimental protocols were approved by the Ethical Committee of the Faculty of Medicine, University of Coimbra, Coimbra, Portugal (CE-136/2016). All procedures performed involving human participants were in accordance with the ethical standards of Ethical Committee of the Faculty of Medicine, University of Coimbra, Coimbra, Portugal (CE-136/2016), and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All participants gave their signed informed consent before entering in the study.
Immunophenotypic Study of Tumor Cells and Tumor-Infiltrating Leukocytes
Cells from the biopsies were mechanically extracted in PBS and aliquoted in different tubes for immunophenotyping.
For the immunophenotypic study of tumor cells, monocytes, and macrophages, 250 μl of biopsy sample was stained with the monoclonal antibodies (mAbs) described in Supplementary Table S1 (tube 1) and incubated for 10 minutes at room temperature (RT) in the dark. After this incubation period, samples were incubated with 2 ml of FACS Lysing solution [Becton Dickinson Biosciences (BD), San Jose, CA] for 10 min in the dark at RT and then centrifuged for 5 minutes at 540g. The supernatant was discarded, and the cell pellet was washed twice in 1 ml of PBS, resuspended in 0.5 ml of PBS, and immediately acquired.
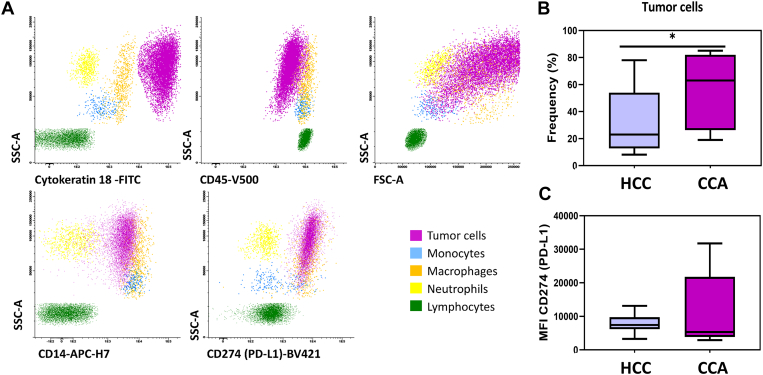
Macrophages and monocytes were identified and distinguished based on CD14 and CD45 positivity and their typical forward scatter (FSC) and side scatter (SSC) light dispersion properties, whereas tumor cells were identified by the absence of expression of CD45, the expression of cytokeratin 18 and specific FSC and SSC light dispersion properties (Figure 1A). The expression of membranous markers such as CD200R, CD80, CD206, CD163, and PD-L1 (CD274) on monocytes and macrophages infiltrating the tumor was assed. Moreover, the expression of membranous PD-L1 (CD274) on tumor cells was also assessed by flow cytometry.
Figure 1.
Phenotypic characterization of tumor biopsies from HCC and CCA patients. (A) Bivariate dot plot histograms illustrating the identification of tumor cells (purple events), monocytes (blue events), macrophages (orange events), neutrophils (yellow events), and lymphocytes (green events) infiltrating tumor biopsies. (B) Boxplot with the frequency of tumor cells identified in each group of patients (HCC and CCA) and (C) boxplot with the expression levels of CD274 (PD-L1) in tumor cells, measured as MFI. The results are given by median with interquartile range. Statistical significant differences were considered when P < .05; ∗between the groups indicated in the figure.
To evaluate cytokine production by T cells infiltrating the tumor, lymphocytes were stimulated by adding phorbol myristate acetate (PMA; 0.25 μg/ml, Sigma-Aldrich, Saint Louis, MO) and ionomycin (1 μg/ml, Boehringer Mannheim, Germany) to 250 μl of sample previously diluted 1:1 (v/v) in RPMI 1640 complete culture medium (Invitrogen, Life Technologies, Carlsbad, CA). Brefeldin-A (10 μg/ml, Sigma-Aldrich) was added to prevent the release of the newly synthesized cytokines. All samples were then incubated in a sterile environment and 5% CO2 humid atmosphere, at 37°C, for 4 hours.
Immunophenotypic analysis of tumor-infiltrating T cells and natural killer (NK), cultured in the presence of PMA plus ionomycin, was performed by using a seven-color mAb combination, detailed in Supplementary Table S1 (tube 2). In short, cells were stained with the mAbs for surface proteins antigens (CD45, CD4, CD56, CD3, and CD8) and, after an incubation period of 10 minutes in the dark at RT, washed with PBS. For intracellular staining, Fix&Perm (Caltag, Hamburg, Germany) reagent was used in accordance with the instructions of the manufacturer, and samples were stained with the mAbs for IFN-γ and IL-17 (Supplementary Table S1, tube 2). After washing twice with PBS, the cell pellet was resuspended in 500 μl of PBS and immediately acquired.
T cells were identified on the basis of CD3 and CD45 positivity and intermediate FSC and SSC properties. Within this cell population, CD4+ and CD8+ T-cell subsets (phenotypically characterized as CD3+CD4+CD8− and CD3+CD4−CD8+, respectively) were identified. NK cells were identified on the basis of CD45 and CD56 positivity, absence of CD3 expression, and intermediate FSC and SSC properties.
Flow Cytometry Data Acquisition and Analysis
Data acquisition was performed in a FACSCanto II flow cytometer (BD) and analyzed with Infinicyt 1.8 software (Cytognos SL, Salamanca, Spain).
Cell Purification by Fluorescence-Activated Cell Sorting
Macrophages, monocytes, and CD4+ and CD8+ T-cell populations from the biopsies were purified by fluorescence-activated cell sorting (FACS) using a FACSAria III flow cytometer (BD) according to their typical phenotype. Thus, the seven-color mAb combination used (Supplementary Table S1, tube 3) allowed the identification of CD4+ T cells (CD3+CD4+CD8−) and CD8+ T cells (CD3+CD4−CD8+). The remaining mAbs found in the panel were used to identify macrophages and monocytes (CD14+ CD45+CD33+). Cell suspensions were centrifuged for 5 minutes at 300g, and the pellet was resuspended in 350 μl of RLT Lysis Buffer (Qiagen, Hilden, Germany).
The purified cell populations were subsequently stored at −80°C for the quantification of mRNA expression.
Evaluation of mRNA Expression by qRT-PCR
Total RNA was extracted and purified with RNeasy Micro Kit (Qiagen) according to the manufacturer's instructions. Reverse transcription was performed with SensiScript Reverse Transcription Kit (Qiagen) according to supplier's instructions and with Random Hexamer Primer (Thermo Fisher Scientific, San Jose, CA). Relative quantification of gene expression was performed in a QuantStudio (Thermo Fisher Scientific) by a real-time qRT-PCR. qRT-PCR was done with PowerUp SYBR Green Master Mix (Thermo Fisher Scientific) using optimized primers for IFN-γ, IL-17, FOXP3, IL-10, TNFα, and endogenous control glyceraldehyde 3-phosphate dehydrogenase (Qiagen) according to the manufacturer's instructions.
Statistical Analysis
For all variables under study, their mean values, standard deviation, median, and range were calculated. To determine the statistical significance of the differences observed between groups, the nonparametric Mann-Whitney comparison test was performed using the Statistical Package for Social Sciences software (SPSS, version 19, IBM, Armonk, NY). Statistically significant differences were considered when P < .05. The Spearman rank test was used to evaluate the correlation among variables.
Results
Phenotypic Characterization of Biopsies' Populations
From the data obtained by flow cytometry immunophenotyping, we observed a significantly higher frequency of tumor cells (cytokeratin18+ CD45−) in the analyzed biopsies from CCA patients in comparison to HCC (Figure 1B). In both groups of cancer patients, tumor cells were positive for PD-L1 (CD274) and presented no significant differences among PD-L1 protein expression [measured as mean fluorescence intensity (MFI)] (Figure 1C).
Regarding tumor-infiltrating major leukocyte subsets, we observed a higher frequency of lymphocytes infiltrating HCC tumors in comparison to CCA (global mean), while no significant differences were observed among the different risk stratification categories or tumor grades either in HCC or in CCA (Table 2).
Table 2.
Frequency (%) of Tumor-Infiltrating Leukocyte Subsets in HCC and CCA BIOPSIES, Classified in Different Tumor Grades and Risk Stratification Categories
| % | Monocytes |
Macrophages |
Lymphocytes |
Neutrophils |
||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| CCA (global mean) | (n = 8) | 1.44 ± 1.29 | 2.07 ± 1.83 | 7.7 ± 5.6a | 14.8 ± 16.7 | |
| CCA | Risk stratification | Low | 0.40 ± 0.42 | 2.25 ± 2.90 | 8.0 ± 8.5 | 6.8 ± 2.5 |
| High | 1.97 ± 1.28 | 1.98 ± 1.65 | 7.5 ± 5.4 | 18.9 ± 20.0 | ||
| Grades | G1-G2 | 1.17 ± 1.16 | 2.08 ± 1.81 | 5.8 ± 5.5 | 19.8 ± 18.8 | |
| G3-G4 | 2.00 ± 1.84 | 2.05 ± 2.62 | 11.4 ± 5.2 | 4.9 ± 5.9 | ||
| HCC (global mean) | (n = 21) | 2.56 ± 3.16 | 1.24 ± 1.23 | 33.1 ± 20.8a | 7.2 ± 5.7 | |
| HCC | Risk stratification | Low | 3.52 ± 4.16 | 1.36 ± 1.36 | 30.4 ± 21.9 | 5.5 ± 4.4 |
| High | 1.49 ± 0.67 | 1.08 ± 1.13 | 36.1 ± 20.7 | 8.9 ± 6.6 | ||
| Grades | G1-G2 | 2.75 ± 3.74 | 1.08 ± 1.24 | 28.7 ± 19.9 | 7.4 ± 6.6 | |
| G3-G4 | 2.10 ± 0.94 | 1.83 ± 1.22 | 43.6 ± 21.2 | 6.7 ± 3.4 | ||
Independent-samples Mann-Whitney U test and Kruskal-Wallis multiple comparison tests were performed with a significance level of .05 (P < .05). The results are given as mean ± SD. Global mean indicates mean values in CCA and HCC groups without discrimination among tumor grades or stages.
CCA vs. HCC.
Immunophenotyping of Tumor-Infiltrating Lymphocytes
As shown in Table 3, the frequency of NK cells infiltrating HCC tumors was significantly higher compared to the frequency of NK-infiltrating CCA tumor biopsies. Moreover, we observed that the frequency of CD8+ T cells with NK activity (CD56+) infiltrating HCC tumor biopsies was significantly higher in comparison to CCA (Table 3). In addition, the expression of IL-17 by CD8+ T cells, measured as MFI, was significantly higher in HCC tumors as compared to CCA. However, no significant differences were observed among the different risk stratification categories or tumor grades for tumor-infiltrating lymphocyte subsets in HCC patients (Table 4). Due to the small number of cases presenting CCA, we did not subdivide this group of patients into other categories.
Table 3.
Frequency (%) of Tumor-Infiltrating Lymphocyte Subsets in HCC and CCA Biopsies
| CCA n = 8 |
HCC n = 21 |
Statistical Significance |
||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | P Value | ||
| %LyT | 10.8 ± 8.7 | 16.7 ± 12.7 | .353 | |
| % LyT CD4 | 49 ± 11 | 57 ± 20 | .312 | |
| % IFNγ | 46.3 ± 22.3 | 60.9 ± 26.8 | .282 | |
| MFI IFNγ | 6067 ± 1883 | 8999 ± 4937 | .179 | |
| % IL17 | 3.08 ± 1.81 | 2.47 ± 1.62 | .521 | |
| MFI IL17 | 283 ± 84 | 387 ± 150 | .152 | |
| %IFNγ+IL17+ | 1.42 ± 0.86 | 1.53 ± 0.80 | .750 | |
| % LyT CD8 | 36 ± 11 | 26 ± 13 | .207 | |
| % IFNγ | 62 ± 23 | 66 ± 25 | .701 | |
| MFI IFNγ | 3914 ± 1355 | 4706 ± 2151 | .639 | |
| % IL17 | 1.13 ± 1.63 | 1.06 ± 1.24 | .831 | |
| MFI IL17 | 223 ± 42 | 355 ± 123 | .045 | |
| %CD56+ | 8.5 ± 6.6 | 21.7 ± 14.2 | .041 | |
| %IFNγ+IL17+ | 0.36 ± 0.38 | 1.01 ± 1.07 | .313 | |
| % NK | 0.60 ± 0.37 | 2.79 ± 3.82 | .041 | |
| % IFNγ | 41 ± 15 | 50 ± 25 | .579 | |
| MFI IFNγ | 2049 ± 679 | 2456 ± 866 | .282 | |
| % CD8+ | 5.2 ± 5.1 | 2.6 ± 2.6 | .494 |
Protein expression levels of IFN-γ and IL-17 (measured as MFI) are also indicated. Independent-samples Mann-Whitney U test was performed with a significance level of .05 (P < .05). The results are given as mean ± SD.
Table 4.
Frequency (%) of Tumor-Infiltrating Lymphocyte Subsets in HCC Biopsies, Classified into Tumors That Were in Grade G1 or G2 (G1-G2) and Tumors That Were in Grade G3 or G4 (G3-G4), and Dichotomized into Low Risk and High Risk.
| Hepatocellular carcinoma |
||||||||
|---|---|---|---|---|---|---|---|---|
| Histologic grade |
Stratification risk |
|||||||
| G1-G2 |
G3-G4 |
p-value |
Low |
High |
p-value |
|||
| Mean ± SD | Mean ± SD | Sig. | Mean ± SD | Mean ± SD | Sig. | |||
| %LyT | 14.8 ± 12.6 | 20.0 ± 13.6 | 0.3710 | 18 ± 14 | 25 ± 19 | 0.6889 | ||
| % LyT CD4 | 52 ± 20 | 66 ± 19 | 0.1645 | 45 ± 22 | 60 ± 18 | 0.2721 | ||
| % IFNγ | 58.2 ± 28.6 | 65.2 ± 26.1 | 0.6064 | 55 ± 17 | 50 ± 37 | 0.8981 | ||
| MFI IFNγ | 9306 ± 5885 | 8507 ± 3466 | 0.6993 | 9535 ± 5151 | 7676 ± 4155 | 0.6993 | ||
| % IL17 | 1.85 ± 1.12 | 3.45 ± 1.93 | 1 | 2.86 ± 3.01 | 1.31 ± 1.63 | 0.2977 | ||
| MFI IL17 | 376 ± 118 | 404 ± 206 | 0.3543 | 367 ± 98 | 439 ± 180 | 0.5237 | ||
| %IFNγ+IL17+ | 1.35 ± 0.75 | 1.78 ± 0.88 | 0.5237 | 1.89 ± 2.42 | 1.00 ± 1.27 | 0.5035 | ||
| % LyT CD8 | 28 ± 12 | 22 ± 16 | 0.8591 | 25 ± 14 | 25 ± 16 | 0.9546 | ||
| % IFNγ | 63 ± 29 | 71 ± 19 | 0.7972 | 58 ± 18 | 54 ± 32 | 0.7972 | ||
| MFI IFNγ | 5081 ± 2561 | 4105 ± 1292 | 0.6993 | 5233 ± 2401 | 3926 ± 1366 | 0.2977 | ||
| % IL17 | 0.99 ± 1.39 | 1.17 ± 1.09 | 0.8329 | 1.00 ± 1.13 | 0.56 ± 1.03 | 0.3543 | ||
| MFI IL17 | 325 ± 81 | 405 ± 184 | 0.5476 | 338 ± 67 | 433 ± 184 | 0.7143 | ||
| %IFNγ+IL17+ | 1.07 ± 1.28 | 0.93 ± 0.81 | 0.4762 | 0.70 ± 0.68 | 0.54 ± 0.83 | 0.5167 | ||
| %CD56+ | 23.5 ± 12.2 | 18.5 ± 18.4 | 0.6787 | 22 ± 9 | 22 ± 23 | 0.5287 | ||
| % NK | 3.72 ± 4.54 | 1.12 ± 0.76 | 0.2544 | 2.83 ± 3.61 | 2.82 ± 4.51 | 0.6070 | ||
| % IFNγ | 52 ± 22 | 48 ± 32 | 1 | 54 ± 21 | 45 ± 35 | 0.8981 | ||
| MFI IFNγ | 2586 ± 640 | 2247 ± 1203 | 0.3636 | 2699 ± 892 | 2265 ± 1186 | 0.4376 | ||
| % CD8+ | 2.0 ± 1.5 | 3.7 ± 4.0 | 0.4396 | 6.2 ± 6.1 | 4.7 ± 7.5 | 0.3880 | ||
Protein expression levels of IFN-γ and IL-17 (measured as MFI) are also indicated. Independent-samples Mann-Whitney U test was performed to compare G1-G2 vs. G3-G4 and low- vs. high-classification groups with a significance level of .05 (P < .05). The results are given as mean ± SD.
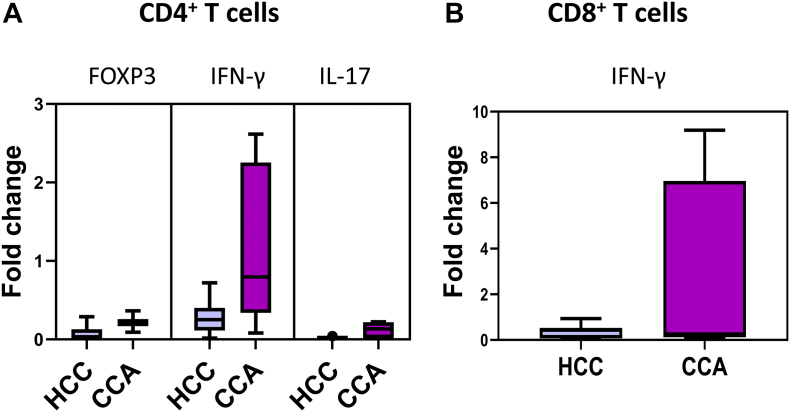
Additionally, CD4+ T cells infiltrating tumor biopsies presented higher expression levels of IFNγ mRNA, measured by qRT-PCR, in comparison with FOXP3 and IL-17 expression levels. Moreover, in HCC patients, a nonsignificant decrease was observed in the expression levels of IFN-γ mRNA, expressed by both CD4+ and CD8+ T cells infiltrating tumor biopsies, in comparison to CCA infiltrating CD4+ and CD8+ T cells (Figure 2).
Figure 2.
Functional characterization of tumor-infiltrating T lymphocytes. (A) Boxplots with the mRNA expression levels of FOXP3, IFNγ, and IL-17 by tumor-infiltrating CD4+ T cells (purified by cell sorting) in HCC and CCA patients. (B) Boxplot with the mRNA expression levels of IFNγ by tumor-infiltrating CD8+ T cells (purified by cell sorting) in HCC and CCA patients.
Immunophenotyping of Tumor-Infiltrating Monocytes and Macrophages
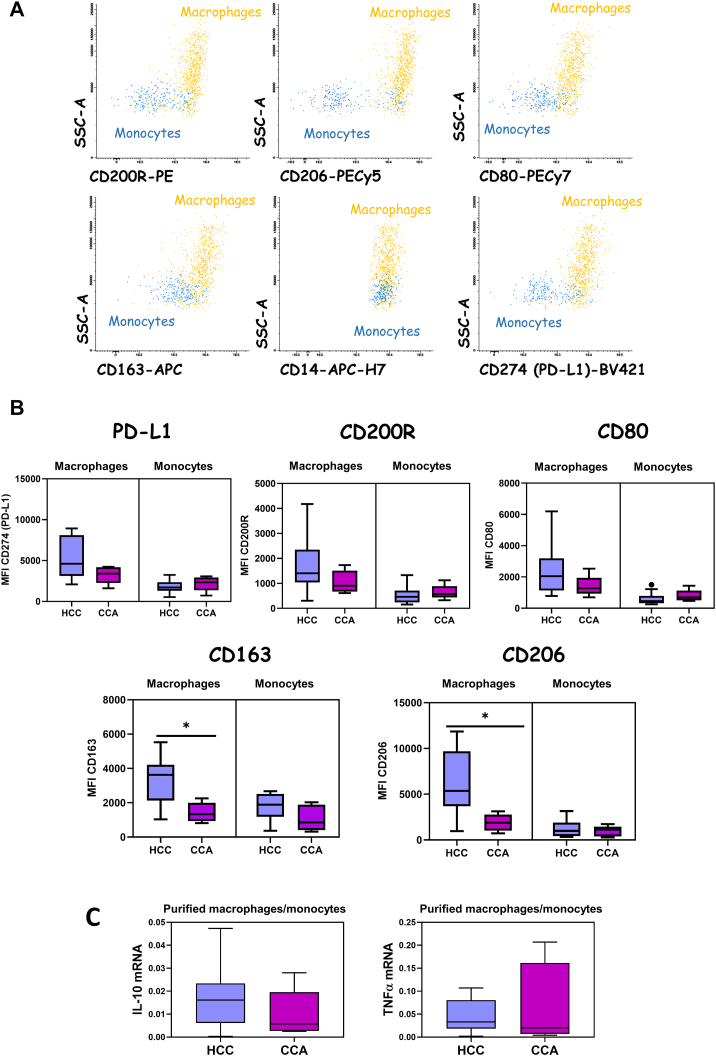
Macrophages and monocytes were identified and distinguished based on CD14 and CD45 positivity and their typical FSC and SSC light dispersion properties, as indicated in Figure 3A. After characterization of the populations of interest, we observed a significantly higher expression of CD206 and CD163 in HCC-infiltrating macrophages in comparison to CCA (Figure 3B). Additionally, a nonsignificant higher expression of PD-L1 (CD274), CD200R, and CD80 markers in HCC-infiltrating macrophages in comparison to CCA was observed (Figure 3B), while no significant differences were found for tumor-infiltrating monocytes among cancer patient groups. After the purification of macrophages and monocytes by cell sorting, we measured the mRNA levels of IL-10 and TNFα by qRT-PCR. The expression levels of IL-10 mRNA among tumor-infiltrating macrophages/monocytes were higher in the HCC group when compared to the CCA group, without reaching statistical significance (Figure 3C). On the other hand, no differences were observed on TNFα mRNA expression among macrophages/monocytes infiltrating CCA or HCC tumor biopsies (Figure 3C).
Figure 3.
Phenotypic characterization of tumor-infiltrating monocytes and macrophages in HCC and CCA patients. (A) Bivariate dot plot histograms illustrating the phenotypic characterization of monocytes (blue events) and macrophages (orange events) infiltrating tumor biopsies. (B) Comparison among HCC and CCA tumor-infiltrating macrophages and monocytes expression levels of CD274 (PD-L1), CD200R, CD206, CD80, and CD163 markers, measured as MFI. The results are given by median with interquartile range. Statistically significant differences were considered when P < .05; ∗between the groups indicated in the figure. (C) Boxplots with the mRNA expression levels of IL-10 and TNFα by tumor-infiltrating macrophages (purified by cell sorting) in HCC and CCA. The results are given as median with interquartile range.
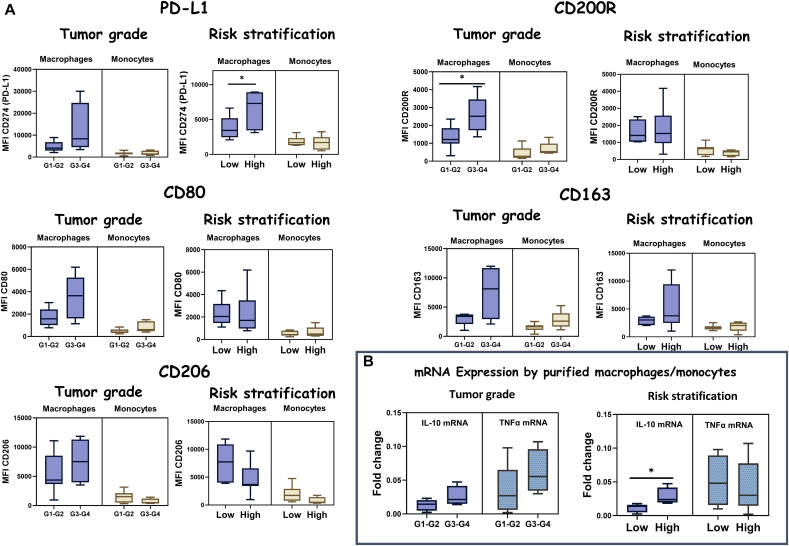
For HCC patients, we observed higher expression levels (MFI) of markers such as PD-L1 (CD274), CD200R, CD80, CD163, and CD206 in macrophages infiltrating tumors that were in grade G3 or G4 (n = 7) in comparison with tumors that were in grade G1 or G2 (n = 14) (Figure 4A). In fact, a significant increase in the expression levels of CD200R (MFI) in macrophages from tumors that were in grade G3 or G4, in comparison to tumors that were in grade G1 or G2, was observed (Figure 4A). In the same line, an increase of CD163 and CD80 expression (MFI) in both monocytes and macrophages infiltrating tumors that were in grade G3 or G4 in comparison to tumors that were in grade G1 or G2 was detected, although without reaching statistical significance (Figure 4A). When comparing tumors classified into different risk stratification, we observed significantly higher expression levels of PD-L1 (CD274) by macrophages infiltrating tumors that were considered with a high degree of aggressiveness in comparison to macrophages infiltrating tumors classified as low risk (Figure 4A). However, a nonsignificant decrease was detected in the expression of CD206 by tumor-infiltrating macrophages in high-risk tumors as compared to low risk, and no differences were observed on the other markers when comparing MFIs.
Figure 4.
Phenotypic characterization of tumor-infiltrating monocytes and macrophages in HCC tumors. (A) Comparison of tumor-infiltrating macrophages and monocytes expression levels of CD274 (PD-L1), CD200R, CD80, CD206, and CD163 markers, measured as MFI, among HCC tumors that were in grade G3 or G4 and tumors that were in grade G1 or G2 (Tumor grade) and comparing tumors that were considered as low risk or low degree of aggressiveness (low) with tumors that were considered as high risk or high degree of aggressiveness (high) (risk stratification). (B, left) Boxplots with the mRNA expression levels of IL-10 and TNFα among purified macrophages/monocytes from HCC biopsies comparing tumors that were in grade G1 or G2 with tumors that were in grade G3 or G4. (Right) Boxplots with the mRNA expression levels of IL-10 and TNFα among purified macrophages/monocytes from HCC biopsies comparing tumors that were considered as low risk or low degree of aggressiveness (low) with tumors that were considered as high risk or high degree of aggressiveness (high).
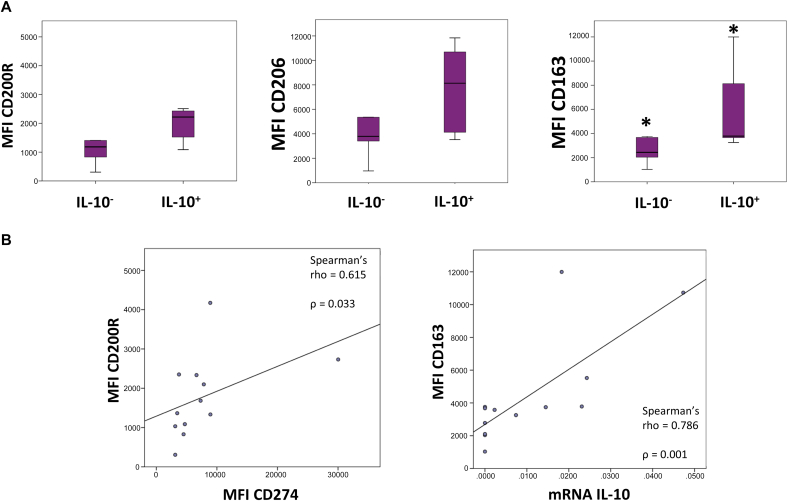
Furthermore, we observed a higher IL-10 mRNA expression among macrophages/monocytes infiltrating tumors that were in grade G3 or G4 in comparison to tumors that were in G1 or G2 in HCC patients, and minor differences in the case of TNFα mRNA expression, which was higher in macrophages infiltrating HCC tumors that were in grade G3 or G4 in comparison to tumors in G1 or G2 (Figure 4B). Regarding the dichotomized classification into low risk and high risk based on the aggressiveness of the tumor, we observed a significant increase in IL-10 mRNA expression among macrophages/monocytes infiltrating HCC tumors that were considered to have a high degree of aggressiveness as compared to those with a low degree of aggressiveness or low risk (Figure 4B). In contrast, regarding TNFα mRNA expression, we observed slightly decreased levels of expression of TNFα mRNA among macrophages/monocytes infiltrating HCC tumors classified as high risk or high degree of aggressiveness as compared to low risk (Figure 4B). Moreover, comparing HCC macrophages that presented amplification for IL-10 mRNA expression by qPCR (IL-10+) and those who did not (IL-10−), we observed elevated levels of expression of markers associated with protumor macrophages such as CD200R, CD206, and CD163, measured as MFI, in IL-10+ macrophages in comparison to IL-10− macrophages (Figure 5A). In addition, we observed a positive correlation between IL-10 mRNA expression in macrophages and the expression of CD163 in macrophages infiltrating HCC tumors, assessed by Spearman's rank correlation (rho = 0.786), with P = .001 (Figure 5B). Moreover, a positive correlation among CD274 (PD-L1) expression (MFI) and CD200R expression (MFI) in HCC tumor-infiltrating macrophages was observed assessed by Spearman's rank correlation (rho = 0.615), with P = .033 (Figure 5B).
Figure 5.
Tumor-infiltrating macrophages in HCC. (A) Expression levels of CD200R, CD206, and CD163 markers, measured as MFI, by HCC tumor-infiltrating macrophages that presented amplification for IL-10 mRNA expression by qRT-PCR (IL-10+) in comparison to those that did not (IL-10−). The results are given by median with interquartile range. (B, left) Positive correlation among CD274 (PD-L1) MFI and CD200R MFI in HCC tumor-infiltrating macrophages (rho = 0.615) as assessed by Spearman's rank correlation, with P = .033. (Right) Positive correlation between CD163 expression (MFI) and IL-10 mRNA expression by HCC tumor-infiltrating macrophages (rho = 0.786) as assessed by Spearman's rank correlation, with P = .001. Statistical significant differences were considered when P < .05; ∗between the groups indicated in the figure.
Discussion
To evaluate the potential of TIL subsets in the pathogenic progression of CCA and HCC, we examined the phenotype and clinical relevance of TIL subsets in tumor biopsies from CCA and HCC patients, collected immediately after surgical procedure and classified into different histopathological grades and aggressiveness degree (risk stratification), by multicolor flow cytometry and qRT-PCR. Despite the great variability detected among different individuals, we have observed different types of common patterns associated with the pathogenesis of these tumors.
Regarding tumor-infiltrating monocytes and macrophages in HCC, we have not detected differences in the total frequency of infiltrating monocytes and macrophages in tumors classified within different grades or risk stratification. However, after the phenotypic and functional characterization, interesting findings came out. In general, the phenotype of TAMs can be categorized into two subpopulations, and each of them has diverse effects on the tumor microenvironment: M1 macrophages, which have been associated with antitumor activity, and M2 macrophages, which exert protumor effects [20], [21]. In our study, we have observed higher expression levels of markers associated to M2 phenotype (CD206 and CD163), together with a higher expression of PD-L1 (CD274) in macrophages infiltrating HCC tumors that were in grade G3 or G4 compared to the expression levels of macrophages infiltrating HCC tumors in grade G1 or G2. Moreover, taking into account the dichotomized classification into low risk and high risk based on the aggressiveness of the tumor, we have observed significantly higher expression levels of PD-L1 by macrophages infiltrating tumors that were considered to have a high degree of aggressiveness or high risk as compared to macrophages infiltrating tumors classified as low risk. Furthermore, we observed as a highlighted result that the marker CD200R was significantly increased in macrophages infiltrating HCC tumors in grade G3 or G4 compared to the expression levels of macrophages infiltrating HCC tumors in grade G1 or G2. In addition, we identified a positive correlation among CD274 (PD-L1) expression and CD200R expression, measured as MFI, in HCC tumor-infiltrating macrophages, assessed by Spearman's rank correlation. CD200 is a cell membrane protein that interacts with CD200 receptor (CD200R) of myeloid lineage cells. During tumor initiation and progression, CD200-positive tumor cells can interact with macrophages through CD200-CD200R complex, leading to different mechanism such as the inhibition of immune cells, the modulation of cytokine profiles from Th1 to Th2, the differentiation of T cells into regulatory T cells, and the facilitation of anti-inflammatory IL-10 and TGF-β production [22], [23], [24]. Our results are in line with previous studies which had already suggested a promising role of CD200R as a prognostic marker in predicting elevated recurrence and reduced survival, and a potential therapeutic target in treating HCC [23]. Regarding the functional characterization of HCC-infiltrating macrophages, macrophages infiltrating HCC tumors that were in grade G3 or G4 presented higher expression levels of IL-10 mRNA when compared to tumors in G1 or G2, without reaching statistical significance. Accordingly, in HCC tumors classified as high risk or high degree of aggressiveness, we observed a significantly higher expression of IL-10 mRNA by infiltrating macrophages when compared to macrophages infiltrating tumors classified as low risk or low degree of aggressiveness, supporting the idea that M2 macrophages possess an important role in advanced HCC tumors. Despite the fact that immunophenotyping did not allow a strict classification into M1 or M2 macrophages, we observed that HCC macrophages that presented amplification for IL-10 mRNA expression by qPCR (IL-10+) exhibited a higher expression of markers associated with protumor macrophages such as CD200R, CD206, and CD163 when compared to those who did not present amplification for IL-10 mRNA (IL-10−). Moreover, we found a positive correlation between CD163 protein expression, measured as MFI, and IL-10 mRNA expression for HCC-infiltrating macrophages. However, for HCC, TNFα expression seems to be increased in HCC tumors that were in grade G3 or G4 in comparison to tumors in grade G1 or G2. Therefore, our data suggest that TNFα expression levels cannot be used as an indicator of M1 phenotype. In fact, it has been previously reported that individual TAMs frequently coexpressed canonical markers of both M1 and M2 activation as IL-10 and TNFα [25]. Three distinctive HCC subtypes with immunocompetent, immunodeficient, and immunosuppressive features have been previously defined by multiomics approaches. Our results suggest that HCC tumors that were in grade G3 or G4 displayed a phenotype that could be classified into HCC subtype 3 (immunosuppressive subtype), characterized by normal lymphocyte infiltration, macrophages with higher expression levels of PD-L1, and increased frequencies of immunosuppressive cells [26]. In this type of scenario, immune checkpoint inhibitors therapy may be a reasonable approach for patients under this classification.
Regarding tumor-infiltrating monocytes and macrophages in CCA, we have not detected differences in the total frequency of infiltrating monocytes and macrophages in different grades or risk groups. Due to the small number of cases presenting CCA, we did not subdivide this group of patients, but we compared the results obtained with the HCC patients' group. In this regard, we have observed significantly higher expression of markers associated to M2 phenotype (CD206 and CD163), together with a higher expression of PD-L1 (CD274), in patients with HCC in comparison to CCA patients. Moreover, macrophages from HCC biopsies presented higher expression of IL-10 mRNA when compared to CCA tumor-infiltrating macrophages, indicating a higher infiltration of protumor macrophages in HCC patients in comparison to CCA. In contrast, we did not observe differences in the levels of TNFα mRNA expression by macrophages between the two groups of cancer patients.
Regarding tumor-infiltrating lymphocytes, we have detected a higher frequency of lymphocytes infiltrating HCC tumor biopsies in comparison to CCA. Moreover, among lymphocyte subsets, we have observed a higher frequency of NK cells as well as CD8+ T cells with NK activity (CD56+) infiltrating HCC tumors in comparison to CCA tumor biopsies. A successful antitumor immune response requires recruitment of specific T cells followed by recognition of tumor antigens and of NK cells, and the generation of antitumor cytotoxicity. In this sense, it has been previously reported that HCC tumors contained significant numbers of both T cells and NK cells at various stages (II/III/IV) [27]. While several reports demonstrated the inefficacy of NK cells in controlling tumor growth and invasion, NK cells' role in the prevention of metastasis has been described in different types of cancer, and a higher number of tumor-infiltrating NK cells have been associated with a better prognosis [28], [29]. Therefore, this higher infiltration of NK cells and CD8+ CD56+ T cells in HCC tumors could contribute to the better prognosis of HCC patients in comparison to CCA [19]. Interestingly, CD4+ T cells infiltrating tumor biopsies presented higher expression levels of IFNγ mRNA, measured by qRT-PCR, in comparison with FOXP3 and IL-17 mRNA expression levels in both HCC and CCA. Moreover, in HCC patients, a nonsignificant decrease in the expression levels of IFN-γ mRNA, expressed by both CD4+, and CD8+ T cells infiltrating tumor biopsies, in comparison to CCA infiltrating CD4+ and CD8+ T cells was observed, without reaching statistical significance.
Finally, we have detected a higher frequency of tumor cells in CCA tumor biopsies in comparison to HCC. In addition, in both groups of patients, tumor cells presented high expression levels of PD-L1 (CD274). It is well established that PD-L1 binding to PD-1 inhibits cytotoxic T-cell activity and promotes other immunoinhibitory effects [30]. Moreover, it has been previously reported that PD-L1 is implicated in immune suppression in HCC by its presence in tumors and adjacent tissue, and high PD-L1 expression in HCC has been positively correlated with reduced overall survival [31]. Consequently, these results suggest that inhibiting PD-L1/PD-1 interaction may increase the antitumor immunity in both HCC and CCA.
Previous works already detected functional impairments in different immune cell subsets in HCC and CCA patients [32], [33]. Therefore, the study of the phenotype and functions of immune cell subsets in the tumor microenvironment may help to understand the complex processes that drive to tumor progression and may work for identifying new therapeutic targets for further studies.
Conclusions
To conclude, we highlight several potential implications. First, the confirmation of PD-L1 expression in HCC and CCA tumor cells as well as in HCC tumor-infiltrating macrophages, supporting the continued development of immune checkpoint inhibitors targeting the PD-1/PD-L1 pathway in both HCC and CCA. Second, the reported expression levels of CD200R in HCC infiltrating macrophages may serve as a benchmark to evaluate novel therapies that seek to target CD200/CD200R axis. Third, we have observed a significantly higher expression of markers associated with protumor macrophages in HCC tumors that were classified into high grades or into high-risk tumors, suggesting an important role of these macrophages in tumor progression. And, therefore, future therapies should consider protumor macrophages as one of the main targets for the development of new combined immunotherapies.
Moreover, this work has demonstrated the utility of multicolor flow cytometry of freshly processed tumor samples to reveal functional and phenotypic differences in TIL cell subpopulations among different cancer patients (CCA vs. HCC), different histopathological grades, and a new risk stratification classification for HCC patients, in addition to providing evidence to better understand the tumor immune microenvironment on both carcinomas. Future work is needed to evaluate its relevance in the design of treatment strategies and in the optimal selection of patients for immunotherapy.
The following are the supplementary data related to this article.
Panel of Monoclonal Antibody Reagents (with Clones and Commercial Source) Used for the Immunophenotypic Characterization and for Cell Purification by Fluorescence-Activated Cell Sorting of Tumor Cells and Immune Cells
Funding
This work was supported by the European Commission's Horizon 2020 research and innovation program for the Marie Sklodowska-Curie (grant agreement number 675132) (MSCAITN-ETN MASSTRPLAN) to Centro Hospitalar e Universitário de Coimbra, Coimbra (Portugal), and by COMPETE 2020–Programa Operacional Competitividade e Internacionalização (POCI) [POCI-01-0145-FEDER-007440, UID/NEU/04539/2013] to CNC.IBILI.
Author Contributions
C. M. S. processed the samples; performed the cell sorting, the flow cytometry and the molecular biology analyses; analyzed the results; and was a major contributor in the writing of the manuscript. R. M., A. M. A., R. C. O., J. G. T., M. F. B., and E. F. provided the biological samples, performed patients' selection, revised the clinical data, and reviewed the manuscript. P. L. supervised the data analysis, helped in sample processing, and reviewed the manuscript. M. C. supervised the molecular biology analyses. M. R. D. supervised the work and reviewed the manuscript. Finally, A. P. conceived the main idea of the work, interpreted the results, and reviewed the manuscript. C. M. S. is a PhD student in the Biochemistry program at University of Aveiro, and this work is part of her doctoral thesis. All authors read and approved the final manuscript.
Competing Interests
The author(s) declare no competing interests.
Data Availability
The authors declare that the main data supporting the results of the present study are available within the article and its Supplementary Information files. Extra data are available from the corresponding author upon request.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Ghouri YA, Mian I, Blechacz B. Cancer review: cholangiocarcinoma. J Carcinog. 2015;14(1) doi: 10.4103/1477-3163.151940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–522. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 6.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 7.Yao W, He J, Yang Y, Wang J, Qian Y, Yang T, Ji L. The prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: a systematic review and meta-analysis. Sci Rep. 2017;7:7525. doi: 10.1038/s41598-017-08128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radvanyi LG. Tumor-infiltrating lymphocyte therapy: addressing prevailing questions. Cancer J. 2015;21:450–464. doi: 10.1097/PPO.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 9.Miksch R, Schoenberg M, Weniger M, Bösch F, Ormanns S, Mayer B, Werner J, Bazhin A, D’Haese J. Prognostic impact of tumor-infiltrating lymphocytes and neutrophils on survival of patients with upfront resection of pancreatic cancer. Cancers (Basel) 2019;11:39. doi: 10.3390/cancers11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bremnes RM, Busund L-T, Kilvær TL, Andersen S, Richardsen E, Paulsen EE, Hald S, Khanehkenari MR, Cooper WA, Kao SC. The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non–small cell lung cancer. J Thorac Oncol. 2016;11:789–800. doi: 10.1016/j.jtho.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Badalamenti G, Fanale D, Incorvaia L, Barraco N, Listì A, Maragliano R, Vincenzi B, Calò V, Iovanna JL, Bazan V. Role of tumor-infiltrating lymphocytes in patients with solid tumors: Can a drop dig a stone? Cell Immunol. 2018 doi: 10.1016/j.cellimm.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Dong P, Ma L, Liu L, Zhao G, Zhang S, Dong L, Xue R, Chen S, Dong P, Ma L. CD86+/CD206+, diametrically polarized tumor-associated macrophages, predict hepatocellular carcinoma patient prognosis. Int J Mol Sci. 2016;17:320. doi: 10.3390/ijms17030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitano Y, Okabe H, Yamashita Y, Nakagawa S, Saito Y, Umezaki N, Tsukamoto M, Yamao T, Yamamura K, Arima K. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer. 2018;118:171–180. doi: 10.1038/bjc.2017.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atanasov G, Dietel C, Feldbrügge L, Benzing C, Krenzien F, Brandl A, Mann E, Englisch JP, Schierle K, Robson SC. Tumor necrosis and infiltrating macrophages predict survival after curative resection for cholangiocarcinoma. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2017.1331806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ünal E, İdilman İS, Akata D, Özmen MN, Karçaaltıncaba M. Microvascular invasion in hepatocellular carcinoma. Diagn Interv Radiol. 2016;22:125–132. doi: 10.5152/dir.2015.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamarajah SK, Frankel TL, Sonnenday C, Cho CS, Nathan H. Critical evaluation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with hepatocellular carcinoma (HCC): a Surveillance, Epidemiology, End Results (SEER) analysis. J Surg Oncol. 2018;117:644–650. doi: 10.1002/jso.24908. [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Park YN. Hepatocellular carcinomas expressing ‘stemness’-related markers: clinicopathological characteristics. Dig Dis. 2014;32:778–785. doi: 10.1159/000368021. [DOI] [PubMed] [Google Scholar]
- 18.Schlageter M, Terracciano LM, D'Angelo S, Sorrentino P. Histopathology of hepatocellular carcinoma. World J Gastroenterol. 2014;20 doi: 10.3748/wjg.v20.i43.15955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Il Lee J, Lee J-W, Kim JM, Kim JK, Chung HJ, Kim YS. Prognosis of hepatocellular carcinoma expressing cytokeratin 19: comparison with other liver cancers. World J Gastroenterol. 2012;18:4751. doi: 10.3748/wjg.v18.i34.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirabe K, Mano Y, Muto J, Matono R, Motomura T, Toshima T, Takeishi K, Uchiyama H, Yoshizumi T, Taketomi A. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma. Surg Today. 2012;42:1–7. doi: 10.1007/s00595-011-0058-8. [DOI] [PubMed] [Google Scholar]
- 21.Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402–1410. doi: 10.1016/j.imbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Liao K-L, Bai X-F, Friedman A. The role of CD200-CD200R in tumor immune evasion. J Theor Biol. 2013;328:65–76. doi: 10.1016/j.jtbi.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Sun H, Xu J, Huang M, Huang Q, Sun R, Xiao W, Sun C. CD200R, a co-inhibitory receptor on immune cells, predicts the prognosis of human hepatocellular carcinoma. Immunol Lett. 2016;178:105–113. doi: 10.1016/j.imlet.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Coles SJ, Wang ECY, Man S, Hills RK, Burnett AK, Tonks A, Darley RL. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia. 2011;25:792–799. doi: 10.1038/leu.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller S, Kohanbash G, Liu SJ, Alvarado B, Carrera D, Bhaduri A, Watchmaker PB, Yagnik G, Di Lullo E, Malatesta M. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 2017;18:234. doi: 10.1186/s13059-017-1362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Lou Y, Yang J, Wang J, Feng J, Zhao Y, Wang L, Huang X, Fu Q, Ye M. Integrated multiomic analysis reveals comprehensive tumour heterogeneity and novel immunophenotypic classification in hepatocellular carcinomas. Gut. 2019 doi: 10.1136/gutjnl-2019-318912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Poon RT, Hughes J, Feng X, Yu WC, Fan ST. Chemokine receptors support infiltration of lymphocyte subpopulations in human hepatocellular carcinoma. Clin Immunol. 2005;114:174–182. doi: 10.1016/j.clim.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur J Immunol. 2014;44:1582–1592. doi: 10.1002/eji.201344272. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J-H, Ma W-J, Wang G-G, Jiang X, Chen X, Wu L, Liu Z-S, Zeng X-T, Zhou F-L, Yuan Y-F. Clinicopathologic significance and prognostic value of programmed cell death ligand 1 (PD-L1) in patients with hepatocellular carcinoma: A meta-analysis. Front Immunol. 2018;9:2077. doi: 10.3389/fimmu.2018.02077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai L, Zhang Z, Zhou L, H Wang J Fu, Zhang S, Shi M, Zhang H, Y Yang H Wu. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129:428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Martín-Sierra C, Martins R, Laranjeira P, Abrantes AM, Oliveira RC, Tralhão JG, Botelho MF, Furtado E, Domingues R, Paiva A. Functional impairment of circulating FcεRI + monocytes and myeloid dendritic cells in hepatocellular carcinoma and cholangiocarcinoma patients. Cytom Part B Clin Cytom. 2019 doi: 10.1002/cyto.b.21777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Panel of Monoclonal Antibody Reagents (with Clones and Commercial Source) Used for the Immunophenotypic Characterization and for Cell Purification by Fluorescence-Activated Cell Sorting of Tumor Cells and Immune Cells
Data Availability Statement
The authors declare that the main data supporting the results of the present study are available within the article and its Supplementary Information files. Extra data are available from the corresponding author upon request.