Abstract
Current treatment of cutaneous leishmaniasis includes pentavalent antimonials as first-line drugs, but this therapy has shown severe adverse effects. An alternative to minimize this issue is based on combination therapy scheme with other drugs. In this study we analyzed the potential of the association of meglumine antimoniate (MA) with the oxiranes epoxy-α-lapachone (LAP) or epoxymethyl-lawsone (LAW). Results demonstrated that association between these drugs enhanced leishmanicidal activity on Leishmania (Leishmania) amazonensis infection. The compounds were tested in monotherapy or in combinations (3:1; 1:1 and 1:3) and reduced intracellular parasite numbers, measured by the endocytic index, in all tested conditions. The most effective combination regimens were MA/LAP or MA/LAW in 3:1 ratio, which achieved a reduction of 98.3% and 93.6% in the endocytic index, respectively. BALB/c mice challenged with L. (L.) amazonensis showed significant reduction in lesion size and parasite load in both footpad and lymph nodes, after four weeks of treatment. Although, MA, LAP or LAW monotherapy were able to control the evolution of lesions when compared to untreated animals (30%, 40% and 40% of reduction, respectively), the combination of MA/LAP and LAW in 3:1 ratio showed better results reducing 61.7 and 54.4%, respectively. The results indicate that the association of meglumine antimoniate to oxiranes lead to an increment in the antileishmanial activity and represent a promising approach for the cutaneous leishmaniasis treatment.
Keywords: Combination treatment, Meglumine antimoniate, Oxiranes, Epoxy-α-lapachone, Epoxymethyl-lawsone, Experimental leishmaniasis, Leishmania (Leishmania) amazonesis
Graphical abstract
Highlights
-
•
Meglumine antimoniate with oxiranes enhanced effect against Leishmania infection.
-
•
The most effective treatment in vitro infection was observed in a 3:1 ratio.
-
•
Mice treatment with drugs caused reductions in lesion size and parasite load.
-
•
Antimony-based combination has the potential for leishmaniasis treatment.
1. Introduction
Cutaneous leishmaniasis (CL) is an infectious disease, transmitted by female sandflies, which presents high incidence and morbidity rates in developing countries, where access to health services is often precarious. This poverty related disease lead to great social and economic burden (Alvar et al., 2006). The World Health Organization estimates that global prevalence is approximately 12 million cases per year, occurring in 98 countries, but nearly 90% of the cutaneous and mucocutaneous cases are concentrated in seven developing countries, including Brazil (World Health Organization (WHO), 2010). Leishmaniasis is considered an important neglected tropical disease due to its extensive distribution in tropical areas of the globe and risk to produce deformities (Organizaciòn Panamericana de la Salud, 2018).
Despite almost seven decades of researches assaying alternative therapies to replace pentavalent antimonials, these drugs remain the first-line treatment for most forms of leishmaniasis worldwide (World Health Organization (WHO), 2017). Pentavalent antimonials therapy is associated with mild to severe adverse effects, being often accompanied by pain and swelling at site of the intramuscular application and several systemic symptoms, which include nausea, vomiting, weakness and myalgia, abdominal colic, diarrhea, skin rashes and variable transient increase of transaminases and/or amylases (Herwaldt and Berman, 1992; Oliveira et al., 2011). The most serious adverse effect associated with pentavalent antimonials therapy is undoubtedly cardiotoxicity, characterized by ventricular repolarization disorders (as alterations in T wave and ST segment) and arrhythmia, which can lead to sudden death (Herwaldt and Berman, 1992; Oliveira et al., 2011). To date, the precise mechanism of action whereby pentavalent antimonials kill Leishmania parasites remains unknown. However, it seems to be multifactorial, as with other heavy metals, rather than being due to a single route (Frézard et al., 2008, 2009). At the molecular level, it is proposed that the antimonial react with sulfhydryl groups present in proteins, causing structural changes that would lead to an impairment of its function (Frézard et al., 2009). There is evidence that these compounds may inhibit certain phases of the energetic metabolism cycle of amastigotes. Experimental observations indicated that in vitro exposure of L. (L.) mexicana to sodium stibogluconate resulted in a dose-dependent reduction of both cellular viability and the production of CO2 from glucose and palmitate in parasite cultures (Berman et al., 1985). Data indicate that the depletion of intracellular ATP levels is due to an inhibition of glycolytic enzymes and other components of the fatty acid pathway, but not from the hexose monophosphate pathway and the citric acid cycle (Berman et al., 1985). Furthermore, studies have shown that the trivalent form (Sb3+) is able to cause inhibition of the trypanothione reductase activity in L. (L.) donovani, an essential enzyme for the parasite survival within macrophages, and it may be responsible for part of the leishmanicidal effects (Baiocco et al., 2009; Wyllie et al., 2004). Additional microbicidal mechanisms of antimonials might include: (i) induction of apoptosis in amastigotes by Sb3+, as observed by DNA fragmentation and phosphatidylserine exposure on the outer surface of the parasite plasma membrane (Sudhandiran and Shaha, 2003); (ii) inhibition of topoisomerases (Lucumi et al., 1998); (iii) formation of complexes with ribonucleosides (Demicheli et al., 2002); and (iv) interference in the translocation of preformed purines (Carter et al., 2000).
The second-line drugs applied when antimonials cannot be resorted, amphotericin B and pentamidine isethionate present even higher toxicity and thus their use are only recommended in cases of contraindication, intolerance, low therapeutic response or resistance to antimonials (Berman, 1988). These drugs are associated to a range of adverse effects, being the most serious nephrotoxicity, detected by a decreasing of glomerular filtration and insulin-dependent diabetes, respectively (Health Ministry of Brazil, 2006; Health Ministry of Brazil, 2010; Panda et al., 2017). Additionally, other antileishmanial medicines such as paramomycin, miltefosine, pentoxifylline and ketoconazole are also available in some countries; however, some are still under clinical investigation (World Health Organization (WHO), 2010).
Drug combination therapy is a successful strategy in the treatment of several infectious diseases such as malaria, tuberculosis and AIDS, and has been applied for leishmaniasis treatment, mainly in endemic countries (Panda et al., 2017; World Health Organization (WHO), 2010). Treatments using combined drugs have potential advantages, such as (i) shorten the course of treatment; (ii) decrease the risk of drug-resistant parasites; and, (iii) reduce the total dose of medicines needed, thereby decreasing both incidence of adverse effects and costs (Blum and Hatz, 2009; World Health Organization (WHO), 2010).
Approaches involving pentavalent antimonials associated to other drugs have been conducted, but some results are still controversial (Arana et al., 1994; Llanos-Cuentas et al., 1997; Martinez and Marr, 1992; Martinez et al., 1997; Miranda-Verastegui et al., 2009). Even though combination therapies against visceral leishmaniasis produce cumulative or synergic effects that could reduce both parasite resistance and treatment failure (Bhattacharjee et al., 2015; Hendrickx et al., 2017), the therapeutic regimen must be based on the individual benefit/risk ratio of drugs, the infrastructure of the health service providing the treatment, the local availability of antileishmanial drugs and epidemiological issues (Griensven et al., 2010; World Health Organization (WHO), 2010).
For CL, topical treatment should be used as a first line treatment whenever possible, but in many cases of New World CL, systemic treatment might be indicated due to the risk of mucosal spread. Besides, the risk of severe adverse effects is acceptable for patients suffering from numerous, face-disfiguring or complicated lesions, but not for those with a mild form of the disease (Blum and Hatz, 2009; World Health Organization (WHO), 2010).
Naphtoquinones are natural compounds considered promising scaffolds for the development of new drugs, since they present many pharmacological activities including antibacterial (Nasiri et al., 2013; Sharma et al., 2013a), antifungal (Ibis et al., 2014; Nittayananta et al., 2013; Sritrairat et al., 2011), antiviral (Kapadia et al., 2013; Mahapatra et al., 2012), antitumor (Bhasin et al., 2013; Bustamante et al., 2013; Jiménez-Alonso et al., 2008; Klaus et al., 2010; Oramas-Royo et al., 2013; Pérez et al., 2007), antimalarial (García-Barrantes et al., 2013; Schuck et al., 2013; Sharma et al., 2013b) and antileishmanial activities (Lima et al., 2004; Sharma et al., 2012). Examples of 1,4-naphthoquinones that showed activity against species of Leishmania and Trypanosoma cruzi are lapachol, isolated from Brazilian trees belonging to the Tabebuia genus, and its derivatives α-lapachone and β-lapachone (Ferreira et al., 2006; Jorqueira et al., 2006). However, lapachol and several of its derivatives exhibited significant toxicity, therefore presenting an obstacle for clinical treatment. In order to develop less toxic derivatives for mammalian cells, the chemical modification of the quinonoid center of α-lapachone and 2-hydroxy-1,4-naphthoquinone (lawsone), followed by epoxidation, generating the oxiranes epoxy-α-lapachone (LAP) and epoxymethyl-lawsone (LAW) respectively. Recently, we have demonstrated that LAP and LAW (Fig. 1), were effective against Leishmania in both in vitro and in vivo experimental infections and showed low toxicity for mammalian cells (Oliveira et al., 2018; Souza-Silva et al., 2014, 2015).
Fig. 1.
Chemical structure of tested drugs. (A) 2,2-dimethyl-3,4-dihydrospiro[benzo[g]chromene-10,2′-oxiran]-5-one, also known as epoxy-α-lapachone (C16H16O3, 256.296 g/mol; CID 12000280) (https://pubchem.ncbi.nlm.nih.gov/compound/12000280#section=Top), (B) 2-methoxy-4H-spiro[naphthalene-1,2′-oxiran]-4-one, also known as epoxymethyl-lawsone (C12H10O3, 202.21 g/mol), and (C) meglumine antimoniate known commercially as Glucantime (C7H18NO8Sb, 365.98 g/mol) (Adapted from Frézard et al., 2009).
LAP led to a dose- and time-dependent decrease in the growth rate of Leishmania (Viannia) braziliensis and L. (L.) amazonensis promastigote cultures, killed amastigotes inside human macrophages (Souza-Silva et al., 2014), and reduced the mean lesion size in BALB/c mice infected by L. (L.) amazonensis (Souza-Silva et al., 2015). LAW affected promastigotes viability after 24 h of exposure and demonstrated an activity on intracellular amastigotes similar to the reference drug meglumine antimoniate. LAW was also able to hinder lesion growth in L. (L.) amazonensis infected BALB/c mice (Oliveira et al., 2018).
Studies aim to identify promising leishmanicidal drug associations with high activity in experimental infection, since it may lead to improved treatment efficacy of treatments and/or reduce dose regimen and toxicity (Gangneux et al., 1997; Pal et al., 2004). Thereby, following this research line, the objective of the present study is to determine potential of the combination therapy using meglumine antimoniate (MA) associated with epoxy-α-lapachone or epoxymethyl-lawsone against experimental infection. Herein, we demonstrate an enhanced leishmanicidal effect produced by such combination treatments on L. (L.) amazonensis, either in macrophage cultures infected by this parasite or in experimentally challenged BALB/c mice.
2. Material and methods
2.1. Chemicals and culture reagents
Dimethyl sulfoxide (DMSO), penicillin, streptomycin, Lab-Tek chamber slides, Greiner CELLSTAR™ 96 well plates, RPMI 1640 medium and Schneider's Drosophila medium were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Fetal calf serum (FCS) was acquired from Cultilab S/A (Brazil). CellTiter-Glo™ luminescent cell viability assay and GoTaq™ qPCR Master Mix were purchased from Promega Corporation (USA). Meglumine antimoniate (Glucantime™) was kindly provided by Dr. Armando de Oliveira Schubach team (INI/Fiocruz). Propylene glycol was purchased from Vetec Quimica. Epoxy-α-lapachone and epoxymethyl-lawsone compounds were synthesized by the Department of Organic Chemistry of the Instituto de Química, Universidade Federal Fluminense.
2.2. Cell culture
Peritoneal macrophages were removed from BALB/c mice as previously described (Oliveira et al., 2018) and recovered in RPMI 1640 medium containing 10% FCS by centrifugation (2 × , 1.800×g, 10 min, 4 °C). After, cells were seeded in Lab-Tek chamber slides at a density of 5 × 105 cells/well and incubated (37 °C, 5% of CO2) for 24 h. Adherent macrophages were used in the cytotoxicity and parasite infection assays.
2.3. Parasite cultures
Promastigotes of Leishmania (Leishmania) amazonensis (strain MHOM/BR/73/LTB0016) were obtained from the Leishmania collection (Coleção de Leishmania do Instituto Oswaldo Cruz – CLIOC) of the Instituto Oswaldo Cruz (Fiocruz). Cell cultures were maintained at 28 °C in Schneider's medium (pH 7.2) containing 1 mM L-glutamine, 10% FCS, 100 IU/mL penicillin, and 100 μg/mL streptomycin. Subpassages were made to maintain the parasites in the logarithmic growth phase.
2.4. Activity against intracellular amastigotes
Macrophages were co-incubated (4 h, 37 °C) with promastigotes in a proportion of 5:1 (parasite:cell) in Lab-Tek chamber slides, followed by washing with PBS and addition of RPMI medium containing 5% FCS. After, cultures were incubed (37 °C, 24 h) with meglumine antimoniate, epoxy-α-lapachone and epoxymethyl-lawsone in monotherapy at the concentrations corresponding to its IC50 values previously determined (2.0 μM, 5.0 μM and 7.5 μM, respectively), and in combinations of MA:LAP or MA:LAW in fixed ratios 3:1, 1:1, 1:3, where 3:1 refers to 3 parts of MA IC50 value with 1 part of LAP IC50 or LAW IC50 value (75% of MA + 25% of LAP or LAW = total dose). Similarly, 1:1 and 1:3 ratios correspond to 1 part of MA IC50 plus LAP IC50 or LAW IC50 (50%: 50%), and 1 part of MA IC50 plus 3 parts of LAP IC50 or LAW IC50 (25%: 75%), respectively. Then, slides were fixed with 100% methanol and Giemsa-stained. The endocytic index was determined by counting intracellular parasites in at least 300 random cells. The results are expressed as the mean and standard deviation of three independent assays.
2.5. Toxicity of compounds and its combinations to BALB/c mice macrophages
Macrophages seeded on 96-well plates (2 × 105 per well) were exposed (37 °C, 72 h) to meglumine antimoniate, epoxy-α-lapachone and epoxymethyl-lawsone in monotherapy at a concentration equivalent to half of IC50 previously defined (2.0 μM, 5.0 μM and 7.5 μM, respectively) and in combinations that followed the ratio 3 : 1, 1 : 1 and 1 : 3, as described above. Then, macrophages viability was determined by addition of CellTiter-Glo™ (20μL/well) and incubation (25 °C, 3 min) under agitation. Luminescence was measured using a FlexStation 3 reader (Molecular Devices, Sunnyvale, CA, USA).
2.6. Experimental murine infection
BALB/c mice with 5- to 7-week-old weighing approximately 22 g were inoculated in the footpad of the left hind limb with 1.0 × 105 promastigotes of L. (L.) amazonensis in the stationary growth phase in a total volume of 50 μL diluted in 10 mM of phosphate-buffered saline pH 7.2 (PBS).
2.7. Mice treatment schedules
To determine doses to be applied in the combination assays, BALB/c mice were previously treated (five animals per group) with three different doses of each drug, defined here as low, intermediate and high dose, as follow: meglumine antimoniate (0.23 mg of Sb5+/Kg/day, 2.27 mg of Sb5+/Kg/day and 22.7 mg of Sb5+/Kg/day); epoxy-α-lapachone (0.23 mg/kg/day, 2.27 mg/kg/day and 22.7 mg/kg/day); and epoxymethyl-lawsone (0.11 mg/kg/day, 1.14 mg/kg/day and 11.4 mg/kg/day), starting six weeks after infection, as previously described (Souza-Silva et al., 2015; Oliveira et al., 2018). Briefly, oxiranes were diluted in a mixture of DMSO: propylene glycol: saline (1 : 12: 7) because of their low solubility in water, then drugs were administrated daily by subcutaneous route (100 μL per animal), from Monday to Friday until 20 doses. Negative-control group was treated with vehicle used to dissolve the oxirane compounds. The lesions were evaluated weekly by measuring the height and width of the paw, and lesion volumes were obtained by multiplying these measures in mm3 with a digital caliper. The intermediate doses of each drug were selected as its efficacy was similar to a high dose. Meglumine antimoniate, epoxy-α-lapachone and epoxymethyl-lawsone were tested in monotherapy (2.27 mg of Sb5+/Kg/day, 2.27 mg/kg/day and 1.14 mg/kg/day, respectively) and in combinations following fixed-proportion of 3 : 1; 1 : 1 and 1 : 3, as already described in 2.4.
2.8. Parasite kinetoplast DNA quantification by real-time PCR (qPCR)
DNA from the footpad and draining lymph nodes of 3 animals per group were extracted following a standard phenol/chloroform protocol (Sambrook and Russel, 2001). DNA was quantified in a NanoDrop 2000c spectrophotometer (ThermoScientific) and diluted to 10 ng/μL of total DNA. Real time PCR was performed in QuantStudio™ 3 Real Time PCR System equipment using fast cycle with GoTaq™ qPCR Master Mix, 2 μL of DNA sample at a final volume of 10 μL. Primers were target for the parasite kDNA1 (forward: 5′-GGGTAGGGGCGTTCTGC-3′, reverse: 5′-TACACCAACCCCCAGTTTGC-3’; accession number: M94088), and mouse β-actin (forward: 5′-AGAGGGAAATCGTGCGTGAC-3′, reverse: 5′-CAATAGTGATGACCTGGCCGT-3’; accession number: X03672) was used as an endogenous control. Parasite load was estimated from a standard curve (1.7 × 10−1 to 1.7 × 104 parasites) of known amounts of Leishmania parasite obtained from in vitro culture, considering size of the genome sequence of L. (L.) amazonensis and kinetoplast DNA replication (Shapiro and Englund, 1995; Real et al., 2013; Almeida-Souza et al., 2016).
2.9. Ethical aspects
Mice experimental procedures performed here were approved by the Committee for the Ethical Use of Animals of Instituto Oswaldo Cruz (L-052/2015). The animals were obtained from the Instituto de Ciência e Tecnologia em Biomodelos – Fiocruz.
2.10. Statistical analysis
Mann-Whitney, one-way ANOVA tests (Bonferroni post-test and Tukey HSD) were applied to compare results and data matrices were considered statistically different when the P value was less than 0.05. Statistical analyses were performed using GraphPad Prism version 5.03 (GraphPad Software, San Diego, CA) and R software version 3.5.2.
3. Results and discussion
Leishmaniases represent a major public health issue in the Americas due to their distribution in several countries and high prevalence (Organizaciòn Panamericana de la Salud, 2013). Treatment is a pivotal step to control the disease; however, effectiveness of current options has been questioned due to the increasing numbers of resistance and toxicity, pointing toward the need of novel drugs (Croft et al., 2006). In recent years, efforts have been performed to improve antimonial chemotherapy. Several studies added further information on its chemical structure (Frézard et al., 2008), mechanisms of action, new methods of preparation and potential incorporation into different formulations (reviewed in Frézard et al., 2009). In this context, a promising strategy is the association of pentavalent antimonials with oxiranes, compounds that showed significant antileishmanial activity; such association could reduce drug doses and toxicity, while maintain or improve the baseline efficacy of pentavalent antimonials. In addition, a combination of two drugs with rather different mechanisms of action over the parasites may have the benefit of hindering the emergence of resistant strains. Therefore, we devised in the present study a new approach for the CL chemotherapy based on the combination treatment of meglumine antimoniate with epoxy-α-lapachone or epoxymethyl-lawsone.
3.1. Effects of drug combinations against intracellular amastigotes of Leishmania (L.) amazonensis
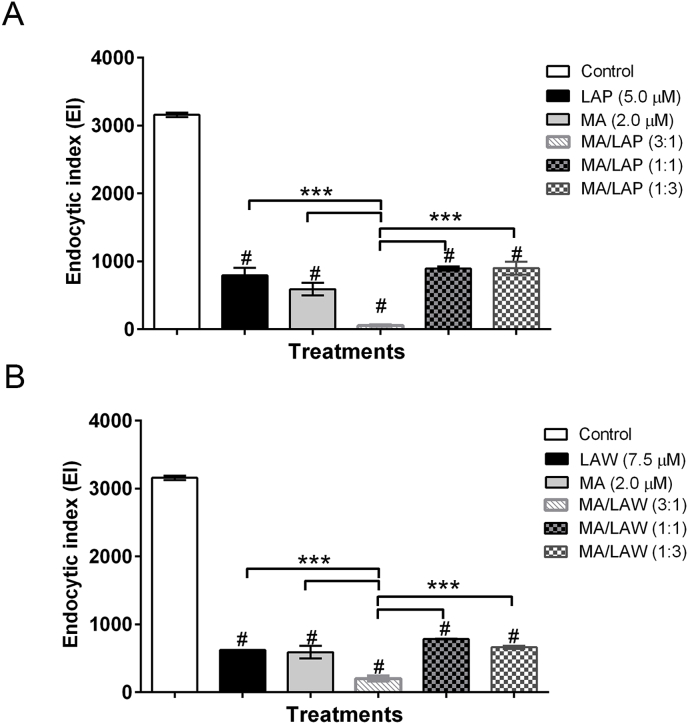
Initially, we investigated the combination effects of MA with oxiranes LAP and LAW on peritoneal macrophages of BALB/c mice infected in vitro with L. (L.) amazonensis. The drugs were tested alone (at a concentration equivalent to half of IC50 values previously determined) or in combinations following fixed-ratios of 3 : 1; 1 : 1 and 1 : 3. All drugs as well as its combinations exhibited impact over the macrophage-infecting parasites, as determined by the endocytic index (EI): treated cultures had EI values lower than untreated cultures (Fig. 2). The effects of MA or LAW monotherapy on parasites showed similar values; the EI was reduced 82% and 80%, respectively. LAP alone showed the lowest impact over the parasites (EI reduced 75%). Some of the drug combinations were sensibly more effective over the intracellular parasites: MA/LAP or MA/LAW at 3 : 1 ratio led to 98.3% and 93.6% reduction on the EI of the control, respectively. In relation to respective drugs alone, the ratio combinations showed reductions of 90.5% and 93.2% (MA/LAP) and 64.6% and 67.7% (MA/LAW). No significant difference in 1:3 and 1:1 ratios were detected. Cytotoxicity assays performed using either the drugs monotherapy or its combinations, with ratios established above, indicated no significant change in the viability of treated macrophage cultures when compared to untreated controls. Control groups were cultivated with medium only or 0.8% DMSO, used as solvent for compounds, (data not shown). This set of results established the feasibility of proceeding to in vivo experiments, aiming to analyze the effects of combination treatments on the progression of lesions in experimentally challenged mice.
Fig. 2.
Effects of combination treatments with epoxy-α-lapachone and meglumine antimoniate (A) and epoxymethyl-lawsone and meglumine antimoniate (B) on the endocytic index in peritoneal murine macrophages infected in vitro with Leishmania (L.) amazonensis. Cultures of peritoneal macrophages isolated from BALB/c mice were infected with L. (L.) amazonensis and incubated (24 h, 37 °C) with either meglumine antimoniate (MA), epoxy-α-lapachone (LAP) or epoxymethyl-lawsone (LAW) in monotherapy at the concentrations corresponding to its IC50 values previously determined (2.0 μM, 5.0 μM and 7.5 μM, respectively), and in combinations of MA: LAP [3 : 1 (1.5 μM + 1.25 μM), 1 : 1 (1.0 μM + 2.5 μM), 1 : 3 (0.5 μM + 3.75 μM)] and MA: LAW in fixed ratios [3 : 1 (1.50 μM + 1.88 μM), 1 : 1 (1.0 μM + 3.75 μM), 1 : 3 (0.5 μM + 5.63 μM)]. Control cultures (white bars) were treated with 0.8% DMSO. The data are expressed as the mean and standard deviation (±) of three independent assays. Difference between groups was analyzed with one-way ANOVA, Bonferroni post-test and Tukey HSD. (#) Statistically significance between control and treated groups (p ≤ 0.001). (***) Difference between treated groups and the most active combination (3:1) (p ≤ 0.001).
Previous data from our research group indicated that treatment of BALB/c mice challenged with L. (L.) amazonensis with either MA, LAP and LAW (in three distinct dosages) led to a measurable reduction in the mean size of parasite-associated lesions, pointing to a control of the in vivo infection. The tenth week post-infection was settled as the chosen time point for lesion size measurements. Considering that no dose-response pattern was observed for any of the applied dosages of LAW and LAP and that the highest dosage of these compounds elicited significant signs of tissue toxicity (Oliveira et al., 2017a), the intermediate dosage was elected to perform the combination treatment assays.
3.2. Effects of drug combinations treatment on experimental cutaneous lesions caused by Leishmania (L.) amazonensis
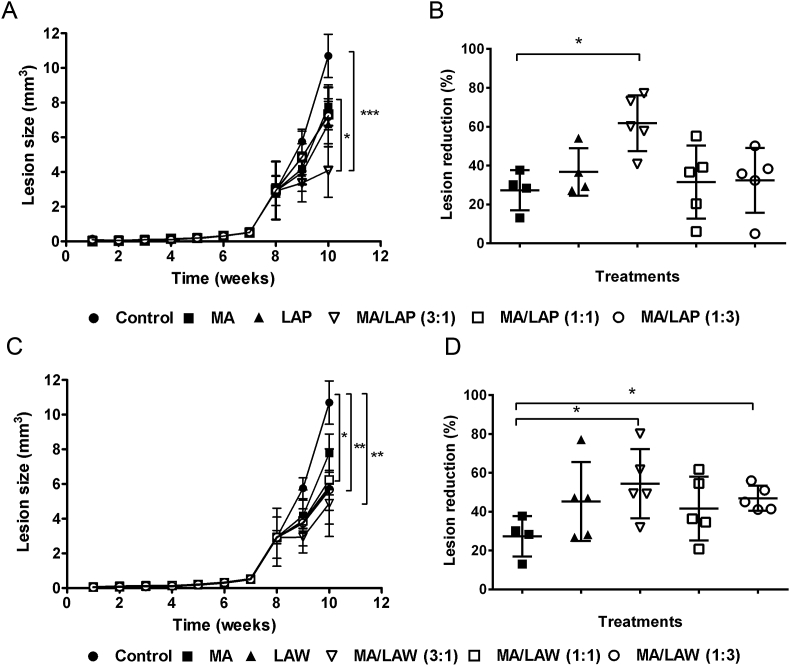
As previously observed, the administration of MA, LAP or LAW prevented lesion development, leading to a reduction in mean lesion size in treated challenged mice when compared to untreated animals after the fourth week of treatment. Similar results were observed in mice treated with combinations of the compounds (Fig. 3A and C). The mean lesion volume measured in untreated animals 10 weeks post-infection was 10.7 ± 1.25 mm3. MA presented the lowest effect on reducing mean lesions size, leading to a 30% decrease in lesions size, whereas both LAP and LAW monotherapy presented better effects, decreasing 40% of lesions size (Fig. 3B and D). Results of drug combination revealed a better profile of MA/LAP or MA/LAW at 3 : 1 ratio (75% of MA and 25% of LAP or LAW) achieving a lesion reduction of 61.7 and 54.4%, respectively. Collectively this data indicates that oxiranes potentiate the leishmanicidal effects of MA. Additionally, the combination of MA/LAW at 1 : 3 ratio also presented statistical difference compared to MA monotherapy (Fig. 3D).
Fig. 3.
Treatment of mice challenged with Leishmania (L.) amazonensis. BALB/c mice were treated daily with meglumine antimoniate (MA), epoxy-α-lapachone (LAP) or epoxymethyl-lawsone (LAW) in monotherapy at doses previously defined (2.27 mg of Sb5+/Kg/day; 2.27 mg/kg/day and 1.14 mg/kg/day, respectively), and in combinations of MA: LAP [3:1 (1.70 mg + 0.57 mg), 1 : 1 (1.14 mg + 1.14 mg) and 1 : 3 (0.57 mg + 1.70 mg)] and MA: LAW [3:1 (1.70 mg + 0.29 mg), 1 : 1 (1.14 mg + 0.57 mg) and 1 : 3 (0.57 mg + 0.86 mg)]. Treatments were administrated daily until 20 doses and lesion sizes (mm3) were measured weekly (A and C). The results are represented as mean and standard deviation (±) from three independent experiments. The endpoint was on the 10th week and the differences (%) of each treatment are shown (C and D). Difference between groups was analyzed using Mann-Whitney test. *Statistically significant p ≤ 0.05, **Statistically significant p ≤ 0.01 and ***Statistically significant p ≤ 0.001.
3.3. Parasite kinetoplast DNA quantification in footpad and lymph nodes of treated mice
The effectiveness of the drugs and its combinations in experimental cutaneous leishmaniasis was also evaluated by measuring the parasite kinetoplast DNA (kDNA) quantification from footpad and lymph nodes after the last week of treatment (tenth week) by a quantitative real-time PCR (qPCR) assay (Fig. 4). The performance of the qPCR for Leishmania kDNA quantification was related with the presence of parasite in the lesion sites and lymph nodes and defined here as parasite load. The results show significant decrease of parasite load in footpad lesion and lymph nodes for all treatment modalities compared to control. LAP, LAW and MA treatments presented similar parasite load reductions in footpad (65.22, 69.75, and 84.25%) and in lymph nodes (74.59, 81.54, and 79.09%).
Fig. 4.
Parasite load in footpad lesion and lymph node in BALB/c mice. Animals infected with Leishmania (L.) amazonensis were treated or not (control) with oxiranes epoxy-α-lapachone (LAP) or epoxymethyl-lawsone (LAW) and meglumine antimoniate alone or in combinations at fixed-ratio of 3:1, 1 : 1 and 1 : 3. The Leishmania kDNA were quantified by real-time PCR (qPCR) assay. Parasite load quantification was performed in footpad (A and C) and lymph nodes (B and D) at the endpoint of 10 weeks of treatment. The results are expressed (parasite load/20 ng total DNA) as the mean and standard deviation of three independent assays. Difference between groups was analyzed using Mann-Whitney test. *Statistically significant p ≤ 0.05, **Statistically significant p ≤ 0.01 and ***Statistically significant p ≤ 0.001.
Although both treatment combinations showed a statistically significant reduction in relation to the control, MA with LAP showed the best results in the reduction of footpad lesion parasite load compared to MA and LAW combination. MA/LAP at 1 : 1 and at 1 : 3 ratios exhibited highest parasite elimination (96.81 and 96.65%), presenting significant decrease in relation to the drugs alone (p < 0.001). Further, kDNA-qPCR analysis of the mice lymph nodes showed that the treatment with drugs, as well as its combinations, was effective in eliminating parasites in these areas when compared to the control group. However, no significant difference among groups was detected (p > 0.05).
The search for new drugs to treat leishmaniasis is still incipient. Drug development faces many obstacles and new drug candidates against CL often did not progress further to clinical trials. The few advances so far are limited to the development of new formulations for clinical available drugs or drug repurposing (Oliveira et al., 2017b). Despite the high incidence of adverse effects and the increasing numbers of unresponsive strains, pentavalent antimonials remain the basis of chemotherapy and in short-term there is no perspective of new chemical entity or formulation approval (Oliveira et al., 2017b).
Enhancement of effects due to combination therapies can occur basically in two ways: (i) one drug may increase the activity of the other, or (ii) the effect of two drugs may combine to produce an activity distinct to that observed for each one individually (Pritchard et al., 2013). The global increment in leishmancidal activity observed for treatment with MA/LAP or MA/LAW at 3:1 ratio, considering the reduction of lesion size was not confirmed by parasite load quantification. According to this measurement, the best results related to combination treatment were found in MA/LAP at 1 : 1 and 1 : 3 ratio. The decrease of the parasitic kDNA in lymphatic nodes of all treated animals confirms the systemic leishmanicidal effect of the oxiranes tested. However, differences among its combinations with reference drug MA were not possible to demonstrate. Although the direct toxic effect of these compounds on Leishmania parasite is well documented (Oliveira et al., 2018; Souza-Silva et al., 2014, 2015), the possibility that their action is also related to the modulation of cells or components of the immune system may not be discarded.
This work showed an additive effect of the combined treatment between oxiranes and meglumine antimoniate that can be exploited, since these drugs present marked structural differences, which ensure distinct mechanisms of action, and may enhance their individual effects; however, further studies to detect synergistic effects between drugs are needed.
The combination treatments proposed here may represent a new alternative for leishmaniasis chemotherapy, keeping the first-line drugs, but with possible reduction in both the incidence of adverse effects and risk of emergence of antimonial-unresponsive strains.
4. Conclusions
The results presented herein indicate that the association of meglumine antimoniate with oxiranes may lead to an increment of global antileishmanial potential in vitro and in vivo, as compared to the use of these compounds individually. Thereby, this strategy may be a new approach for the cutaneous leishmaniasis treatment. Finally, we emphasize the need for further studies to thoroughly understand the basis of the effects of such combinations.
Conflict of interest disclosure
The authors declare no competing financial interest.
Acknowledgments
This research has a financial support of the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (E-26/111.354/2013; E-26/010.001261/2015), Fundação de Amparo à Pesquisa e Desenvolvimento Científico e Tecnológico do Maranhão (DCR-03438-16) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. Dr. Luiz F. G. Oliveira is posdoctoral researcher fellow of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) institution, Dr. Franklin Souza-Silva and Fernando A. Souza are postdoctoral researcher fellows of CAPES and Dr. Carlos R. Alves and Vitor F. Ferreira are researcher fellows of CNPq institution.
Contributor Information
Luiz Filipe Gonçalves-Oliveira, Email: luizfilipeol07@gmail.com.
Franklin Souza-Silva, Email: franklin.frankss@gmail.com.
Luzia Monteiro de Castro Côrtes, Email: luzia@ioc.fiocruz.br.
Laura Barral Veloso, Email: luzia@ioc.fiocruz.br.
Bernardo Acácio Santini Pereira, Email: baspereira@gmail.com.
Lea Cysne-Finkelstein, Email: lcysne@ioc.fiocruz.br.
Guilherme Curty Lechuga, Email: guilherme.lechuga@yahoo.com.br.
Saulo Cabral Bourguignon, Email: saulocb.uff@gmail.com.
Fernando Almeida-Souza, Email: fernandoalsouza@gmail.com.
Kátia da Silva Calabrese, Email: calabrese@ioc.fiocruz.br.
Vitor Francisco Ferreira, Email: vitorferreira@id.uff.br.
Carlos Roberto Alves, Email: calves@ioc.fiocruz.br.
References
- Almeida-Souza F., Cardoso FdO., Souza B.V., do Valle T.Z., de Sá J.C., Oliveira Idos.S., de Souza Cda S., Moragas Telles C.J., Chagas Mdo S., Behrens M.D., Abreu-Silva A.L., Calabrese Kda S. Morinda citrifolia Linn. Reduces parasite load and modulates cytokines and extracellular matrix proteins in C57BL/6 mice infected with Leishmania (Leishmania) amazonensis. PLoS Neglected Trop. Dis. 2016;10(8) doi: 10.1371/journal.pntd.0004900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvar J., Yactayo S., Bern C. Leishmaniasis and poverty. Trends Parasitol. 2006;22:552–557. doi: 10.1016/j.pt.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Arana B.A., Navin T.R., Arana F.E., Berman J.D., Rosenkaimer F. Efficacy of a short course (10 days) of high-dose meglumine antimonate with or without interferon-gamma in treating cutaneous leishmaniasis in Guatemala. Clin. Infect. Dis. 1994;18:381–384. doi: 10.1093/clinids/18.3.381. [DOI] [PubMed] [Google Scholar]
- Baiocco P., Colotti G., Franceschini S., Ilari A. Molecular basis of antimony treatment in leishmaniasis. J. Med. Chem. 2009;52(8):2603–2612. doi: 10.1021/jm900185q. [DOI] [PubMed] [Google Scholar]
- Berman J.D., Waddell D., Hanson B.D. Biochemical mechanisms of the antileishmanial activity of sodium stibogluconate. Antimicrob. Agents Chemother. 1985;27(6):916–920. doi: 10.1128/aac.27.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J.D. Chemotherapy for leishmaniasis: biochemical mechanisms, clinical efficacy, and future strategies. Rev. Infect. Dis. 1988;10:560–586. doi: 10.1093/clinids/10.3.560. [DOI] [PubMed] [Google Scholar]
- Bhasin D., Chettiar S.N., Etter J.P., Mok M., Li P.K. Anticancer activity and SAR studies of substituted 1,4-naphthoquinones. Bioorg. Med. Chem. 2013;21(15):4662–4669. doi: 10.1016/j.bmc.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A., Majumder S., Majumdar S.B., Choudhuri S.K., Roy S., Majumdar S. Co-administration of glycyrrhizic acid with the antileishmanial drug sodium antimony gluconate (SAG) cures SAG-resistant visceral leishmaniasis. Int. J. Antimicrob. Agents. 2015;45(3):268–277. doi: 10.1016/j.ijantimicag.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Blum J.A., Hatz C.F. Treatment of cutaneous leishmaniasis in travelers 2009. J. Travel Med. 2009;16(2):123–131. doi: 10.1111/j.1708-8305.2008.00286.x. [DOI] [PubMed] [Google Scholar]
- Bustamante F.L.S., Metello J.M., de Castro F.A.V., Pinheiro C.B., Pereira M.D., Lanznaster M. Lawsone dimerization in cobalt(III) complexes toward the design of new prototypes of bioreductive prodrugs. Inorg. Chem. 2013;52(3):1167–1169. doi: 10.1021/ic302175t. [DOI] [PubMed] [Google Scholar]
- Carter N.S., Drew M.E., Sanchez M., Vasudevan G., Landfear S.M., Ullman B. Cloning of a novel inosineguanosine transporter gene from Leishmania donovani by functional rescue of a transport-deficient mutant. J. Biol. Chem. 2000;275(27):20935–20941. doi: 10.1074/jbc.M002418200. [DOI] [PubMed] [Google Scholar]
- Croft S.L., Sundar S., Fairlamb A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006;19(1):111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demicheli C., Frezard F., Lecouvey M., Garnier-Suillerot A. Antimony(V) complex formation with adenine nucleosides in aqueous solution. Biochim. Biophys. Acta. 2002;1570(3):192–198. doi: 10.1016/s0304-4165(02)00198-8. [DOI] [PubMed] [Google Scholar]
- Ferreira V.F., Jorqueira A., Souza A.M., da Silva M.N., de Souza M.C., Gouvêa R.M., Rodrigues C.R., Pinto A.V., Castro H.C., Santos D.O., Araújo H.P., Bourguignon S.C. Trypanocidal agents with low cytotoxicity to mammalian cell line: a comparison of the theoretical and biological features of lapachone derivatives. Bioorg. Med. Chem. 2006;14:5459–5466. doi: 10.1016/j.bmc.2006.04.046. [DOI] [PubMed] [Google Scholar]
- Frézard F., Martins P.S., Barbosa M.C.M., Pimenta A.M.C., Ferreira W.A., de Melo J.E., Mangrum J.B., Demicheli C. New insights into the chemical structure and composition of the pentavalent antimonial drugs, meglumine antimonate and sodium stibogluconate. J. Inorg. Biochem. 2008;102:656–665. doi: 10.1016/j.jinorgbio.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Frézard F., Demicheli C., Ribeiro R.R. Pentavalent antimonials: new perspectives for old drugs. Molecules. 2009;14:2317–2336. doi: 10.3390/molecules14072317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangneux J.P., Sulahian A., Garin Y.J., Derouin F. Efficacy of aminosidine administered alone or in combination with meglumine antimoniate for the treatment of experimental visceral leishmaniasis caused by Leishmania infantum. J. Antimicrob. Chemother. 1997;40(2):287–289. doi: 10.1093/jac/40.2.287. [DOI] [PubMed] [Google Scholar]
- García-Barrantes P.M., Lamoureux G.V., Pérez A.L., García-Sánchez R.N., Martínez A.R., San Feliciano A. Synthesis and biological evaluation of novel ferrocene-naphthoquinones as antiplasmodial agents. Eur. J. Med. Chem. 2013;70:548–557. doi: 10.1016/j.ejmech.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Griensven J., Balasegaram M., Meheus F., Alvar J., Lynen L., Boelaert M. Combination therapy for visceral leishmaniasis. Lancet Infect. Dis. 2010;10:184–194. doi: 10.1016/S1473-3099(10)70011-6. [DOI] [PubMed] [Google Scholar]
- Health Ministry of Brazil, Department of Epidemiological Surveillance Manual de vigilância e controle da leishmaniose visceral. 2006. http://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_controle_leishmaniose_visceral.pdf
- Health Ministry of Brazil, Department of Epidemiological Surveillance Manual de Vigilância e Controle da Leishmaniose Tegumentar Americana. 2010. http://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_leishmaniose_tegumentar_americana.pdf
- Hendrickx S., Van den Kerkhof M., Mabille D., Cos P., Delputte P., Maes L., Caljon G. Combined treatment of miltefosine and paromomycin delays the onset of experimental drug resistance in Leishmania infantum. PLoS Neglected Trop. Dis. 2017;11(5) doi: 10.1371/journal.pntd.0005620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwaldt B., Berman J. Recommendations for treating Leishmaniasis with sodium stibogluconate (Pentostam) and review of pertinent clinical studies. Am. J. Trop. Med. Hyg. 1992;46:296–306. doi: 10.4269/ajtmh.1992.46.296. [DOI] [PubMed] [Google Scholar]
- Ibis C., Tuyun A.F., Bahar H., Ayla S.S., Stasevych M.V., Musyanovych R.Y., Komarovska-Porokhnyavets O., Novikov V. Nucleophilic substitution reactions of 1,4-naphthoquinone and biologic properties of novel S-, S,S-, N-, and N,S-substituted 1,4-naphthoquinone derivatives. Med. Chem. Res. 2014;23(4):2140–2149. [Google Scholar]
- Jiménez-Alonso S., Orellana H.C., Estévez-Braun A., Ravelo A.G., Pérez-Sacau E., Machín F. Design and synthesis of a novel series of pyranonaphthoquinones as topoisomerase II catalytic inhibitors. J. Med. Chem. 2008;51(21):6761–6772. doi: 10.1021/jm800499x. [DOI] [PubMed] [Google Scholar]
- Jorqueira A., Gouvêa R.M., Ferreira V.F., Silva M.N., Souza M.C.B.V., Zuma A.A., Cavalcanti D.F., Araújo H.P., Santos D.O., Bourguignon S.C. Oxyrane derivative of α-lapachone is potent growth inhibitor of Trypanosoma cruzi epimastigote forms. Parasitol. Res. 2006;99:429–433. doi: 10.1007/s00436-006-0153-8. [DOI] [PubMed] [Google Scholar]
- Kapadia G.J., Rao G.S., Sridhar R., Ichiishi E., Takasaki M., Suzuki N., Konoshima T., Iida A., Tokuda H. Chemoprevention of skin cancer: effect of Lawsonia inermis L. (Henna) leaf powder and its pigment artifact, lawsone in the Epstein- Barr virus early antigen activation assay and in two-stage mouse skin carcinogenesis models. Anti Cancer Agents Med. Chem. 2013;13(10):1500–1507. doi: 10.2174/18715206113139990096. [DOI] [PubMed] [Google Scholar]
- Klaus V., Hartmann T., Gambini J., Graf P., Stahl W., Hartwig A., Klotz L. 1,4-Naphthoquinones as inducers of oxidative damage and stress signaling in HaCaT human keratinocytes. Arch. Biochem. Biophys. 2010;496(2):93–100. doi: 10.1016/j.abb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Lima N.M., Correia C.S., Leon L.L., Machado G.M., Madeira Mde F., Santana A.E., Goulart M.O. Antileishmanial activity of lapachol analogues. Mem. Inst. Oswaldo Cruz. 2004;99(7):757–761. doi: 10.1590/s0074-02762004000700017. [DOI] [PubMed] [Google Scholar]
- Llanos-Cuentas A., Echevarria J., Cruz M., La Rosa A., Campos P., Campos M., Franke E., Berman J., Modabber F., Marr J. Efficacy of sodium stibogluconate alone and in combination with allopurinol for treatment of mucocutaneous leishmaniasis. Clin. Infect. Dis. 1997;25:677–684. doi: 10.1086/513776. [DOI] [PubMed] [Google Scholar]
- Lucumi A., Robledo S., Gama V., Saravia N.G. Sensitivity of Leishmania Viannia panamensis to pentavalent antimony is correlated with the formation of cleavable DNA-protein complexes. Antimicrob. Agents Chemother. 1998;42(8):1990–1995. doi: 10.1128/aac.42.8.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra A., Tshikalange T., Meyer J., Lall N. Synthesis and HIV-1 reverse transcriptase inhibition activity of 1,4-naphthoquinone derivatives. Chem. Nat. Compd. 2012;47(6):883–887. [Google Scholar]
- Martinez S., Marr J.J. Allopurinol in the treatment of American cutaneous leishmaniasis. N. Engl. J. Med. 1992;326:741–744. doi: 10.1056/NEJM199203123261105. [DOI] [PubMed] [Google Scholar]
- Martinez S., Gonzalez M., Vernaza M.E. Treatment of cutaneous leishmaniasis with allopurinol and stibogluconate. Clin. Infect. Dis. 1997;24:165–169. doi: 10.1093/clinids/24.2.165. [DOI] [PubMed] [Google Scholar]
- Miranda-Verastegui C., Tulliano G., Gyorkos T.W., Calderon W., Rahme E., Ward B., Cruz M., Llanos-Cuentas A., Matlashewski G. First-line therapy for human cutaneous leishmaniasis in Peru using the TLR7 agonist imiquimod in combination with pentavalent antimony. PLoS Neglected Trop. Dis. 2009;3:e491. doi: 10.1371/journal.pntd.0000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasiri H.R., Madej M.G., Panisch R., Lafontaine M., Bats J.W., Lancaster C.R.D., Schwalbe H. Design, synthesis, and biological testing of novel naphthoquinones as substrate-based inhibitors of the quinol/fumarate reductase from Wolinella succinogenes. J. Med. Chem. 2013;56(23):9530–9541. doi: 10.1021/jm400978u. [DOI] [PubMed] [Google Scholar]
- Nittayananta W., Pangsomboon K., Panichayupakaranant P., Chanowanna N., Chelae S., Vuddhakul V., Sukhumungoon P., Pruphetkaew N. Effects of lawsone methyl ether mouthwash on oral Candida in HIV-infected subjects and subjects with denture stomatitis. J. Oral Pathol. Med. 2013;42(9):698–704. doi: 10.1111/jop.12060. [DOI] [PubMed] [Google Scholar]
- Oliveira L., Schubach A., Martins M., Passos S., Oliveira R., Marzochi M., Andrade C. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the new world. Acta Trop. 2011;118:87–96. doi: 10.1016/j.actatropica.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Oliveira L.F.G., Souza-Silva F., Côrtes L.M.C., Cysne-Finkelstein L., Pereira M.C.S., Oliveira-Junior F.O.O., Pinho R.T., Corte Real S., Bourguignon S.C., Ferreira V.F., Alves C.R. Antileishmanial activity of 2-methoxy-4H-spiro-[naphthalene-1,2′-oxiran]-4-one (Epoxymethoxy-lawsone): a promising new drug candidate for leishmaniasis treatment. Molecules. 2018;23(4):864. doi: 10.3390/molecules23040864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira L.F.G., Souza-Silva F., Cysne-Finkelstein L., Rabelo K., Amorim J.F., Azevedo A.S., Bourguignon S.C., Ferreira V.F., Paes M.V., Alves C.R. Evidence for tissue toxicity in BALB/c exposed to a long-term treatment with oxiranes compared to meglumine antimoniate. BioMed Res. Int. 2017;2017:9840210. doi: 10.1155/2017/9840210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira L.F.G., Pereira B.A.S., Gilbert B., Corrêa A.L., Rocha L., Alves C.R. Natural products and phytotherapy: an innovative perspective in leishmaniasis treatment. Phytochem. Rev. 2017;16:219. [Google Scholar]
- Oramas-Royo S., Torrejón C., Cuadrado I., Hernández-Molina R., Hortelano S., Estévez-Braun A., de las Heras B. Synthesis and cytotoxic activity of metallic complexes of lawsone. Bioorg. Med. Chem. 2013;21(9):2471–2477. doi: 10.1016/j.bmc.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Organizaciòn Panamericana de la Salud (OPAS) Organización Panamericana de la Salud; Washington, DC, USA: 2013. Leishmaniasis en las Américas: recomendaciones para el tratamento.http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&gid=22226&Itemid=270&lang=en 2013. Available online. [Google Scholar]
- Organizaciòn Panamericana de la Salud (OPAS) Leishmaniasis. Epidemiological report of the Americas.: Washington, DC, USA. 2018. http://iris.paho.org/xmlui/handle/123456789/34856 Available online.
- Pal S., Ravindran R., Ali N. Combination therapy using sodium antimony gluconate in stearylamine-bearing liposomes against established and chronic Leishmania donovani infection in BALB/c mice. Antimicrob. Agents Chemother. 2004;38:3591–3593. doi: 10.1128/AAC.48.9.3591-3593.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S., Swaminathan S., Hyder K.A., Christophel E.A., Pendse R.N., Sreenivas A.N., Laksono S.J., Srivastava R., Nair G.B., Aditama T.Y., Singhasivanon P., Thapa A.B., Sarkar S.K. Drug resistance in malaria, tuberculosis, and HIV in South East Asia: biology, programme, and policy considerations. BMJ. 2017;358:j3545. doi: 10.1136/bmj.j3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez E., Díaz R., Estévez A., Ravelo A., García J., Pardo L., Campillo M. Synthesis and pharmacophore modeling of naphthoquinones derivatives with cytotoxic activity in human promyelocytic leucemia HL-60 cell line. J. Med. Chem. 2007;50(4):696–706. doi: 10.1021/jm060849b. [DOI] [PubMed] [Google Scholar]
- Pritchard J.R., Bruno P.M., Gilbert L.A., Capron K.L., Lauffenburger D.A., Hemann M.T. Defining principles of combination drug mechanisms of action. Proc. Natl. Acad. Sci. U.S.A. 2013;110(2):E170–E179. doi: 10.1073/pnas.1210419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real F., Vidal R.O., Carazzolle M.F., Mondego J.M., Costa G.G., Herai R.H., Würtele M., de Carvalho L.M., Carmona e Ferreira R., Mortara R.A., Barbiéri C.L., Mieczkowski P., da Silveira J.F., Briones M.R., Pereira G.A., Bahia D. The genome sequence of Leishmania (Leishmania) amazonensis: functional annotation and extended analysis of gene models. DNA Res. 2013;20(6):567–581. doi: 10.1093/dnares/dst031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Russel D.W. vol. 1. Cold Spring Harbor Laboratory Press; 2001. p. 2344. (Molecular Cloning - A Laboratory Manual). ISBN 0879695773, 9780879695774. [Google Scholar]
- Shapiro T.A., Englund P.T. The structure and replication of kinetoplast DNA. Annu. Rev. Microbiol. 1995;49:117–143. doi: 10.1146/annurev.mi.49.100195.001001. [DOI] [PubMed] [Google Scholar]
- Schuck D.C., Ferreira S.B., Cruz L.N., da Rocha D.R., Moraes M.S., Nakabashi M., Rosenthal P.J., Ferreira V.F., Garcia C.R.S. Biological evaluation of hydroxynaphthoquinones as anti-malarials. Malar. J. 2013;12(234):1–6. doi: 10.1186/1475-2875-12-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., Shukla A.K., Das M., Dubey V.K. Evaluation of plumbagin and its derivative as potential modulators of redox thiol metabolism of Leishmania parasite. Parasitol. Res. 2012;110(1):341–348. doi: 10.1007/s00436-011-2498-x. [DOI] [PubMed] [Google Scholar]
- Sharma U., Katoch D., Sood S., Kumar N., Singh B., Thakur A., Gulati A. Synthesis, antibacterial and antifungal activity of 2-amino-1,4-naphthoquinone using silica-supported perchloric acid (HClO4-SiO2) as a mild recyclable and highly efficient heterogeneous catalyst. Indian J. Chem. 2013;52B 1431-1140. [Google Scholar]
- Sharma A., Santos I.O., Gaur P., Ferreira V.F., Garcia C.R., Rocha D.R. Addition of thiols to o-quinone methide: new 2-hydroxy-3-phenylsulfanylmethyl[1,4]naphthoquinones and their activity against the human malaria parasite Plasmodium falciparum (3D7) Eur. J. Med. Chem. 2013;59:48–53. doi: 10.1016/j.ejmech.2012.10.052. [DOI] [PubMed] [Google Scholar]
- Souza-Silva F., do Nascimento S.B., Bourguignon S.C., Pereira B.A., Carneiro P.F., da Silva W.S., Alves C.R., de Pinho R.T. Evidences for leishmanicidal activity of the naphthoquinone derivative epoxy-α-lapachone. Exp. Parasitol. 2014;147:81–84. doi: 10.1016/j.exppara.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Souza-Silva F., Bourguignon S.C., Pereira B.A., Côrtes L.M., de Oliveira L.F., Henriques-Pons A., Finkelstein L.C., Ferreira V.F., Carneiro P.F., de Pinho R.T., Caffarena E.R., Alves C.R. Epoxy-α-lapachone has in vitro and in vivo anti-leishmania (Leishmania) amazonensis effects and inhibits serine proteinase activity in this parasite. Antimicrob. Agents Chemother. 2015;59(4):1910–1918. doi: 10.1128/AAC.04742-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sritrairat N., Nukul N., Inthasame P., Sansuk A., Prasirt J., Leewatthanakorn T., Piamsawad U., Dejrudee A., Panichayupakaranant P., Pangsomboon K., Chanowanna N., Hintao J., Teanpaisan R., Chaethong W., Yongstar P., Pruphetkaew N., Chongsuvivatwong V., Nittayananta W. Antifungal activity of lawsone methyl ether in comparison with chlorhexidine. J. Oral Pathol. Med. 2011;40(1):90–96. doi: 10.1111/j.1600-0714.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- Sudhandiran G., Shaha C. Antimonial-induced increase in intracellular Ca2+ through non-selective cation channels in the host and the parasite is responsible for apoptosis of intracellular Leishmania donovani amastigotes. J. Biol. Chem. 2003;278(27):25120–25132. doi: 10.1074/jbc.M301975200. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) WHO; Geneva, Switzerland: 2010. Control of Leishmaniases: Report of the Meeting of the WHO Expert Committee on the Control of Leishmaniases. [Google Scholar]
- World Health Organization (WHO) Leishmaniasis. Fact sheet. Updated april 2017. 2017. http://www.who.int/mediacentre/factsheets/fs375/en/ Available online.
- Wyllie S., Cunningham M.L., Fairlamb A.H. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J. Biol. Chem. 2004;279(38):39925–39932. doi: 10.1074/jbc.M405635200. [DOI] [PubMed] [Google Scholar]