Abstract
Objective
Determine the occurrence rate of cochlear implant (CI) electrode tip fold-over and electrode scalar deviation as reported in patient cases with different commercial electrode types.
Data-sources
PubMed search for identifying peer-reviewed articles published till 2018 on CI electrode tip fold-over and scalar deviation. Key-words for searching were “Cochlear electrode tip fold-over”, “Cochlear electrode scalar position” and “Cochlear electrode scalar location”.
Articles-selection
Only if electrode related issues were investigated in patient cases. 38 articles met the inclusion-criteria.
Results
13 articles on electrode tip fold-over issue covering 3177 implanted ears, out of which 50 ears were identified with electrode tip fold-over with an occurrence rate of 1.57%. Out of 50 ears, 43 were implanted with pre-curved electrodes and the remaining 7 with lateral-wall electrodes. One article reported on both tip fold-over and scalar deviation. 26 articles reported on the electrode scalar deviation covering an overall number of 2046 ears out of which, 458 were identified with electrode scalar deviation at a rate of 22.38%. After removing the studies that did not report on the number of electrodes per electrode type, it was 1324 ears implanted with pre-curved electrode and 507 ears with lateral-wall electrode. Out of 1324 pre-curved electrode implanted ears, 424 were reported with scalar deviation making an occurrence rate of 32%. Out of 507 lateral-wall electrode implanted ears, 43 were associated with scalar deviation at an occurrence rate of 6.7%.
Conclusion
This literature review revealing the fact of higher rate of electrode insertion trauma associated with pre-curved electrode type irrespective of CI brand is one step closer to obsolete it from the clinical practice in the interest of patient's cochlear health.
Keywords: Electrode tip fold-over, Scalar deviation, Pre-curved electrode, Straight electrode
1. Introduction
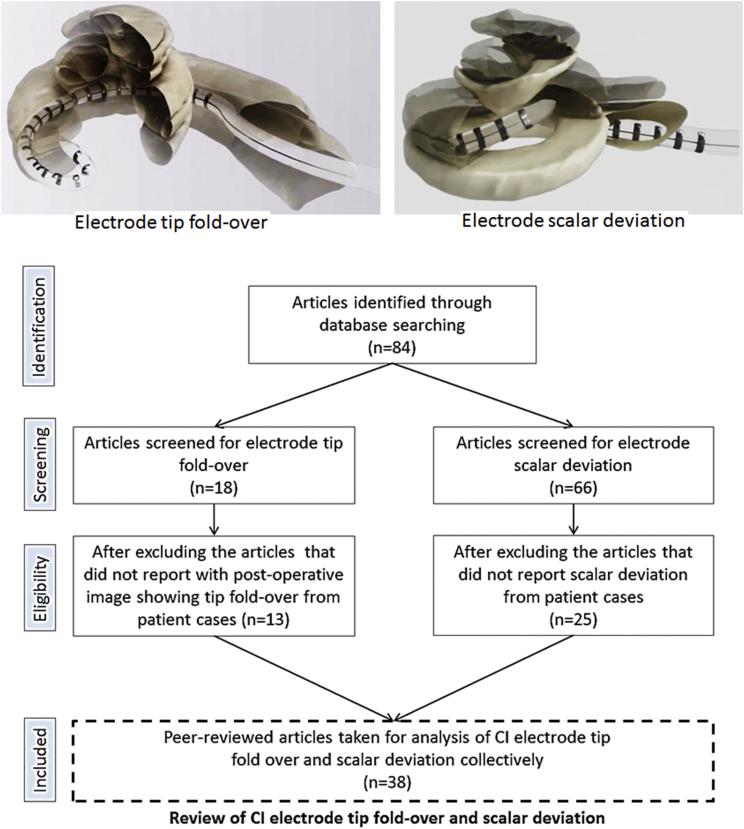
The preferred location for the surgical placement of Cochlear Implant (CI) electrode is the Scala Tympani (ST) (Aschendorff et al., 2007). While the Round Window (RW) approach of electrode insertion has been widely accepted as the standard of CI surgery (O'Connell et al., 2016) still cochleostomy approach is practiced by some group of surgeons (Badr et al., 2018). As per Eshraghi's scale of electrode insertion intra-cochlear trauma (Eshraghi et al., 2003), an electrode insertion into the cochlea would result in any of the following degree of trauma. Grade 0 corresponds to zero trauma or atraumatic insertion whereas, grade 1 corresponds to lifting of the basilar membrane (BM), grade 2 refers to rupture of BM or Spiral ligament (SL), grade 3 corresponds to the electrode dislocation from ST to Scala Vestibuli (SV) and grade 4 refers to the fracture of osseous spiral lamina (OSL) or modiolus wall. While every CI surgeon would aim for atraumatic electrode insertion, grade 2–4 is considered irreversible as the damage to the osseous spiral lamina or to the basilar membrane could mix-up the perilymph from ST with the endolymph of Scala Media (SM) (Bas et al., 2012). Electrode tip fold-over or rollover is another form of intra-cochlear complication which is a result of electrode tip getting stuck with any of the intra-cochlear structures which the surgeons may not feel and further pushing of the electrode makes the electrode tip to be folded over as shown in Fig. 1. While electrode tip fold-over can be corrected in the same surgery if identified by intra-operative images (McJunkin et al., 2018), it can also be corrected in a revision surgery (Sabban et al., 2018) if identified post-operatively if patients complain of vertigo, pitch confusion or tinnitus.
Fig. 1.
Literature review process.
Visualizing the intra-cochlear electrode position in live patients can be done by the following methods of clinical-imaging (Aschendorff et al., 2007), Neural Response Threshold (NRT) (Mittmann et al., 2017) and Electro-Cochleography (ECochG) (Koka et al., 2018). While a plain film x-ray is enough to reveal the electrode tip fold-over/rollover issues (Dirr et al., 2013), clinical CT (computed Tomography) has been in use to detect electrode translocation. Grade 1 and 2 degree of trauma cannot be detected conclusively by the clinical CT due to the image artefact but grade 3 and 4 can be conclusively detected. There is enough number of publications since 2007 that reported on electrode tip fold-over (McJunkin et al., 2018; Sabban et al., 2018; Dirr et al., 2013; Timm et al.; Sipari et al., 2018; Gabrielpillai et al., 2018; Jia et al., 2018; Garaycochea et al., 2017; Aschendorff et al., 2017; Zuniga et al., 2017; Fischer et al., 2015; Cosetti et al., 2012; Grolman et al., 2009) and electrode scalar deviation (McJunkin et al., 2018; Mittmann et al., 2017; Koka et al., 2018; Dirr et al., 2013; Sipari et al., 2018; Jia et al., 2018; Aschendorff et al., 2017; Shaul et al., 2018; Ketterer et al., 2017; An et al., 2017; O'Connell et al., 2017a; O'Connell et al., 2017b; Lathuillière et al., 2017; O'Connell et al., 2016a; O'Connell et al., 2016b; Wanna et al., 2015; Nordfalk et al., 2016; Mittmann et al., 2015a; Mittmann et al., 2015b; Mittmann et al., 2015c; Boyer et al., 2015; Wanna et al., 2014; Nordfalk et al., 2014; Aschendorff et al., 2011; Wanna et al., 2011; Lane et al., 2007) detected with x-ray, clinical CT, NRT and ECochG methods. All the commercially available CI electrodes vary in its size, shape and the method of insertion, the operating surgeon should be well informed about all these variation before the surgery. Reviews on the CI electrode design are given in detail by Briggs et al. (2011), Boyle et al. (Boyle, 2016) and Dhanasingh et al. (Dhanasingh and Jolly, 2017) covering various commercially available CI electrodes.
In this review study, Slim-Modiolar (SM), Contour Advance (CA), Contour (C), Mid-Scala (MS) and Helix (H) were all grouped under pre-curved electrode type. The remaining electrodes from all the CI brands were grouped under the straight lateral wall (LW) type for example Slim-Straight (SS), 1J, Standard (Std), Medium (M), Compressed (S) and FLEX electrodes (FLEX SOFT, FLEX28, FLEX24) as (F). To the best of authors ability and responsibility, every published literature that reported mainly on electrode tip fold-over and electrode scalar deviation in patient cases were identified without any bias hoping that this could be of any minor assistance to the operating surgeons to know more about the incidence rate of tip fold-over and scalar deviation and its negative effects (Sabban et al., 2018; Shaul et al., 2018; Ketterer et al., 2017). As for the CI companies, this could already be a final alarm for them to come-up with newer electrode design that results in zero tip fold-overs and scalar deviations, which will highly benefit the patients at the end.
2. Materials and methods
A thorough search on PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) was performed using the key words “Cochlear electrode tip fold over”, “Cochlear electrode tip roll over”, “Cochlear electrode scalar position” and “Cochlear electrode scalar location”. Articles published only in English language were taken for the review. Break down analysis corresponds to finding the incidence rate of tip fold-over and scalar deviation within the pre-curved and straight electrode type.
2.1. Inclusion criteria
Inclusion criteria for this study were as follows with studies that reported on (1) electrode tip fold-over and (2) electrode scalar deviation (grade 3 trauma) detected using post-operative imaging or intra-operative electrophysiological assessment only in patient cases and not from cadaveric temporal bone studies.
2.2. Exclusion criteria
Articles reported on electrode tip fold-over and scalar deviation in cadaveric temporal bones and the duplicate articles that came in the list when using different key words were excluded.
3. Results
84 peer reviewed publications reported on electrode tip fold-over and scalar deviation from patient cases were identified using the search method given in Fig. 1. Fig. 1 also pictorially represents how an electrode tip fold-over and scalar deviation would look like inside the cochlea. After removing the inappropriate articles that did not have to do anything with electrode tip fold-over or electrode scalar deviation, duplicate articles and reports from cadaveric temporal bones a total of 38 articles were found eligible for the review analysis.
A total of 13 peer-reviewed publications report on electrode tip fold-over in patient cases was identified from the data base. All these articles either applied post-operative computed tomography (CT)/plain film x-ray or intra-operative fluoroscopy in the identification of electrode tip fold-over.Table 1 summarizes these 13 studies with different electrode types from all 3 major CI brands. Altogether, 3177 implanted ears were taken for analysis and out of which 50 ears were identified with electrode tip fold-over making an overall occurrence rate of 1.57% irrespective of CI brand and electrode type. Out of these 50 implanted ears, 43 ears were implanted with pre-curved electrode type and 7 ears with LW electrode type irrespective of the CI brand. Gabrielpillai et al. (2018), Zuniga et al. (2017), Dirr et al. (2013), did not provide number of cases per electrode type that made it difficult to perform the electrode type break-down analysis. With these 3 studies neglected, the total number of pre-curved electrode and lateral wall electrode implanted ears accounted to 553 and 387 respectively. Out of these numbers, 26 and 3 ears were reported with tip fold-over making an incidence rate of 4.7% and 0.80% respectively with the pre-curved and lateral wall electrode types.
Table 1.
Summarizing the 13 studies on tip fold-over.
| Study | No. of cases taken for analysis/method | No. of electrode per type/brand |
No. of cases reported tip fold-over | ||
|---|---|---|---|---|---|
| A | B | C | |||
| Timm et al (2018) | 275 (CT) | – | – | 275 (F28, F24, F20, F16) | 0 |
| Sipari et al. (2018) | 23 (CT) | – | MS (23) | – | 2 |
| Gabrielpillai et al. (2018) | 1722 (CT) | No info on brand segments | 7 (CA), 6 (SM), 2(SS) | ||
| Jia et al. (2018) | 65 (Intra-op CT) (Contains 3 electrodes from Oticon) |
CA (12), SM (1), SS (31) | 1J (2), MS (3) | F28 (13) | 1 (SM) |
| Sabban et al. (2018) | 2 (x-ray & CT) | – | MS | – | 2 |
| McJunkin et al. (2018) | 117 (intra-op x-ray) | SM | – | – | 9 |
| Garaycochea et al. (2017) | 1 (Intra-op fluoroscopy) | SM | – | – | 1 (100%) |
| Aschendorff et al. (2017) | 45 (intra-op x-ray/fluoroscopy) | SM | – | – | 2 cases. 1st case corrected in the same surgery. 2nd case underwent revision surgery |
| Zuniga et al. (2017) | 303 (CT) | CA, SS | MS, 1J | – | 3(CA), 1(MS), 1 (SS) and 1(1J) |
| No info on brand segments | |||||
| Fischer et al., 2015 | 63 (CT) | – | – | F24,F28, Std | 1 |
| Dirr et al. (2013) | 215 (Post-op x-ray) | CA, SS | – | Std, M, S, FL, F28 | 2 (F) |
| No info on brand segments | |||||
| Cosetti et al. (2012) | 277 (Intra-op x-ray) | CA | – | – | 5 |
| Grolman et al. (2009) | 72 (Intra-op rotational x-ray) | CA | – | – | 4 |
| Total | 3180 | 50 (43 pre-curved electrode + 7 lateral wall electrode) | |||
| Total, after excluding 3 studies that did not specify number per electrode type | 940 |
Pre-curved (553) Lateral wall (387) |
Pre-curved (26) Lateral wall (3) |
||
CA: Contour Advance; SM: Slim-Modiolar; MS: Mid-Scala; SS: Slim Straight; Std: Standard; M: Medium; S: Compressed; FL: FLEX SOFT, F28: FLEX28; F24: FLEX24; F20: FLEX20; F16: FLEX16.
Electrode scalar deviation is another important and the most traumatic event that could happen inside the cochlea during electrode placement. A total of 26 peer-reviewed publications were found reporting on intra-cochlear electrode array scalar deviation in patient cases with the current commercially available electrode types (McJunkin et al., 2018; Mittmann et al., 2017; Koka et al., 2018; Dirr et al., 2013; Sipari et al., 2018; Jia et al., 2018; Aschendorff et al., 2017; Fischer et al., 2015; Shaul et al., 2018; Ketterer et al., 2017; An et al., 2017; O'Connell et al., 2017a; O'Connell et al., 2017b; Lathuillière et al., 2017; O'Connell et al., 2016a; O'Connell et al., 2016b; Wanna et al., 2015; Nordfalk et al., 2016; Mittmann et al., 2015a; Mittmann et al., 2015b; Mittmann et al., 2015c; Boyer et al., 2015; Wanna et al., 2014; Nordfalk et al., 2014; Aschendorff et al., 2011; Wanna et al., 2011; Lane et al., 2007) and were summarized in Table 2.
Table 2.
26 studies reported on electrode scalar deviation.
| Study | No. of cases taken for analysis | Analyzing method | No. of electrode from type/brand |
No. of cases reported with scalar deviation |
|||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | MH | MS | LW | ||||
| Shaul et al. (2018) | 110 | Imaging | CA (92), SM (18) | – | – | 18 (19.5%) | – | – | |
| Sipari et al. (2018) | 23 | Imaging | – | MS (23) | – | – | 5 (22%) | – | |
| Koka et al. (2018) | 32 | Imaging/EcochG | – | MS (32) | – | – | 7 (22%) | – | |
| Jia et al. (2018) | 65 | Imaging | CA (12), SM(1), SS (31) | 1J (2), MS (3) | F28 (16), | 1 (8%) | 1 (20%) | – | |
| McJunkin et al. (2018) | 23 | Imaging | SM (23) | – | – | 6 (26%) | – | – | |
| Ketterer et al., 2017 | 368 | Imaging | CA (368) | – | – | 118 (32%) | – | – | |
| An et al., 2017 | 26 | Imaging | SS (5) | – | F28 (21) | – | – | 1 (4.7%) F28, 1(20%) SS |
|
| Aschendorff et al. (2017) | 45 | Imaging | SM (45) | – | – | 0 (0%) | – | – | |
| O'Connell et al., 2017a | 48 | Imaging | – | – | F24, F28, Std (48) | – | – | 0 (0%) | |
| O'Connell et al., 2017b | 18 | EcochG | – | MS (18) | – | – | 6(33%) | – | |
| Mittmann et al. (2017) | 50 | NRT | SS (50) | – | – | – | – | 2 (4%) SS | |
| Lathuillière et al., 2017 | 24 | Imaging | CA (24), | – | – | 3 (13%) | – | – | |
| O'Connell et al., 2016a | 56 | Imaging | CA (36), SS (20) | – | – | 19 (52%) | – | 2 (10%) SS | |
| O'Connell et al., 2016b | 220 | Imaging | CA (115), SS (19), | 1J(21), MS (14) | F28 (28), Std (17), F24 (4) & M (2) | 59 (51%) | 8(57%) | 4 (4.4%) F | |
| Wanna et al. (2015) (Wanna et al., 2015) | 45 | Imaging | CA (15) | MS (3) | 4 (26%) | 1(33%) | 2 (7%)- 1J &SS | ||
| SS, 1J & F collectively (27) | |||||||||
| Nordfalk et al. (2016) (Nordfalk et al., 2016) | 39 | Imaging | – | – | F28 (18), FL (17), F24 (4) | – | – | 0 (0%) F | |
| Mittmann et al., 2015a | 23 | NRT | CA (23) | – | – | 6 (26%) | – | – | |
| Mittmann et al., 2015b | 85 | NRT | CA (85) | – | – | 16 (18%) | – | – | |
| Boyer et al. (2015) | 61 | Imaging | CA (31), | – | FL, F28, F24, Std (30) | 8 (25%) | – | 0 (0%) FLEX, 1 (3%) Std. |
|
| Fischer et al., 2015 | 63 | Imaging | – | – | F28 (40), F24 (2), FL (7), Std (14) | – | – | 5 (12.5%) F28 | |
| Wanna et al. (2014) | 116 | Imaging | CA, MS (69) | (47) LW from all 3 CI brands | 29 (42%) with MH | – | 5 (10.6%) All LW | ||
| Dirr et al. (2013) | 215 | imaging | 107 | 108 | – | – | 1(0.92%) F | ||
| Nordfalk et al. (2014) | 13 | Imaging | CA (7) | 1J (3) | Std (2), F24 (1) | 3 (43%) with CA, | – | 1 (50%) Std, 1 (33%) 1J |
|
| Aschendorff et al. (2011) | 223 | Imaging | C (21), CA (202) | – | – | 19 (90%) C, 70 (35%) CA. | – | – | |
| Wanna et al. (2011) | 32 | Imaging | 20 | 10 | 2 | 7 (35%) | 4(40%) | 0 (0%) F | |
| Lane et al. (2007) | 23 | Imaging | C/CA (13) | H (1) | – | 6 (46%) C | – | 7 (78%) LW | |
| LW electrodes from brand A & B (9) | |||||||||
| Total | 2046 | 1415 | 272 | 359 | 392 | 32 | 34 | ||
| Total, after excluding Dirr et al that did not specify number per electrode type | 1831 |
Pre-curved (1324) Lateral wall (507) |
Pre-curved (424) Lateral wall (34) |
||||||
NRT: neural response telemetry, EcochG: electrocochleography.
A total of 2046 implanted ears reported from 26 peer-reviewed published articles were taken for analysis. Out of 2046 implanted ears, a total of 458 ears were reported with electrode scalar deviation making an overall incidence rate of 22.38% irrespective of CI electrode types and brands. Dirr et al. (2013) did not provide electrode type specific number and with the exclusion of that data a total of 1831 implanted ears were available for further analysis. Out of 1831 implanted ears, pre-curved electrode including the Mid-Scala electrode were implanted in 1324 ears and the remaining 507 ears were implanted with lateral wall electrode type irrespective of the CI brand. Bringing C, CA, H and MS all under pre-curved electrode type (392 + 32), a total of 424 implanted ears had scalar deviation making an incidence rate of 32% with this electrode type. The lateral wall electrode implanted ears showed scalar deviation rate of only 6.7% (34/507).
Lee et al. (2011) in 2011 reported on electrode scalar deviation identified by the histopathologic method from patients who were implanted with the older generation of electrodes when they were alive and these electrodes are currently not available in the market as given in Table 3. Lateral wall electrodes irrespective of CI brands are given in Table 3 were involved in the scalar deviation.
Table 3.
One study reporting on electrode scalar deviation from older generation of electrodes which are currently not available in the market.
| Study | No. of cases taken for analysis | Analyzing method | No. of electrode from type/brand |
No. of cases reported with scalar deviation |
|||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | MH | MS | LW | |||
| Lee et al. (2011) | 38 | Histology | Nucleus 22 (16) (LW), 24R (1) (MH), 24 M (4) (double array LW), 24 RST (1) (LW) | Clarion (1) (LW) | MED-EL 40+ (1) (LW) | Ineraid (10) (LW) | 0 | – | Nucleus 22 (8) Clarion (1) Ineraid (4) 40+ (1) |
4. Discussion
Atraumatic (Grade 0) intra-cochlear electrode placement ensures better audiological performance irrespective of electrode type following the CI surgery (O'Connell et al., 2016; Shaul et al., 2018; Ketterer et al., 2017; O'Connell et al., 2017a; O'Connell et al., 2016a; Wanna et al., 2015; Wanna et al., 2011; Holden et al., 2013) especially in patients with low frequency residual hearing. With electrode types varying in its design and insertion methods among the CI brands (Dhanasingh and Jolly, 2017), it is a challenging task for the operating surgeon in consistently and atraumatically handle the electrode in every patient in which the cochlear anatomy is also varying which results in considerable rates of intra-cochlear trauma.
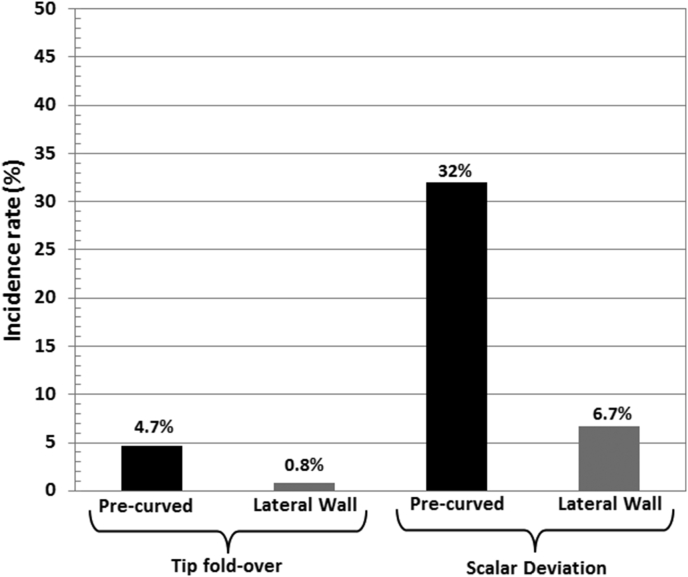
Table 1, Table 2 summarizes the 38 peer reviewed publications that collectively reported on electrode tip fold-over and scalar deviation issues respectively with the electrode types from all 3 major CI manufacturers in patient cases. Fig. 2 simply shows incidence rate of both tip fold-over and scalar deviation by pre-curved and lateral wall electrodes separately. While the rate of tip fold-over with pre-curved and lateral wall electrode type accounts to 4.7% and 0.8% of implanted ears respectively, rate of scalar deviation with these two electrode types accounts to 32% and 6.7% respectively. Both tip fold and scalar deviation is approximately 5 times higher with the pre-curved electrodes in comparison to the lateral wall electrodes.
Fig. 2.
Histogram chart showing the % of electrode related issues with two general types of CI electrodes.
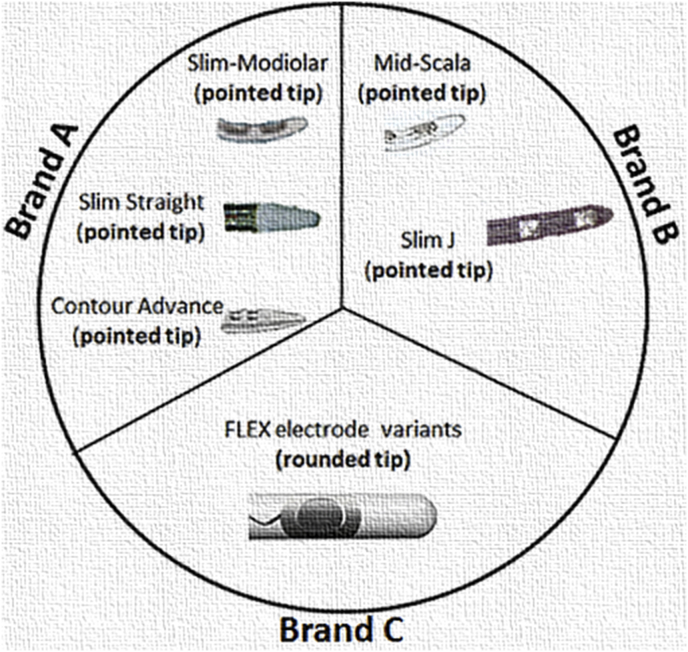
Understanding how electrode tip fold-over happens is essential. With pre-curved electrode type, pre-mature pulling of the stylet rod out of the electrode could be thought as one of the several other reasons whereas with lateral wall electrode type, the tip geometry alone can be blamed. Fig. 3 shows the tip geometry of all the electrodes taken here for the analysis. Considering the rough/porous surface of the modiolus wall inside the cochlea, the chances for pointed/conical tip to get stuck is highly there and this could explain how the tip fold-over could happen inside the cochlea for both electrode types. Though there are colored markers available along the pre-curved electrode array length indicating the insertion depths to which the pre-curved electrodes should be inserted with the stylet in place, variations in the cochlear size and shape jeopardizes the benefits of colored markers. If tip fold-over can be identified intra-operatively then there are higher chances to correct it in the same surgery, if not the tip fold-over could create pitch confusions, vertigo and tinnitus as reported by Sabban et al., in 2018 (Sabban et al., 2018). Trakimas et al., in 2018 (Trakimas et al., 2018) reported new bone and fibrous tissue formation right at the place where the intra-cochlear electrode bending, kinking and tip fold-over happened as seen from the CI implanted patients who died and donated their bodies for research.
Fig. 3.
Electrode tip geometry from all three major CI brands.
Reasons for electrode scalar deviation can be the following. The stiffness of the electrode can be substantially blamed for such an electrode insertion trauma. Again the colored markers in the array indicating the insertion depth to which the pre-curved electrode with the metal stylet rod or polymer sheath should be inserted inside the cochlea, the cochlear size and shape variation could mislead and as a result, pre-curved electrode made straight with the metal stylet inside the array could have already pierced through the spiral ligament at the end of the straight portion of the basal turn. Also if there is a disorientation with stimulating channels slightly facing the basilar membrane at the beginning of the electrode insertion and as stylet rod/polymer rod is pulled out to make the pre-curved electrode to hug the modiolus wall after reaching the prescribed insertion depth, the stiff electrode with the conical/pointed tip of the electrode can break the osseous spiral lamina or the organ of corti and enter the SV. These are beyond what a surgeon can control during the surgical procedure. Shaul et al., in 2018 (Shaul et al., 2018), Ketterer et al., in 2017 (Ketterer et al., 2017), O'Connell et al., in 2016 (O'Connell et al., 2016a; O'Connell et al., 2016b), Wanna et al., in 2011 (Wanna et al., 2011) and Holden et al., in 2013 (Holden et al., 2013) reported on drop in audiological performance among the CI patients with electrode scalar deviation in comparison to CI patients with electrode completely residing in ST.
Study by Lee et al. (2011) included 38 cadaveric cochlear samples from patients who were previously implanted with CI was not included in the analysis of scalar deviation as it had the older generation of electrodes which are currently not available in the market anymore. They reported scalar deviation with 17 cases which were all implanted with the lateral wall electrode type of the older generation. Moreover, the patients who were implanted with these older generations of electrodes were reported with pre-operative complications like labyrinthitis ossificans, otosclerosis, temporal bone fracture and soft tissue obstruction which could well be responsible for the electrode scalar deviation.
While straight lateral wall electrode type is expected to be placed along the lateral wall of cochlea, chances of touching the basilar membrane is highly there and this will be classified as Grade 1 trauma per Eshraghi's scale. Though this is interesting and important information to know, how this can be visualized from the clinical CT images of patients which are highly inconclusive is a question, whereas Grade 3 trauma of scalar translocation can be easily recognized from the clinical CT images. If closer proximity of the stimulating contacts to modiolus wall is considered a big advantage of pre-curved electrode, how often does a pre-curved electrode type places the stimulating contacts closer to modiolus wall is again a big question and this was raised by Wang et al. (2017). In their study they pointed out clearly that “Our results show that perimodiolar EAs, more often than not, do not sit adjacent to the modiolus where they are likely most effective”. If pre-curved electrode do not provide the stimulating contacts a closer proximity to modiolus wall and in addition, it involves in scalar deviation at a rate which is higher than straight lateral wall electrode, it poses the question to the operating surgeon why not select lateral wall electrode type in all cases in which there are no anatomical complications. Pre-curved electrodes may be of good choice for example cases with otosclerosis in which the stimulating contacts placed relatively closer to modiolus wall proved beneficial (Battmer et al., 2008). Not to forget the issue of electrode array migration out of the cochlea associated with lateral wall electrode irrespective of CI brand as reported by Dietz et al., in 2015 (Dietz et al., 2016) and van der Marel et al., in 2012 (van der Marel et al., 2012).Techniques like fixation clip (Cohen and Kuzma, 1995; Müller et al., 1998) can be thought to minimize or eliminate the electrode array migration issue. Recently, intra-operative electrocochleography method has been used as a research tool in finding the scalar deviation as reported by Koka et al. (2018), hoping that it could reliably inform the operating surgeon before the scalar deviation happens. Scalar deviation is mainly detected using the post-operative CT imaging and this method has been validated using histology section as reported by Iso Mutajärvi et al., in 2017 (Iso-Mustajärvi et al., 2017). Therefore the reliability of the scalar deviation detection method should not pose serious question on how representative the data used in this review.
5. Conclusion
Electrode tip fold-over and electrode scalar deviation are gaining importance among clinicians as more and more reports are coming out demonstrating relatively lower CI outcomes with electrode insertion trauma especially scalar deviation. With the data analyzed from the peer-reviewed publications in this review, it is evident that pre-curved electrode type encounters intra-cochlear trauma relatively at a higher rate compared with lateral wall electrode type. While CI brings some hearing benefit in all patients with or without any electrode related intra-cochlear trauma and irrespective of electrode type, the hearing benefit increases with the electrode completely positioned in the ST and this needs to be taken into a serious consideration in the interest of the patients while selecting the electrode type.
Declaration
Both the authors are employees of MED-EL Austria, at the time of writing this manuscript. Authors declare that this review was done to the best of their ability and responsibility avoiding any bias and considering all the critical comments by the reviewers.
Acknowledgement
Authors extend their sincere gratitude to all those CI surgeons from all over the world who shared their personal experience handling various CI electrode types and that stimulated to write this article. Mr. Michael Todd (MED-EL) and Mr. Sebastian Falkner (MED-EL) are acknowledged for English corrections and graphical work respectively.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.joto.2019.01.002.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- An S.Y., An C.H., Lee K.Y., Jang J.H., Choung Y.H., Lee S.H. Diagnostic role of cone beam computed tomography for the position of straight array. Acta Otolaryngol. 2017 Nov 26:1–7. doi: 10.1080/00016489.2017.1404639. [DOI] [PubMed] [Google Scholar]
- Aschendorff A., Kromeier J., Klenzner T., Laszig R. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear. 2007 Apr;28(2 Suppl. l):75S–79S. doi: 10.1097/AUD.0b013e318031542e. [DOI] [PubMed] [Google Scholar]
- Aschendorff A., Klenzner T., Arndt S., Beck R., Schild C., Roddiger L., Maier W., Laszig R. Insertion results for contour and contour Advance electrodes: are there individual learning curves? HNO. 2011 May;59(5):448–452. doi: 10.1007/s00106-011-2319-7. [DOI] [PubMed] [Google Scholar]
- Aschendorff A., Briggs R., Brademann G., Helbig S., Hornung J., Lenarz T., Marx M., Ramos A., Stöver T., Escudé B., James C.J. Clinical investigation of the nucleus slim modiolar electrode. Audiol. Neuro. Otol. 2017;22(3):169–179. doi: 10.1159/000480345. [DOI] [PubMed] [Google Scholar]
- Badr A., Shabana Y., Mokbel K., Elsharabasy A., Ghonim M., Sanna M. Atraumatic scala tympani cochleostomy; Resolution of the dilemma. J. Int. Adv. Otol. 2018 Aug;14(2):190–196. doi: 10.5152/iao.2018.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas E., Dinh C.T., Garnham C., Polak M., Van De Water T.R. Conservation of hearing and protection of hair cells in cochlear implant patients' with residual hearing. Anat. Rec. 2012 Nov;295(11):1909–1927. doi: 10.1002/ar.22574. [DOI] [PubMed] [Google Scholar]
- Battmer R., Pesch J., Stöver T., Lesinski-Schiedat A., Lenarz M., Lenarz T. Elimination of facial nerve stimulation by reimplantation in cochlear implant subjects. Otol. Neurotol. 2008 Oct;27(7):918–922. doi: 10.1097/01.mao.0000235374.85739.c6. [DOI] [PubMed] [Google Scholar]
- Boyer E., Karkas A., Attye A., Lefournier V., Escude B., Schmerber S. Scalar localization by cone-beam computed tomography of cochlear implant carriers: a comparative study between straight and periomodiolar precurved electrode arrays. Otol. Neurotol. 2015 Mar;36(3):422–429. doi: 10.1097/MAO.0000000000000705. [DOI] [PubMed] [Google Scholar]
- Boyle P.J. The rational for a mid-scala electrode array. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016 Jun;133(Suppl. 1):S61–S62. doi: 10.1016/j.anorl.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Briggs R.J., Tykocinski M., Lazsig R., Aschendorff A., Lenarz T., Stöver T., Fraysse B., Marx M., Roland J.T., Jr., Roland P.S., Wright C.G., Gantz B.J., Patrick J.F., Risi F. Development and evaluation of the modiolar research array—multi centre collaborative study in human temporal bones. Cochlear Implants Int. 2011 Aug;12(3):129–139. doi: 10.1179/1754762811Y0000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N.L., Kuzma J. Titanium clip for cochlear implant electrode fixation. Ann. Otol. Rhinol. Laryngol. Suppl. 1995 Sep;166:402–403. [PubMed] [Google Scholar]
- Cosetti M.K., Troob S.H., Latzman J.M., Shapiro W.H., Roland J.T., Jr., Waltzman S.B. An evidence-based algorithm for intraoperative monitoring during cochlear implantation. Otol. Neurotol. 2012 Feb;33(2):169–176. doi: 10.1097/MAO.0b013e3182423175. [DOI] [PubMed] [Google Scholar]
- Dhanasingh A., Jolly C. An overview of cochlear implant electrode array designs. Hear. Res. 2017 Dec;356:93–103. doi: 10.1016/j.heares.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Dietz A., Wennström M., Lehimäki A., Löppönen H., Valtonen H. Electrode migration after cochlear implant surgery: more common than expected? Eur. Arch. Oto-Rhino-Laryngol. 2016 Jun;273(6):1411–1418. doi: 10.1007/s00405-015-3716-4. [DOI] [PubMed] [Google Scholar]
- Dirr F., Hempel J.M., Krause E., Müller J., Berghaus A., ertl-Wagner B., Braun T. Value of routine plain x-ray position checks after cochlear implantation. Otol. Neurotol. 2013 Dec;34(9):1666–1669. doi: 10.1097/MAO.0b013e3182a09cc3. [DOI] [PubMed] [Google Scholar]
- Eshraghi A.A., Yang N.W., Balkany T.J. Comparative study of cochlear damage with three perimodiolar electrode design. Laryngoscope. 2003;113:415–419. doi: 10.1097/00005537-200303000-00005. [DOI] [PubMed] [Google Scholar]
- Fischer N., Pinggera L., Weichbold V., Dejaco D., Schmutzhard J., Widmann G. Radiologic and functional evaluation of electrode dislocation from the scala tympani to the scala vestibuli in patients with cochlear implants. AJNR Am. J. Neuroradiol. 2015;36(2):372–377. doi: 10.3174/ajnr.A4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielpillai J., Burck I., Baumann U., Stöver T., Helbig S. Incidence of tip foldover during cochlear implantation. Otol. Neurotol. 2018 Oct;39(9):1115–1121. doi: 10.1097/MAO.0000000000001915. [DOI] [PubMed] [Google Scholar]
- Garaycochea O., Manrique-Huarte R., Manrique M. Intra-operative radiological diagnosis of a tip roll-over electrode array discplacement using fluoroscopy, when electrophysiological testing is normal: the importantce of both techniques in cochlear implant surgery. Braz. J. Otorhinolaryngol. 2017 Jun 1 doi: 10.1016/j.bjorl.2017.05.003. Pii: S1808-8694 (17)30079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolman W., Maat A., Verdam F., Simis Y., Carelsen B., Freling N., Tange R.A. Spread of excitation measurements for the detection of electrode array fold-overs: a prospective study comparing 3-dimensional rotational x-ray and intraoperative spread of excitation measurements. Otol. Neurotol. 2009 Jan;30(1):27–33. doi: 10.1097/mao.0b013e31818f57ab. [DOI] [PubMed] [Google Scholar]
- Holden L.K., Finley C.C., Firszt J.B., Holden T.A., Brenner C., Potts L.G., Gotter B.D., Vanderhoof S.S., Mispagel K., Heydebrand G., Skinner M.W. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013;34(3):342–360. doi: 10.1097/AUD.0b013e3182741aa7. May-Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso-Mustajärvi M., Matikka H., Risi F., Sipari S., Koski T., Willberg T., Lehtimäki A., Tervaniemi J., Löppönen H., Dietz A. A new slim modiolar electrode array for cochlear implantation: a radiological and histological study. Otol. Neurotol. 2017 Oct;38(9) doi: 10.1097/MAO.0000000000001542. e327-3334. [DOI] [PubMed] [Google Scholar]
- Jia H., Torres R., Nguyen Y., De Seta D., Ferrary E., Wu H., Sterkers O., Bernardeschi D., Mosnier I. Intraoperative cone-beam CT for assessment of intracochlear positioning of electrode arrays in adult recipients of cochlear implants. AJNR Am. J. Neuroradiol. 2018 Apr;39(4):768–774. doi: 10.3174/ajnr.A5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterer M.C., Aschendorff A., Arndt S., Hassepass F., Wesarg T., Laszig R., Beck R. European Archives of Oto-Rhino-Laryngology; 2017. The Influence of Cochlear Morphology on the Final Electrode Array Position; pp. 1–10. [DOI] [PubMed] [Google Scholar]
- Koka K., Riggs W.J., Dwyer R., Holder J.T., Noble J.H., Dawant B.M., Ortmann A., Valenzuela C.V., Mattingly J.K., Harris M.M., O'Connell B.P., Litvak L.M., Adunka O.F., Buchman C.A., Labadie R.F. Intra-cochlear electrocochleography during cochlear implant electrode insertion is predictive of final scalar location. Otol. Neurotol. 2018 Sep;39(8):e654–e659. doi: 10.1097/MAO.0000000000001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J.I., Witte R.J., Driscoll C.L., Shallop J.K., Beatty C.W., Primak A.N. Scalar localization of the electrode array after cochlear implantation: clinical experience using 64-slice multidetector computed tomography. Otol. Neurotol. 2007 Aug;28(5):658–662. doi: 10.1097/MAO.0b013e3180686e26. [DOI] [PubMed] [Google Scholar]
- Lathuillière M., Merklen F., Piron J.P., Sicard M., Villemus F., Menjot de Champfleur N., Venail F., Uziel A., Mondain M. Cone-beam computed tomography in children with cochlear implants: the effect of electrode array position on ECAP. Int. J. Pediatr. Otorhinolaryngol. 2017 Jan;92:27–31. doi: 10.1016/j.ijporl.2016.10.033. [DOI] [PubMed] [Google Scholar]
- Lee J., Nadol J.B., Eddington D.K. Factors associated with incomplete insertion of electrodes in cochlear implant surgery: a histopathologic study. Audiol. Neurotol. 2011;16:69–81. doi: 10.1159/000316445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McJunkin J.L., Durakovic N., Herzog J., Buchman C.A. Early outcomes with a slim modiolar cochlear implant electrode array. Otol. Neurotol. 2018 Jan;39(1):e28–e33. doi: 10.1097/MAO.0000000000001652. [DOI] [PubMed] [Google Scholar]
- Mittmann P., Ernst A., Todt I. Intraoperative electro-physiologic variations caused by the scalar position of cochlear Implant electrodes. Otol. Neurotol. 2015 Jul;36(6):1010–1014. doi: 10.1097/MAO.0000000000000736. [DOI] [PubMed] [Google Scholar]
- Mittmann P., Todt I., Wesarg T., Arndt S., Ernst A., Hassepass F. Electrophysiological detection of scalar-changing perimodiolar cochlear electrode arrays: a six-month follow-up study. Audiol. Neuro. Otol. 2015 Nov;20(6):400–405. doi: 10.1159/000441346. [DOI] [PubMed] [Google Scholar]
- Mittmann P., Todt I., Wesarg T., Arndt S., Ernst A., Hassepass F. Electrophysiological detection of intracochlear scalar changing perimodiolar cochlear implant electrodes: a blinded study. Otol. Neurotol. 2015 Aug;36(7):1166–1171. doi: 10.1097/MAO.0000000000000766. [DOI] [PubMed] [Google Scholar]
- Mittmann P., Todt I., Ernst A., Rademacher G., Mutze S., Göricke S., Schlamann M., Lang S., Arweiler-Harbeck D., Christov F. Radiological and NRT-ratio-based estimation of slim straight cochlear implant electrode positions: a multicenter study. Ann. Otol. Rhinol. Laryngol. 2017 Jan;126(1):73–78. doi: 10.1177/0003489416675355. Epub 2016 Oct 25. [DOI] [PubMed] [Google Scholar]
- Müller J., Schön F., helms J. Reliable fixation of cochlear implant electrode mountings in children and adults—initial experiences with a new titanium clip. Laryngo-Rhino-Otol. 1998 Apr;77(4):238–240. doi: 10.1055/s-2007-996968. [DOI] [PubMed] [Google Scholar]
- Nordfalk K.F., Rasmussen K., Hopp E., Greisiger R., Jablonski G.E. Scalar position in cochlear implant surgery and outcome in residual hearing and the vestibular system. Int. J. Audiol. 2014 Feb;53(2):121–127. doi: 10.3109/14992027.2013.854413. [DOI] [PubMed] [Google Scholar]
- Nordfalk K.F., Rasmussen K., Hopp E., Bunne M., Silvola J.T., Jablonski G.E. Insertion depth in cochlear implantation and outcome in residual hearing and vestibular function. Ear Hear. 2016;37(2):e129–e137. doi: 10.1097/AUD.0000000000000241. Mar-Apr. [DOI] [PubMed] [Google Scholar]
- O'Connell B.P., Hunter J.B., Gifford R.H., Rivas A., Haynes D.S., Noble J.H., Wanna G.B. Electrode location and audiologic performance after cochlear implantation: a comparative study between nucleus CI422 and CI512 electrode arrays. Otol. Neurotol. 2016 Sep;37(8):1032–1035. doi: 10.1097/MAO.0000000000001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell B.P., Cakir A., Hunter J.B., Francis D.O., Noble J.H., Labadie R.F., Zuniga G., Dawant B.M., Rivas A., Wanna G.B. Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otol. Neurotol. 2016 Sep;37(8):1016–1023. doi: 10.1097/MAO.0000000000001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell B.P., Hunter J.B., Haynes D.S., Holder J.T., Dedmon M.M., Noble J.H., Dawant B.M., Wanna G.B. Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope. 2017 Oct;127(10):2352–2357. doi: 10.1002/lary.26467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell B.P., Holder J.T., Dwyer R.T., Gifford R.H., Noble J.H., Bennett M.L., Rivas A., Wanna G.B., Haynes D.S., Labadie R.F. Intra- and postoperative electrocochleography may be predictive of final electrode position and postoperative hearing preservation. Front. Neurosci. 2017;11:291. doi: 10.3389/fnins.2017.00291. May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell B.P., Hunter J.B., Wanna G.B. The importance of electrode location in cochlear implantation. Laryngoscope Investig. Otolaryngol. 2016;1(6):169–174. doi: 10.1002/lio2.42. Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabban D., Parodi M., Blanchard M., Ettienne V., Rouillon I., Loundon N. Intra-cochlear electrode tip fold-over. Cochlear Implants Int. 2018:1–5. doi: 10.1080/14670100.2018.1427823. Jan 24. [DOI] [PubMed] [Google Scholar]
- Shaul C., gragovic A.S., Stringer A.L., O'Leary S.J., Briggs R.J. Scalar localization of peri-modiolar electrodes and speech perception outcomes. J. Laryngol. Otol. 2018;132:1000–1006. doi: 10.1017/S0022215118001871. [DOI] [PubMed] [Google Scholar]
- Sipari S., Iso-Mustajärvi M., Löppönen H., Dietz A. The insertion results of a mid-scala electrode assessed by MRI and CBCT image fusion. Otol. Neurotol. 2018 Dec;39(10):e1019–e1025. doi: 10.1097/MAO.0000000000002045. [DOI] [PubMed] [Google Scholar]
- Timm ME, Majdani O, Weller T, Windeler M, Lenarz T, Büchner A, Salcher RB. Patient specific selection of lateral wall cochlear implant electrodes based on anatomical ranges. PLoS One 13(10):30206435. [DOI] [PMC free article] [PubMed]
- Trakimas D.R., Kozin E.D., Ghanad I., Nadol J.B., Jr., Remenschneider A.K. Human otopathologic findings in cases of folded cochlear implant electrodes. Otol. Neurotol. 2018 Sep;39(8):970–978. doi: 10.1097/MAO.0000000000001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Marel K.S., Verbist B.M., Briaire J.J., Joemai R.M.S., Frijns J.H.M. Electrode migration in cochlear implant patients: not an exception. Audiol. Neurotol. 2012;17:275–281. doi: 10.1159/000338475. [DOI] [PubMed] [Google Scholar]
- Wang J., Dawant B.M., Labadie R.F., Noble J.H. Retrospective evaluation of a technique for patient-customized placement of precurved cochlear implant electrode arrays. Otolaryngol. Head Neck Surg. 2017 Jul;157(1):107–112. doi: 10.1177/0194599817697298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanna G.B., Noble J.H., McRackan T.R., Dawant B.M., Dietrich M.S., Watkins L.D., Rivas A., Schuman T.A., Labadie R.F. Assessment of electrode placement and audiological outcomes in bilateral cochlear implantation. Otol. Neurotol. 2011 Apr;32(3):428–432. doi: 10.1097/MAO.0b013e3182096dc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanna G.B., Noble J.H., Carlson M.L., Gifford R.H., Dietrich M.S., Haynes D.S., Dawant B.M., Labadie R.F. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope. 2014 Nov;124(Suppl. 6):S1–S7. doi: 10.1002/lary.24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanna G.B., Noble J.H., Gifford R.H., Dietrich M.S., Sweeney A.D., Zhang D., Dawant B.M., Rivas A., Labadie R.F. Impact of intrascalar electrode location, electrode type, and angular insertion depth on residual hearing in cochlear implant patients: preliminary Results. Otol. Neurotol. 2015 Sep;36(8):1343–1348. doi: 10.1097/MAO.0000000000000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga M.G., Rivas A., Hedley-Williams A., Gifford R.H., Dwyer R., Dawant B.M., Sunderhaus L.W., Hovis K.L., Wanna G.B., Noble J.H., Labadie R.F. tip fold-over in cochlear implantation: case series. Otol. Neurotol. 2017 Feb;38(2):199–206. doi: 10.1097/MAO.0000000000001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.