Abstract
Background:
Neurological disorders constitute a growing worldwide concern due to the progressive aging of the population and the risky behavior they represent. Herbal medicines have scientific relevance in the treatment of these pathol-ogies. One of these substances, Astragaloside IV (AS-IV), is the main active compound present in the root of Astragalus membranaceus (Fisch.) Bge, a Chinese medicinal herb with neuroprotective properties.
Objective:
In the present study we performed a systematic review that sought to comprehend the neuroprotective effect pre-sented by AS-IV in experimental models of neurological disorders.
Method:
This study is a systematic review, where an electronic search in United States National Library of Medicine (Pub-Med), Science Direct, Cochrane Library, Scientific Electronic Library Online (SciELO), Scopus, Web of Science, Medline via Proquest and Periodicos Capes databases covering the years between 2007 and 2017, using “Astragaloside IV” and “Neurodegenerative diseases”; “Astragaloside IV” and “ Neurological disorders” as reference terms was made.
Results:
A total of 16 articles were identified, in which the efficacy of AS-IV was described in experimental models of Par-kinson’s disease, Alzheimer’s disease, cerebral ischemia and autoimmune encephalomyelitis, by improving motor deficits and/or neurochemical activity, especially antioxidant systems, reducing inflammation and oxidative stress.
Conclusion:
The findings of the present study indicate that the administration of AS-IV can improve behavioral and neuro-chemical deficits largely due to its antioxidant, antiapoptotic and anti-inflammatory properties, emerging as an alternative therapeutic approach for the treatment of neurological disorders.
Keywords: Neurological disorders, Parkinson’s disease, Alzheimer’s disease, Cerebral ischemia, Astragaloside IV, Brain
1. INTRODUCTION
In neurological disorders, functional or sensory loss occurs due to lesions in neuronal cells, in addition to other environmental or genetic factors that also contribute to this condition. Oxidative stress, caused by the attack of free radicals on these cells, is an important factor associated with neurodegeneration. Excess of reactive oxygen species (ROS)
and imbalanced metabolism are involved in a number of neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease as well as other neurological conditions such as traumatic lesion and stroke [1-3].
Neurological disturbances constitute a growing worldwide concern due to the progressive aging of the population and the risky behavior they represent. These are pathologies that affect the central (CNS) and peripheral (PNS) nervous systems, including epilepsy, Alzheimer’s disease and other dementias, cerebrovascular diseases including stroke, migraine and other headache disorders, multiple sclerosis, Parkinson’s disease, neuroinfections, brain tumors, traumatic disorders of the nervous system due to head trauma and neurological disorders as a result of malnutrition [4-6].
Millions of people around the world are affected by neurological disorders. Estimates suggest that more than 6 million people die from stroke every year; more than 50 million people have epilepsy worldwide; an overall average of 47.5 million people live with dementia and about 7.7 million new cases are recorded annually, of which Alzheimer’s disease (AD) is the most common cause of dementia, accounting for 60% to 70% of cases. The prevalence of migraines occurs in more than 10% of the world population [7].
The economic impact of treatment is also high with disproportionately scarce neurological services and resources, which can be determinant for patient survival. Studies show that more than 80% of stroke patients die in low- and middle-income countries [8]. In the United States (USA) alone, the combined annual costs of these diseases total nearly $800 billion, which is likely to increase in the coming years due to the aging of the population, resulting in a severe economic burden [9].
Recent advances in understanding the pathophysiological mechanisms related to neurological disorders point to new strategies in drug development. Animal models have contributed considerably to these advances and play an even greater role in the evaluation of possible drugs with therapeutic potential, not only to alleviate these pathologies but to modify the disease process [10]. Rodents are suitable models for such studies because of their very well-characterized brain organization and the magnitude of information focusing on altered states of the nervous system [11-18].
In the recent decades, interest in natural products has increased significantly, with a concomitant expansion of the use of herbal medicines [19], some of which present a neuroprotective effect. Astragaloside IV (AS-IV), 3-O-beta-d-xylopyranosyl-6-O-beta-d-glucopyranosyl-cycloastragenol (C41H68O14, Molecular Weight 784.97) (Fig. 1), a triterpenoid saponin present in the root of the Astragalus membranaceus (Fisch.) Bge, is a phytotherapeutic described as the dry root of legumes belonging to the Fabaceae family, subfamily Faboideae and native to China. Used in traditional Chinese culture [20], it was first described in the Chinese book Shen Nong Ben Cao Jing in AD 200 with a range of beneficial effects and without toxicity [21, 22].
Fig. (1).
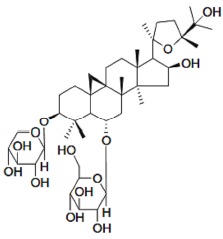
Chemical structure of Astragaloside IV.
The biological and pharmacological properties of AS-IV include a potent protective effect in pathologies due to its wide range of beneficial actions, such as antioxidant, antiviral, antibacterial [23, 24], anti-inflammatory, antifibrotic, antiasthmatic, antidiabetic, immunoregulation and cardioprotective effects. It has been seen as avoiding myocardial failure in rats [20, 25], being capable of increasing metabolism, improving the immune system, digestion and promoting the healing of wounds and injuries [26]. It possesses a moderate penetration in the blood-brain barrier (BBB) [27, 28].
Herbal medicines have scientific relevance in the treatment of neurological disorders, as they contain multiple compounds and phytochemicals with potential neuroprotective effects, with consequent benefits to promote health in different neuropsychiatric and neurodegenerative disorders [29, 30]. In light of this, AS-IV is important due to its above-cited action on nerve regeneration and functional recovery in animals, in addition to its neuroprotective activity [31, 32]. The present study aimed to evaluate reports on the neuroprotective effect of AS-IV in experimental neurodegenerative disorder models based on a systematic review of the literature.
2. METHODS
2.1. Databases and Keywords
The present study comprises a systematic review of the literature concerning the effects of AS-IV on experimental models of neurological disorders and neurodegenerative diseases that were published between the years of 2007 and 2017. It was developed based on previously established stages of search, identification, selection and eligibility strategies. The search strategy in the databases was performed using the terminologies referring to the search, filters and descriptors for articles published in eight databases: PubMed, Science Direct, Cochrane Library databases, SciELO, Scopus, Web of Science, Medline via Proquest and Periodicos Capes (the latter is a Brazilian database that gathers several International Journals). The terms used for the research were previously selected considering the technical vocabulary used to index articles in the field of health sciences, the Medical Subject Headings (MeSH) of the US National Library of Medicine (NLM) through which the descriptors “Astragaloside IV”, “Neurodegenerative diseases”, and “Neurological disorders” were identified. These keywords were used together in the search, seeking to detect all studies that analyzed the effects of Astragaloside IV supplementation in experimental models of both neurological disorders and neurodegenerative diseases.
2.2. Eligibility/exclusion Criteria
The search for studies was carried out independently by two expert evaluators in the context discussed, using titles, abstracts, or both, and resolving discrepancies through a subsequent consensus meeting. The terms were then searched together. A language restriction was considered for the selection, with only articles published in English being analyzed. Screening of the studies was performed based on the titles and abstracts, and the publications were subsequently read in full and compared.
Experimental studies using AS-IV in in vitro or in vivo analyses with the following pathological conditions were included: Alzheimer’s disease (AD), Parkinson’s disease (PD), cerebral ischemia and encephalomyelitis, evaluating its neuroprotective action (antioxidant activity); and studies that performed the in vivo analysis evaluating reduced symptoms of the pathology through behavioral analysis. The following exclusion criteria were considered: 1) The article was not original; 2) Experimental models other than mice, rats or cell culture were used; 3) Studies that analyzed the AS-IV action along with other compounds without an isolated group for AS-IV; 4) Absence of control group (the control group had to be comparable to the group supplemented with AS-IV).
The following eligibility criteria were considered: 1) Experiments performed on mice, rats or cell culture induced on the neurological lesion; 2) Treated with AS-IV before or after damage; 3) Analysis of the AS-IV effect on injury and action mechanisms; and 4) All animals or models used should present clinical symptoms of the pathologies.
The studies evaluated in this systematic review presented were inconsistent in important aspects, such as the inducing agent of neurodegenerative diseases, the duration of AS-IV treatment and the behavioral tests used. The studies used different compounds to simulate neuronal damage in these experimental models and all of them worked through different mechanisms of action. In addition, these compounds were administered in different doses and through different routes, aspects that reinforce the heterogeneity of the studies. Based on the methodological heterogeneities among studies with different neurodegenerative diseases analyzed in different experimental models, grouping statistics were not considered. Thus, meta-analyses were not performed for the accessed data.
3. RESULTS
According to the initial screening of the studies using the previously mentioned descriptors, 167 articles were found published in the 10 years between 2007 and 2017, of which 34 appeared in Medline via Proquest, 33 in Science Direct, 11 in Scopus, 72 in Periodicos Capes, 10 in the PubMed database and 7 in Web of Science, and none were found in the Cochrane Library or in SciELO.
The first criterion adopted was to consider only articles published in the 10 year period mentioned, which resulted in the identification of the 165 articles. Next, search filters were implemented, which excluded 69 articles (41 excluded by duplication, 21 excluded for being reviews and 7 book chapters), resulting in 96 studies on the subject.
After analysis of the titles, 18 articles were excluded, with 22 articles excluded after reading the abstract because they had no relation to the specific focus of our study. Thus, 56 articles were read in their entirety. After analyzing these articles, 42 studies failed for at least 1 criterion and were excluded (such as studies that used experimental models that were not mice, rats or cell culture, or that analyzed AS-IV action along with other compounds without isolating the AS-IV group), totaling 16 articles at the end of the analysis (see the flowchart in Fig. 2, which graphically details the systematic review process for inclusion/exclusion of articles).
Fig. (2).
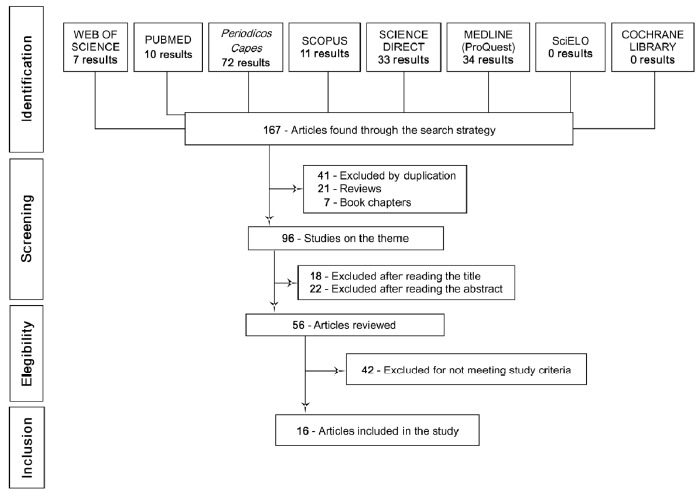
Flowchart showing the selection process of the studies used in this systematic review.
The studies showed variations regarding several characteristics. Through the search we found studies analyzing the AS-IV action on different neuropathological conditions: PD (2), AD (3), ischemia (8) and encephalomyelitis - experimental model of lateral sclerosis (1), including different in vivo (9), in vitro (3) and in vivo and in vitro tests (2).
The similar and individual methodological criteria used in the 14 articles included in the study are described below. The studies employed different experimental procedures and observations, thus the total number of the evaluations undertaken differed from the number of studies mentioned, given our intention to verify how many studies have evaluated, as will be described below.
Regarding the gender of the animals (rats) the in vivo studies used in the research, 9 studies used males, 1 used a female and 1 study used both genders. The neuropathology experimental models were induced through the administration of different substances: for PD, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (1) and 6-hydroxydopamine (6-OHDA) (1); for AD, by intracerebral beta-amyloid induction (2) and in cells transfected with pEGFP-N1-BACE1 (1); for ischemia by middle cerebral artery occlusion (MCAO) (5) and bilateral common carotid artery occlusion (BCAO) (2); and for encephalomyelitis by complex injection of the Freund+ MOG35-55 + Mycobacterium tuberculosis H37RA+ Pertussis toxin (1).
Concerning the behavioral analysis, the studies used the following: the Morris aquatic labyrinth (MAL) test (3), neurological (1) and neurobehavioral activity (4); focus on cell cultures such as cell line human neuroblast from neural tissue SH-SY5Y (1), human neuroblastoma cell line - SH-N-SH (1) and cells from the substantia nigra (1). Other studies evaluated the cellular defense system through the enzymes superoxide dismutase (SOD) (6), malondialdehyde (MDA) (5), catalase (CAT) (1) and glutathione peroxidase (GSH-Px) (2), in addition to caspase-3 (3), expression of BAX (3), cytochrome c (1), nitric oxide synthase (NOS) (3) and ROS (4).
Among the most used tests were the tests for 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (4), Western blot (9), enzyme-linked immunosorbent assay (ELISA) (4), polymerase chain reaction (PCR) (3), immunohistochemistry (7), immunofluorescence (3) and tumor necrosis factor alpha (TNF-α) (3).
About the duration of the AS-IV treatment, the minimum time in the in vitro study was 24 hours and the minimum and maximum dosages used in these studies were +10 μM-500 μM; for studies performed in vivo, the minimum and maximum dosages were 10-200 mg/kg. Supplementation duration in the studies was between 7 and 28 days, and in vivo and in vitro studies up were to 3 months. The characteristics of the selected studies are shown in Table 1.
Table 1. In vivo and in vitro models of studies of neurological disorders and the implications of Astragaloside IV in the treatment.
|
Author/
Year |
Pathology | Experimental Model | Experimental Groups | Posology | Analyses and Tests Carried out | Findings |
|---|---|---|---|---|---|---|
| Zhang et al. (2012) [37] |
In vitro Model for PD induced by MPP+ In vitro |
SH-SY5Y Cells | (1) Control; (2) 3mM MPP+; (3) +10µM AS-IV; (4) +25µM AS-IV; (5) +50µM AS-IV. |
- Cells treated with 3 mM of MPP+
(24 h); - Pre-treated with 25 or 50 lM AS-IV for (2 h); - Exposed to 3 mM MPP+ (24 h). |
- Cell viability (MTT Test); - Hoechst 33258; -Detection of apoptosis; - Western blot; - Bax Expression; - Measurement of intracellular ROS. |
Notably the AS-IV treatment increased cell survival, reversed the intracellular generation of reactive O2 species and nuclear condensation, inhibited the Bax-mediated path-way; in addition, it suppressed the activity of caspase-3. |
| Chan et al. (2009) [31] |
In vitro Model for PD induced by 6-OHDA In vitro |
Substantia nigra cells | (1) Control (0.3 mg/ml of ascorbic acid in HBSS); (2) 6-OHDA; (3) AS-IV (50 µM + 6-OHDA); (4) AS-IV (100 µM + 6-OHDA); (5) AS-IV (200 µM + 6-OHDA); |
- Cell treatment with 200 µM of 6-OHDA (24 h); -Pretreatment with AS-IV (1 h). |
- Analysis of neuronal toxicity; - Measurement of neurite growth; - Immunopositive TH; - Immunofluorescence. |
Treatment with AS-IV mitigated the loss of dopaminergic neurons, presented intact germination and increased immuno- reactive TH and NOS, especially in the group that received concen- trations of 100 mM. The study suggests that the neuroprotector and neuro-excitatory effects are specific to dopaminergic neurons and have therapeutic potential in the treat- ment of Parkinson's Disease. |
| Liu et al., (2017) [83] |
In vitro Model for cellular stress in PD induced by H2O2 In vitro |
Apoptosis of SH-SY5Y cells |
(1) Control; (2) H2O2 (300 μmol/l); (3) AS IV (50, 100, 200 μmol/l); (4) H2O2 (300 μmol/l); + ASIV(200 μmol/l); (5) H2O2 + Vit. C. |
- SH-SY5Y cells treated with AS-IV (50-200 μmol/l) during 24 h; - Exposed to H2O2 (300 μmol/l) during 4 h. |
- MTT assay; - Flow cytometry - Measurement of intracellular ROS levels; -Immunofluorescence staining; - DAPI staining; - Western blot analysis. |
Treatment with AS-IV decreased the H2O2-induced cellular damage, prevented morphologi- cal changes and decreased ROS produc-tion and apoptosis rate. In addition, the neuroprotective effects of AS-IV against H2O2-induced apoptosis of SH-SY5Y cells were associated with Bax/ Bcl-2 ratio deregulation, decreased α-synuclein levels and increased TH levels via the p38 MAPK signaling pathway. |
| Liu et al., 2016 [84] |
Experimental autoimmune encephalomyelitis (EAE) In vivo and In vitro |
BV2 cells and female C57BL/6 mice; |
Experiment 1 (1) Control; (2) LPS (positive or negative); (3) LPS+AS1 (10,25,50,100); (4) LPS+Dex (0-10). Experiment 2 (1) Control; (2) EAE; (3) EAE + ASI; (4) EAE + RU486; (5) EAE + ASI + RU486; (6) EAE + Dex (Dexamethasone). Experiment 3 (1) Control; (2) Dex; (3) ASI 10 µM; (4) ASI 25 µM; (5) ASI 50 µM; (6) ASI 100 µM; (7) RU486; (8) RU486+Dex ; (9) RU486+ ASI 25 µM. Experiment 4 (1) Control; (2) ASI 10 µM; (3) ASI 20 µM; (4) ASI 40 µM; (5) ASI 80 µM; (6) ASI 100 µM; (7) Dex 1 nM. |
- EAE was induced in 6-weekold female C57BL/6 mice; - Each mouse was immunized subcutaneously with 300 μg of MOG35–55 that emulsified in complete Freund’s adjuvant containing 400 μg of heat-inactivated Mycobacterium tuberculosis H37RA. -The control mice were injected with adjuvant without MOG35–55. -Intraperitoneal injection of pertussis toxin (200 ng/mouse) was given immediately and again 48 h later. |
- Immunohistochemistry; - Western blotting analysis; - Proinflammatory factors assay; - Luciferase activity assay; - Quantitative PCR; - Molecular docking experiment; - GR competitive ligand-binding assay. |
ASI modulated GR-mediated signaling pathway, including dephosphorylation of PI3K, Akt, IκB and NFκB, decreasing downstream production of proinflammatory mediators. Suppression of microglial BV-2 activation by ASI was abrogated by GR inhibitor, RU486 or GR siRNA. Similarly, RU486 counteracted the alleviative effect of ASI on microgliosis and neuronal injury in vivo. ASI inhibited microglia activation at least partially by activating the glucocorticoid pathway, suggesting its possible therapeutic potential for neuroinflammation in neurological diseases. |
| Li et al. (2017) [71] | Model for ischemia/reperfusion In vivo |
Male ICR Mice | (1) Control; (2) Ischemia Model; (3) AS-IV (10 mg/kg); (4) AS-IV (20 mg/kg). |
- Oral administration of AS-IV (10 and 20 mg/kg) once a day; - Treatment groups were given i.g. 7 days before surgery ending on the day of euthanasia; - AS-IV was administered 2 h prior to ischemia on the day of surgery. |
- MAL; - IL-1β measurement; - TNF-α; - ROS; - SOD; - MDA; - Western blot; - Immunohistochemistry. |
AS-IV significantly improved cognitive changes induced by transient ischemia and reperfusion injury. It regulated inflammatory responses by inhibiting TLR4 signaling and avoids microglial overactivation. It attenuated memory deficits and neuroinflammation, increasing SOD activity and decreasing ROS and MDA levels. |
| Qu et al. (2009) [28] | Model for transient focal cerebral ischemia In vivo |
Adult male Sprague-Dawley rats | (1) Control; (2) Ischemia model; (3) AS-IV (10 mg/kg); (4) AS-IV (20 mg/kg). |
- In treatment groups, the animals received AS-IV injections (i.p.) immediately after occlusion of the middle cerebral artery. | - BBB Integrity Assessment; - TEM; - Immunohistochemistry. |
The study proposes that the regulation of junctional proteins in endothelial cells may be a mechanism of protection of AS-IV resulting from the protection of the blood-brain barrier. |
| Cao et al. (2014) [85] |
Model for cerebral ischemia In vivo |
Adult male Sprague-Dawley rats | (1) Control; (2) QDBSþI / R model group; (3) AS-IV, (4) HSYA; (5) AS-IV+HSYA; (6) HH. |
- The volume injected was 3 mL/kg throughout the period for both HQI and HH. - The animals were given carbohydrate hydrochloride by intraperitoneal injection (400 mg/kg). |
-Neurological evaluation; - Measurement of infarct volume; - Histological study; - Mitochondrial ROS generation; - Measurement of (SOD, CAT, GSH-Px enzymes) and MDA. - Western blot. |
The results showed that AS-IV and HSYA had a synergistic effect on brain protection for measurement of (total) infarct volume and antioxidant defense system. |
| Li et al. (2012) [74] | Model for cerebral focal ischemia induced by occlusion of the right middle cerebral artery with reperfusion In vivo |
Adult male Sprague-Dawley rats | (1) Control; (2) Ischemia model; (3) AS-IV (10 mg/kg); (4) AS-IV (20 mg/kg). |
- Animals received AS-IV (10 or 20 mg/kg) when reperfusion was initiated. - For both drug groups, the animals received intraperitoneal injections of AS-IV immediately and 12 hours after the onset of the reperfusion. |
- MPO; - ELISA; -Neurobehavioral evaluation and infarction evaluation; - CD11b/CD18 measurement; - Western blot; - Immunohistochemistry; - TNF-α and IL-1β levels. |
The protective effect of AS-IV occurred through the prevention of neutrophil accumulation in the cerebral parenchyma, demonstrating a significant reduction of MPO concentration in brain tissue; in addition it decreased the percentage of neutrophils positive for CD11b/ CD18, reducing the expression of ICAM-1, which is partially achieved by attenuation of the production of TNF-α and IL-1β and inhibition level of NF-jB. The study proposes an anti-inflammatory mechanism favored by AS-IV by suppression of these molecules related to the adhesion of neurotrophs, which exerts neuroprotection against the lesion. |
| Cao et al. (2015) [86] |
Model for Cerebral ischemia/ reperfusion In vivo |
Adult Sprague-Dawley rats (6 males and 6 females) | (1) Control; (2) AS-IV (10 mg/kg); (3) AS-IV (40 mg/kg); (4) AS-IV (100 mg/kg). |
- The animals were euthanized 2 h after ischemia and after 24 h of reperfusion. |
- Behavioral test; - Dissection of the ischemic area; - [3H] PK11195 and Scatchard binding analysis. |
AS-IV protects ischemic brain tissue by inhibiting PBR expression after cerebral ischemia. This finding suggests that AS-IV could change the plasticity of the ischemic area, which creates an opportunity for clinical prevention and treatment of brain lesions after cerebral focal ischemia. |
| Yang et al. (2012) [82] |
Model for Cerebral ischemia/ reperfusion In vivo |
Male Sprague-Dawley rats | (1) Control; (2) IR Group; (3) AS-IV (20mg/kg); (4) AS-IV (20mg/kg) -TMPZ (10 mg/kg); (5) Nimodipine (10 mg / kg). |
- All drugs were injected intraperitoneally into each group with the same volume (2 ml) at 0, 12 h, 1 d, 2 d, 3 d, up to 7 d after reperfusion. | - Micro-PET images; - Modeling and administration of MCAO rats; - Evaluation of triphenyltetrazolium chloride. |
Both the AS-IV-TMPZ and the isolated AS-IV treatment groups reversed the induced changes and the parameters evaluated, meaning the downregulation of Caspase-3 mRNA, MDA and iNOS, and the regulation of SOD activity and Bcl-2 expression, in addition to reversing the decrease in glucose metabolism, resulting in a reduction of myocardial infarction volume in ischemia-reperfusion injury. |
| Li et al. (2013) [78] | Model for Cerebral ischemia/ reperfusion In vivo |
Male Sprague-Dawley rats | (1) Control; (2) Assay (Ischemia); (3) AS-IV (10 mg/kg); (4) AS-IV (20 mg/kg). |
- Rats were anesthetized by intraperitoneal injection of 5% chloral hydrate at a dose of 400 mg/kg. | -Neurobehavior Assessment; - Evaluation of Functionality; - Determination of water content; oxidative stress and iNOS; - Isolation of RNA and quantitative PCR; - Immunohistochemistry. |
The permeability of BBB improved significantly in AS-IV treated groups compared to the vehicle addition group. The study proposes that the potential anti-edema action of AS-IV be correlated with the regulation of MMP-9 and AQP4. |
| Sun et al. (2014) [45] |
Model for AD induced by Aβ1-42 In vitro |
SK-N-SH Cells | (1) Control; (2) Ab1-42 (Cells treated with 5 mM); (3) AS-IV + Ab1-42 (10 mM); (4) AS-IV + Ab1-42 (25 mM); (5) AS-IV + Ab1-42 (50 mM). |
- Ab1-42 (cells pre-treated with AS-IV 2 h prior to 5 mM Ab1-42). All experiments were performed after incubation for 24 h. |
- Cell viability; - Measurement of ROS and mitochondrial superoxide; - Apoptotic parameters; - ATP level; - mPTP assay; - oxidized cytochrome C activity; - Release of Cit C. - Protein extraction;- Western blot. |
Pretreatment of AS-IV significantly increased the viability of neuronal cells, reduced apoptosis, decreased intracellular ROS generation and decreased mitochondrial superoxide in the presence of Ab1-42. Moreover, the pre-treatment of AS-IV inhibited the opening of mPTP, the recovered mitochondrial membrane potential (DYm) and improved ATP generation, It improved cytochrome C oxidase activity and blocked the release of cytochrome C from mitochondria. AS-IV also reduced expression of Bax and cleaved caspase-3 |
| Kim et al. (2015) [72] |
Model for Chronic cerebral hypoperfusion In vivo |
Male Sprague-Dawley rats | (1) Normal group; (2) Control; (3) Drug vehicle (4) AS-IV (10 mg/kg); (5) AS-IV (20 mg/kg). |
- AS-IV was administered by intrapetritonial injection 1 x per day for 28 days, starting after the week of pBCAO; - After intravenous injection of AS-IV in rats, extracts of brain tissue contained ~ 0.1 μg/g AS-IV at the dose of 1.5 mg/kg of intravenous injection. |
- pBCAO; - MAL; - TUNEL; - Bax; - Caspase-3; - Cresyl violet; - SOD; - MDA. - ELISA; - Immunohistochemistry. - Western blot. |
AS-IV treatment (at a dose of 20 mg/kg) significantly improved learning and memory deficits, attenuating apoptosis, SOD levels and lipid peroxidation markers, including MDA and 4-hydroxy-2-non-renal; it significantly inhibited astrocytic and microglial reactivity in the hippocampus. |
| Haiyan et al. (2016) [54] | Model for Alzheimer induced by amyloid-β intracerebral In vivo and In vitro |
Embryos of Sprague-Dawley rats |
Experiment I (1) Control; (2) Vehicle; (3) Donepezil (DH); (4) Non-induced NSC transplants (TP); (5) AS-IV induced NSC transplants (TP-ASI). Experiment II (1) Control; (2) 10−5MASI; (3) 10−6MASI; (4) 10−7MASI. Experiment III (1) Control; (2) Positive Control; (3) AS-IV. |
- Cells were transferred to 96-well plates coated with poly-L-lysine and treated with 10-5, 10-6 and 10-7 M AS-IV for 3 days; - The models were produced by bilateral intra-hippocampal injection of 5 μg of Aβ for each side under anesthesia; - Rats were intraperitoneally injected with 5 mg/kg/day of AS-IV for 4 weeks. Injection of 5 μg of Aβ for each side under anesthesia; - 4 weeks after transplant, rats were trained in two trials per day for 6 days. |
- MTT assay; - MAL - Immunostaining; - Western blot. |
Treatment with AS-IV resulted in an increase in the number of β-tubulin III reactive cells in the hippocampus. Further research has shown that AS-IV inhibited In vitro and In vivo expression of PS1. Elevated dose of AS-IV reduced the intracellular Notch domain, while low AS-IV doses increased Notch-1 and NICD. Thus, treatment with AS-IV resulted in improvements in learning and memory of AD models, promoting the proliferation of NSC and differentiation in part through the Notch signal pathway. |
| Wang et al. (2017) [62] |
Model for AD obtained by cells transfected with pEGFP-N1-BACE1 In vivo and In vitro |
SH-SY5Y Cells | Analysis I (1) Control; (2) 0.1 ug/ml; (3) 1 ug/ml; (4) 10 ug/ml; (5) 100 and 200 ug/ml. Analysis II (1) Control; (2) 50 µM AS-IV; (3) 100 µM AS-IV; (4) 250 µM AS-IV; (5) 500 µM AS-IV. Analysis III (1) Control; (2) AS-IV + GW9662; (3) AS-IV. Analysis IV (1) Control; (2) PEGFP-N1; (3) PEGFP-N1+ BACE1; (4) RSG; (5) AS-IV. |
- Cells transfected with pEGFP-N1-BACE1 were treated with AS-IV for 24 h or AS-IV plus the PPAR-γ antagonist GW9662 in vitro. - APP/PS1 rats were intragastrically treated with AS-IV or AS-IV plus GW9662 every 48 h for 3 months. |
- Immunofluorescence; - Western blot; - PCR; - ELISA; - Immunohistochemistry; - MTT assay. |
The results showed that the AS-IV treatment increased PPARγ activity and in vitro inhibited BACE1. In vivo treatment with AS-IV increased PPARγ and BACE1 expression and reduced neuritic plaque formation and Aβ levels in the brains of APP/PS1 rats. Therefore, AS-IV may be a future agent for modulation of Aβ-related pathology in AD. |
| He et al. (2013) [65] | Experimental autoimmune Encephalomyelitis (Model for Multiple sclerosis). In vivo and In vitro |
6-week old female C57BL rats | (1) Control; (2) EAE; (3) EAE+AS-IV (20 mg/kg). |
- Each rat received 100 μl subcutaneous injection of Freund's complete + 300 μg of MOG 35-55 and 400 μg of Mycobacterium tuberculosis H37RA; - Pertussis toxin (200 ng) was administered via i.p. on the day of immunization and again 2 days later; - AS-IV (20 mg/kg) was administered daily on the day prior to MOG 35-55 immunization and continued for 2 weeks; - MPD (positive control), 20 mg/kg i.p. consecutively from day 8 to day 10 after immunization. |
- Histopathology; - Immunohistochemistry; - Westerblot; - ROS levels; - Evans Blue Extravasation; - Quantification of cytokines (IFNγ, TNFa, IL6, IL4 And IL17A); - ELISA; - Biochemical analysis (GSH-Px, MDA and T-SOD); - PCR. |
The results of the study show that relief of the EAE progression by AS-IV was correlated with its anti-oxidant and anti-apoptotic characteristics. This can be explained because it prevented the generation of ROS by inhibiting the infiltration of T cells in the CNS, BBB leakage and neuroinflammation. It reduced tau phosphorylation in response to the action of hydrogen peroxide by modulating the Bcl-2/Bax ratio, and it also inhibited the activation of microglia both in vivo and in vitro and the upward regulation of iNOS induced by IFNγ stimulation. |
(NOS) expression, especially in the group that received the concentration of 100 millimolar (mM) [31].
The study designs varied greatly by data used for the pathology and the type of lesion in the animal, experimental model, experimental groups, substance dosages, performed analyses and tests and how the findings (results that the authors obtained with their studies) had been collected. One study used other phytotherapeutic agents in conjunction with the AS-IV group. There were, however, also isolated groups for both substances.
Table 1 indicates the findings related to neurodegenerative diseases and the implications of AS-IV. The studies not only point to its neuroprotective effect, but also to an anti-oxidant, anti-apoptotic, anti-inflammatory action, as well as improvement in mitochondrial damage and behavioral deficiencies.
4. DISCUSSION
Neurodegenerative diseases are debilitating conditions that compromise the quality of life of patients and affect society. The consequences and the social and economic impact generated by these pathologies establish an important theme of study, making the elucidation of their mechanisms and the search for natural medicines that can contain or repair disease damage and sequelae necessary.
As reported, herbal medicines and their multiple bioactive compounds have scientific relevance in treating neurodegenerative diseases, acting on the symptomatology presented by the pathologies. The present study investigates the neuroprotective action of AS-IV, a triterpenoid saponin present in the root of the Astragalus membranaceus, described in the literature as a compound capable of acting towards the repair of damage in pathologies such as PD, AD and cerebral ischemia, as evidenced in the systematic search.
The abovementioned neurodegenerative pathologies involve similar pathophysiological aspects, as observed in the experimental models studied. AS-IV acts on the antioxidant defense system, maintaining an adequate level of enzymes that act in the maintenance of this system, on apoptotic pathways, maintaining the ideal proportions between Bax and Bcl-2, as will be described later. This blocks the activation of effector caspases in apoptotic cell death caused by neurotoxins such as MPTP, 6-OHDA, toxin pertussis and amyloid-β, as well as acting on inflammatory markers common to these pathologies.
4.1. Parkinson’s Disease (PD)
AS-IV has a neuroprotective effect and it has been investigated in experimental models of PD. One of the possible explanations for its mechanism of action in this pathology may be associated with oxidative stress as the main inducer in the loss of dopaminergic neurons in the CNS [33, 34].
This evidence is demonstrated through experimental studies that induce PD in substantia nigra cell culture through the action of neuronal toxins such as 6-OHDA, which has the characteristic of inducing neurotoxicity, ROS generation and mitochondrial depletion, as well as inhibiting tyrosine hydroxylase (TH), which is the rate-limiting enzyme for dopamine synthesis [35]. A study carried out by Chan et al. (2009) showed that AS-IV administered in concentrations of 50 ~ 200 µM for 25 h attenuated the loss of dopaminergic neurons. The treated group presented intact germination, neurite growth and increased TH and nitric oxide synthase
Another study developed with the purpose of understanding the effects of AS-IV in PD was carried out in a model for induction by MPP+ (active form of MPTP drug, classic model to establish PD in vitro) [36]. The study by Zhang et al. (2012) in observing AS-IV action on SH-SY5Y cell culture containing 25 ~ 50 uM Astragaloside for 26 h found a remarkable increase in cell survival, as well as an inversion of the intracellular generation of ROS and nuclear condensation, in addition to inhibiting the Bax-mediated pathway and suppressing caspase-3 activity [37]. Modification of the apoptotic cascade and ROS regulation may be crucial events in preventing PD pathology [37]. Cell survival in the phase that the apoptosis cascade begins depends considerably on the balance between pro- and antiapoptotic Bcl-2 family proteins; therefore, the Bax/Bcl-2 ratio may be a determinant factor for cell growth and protection [38].
Previous findings regarding Bcl-2 protein in PD models have indicated that members of the pro-apoptotic family participate in neuronal death [39]. According to Zhang et al. (2012), the proportion of pro-apoptotic Bax to antiapoptotic Bcl-2 increased as a response to MPP+, with the treatment with AS-IV significantly reducing Bax expression [37], while also increasing Bcl-2 expression in the in vitro model for PD. The effect of AS-IV on apoptosis induced by MPP+ can be partly explained by this regulatory mechanism.
In our recently published systematic review, we described the action mechanism of various phytotherapeutic compounds and extracts which also had a neuroprotective effect on PD, capable of protecting neural cells against the toxicity of both 6-OHDA and MPP+ toxins due to the presence of multiple bioactive antioxidant compounds such as flavonoids, saponins and phenolic compounds [40].
4.2. Alzheimer’s Disease (AD)
Alzheimer’s disease (AD) leads to a progressive degeneration of neurons that results in damage to memory, thinking and behavior [41]. This degeneration occurs due to several factors, among which are the aggregates of the amyloid protein which is produced by the amyloid precursor protein (APP) [42]. Thus, as the major component of senile plaques, beta-amyloid (Aβ) is an important histopathological indication for AD [43].
Previous studies have demonstrated that Aβ can induce apoptotic and necrotic cell death [44]. A study carried out by Sun et al. (2014) described that SK-N-SH cells treated with Aβ1-42 5 mM for 24 h presented lower viability and higher levels of cellular apoptosis. Pretreatment of AS-IV in concentrations of 25 and 50 mM significantly decreased ROS and SOD generation and reduced the number of apoptotic cells, thereby providing a better cell viability in the presence of Aβ1-42 [45]. Moreover, pretreatment with Astragalus inhibited the mitochondrial permeability transition pore (mPTP) opening, an important neuroprotective action since the pore opening plays a central role in the death of neuronal cells in neurodegenerative diseases, in which the presence of ROS acts as one of the factors that causes its opening [46].
A recent study, with a Tg2576 mouse model, for AD showed that chronic stress could increase levels of corticosterone in conjunction with increases in the β-amyloid peptide levels of brain tissue and deposition of Aβ plaques [47]. In addition, dexamethasone (DEX) administration associated with the damage presented by APP/PS1/MAPT in transgenic rats increased brain levels of APP [48]. According to the researchers, the exposure to dexamethasone (5 mg/kg) at the stress threshold may induce learning deficits and memory impairment, damage of hippocampal neurons, as well as increase in Aβ formation, expression of APP and the cleavage enzyme β-APP, suggesting an interaction between the stress level and Aβ formation [49, 50].
Li et al. (2011) propose the action of Astragalus extract in experimental models with AD induced by different substances. Since glucocorticoids may play a crucial role in dementia progression in AD patients [48], the study administered dexamethasone (DEX) (i.g) for 21 days in male rats and evaluated the action of Astragalus in concentrations of 10, 20 and 40 mg/kg according to spontaneous motor activity (SMA) and the MAL test, an established behavioral test to evaluate memory and learning [51].
The results of this study showed that administration of 40 mg/kg of Astragalus significantly increased SMA in 10 days and the swim time of the animals within the platform quadrant, which expresses the animal’s preference and spatial learning, pointing to positive results regarding its efficacy in the learning process and memory in rats. This study also performed immunohistochemistry for caspase-3,9 and cytochrome c in the hippocampus (CA1-CA3 regions) and neocortex, noting that the treatment with the extract regulated the level of caspases in both regions [48].
Accordingly, Astragalus action can be explained as regulating the release of caspases and cytochrome c (both being apoptosis inducing factors), since cytochrome binds to Apaf-1 and Procaspase-9c when it is released in cytosol, forming a functional apoptosome, and subsequently triggering the sequential activation of caspase-3 and 9 [52]. Various stimuli that induce apoptosis lead to the release of cytochrome c from mitochondria, which plays a pivotal role in a common path of caspase activation [52, 53]. In addition, caspase-3 activation has been shown to be a key step in the apoptosis process and its inhibition can block cell apoptosis.
A study carried out by Hayan et al. (2016) investigated whether treatment with AS-IV could promote the proliferation and differentiation of grafted neural stem cells (NSCs) and investigated the improvement of cognitive impairment caused after administrating intracerebral injection with Aβ protein in rat models with AD [54]. Based on this hypothesis, stem cell-based therapy could be a promising treatment strategy for neurodegenerative diseases such as AD. In this study, these cells (NSCs), originating from the hippocampus of rats and from embryos, were cultured and treated with AS-IV, then grafted onto the hippocampus of rats with AD, resulting in an improvement in cognitive impairment after administrating an intracerebral Aβ protein injection.
AS-IV treatment results in improvements in learning and memory in AD models, promoting NSC proliferation and differentiation in part through the Notch signal pathway, which is one of the major signal systems that govern neurogenesis. This signaling pathway plays a critical role in maintaining and renewing neural progenitor cells (NPCs). The activation of this pathway was sufficient to keep NPCs in their proliferative state, whereas loss-of-function mutations in critical components caused early neuronal differentiation and NPC depletion [55].
Other results found in the aforementioned study indicate that administration of 10-5 mM AS-IV did not increase the rate of cell proliferation; however, it did induce stem cell differentiation into Nestin + GFAP cells. Besides this, the differentiation indices were similar to those of positive controls using 1% serum/saline solution. The results were also consistent with the findings by [56], who demonstrated that Astragaloside plays a double role in peripheral nerve regeneration, with low doses (50 μM) inducing a higher rate of regeneration, and with a high dose not demonstrating this effect (200 µM).
Clinical and epidemiological evidence suggests that there is a direct relationship between type II (DM II) diabetes associated with an increased risk of AD [57]. Drugs used in the treatment of DM II such as thiazolidinediones (TZDs) act as agonists in the nuclear receptor and peroxisome proliferator-activated receptor gamma (PPARγ), tested as potential drugs for treating neurodegenerative disorders such as AD [58, 59].
As mentioned, the major pathological features of AD are the aggregations of Aβ and tau proteins. Aβ is produced from the APP by the sequential action of two proteins, α- and β-secretases. The breakdown of the β-secretase product by Y-secretase produces two substances: toxic Ab42 and non-toxic Ab40 [42]. Sastre and coworkers (2006) reported that PPARγ inhibits the activity of the BACE1 promoter gene in response to the binding of a PPARγ-peroxisome responsive element [60]. The proliferator-response element (PPRE) located in the BACE1 promoter gene is an enzyme responsible for the cleavage of the site in the Aβ precursor protein [60, 61]. Thus, it becomes clear that the PPARγ agonist modulates APP processing through the regulation of β-secretase.
Corroborating the aforementioned study, Wang et al. (2017) evaluated the action of AS-IV treatment which was able to increase PPARγ activity and inhibit BACE1 in vitro. As a result, Aβ levels significantly decreased. The in vivo treatment with AS-IV reduced the expression of PPARγ and BACE1 and reduced the formation of neuritic plaque and Aβ levels in the brain of APP/PS1 rats [62].
Also corroborating this study, Chang et al. (2016) evaluated the action of AS-IV in the cerebral cortex after infusion of Aβ, showing that i.p. administration of 40 mg/kg/day of the herbal compound once a day for 14 days reduced apoptosis levels with mitochondrial dysfunction in cortical cells having been blocked by inhibition of protein kinase phosphoinositol 3-kinase (PI3K), known as AKT [63].
Recent systematic reviews highlight the protective potential of traditional Chinese medicine (MHC) in experimental models and clinical studies of neurodegenerative diseases, since conventional treatments only offer discrete benefits to clinical symptoms and do not prevent neuronal cell degeneration. In contrast, a promising alternative are medicinal plants because they have multiple compounds and phytochemicals with neuroprotective effect [30, 40]. Yang et al. (2017), for instance, suggest that to some extent MHC can be recommended for routine use in patients with AD, since it potentially increases the hippocampal neurogenesis through the activation of multiple signal pathways [64].
4.3. Autoimmune Encephalomyelitis
AS-IV has also been tested in rat models with experimental autoimmune encephalomyelitis [65]. In this model, AS-IV given at a dose of 20 mg/kg significantly attenuated the severity of experimental autoimmune encephalomyelitis (EAE) in rats. The study suggested that AS-IV may be effective for clinical therapy/prevention of multiple sclerosis, able to improve the antioxidant defense system by increasing antiapoptotic and anti-inflammatory pathways and to modulate the differentiation of T cells and their infiltration [65].
Another study carried out posteriorly by the above-mentioned authors evaluated the action of Astragalosides I, II and IV (25 and 50 mg/kg) present in the composition of AST [66]. The results showed a similar pattern in which Astragalosides prevented the infiltration of inflammatory cells in rats and decreased oxidative stress and glial activation in the hippocampus by anti-inflammatory and antioxidant actions. Moreover, an assessment of neuronal injury caused by inflammatory infiltrating T cells (a common feature of EAE) demonstrated that the treatment with AST acted in neutralizing neuronal damage through the expression of proteins associated with apoptosis, Bax regulation and Bcl-2 inhibition [66].
4.4. Cerebral Ischemia
Cerebral ischemia has an inflammatory process as one of its components that aggravates brain damage and plays an important role in the disease progression. As a response to ischemia or trauma, the CNS responds by activating resident microglia/astrocytes and peripheral leukocyte infiltration, resulting in microvessel obstruction, edema formation, cell death and tissue infarction [67, 68]. Activated microglia release inflammatory cytokines including IL-1β, TNF-α and other cytotoxic molecules such as NO and ROS [69, 70].
Li et al. (2017) assessed the expression of Iba1, an activated microglial marker after ischemia. The results indicated an increase in Iba1, IL-1β and TNF-α expression in the control group with lesion, unlike the AS-IV treated group which experienced a decrease [71]. So, AS-IV presents neuroprotective effects during cerebral ischemic brain injury/focal reperfusion, possibly due to its antioxidant, anti-inflammatory and antiapoptotic properties; facts well-described by other authors [65, 71-73].
Some studies suggest that the protective effect against cerebral focal ischemia in rats at different reperfusion time points is related to antioxidation, regulating expressions of neuronal growth factor, tropomyosin kinase A receptors and messenger RNA. Li et al. (2012) and Yin et al. (2010) assessed the potential of AS-IV to protect the cause of cerebral inflammation caused by cerebral focal ischemia and reperfusion with dosages of 10 or 20 mg/kg in rats, observing a protective effect through the remarkable decrease in the percentage of positive neutrophils for CD11b/CD18 and negative expression regulation of the intercellular adhesion molecule-1 (ICAM-1), which is partially achieved by attenuating the production of TNF-γ and IL-1β and inhibition level of nuclear factor-β (NF-β) [74, 75]. In these studies, the anti-inflammatory mechanism evoked by AS-IV occurred by suppressing molecules related to neutrophil adhesion, exerting neuroprotection against ischemia/reperfusion (I/R) injury [74, 75].
In their analysis, Kim et al. (2015) evaluated the effect of AS-IV on learning and memory deficits induced by chronic cerebral hypoperfusion in rats at dosages of 10 and 20 mg/kg daily for 28 days starting at the 5th week after bilateral carotid artery occlusion. It was observed that a dosage of 20 mg/kg significantly improved spatial learning and memory deficits assessed using the MAL test in rats with chronic cerebral hypoperfusion [72]. In addition, the AS-IV significantly attenuated neuronal apoptosis as well as levels of superoxide dismutase and lipid peroxidation markers, including MDA and 4-hydroxy-2-nonenal in the hippocampus. It significantly reduced the expression of 8-hydroxy-2'-deoxyguanosine, an agent that causes oxidative DNA damage; moreover, it significantly inhibited the activation of astrocytes and microglia in the hippocampus. This indicates that AS-IV has the therapeutic potential for preventing dementia caused by cerebral hypoperfusion, suggesting that the enhancement effect of AS-IV on learning and memory deficits may be the result of neuronal apoptosis and oxidative damage suppression in the hippocampus [72]. It should be noted that the signaling pathways of AS-IV during cerebral I/R have not yet been fully reported [73].
Another extremely relevant event for the promotion of brain lesions in the ischemic process is the blood brain barrier (BBB) dysfunction. Interruption of BBB plays an important role in cell damage in neurological diseases, including acute and chronic cerebral ischemia, cerebral trauma, multiple sclerosis, brain tumors and brain infections [76].
The increase in BBB permeability and vascular rupture may possibly be the initiating factors for developing cerebral infarction [77]. A cascade of molecular events occurs during a lesion, and is terminated by BBB interruption by radicals and free proteases, which attack membranes and degrade tight junction proteins in endothelial cells.
Oxygen and nitrogen free radicals and proteases, matrix metalloproteinases (MMP9) and cyclooxygenase are important in early and late BBB interruption as the neuroinflammatory response progresses. The damage caused by BBB rupture contributes to disease progression, as well as to cognitive changes in the affected individual. Thus the protection of the BBB in brain tissues may be potentially beneficial for the neuronal recovery of I/R injury [28, 76]. In the pioneering study by Qu et al. (2009), they evaluated the effect of AS-IV purified extract on BBB on a focal model of cerebral I/R injury at dosages of 10 and 20 mg/kg, observing a significant reduction in BBB permeability in comparison with the control group. In this study, an increase in the expression of ocludin and ZO-1 in the endothelial cells was observed in groups treated with the extract compared to other vehicle groups, what may possibly implicate the potential activity where AS-IV protects the BBB against I/R-induced rupture by upward regulation of the expression of tight junctional proteins [28].
BBB permeability is closely related to cerebral edema and is a critical complication following post-acute intravascular thrombosis. A study by Li et al. (2013) on post-ischemia/reperfusion cerebral edema evaluated animals treated with AS-IV in doses of 10 and 20 mg/kg and BBB permeability. The researchers used a technique similar to a previous study using Evans blue leak [78]. The results showed that AS-IV significantly attenuated brain water content and provided better neurological results in the treated animals compared to the control group. In addition, BBB permeability improved significantly, also indicating the potential protective effect of AS-IV on BBB. MMP9 and aquaporin 4 (AQP4) expressions related to cerebral vasogenic edema or cytotoxic edema were increased in control animals, but were significantly inhibited by the administration of AS-IV, thus proposing the anti-edema effect. The potential mechanisms proposed for the AS-IV action in experimental models of neurodegenerative diseases are represented in Fig. 3.
Fig. (3).
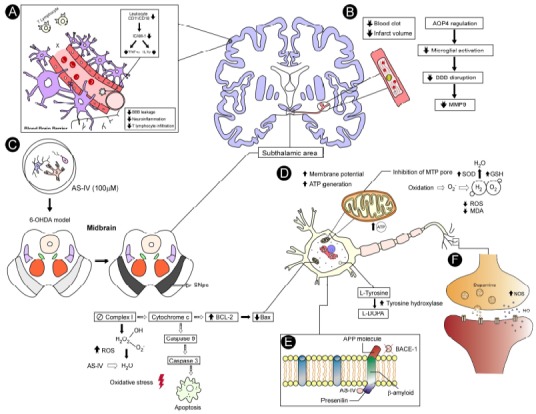
Potential mechanisms proposed for the AS-IV action in experimental models of neurological disorders. In step A, the protective effect of AS-IV (10 and 20 mg/kg) observed in an experimental I/R model as outlined prevented the accumulation of CD11b/CD18 positive neutrophils and reduced the expression of the intracellular adhesion molecule-1 (ICAM-1), which was partially achieved by the strong attenuation of TNF-α and IL-1β anti-inflammatory mechanism production by the suppression of these molecules related to neutrophil adhesion. Another proposed action mechanism reduced BBB permeability and decreased lymphocyte infiltration due to anti-inflammatory action, resulting in in decreased neuroinflammation. In step B, the AS-IV acted on the ischemic region, decreasing the metabolism of glucose and resulting in the reduction of clot area, attenuating the volume of cerebral infarction. The anti-edema action of AS-IV was also correlated with AQP4 regulation, which mediates the flow of water in the CNS with a consequent decrease of the cerebral edema and reduction of microglial activation, followed by reduced BBB interruption and MMP9 (Matrix metaloproteinase) related to vasogenic edema, which was inhibited by AS-IV which also decreased lymphocyte infiltration. In step C, the effect of the AS-IV was observed in a culture of dopaminergic neurons when 100 μM was administered. This model of 6-OHDA showed cell death in the SNpc (substantia nigra pars compacta), while the group treated with AS-IV notably increased cell survival, attenuating the loss of dopaminergic neurons since it presented intact germination; and in step D, the AS-IV prevented mitochondrial dysfunction by increasing the enzyme action of the SOD and GSH antioxidant defense system to convert hydrogen peroxide into H2O and O2, thereby preventing lipid peroxidation and mitochondrial damage. The AS-IV inhibited the apoptotic pathway between both action mechanisms proposed for neurodegenerative diseases. These action mechanisms were briefly summarized and evaluated in the reviewed studies, reporting that AS-IV reduced the activation of the BAX channel (which accelerates programmed cell death) and increased Bcl-2 that represses apoptosis. Furthermore, AS-IV prevented the activation of the procaspases that activate the effector caspase 3, thus preventing oxidative stress, apoptosis and a decrease malondialdehyde (MDA) which catalyzes the formation of numerous ROS. AS-IV also increased mitochondrial potential and ATP generation, inhibiting the membrane pore opening and consequently reverting the mitochondrial oxidation. In step E, AS-IV action in the experimental model induced by Aβ-amyloid illustrated, since it inhibits Aβ-amyloid formation by the APP, thus inhibiting the BACE-1 which cleaves this protein by generating the toxic amyloid, and it also inhibited presenilin-1 expression. In step F the TH enzyme of dopamine, NO and NOS synthesis increased, which elevated dopamine release in the striatum. (The color version of the figure is available in the electronic copy of the article).
In another study, Shao et al. (2014) evaluated cerebral edema in animals with subarachnoid hemorrhage and reported that edema was significantly attenuated following AS-IV administration as compared to the vehicle group. There was as well an improvement in neurobehavioral outcomes and alleviation of brain injury through antioxidant and antiapoptotic effects. These effects were also evaluated in neonates who were pre-treated with AS-IV or solvent by oral probe for three days, and then exposed to isoflurane [79].
A double-blind, randomized, controlled clinical study enrolled a total of 68 patients within 24 hours after hemorrhagic stroke [80]. This study demonstrated that Astragalus membranaceus improved the functional recovery of patients after hemorrhagic stroke in the group treated with oral or nasopharyngeal administration of the substance at a rate of 3 g three times a day for 14 days. Some adverse effects were observed by the authors, such as the occurrence of dizziness, rash or fever, but these events were classified as minor, with the study indicating that Astragalus membranaceus has an excellent safety profile for treatment because it is well tolerated and has no serious adverse events. However, the authors emphasized the need for complementary studies for a better understanding of its protective functions [80].
Exposure to general anesthesia can cause severe neurotoxicity in the development of the brain due to neuronal apoptosis. However, pre-treatment with AS-IV significantly inhibited isoflurane-induced neural apoptosis in the hippocampus, also significantly alleviating oxidative stress and the proinflammatory release of cytokines [81]. AS-IV has been shown to work well with other drugs, as reported [82]. This study investigated the effects of AS-IV and tetramethylpyrazine on a cerebral I/R injury model in rats. The authors concluded that AS-IV and tetramethylpyrazine played a key role in synergistic protection against I/R-induced focal brain damage.
CONCLUSION
Several studies have shown that AS-IV administration is effective in both in vivo and in vitro models for neurodegenerative diseases, such as those of Parkinson (PD) and Alzheimer (AD) diseases, as well as in models for neurological disorders such as cerebral ischemia and autoimmune encephalomyelitis, as highlighted in Fig. 3.
The protective effect of AS-IV in cerebral ischemia occurs through a reduction in the BBB permeability and a decrease in the lymphocyte infiltration with concomitant reduction in neuroinflammation. In addition, AS-IV has prevented the accumulation of CD11b/CD18 positive neutrophils and has reduced the expression of intercellular adhesion molecule-1 (ICAM-1), which was a result of a decreased production of TNF-α and IL-1β. The anti-apoptotic activity of AS-VI resulted in both procaspase and caspase-3 inhibition, thus preventing apoptosis and also oxidative stress, by a decrease in malondialdehyde (MDA), which catalyzes the formation of numerous ROS. In an experimental model induced by Aβ-amyloid, treatment with AS-IV inhibited BACE-1, which resulted in decreased Aβ production and neuritic plaque formation. Furthermore, in a 6-OHDA-induced PD model, treatment with AS-IV significantly increased cell survival through attenuating the loss of dopaminergic neurons in SNpc by increasing the immunoreactivity to TH and NOS and by potentiating the enzymatic activity of the antioxidant defense system, thus preventing both lipid peroxidation and mitochondrial damage processes.
In summary, the main mechanisms involved in the activities of AS-IV include an anti-edema effect, a reduction of lymphocyte infiltration and an attenuation of the loss of dopaminergic neurons. AS-IV can attenuate behavioral and neurochemical deficits as a result of its antioxidant, antiapoptotic, regulation of calcium balance and anti-inflammatory properties. Therefore, AS-IV emerges as an alternative therapeutic approach for the treatment of disorders of the central nervous system.
ACKNOWLEDGEMENTS
All authors have made a substantial, direct and intellectual contribution to the work, and gave final approval for its publication. The English text of this paper has been revised by Sidney Pratt, Canadian, MAT (The Johns Hopkins University), RSAdip-TESL (Cambridge University).
LIST OF ABBREVIATIONS
- AD
Alzheimer’s disease
- BACE-1
Beta-secretase 1
- BBB
Blood brain barrier
- BCAO
Bilateral common carotid artery occlusion
- CA1
Cornu Ammonis 1
- CA3
Cornu Ammonis 3
- CD11b/
Type I transmembrane proteins
- CD18
- CPR
C-reactive protein
- Dex
Dexamethasone
- DNA
Deoxyribonucleic acid
- EAE
Experimental autoimmune encephalomyelitis
- EAE
Experimental autoimmune encephalomyelitis
- ELISA
Enzyme-linked immunosorbent assay
- HH
Huangqi_Honghua combination
- HSYA
Hydroxysafflor yellow A
- IBA1
Ionized calcium-binding adapter molecule 1
- ICAM-1
Intercellular adhesion molecule-1
- ICAM-1
Intercellular adhesion molecule-1
- IFNγ
Interferon gamma
- IL-1β
Interleukin 1 beta
- iNOS
Inducible nitric oxide synthase
- IR
Cerebral ischemia-reperfusion
- LV
Lateral ventricles
- MAD
Malondialdehyde
- MAL
Morris aquatic labyrinth
- MCAO
Middle cerebral artery occlusion
- MMP-9
Matrix metallopeptidase 9
- MOG
Myelin oligodendrocyte glycoprotein
- MPD
Methylprednisolone
- MPO
Myeloperoxidase
- MPO
myeloperoxidase
- Mptp
Mitochondrial permeability transition pore
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MTT+
1-methyl-4-phenylpyridnium
- NF-jB
Level of inhibition of nuclear factor-jB
- NfJ-B
Nuclear factor-jB
- NOS
Nitric Oxide Synthase
- PBR
Peripheral benzodiazepine receptors
- PD
Parkinson’s disease
- QDBS
Qi deficiency and blood stasis
- QDBS
Qi deficiency and blood stasis
- RNA
Ribonucleic acid
- ROS
Reactive Oxygen Species
- SOD
Superoxide dismutase
- TEM
Transmission electronic microscopy
- TLR4
Notch toll-like receptor 4
- TNF-α
Tumor Necrosis Factors Alpha
- TUNEL
Terminal deoxynucleotidyl transferase dUTP
- ZO-1
Zonae occludens-1
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
Work supported by funding from the National Counsel of Technological and Scientific Development (CNPq) and the Coordination for Improvement of High Level Staff (CAPES). This study was part of the requirements for the Master’s degree obtained by IMC and FOVL (PPGSS/ UERN). IMC was a recipient of a CAPES fellowship.
CONFLICT OF INTEREST
The authors certify that they have no affiliation with or financial involvement in any organization with a direct financial interest in the subject matter or materials discussed in the manuscript. This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [http://dx.doi.org/10.2174/157015909787602823]. [PMID: 19721819]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freire M.A.M. Pathophysiology of neurodegeneration following traumatic brain injury. West Indian Med. J. 2012;61(7):751–755. [PMID: 23620976]. [PubMed] [Google Scholar]
- 3.Guimarães J.S., Freire M.A.M., Lima R.R., Souza-Rodrigues R.D., Costa A.M., dos Santos C.D., Picanço-Diniz C.W., Gomes-Leal W. Mechanisms of secondary degeneration in the central nervous system during acute neural disorders and white matter damage. Rev. Neurol. 2009;48(6):304–310. [PMID: 19291655]. [PubMed] [Google Scholar]
- 4.Batista P., Pereira A. Quality of life of patients with neurodegenerative diseases. J. Neurol. Neurosci. 2016;7:1–7. [http://dx.doi.org/10.21767/2171-6625.100074]. [Google Scholar]
- 5.Norrara B., Doerl J.G., Guzen F.P., Cavalcanti J.R.L.P., Freire M.A.M. Commentary: Localized vs. systematic neurodegeneration: A paradigm shift in understanding neurodegenerative diseases. Front. Syst. Neurosci. 2017;11:91. doi: 10.3389/fnsys.2017.00091. [http://dx.doi.org/10.3389/fnsys.2017.00091]. [PMID: 29270113]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos J.R., Gois A.M., Mendonça D.M., Freire M.A.M. Nutritional status, oxidative stress and dementia: the role of selenium in Alzheimer’s disease. Front. Aging Neurosci. 2014;6:206. doi: 10.3389/fnagi.2014.00206. [http://dx.doi.org/10.3389/fnagi.2014.00206]. [PMID: 25221506]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Statistics W.H.O. Monitoring Health for the SDGs Sustainable Development Goals. Geneva: WHO; 2016. http://www.who.int/gho/publications/world_health_statistics/ [Google Scholar]
- 8.WHO World Health Organization. Neurological disorders: public health challenges. WHO, Geneva. Available at: http://www.who
- 9.Shaw G. The economic burden of neurologic disease - $800 billion annually in the US. Neurol. Today. 2017;17:1–14. [http://dx.doi.org/10.1097/01.NT.0000521169.52982.7f]. [Google Scholar]
- 10.Van Dam D., De Deyn P.P. Drug discovery in dementia: the role of rodent models. Nat. Rev. Drug Discov. 2006;5(11):956–970. doi: 10.1038/nrd2075. [http://dx.doi.org/10.1038/nrd2075]. [PMID: 17080031]. [DOI] [PubMed] [Google Scholar]
- 11.Hellewell S.C., Ziebell J.M., Lifshitz J., Morganti-Kossmann M.C. Impact acceleration model of diffuse traumatic brain injury. Methods Mol. Biol. 2016;1462:253–266. doi: 10.1007/978-1-4939-3816-2_15. [http://dx.doi.org/10.1007/978-1-4939-3816-2_15]. [PMID: 27604723]. [DOI] [PubMed] [Google Scholar]
- 12.Levy H., Assaf Y., Frenkel D. Characterization of brain lesions in a mouse model of progressive multiple sclerosis. Exp. Neurol. 2010;226(1):148–158. doi: 10.1016/j.expneurol.2010.08.017. [http://dx.doi.org/10.1016/j.expneurol.2010.08.017]. [PMID: 20736006]. [DOI] [PubMed] [Google Scholar]
- 13.Plantman S., Ng K.C., Lu J., Davidsson J., Risling M. Characterization of a novel rat model of penetrating traumatic brain injury. J. Neurotrauma. 2012;29(6):1219–1232. doi: 10.1089/neu.2011.2182. [http://dx.doi.org/10.1089/neu.2011.2182]. [PMID: 22181060]. [DOI] [PubMed] [Google Scholar]
- 14.Santiago L.F., Rocha E.G., Freire M.A.M., Dias I.A., Lent R., Houzel J.C., Picanço-Diniz C.W., Pereira A., Jr, Franca J.G. The organizational variability of the rodent somatosensory cortex. Rev. Neurosci. 2007;18(3-4):283–294. doi: 10.1515/revneuro.2007.18.3-4.283. [http://dx.doi.org/10.1515/REVNEURO.2007.18.3-4.283]. [PMID: 18019610]. [DOI] [PubMed] [Google Scholar]
- 15.Lopes R.S., Cardoso M.M., Sampaio A.O., Barbosa M.S., Jr, Souza C.C., Silva D.A. M.C.; Ferreira, E.M.; Freire, M.A.M.; Lima, R.R.; Gomes-Leal, W. Indomethacin treatment reduces microglia activation and increases numbers of neuroblasts in the subventricular zone and ischaemic striatum after focal ischaemia. J. Biosci. 2016;41(3):381–394. doi: 10.1007/s12038-016-9621-1. [http://dx.doi.org/10.1007/s12038-016-9621-1]. [PMID: 27581930]. [DOI] [PubMed] [Google Scholar]
- 16.Freire M.A.M., Oliveira R.B., Picanço-Diniz C.W., Pereira A., Jr Differential effects of methylmercury intoxication in the rat’s barrel field as evidenced by NADPH diaphorase histochemistry. Neurotoxicology. 2007;28(1):175–181. doi: 10.1016/j.neuro.2006.06.007. [http://dx.doi.org/10.1016/j.neuro.2006.06.007]. [PMID: 16930717]. [DOI] [PubMed] [Google Scholar]
- 17.Norrara B., Fiuza F.P., Arrais A.C., Costa I.M., Santos J.R., Engelberth R.C.G.J., Cavalcante J.S., Guzen F.P., Cavalcanti J.R.L.P., Freire M.A.M. Pattern of tyrosine hydroxylase expression during aging of mesolimbic pathway of the rat. J. Chem. Neuroanat. 2018;92:83–91. doi: 10.1016/j.jchemneu.2018.05.004. [http://dx.doi.org/10.1016/j.jchemneu.2018.05.004]. [PMID: 29842891]. [DOI] [PubMed] [Google Scholar]
- 18.Pereira A., Jr, Freire M.A.M., Bahia C.P., Franca J.G., Picanço-Diniz C.W. The barrel field of the adult mouse SmI cortex as revealed by NADPH-diaphorase histochemistry. Neuroreport. 2000;11(9):1889–1892. doi: 10.1097/00001756-200006260-00017. [http://dx.doi.org/10.1097/00001756-200006260-00017]. [PMID: 10884038]. [DOI] [PubMed] [Google Scholar]
- 19.Sachan A., Singh S., Singh H.K., Shankar P., Kumar D., Sachan A.K., Nath R., Dixit R. An experimental study to evaluate the effect of mucuna pruriens on learning and memory in mice. Int. J. Innov. Sci. Res. 2015;4:144–148. [Google Scholar]
- 20.Li L., Hou X., Xu R., Liu C., Tu M. Research review on the pharmacological effects of astragaloside IV. Fundam. Clin. Pharmacol. 2017;31(1):17–36. doi: 10.1111/fcp.12232. [http://dx.doi.org/10.1111/fcp.12232]. [PMID: 27567103]. [DOI] [PubMed] [Google Scholar]
- 21.Xu M., Yin J., Xie L., Zhang J., Zou C., Zou J., Liu F., Ju W., Li P. Pharmacokinetics and tolerance of toal astragalosides after intravenous infusion of astragalosides injection in healthy Chinese volunteers. Phytomedicine. 2013;20(12):1105–1111. doi: 10.1016/j.phymed.2013.05.004. [http://dx.doi.org/10.1016/j.phymed.2013.05.004]. [PMID: 23838148]. [DOI] [PubMed] [Google Scholar]
- 22.Ren S., Zhang H., Mu Y., Sun M., Liu P. Pharmacological effects of Astragaloside IV: a literature review. J. Tradit. Chin. Med. 2013;33(3):413–416. doi: 10.1016/s0254-6272(13)60189-2. [http://dx.doi.org/10.1016/S0254-6272(13)60189-2]. [PMID: 24024343]. [DOI] [PubMed] [Google Scholar]
- 23.Wagner H., Bauer R., Xiao P.G., Chen J.M., Michler G. Chinese Drug Monographs and Analysis: Radix Astragali. Huangqi; 1997. pp. 1–17. [Google Scholar]
- 24.Zheng X.Y. Pharmacopoeia of the Peoples Republic of China. (Chinese edn.) 2005;Vol. 1 [Google Scholar]
- 25.Li Z.P., Cao Q. Effects of astragaloside IV on myocardial calcium transport and cardiac function in ischemic rats. Acta Pharmacol. Sin. 2002;23(10):898–904. [PMID: 12370095]. [PubMed] [Google Scholar]
- 26.Yang J., Wang H.X., Zhang Y.J., Yang Y.H., Lu M.L., Zhang J., Li S.T., Zhang S.P., Li G. Astragaloside IV attenuates inflammatory cytokines by inhibiting TLR4/NF-кB signaling pathway in isoproterenol-induced myocardial hypertrophy. J. Ethnopharmacol. 2013;150(3):1062–1070. [http://dx.doi.org/10.1016/j.jep.2013.10.017]. [PMID: 24432369]. [PubMed] [Google Scholar]
- 27.Zhang W.D., Zhang C., Liu R.H., Li H.L., Zhang J.T., Mao C., Moran S., Chen C.L. Preclinical pharmacokinetics and tissue distribution of a natural cardioprotective agent astragaloside IV in rats and dogs. Life Sci. 2006;79(8):808–815. doi: 10.1016/j.lfs.2006.02.032. [http://dx.doi.org/10.1016/j.lfs.2006.02.032]. [PMID: 16564551]. [DOI] [PubMed] [Google Scholar]
- 28.Qu Y.Z., Li M., Zhao Y.L., Zhao Z.W., Wei X.Y., Liu J.P., Gao L., Gao G.D. Astragaloside IV attenuates cerebral ischemia-reperfusion-induced increase in permeability of the blood-brain barrier in rats. Eur. J. Pharmacol. 2009;606(1-3):137–141. doi: 10.1016/j.ejphar.2009.01.022. [http://dx.doi.org/10.1016/j.ejphar.2009.01.022]. [PMID: 19374856]. [DOI] [PubMed] [Google Scholar]
- 29.Kumar G.P., Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn. Rev. 2012;6(12):81–90. doi: 10.4103/0973-7847.99898. [http://dx.doi.org/10.4103/0973-7847.99898]. [PMID: 23055633]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Costa I.M., de Moura Freire M.A., de Paiva Cavalcanti J.R.L., de Araújo D.P., Norrara B., Moreira Rosa I.M.M., de Azevedo E.P., do Rego A.C.M., Filho I.A., Guzen F.P. Supplementation with Curcuma longa reverses neurotoxic and behavioral damage in models of Alzheimer’s disease: A systematic review. Curr. Neuropharmacol. 2019;17(5):406–421. doi: 10.2174/0929867325666180117112610. [http://dx.doi.org/10.2174/0929867325666180117112610]. [PMID: 29338678]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan W.S., Durairajan S.S., Lu J.H., Wang Y., Xie L.X., Kum W.F., Koo I., Yung K.K., Li M. Neuroprotective effects of Astragaloside IV in 6-hydroxydopamine-treated primary nigral cell culture. Neurochem. Int. 2009;55(6):414–422. doi: 10.1016/j.neuint.2009.04.012. [http://dx.doi.org/10.1016/j.neuint.2009.04.012]. [PMID: 19409437]. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X., Chen J. The mechanism of astragaloside IV promoting sciatic nerve regeneration. Neural Regen. Res. 2013;8(24):2256–2265. doi: 10.3969/j.issn.1673-5374.2013.24.005. [PMID: 25206535]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blesa J., Trigo-Damas I., Quiroga-Varela A., Jackson-Lewis V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015;9:91. doi: 10.3389/fnana.2015.00091. [http://dx.doi.org/10.3389/fnana.2015.00091]. [PMID: 26217195]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding Y.M., Jaumotte J.D., Signore A.P., Zigmond M.J. Effects of 6-hydroxydopamine on primary cultures of substantia nigra: specific damage to dopamine neurons and the impact of glial cell line-derived neurotrophic factor. J. Neurochem. 2004;89(3):776–787. doi: 10.1111/j.1471-4159.2004.02415.x. [http://dx.doi.org/10.1111/j.1471-4159.2004.02415.x]. [PMID: 15086533]. [DOI] [PubMed] [Google Scholar]
- 35.Perese D.A., Ulman J., Viola J., Ewing S.E., Bankiewicz K.S.A.A. 6-hydroxydopamine-induced selective parkinsonian rat model. Brain Res. 1989;494(2):285–293. doi: 10.1016/0006-8993(89)90597-0. [http://dx.doi.org/10.1016/0006-8993(89)90597-0]. [PMID: 2528389]. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt N., Ferger B. Neurochemical findings in the MPTP model of Parkinson’s disease. J. Neural Transm. (Vienna) 2001;108(11):1263–1282. doi: 10.1007/s007020100004. [http://dx.doi.org/10.1007/s007020100004]. [PMID: 11768626]. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z.G., Wu L., Wang J.L., Yang J.D., Zhang J., Zhang J., Li L.H., Xia Y., Yao L.B., Qin H.Z., Gao G.D. Astragaloside IV prevents MPP+-induced SH-SY5Y cell death via the inhibition of Bax-mediated pathways and ROS production. Mol. Cell. Biochem. 2012;364(1-2):209–216. doi: 10.1007/s11010-011-1219-1. [http://dx.doi.org/10.1007/s11010-011-1219-1]. [PMID: 22278385]. [DOI] [PubMed] [Google Scholar]
- 38.Kalimuthu S., Se-Kwon K. Cell survival and apoptosis signaling as therapeutic target for cancer: marine bioactive compounds. Int. J. Mol. Sci. 2013;14(2):2334–2354. doi: 10.3390/ijms14022334. [http://dx.doi.org/10.3390/ijms14022334]. [PMID: 23348928]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy O.A., Malagelada C., Greene L.A. Cell death pathways in Parkinson’s disease: proximal triggers, distal effectors, and final steps. Apoptosis. 2009;14(4):478–500. doi: 10.1007/s10495-008-0309-3. [http://dx.doi.org/10.1007/s10495-008-0309-3]. [PMID: 19165601]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.da Costa I.M., Cavalcanti J.R.L.P., de Queiroz D.B., de Azevedo E.P., do Rêgo A.C.M., Araújo F.I., Parente P., Botelho M.A., Guzen F.P. Supplementation with herbal extracts to promote behavioral and neuroprotective effects in experimental models of Parkinson’s disease: A systematic review. Phytother. Res. 2017;31(7):959–970. doi: 10.1002/ptr.5813. [http://dx.doi.org/10.1002/ptr.5813]. [PMID: 28544038]. [DOI] [PubMed] [Google Scholar]
- 41.Cole G.M., Frautschy S.A. Docosahexaenoic acid protects from amyloid and dendritic pathology in an Alzheimer’s disease mouse model. Nutr. Health. 2006;18(3):249–259. doi: 10.1177/026010600601800307. [http://dx.doi.org/10.1177/026010600601800307]. [PMID: 17180870]. [DOI] [PubMed] [Google Scholar]
- 42.Kasper D., Fauci A., Hauser S., Longo D., Jameson J.L., Loscalzo J. Harrison’s Principles of Internal Medicine. 19th ed. 2015. [Google Scholar]
- 43.Machado A., Herrera A.J., de Pablos R.M., Espinosa-Oliva A.M., Sarmiento M., Ayala A., Venero J.L., Santiago M., Villarán R.F., Delgado-Cortés M.J., Argüelles S., Cano J. Chronic stress as a risk factor for Alzheimer’s disease. Rev. Neurosci. 2014;25(6):785–804. doi: 10.1515/revneuro-2014-0035. [http://dx.doi.org/10.1515/revneuro-2014-0035]. [PMID: 25178904]. [DOI] [PubMed] [Google Scholar]
- 44.Dartigues J.F. Alzheimer’s disease: a global challenge for the 21st century. Lancet Neurol. 2009;8(12):1082–1083. doi: 10.1016/S1474-4422(09)70298-4. [http://dx.doi.org/10.1016/S1474-4422(09)70298-4]. [PMID: 19909903]. [DOI] [PubMed] [Google Scholar]
- 45.Sun Q., Jia N., Wang W., Jin H., Xu J., Hu H. Protective effects of astragaloside IV against amyloid beta1-42 neurotoxicity by inhibiting the mitochondrial permeability transition pore opening. PLoS One. 2014;9(6):e98866. doi: 10.1371/journal.pone.0098866. [http://dx.doi.org/10.1371/journal.pone.0098866]. [PMID: 24905226]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halestrap A.P. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem. Soc. Trans. 2006;34(Pt 2):232–237. doi: 10.1042/BST20060232. [http://dx.doi.org/10.1042/BST0340232]. [PMID: 16545083]. [DOI] [PubMed] [Google Scholar]
- 47.Dong H., Yuede C.M., Yoo H.S., Martin M.V., Deal C., Mace A.G., Csernansky J.G. Corticosterone and related receptor expression are associated with increased beta-amyloid plaques in isolated Tg2576 mice. Neuroscience. 2008;155(1):154–163. doi: 10.1016/j.neuroscience.2008.05.017. [http://dx.doi.org/10.1016/j.neuroscience.2008.05.017]. [PMID: 18571864]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li W.Z., Li W.P., Zhang W., Yin Y.Y., Sun X.X., Zhou S.S., Xu X.Q., Tao C.R. Protective effect of extract of Astragalus on learning and memory impairments and neurons’ apoptosis induced by glucocorticoids in 12-month-old male mice. Anat. Rec. (Hoboken) 2011;294(6):1003–1014. doi: 10.1002/ar.21386. [http://dx.doi.org/10.1002/ar.21386]. [PMID: 21538932]. [DOI] [PubMed] [Google Scholar]
- 49.Boissonneault V., Plante I., Rivest S., Provost P. MicroRNA-298 and microRNA-328 regulate expression of mouse beta-amyloid precursor protein-converting enzyme 1. J. Biol. Chem. 2009;284(4):1971–1981. doi: 10.1074/jbc.M807530200. [http://dx.doi.org/10.1074/jbc.M807530200]. [PMID: 18986979]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sotiropoulos I., Catania C., Pinto L.G., Silva R., Pollerberg G.E., Takashima A., Sousa N., Almeida O.F. Stress acts cumulatively to precipitate Alzheimer’s disease-like tau pathology and cognitive deficits. J. Neurosci. 2011;31(21):7840–7847. doi: 10.1523/JNEUROSCI.0730-11.2011. [http://dx.doi.org/10.1523/JNEUROSCI.0730-11.2011]. [PMID: 21613497]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris R.G., Garrud P., Rawlins J.N., O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [http://dx.doi.org/10.1038/297681a0]. [PMID: 7088155]. [DOI] [PubMed] [Google Scholar]
- 52.Mancini M., Nicholson D.W., Roy S., Thornberry N.A., Peterson E.P., Casciola-Rosen L.A., Rosen A. The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling. J. Cell Biol. 1998;140(6):1485–1495. doi: 10.1083/jcb.140.6.1485. [http://dx.doi.org/10.1083/jcb.140.6.1485]. [PMID: 9508780]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mulugeta S., Maguire J.A., Newitt J.L., Russo S.J., Kotorashvili A., Beers M.F. Misfolded BRICHOS SP-C mutant proteins induce apoptosis via caspase-4- and cytochrome c-related mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293(3):L720–L729. doi: 10.1152/ajplung.00025.2007. [http://dx.doi.org/10.1152/ajplung.00025.2007]. [PMID: 17586700]. [DOI] [PubMed] [Google Scholar]
- 54.Haiyan H., Rensong Y., Guoqin J., Xueli Z., Huaying X., Yanwu X. Effect of Astragaloside IV on neural stem cell transplantation in Alzheimer’s disease rat models. Evid. Based Complement. Alternat. Med. 2016:20163106980. doi: 10.1155/2016/3106980. [http://dx.doi.org/10.1155/2016/3106980]. [PMID: 27034688]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hitoshi S., Alexson T., Tropepe V., Donoviel D., Elia A.J., Nye J.S., Conlon R.A., Mak T.W., Bernstein A., van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16(7):846–858. doi: 10.1101/gad.975202. [http://dx.doi.org/10.1101/gad.975202]. [PMID: 11937492]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng C.Y., Yao C.H., Liu B.S., Liu C.J., Chen G.W., Chen Y.S. The role of astragaloside in regeneration of the peripheral nerve system. J. Biomed. Mater. Res. A. 2006;76(3):463–469. doi: 10.1002/jbm.a.30249. [http://dx.doi.org/10.1002/jbm.a.30249]. [PMID: 16315188]. [DOI] [PubMed] [Google Scholar]
- 57.Mittal K., Katare D.P. Shared links between type 2 diabetes mellitus and Alzheimer’s disease: A review. Diabetes Metab. Syndr. 2016;10(2) Suppl. 1:S144–S149. doi: 10.1016/j.dsx.2016.01.021. [http://dx.doi.org/10.1016/j.dsx.2016.01.021]. [PMID: 26907971]. [DOI] [PubMed] [Google Scholar]
- 58.Escribano L., Simón A.M., Gimeno E., Cuadrado-Tejedor M., López de Maturana R., García-Osta A., Ricobaraza A., Pérez-Mediavilla A., Del Río J., Frechilla D. Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology. 2010;35(7):1593–1604. doi: 10.1038/npp.2010.32. [http://dx.doi.org/10.1038/npp.2010.32]. [PMID: 20336061]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mandrekar-Colucci S., Karlo J.C., Landreth G.E. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-γ-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer’s disease. J. Neurosci. 2012;32(30):10117–10128. doi: 10.1523/JNEUROSCI.5268-11.2012. [http://dx.doi.org/10.1523/JNEUROSCI.5268-11.2012]. [PMID: 22836247]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sastre M., Klockgether T., Heneka M.T. Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int. J. Dev. Neurosci. 2006;24(2-3):167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [http://dx.doi.org/10.1016/j.ijdevneu.2005.11.014]. [PMID: 16472958]. [DOI] [PubMed] [Google Scholar]
- 61.Chan A., Tchantchou F., Rogers E.J., Shea T.B. Dietary deficiency increases presenilin expression, gamma-secretase activity, and Abeta levels: potentiation by ApoE genotype and alleviation by S-adenosyl methionine. J. Neurochem. 2009;110(3):831–836. doi: 10.1111/j.1471-4159.2009.06177.x. [http://dx.doi.org/10.1111/j.1471-4159.2009.06177.x]. [PMID: 19457069]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X., Wang Y., Hu J.P., Yu S., Li B.K., Cui Y., Ren L., Zhang L.D. Astragaloside IV, a natural PPARgamma agonist, reduces Abeta production in Alzheimer’s disease through inhibition of BACE1. Mol. Neurobiol. 2017;54(4):2939–2949. doi: 10.1007/s12035-016-9874-6. [http://dx.doi.org/10.1007/s12035-016-9874-6]. [PMID: 27023226]. [DOI] [PubMed] [Google Scholar]
- 63.Chang C.P., Liu Y.F., Lin H.J., Hsu C.C., Cheng B.C., Liu W.P., Lin M.T., Hsu S.F., Chang L.S., Lin K.C. Beneficial effect of Astragaloside on Alzheimer’s disease condition using cultured primary cortical cells under beta-amyloid exposure. Mol. Neurobiol. 2016;53(10):7329–7340. doi: 10.1007/s12035-015-9623-2. [http://dx.doi.org/10.1007/s12035-015-9623-2]. [PMID: 26696494]. [DOI] [PubMed] [Google Scholar]
- 64.Yang W.T., Zheng X.W., Chen S., Shan C.S., Xu Q.Q., Zhu J.Z., Bao X.Y., Lin Y., Zheng G.Q., Wang Y. Chinese herbal medicine for Alzheimer’s disease: Clinical evidence and possible mechanism of neurogenesis. Biochem. Pharmacol. 2017;141:143–155. doi: 10.1016/j.bcp.2017.07.002. [http://dx.doi.org/10.1016/j.bcp.2017.07.002]. [PMID: 28690138]. [DOI] [PubMed] [Google Scholar]
- 65.He Y., Du M., Gao Y., Liu H., Wang H., Wu X., Wang Z. Astragaloside IV attenuates experimental autoimmune encephalomyelitis of mice by counteracting oxidative stress at multiple levels. PLoS One. 2013;8(10):e76495. doi: 10.1371/journal.pone.0076495. [http://dx.doi.org/10.1371/journal.pone.0076495]. [PMID: 24124567]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He Y.X., Du M., Shi H.L., Huang F., Liu H.S., Wu H., Zhang B.B., Dou W., Wu X.J., Wang Z.T. Astragalosides from Radix Astragali benefits experimental autoimmune encephalomyelitis in C57BL /6 mice at multiple levels. BMC Complement. Altern. Med. 2014;14:313. doi: 10.1186/1472-6882-14-313. [http://dx.doi.org/10.1186/1472-6882-14-313]. [PMID: 25150364]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Campanella M., Sciorati C., Tarozzo G., Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33(2):586–592. doi: 10.1161/hs0202.103399. [http://dx.doi.org/10.1161/hs0202.103399]. [PMID: 11823674]. [DOI] [PubMed] [Google Scholar]
- 68.Clark R.K., Lee E.V., Fish C.J., White R.F., Price W.J., Jonak Z.L., Feuerstein G.Z., Barone F.C. Development of tissue damage, inflammation and resolution following stroke: an immunohistochemical and quantitative planimetric study. Brain Res. Bull. 1993;31(5):565–572. doi: 10.1016/0361-9230(93)90124-t. [http://dx.doi.org/10.1016/0361-9230(93)90124-T]. [PMID: 8495380]. [DOI] [PubMed] [Google Scholar]
- 69.Kriz J., Lalancette-Hébert M. Inflammation, plasticity and real-time imaging after cerebral ischemia. Acta Neuropathol. 2009;117(5):497–509. doi: 10.1007/s00401-009-0496-1. [http://dx.doi.org/10.1007/s00401-009-0496-1]. [PMID: 19225790]. [DOI] [PubMed] [Google Scholar]
- 70.Shichita T., Hasegawa E., Kimura A., Morita R., Sakaguchi R., Takada I., Sekiya T., Ooboshi H., Kitazono T., Yanagawa T., Ishii T., Takahashi H., Mori S., Nishibori M., Kuroda K., Akira S., Miyake K., Yoshimura A. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat. Med. 2012;18(6):911–917. doi: 10.1038/nm.2749. [http://dx.doi.org/10.1038/nm.2749]. [PMID: 22610280]. [DOI] [PubMed] [Google Scholar]
- 71.Li M., Li H., Fang F., Deng X., Ma S. Astragaloside IV attenuates cognitive impairments induced by transient cerebral ischemia and reperfusion in mice via anti-inflammatory mechanisms. Neurosci. Lett. 2017;639:114–119. doi: 10.1016/j.neulet.2016.12.046. [http://dx.doi.org/10.1016/j.neulet.2016.12.046]. [PMID: 28011393]. [DOI] [PubMed] [Google Scholar]
- 72.Kim S., Kang I.H., Nam J.B., Cho Y., Chung D.Y., Kim S.H., Kim J.S., Cho Y.D., Hong E.K., Sohn N.W., Shin J.W. Ameliorating the effect of astragaloside IV on learning and memory deficit after chronic cerebral hypoperfusion in rats. Molecules. 2015;20(2):1904–1921. doi: 10.3390/molecules20021904. [http://dx.doi.org/10.3390/molecules20021904]. [PMID: 25625683]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H.L., Zhou Q.H., Xu M.B., Zhou X.L., Zheng G.Q. Astragaloside IV for experimental focal cerebral ischemia: Preclinical evidence and possible mechanisms. Oxid. Med. Cell. Longev. 2017:20178424326. doi: 10.1155/2017/8424326. [http://dx.doi.org/10.1155/2017/8424326]. [PMID: 28303172]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li M., Qu Y.Z., Zhao Z.W., Wu S.X., Liu Y.Y., Wei X.Y., Gao L., Gao G.D. Astragaloside IV protects against focal cerebral ischemia/reperfusion injury correlating to suppression of neutrophils adhesion-related molecules. Neurochem. Int. 2012;60(5):458–465. doi: 10.1016/j.neuint.2012.01.026. [http://dx.doi.org/10.1016/j.neuint.2012.01.026]. [PMID: 22342823]. [DOI] [PubMed] [Google Scholar]
- 75.Yin Y.Y., Li W.P., Gong H.L., Zhu F.F., Li W.Z., Wu G.C. Protective effect of astragaloside on focal cerebral ischemia/reperfusion injury in rats. Am. J. Chin. Med. 2010;38(3):517–527. doi: 10.1142/S0192415X10008020. [http://dx.doi.org/10.1142/S0192415X10008020]. [PMID: 20503469]. [DOI] [PubMed] [Google Scholar]
- 76.Rosenberg G.A. Neurological diseases in relation to the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012;32(7):1139–1151. doi: 10.1038/jcbfm.2011.197. [http://dx.doi.org/10.1038/jcbfm.2011.197]. [PMID: 22252235]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Date I., Takagi N., Takagi K., Tanonaka K., Funakoshi H., Matsumoto K., Nakamura T., Takeo S. Hepatocyte growth factor attenuates cerebral ischemia-induced increase in permeability of the blood-brain barrier and decreases in expression of tight junctional proteins in cerebral vessels. Neurosci. Lett. 2006;407(2):141–145. doi: 10.1016/j.neulet.2006.08.050. [http://dx.doi.org/10.1016/j.neulet.2006.08.050]. [PMID: 16973272]. [DOI] [PubMed] [Google Scholar]
- 78.Li M., Ma R.N., Li L.H., Qu Y.Z., Gao G.D. Astragaloside IV reduces cerebral edema post-ischemia/reperfusion correlating the suppression of MMP-9 and AQP4. Eur. J. Pharmacol. 2013;715(1-3):189–195. doi: 10.1016/j.ejphar.2013.05.022. [http://dx.doi.org/10.1016/j.ejphar.2013.05.022]. [PMID: 23747593]. [DOI] [PubMed] [Google Scholar]
- 79.Shao A., Guo S., Tu S., Ammar A.B., Tang J., Hong Y., Wu H., Zhang J. Astragaloside IV alleviates early brain injury following experimental subarachnoid hemorrhage in rats. Int. J. Med. Sci. 2014;11(10):1073–1081. doi: 10.7150/ijms.9282. [http://dx.doi.org/10.7150/ijms.9282]. [PMID: 25136262]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen C.C., Lee H.C., Chang J.H., Chen S.S., Li T.C., Tsai C.H., Cho D.Y., Hsieh C.L. Chinese herb Astragalus membranaceus enhances recovery of hemorrhagic stroke: Double-blind, placebo-controlled, randomized study. Evid. Based Complement. Alternat. Med. 2012;2012:708452. doi: 10.1155/2012/708452. [PMID: 22474516]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun J., Chen X.L., Zheng J.Y., Zhou J.W., Ma Z.L. Astragaloside IV protects new born rats from anesthesia-induced apoptosis in the developing brain. Exp. Ther. Med. 2016;12(3):1829–1835. doi: 10.3892/etm.2016.3519. [http://dx.doi.org/10.3892/etm.2016.3519]. [PMID: 27588101]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang J., Li J., Lu J., Zhang Y., Zhu Z., Wan H. Synergistic protective effect of astragaloside IV-tetramethylpyrazine against cerebral ischemic-reperfusion injury induced by transient focal ischemia. J. Ethnopharmacol. 2012;140(1):64–72. doi: 10.1016/j.jep.2011.12.023. [http://dx.doi.org/10.1016/j.jep.2011.12.023]. [PMID: 22207211]. [DOI] [PubMed] [Google Scholar]
- 83.Liu X., Zhang J., Wang S., Qiu J., Yu C. Astragaloside IV attenuates the H2O2-induced apoptosis of neuronal cells by inhibiting α-synuclein expression via the p38 MAPK pathway. Int. J. Mol. Med. 2017;40(6):1772–1780. doi: 10.3892/ijmm.2017.3157. [http://dx.doi.org/10.3892/ijmm.2017.3157]. [PMID: 29039448]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu H.S., Shi H.L., Huang F., Peterson K.E., Wu H., Lan Y.Y., Zhang B.B., He Y.X., Woods T., Du M., Wu X.J., Wang Z.T. Astragaloside IV inhibits microglia activation via glucocorticoid receptor mediated signaling pathway. Sci. Rep. 2016;6:19137. doi: 10.1038/srep19137. [http://dx.doi.org/10.1038/srep19137]. [PMID: 26750705]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cao J., Chen Z., Zhu Y., Li Y., Guo C., Gao K., Chen L., Shi X., Zhang X., Yang Z., Wen A. Huangqi-Honghua combination and its main components ameliorate cerebral infarction with Qi deficiency and blood stasis syndrome by antioxidant action in rats. J. Ethnopharmacol. 2014;155(2):1053–1060. doi: 10.1016/j.jep.2014.05.061. [http://dx.doi.org/10.1016/j.jep.2014.05.061]. [PMID: 24960183]. [DOI] [PubMed] [Google Scholar]
- 86.Cao Y.L., Chen C.F., Wang A.W., Feng Y.B., Cheng H.X., Zhang W.W., Xin W. Changes of peripheral-type benzodiazepine receptors in the penumbra area after cerebral ischemia-reperfusion injury and effects of astragaloside IV on rats. Genet. Mol. Res. 2015;14(1):277–285. doi: 10.4238/2015.January.23.1. [http://dx.doi.org/10.4238/2015.January.23.1]. [PMID: 25729960]. [DOI] [PubMed] [Google Scholar]


