Abstract
Abstract: Neurodegenerative diseases are among the most serious health problems affecting millions of people worldwide. Such diseases are characterized by a progressive degeneration and / or death of neurons in the central nervous system. Currently, there are no therapeutic approaches to cure or even halt the progression of neurodegenerative diseases. During the last two decades, much attention has been paid to the neuroprotective and anti-neurodegenerative activities of compounds isolated from natural products with high efficacy and low toxicity. Accumulating evidence indicates that berberine, an isoquinoline alkaloid isolated from traditional Chinese medicinal herbs, may act as a promising anti-neurodegenerative agent by inhibiting the activity of the most important pathogenic enzymes, ameliorating intracellular oxidative stress, attenuating neuroinflammation, triggering autophagy and protecting neurons against apoptotic cell death. This review attempts to summarize the current state of knowledge regarding the therapeutic potential of berberine against neurodegenerative diseases, with a focus on the molecular mechanisms that underlie its effects on Alzheimer’s, Parkinson’s and Huntington’s diseases.
Keywords: Neurodegenerative diseases, berberine, neuroprotection, oxidative stress, neuroinflammation, autophagy
1. INTRODUCTION
Berberine is a natural isoquinoline alkaloid that is widely present in the roots, rhizomes, stems, or bark of various medicinal herbs such as Berberis, Coptis chinensis, Phellodendron amurense and Hydrastis Canadensis (Fig. 1) [1-5]. For more than 2,000 years, these plants have been used in Chinese traditional medicine (TCM) to treat a variety of diseases [6]. However, it was not until recently that berberine was isolated and identified as the major pharmacologically active compound of these herbs [6]. Berberine has been shown to possess a wide range of pharmacological activities including anti-microbial, anti-inflammatory, anti-oxidant, hypoglycemic, hypolipidemic, and hepatoprotective properties [7, 8]. It has been tested clinically in the treatment of diarrhea, ulcerative colitis, gastritis, metabolic syndrome, type 2 diabetes mellitus, polycystic ovary syndrome, hypercholesterolemia, hyperlipemia, non-alcoholic fatty liver disease, heart diseases, and so on [6-8].
Fig. (1).
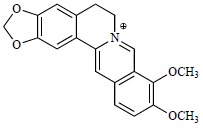
Molecular structure of berberine.
Although pharmacokinetic studies of berberine in animals indicate that berberine is not easily absorbed by the gut wall and is present at a very low level in blood, the concentration of berberine as well as its active metabolites was found to be higher in tissues than that in the blood after oral administration [9-11]. In a recent study, Tan and colleagues have systematically analyzed tissue distribution of berberine and its bioactive metabolites in rats after oral administration (200 mg/kg) and have shown that berberine was quickly distributed in the liver, kidneys, muscle, lungs, brain, heart, pancreas, and fat in descending order of its amount [11]. The concentration of berberine in liver was more than ten times of that in plasma, with a peak (68.19 ng/g) observed at 8h after oral administration [11]. They further showed that berberrubine, thalifendine and jatrorrhizine were the major bioactive metabolites of berberine and the level of AUC(0-t) (area under the concentration-time curve) for the metabolites in the liver was 30-fold higher than that in plasma [11]. The tissue distribution profile of berberine and its metabolites provides at least a partial explanation for the discrepancy between poor bioavailability of berberine and its pharmacological effects.
More recently, berberine has been extensively investigated for its therapeutic efficacy against neurodegenerative diseases [12-17]. Accumulating evidence indicates that berberine can penetrate the blood-brain barrier under normal physiological conditions and exert neuroprotective effects in both cellular and animal models of Alzheimer’s, Parkinson’s and Huntington’s diseases [12-21]. Recent research into berberine’s neuropharmacology suggests that the neuroprotective properties of berberine are probably due to its ability to inhibit the activity of the most important pathogenic enzymes, to reduce intracellular oxidative stress, and to attenuate the inflammatory response [22-30]. In addition, most recent studies have showed that berberine can also combat neurodegenerative diseases by accelerating the clearance of toxic misfolded and aggregated proteins in neuronal cells through induction of autophagy [31, 32]. This review attempts to summarize the current state of knowledge regarding the therapeutic potential of berberine against neurodegenerative diseases, with a focus on the molecular mechanisms that underlie these effects in Alzheimer’s, Parkinson’s and Huntington’s diseases.
2. BERBERINE AND ALZHEIMER’S DISEASE
Alzheimer's disease (AD) is an irreversible, progressive, neurodegenerative disease that gradually erodes memory, cognitive skills, and eventually the ability to carry out simple, everyday tasks [33, 34]. It is the most common cause of dementia, accounting for 60-80% of all cases of dementia in the elderly [35]. According to the Alzheimer's Disease International (ADI), nearly 44 million people are currently afflicted with Alzheimer’s or a related dementia worldwide, and it is predicted that the number of individuals affected by AD will quadruple over the next 50 years due to the coming acceleration of global population ageing [35].
The past three decades have witnessed remarkable advances in our understanding of the pathophysiology of AD; however, the etiology of the disease is still mostly unknown [36, 37]. To date, three major hypotheses (cholinergic hypothesis, amyloid hypothesis and tau hypothesis) have been proposed regarding the pathogenesis of AD, which postulate that deficits in the brain neurotransmitter acetylcholine, extracellular deposition of beta-amyloid (Aβ), and intracellular aggregation of tau protein played significant roles in the pathophysiology of the disease [38-41]. Apart from these major hypotheses, there is growing evidence suggests that neuroinflammation, oxidative stress and high levels of serum cholesterol are also implicated in the etiology of AD [42-46].
Since multiple pathogenetic factors are involved in the disease, the current therapeutic strategies for AD that target only a single neurotoxic factor or pathway have been proved to be inadequate [47]. It is, therefore, extremely valuable to consider developing drugs that target multiple factors implicated in AD pathogenesis [48-52]. Recently, several lines of evidence have suggested that berberine may act as a promising multipotent agent to combat AD due to the multiple activities that it possesses, including acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activity, β-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitory activity, Glycogen synthase kinase 3 (GSK-3) inhibitory activity, anti-inflammatory activity, antioxidant activity, and cholesterol-lowering activity [1, 12-14, 22-29].
2.1. Berberine Alleviates Cholinergic Deficit and Improves Neurotransmission
Acetylcholine, the first neurotransmitter ever to be identified, is synthesized from choline and acetyl-coenzymeA in presynaptic neurons by the enzyme cholineacetyltransferase [53, 54]. Once released, acetylcholine is rapidly broken down by AChE and BChE [55, 56]. For more than three decades, the pathogenesis of AD has been linked to acetylcholine deficiency in the brain [38]. AChE and BChE inhibition have recently been documented as critical targets for the effective management of AD by alleviating cholinergic deficit and improving neurotransmission [57-61]. As a result, three cholinesterase inhibitors (Donepezil, Galantamine and Rivastigmine) were approved by the Food and Drug Administration (FDA), which constituted the first group of drugs for the symptomatic treatment of AD [62-64]. Recently, a number of studies have shown that berberine exhibited significant AChE inhibitory effects with IC50 values ranging between 0.37 and 0.58 mM, which were comparable to that of Galanthamine (0.59-0.62 mM) [14, 22, 23, 65, 66]. Moreover, Ji et al. applied a computational pharmaceutical analysis to reveal that berberine has a much higher Dock score and possesses higher AChE inhibitory activity than the FDA-approved Alzheimer's drug Donepezil [23]. Although some studies indicated that berberine had only weak inhibitory activity against BChE, in a recent study, Jung et al. oberserved a potent inhibitory effect of berberine on BChE with an IC50 value of 3.44 mM [67]. In addition to functioning as a powerful dual inhibitor of AChE and BChE, berberine has also been identified as a promising candidate for Aβ and tau-based therapeutics to prevent or delay the onset of Aβ and tau pathology in AD [22, 26-28, 32].
2.2. Berberine Inhibits Aβ Generation and Senile Plaques Formation
Aβ peptide is a proteolytic product derived from the sequential cleavage of Aβ precursor protein (APP) by β-secretase (also known as BACE1) and γ-secretase, whereas α-secretase precludes its generation through alternative cleavage of APP within the Aβ sequence [68-70]. The progressive accumulation and aggregation of Aβ in the extracellular space leads to the formation of senile plaques, one of the hallmark lesions of AD [71-73]. Therefore, inhibition of Aβ generation and/or aggregation should be a rational therapeutic strategy for AD [74, 75]. Accumulating evidence suggests that berberine can reduce Aβ peptide production by modulating APP processing, and can prevent amyloid fibril formation by inhibiting Aβ aggregation [26, 28, 32, 76, 77]. Asai and colleagues reported that berberine treatment effectively reduced levels of Aβ in human neuroglioma H4 cells that stably express Swedish-type of APP, with an IC50 of around 5 μM [76]. They further demonstrated that this reduction was modulated by berberine through both down-regulation of β-secretase (BACE1) activity and up-regulation of α-secretase activity, leading to a shift in the processing of APP from the amyloidogenic to the non-amyloidogenic pathway [76]. Using Swedish APP-expressing HEK293 cells, Zhu et al. showed that berberine decreases the production of Aβ by inhibiting the expression of BACE1 via activation of the ERK1/2 pathway [78]. Inhibition of ERK1/2 with the MEK1/2 antagonist, U0126, could abolish the effects of berberine on both Aβ and BACE1 [78]. A recent study by Zhang et al. found that the AMP-activated protein kinase (AMPK) pathway was also involved in berberine-modulated metabolism of Aβ. Through the activation of AMPK, berberine down-regulatd the expression of BACE1 and reduced Aβ generation in neuroblastoma cells and primary cultured neurons [79]. Recently, the efficacy of berberine on the modulation of APP processing and Aβ-induced neurotoxicity was further validated in several in vivo models [26, 28, 80]. Using an Al-maltol-induced AD rabbit model, Panahi et al. showed that berberine treatment could significant decrease the activity of BACE1, protect the hippocampus from degeneration, and restore chemically-induced AD-like behavioral derangements [28]. Similarly, Haghani et al. created a rat model of AD by bilateral injection of Aβ in the prefrontal cortex and investigated the effects of berberine on the Aβ-induced cognitive impairment and neurotoxicity [80]. Their results suggested that the administration of berberine could ameliorate neurotoxicity induced by Aβ through prevention of the impairing impacts of Aβ on the learning, memory, as well as electrophysiological properties of the hippocampal pyramidal neurons [80]. Moreover, in a recent study Durairajan et al. demonstated that chronic administration of berberine not only reduced Aβ deposits, but also ameliorated tau hyperphosphorylation, gliosis, and cognitive impairments in a well characterized transgenic mouse model of AD (TgCRND8 mice) [26]. They also found that these effects were achieved mainly through regulation of APP processing via the PI3K/Akt/GSK3 signaling pathway [26]. Berberine treatment led to the activation of PI3K/Akt, which subsequently inactivated glycogen synthase kinase 3(GSK3) via modulation of its phosphorylation status, resulting in reduced levels of p-APP and Aβ [26].
2.3. Berberine Inhibits Tau Hyperphosphorylation and Neurofibrillary Tangles Formation
It is noteworthy that, as an inhibitory target of berberine, GSK3 not only plays an important role in modulating APP processing and influencing the production of Aβ, but also contributes to hyperphosphorylation of tau, which leads to the transformation of normal tau protein into paired helical filament and neurofibrillary tangles, another hallmark lesion of AD [81-83]. The phosphorylation status of tau is the result of a balanced action between protein kinases and protein phosphatases, therefore, it is no surprise to find that over-activation of glycogen synthase kinase 3 beta (GSK-3β) and/or inhibition of protein phosphatase 2A (PP2A) have been frequently reported to induce tau hyperphosphorylation [84-88]. In a recent study, Yu et al. treated tau-expressing HEK293 cells with calyculin A, a potent inhibitor of PP2A and PP1, and created a cellular model of tauopathy to investigate the roles of berberine in tau hyperphosphorylation [89]. They showed that calyculin A treatment not only inhibited PP2A, but also activated GSK3β by phosphorylating it on Tyr216 and dephosphorylating it on Ser9, which subsequently led to tau hyperphosphorylation at multiple sites [89]. Berberine significantly attenuated calyculin A-induced tau hyperphosphorylation and cytotoxicity through restoration of PP2A activity and reversal of GSK3β activation [89]. Consistent with these findings, Liu et al. reported that berberine rescued the decrease in PP2A activity by reversing the phosphorylation of PP2A on Tyr307, which in turn alleviated tau hyperphosphorylation and axonal transport impairment induced by calyculin A [16].
3. BERBERINE AND PARKINSON’S DISEASE
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disorder, which affects approximately 7 million people worldwide [90-92]. There are four cardinal motor symptoms of PD that can be grouped under the acronym TRAP: Tremor at rest, Rigidity, Akinesia (or bradykinesia) and Postural instability [93, 94]. In addition, PD can also give rise to several non-motor dysfunctions that include hyposmia, cognitive difficulties, autonomic disturbances and sleep disorders [95, 96]. It is suggested that the primary symptoms of PD result from the selective loss of dopaminergic neurons in the substantia nigra and the formation of Lewy bodies in many of the remaining neurons due to an abnormal accumulation of alpha-synuclein protein [97-105]. Although we have gained a relatively large amount of knowledge on the pathophysiology of PD, the etiology of the disease remains elusive [106-108]. Due to poor understanding of its pathogenesis, there is no treatment to halt or reverse the progression of PD [106-108].
Most of the current therapies are limited to symptomatic relief of the disease by restoring levels of dopamine in the brain and, in this respect, levodopa, dopamine agonists and monoamine oxidase B (MAO-B) inhibitors represent first-line pharmacological options [109-113]. Levodopa is the metabolic precursor of dopamine and it can cross the blood–brain barrier, whereas dopamine itself cannot [114]. Once entered the central nervous system, levodopa can be taken up by dopaminergic neurons and converted into dopamine by 3,4-Dihydroxyphenylalanine (DOPA) decarboxylase [114]. The administration of levodopa temporarily restores striatal dopaminergic neurotransmission and alleviates symptoms of PD [115]. This alleviation can also be accomplished by dopamine agonists, compounds that directly activate postsynaptic striatal dopamine receptors, thus obviating the need for dopamine production [116]. Monoamine oxidases (MAO) are a family of mitochondrial enzymes, which catalyze the oxidative deamination of monoamine neurotransmitters such as dopamine and serotonin [117-120]. MAO consists of two different subtypes, MAO-A and MAO-B, which are coded by two distinct genes and have different substrate and inhibitor specificity [118, 119]. In the striatum, the predominant form of MAO is MAO-B, which constitutes about 80% of the total MAO activity in the human brain [118, 119]. Since MAO-B breaks down dopamine, inhibiting it will prolong the action of dopamine and improve the symptoms of PD [120-122]. Indeed, MAO-B inhibitors, such as selegiline and rasagaline, have been shown to be effective in treating motor symptoms and delay the need for levodopa in early PD [123-128]. However, due to the undesirable adverse effects of the chemically synthesised drugs, searching for natural product-based MAO-B inhibitors would be of great value for the safe treatment of PD [129].
3.1. Berberine Reduces Degradation of Dopamine and Prevents Neurotoxins Induced Dopaminergic Neuronal Death
Inhibition of MAO-A has been related to antidepressant activity, whereas inhibition of MAO-B has been proven to delay onset, or alleviate symptoms of PD [130]. Recent studies have found that berberine exhibits inhibitory activity on both MAO-A and MAO-B [23, 131-133]. Lee et al. evaluated the inhibitory effect of berberine on MAO-B and oberserved an IC50 value of 98.2 μM [131]. Similar results were obtained by Castillo and colleagues using the conventional Benzylamine (substrate) method and an alternative LED fluorescence (without substrate) method, reporting IC50 values of 98.4 μM and 90 μM, respectively [132]. Moreover, to elucidate the molecular basis of berberine’s inhibitory effects against MAO-B, Ji and coworkers investigated the binding mode of berberine with MAO-B by means of docking simulations [23]. The theoretical binding constant of berberine to MAO-B was estimated to be 66 μM, which was very close to the experimental value [23]. In addition, their results indicate that the binding of berberine to MAO-B is mainly driven by hydrophobic interactions rather than electrostatics interactions [23]. These findings indicate that berberine may reduce degradation of dopamine and alleviate symptoms of PD, at least partly, through its inhibitory effect on MAO-B.
Several in vitro and in vivo studies enable an overview of possibilities and limitations of berberine as a therapeutic agent against PD [15, 134]. Using an in vitro model of PD, Bae et al. have demonstrated that berberine protects dopaminergic neurons from cell death induced by the parkinsonism-inducing neurotoxin 6-hydroxydopamine (6-OHDA) [134]. In addition, Kim and colleagues investigated the in vivo neuroprotective effects of berberine, with the use of the 1-methyl-4-phenyl-1,2,3,6-trerahydropyridine (MPTP) mouse model of PD that recapitulates several features of the disease [15]. Their studies have suggested that berberine can improve motor balance and coordination by preventing MPTP-induced dopaminergic neuronal death in the substantia nigra and fiber loss in the striatum [15]. They have also demonstrated that berberine can alleviate MPTP-induced short-term memory impairment by inhibiting apoptosis in the hippocampus [15]. Collectively, these studies have shed some light on the possibility of utilizing berberine as a therapeutic agent against PD [15, 134]. It is worth mentioning that, although berberine is generally considered safe for human use, studies by Shin et al. from South Korea have raised concerns regarding the adverse effect of chronic L-DOPA-berberine combination therapy in a rat model of PD [135]. In a previous study, they also claimed that berberine aggravated 6-OHDA-induced cytotoxicity in PC12 cells, and led to the degeneration of dopaminergic neuronal cells in the substantia nigra of 6-OHDA-lesioned rats [136]. Although their findings are inconsistent with other studies, which have suggested that berberine protects 6-OHDA or MPTP-induced dopaminergic neuronal loss and suppresses hippocampal apoptosis [15, 134], further studies are required to evaluate the potential adverse effects and limitations of single or combined use of berberine for the treatment of PD.
4. BERBERINE AND HUNTINGTON'S DISEASE
Huntington’s disease (HD) is a hereditary neurodegenerative disorder, which is characterized by involuntary motor movement, cognitive impairments and psychiatric disturbances [137, 138]. It is estimated that the worldwide prevalence of HD is 5-10 cases per 100,000 persons, but varies greatly across ethnic groups and geographical locations, with the highest incidence in Western Europe and the lowest in Asia [139]. The cause of the disease has been unknown until fairly recently [140-142]. It is now clear that HD is caused mainly by an autosomal dominant mutation in either of an individual's two copies of a gene called Huntingtin (HTT), which is located on the short (p) arm of chromosome 4 at position 16.3 [143-146]. HTT is variable in its structure, as it has many polymorphisms that harbor different number of CAG trinucleotide repeats in exon 1 [147]. Since the CAG trinucleotide is the codon for the amino acid glutamine, a string of CAG results in the production of a polyglutamine (polyQ) tract [147]. The length of the glutamine repeats in normal persons varies from 6 to 35 [148]. Expansion of the polyQ tract to 36 or more glutamine repeats causes HTT protein misfolding, aggregation and neuronal death, resulting in Huntington's disease [149]. In addition, the age of HD onset was found to be inversely correlated with the length of the polyQ tract [150, 151]. Once above the pathogenic threshold, there is a strong negative correlation between the polyQ tract length and the age of HD onset [150, 151].
It is noteworthy that recent studies have suggested a direct correlation between HD progression and defects in the autophagy-lysosomal pathway [152-154]. Macroautophagy (generally referred to as autophagy) is an essential homeostatic process responsible for the degradation of larger structures such as organelles and protein aggregates that the ubiquitin-proteasome system (UPS) is unable to handle [155-156]. During this process, targeted cytoplasmic constituents are sequestered in double-membrane structures called autophagosomes, which then fuse with endosomes and lysosomes, allowing their contents to be degraded and recycled [155-156]. It has been demonstrated that autophagy is a major clearance pathway for the removal of intracytosolic aggregate-prone proteins associated with AD (mutant tau) and PD (mutant a-synuclein) [157-165]. Moreover, recent studies suggest that the autophagy-lysosomal pathway also plays a vital role in the clearance of aggregate-prone mutant form of huntingtin (polyQ-HTT) [152-154]. Multiple independent lines of evidence show the impairment of autophagy during the progression of HD [152-154]. Induction of autophagy enhances the clearance of polyQ-HTT aggregates and alleviates mutant huntingtin-mediated toxicity in Drosophila and mouse models of HD [166-168]. These findings provide a strong rationale for the development of autophagy modulators as a novel strategy to treat or retard progression of HD and related protein misfolding disorders [169]. Interestingly, berberine has turned out to be a promising autophagy activator. Using a transgenic HD mouse model, Jiang and colleagues showed that berberine could significantly trigger autophagy to remove polyQ-HTT aggregates and, as a result, remarkably ameliorated the neurological phenotypes in the HD mice [31].
5. GENERAL NEUROPROTECTIVE ROLE OF BERBERINE
5.1. Berberine Triggers Autophagy and Enhances the Clearance of Toxic Aggregated Proteins
Recently, berberine has been found to be a potent trigger of autophagy in a wide range of cell types including liver, kidney, breast, myocardium, macrophages, lung, as well as neuron cells [31, 32, 170-189]. Berberine is effective in promoting autophagy, which can attenuate left ventricular remodeling, cardiac dysfunction and cardiomyocyte apoptosis in a rat model of cardiac hypertrophy [175, 176]. By upregulating the levels of autophagy, berberine can also inhibit oxidized low-density lipoprotein (ox-LDL)-induced inflammation in macrophages and provide a therapeutic potential in the treatment atherosclerosis [177-180]. In addition, berberine was reported to stimulate autophagy by inhibiting PI3K/Akt-mTOR signaling cascade, and significantly reduced the degree of pulmonary fibrosis in animal models [181]. Moreover, a growing body of evidence has suggested that berberine also possesses anti-tumor properties and can enhance the effect of certain chemotherapeutic agents and radiotherapy on cancer cells, not only via apoptosis but also through the induction of autophagy [182-189].
In a most recent study, Jiang and colleagues examined the effects of berberine on the accumulation of toxic polyQ-HTT in cellular and an animal model of HD [31]. Their results showed that berberine could significantly upregulate autophagy to remove polyQ-HTT both in HEK293 cells transfected with a mutant HTT containing 120 CAG repeats in exon1, and in a transgenic HD mouse model expressing mutant HTT containing 82 glutamine repeats in the polyQ tract [31]. The clearance of polyQ-HTT aggregates by autophagy significantly ameliorates the neurological phenotypes in the HD mice, with greatly improved rotarod performance, muscle strength, motor coordination and life span [31].
What is more, using APP/tau/PS1 triple-transgenic mouse model of Alzheimer's disease, Huang et al. have showed that berberine also triggers autophagy and facilitates Aβ clearance in the hippocampus of AD mice. They have further showed that berberine enhances autophagy in the brains of triple-transgenic AD mice possibly through the induction of the class III PI3K/Beclin-1 pathway [32]. Interestingly, although a great many studies have indicated that autophagy can be triggered by activating of the AMPK/mTOR pathway, Zhang et al. have recently demonstrated that berberine reduces the expression of BACE1 and inhibits the generation of Aβ just via activating AMPK, with no effect on mTOR signaling [79].
These findings should promote further investigations aimed at elucidating the therapeutic role of berberine in other neurodegenerative diseases that are also caused by misfolded proteins [32, 174, 178].
5.2. Berberine Inhibits Neuroinflammation
Neuroinflammation is inflammation of the nervous tissue. It may be initiated by a variety of factors, including traumatic brain injury, microbial infections, toxic metabolites, autoimmunity and aging [190-192]. Despite different triggering events, a common feature of neuroinflammation is chronic activation of microglia and astrocytes [193, 194]. In the diseased central nervous system (CNS), microglia and astrocytes are dramatically activated and secrete various pro-inflammatory mediators and neurotoxic cytokines that will cause further damage in neuronal cells, thus creating a vicious self-propagating cycle of neuronal damage and neuroinflammation and contributing to the chronic progression of neurodegenerative diseases [195-197]. It has been hypothesized that inhibiting neuroinflammation could diminish neuronal loss, and this hypothesis is supported by observations that long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) is associated with a significantly lower incidence of neurodegenerative diseases [198-200].
Recently, it was demonstrated that berberine could suppress neuroinflammation, and could be considered as a drug candidate for inflammatory-related CNS disorders [201-205]. Nam et al. have found that berberine was effective at inhibiting inflammatory activation of rat brain microglia by suppression of pro-inflammatory cytokines, nitric oxide (NO), and nuclear factor-kappaB (NF-κB) activity [201]. In addition, berberine was also shown to exert significant inhibitory effects on the LPS- or interferon-g-induced production of inflammatory mediators and cytokines, such as inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), Tumor necrosis factor-a (TNF-a), Interleukin(IL)-1β, and IL-6 in BV-2 microglia cells, through activation of the AMPK signalling pathway [202, 203]. In consistent with these findings, Jia et al. have found that berberine treatment suppressed Aβ-induced inflammatory responses in murine primary microglial cells and BV2 microglia cell line by inhibiting the mitogen-activated protein kinase (MAPK) and the NF-κB signalling pathways [204]. In a recent study, Chen and coworkers has shown that berberine could also attenuate traumatic brain injury (TBI)-induced neuronal damage by inhibiting leukocyte infiltration, microglial activation, and the production of inflammatory mediators [205].
5.3. Berberine Ameliorates Oxidative Stress
Accumulating data shows that oxidative stress plays a crucial role in the onset and progression of neurodegeneration [206-208]. Under the circumstance of oxidative stress, excessive amounts of reactive oxygen species (ROS) and/or reactive nitrogen species (RNS) cause lipid peroxidation, protein oxidation, protein nitration, and glycoloxidation, resulting in plasma membrane damage, cytoskeletal disruption as well as nucleic acids mutations in the nervous tissue [209, 210]. In recent years, increasing attention has been paid to the use of antioxidant compounds to combat neurodegenerative diseases [211-214].
It was reported that berberine possesses potent and diverse antioxidant activities [215-226]. Berberine can effectively reduce intracellular superoxide levels in Lipopolysaccharide (LPS)-stimulated human monocyte-derived macrophages by inhibiting the expression of gp91phox, the catalytic subunit of NADPH oxidase [227]. Berberine has also been shown to reduce NO production in LPS-treated murine macrophages by downregulating the expression of iNOS [228]. In addition, it was found that berberine can also restore the activities of several antioxidant enzymes both in vitro and in vivo, including superoxide dismutase (SOD), catalase, glutathione S-transferase (GST), and glutathione peroxidase (GSH-Px) [229, 230].
Using NSC34, a motor neuron-like cell line, Hsu et al. investigated the in vitro neuroprotective effects of berberine. They found that berberine protected NSC34 cells against hydrogen peroxide induced cytotoxicity not only by attenuating ROS production and restoreing GSH and SOD activity, but also by activating anti-oxidant proteins nuclear erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) [231]. They further explored the protective mechanism of berberine on high glucose-induced oxidative stress and neurite damage of SH-SY5Y cells. Berberine was found to effectively ameliorate high glucose-induced ROS production, nuclear condensation and neuronal cell death, at least in parts, by activating the PI3K/Akt/Nrf2/HO-1-dependent cytoprotective and antioxidant pathways [232]. All the above mentioned studies have established berberine as a potent anti-oxidant molecule, therefore, may have therapeutic effect on neurodegenerative diseases.
5.4. Berberine Protects Neuronal Cells Against Apoptosis
Although a large number of studies have reported that berberine can induce apoptosis in various cancer cells [233, 234], accumulating evidence indicates that berberine plays an important role in inhibiting oxidative stress, high glucose stress or ischemia/reperfusion-induced apoptosis in different types of normal cells [235-243], particularly neuronal cells [244-256].
Using the MPTP mouse model of PD, Kim et al. have demonstrated that berberine upregulates the anti-apoptotic protein Bcl-2 and downregulates the pro-apoptotic protein Bax [246]. As a result, it enhanced motor balance and coordination by preventing dopaminergic neuronal death in the substantia nigra of mice with PD, and ameliorated short-term memory impairment by inhibiting neuronal injury and apoptosis in the hippocampus [246]. Berberine has also been shown to attenuate apoptosis of pyramidal neurons in the CA1 area, and ameliorate learning and memory impairment of streptozotocin (STZ)-diabetic rats [247].
Furthermore, berberine exhibits neuroprotective effects against ischemic brain injury by inhibiting neuronal apoptosis in both in vitro and in vivo models [248-255]. It has been reported that berberine improved neurological outcome and attenuated ischemia/reperfusion (I/R)-induced neuron death by suppressing intracellular ROS generation, or by modulating of PI3K/Akt/GSK3β and JNK signaling pathways, decreasing hypoxia-inducible factor 1α (HIF-1α), caspase 9, caspase 3 activity and increasing Bcl-2/Bax ratio, and as a result, inhibited caspase-dependent and -independent apoptosis pathways [248-255]. In addition, a recent study by Zhang et al. has suggested that berberine could also act as a preconditioning stimulus to ameliorate hypoxia/ischemia-induced cerebral damage via triggering prosurvival autophagy and suppressing anoxia-induced apoptosis [256]. These findings, therefore, provide a novel perspective for berberine to combat neurodegenerative diseases.
CONCLUSION AND FUTURE PERSPECTIVES
Neurodegenerative diseases are among the most serious health problems affecting millions of people worldwide [257]. Such diseases are characterized by a progressive degeneration and/or death of neurons in the central nervous system [258]. Currently, there are no therapeutic approaches to cure or even halt the progression of neurodegenerative diseases [259]. During the last two decades, much attention has been paid to the neuroprotective and anti-neurodegenerative activities of compounds isolated from natural products with high efficacy and low toxicity [260-263]. Accumulating evidence indicates that berberine, an isoquinoline alkaloid isolated from traditional Chinese medicinal herbs may act as a promising anti-neurodegenerative agent.
Here, we reviewed the current state of knowledge regarding the therapeutic potential of berberine against neurodegenerative diseases, with a focus on the molecular mechanisms that underlie its effects on Alzheimer’s, Parkinson’s and Huntington’s diseases. In summary, previous studies have clearly showed that berberine exerts neuroprotective effects primarily by inhibiting the activity of several important pathogenic enzymes (such as AChE, BChE, BACE1, GSK-3 and MAO-B), ameliorating intracellular oxidative stress, attenuating neuroinflammation and inhibiting apoptotic cell death. Berberine can also combat neurodegenerative diseases by accelerating the clearance of toxic misfolded and aggregated proteins in neuronal cells through induction of autophagy (Fig. 2). In addition, berberine has been tested clinically in the treatment of diarrhea, metabolic disorders, cardiovascular diseases, type 2 diabetes mellitus, polycystic ovary syndrome, hypercholesterolemia, hyperlipemia, and non-alcoholic fatty liver disease for many years, and it is generally considered to be effective and safe at doses used in clinical situations, with no cytotoxic, genotoxic or mutagenic activity being detected [264-275]. Furthermore, berberine can be administered orally and it can easily pass through the blood-brain barrier [276].
Fig. (2).
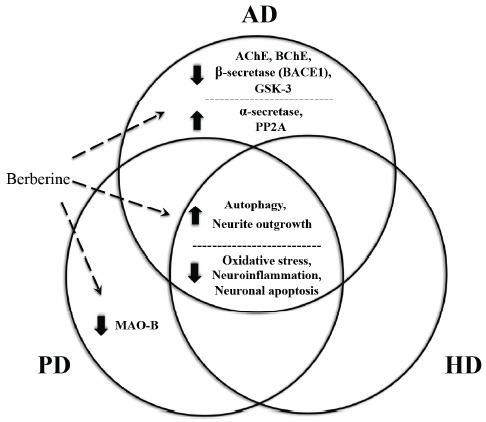
Common and distinct molecular mechanisms underlying berberine’s capability of combating AD, PD and HD. Excessive oxidative stress, neuroinflammation, apoptosis and insufficient autophagy are common pathologic features that are shared by neurodegenerative diseases and can be targeted by berberine in common. In addition, berberine can inhibit the activity of several important but distinct pathogenic enzymes in AD, PD and HD, including AChE, BChE, BACE1, GSK-3 and MAO-B.
Although clinical trials of berberine have been performed in several diseases including, diarrhea, metabolic syndrome, diabetes, congestive heart failure, and radiation-induced acute intestinal symptoms and these studies reinforce its safety profile with zero death rate and no or minor gastrointestinal adverse effects, clinical studies of berberine against CNS disorders are still lacking [277-281]. Extensive preclinical data summarized in this review should provide a convincing basis for conducting clinical trials of berberine against neurodegenerative diseases in the near future. On the other hand, the clinical application of berberine is greatly limited due to poor gastrointestinal absorption and bioavailability after oral administration, novel dosage forms of berberine that can overcome these barriers is needed. Recently, nanotechnology drug delivery approach of berberine has been shown to improve the drug pharmacokinetic performance and the intended target outcome [282-286]. Future studies should also be focused on testing different type of nanoparticles and nanoformulation of berberine to improve the bioavailability, pharmacological activities, efficacy and selectivity of berberine and/or its derivatives in clinical settings.
ACKNOWLEDGEMENTS
Dahua Fan and Liping Liu performed the literature review and wrote the manuscript under the advisement of Zhengzhi Wu and Meiqun Cao. Dahua Fan and Zhengzhi Wu graphed the illustrations and contributed to the content of the manuscript. Meiqun Cao edited the manuscript. All authors reviewed the manuscript before submission. All authors read and approved the final manuscript.
LIST OF ABBREVIATIONS
- 6-OHDA
6-hydroxydopamine
- AChE
Acetylcholinesterase
- AD
Alzheimer’s Disease
- ADI
Alzheimer's Disease International
- AMPK
AMP-Activated Protein Kinase
- APP
Amyloid-β Precursor Protein
- Aβ
Amyloid-β
- BACE1
β-site Amyloid Precursor Protein Cleaving Enzyme 1
- BChE
Butyrylcholinesterase
- CNS
Central Nervous System
- COX-2
Cyclooxygenase 2
- DOPA
3,4-Dihydroxyphenylalanine
- FDA
Food and Drug Administration
- GSH-Px
Glutathione Peroxidase
- GSK-3
Glycogen Synthase Kinase 3
- GST
Glutathione S-Transferase
- HD
Huntington’s Diseases
- HIF-1α
Hypoxia-Inducible Factor 1α
- HO-1
Heme Oxygenase-1
- HTT
Huntingtin
- IL
Interleukin
- iNOS
inducible Nitric Oxide Synthase
- LPS
Lipopolysaccharide
- MAO
Monoamine Oxidase
- MAPK
Mitogen-Activated Protein Kinase
- MPTP
1-Methyl-4-Phenyl-1,2,3,6-Trerahydropyridine
- NF-κB
Nuclear Factor-kappaB
- NO
Nitric Oxide
- Nrf2
Nuclear Erythroid 2-Related Factor 2
- NSAIDs
Non-Steroidal Anti-Inflammatory Drugs
- ox-LDL
Oxidized Low-Density Lipoprotein
- PD
Parkinson’s Diseases
- polyQ
polyglutamine
- PP2A
protein Phosphatase 2A
- RNS
Reactive Nitrogen Species
- ROS
Reactive Oxygen Species
- SOD
Superoxide Dismutase
- STZ
Streptozotocin
- TCM
Chinese Traditional Medicine
- TNF-a
Tumor Necrosis Factor-a
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This review summarizes published studies using human and animal material. Research studies in this review were approved by the appropriate ethics committees.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
FUNDING
The authors received funding from the National Natural Science Foundation of China (No. 81603671, 81574038, 81503415), the China Postdoctoral Science Foundation Grant (2016M600709) and the Science and Technology Innovation Foundation of Shenzhen (JCYJ20160428105749954, JCYJ20150403101028198, JCYJ20160422152408705).
REFERENCES
- 1.Kong W., Wei J., Abidi P., Lin M., Inaba S., Li C., Wang Y., Wang Z., Si S., Pan H., Wang S., Wu J., Wang Y., Li Z., Liu J., Jiang J.D. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004;10(12):1344–1351. doi: 10.1038/nm1135. [http://dx.doi.org/10.1038/nm1135]. [PMID: 15531889]. [DOI] [PubMed] [Google Scholar]
- 2.Imanshahidi M., Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother. Res. 2008;22(8):999–1012. doi: 10.1002/ptr.2399. [http://dx.doi.org/10.1002/ptr.2399]. [PMID: 18618524]. [DOI] [PubMed] [Google Scholar]
- 3.Liu B., Li W., Chang Y., Dong W., Ni L. Extraction of berberine from rhizome of Coptis chinensis Franch using supercritical fluid extraction. J. Pharm. Biomed. Anal. 2006;41(3):1056–1060. doi: 10.1016/j.jpba.2006.01.034. [http://dx.doi.org/10.1016/j.jpba.2006.01.034]. [PMID: 16500064]. [DOI] [PubMed] [Google Scholar]
- 4.Lee B., Sur B., Shim I., Lee H., Hahm D.H. Phellodendron amurense and its major alkaloid compound, berberine ameliorates scopolamine-induced neuronal impairment and memory dysfunction in rats. Korean J. Physiol. Pharmacol. 2012;16(2):79–89. doi: 10.4196/kjpp.2012.16.2.79. [http://dx.doi.org/10.4196/kjpp.2012.16.2.79]. [PMID: 22563252]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gentry E.J., Jampani H.B., Keshavarz-Shokri A., Morton M.D., Velde D.V., Telikepalli H., Mitscher L.A., Shawar R., Humble D., Baker W. Antitubercular natural products: Berberine from the roots of commercial Hydrastis canadensis powder. Isolation of inactive 8-oxotetrahydrothalifendine, canadine, β-hydrastine, and two new quinic acid esters, hycandinic acid esters-1 and -2. J. Nat. Prod. 1998;61(10):1187–1193. doi: 10.1021/np9701889. [http://dx.doi.org/10.1021/np9701889]. [PMID: 9784149]. [DOI] [PubMed] [Google Scholar]
- 6.Imenshahidi M., Hosseinzadeh H. Berberis vulgaris and berberine: An update review. Phytother. Res. 2016;30(11):1745–1764. doi: 10.1002/ptr.5693. [http://dx.doi.org/10.1002/ptr.5693]. [PMID: 27528198]. [DOI] [PubMed] [Google Scholar]
- 7.Amritpal S., Sanjiv D., Navpreet K., Jaswinder S. Berberine: Alkaloid with wide spectrum of pharmacological activities. J. Nat. Prod. 2010;3:64–75. [Google Scholar]
- 8.Kumar A. Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015;761:288–297. doi: 10.1016/j.ejphar.2015.05.068. [http://dx.doi.org/10.1016/j.ejphar.2015.05.068]. [PMID: 26092760]. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A. Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015;761:288–297. doi: 10.1016/j.ejphar.2015.05.068. [http://dx.doi.org/10.1016/j.ejphar.2015.05.068]. [PMID: 26092760]. [DOI] [PubMed] [Google Scholar]
- 10.Ye M., Fu S., Pi R., He F. Neuropharmacological and pharmacokinetic properties of berberine: A review of recent research. J. Pharm. Pharmacol. 2009;61(7):831–837. doi: 10.1211/jpp/61.07.0001. [DOI] [PubMed] [Google Scholar]
- 11.Tan X.S., Ma J.Y., Feng R., Ma C., Chen W.J., Sun Y.P., Fu J., Huang M., He C.Y., Shou J.W., He W.Y., Wang Y., Jiang J.D. Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS One. 2013;8(10):e77969. doi: 10.1371/journal.pone.0077969. [http://dx.doi.org/10.1371/journal.pone.0077969]. [PMID: 24205048]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J., Yang J.Q., He B.C., Zhou Q.X., Yu H.R., Tang Y., Liu B.Z. Berberine and total base from rhizoma coptis chinensis attenuate brain injury in an aluminum-induced rat model of neurodegenerative disease. Saudi Med. J. 2009;30(6):760–766. [PMID: 19526156]. [PubMed] [Google Scholar]
- 13.Habtemariam S. The therapeutic potential of Berberis darwinii stem-bark: Quantification of berberine and in vitro evidence for Alzheimer’s disease therapy. Nat. Prod. Commun. 2011;6(8):1089–1090. [PMID: 21922905]. [PubMed] [Google Scholar]
- 14.Jiang H., Wang X., Huang L., Luo Z., Su T., Ding K., Li X. Benzenediol-berberine hybrids: Multifunctional agents for Alzheimer’s disease. Bioorg. Med. Chem. 2011;19(23):7228–7235. doi: 10.1016/j.bmc.2011.09.040. [http://dx.doi.org/10.1016/j.bmc.2011.09.040]. [PMID: 22041172]. [DOI] [PubMed] [Google Scholar]
- 15.Kim M., Cho K.H., Shin M.S., Lee J.M., Cho H.S., Kim C.J., Shin D.H., Yang H.J. Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson’s disease. Int. J. Mol. Med. 2014;33(4):870–878. doi: 10.3892/ijmm.2014.1656. [http://dx.doi.org/10.3892/ijmm.2014.1656]. [PMID: 24535622]. [DOI] [PubMed] [Google Scholar]
- 16.Liu X., Zhou J., Abid M.D., Yan H., Huang H., Wan L., Feng Z., Chen J. Berberine attenuates axonal transport impairment and axonopathy induced by Calyculin A in N2a cells. PLoS One. 2014;9(4):e93974. doi: 10.1371/journal.pone.0093974. [http://dx.doi.org/10.1371/journal.pone.0093974]. [PMID: 24713870]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee T., Heo H., Kim K.Y., Kim K.Y. Effect of berberine on cell survival in the developing rat brain damaged by MK-801. Exp. Neurobiol. 2010;19(3):140–145. doi: 10.5607/en.2010.19.3.140. [http://dx.doi.org/10.5607/en.2010.19.3.140]. [PMID: 22110353]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar A. Ekavali; Mishra, J.; Chopra, K.; Dhull, D.K. Possible role of P-glycoprotein in the neuroprotective mechanism of berberine in intracerebroventricular streptozotocin-induced cognitive dysfunction. Psychopharmacology (Berl.) 2016;233(1):137–152. doi: 10.1007/s00213-015-4095-7. [http://dx.doi.org/10.1007/s00213-015-4095-7]. [PMID: 26446867]. [DOI] [PubMed] [Google Scholar]
- 19.Kalalian-Moghaddam H., Baluchnejadmojarad T., Roghani M., Goshadrou F., Ronaghi A. Hippocampal synaptic plasticity restoration and anti-apoptotic effect underlie berberine improvement of learning and memory in streptozotocin-diabetic rats. Eur. J. Pharmacol. 2013;698(1-3):259–266. doi: 10.1016/j.ejphar.2012.10.020. [http://dx.doi.org/10.1016/j.ejphar.2012.10.020]. [PMID: 23099256]. [DOI] [PubMed] [Google Scholar]
- 20.Campisi A., Acquaviva R., Mastrojeni S., Raciti G., Vanella A., De Pasquale R., Puglisi S., Iauk L. Effect of berberine and Berberis aetnensis C. Presl. alkaloid extract on glutamate-evoked tissue transglutaminase up-regulation in astroglial cell cultures. Phytother. Res. 2011;25(6):816–820. doi: 10.1002/ptr.3340. [http://dx.doi.org/10.1002/ptr.3340]. [PMID: 21086546]. [DOI] [PubMed] [Google Scholar]
- 21.Hsu Y.Y., Tseng Y.T., Lo Y.C. Berberine, a natural antidiabetes drug, attenuates glucose neurotoxicity and promotes Nrf2-related neurite outgrowth. Toxicol. Appl. Pharmacol. 2013;272(3):787–796. doi: 10.1016/j.taap.2013.08.008. [http://dx.doi.org/10.1016/j.taap.2013.08.008]. [PMID: 23954465]. [DOI] [PubMed] [Google Scholar]
- 22.Shan W.J., Huang L., Zhou Q., Meng F.C., Li X.S. Synthesis, biological evaluation of 9-N-substituted berberine derivatives as multi-functional agents of antioxidant, inhibitors of acetylcholinesterase, butyrylcholinesterase and amyloid-β aggregation. Eur. J. Med. Chem. 2011;46(12):5885–5893. doi: 10.1016/j.ejmech.2011.09.051. [http://dx.doi.org/10.1016/j.ejmech.2011.09.051]. [PMID: 22019228]. [DOI] [PubMed] [Google Scholar]
- 23.Ji H.F., Shen L. Molecular basis of inhibitory activities of berberine against pathogenic enzymes in Alzheimer's disease. Sci. World J. 2012;2012 doi: 10.1100/2012/823201. [http://dx.doi.org/10.1100/2012/823201] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abd El-Wahab A.E., Ghareeb D.A., Sarhan E.E., Abu-Serie M.M., El Demellawy M.A. In vitro biological assessment of Berberis vulgaris and its active constituent, berberine: Antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement. Altern. Med. 2013;13:218. doi: 10.1186/1472-6882-13-218. [http://dx.doi.org/10.1186/1472-6882-13-218]. [PMID: 24007270]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mak S., Luk W.W., Cui W., Hu S., Tsim K.W., Han Y. Synergistic inhibition on acetylcholinesterase by the combination of berberine and palmatine originally isolated from Chinese medicinal herbs. J. Mol. Neurosci. 2014;53(3):511–516. doi: 10.1007/s12031-014-0288-5. [http://dx.doi.org/10.1007/s12031-014-0288-5]. [PMID: 24793543]. [DOI] [PubMed] [Google Scholar]
- 26.Durairajan S.S., Liu L.F., Lu J.H., Chen L.L., Yuan Q., Chung S.K., Huang L., Li X.S., Huang J.D., Li M. Berberine ameliorates β-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer’s disease transgenic mouse model. Neurobiol. Aging. 2012;33(12):2903–2919. doi: 10.1016/j.neurobiolaging.2012.02.016. [http://dx.doi.org/10.1016/j.neurobiolaging.2012.02.016]. [PMID: 22459600]. [DOI] [PubMed] [Google Scholar]
- 27.Jia L., Liu J., Song Z., Pan X., Chen L., Cui X., Wang M. Berberine suppresses amyloid-beta-induced inflammatory response in microglia by inhibiting nuclear factor-kappaB and mitogen-activated protein kinase signalling pathways. J. Pharm. Pharmacol. 2012;64(10):1510–1521. doi: 10.1111/j.2042-7158.2012.01529.x. [http://dx.doi.org/10.1111/j.2042-7158.2012.01529.x]. [PMID: 22943182]. [DOI] [PubMed] [Google Scholar]
- 28.Panahi N., Mahmoudian M., Mortazavi P., Hashjin G.S. Effects of berberine on β-secretase activity in a rabbit model of Alzheimer’s disease. Arch. Med. Sci. 2013;9(1):146–150. doi: 10.5114/aoms.2013.33354. [http://dx.doi.org/10.5114/aoms.2013.33354]. [PMID: 23516061]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu F., Qian C. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1beta and inducible nitric oxide synthase in the rat model of Alzheimer’s disease. BMC Neurosci. 2006;7:78. doi: 10.1186/1471-2202-7-78. [http://dx.doi.org/10.1186/1471-2202-7-78]. [PMID: 17137520]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C.C., Hung T.H., Lee C.Y., Wang L.F., Wu C.H., Ke C.H., Chen S.F. Berberine protects against neuronal damage via suppression of glia-mediated inflammation in traumatic brain injury. PLoS One. 2014;9(12):e115694. doi: 10.1371/journal.pone.0115694. [http://dx.doi.org/10.1371/journal.pone.0115694]. [PMID: 25546475]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang W., Wei W., Gaertig M.A., Li S., Li X.J. Therapeutic effect of berberine on Huntington’s disease transgenic mouse model. PLoS One. 2015;10(7):e0134142. doi: 10.1371/journal.pone.0134142. [http://dx.doi.org/10.1371/journal.pone.0134142]. [PMID: 26225560]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang M., Jiang X., Liang Y., Liu Q., Chen S., Guo Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp. Gerontol. 2017;91:25–33. doi: 10.1016/j.exger.2017.02.004. [http://dx.doi.org/10.1016/j.exger.2017.02.004]. [PMID: 28223223]. [DOI] [PubMed] [Google Scholar]
- 33.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C., Gamst A., Holtzman D.M., Jagust W.J., Petersen R.C., Snyder P.J., Carrillo M.C., Thies B., Phelps C.H. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [http://dx.doi.org/10.1016/j.jalz.2011.03.008]. [PMID: 21514249]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K., DeKosky S.T., Gauthier S., Selkoe D., Bateman R., Cappa S., Crutch S., Engelborghs S., Frisoni G.B., Fox N.C., Galasko D., Habert M.O., Jicha G.A., Nordberg A., Pasquier F., Rabinovici G., Robert P., Rowe C., Salloway S., Sarazin M., Epelbaum S., de Souza L.C., Vellas B., Visser P.J., Schneider L., Stern Y., Scheltens P., Cummings J.L. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–629. doi: 10.1016/S1474-4422(14)70090-0. [http://dx.doi.org/10.1016/S1474-4422(14)70090-0]. [PMID: 24849862]. [DOI] [PubMed] [Google Scholar]
- 35.2017 Alzheimer’s disease facts and figures. Alzheimers Dement. 2017;13(4):325–373. [http://dx.doi.org/10.1016/j.jalz.2017.02.001]. [Google Scholar]
- 36.Jiang T., Yu J.T., Tian Y., Tan L. Epidemiology and etiology of Alzheimer’s disease: from genetic to non-genetic factors. Curr. Alzheimer Res. 2013;10(8):852–867. doi: 10.2174/15672050113109990155. [http://dx.doi.org/10.2174/15672050113109990155]. [PMID: 23919770]. [DOI] [PubMed] [Google Scholar]
- 37.Talwar P., Sinha J., Grover S., Rawat C., Kushwaha S., Agarwal R., Taneja V., Kukreti R. Dissecting complex and multifactorial nature of alzheimer’s disease pathogenesis: A clinical, genomic, and systems biology perspective. Mol. Neurobiol. 2016;53(7):4833–4864. doi: 10.1007/s12035-015-9390-0. [http://dx.doi.org/10.1007/s12035-015-9390-0]. [PMID: 26351077]. [DOI] [PubMed] [Google Scholar]
- 38.Coyle J.T., Price D.L., DeLong M.R. Alzheimer’s disease: A disorder of cortical cholinergic innervation. Science. 1983;219(4589):1184–1190. doi: 10.1126/science.6338589. [http://dx.doi.org/10.1126/science.6338589]. [PMID: 6338589]. [DOI] [PubMed] [Google Scholar]
- 39.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [http://dx.doi.org/10.1126/science.1072994]. [PMID: 12130773]. [DOI] [PubMed] [Google Scholar]
- 40.Grundke-Iqbal I., Iqbal K., Tung Y.C., Quinlan M., Wisniewski H.M., Binder L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA. 1986;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [http://dx.doi.org/10.1073/pnas.83.13.4913]. [PMID: 3088567]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ittner L.M., Götz J. Amyloid-β and tau--a toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011;12(2):65–72. doi: 10.1038/nrn2967. [http://dx.doi.org/10.1038/nrn2967]. [PMID: 21193853]. [DOI] [PubMed] [Google Scholar]
- 42.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., Finch C.E., Frautschy S., Griffin W.S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I.R., McGeer P.L., O’Banion M.K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F.L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [http://dx.doi.org/10.1016/S0197-4580(00)00124-X]. [PMID: 10858586]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., Herrup K., Frautschy S.A., Finsen B., Brown G.C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G.C., Town T., Morgan D., Shinohara M.L., Perry V.H., Holmes C., Bazan N.G., Brooks D.J., Hunot S., Joseph B., Deigendesch N., Garaschuk O., Boddeke E., Dinarello C.A., Breitner J.C., Cole G.M., Golenbock D.T., Kummer M.P. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [http://dx.doi.org/10.1016/S1474-4422(15)70016-5]. [PMID: 25792098]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [http://dx.doi.org/10.1038/nature05292]. [PMID: 17051205]. [DOI] [PubMed] [Google Scholar]
- 45.Puglielli L., Tanzi R.E., Kovacs D.M. Alzheimer’s disease: The cholesterol connection. Nat. Neurosci. 2003;6(4):345–351. doi: 10.1038/nn0403-345. [http://dx.doi.org/10.1038/nn0403-345]. [PMID: 12658281]. [DOI] [PubMed] [Google Scholar]
- 46.Di Paolo G., Kim T.W. Linking lipids to Alzheimer’s disease: Cholesterol and beyond. Nat. Rev. Neurosci. 2011;12(5):284–296. doi: 10.1038/nrn3012. [http://dx.doi.org/10.1038/nrn3012]. [PMID: 21448224]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anand R., Gill K.D., Mahdi A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology, 2014;76(Pt A):27–50. doi: 10.1016/j.neuropharm.2013.07.004. [http://dx.doi.org/10.1016/j.neuropharm.2013.07.004] [PMID: 23891641]. [DOI] [PubMed] [Google Scholar]
- 48.Guzior N., Wieckowska A., Panek D., Malawska B. Recent development of multifunctional agents as potential drug candidates for the treatment of Alzheimer’s disease. Curr. Med. Chem. 2015;22(3):373–404. doi: 10.2174/0929867321666141106122628. [http://dx.doi.org/10.2174/0929867321666141106122628]. [PMID: 25386820]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.León R., Garcia A.G., Marco-Contelles J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med. Res. Rev. 2013;33(1):139–189. doi: 10.1002/med.20248. [http://dx.doi.org/10.1002/med.20248]. [PMID: 21793014]. [DOI] [PubMed] [Google Scholar]
- 50.Agis-Torres A., Sölhuber M., Fernandez M., Sanchez-Montero J.M. Multi-target-directed ligands and other therapeutic strategies in the search of a real solution for Alzheimer’s disease. Curr. Neuropharmacol. 2014;12(1):2–36. doi: 10.2174/1570159X113116660047. [http://dx.doi.org/10.2174/1570159X113116660047]. [PMID: 24533013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dias K.S., Viegas C., Jr Multi-target directed drugs: a modern approach for design of new drugs for the treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2014;12(3):239–255. doi: 10.2174/1570159X1203140511153200. [http://dx.doi.org/10.2174/1570159X1203140511153200]. [PMID: 24851088]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosini M., Simoni E., Caporaso R., Minarini A. Multitarget strategies in Alzheimer’s disease: Benefits and challenges on the road to therapeutics. Future Med. Chem. 2016;8(6):697–711. doi: 10.4155/fmc-2016-0003. [http://dx.doi.org/10.4155/fmc-2016-0003]. [PMID: 27079260]. [DOI] [PubMed] [Google Scholar]
- 53.Blusztajn J.K., Wurtman R.J. Choline and cholinergic neurons. Science. 1983;221(4611):614–620. doi: 10.1126/science.6867732. [http://dx.doi.org/10.1126/science.6867732]. [PMID: 6867732]. [DOI] [PubMed] [Google Scholar]
- 54.Tuček S. Regulation of acetylcholine synthesis in the brain. J. Neurochem. 1985;44(1):11–24. doi: 10.1111/j.1471-4159.1985.tb07106.x. [http://dx.doi.org/10.1111/j.1471-4159.1985.tb07106.x]. [PMID: 3880580]. [DOI] [PubMed] [Google Scholar]
- 55.Arendt T., Brückner M.K., Lange M., Bigl V. Changes in acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease resemble embryonic development--a study of molecular forms. Neurochem. Int. 1992;21(3):381–396. doi: 10.1016/0197-0186(92)90189-x. [http://dx.doi.org/10.1016/0197-0186(92)90189-X]. [PMID: 1303164]. [DOI] [PubMed] [Google Scholar]
- 56.Talesa V.N. Acetylcholinesterase in Alzheimer’s disease. Mech. Ageing Dev. 2001;122(16):1961–1969. doi: 10.1016/s0047-6374(01)00309-8. [http://dx.doi.org/10.1016/S0047-6374(01)00309-8]. [PMID: 11589914]. [DOI] [PubMed] [Google Scholar]
- 57.Darvesh S., Hopkins D.A., Geula C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003;4(2):131–138. doi: 10.1038/nrn1035. [http://dx.doi.org/10.1038/nrn1035]. [PMID: 12563284]. [DOI] [PubMed] [Google Scholar]
- 58.Enz A., Amstutz R., Boddeke H., Gmelin G., Malanowski J. Brain selective inhibition of acetylcholinesterase: A novel approach to therapy for Alzheimer’s disease. Prog. Brain Res. 1993;98:431–438. doi: 10.1016/s0079-6123(08)62429-2. [DOI] [PubMed] [Google Scholar]
- 59.Lane R.M., Kivipelto M., Greig N.H. Acetylcholinesterase and its inhibition in Alzheimer disease. Clin. Neuropharmacol. 2004;27(3):141–149. doi: 10.1097/00002826-200405000-00011. [http://dx.doi.org/10.1097/00002826-200405000-00011]. [PMID: 15190239]. [DOI] [PubMed] [Google Scholar]
- 60.Muñoz-Torrero D. Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer’s disease. Curr. Med. Chem. 2008;15(24):2433–2455. doi: 10.2174/092986708785909067. [http://dx.doi.org/10.2174/092986708785909067]. [PMID: 18855672]. [DOI] [PubMed] [Google Scholar]
- 61.Greig N.H., Utsuki T., Ingram D.K., Wang Y., Pepeu G., Scali C., Yu Q.S., Mamczarz J., Holloway H.W., Giordano T., Chen D., Furukawa K., Sambamurti K., Brossi A., Lahiri D.K. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA. 2005;102(47):17213–17218. doi: 10.1073/pnas.0508575102. [http://dx.doi.org/10.1073/pnas.0508575102]. [PMID: 16275899]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ritchie C.W., Ames D., Clayton T., Lai R. Metaanalysis of randomized trials of the efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer disease. Am. J. Geriatr. Psychiatry. 2004;12(4):358–369. doi: 10.1176/appi.ajgp.12.4.358. [http://dx.doi.org/10.1097/00019442-200407000-00003]. [PMID: 15249273]. [DOI] [PubMed] [Google Scholar]
- 63.Kaduszkiewicz H., Zimmermann T., Beck-Bornholdt H.P., van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ. 2005;331(7512):321–327. doi: 10.1136/bmj.331.7512.321. [http://dx.doi.org/10.1136/bmj.331.7512.321]. [PMID: 16081444]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hansen R.A., Gartlehner G., Webb A.P., Morgan L.C., Moore C.G., Jonas D.E. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. Clin. Interv. Aging. 2008;3(2):211–225. [PMID: 18686744]. [PMC free article] [PubMed] [Google Scholar]
- 65.Huang L., Luo Z., He F., Lu J., Li X. Synthesis and biological evaluation of a new series of berberine derivatives as dual inhibitors of acetylcholinesterase and butyrylcholinesterase. Bioorg. Med. Chem. 2010;18(12):4475–4484. doi: 10.1016/j.bmc.2010.04.063. [http://dx.doi.org/10.1016/j.bmc.2010.04.063]. [PMID: 20471843]. [DOI] [PubMed] [Google Scholar]
- 66.Mak S., Luk W.W., Cui W., Hu S., Tsim K.W., Han Y. Synergistic inhibition on acetylcholinesterase by the combination of berberine and palmatine originally isolated from Chinese medicinal herbs. J. Mol. Neurosci. 2014;53(3):511–516. doi: 10.1007/s12031-014-0288-5. [http://dx.doi.org/10.1007/s12031-014-0288-5]. [PMID: 24793543]. [DOI] [PubMed] [Google Scholar]
- 67.Jung H.A., Min B.S., Yokozawa T., Lee J.H., Kim Y.S., Choi J.S. Anti-Alzheimer and antioxidant activities of Coptidis Rhizoma alkaloids. Biol. Pharm. Bull. 2009;32(8):1433–1438. doi: 10.1248/bpb.32.1433. [http://dx.doi.org/10.1248/bpb.32.1433]. [PMID: 19652386]. [DOI] [PubMed] [Google Scholar]
- 68.Vassar R., Bennett B.D., Babu-Khan S., Kahn S., Mendiaz E.A., Denis P., Teplow D.B., Ross S., Amarante P., Loeloff R., Luo Y., Fisher S., Fuller J., Edenson S., Lile J., Jarosinski M.A., Biere A.L., Curran E., Burgess T., Louis J.C., Collins F., Treanor J., Rogers G., Citron M. β-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [http://dx.doi.org/10.1126/science.286.5440.735]. [PMID: 10531052]. [DOI] [PubMed] [Google Scholar]
- 69.Sinha S., Anderson J.P., Barbour R., Basi G.S., Caccavello R., Davis D., Doan M., Dovey H.F., Frigon N., Hong J., Jacobson-Croak K., Jewett N., Keim P., Knops J., Lieberburg I., Power M., Tan H., Tatsuno G., Tung J., Schenk D., Seubert P., Suomensaari S.M., Wang S., Walker D., Zhao J., McConlogue L., John V. Purification and cloning of amyloid precursor protein β-secretase from human brain. Nature. 1999;402(6761):537–540. doi: 10.1038/990114. [http://dx.doi.org/10.1038/990114]. [PMID: 10591214]. [DOI] [PubMed] [Google Scholar]
- 70.Tanzi R.E., Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: A genetic perspective. Cell. 2005;120(4):545–555. doi: 10.1016/j.cell.2005.02.008. [http://dx.doi.org/10.1016/j.cell.2005.02.008]. [PMID: 15734686]. [DOI] [PubMed] [Google Scholar]
- 71.Iwatsubo T., Odaka A., Suzuki N., Mizusawa H., Nukina N., Ihara Y. Visualization of A β 42(43) and A β 40 in senile plaques with end-specific A β monoclonals: Evidence that an initially deposited species is A β 42(43). Neuron. 1994;13(1):45–53. doi: 10.1016/0896-6273(94)90458-8. [http://dx.doi.org/10.1016/0896-6273(94)90458-8]. [PMID: 8043280]. [DOI] [PubMed] [Google Scholar]
- 72.Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T.D., Hardy J., Hutton M., Kukull W., Larson E., Levy-Lahad E., Viitanen M., Peskind E., Poorkaj P., Schellenberg G., Tanzi R., Wasco W., Lannfelt L., Selkoe D., Younkin S. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat. Med. 1996;2(8):864–870. doi: 10.1038/nm0896-864. [http://dx.doi.org/10.1038/nm0896-864]. [PMID: 8705854]. [DOI] [PubMed] [Google Scholar]
- 73.Haass C., Selkoe D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 2007;8(2):101–112. doi: 10.1038/nrm2101. [http://dx.doi.org/10.1038/nrm2101]. [PMID: 17245412]. [DOI] [PubMed] [Google Scholar]
- 74.Citron M. Alzheimer’s disease: strategies for disease modification. Nat. Rev. Drug Discov. 2010;9(5):387–398. doi: 10.1038/nrd2896. [http://dx.doi.org/10.1038/nrd2896]. [PMID: 20431570]. [DOI] [PubMed] [Google Scholar]
- 75.Huang Y., Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):1204–1222. doi: 10.1016/j.cell.2012.02.040. [http://dx.doi.org/10.1016/j.cell.2012.02.040]. [PMID: 22424230]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Asai M., Iwata N., Yoshikawa A., Aizaki Y., Ishiura S., Saido T.C., Maruyama K. Berberine alters the processing of Alzheimer’s amyloid precursor protein to decrease Abeta secretion. Biochem. Biophys. Res. Commun. 2007;352(2):498–502. doi: 10.1016/j.bbrc.2006.11.043. [http://dx.doi.org/10.1016/j.bbrc.2006.11.043]. [PMID: 17125739]. [DOI] [PubMed] [Google Scholar]
- 77.Shi A., Huang L., Lu C., He F., Li X. Synthesis, biological evaluation and molecular modeling of novel triazole-containing berberine derivatives as acetylcholinesterase and β-amyloid aggregation inhibitors. Bioorg. Med. Chem. 2011;19(7):2298–2305. doi: 10.1016/j.bmc.2011.02.025. [http://dx.doi.org/10.1016/j.bmc.2011.02.025]. [PMID: 21397508]. [DOI] [PubMed] [Google Scholar]
- 78.Zhu F., Wu F., Ma Y., Liu G., Li Z., Sun Y., Pei Z. Decrease in the production of β-amyloid by berberine inhibition of the expression of β-secretase in HEK293 cells. BMC Neurosci. 2011;12:125. doi: 10.1186/1471-2202-12-125. [http://dx.doi.org/10.1186/1471-2202-12-125]. [PMID: 22152059]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang H., Zhao C., Cao G., Guo L., Zhang S., Liang Y., Qin C., Su P., Li H., Zhang W. Berberine modulates amyloid-β peptide generation by activating AMP-activated protein kinase. Neuropharmacology. 2017;125:408–417. doi: 10.1016/j.neuropharm.2017.08.013. [http://dx.doi.org/10.1016/j.neuropharm.2017.08.013]. [PMID: 28822725]. [DOI] [PubMed] [Google Scholar]
- 80.Haghani M., Shabani M., Tondar M. The therapeutic potential of berberine against the altered intrinsic properties of the CA1 neurons induced by Aβ neurotoxicity. Eur. J. Pharmacol. 2015;758:82–88. doi: 10.1016/j.ejphar.2015.03.016. [http://dx.doi.org/10.1016/j.ejphar.2015.03.016]. [PMID: 25861937]. [DOI] [PubMed] [Google Scholar]
- 81.Hernández F., Gómez de Barreda E., Fuster-Matanzo A., Lucas J.J., Avila J. GSK3: A possible link between beta amyloid peptide and tau protein. Exp. Neurol. 2010;223(2):322–325. doi: 10.1016/j.expneurol.2009.09.011. [http://dx.doi.org/10.1016/j.expneurol.2009.09.011]. [PMID: 19782073]. [DOI] [PubMed] [Google Scholar]
- 82.Hooper C., Killick R., Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J. Neurochem. 2008;104(6):1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [http://dx.doi.org/10.1111/j.1471-4159.2007.05194.x]. [PMID: 18088381]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Llorens-Martín M., Jurado J., Hernández F., Avila J. GSK-3β, a pivotal kinase in Alzheimer disease. Front. Mol. Neurosci. 2014;7:46. doi: 10.3389/fnmol.2014.00046. [PMID: 24904272]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leclerc S., Garnier M., Hoessel R., Marko D., Bibb J.A., Snyder G.L., Greengard P., Biernat J., Wu Y.Z., Mandelkow E.M., Eisenbrand G., Meijer L. Indirubins inhibit glycogen synthase kinase-3 beta and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors? J. Biol. Chem. 2001;276(1):251–260. doi: 10.1074/jbc.M002466200. [http://dx.doi.org/10.1074/jbc.M002466200]. [PMID: 11013232]. [DOI] [PubMed] [Google Scholar]
- 85.Sontag E., Nunbhakdi-Craig V., Lee G., Bloom G.S., Mumby M.C. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996;17(6):1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [http://dx.doi.org/10.1016/S0896-6273(00)80250-0]. [PMID: 8982166]. [DOI] [PubMed] [Google Scholar]
- 86.Sontag E., Nunbhakdi-Craig V., Lee G., Brandt R., Kamibayashi C., Kuret J., White C.L., III, Mumby M.C., Bloom G.S. Molecular interactions among protein phosphatase 2A, tau, and microtubules. Implications for the regulation of tau phosphorylation and the development of tauopathies. J. Biol. Chem. 1999;274(36):25490–25498. doi: 10.1074/jbc.274.36.25490. [http://dx.doi.org/10.1074/jbc.274.36.25490]. [PMID: 10464280]. [DOI] [PubMed] [Google Scholar]
- 87.Gong C.X., Lidsky T., Wegiel J., Zuck L., Grundke-Iqbal I., Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer’s disease. J. Biol. Chem. 2000;275(8):5535–5544. doi: 10.1074/jbc.275.8.5535. [http://dx.doi.org/10.1074/jbc.275.8.5535]. [PMID: 10681533]. [DOI] [PubMed] [Google Scholar]
- 88.Kins S., Crameri A., Evans D.R., Hemmings B.A., Nitsch R.M., Gotz J. Reduced protein phosphatase 2A activity induces hyperphosphorylation and altered compartmentalization of tau in transgenic mice. J. Biol. Chem. 2001;276(41):38193–38200. doi: 10.1074/jbc.M102621200. [PMID: 11473109]. [DOI] [PubMed] [Google Scholar]
- 89.Yu G., Li Y., Tian Q., Liu R., Wang Q., Wang J.Z., Wang X. Berberine attenuates calyculin A-induced cytotoxicity and Tau hyperphosphorylation in HEK293 cells. J. Alzheimers Dis. 2011;24(3):525–535. doi: 10.3233/JAD-2011-101779. [http://dx.doi.org/10.3233/JAD-2011-101779]. [PMID: 21297267]. [DOI] [PubMed] [Google Scholar]
- 90.Ascherio A., Schwarzschild M.A. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [http://dx.doi.org/10.1016/S1474-4422(16)30230-7]. [PMID: 27751556]. [DOI] [PubMed] [Google Scholar]
- 91.Pringsheim T., Jette N., Frolkis A., Steeves T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014;29(13):1583–1590. doi: 10.1002/mds.25945. [http://dx.doi.org/10.1002/mds.25945]. [PMID: 24976103]. [DOI] [PubMed] [Google Scholar]
- 92.Przedborski S. The two-century journey of Parkinson disease research. Nat. Rev. Neurosci. 2017;18(4):251–259. doi: 10.1038/nrn.2017.25. [http://dx.doi.org/10.1038/nrn.2017.25]. [PMID: 28303016]. [DOI] [PubMed] [Google Scholar]
- 93.Postuma R.B., Berg D., Adler C.H., Bloem B.R., Chan P., Deuschl G., Gasser T., Goetz C.G., Halliday G., Joseph L., Lang A.E., Liepelt-Scarfone I., Litvan I., Marek K., Oertel W., Olanow C.W., Poewe W., Stern M. The new definition and diagnostic criteria of Parkinson’s disease. Lancet Neurol. 2016;15(6):546–548. doi: 10.1016/S1474-4422(16)00116-2. [http://dx.doi.org/10.1016/S1474-4422(16)00116-2]. [PMID: 27302120]. [DOI] [PubMed] [Google Scholar]
- 94.Postuma R.B., Berg D., Stern M., Poewe W., Olanow C.W., Oertel W., Obeso J., Marek K., Litvan I., Lang A.E., Halliday G., Goetz C.G., Gasser T., Dubois B., Chan P., Bloem B.R., Adler C.H., Deuschl G. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015;30(12):1591–1601. doi: 10.1002/mds.26424. [http://dx.doi.org/10.1002/mds.26424]. [PMID: 26474316]. [DOI] [PubMed] [Google Scholar]
- 95.Schapira A.H.V., Chaudhuri K.R., Jenner P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017;18(7):435–450. doi: 10.1038/nrn.2017.62. [http://dx.doi.org/10.1038/nrn.2017.62]. [PMID: 28592904]. [DOI] [PubMed] [Google Scholar]
- 96.Chaudhuri K.R., Healy D.G., Schapira A.H. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 2006;5(3):235–245. doi: 10.1016/S1474-4422(06)70373-8. [http://dx.doi.org/10.1016/S1474-4422(06)70373-8]. [PMID: 16488379]. [DOI] [PubMed] [Google Scholar]
- 97.Surmeier D.J., Obeso J.A., Halliday G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017;18(2):101–113. doi: 10.1038/nrn.2016.178. [http://dx.doi.org/10.1038/nrn.2016.178]. [PMID: 28104909]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hirsch E.C., Jenner P., Przedborski S. Pathogenesis of Parkinson’s disease. Mov. Disord. 2013;28(1):24–30. doi: 10.1002/mds.25032. [http://dx.doi.org/10.1002/mds.25032]. [PMID: 22927094]. [DOI] [PubMed] [Google Scholar]
- 99.Dexter D.T., Jenner P. Parkinson disease: From pathology to molecular disease mechanisms. Free Radic. Biol. Med. 2013;62:132–144. doi: 10.1016/j.freeradbiomed.2013.01.018. [http://dx.doi.org/10.1016/j.freeradbiomed.2013.01.018]. [PMID: 23380027]. [DOI] [PubMed] [Google Scholar]
- 100.Dauer W., Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [http://dx.doi.org/10.1016/S0896-6273(03)00568-3]. [PMID: 12971891]. [DOI] [PubMed] [Google Scholar]
- 101.Braak H., Del Tredici K., Rüb U., de Vos R.A., Jansen Steur E.N., Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [http://dx.doi.org/10.1016/S0197-4580(02)00065-9]. [PMID: 12498954]. [DOI] [PubMed] [Google Scholar]
- 102.Damier P., Hirsch E.C., Agid Y., Graybiel A.M. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122(Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [http://dx.doi.org/10.1093/brain/122.8.1437]. [PMID: 10430830]. [DOI] [PubMed] [Google Scholar]
- 103.Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. α-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [http://dx.doi.org/10.1038/42166]. [PMID: 9278044]. [DOI] [PubMed] [Google Scholar]
- 104.Baba M., Nakajo S., Tu P.H., Tomita T., Nakaya K., Lee V.M., Trojanowski J.Q., Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 1998;152(4):879–884. [PMID: 9546347]. [PMC free article] [PubMed] [Google Scholar]
- 105.Spillantini M.G., Crowther R.A., Jakes R., Hasegawa M., Goedert M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA. 1998;95(11):6469–6473. doi: 10.1073/pnas.95.11.6469. [http://dx.doi.org/10.1073/pnas.95.11.6469]. [PMID: 9600990]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen T., Pu J., Si X., Ye R., Zhang B. An update on potential therapeutic strategies for Parkinson’s disease based on pathogenic mechanisms. Expert Rev. Neurother. 2016;16(6):711–722. doi: 10.1080/14737175.2016.1179112. [http://dx.doi.org/10.1080/14737175.2016.1179112]. [PMID: 27138872]. [DOI] [PubMed] [Google Scholar]
- 107.Lindholm D., Mäkelä J., Di Liberto V., Mudò G., Belluardo N., Eriksson O., Saarma M. Current disease modifying approaches to treat Parkinson’s disease. Cell. Mol. Life Sci. 2016;73(7):1365–1379. doi: 10.1007/s00018-015-2101-1. [http://dx.doi.org/10.1007/s00018-015-2101-1]. [PMID: 26616211]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pires A.O., Teixeira F.G., Mendes-Pinheiro B., Serra S.C., Sousa N., Salgado A.J. Old and new challenges in Parkinson’s disease therapeutics. Prog. Neurobiol. 2017;156:69–89. doi: 10.1016/j.pneurobio.2017.04.006. [http://dx.doi.org/10.1016/j.pneurobio.2017.04.006]. [PMID: 28457671]. [DOI] [PubMed] [Google Scholar]
- 109.Connolly B.S., Lang A.E. Pharmacological treatment of Parkinson disease: A review. JAMA. 2014;311(16):1670–1683. doi: 10.1001/jama.2014.3654. [http://dx.doi.org/10.1001/jama.2014.3654]. [PMID: 24756517]. [DOI] [PubMed] [Google Scholar]
- 110.Fahn S., Poewe W. Levodopa: 50 years of a revolutionary drug for Parkinson disease. Mov. Disord. 2015;30(1):1–3. doi: 10.1002/mds.26122. [http://dx.doi.org/10.1002/mds.26122]. [PMID: 25488146]. [DOI] [PubMed] [Google Scholar]
- 111.Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [http://dx.doi.org/10.1002/mds.23429]. [PMID: 21069833]. [DOI] [PubMed] [Google Scholar]
- 112.Fernandez H.H., Chen J.J. Monoamine oxidase-B inhibition in the treatment of Parkin-son's disease. Pharmacotherapy, 2007;27(12P2):174S–185S. doi: 10.1592/phco.27.12part2.174S. [DOI] [PubMed] [Google Scholar]
- 113.Youdim M.B., Edmondson D., Tipton K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006;7(4):295–309. doi: 10.1038/nrn1883. [http://dx.doi.org/10.1038/nrn1883]. [PMID: 16552415]. [DOI] [PubMed] [Google Scholar]
- 114.LeWitt P.A. Levodopa therapy for Parkinson’s disease: Pharmacokinetics and pharmacodynamics. Mov. Disord. 2015;30(1):64–72. doi: 10.1002/mds.26082. [http://dx.doi.org/10.1002/mds.26082]. [PMID: 25449210]. [DOI] [PubMed] [Google Scholar]
- 115.Fahn S., Oakes D., Shoulson I., Kieburtz K., Rudolph A., Lang A., Olanow C.W., Tanner C., Marek K. Levodopa and the progression of Parkinson’s disease. N. Engl. J. Med. 2004;351(24):2498–2508. doi: 10.1056/NEJMoa033447. [http://dx.doi.org/10.1056/NEJMoa033447]. [PMID: 15590952]. [DOI] [PubMed] [Google Scholar]
- 116.Poletti M., Bonuccelli U. Acute and chronic cognitive effects of levodopa and dopamine agonists on patients with Parkinson’s disease: A review. Ther. Adv. Psychopharmacol. 2013;3(2):101–113. doi: 10.1177/2045125312470130. [http://dx.doi.org/10.1177/2045125312470130]. [PMID: 24167681]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Glover V., Sandler M., Owen F., Riley G.J. Dopamine is a monoamine oxidase B substrate in man. Nature. 1977;265(5589):80–81. doi: 10.1038/265080a0. [http://dx.doi.org/10.1038/265080a0]. [PMID: 834248]. [DOI] [PubMed] [Google Scholar]
- 118.Westlund K.N., Denney R.M., Kochersperger L.M., Rose R.M., Abell C.W. Distinct monoamine oxidase A and B populations in primate brain. Science. 1985;230(4722):181–183. doi: 10.1126/science.3875898. [http://dx.doi.org/10.1126/science.3875898]. [PMID: 3875898]. [DOI] [PubMed] [Google Scholar]
- 119.Bach A.W., Lan N.C., Johnson D.L., Abell C.W., Bembenek M.E., Kwan S.W., Seeburg P.H., Shih J.C. cDNA cloning of human liver monoamine oxidase A and B: Molecular basis of differences in enzymatic properties. Proc. Natl. Acad. Sci. USA. 1988;85(13):4934–4938. doi: 10.1073/pnas.85.13.4934. [http://dx.doi.org/10.1073/pnas.85.13.4934]. [PMID: 3387449]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shih J.C., Chen K., Ridd M.J. Monoamine oxidase: From genes to behavior. Annu. Rev. Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [http://dx.doi.org/10.1146/annurev.neuro.22.1.197]. [PMID: 10202537]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hauser R.A., Li R., Pérez A., Ren X., Weintraub D., Elm J., Goudreau J.L., Morgan J.C., Fang J.Y., Aminoff M.J., Christine C.W., Dhall R., Umeh C.C., Boyd J.T., Stover N., Leehey M., Zweig R.M., Nicholas A.P., Bodis-Wollner I., Willis A., Kieburtz K., Tilley B.C. Longer duration of MAO-B inhibitor exposure is associated with less clinical decline in parkinson’s disease: An analysis of NET-PD LS1. J. Parkinsons Dis. 2017;7(1):117–127. doi: 10.3233/JPD-160965. [http://dx.doi.org/10.3233/JPD-160965]. [PMID: 27911341]. [DOI] [PubMed] [Google Scholar]
- 122.Teo K.C., Ho S.L. Monoamine oxidase-B (MAO-B) inhibitors: Implications for disease-modification in Parkinson’s disease. Transl. Neurodegener. 2013;2(1):19. doi: 10.1186/2047-9158-2-19. [http://dx.doi.org/10.1186/2047-9158-2-19]. [PMID: 24011391]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rabey J.M., Sagi I., Huberman M., Melamed E., Korczyn A., Giladi N., Inzelberg R., Djaldetti R., Klein C., Berecz G. Rasagiline mesylate, a new MAO-B inhibitor for the treatment of Parkinson’s disease: A double-blind study as adjunctive therapy to levodopa. Clin. Neuropharmacol. 2000;23(6):324–330. doi: 10.1097/00002826-200011000-00005. [http://dx.doi.org/10.1097/00002826-200011000-00005]. [PMID: 11575866]. [DOI] [PubMed] [Google Scholar]
- 124.Tetrud J.W., Langston J.W. The effect of deprenyl (selegiline) on the natural history of Parkinson’s disease. Science. 1989;245(4917):519–522. doi: 10.1126/science.2502843. [http://dx.doi.org/10.1126/science.2502843]. [PMID: 2502843]. [DOI] [PubMed] [Google Scholar]
- 125.Lees A.J. Comparison of therapeutic effects and mortality data of levodopa and levodopa combined with selegiline in patients with early, mild Parkinson’s disease. BMJ. 1995;311(7020):1602–1607. doi: 10.1136/bmj.311.7020.1602. [http://dx.doi.org/10.1136/bmj.311.7020.1602]. [PMID: 8555803]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Olanow C.W., Rascol O., Hauser R., Feigin P.D., Jankovic J., Lang A., Langston W., Melamed E., Poewe W., Stocchi F., Tolosa E., Investigators A.S. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N. Engl. J. Med. 2009;361(13):1268–1278. doi: 10.1056/NEJMoa0809335. [http://dx.doi.org/10.1056/NEJMoa0809335]. [PMID: 19776408]. [DOI] [PubMed] [Google Scholar]
- 127.A controlled, randomized, delayed-start study of rasagiline in early Parkinson disease. Arch. Neurol. 2004;61(4):561–566. doi: 10.1001/archneur.61.4.561. [http://dx.doi.org/10.1001/archneur.61.4.561]. [PMID: 15096406]. [DOI] [PubMed] [Google Scholar]
- 128.Rascol O., Brooks D.J., Melamed E., Oertel W., Poewe W., Stocchi F., Tolosa E. Rasagiline as an adjunct to levodopa in patients with Parkinson’s disease and motor fluctuations (LARGO, Lasting effect in Adjunct therapy with Rasagiline Given Once daily, study): a randomised, double-blind, parallel-group trial. Lancet. 2005;365(9463):947–954. doi: 10.1016/S0140-6736(05)71083-7. [http://dx.doi.org/10.1016/S0140-6736(05)71083-7]. [PMID: 15766996]. [DOI] [PubMed] [Google Scholar]
- 129.Robakis D., Fahn S. Defining the role of the monoamine oxidase-B inhibitors for Parkinson’s disease. CNS Drugs. 2015;29(6):433–441. doi: 10.1007/s40263-015-0249-8. [http://dx.doi.org/10.1007/s40263-015-0249-8]. [PMID: 26164425]. [DOI] [PubMed] [Google Scholar]
- 130.Youdim M.B., Edmondson D., Tipton K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006;7(4):295–309. doi: 10.1038/nrn1883. [http://dx.doi.org/10.1038/nrn1883]. [PMID: 16552415]. [DOI] [PubMed] [Google Scholar]
- 131.Lee S.S., Kai M., Lee M.K. Effects of natural isoquinoline alkaloids on monoamine oxidase activity in mouse brain: Inhibition by berberine and palmatine. Med. Sci. Res. 1999;27(11):749–751. [Google Scholar]
- 132.Castillo J., Hung J., Rodriguez M., Bastidas E., Laboren I., Jaimes A. LED fluorescence spectroscopy for direct determination of monoamine oxidase B inactivation. Anal. Biochem. 2005;343(2):293–298. doi: 10.1016/j.ab.2005.05.027. [http://dx.doi.org/10.1016/j.ab.2005.05.027]. [PMID: 16004952]. [DOI] [PubMed] [Google Scholar]
- 133.Zhang J., Yang J.Q., He B.C., Zhou Q.X., Yu H.R., Tang Y., Liu B.Z. Berberine and total base from rhizoma coptis chinensis attenuate brain injury in an aluminum-induced rat model of neurodegenerative disease. Saudi Med. J. 2009;30(6):760–766. [PMID: 19526156]. [PubMed] [Google Scholar]
- 134.Bae J., Lee D., Kim Y.K., Gil M., Lee J.Y., Lee K.J. Berberine protects 6-hydroxydopamine-induced human dopaminergic neuronal cell death through the induction of heme oxygenase-1. Mol. Cells. 2013;35(2):151–157. doi: 10.1007/s10059-013-2298-5. [http://dx.doi.org/10.1007/s10059-013-2298-5]. [PMID: 23329300]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shin K.S., Choi H.S., Zhao T.T., Suh K.H., Kwon I.H., Choi S.O., Lee M.K. Neurotoxic effects of berberine on long-term L-DOPA administration in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Arch. Pharm. Res. 2013;36(6):759–767. doi: 10.1007/s12272-013-0051-4. [http://dx.doi.org/10.1007/s12272-013-0051-4]. [PMID: 23539311]. [DOI] [PubMed] [Google Scholar]
- 136.Kwon I.H., Choi H.S., Shin K.S., Lee B.K., Lee C.K., Hwang B.Y., Lim S.C., Lee M.K. Effects of berberine on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and a rat model of Parkinson’s disease. Neurosci. Lett. 2010;486(1):29–33. doi: 10.1016/j.neulet.2010.09.038. [http://dx.doi.org/10.1016/j.neulet.2010.09.038]. [PMID: 20851167]. [DOI] [PubMed] [Google Scholar]
- 137.Bates G.P., Dorsey R., Gusella J.F., Hayden M.R., Kay C., Leavitt B.R., Nance M., Ross C.A., Scahill R.I., Wetzel R., Wild E.J., Tabrizi S.J. Huntington disease. Nat. Rev. Dis. Primers. 2015;1:15005. doi: 10.1038/nrdp.2015.5. [http://dx.doi.org/10.1038/nrdp.2015.5]. [PMID: 27188817]. [DOI] [PubMed] [Google Scholar]
- 138.Walker F.O. Huntington’s disease. Lancet. 2007;369(9557):218–228. doi: 10.1016/S0140-6736(07)60111-1. [http://dx.doi.org/10.1016/S0140-6736(07)60111-1]. [PMID: 17240289]. [DOI] [PubMed] [Google Scholar]
- 139.Rawlins M.D., Wexler N.S., Wexler A.R., Tabrizi S.J., Douglas I., Evans S.J., Smeeth L. The prevalence of Huntington’s disease. Neuroepidemiology. 2016;46(2):144–153. doi: 10.1159/000443738. [http://dx.doi.org/10.1159/000443738]. [PMID: 26824438]. [DOI] [PubMed] [Google Scholar]
- 140.Ross C.A., Tabrizi S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10(1):83–98. doi: 10.1016/S1474-4422(10)70245-3. [http://dx.doi.org/10.1016/S1474-4422(10)70245-3]. [PMID: 21163446]. [DOI] [PubMed] [Google Scholar]
- 141.Labbadia J., Morimoto R.I. Huntington’s disease: Underlying molecular mechanisms and emerging concepts. Trends Biochem. Sci. 2013;38(8):378–385. doi: 10.1016/j.tibs.2013.05.003. [http://dx.doi.org/10.1016/j.tibs.2013.05.003]. [PMID: 23768628]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Identification of genetic factors that modify clinical onset of Huntington’s disease. Cell. 2015;162(3):516–526. doi: 10.1016/j.cell.2015.07.003. [http://dx.doi.org/10.1016/j.cell.2015.07.003]. [PMID: 26232222]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.DiFiglia M., Sapp E., Chase K.O., Davies S.W., Bates G.P., Vonsattel J.P., Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277(5334):1990–1993. doi: 10.1126/science.277.5334.1990. [http://dx.doi.org/10.1126/science.277.5334.1990]. [PMID: 9302293]. [DOI] [PubMed] [Google Scholar]
- 144.Scherzinger E., Sittler A., Schweiger K., Heiser V., Lurz R., Hasenbank R., Bates G.P., Lehrach H., Wanker E.E. Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: implications for Huntington’s disease pathology. Proc. Natl. Acad. Sci. USA. 1999;96(8):4604–4609. doi: 10.1073/pnas.96.8.4604. [http://dx.doi.org/10.1073/pnas.96.8.4604]. [PMID: 10200309]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bates G. Huntingtin aggregation and toxicity in Huntington’s disease. Lancet. 2003;361(9369):1642–1644. doi: 10.1016/S0140-6736(03)13304-1. [http://dx.doi.org/10.1016/S0140-6736(03)13304-1]. [PMID: 12747895]. [DOI] [PubMed] [Google Scholar]
- 146.Cicchetti F., Lacroix S., Cisbani G., Vallières N., Saint-Pierre M., St-Amour I., Tolouei R., Skepper J.N., Hauser R.A., Mantovani D., Barker R.A., Freeman T.B. Mutant huntingtin is present in neuronal grafts in Huntington disease patients. Ann. Neurol. 2014;76(1):31–42. doi: 10.1002/ana.24174. [http://dx.doi.org/10.1002/ana.24174]. [PMID: 24798518]. [DOI] [PubMed] [Google Scholar]
- 147.Mangiarini L., Sathasivam K., Seller M., Cozens B., Harper A., Hetherington C., Lawton M., Trottier Y., Lehrach H., Davies S.W., Bates G.P. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87(3):493–506. doi: 10.1016/s0092-8674(00)81369-0. [http://dx.doi.org/10.1016/S0092-8674(00)81369-0]. [PMID: 8898202]. [DOI] [PubMed] [Google Scholar]
- 148.Andrew S.E., Goldberg Y.P., Kremer B., Telenius H., Theilmann J., Adam S., Starr E., Squitieri F., Lin B., Kalchman M.A. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington’s disease. Nat. Genet. 1993;4(4):398–403. doi: 10.1038/ng0893-398. [http://dx.doi.org/10.1038/ng0893-398]. [PMID: 8401589]. [DOI] [PubMed] [Google Scholar]
- 149.Morley J.F., Brignull H.R., Weyers J.J., Morimoto R.I. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2002;99(16):10417–10422. doi: 10.1073/pnas.152161099. [http://dx.doi.org/10.1073/pnas.152161099]. [PMID: 12122205]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Langbehn D.R., Brinkman R.R., Falush D., Paulsen J.S., Hayden M.R. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin. Genet. 2004;65(4):267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [http://dx.doi.org/10.1111/j.1399-0004.2004.00241.x]. [PMID: 15025718]. [DOI] [PubMed] [Google Scholar]
- 151.Rué L., Bañez-Coronel M., Creus-Muncunill J., Giralt A., Alcalá-Vida R., Mentxaka G., Kagerbauer B., Zomeño-Abellán M.T., Aranda Z., Venturi V., Pérez-Navarro E., Estivill X., Martí E. Targeting CAG repeat RNAs reduces Huntington’s disease phenotype independently of huntingtin levels. J. Clin. Invest. 2016;126(11):4319–4330. doi: 10.1172/JCI83185. [http://dx.doi.org/10.1172/JCI83185]. [PMID: 27721240]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sarkar S., Rubinsztein D.C. Huntington’s disease: Degradation of mutant huntingtin by autophagy. FEBS J. 2008;275(17):4263–4270. doi: 10.1111/j.1742-4658.2008.06562.x. [http://dx.doi.org/10.1111/j.1742-4658.2008.06562.x]. [PMID: 18637946]. [DOI] [PubMed] [Google Scholar]
- 153.Martinez-Vicente M., Talloczy Z., Wong E., Tang G., Koga H., Kaushik S., de Vries R., Arias E., Harris S., Sulzer D., Cuervo A.M. Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nat. Neurosci. 2010;13(5):567–576. doi: 10.1038/nn.2528. [http://dx.doi.org/10.1038/nn.2528]. [PMID: 20383138]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Martin D.D., Ladha S., Ehrnhoefer D.E., Hayden M.R. Autophagy in Huntington disease and huntingtin in autophagy. Trends Neurosci. 2015;38(1):26–35. doi: 10.1016/j.tins.2014.09.003. [http://dx.doi.org/10.1016/j.tins.2014.09.003]. [PMID: 25282404]. [DOI] [PubMed] [Google Scholar]
- 155.Mizushima N., Klionsky D.J. Protein turnover via autophagy: Implications for metabolism. Annu. Rev. Nutr. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [http://dx.doi.org/10.1146/annurev.nutr.27.061406.093749]. [PMID: 17311494]. [DOI] [PubMed] [Google Scholar]
- 156.Mizushima N., Komatsu M. Autophagy: Renovation of cells and tissues. Cell. 2011;147(4):728–741. doi: 10.1016/j.cell.2011.10.026. [http://dx.doi.org/10.1016/j.cell.2011.10.026]. [PMID: 22078875]. [DOI] [PubMed] [Google Scholar]
- 157.Mizushima N., Levine B., Cuervo A.M., Klionsky D.J. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [http://dx.doi.org/10.1038/nature06639]. [PMID: 18305538]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., Yokoyama M., Mishima K., Saito I., Okano H., Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–889. doi: 10.1038/nature04724. [http://dx.doi.org/10.1038/nature04724]. [PMID: 16625204]. [DOI] [PubMed] [Google Scholar]
- 159.Nixon R.A., Wegiel J., Kumar A., Yu W.H., Peterhoff C., Cataldo A., Cuervo A.M. Extensive involvement of autophagy in Alzheimer disease: An immuno-electron microscopy study. J. Neuropathol. Exp. Neurol. 2005;64(2):113–122. doi: 10.1093/jnen/64.2.113. [http://dx.doi.org/10.1093/jnen/64.2.113]. [PMID: 15751225]. [DOI] [PubMed] [Google Scholar]
- 160.Boland B., Kumar A., Lee S., Platt F.M., Wegiel J., Yu W.H., Nixon R.A. Autophagy induction and autophagosome clearance in neurons: Relationship to autophagic pathology in Alzheimer’s disease. J. Neurosci. 2008;28(27):6926–6937. doi: 10.1523/JNEUROSCI.0800-08.2008. [http://dx.doi.org/10.1523/JNEUROSCI.0800-08.2008]. [PMID: 18596167]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee J.H., Yu W.H., Kumar A., Lee S., Mohan P.S., Peterhoff C.M., Wolfe D.M., Martinez-Vicente M., Massey A.C., Sovak G., Uchiyama Y., Westaway D., Cuervo A.M., Nixon R.A. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141(7):1146–1158. doi: 10.1016/j.cell.2010.05.008. [http://dx.doi.org/10.1016/j.cell.2010.05.008]. [PMID: 20541250]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nixon R.A., Yang D.S. Autophagy failure in Alzheimer’s disease--locating the primary defect. Neurobiol. Dis. 2011;43(1):38–45. doi: 10.1016/j.nbd.2011.01.021. [http://dx.doi.org/10.1016/j.nbd.2011.01.021]. [PMID: 21296668]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cuervo A.M., Stefanis L., Fredenburg R., Lansbury P.T., Sulzer D. Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science. 2004;305(5688):1292–1295. doi: 10.1126/science.1101738. [http://dx.doi.org/10.1126/science.1101738]. [PMID: 15333840]. [DOI] [PubMed] [Google Scholar]
- 164.Martinez-Vicente M., Talloczy Z., Kaushik S., Massey A.C., Mazzulli J., Mosharov E.V., Hodara R., Fredenburg R., Wu D.C., Follenzi A., Dauer W., Przedborski S., Ischiropoulos H., Lansbury P.T., Sulzer D., Cuervo A.M. Dopamine-modified α-synuclein blocks chaperone-mediated autophagy. J. Clin. Invest. 2008;118(2):777–788. doi: 10.1172/JCI32806. [PMID: 18172548]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang H., Duan C., Yang H. Defective autophagy in Parkinson’s disease: Lessons from genetics. Mol. Neurobiol. 2015;51(1):89–104. doi: 10.1007/s12035-014-8787-5. [http://dx.doi.org/10.1007/s12035-014-8787-5]. [PMID: 24990317]. [DOI] [PubMed] [Google Scholar]
- 166.Sarkar S., Perlstein E.O., Imarisio S., Pineau S., Cordenier A., Maglathlin R.L., Webster J.A., Lewis T.A., O’Kane C.J., Schreiber S.L., Rubinsztein D.C. Small molecules enhance autophagy and reduce toxicity in Huntington’s disease models. Nat. Chem. Biol. 2007;3(6):331–338. doi: 10.1038/nchembio883. [http://dx.doi.org/10.1038/nchembio883]. [PMID: 17486044]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Floto R.A., Sarkar S., Perlstein E.O., Kampmann B., Schreiber S.L., Rubinsztein D.C. Small molecule enhancers of rapamycin-induced TOR inhibition promote autophagy, reduce toxicity in Huntington’s disease models and enhance killing of mycobacteria by macrophages. Autophagy. 2007;3(6):620–622. doi: 10.4161/auto.4898. [http://dx.doi.org/10.4161/auto.4898]. [PMID: 17786022]. [DOI] [PubMed] [Google Scholar]
- 168.Tsvetkov A.S., Miller J., Arrasate M., Wong J.S., Pleiss M.A., Finkbeiner S. A small-molecule scaffold induces autophagy in primary neurons and protects against toxicity in a Huntington disease model. Proc. Natl. Acad. Sci. USA. 2010;107(39):16982–16987. doi: 10.1073/pnas.1004498107. [http://dx.doi.org/10.1073/pnas.1004498107]. [PMID: 20833817]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schapira A.H., Olanow C.W., Greenamyre J.T., Bezard E. Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: Future therapeutic perspectives. Lancet. 2014;384(9942):545–555. doi: 10.1016/S0140-6736(14)61010-2. [http://dx.doi.org/10.1016/S0140-6736(14)61010-2]. [PMID: 24954676]. [DOI] [PubMed] [Google Scholar]
- 170.Lin Y., Sheng M., Weng Y., Xu R., Lu N., Du H., Yu W. Berberine protects against ischemia/reperfusion injury after orthotopic liver transplantation via activating Sirt1/FoxO3α induced autophagy. Biochem. Biophys. Res. Commun. 2017;483(2):885–891. doi: 10.1016/j.bbrc.2017.01.028. [http://dx.doi.org/10.1016/j.bbrc.2017.01.028]. [PMID: 28077277]. [DOI] [PubMed] [Google Scholar]
- 171.He Q., Mei D., Sha S., Fan S., Wang L., Dong M. ERK-dependent mTOR pathway is involved in berberine-induced autophagy in hepatic steatosis. J. Mol. Endocrinol. 2016;57(4):251–260. doi: 10.1530/JME-16-0139. [http://dx.doi.org/10.1530/JME-16-0139]. [PMID: 27658958]. [DOI] [PubMed] [Google Scholar]
- 172.Domitrović R., Cvijanović O., Pernjak-Pugel E., Skoda M., Mikelić L., Crnčević-Orlić Z. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem. Toxicol. 2013;62:397–406. doi: 10.1016/j.fct.2013.09.003. [http://dx.doi.org/10.1016/j.fct.2013.09.003]. [PMID: 24025684]. [DOI] [PubMed] [Google Scholar]
- 173.Jin Y., Liu S., Ma Q., Xiao D., Chen L. Berberine enhances the AMPK activation and autophagy and mitigates high glucose-induced apoptosis of mouse podocytes. Eur. J. Pharmacol. 2017;794:106–114. doi: 10.1016/j.ejphar.2016.11.037. [http://dx.doi.org/10.1016/j.ejphar.2016.11.037]. [PMID: 27887947]. [DOI] [PubMed] [Google Scholar]
- 174.Zhang Q., Bian H., Guo L., Zhu H. Pharmacologic preconditioning with berberine attenuating ischemia-induced apoptosis and promoting autophagy in neuron. Am. J. Transl. Res. 2016;8(2):1197–1207. [PMID: 27158406]. [PMC free article] [PubMed] [Google Scholar]
- 175.Li M.H., Zhang Y.J., Yu Y.H., Yang S.H., Iqbal J., Mi Q.Y., Li B., Wang Z.M., Mao W.X., Xie H.G., Chen S.L. Berberine improves pressure overload-induced cardiac hypertrophy and dysfunction through enhanced autophagy. Eur. J. Pharmacol. 2014;728:67–76. doi: 10.1016/j.ejphar.2014.01.061. [http://dx.doi.org/10.1016/j.ejphar.2014.01.061]. [PMID: 24508518]. [DOI] [PubMed] [Google Scholar]
- 176.Zhang Y.J., Yang S.H., Li M.H., Iqbal J., Bourantas C.V., Mi Q.Y., Yu Y.H., Li J.J., Zhao S.L., Tian N.L., Chen S.L. Berberine attenuates adverse left ventricular remodeling and cardiac dysfunction after acute myocardial infarction in rats: Role of autophagy. Clin. Exp. Pharmacol. Physiol. 2014;41(12):995–1002. doi: 10.1111/1440-1681.12309. [http://dx.doi.org/10.1111/1440-1681.12309]. [PMID: 25224725]. [DOI] [PubMed] [Google Scholar]
- 177.Fan X., Wang J., Hou J., Lin C., Bensoussan A., Chang D., Liu J., Wang B. Berberine alleviates ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway. J. Transl. Med. 2015;13:92. doi: 10.1186/s12967-015-0450-z. [http://dx.doi.org/10.1186/s12967-015-0450-z]. [PMID: 25884210]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chang C.F., Lee Y.C., Lee K.H., Lin H.C., Chen C.L., Shen C.J., Huang C.C. Therapeutic effect of berberine on TDP-43-related pathogenesis in FTLD and ALS. J. Biomed. Sci. 2016;23(1):72. doi: 10.1186/s12929-016-0290-z. [http://dx.doi.org/10.1186/s12929-016-0290-z]. [PMID: 27769241]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kou J.Y., Li Y., Zhong Z.Y., Jiang Y.Q., Li X.S., Han X.B., Liu Z.N., Tian Y., Yang L.M. Berberine-sonodynamic therapy induces autophagy and lipid unloading in macrophage. Cell Death Dis., 2017;e8(1):2558. doi: 10.1038/cddis.2016.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhou H., Feng L., Xu F., Sun Y., Ma Y., Zhang X., Liu H., Xu G., Wu X., Shen Y., Sun Y., Wu X., Xu Q. Berberine inhibits palmitate-induced NLRP3 inflammasome activation by triggering autophagy in macrophages: A new mechanism linking berberine to insulin resistance improvement. Biomed. Pharmacother. 2017;89:864–874. doi: 10.1016/j.biopha.2017.03.003. [http://dx.doi.org/10.1016/j.biopha.2017.03.003]. [PMID: 28282788]. [DOI] [PubMed] [Google Scholar]
- 181.Chitra P., Saiprasad G., Manikandan R., Sudhandiran G. Berberine inhibits Smad and non-Smad signaling cascades and enhances autophagy against pulmonary fibrosis. J. Mol. Med. (Berl.) 2015;93(9):1015–1031. doi: 10.1007/s00109-015-1283-1. [http://dx.doi.org/10.1007/s00109-015-1283-1]. [PMID: 25877860]. [DOI] [PubMed] [Google Scholar]
- 182.Peng P.L., Kuo W.H., Tseng H.C., Chou F.P. Synergistic tumor-killing effect of radiation and berberine combined treatment in lung cancer: the contribution of autophagic cell death. Int. J. Radiat. Oncol. Biol. Phys. 2008;70(2):529–542. doi: 10.1016/j.ijrobp.2007.08.034. [http://dx.doi.org/10.1016/j.ijrobp.2007.08.034]. [PMID: 18207031]. [DOI] [PubMed] [Google Scholar]
- 183.Wang N., Feng Y., Zhu M., Tsang C.M., Man K., Tong Y., Tsao S.W. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: The cellular mechanism. J. Cell. Biochem. 2010;111(6):1426–1436. doi: 10.1002/jcb.22869. [http://dx.doi.org/10.1002/jcb.22869]. [PMID: 20830746]. [DOI] [PubMed] [Google Scholar]
- 184.Hou Q., Tang X., Liu H., Tang J., Yang Y., Jing X., Xiao Q., Wang W., Gou X., Wang Z. Berberine induces cell death in human hepatoma cells in vitro by downregulating CD147. Cancer Sci. 2011;102(7):1287–1292. doi: 10.1111/j.1349-7006.2011.01933.x. [http://dx.doi.org/10.1111/j.1349-7006.2011.01933.x]. [PMID: 21443647]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yu R., Zhang Z.Q., Wang B., Jiang H.X., Cheng L., Shen L.M. Berberine-induced apoptotic and autophagic death of HepG2 cells requires AMPK activation. Cancer Cell Int. 2014;14:49. doi: 10.1186/1475-2867-14-49. [http://dx.doi.org/10.1186/1475-2867-14-49]. [PMID: 24991192]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lee K.H., Lo H.L., Tang W.C., Hsiao H.H., Yang P.M. A gene expression signature-based approach reveals the mechanisms of action of the Chinese herbal medicine berberine. Sci. Rep. 2014;4:6394. doi: 10.1038/srep06394. [http://dx.doi.org/10.1038/srep06394]. [PMID: 25227736]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang J., Qi Q., Feng Z., Zhang X., Huang B., Chen A., Prestegarden L., Li X., Wang J. Berberine induces autophagy in glioblastoma by targeting the AMPK/mTOR/ULK1-pathway. Oncotarget. 2016;7(41):66944–66958. doi: 10.18632/oncotarget.11396. [http://dx.doi.org/10.18632/oncotarget.11396]. [PMID: 27557493]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Halicka H.D., Garcia J., Li J., Zhao H., Darzynkiewicz Z. Synergy of 2-deoxy-D-glucose combined with berberine in inducing the lysosome/autophagy and transglutaminase activation-facilitated apoptosis. Apoptosis. 2017;22(2):229–238. doi: 10.1007/s10495-016-1315-5. [http://dx.doi.org/10.1007/s10495-016-1315-5]. [PMID: 27796611]. [DOI] [PubMed] [Google Scholar]
- 189.La X., Zhang L., Li Z., Yang P., Wang Y. Berberine-induced autophagic cell death by elevating GRP78 levels in cancer cells. Oncotarget. 2017;8(13):20909–20924. doi: 10.18632/oncotarget.14959. [http://dx.doi.org/10.18632/oncotarget.14959]. [PMID: 28157699]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tansey M.G., McCoy M.K., Frank-Cannon T.C. Neuroinflammatory mechanisms in Parkinson’s disease: Potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp. Neurol. 2007;208(1):1–25. doi: 10.1016/j.expneurol.2007.07.004. [http://dx.doi.org/10.1016/j.expneurol.2007.07.004]. [PMID: 17720159]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Glass C.K., Saijo K., Winner B., Marchetto M.C., Gage F.H. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [http://dx.doi.org/10.1016/j.cell.2010.02.016]. [PMID: 20303880]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Block M.L., Hong J.S. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog. Neurobiol. 2005;76(2):77–98. doi: 10.1016/j.pneurobio.2005.06.004. [http://dx.doi.org/10.1016/j.pneurobio.2005.06.004]. [PMID: 16081203]. [DOI] [PubMed] [Google Scholar]
- 193.Perry V.H., Nicoll J.A., Holmes C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010;6(4):193–201. doi: 10.1038/nrneurol.2010.17. [http://dx.doi.org/10.1038/nrneurol.2010.17]. [PMID: 20234358]. [DOI] [PubMed] [Google Scholar]
- 194.Perry V.H., Holmes C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014;10(4):217–224. doi: 10.1038/nrneurol.2014.38. [http://dx.doi.org/10.1038/nrneurol.2014.38]. [PMID: 24638131]. [DOI] [PubMed] [Google Scholar]
- 195.Gao H.M., Hong J.S. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29(8):357–365. doi: 10.1016/j.it.2008.05.002. [http://dx.doi.org/10.1016/j.it.2008.05.002]. [PMID: 18599350]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., Herrup K., Frautschy S.A., Finsen B., Brown G.C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G.C., Town T., Morgan D., Shinohara M.L., Perry V.H., Holmes C., Bazan N.G., Brooks D.J., Hunot S., Joseph B., Deigendesch N., Garaschuk O., Boddeke E., Dinarello C.A., Breitner J.C., Cole G.M., Golenbock D.T., Kummer M.P. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [http://dx.doi.org/10.1016/S1474-4422(15)70016-5]. [PMID: 25792098]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hirsch E.C., Hunot S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009;8(4):382–397. doi: 10.1016/S1474-4422(09)70062-6. [http://dx.doi.org/10.1016/S1474-4422(09)70062-6]. [PMID: 19296921]. [DOI] [PubMed] [Google Scholar]
- 198.Klegeris A., McGeer P.L. Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Curr. Alzheimer Res. 2005;2(3):355–365. doi: 10.2174/1567205054367883. [http://dx.doi.org/10.2174/1567205054367883]. [PMID: 15974901]. [DOI] [PubMed] [Google Scholar]
- 199.Szekely C.A., Zandi P.P. Non-steroidal anti-inflammatory drugs and Alzheimer’s disease: The epidemiological evidence. CNS Neurol. Disord. Drug Targets. 2010;9(2):132–139. doi: 10.2174/187152710791012026. [http://dx.doi.org/10.2174/187152710791012026]. [PMID: 20205647]. [DOI] [PubMed] [Google Scholar]
- 200.Esposito E., Di Matteo V., Benigno A., Pierucci M., Crescimanno G., Di Giovanni G. Non-steroidal anti-inflammatory drugs in Parkinson’s disease. Exp. Neurol. 2007;205(2):295–312. doi: 10.1016/j.expneurol.2007.02.008. [http://dx.doi.org/10.1016/j.expneurol.2007.02.008]. [PMID: 17433296]. [DOI] [PubMed] [Google Scholar]
- 201.Nam K.N., Kim J.H., Jung H.J., Park J.M., Moon S.K., Kim Y.S., Sun Y.K., Lee E.H. Berberine inhibits inflammatory activation of rat brain microglia. Neural Regen. Res. 2010;12(1):1384–1390. [Google Scholar]
- 202.Lu D.Y., Tang C.H., Chen Y.H., Wei I.H. Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J. Cell. Biochem. 2010;110(3):697–705. doi: 10.1002/jcb.22580. [http://dx.doi.org/10.1002/jcb.22580]. [PMID: 20512929]. [DOI] [PubMed] [Google Scholar]
- 203.Zhang Z., Li X., Li F., An L. Berberine alleviates postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. Int. Immunopharmacol. 2016;38:426–433. doi: 10.1016/j.intimp.2016.06.031. [http://dx.doi.org/10.1016/j.intimp.2016.06.031]. [PMID: 27376853]. [DOI] [PubMed] [Google Scholar]
- 204.Jia L., Liu J., Song Z., Pan X., Chen L., Cui X., Wang M. Berberine suppresses amyloid-beta-induced inflammatory response in microglia by inhibiting nuclear factor-kappaB and mitogen-activated protein kinase signalling pathways. J. Pharm. Pharmacol. 2012;64(10):1510–1521. doi: 10.1111/j.2042-7158.2012.01529.x. [http://dx.doi.org/10.1111/j.2042-7158.2012.01529.x]. [PMID: 22943182]. [DOI] [PubMed] [Google Scholar]
- 205.Chen C.C., Hung T.H., Lee C.Y., Wang L.F., Wu C.H., Ke C.H., Chen S.F. Berberine protects against neuronal damage via suppression of glia-mediated inflammation in traumatic brain injury. PLoS One. 2014;9(12):e115694. doi: 10.1371/journal.pone.0115694. [http://dx.doi.org/10.1371/journal.pone.0115694]. [PMID: 25546475]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Niedzielska E., Smaga I., Gawlik M., Moniczewski A., Stankowicz P., Pera J., Filip M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016;53(6):4094–4125. doi: 10.1007/s12035-015-9337-5. [http://dx.doi.org/10.1007/s12035-015-9337-5]. [PMID: 26198567]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kim G.H., Kim J.E., Rhie S.J., Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015;24(4):325–340. doi: 10.5607/en.2015.24.4.325. [http://dx.doi.org/10.5607/en.2015.24.4.325]. [PMID: 26713080]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [http://dx.doi.org/10.1038/nature05292]. [PMID: 17051205]. [DOI] [PubMed] [Google Scholar]
- 209.Barnham K.J., Masters C.L., Bush A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004;3(3):205–214. doi: 10.1038/nrd1330. [http://dx.doi.org/10.1038/nrd1330]. [PMID: 15031734]. [DOI] [PubMed] [Google Scholar]
- 210.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [http://dx.doi.org/10.2174/157015909787602823]. [PMID: 19721819]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fernandes C., Oliveira C., Benfeito S., Soares P., Garrido J., Borges F. Nanotechnology and antioxidant therapy: An emerging approach for neurodegenerative diseases. Curr. Med. Chem. 2014;21(38):4311–4327. doi: 10.2174/0929867321666140915141836. [http://dx.doi.org/10.2174/0929867321666140915141836]. [PMID: 25245378]. [DOI] [PubMed] [Google Scholar]
- 212.Danta C.C., Piplani P. The discovery and development of new potential antioxidant agents for the treatment of neurodegenerative diseases. Expert Opin. Drug Discov. 2014;9(10):1205–1222. doi: 10.1517/17460441.2014.942218. [http://dx.doi.org/10.1517/17460441.2014.942218]. [PMID: 25056182]. [DOI] [PubMed] [Google Scholar]
- 213.Guerra-Araiza C., Álvarez-Mejía A.L., Sánchez-Torres S., Farfan-García E., Mondragón-Lozano R., Pinto-Almazán R., Salgado-Ceballos H. Effect of natural exogenous antioxidants on aging and on neurodegenerative diseases. Free Radic. Res. 2013;47(6-7):451–462. doi: 10.3109/10715762.2013.795649. [http://dx.doi.org/10.3109/10715762.2013.795649]. [PMID: 23594291]. [DOI] [PubMed] [Google Scholar]
- 214.Sarrafchi A., Bahmani M., Shirzad H., Rafieian-Kopaei M. Oxidative stress and Parkinson’s disease: New hopes in treatment with herbal antioxidants. Curr. Pharm. Des. 2016;22(2):238–246. doi: 10.2174/1381612822666151112151653. [http://dx.doi.org/10.2174/1381612822666151112151653]. [PMID: 26561062]. [DOI] [PubMed] [Google Scholar]
- 215.Shirwaikar A., Shirwaikar A., Rajendran K., Punitha I.S. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol. Pharm. Bull. 2006;29(9):1906–1910. doi: 10.1248/bpb.29.1906. [http://dx.doi.org/10.1248/bpb.29.1906]. [PMID: 16946507]. [DOI] [PubMed] [Google Scholar]
- 216.Bhutada P., Mundhada Y., Bansod K., Tawari S., Patil S., Dixit P., Umathe S., Mundhada D. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav. Brain Res. 2011;220(1):30–41. doi: 10.1016/j.bbr.2011.01.022. [http://dx.doi.org/10.1016/j.bbr.2011.01.022]. [PMID: 21262264]. [DOI] [PubMed] [Google Scholar]
- 217.Li Z., Geng Y.N., Jiang J.D., Kong W.J. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid. Based Complement. Alternat. Med. 2014;2014:289264. doi: 10.1155/2014/289264. [PMID: 24669227]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Thirupurasundari C.J., Padmini R., Devaraj S.N. Effect of berberine on the antioxidant status, ultrastructural modifications and protein bound carbohydrates in azoxymethane-induced colon cancer in rats. Chem. Biol. Interact. 2009;177(3):190–195. doi: 10.1016/j.cbi.2008.09.027. [http://dx.doi.org/10.1016/j.cbi.2008.09.027]. [PMID: 18951886]. [DOI] [PubMed] [Google Scholar]
- 219.Zhou J.Y., Zhou S.W. Protective effect of berberine on antioxidant enzymes and positive transcription elongation factor b expression in diabetic rat liver. Fitoterapia. 2011;82(2):184–189. doi: 10.1016/j.fitote.2010.08.019. [http://dx.doi.org/10.1016/j.fitote.2010.08.019]. [PMID: 20828602]. [DOI] [PubMed] [Google Scholar]
- 220.Zhang S., Zhang B., Dai W., Zhang X. Oxidative damage and antioxidant responses in Microcystis aeruginosa exposed to the allelochemical berberine isolated from golden thread. J. Plant Physiol. 2011;168(7):639–643. doi: 10.1016/j.jplph.2010.10.005. [http://dx.doi.org/10.1016/j.jplph.2010.10.005]. [PMID: 21131096]. [DOI] [PubMed] [Google Scholar]
- 221.Tan Y., Tang Q., Hu B.R., Xiang J.Z. Antioxidant properties of berberine on cultured rabbit corpus cavernosum smooth muscle cells injured by hydrogen peroxide. Acta Pharmacol. Sin. 2007;28(12):1914–1918. doi: 10.1111/j.1745-7254.2007.00705.x. [http://dx.doi.org/10.1111/j.1745-7254.2007.00705.x]. [PMID: 18031604]. [DOI] [PubMed] [Google Scholar]
- 222.Siow Y.L., Sarna L. Redox regulation in health and disease – therapeutic potential of Berberine. Food Res. Int. 2011;44:2409–2417. [http://dx.doi.org/10.1016/j.foodres.2010.12.038]. [Google Scholar]
- 223.Germoush M.O., Mahmoud A.M. Berberine mitigates cyclophosphamide-induced hepatotoxicity by modulating antioxidant status and inflammatory cytokines. J. Cancer Res. Clin. Oncol. 2014;140(7):1103–1109. doi: 10.1007/s00432-014-1665-8. [http://dx.doi.org/10.1007/s00432-014-1665-8]. [PMID: 24744190]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Luo A., Fan Y. Antioxidant activities of berberine hydrochloride. Int. J. Nanomedicine. 2016;11:1687–1700. [PMID: 27217747]. [Google Scholar]
- 225.Liu W.H., Hei Z.Q., Nie H., Tang F.T., Huang H.Q., Li X.J., Deng Y.H., Chen S.R., Guo F.F., Huang W.G., Chen F.Y., Liu P.Q. Berberine ameliorates renal injury in streptozotocin-induced diabetic rats by suppression of both oxidative stress and aldose reductase. Chin. Med. J. (Engl.) 2008;121(8):706–712. [http://dx.doi.org/10.1097/00029330-200804020-00009]. [PMID: 18701023]. [PubMed] [Google Scholar]
- 226.Hsieh Y.S., Kuo W.H., Lin T.W., Chang H.R., Lin T.H., Chen P.N., Chu S.C. Protective effects of berberine against low-density lipoprotein (LDL) oxidation and oxidized LDL-induced cytotoxicity on endothelial cells. J. Agric. Food Chem. 2007;55(25):10437–10445. doi: 10.1021/jf071868c. [http://dx.doi.org/10.1021/jf071868c]. [PMID: 18001034]. [DOI] [PubMed] [Google Scholar]
- 227.Sarna L.K., Wu N., Hwang S.Y., Siow Y.L. O, K. Berberine inhibits NADPH oxidase mediated superoxide anion production in macrophages. Can. J. Physiol. Pharmacol. 2010;88(3):369–378. doi: 10.1139/Y09-136. [http://dx.doi.org/10.1139/Y09-136]. [PMID: 20393601]. [DOI] [PubMed] [Google Scholar]
- 228.Jeong H.W., Hsu K.C., Lee J.W., Ham M., Huh J.Y., Shin H.J., Kim W.S., Kim J.B. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am. J. Physiol. Endocrinol. Metab. 2009;296(4):E955–E964. doi: 10.1152/ajpendo.90599.2008. [http://dx.doi.org/10.1152/ajpendo.90599.2008]. [PMID: 19208854]. [DOI] [PubMed] [Google Scholar]
- 229.Yokozawa T., Ishida A., Kashiwada Y., Cho E.J., Kim H.Y., Ikeshiro Y. Coptidis Rhizoma: protective effects against peroxynitrite-induced oxidative damage and elucidation of its active components. J. Pharm. Pharmacol. 2004;56(4):547–556. doi: 10.1211/0022357023024. [http://dx.doi.org/10.1211/0022357023024]. [PMID: 15099450]. [DOI] [PubMed] [Google Scholar]
- 230.Thirupurasundari C.J., Padmini R., Devaraj S.N. Effect of berberine on the antioxidant status, ultrastructural modifications and protein bound carbohydrates in azoxymethane-induced colon cancer in rats. Chem. Biol. Interact. 2009;177(3):190–195. doi: 10.1016/j.cbi.2008.09.027. [http://dx.doi.org/10.1016/j.cbi.2008.09.027]. [PMID: 18951886]. [DOI] [PubMed] [Google Scholar]
- 231.Hsu Y.Y., Chen C.S., Wu S.N., Jong Y.J., Lo Y.C. Berberine activates Nrf2 nuclear translocation and protects against oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent mechanism in NSC34 motor neuron-like cells. Eur. J. Pharm. Sci. 2012;46(5):415–425. doi: 10.1016/j.ejps.2012.03.004. [http://dx.doi.org/10.1016/j.ejps.2012.03.004]. [PMID: 22469516]. [DOI] [PubMed] [Google Scholar]
- 232.Hsu Y.Y., Tseng Y.T., Lo Y.C. Berberine, a natural antidiabetes drug, attenuates glucose neurotoxicity and promotes Nrf2-related neurite outgrowth. Toxicol. Appl. Pharmacol. 2013;272(3):787–796. doi: 10.1016/j.taap.2013.08.008. [http://dx.doi.org/10.1016/j.taap.2013.08.008]. [PMID: 23954465]. [DOI] [PubMed] [Google Scholar]
- 233.Pandey M.K., Sung B., Kunnumakkara A.B., Sethi G., Chaturvedi M.M., Aggarwal B.B. Berberine modifies cysteine 179 of IkappaBalpha kinase, suppresses nuclear factor-kappaB-regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 2008;68(13):5370–5379. doi: 10.1158/0008-5472.CAN-08-0511. [http://dx.doi.org/10.1158/0008-5472.CAN-08-0511]. [PMID: 18593939]. [DOI] [PubMed] [Google Scholar]
- 234.Zhang X., Gu L., Li J., Shah N., He J., Yang L., Hu Q., Zhou M. Degradation of MDM2 by the interaction between berberine and DAXX leads to potent apoptosis in MDM2-overexpressing cancer cells. Cancer Res. 2010;70(23):9895–9904. doi: 10.1158/0008-5472.CAN-10-1546. [http://dx.doi.org/10.1158/0008-5472.CAN-10-1546]. [PMID: 20935220]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhou Y., Liu S.Q., Yu L., He B., Wu S.H., Zhao Q., Xia S.Q., Mei H.J. Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis. 2015;20(9):1187–1199. doi: 10.1007/s10495-015-1152-y. [http://dx.doi.org/10.1007/s10495-015-1152-y]. [PMID: 26184498]. [DOI] [PubMed] [Google Scholar]
- 236.Wang Z., Chen Z., Chen T., Yi T., Zheng Z., Fan H., Chen Z. Berberine attenuates inflammation associated with delayed-type hypersensitivity via suppressing Th1 response and inhibiting apoptosis. Inflammation. 2017;40(1):221–231. doi: 10.1007/s10753-016-0472-6. [http://dx.doi.org/10.1007/s10753-016-0472-6]. [PMID: 27832398]. [DOI] [PubMed] [Google Scholar]
- 237.Lv X., Yu X., Wang Y., Wang F., Li H., Wang Y., Lu D., Qi R., Wang H. Berberine inhibits doxorubicin-triggered cardiomyocyte apoptosis via attenuating mitochondrial dysfunction and increasing Bcl-2 expression. PLoS One. 2012;7(10):e47351. doi: 10.1371/journal.pone.0047351. [http://dx.doi.org/10.1371/journal.pone.0047351]. [PMID: 23077597]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chen K., Li G., Geng F., Zhang Z., Li J., Yang M., Dong L., Gao F. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K-Akt signaling in diabetic rats. Apoptosis. 2014;19(6):946–957. doi: 10.1007/s10495-014-0977-0. [http://dx.doi.org/10.1007/s10495-014-0977-0]. [PMID: 24664781]. [DOI] [PubMed] [Google Scholar]
- 239.Wang Y., Liu J., Ma A., Chen Y. Cardioprotective effect of berberine against myocardial ischemia/reperfusion injury via attenuating mitochondrial dysfunction and apoptosis. Int. J. Clin. Exp. Med. 2015;8(8):14513–14519. [PMID: 26550442]. [PMC free article] [PubMed] [Google Scholar]
- 240.Li W., Liu Y., Wang B., Luo Y., Hu N., Chen D., Zhang X., Xiong Y. Protective effect of berberine against oxidative stress-induced apoptosis in rat bone marrow-derived mesenchymal stem cells. Exp. Ther. Med. 2016;12(6):4041–4048. doi: 10.3892/etm.2016.3866. [http://dx.doi.org/10.3892/etm.2016.3866]. [PMID: 28101183]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Guo J., Wang L., Wang L., Qian S., Zhang D., Fang J., Pan J. Berberine protects human umbilical vein endothelial cells against LPS-induced apoptosis by blocking JNK-mediated signaling. Evid. Based Complement. Alternat. Med. 2016;2016:6983956. doi: 10.1155/2016/6983956. [http://dx.doi.org/10.1155/2016/6983956]. [PMID: 27478481]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Adil M., Kandhare A.D., Dalvi G., Ghosh P., Venkata S., Raygude K.S., Bodhankar S.L. Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Ren. Fail. 2016;38(6):996–1006. doi: 10.3109/0886022X.2016.1165120. [http://dx.doi.org/10.3109/0886022X.2016.1165120]. [PMID: 27056079]. [DOI] [PubMed] [Google Scholar]
- 243.Zhang X., Liang D., Lian X., Jiang Y., He H., Liang W., Zhao Y., Chi Z.H. Berberine activates Nrf2 nuclear translocation and inhibits apoptosis induced by high glucose in renal tubular epithelial cells through a phosphatidylinositol 3-kinase/Akt-dependent mechanism. Apoptosis. 2016;21(6):721–736. doi: 10.1007/s10495-016-1234-5. [http://dx.doi.org/10.1007/s10495-016-1234-5]. [PMID: 26979714]. [DOI] [PubMed] [Google Scholar]
- 244.Hu J., Chai Y., Wang Y., Kheir M.M., Li H., Yuan Z., Wan H., Xing D., Lei F., Du L. PI3K p55γ promoter activity enhancement is involved in the anti-apoptotic effect of berberine against cerebral ischemia-reperfusion. Eur. J. Pharmacol. 2012;674(2-3):132–142. doi: 10.1016/j.ejphar.2011.11.014. [http://dx.doi.org/10.1016/j.ejphar.2011.11.014]. [PMID: 22119079]. [DOI] [PubMed] [Google Scholar]
- 245.Chai Y.S., Hu J., Lei F., Wang Y.G., Yuan Z.Y., Lu X., Wang X.P., Du F., Zhang D., Xing D.M., Du L.J. Effect of berberine on cell cycle arrest and cell survival during cerebral ischemia and reperfusion and correlations with p53/cyclin D1 and PI3K/Akt. Eur. J. Pharmacol. 2013;708(1-3):44–55. doi: 10.1016/j.ejphar.2013.02.041. [http://dx.doi.org/10.1016/j.ejphar.2013.02.041]. [PMID: 23499694]. [DOI] [PubMed] [Google Scholar]
- 246.Kim M., Cho K.H., Shin M.S., Lee J.M., Cho H.S., Kim C.J., Shin D.H., Yang H.J. Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson’s disease. Int. J. Mol. Med. 2014;33(4):870–878. doi: 10.3892/ijmm.2014.1656. [http://dx.doi.org/10.3892/ijmm.2014.1656]. [PMID: 24535622]. [DOI] [PubMed] [Google Scholar]
- 247.Kalalian-Moghaddam H., Baluchnejadmojarad T., Roghani M., Goshadrou F., Ronaghi A. Hippocampal synaptic plasticity restoration and anti-apoptotic effect underlie berberine improvement of learning and memory in streptozotocin-diabetic rats. Eur. J. Pharmacol. 2013;698(1-3):259–266. doi: 10.1016/j.ejphar.2012.10.020. [http://dx.doi.org/10.1016/j.ejphar.2012.10.020]. [PMID: 23099256]. [DOI] [PubMed] [Google Scholar]
- 248.Zhou X.Q., Zeng X.N., Kong H., Sun X.L. Neuroprotective effects of berberine on stroke models in vitro and in vivo. Neurosci. Lett. 2008;447(1):31–36. doi: 10.1016/j.neulet.2008.09.064. [http://dx.doi.org/10.1016/j.neulet.2008.09.064]. [PMID: 18838103]. [DOI] [PubMed] [Google Scholar]
- 249.Cui H.S., Matsumoto K., Murakami Y., Hori H., Zhao Q., Obi R. Berberine exerts neuroprotective actions against in vitro ischemia-induced neuronal cell damage in organotypic hippocampal slice cultures: involvement of B-cell lymphoma 2 phosphorylation suppression. Biol. Pharm. Bull. 2009;32(1):79–85. doi: 10.1248/bpb.32.79. [http://dx.doi.org/10.1248/bpb.32.79]. [PMID: 19122285]. [DOI] [PubMed] [Google Scholar]
- 250.Zhang Q., Qian Z., Pan L., Li H., Zhu H. Hypoxia-inducible factor 1 mediates the anti-apoptosis of berberine in neurons during hypoxia/ischemia. Acta Physiol. Hung. 2012;99(3):311–323. doi: 10.1556/APhysiol.99.2012.3.8. [http://dx.doi.org/10.1556/APhysiol.99.2012.3.8]. [PMID: 22982719]. [DOI] [PubMed] [Google Scholar]
- 251.Zhang X., Zhang X., Wang C., Li Y., Dong L., Cui L., Wang L., Liu Z., Qiao H., Zhu C., Xing Y., Cao X., Ji Y., Zhao K. Neuroprotection of early and short-time applying berberine in the acute phase of cerebral ischemia: Up-regulated pAkt, pGSK and pCREB, down-regulated NF-κB expression, ameliorated BBB permeability. Brain Res. 2012;1459:61–70. doi: 10.1016/j.brainres.2012.03.065. [http://dx.doi.org/10.1016/j.brainres.2012.03.065]. [PMID: 22560097]. [DOI] [PubMed] [Google Scholar]
- 252.Kim M., Shin M.S., Lee J.M., Cho H.S., Kim C.J., Kim Y.J., Choi H.R., Jeon J.W. Inhibitory effects of isoquinoline alkaloid berberine on ischemia-induced apoptosis via activation of phosphoinositide 3-kinase/protein kinase B signaling pathway. Int. Neurourol. J. 2014;18(3):115–125. doi: 10.5213/inj.2014.18.3.115. [http://dx.doi.org/10.5213/inj.2014.18.3.115]. [PMID: 25279238]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Simões Pires E.N., Frozza R.L., Hoppe J.B. Menezes, Bde.M.; Salbego, C.G. Berberine was neuroprotective against an in vitro model of brain ischemia: Survival and apoptosis pathways involved. Brain Res. 2014;1557:26–33. doi: 10.1016/j.brainres.2014.02.021. [http://dx.doi.org/10.1016/j.brainres.2014.02.021]. [PMID: 24560603]. [DOI] [PubMed] [Google Scholar]
- 254.Sadeghnia H.R., Kolangikhah M., Asadpour E., Forouzanfar F., Hosseinzadeh H. Berberine protects against glutamate-induced oxidative stress and apoptosis in PC12 and N2a cells. Iran. J. Basic Med. Sci. 2017;20(5):594–603. doi: 10.22038/IJBMS.2017.8847. [PMID: 28656094]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Liang Y., Huang M., Jiang X., Liu Q., Chang X., Guo Y. The neuroprotective effects of berberine against amyloid β-proteininduced apoptosis in primary cultured hippocam-pal neurons via mitochondria-related caspase pathway. Neurosci Lett. 2017;S0304-3940:30540–30542.. doi: 10.1016/j.neulet.2017.06.048. [DOI] [PubMed] [Google Scholar]
- 256.Zhang Q., Bian H., Guo L., Zhu H. Pharmacologic preconditioning with berberine attenuating ischemia-induced apoptosis and promoting autophagy in neuron. Am. J. Transl. Res. 2016;8(2):1197–1207. [PMID: 27158406]. [PMC free article] [PubMed] [Google Scholar]
- 257.Heemels M.T. Neurodegenerative diseases. Nature. 2016;539(7628):179. doi: 10.1038/539179a. [http://dx.doi.org/10.1038/539179a]. [PMID: 27830810]. [DOI] [PubMed] [Google Scholar]
- 258.Yates D. Neurodegenerative disease: Straining the brain. Nat. Rev. Neurosci. 2016;17(12):738. doi: 10.1038/nrn.2016.161. [http://dx.doi.org/10.1038/nrn.2016.161]. [PMID: 27853142]. [DOI] [PubMed] [Google Scholar]
- 259.Zhang K., Rothstein J.D. Neurodegenerative disease: Two-for-one on potential therapies. Nature. 2017;544(7650):302–303. doi: 10.1038/nature21911. [http://dx.doi.org/10.1038/nature21911]. [PMID: 28405020]. [DOI] [PubMed] [Google Scholar]
- 260.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79(3):629–661. doi: 10.1021/acs.jnatprod.5b01055. [http://dx.doi.org/10.1021/acs.jnatprod.5b01055]. [PMID: 26852623]. [DOI] [PubMed] [Google Scholar]
- 261.Butler M.S., Robertson A.A., Cooper M.A. Natural product and natural product derived drugs in clinical trials. Nat. Prod. Rep. 2014;31(11):1612–1661. doi: 10.1039/c4np00064a. [http://dx.doi.org/10.1039/C4NP00064A]. [PMID: 25204227]. [DOI] [PubMed] [Google Scholar]
- 262.Chen X., Decker M. Multi-target compounds acting in the central nervous system designed from natural products. Curr. Med. Chem. 2013;20(13):1673–1685. doi: 10.2174/0929867311320130007. [http://dx.doi.org/10.2174/0929867311320130007]. [PMID: 23410166]. [DOI] [PubMed] [Google Scholar]
- 263.Ansari N., Khodagholi F. Natural products as promising drug candidates for the treatment of Alzheimer’s disease: Molecular mechanism aspect. Curr. Neuropharmacol. 2013;11(4):414–429. doi: 10.2174/1570159X11311040005. [http://dx.doi.org/10.2174/1570159X11311040005]. [PMID: 24381531]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Khin-Maung-U. Myo-Khin.; Nyunt-Nyunt-Wai.; Aye-Kyaw.; Tin-U, Clinical trial of berberine in acute watery diarrhoea. Br. Med. J. (Clin. Res. Ed.) 1985;291(6509):1601–1605. doi: 10.1136/bmj.291.6509.1601. [http://dx.doi.org/10.1136/bmj.291.6509.1601]. [PMID: 3935203]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Chen C., Tao C., Liu Z., Lu M., Pan Q., Zheng L., Li Q., Song Z., Fichna J. A randomized clinical trial of berberine hydrochloride in patients with diarrhea-predominant irritable bowel syndrome. Phytother. Res. 2015;29(11):1822–1827. doi: 10.1002/ptr.5475. [http://dx.doi.org/10.1002/ptr.5475]. [PMID: 26400188]. [DOI] [PubMed] [Google Scholar]
- 266.Caliceti C., Franco P., Spinozzi S., Roda A., Cicero A.F. Berberine: Berberine: New insights from pharmacological aspects to clinical evidences in the management of metabolic disorders. Curr. Med. Chem. 2016;23(14):1460–1476. doi: 10.2174/0929867323666160411143314. [http://dx.doi.org/10.2174/0929867323666160411143314]. [PMID: 27063256]. [DOI] [PubMed] [Google Scholar]
- 267.Derosa G., Maffioli P., Cicero A.F. Berberine on metabolic and cardiovascular risk factors: An analysis from preclinical evidences to clinical trials. Expert Opin. Biol. Ther. 2012;12(8):1113–1124. doi: 10.1517/14712598.2012.704014. [http://dx.doi.org/10.1517/14712598.2012.704014]. [PMID: 22780092]. [DOI] [PubMed] [Google Scholar]
- 268.Cicero A.F., Tartagni E. Antidiabetic properties of berberine: From cellular pharmacology to clinical effects. Hosp Pract (1995) 2012;40(2):56–63. doi: 10.3810/hp.2012.04.970. [http://dx.doi.org/10.3810/hp.2012.04.970] [PMID: 22615079]. [DOI] [PubMed] [Google Scholar]
- 269.Chang W., Chen L., Hatch G.M. Berberine as a therapy for type 2 diabetes and its complications: From mechanism of action to clinical studies. Biochem. Cell Biol. 2015;93(5):479–486. doi: 10.1139/bcb-2014-0107. [http://dx.doi.org/10.1139/bcb-2014-0107]. [PMID: 25607236]. [DOI] [PubMed] [Google Scholar]
- 270.Wei W., Zhao H., Wang A., Sui M., Liang K., Deng H., Ma Y., Zhang Y., Zhang H., Guan Y. A clinical study on the short-term effect of berberine in comparison to metformin on the metabolic characteristics of women with polycystic ovary syndrome. Eur. J. Endocrinol. 2012;166(1):99–105. doi: 10.1530/EJE-11-0616. [http://dx.doi.org/10.1530/EJE-11-0616]. [PMID: 22019891]. [DOI] [PubMed] [Google Scholar]
- 271.An Y., Sun Z., Zhang Y., Liu B., Guan Y., Lu M. The use of berberine for women with polycystic ovary syndrome undergoing IVF treatment. Clin. Endocrinol. (Oxf.) 2014;80(3):425–431. doi: 10.1111/cen.12294. [http://dx.doi.org/10.1111/cen.12294]. [PMID: 23869585]. [DOI] [PubMed] [Google Scholar]
- 272.Dong H., Zhao Y., Zhao L., Lu F. The effects of berberine on blood lipids: A systemic review and meta-analysis of randomized controlled trials. Planta Med. 2013;79(6):437–446. doi: 10.1055/s-0032-1328321. [http://dx.doi.org/10.1055/s-0032-1328321]. [PMID: 23512497]. [DOI] [PubMed] [Google Scholar]
- 273.Pirillo A., Catapano A.L. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis. 2015;243(2):449–461. doi: 10.1016/j.atherosclerosis.2015.09.032. [http://dx.doi.org/10.1016/j.atherosclerosis.2015.09.032]. [PMID: 26520899]. [DOI] [PubMed] [Google Scholar]
- 274.Zeng X.H., Zeng X.J., Li Y.Y. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2003;92(2):173–176. doi: 10.1016/s0002-9149(03)00533-2. [http://dx.doi.org/10.1016/S0002-9149(03)00533-2]. [PMID: 12860219]. [DOI] [PubMed] [Google Scholar]
- 275.Lan J., Zhao Y., Dong F., Yan Z., Zheng W., Fan J., Sun G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 2015;161:69–81. doi: 10.1016/j.jep.2014.09.049. [http://dx.doi.org/10.1016/j.jep.2014.09.049]. [PMID: 25498346]. [DOI] [PubMed] [Google Scholar]
- 276.Wang X., Wang R., Xing D., Su H., Ma C., Ding Y., Du L. Kinetic difference of berberine between hippocampus and plasma in rat after intravenous administration of Coptidis rhizoma extract. Life Sci. 2005;77(24):3058–3067. doi: 10.1016/j.lfs.2005.02.033. [http://dx.doi.org/10.1016/j.lfs.2005.02.033]. [PMID: 15996686]. [DOI] [PubMed] [Google Scholar]
- 277.Wei W., Zhao H., Wang A., Sui M., Liang K., Deng H., Ma Y., Zhang Y., Zhang H., Guan Y. A clinical study on the short-term effect of berberine in comparison to metformin on the metabolic characteristics of women with polycystic ovary syndrome. Eur. J. Endocrinol. 2012;166(1):99–105. doi: 10.1530/EJE-11-0616. [http://dx.doi.org/10.1530/EJE-11-0616]. [PMID: 22019891]. [DOI] [PubMed] [Google Scholar]
- 278.Zhang H., Wei J., Xue R., Wu J.D., Zhao W., Wang Z.Z., Wang S.K., Zhou Z.X., Song D.Q., Wang Y.M., Pan H.N., Kong W.J., Jiang J.D. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010;59(2):285–292. doi: 10.1016/j.metabol.2009.07.029. [http://dx.doi.org/10.1016/j.metabol.2009.07.029]. [PMID: 19800084]. [DOI] [PubMed] [Google Scholar]
- 279.Zeng X.H., Zeng X.J., Li Y.Y. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2003;92(2):173–176. doi: 10.1016/s0002-9149(03)00533-2. [http://dx.doi.org/10.1016/S0002-9149(03)00533-2]. [PMID: 12860219]. [DOI] [PubMed] [Google Scholar]
- 280.Sack R.B., Froehlich J.L. Berberine inhibits intestinal secretory response of Vibrio cholerae and Escherichia coli enterotoxins. Infect. Immun. 1982;35(2):471–475. doi: 10.1128/iai.35.2.471-475.1982. [PMID: 7035365]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Li G.H., Wang D.L., Hu Y.D., Pu P., Li D.Z., Wang W.D., Zhu B., Hao P., Wang J., Xu X.Q., Wan J.Q., Zhou Y.B., Chen Z.T. Berberine inhibits acute radiation intestinal syndrome in human with abdomen radiotherapy. Med. Oncol. 2010;27(3):919–925. doi: 10.1007/s12032-009-9307-8. [http://dx.doi.org/10.1007/s12032-009-9307-8]. [PMID: 19757213]. [DOI] [PubMed] [Google Scholar]
- 282.Xue M., Yang M.X., Zhang W., Li X.M., Gao D.H., Ou Z.M., Li Z.P., Liu S.H., Li X.J., Yang S.Y. Characterization, pharmacokinetics, and hypoglycemic effect of berberine loaded solid lipid nanoparticles. Int. J. Nanomedicine. 2013;8:4677–4687. doi: 10.2147/IJN.S51262. [http://dx.doi.org/10.2147/IJN.S51262]. [PMID: 24353417]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Xue M., Zhang L., Yang M.X., Zhang W., Li X.M., Ou Z.M., Li Z.P., Liu S.H., Li X.J., Yang S.Y. Berberine-loaded solid lipid nanoparticles are concentrated in the liver and ameliorate hepatosteatosis in db/db mice. Int. J. Nanomedicine. 2015;10:5049–5057. doi: 10.2147/IJN.S84565. [http://dx.doi.org/10.2147/IJN.S84565]. [PMID: 26346310]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lin Y.H., Lin J.H., Chou S.C., Chang S.J., Chung C.C., Chen Y.S., Chang C.H. Berberine-loaded targeted nanoparticles as specific Helicobacter pylori eradication therapy: In vitro and in vivo study. Nanomedicine (Lond.) 2015;10(1):57–71. doi: 10.2217/nnm.14.76. [http://dx.doi.org/10.2217/nnm.14.76]. [PMID: 25177920]. [DOI] [PubMed] [Google Scholar]
- 285.Chang C.H., Huang W.Y., Lai C.H., Hsu Y.M., Yao Y.H., Chen T.Y., Wu J.Y., Peng S.F., Lin Y.H. Development of novel nanoparticles shelled with heparin for berberine delivery to treat Helicobacter pylori. Acta Biomater. 2011;7(2):593–603. doi: 10.1016/j.actbio.2010.08.028. [http://dx.doi.org/10.1016/j.actbio.2010.08.028]. [PMID: 20813208]. [DOI] [PubMed] [Google Scholar]
- 286.Yu F., Ao M., Zheng X., Li N., Xia J., Li Y., Li D., Hou Z., Qi Z., Chen X.D. PEG-lipid-PLGA hybrid nanoparticles loaded with berberine-phospholipid complex to facilitate the oral delivery efficiency. Drug Deliv. 2017;24(1):825–833. doi: 10.1080/10717544.2017.1321062. [http://dx.doi.org/10.1080/10717544.2017.1321062]. [PMID: 28509588]. [DOI] [PMC free article] [PubMed] [Google Scholar]


