Abstract
Background
As the debate continues about whether obesity in metabolically healthy individuals is associated with poor outcomes or not, investigating the association between the obesity phenotypes and markers of subclinical myocardial injury will help identify those at risk for future cardiovascular events (cardiovascular disease [CVD]).
Hypothesis
We hypothesize that obesity phenotypes including metabolically healthy obesity (MHO) is associated with subclinical myocardial injury (SC‐MI).
Methods
This analysis included 3423 participants (57.85 ± 13.06 years, 53.3% women) without known CVD from National Health and Nutrition Examination Survey (NHANES) III. Multivariable logistic regression models were used to examine the cross‐sectional association between four obesity phenotypes (metabolically healthy nonobese (MHNO) [reference], metabolically unhealthy nonobese (MUNO), MHO, and metabolically unhealthy obese (MUO) with SC‐MI. SC‐MI was defined from the 12‐lead electrocardiogram as cardiac infarction/injury score ≥ 10 units. Metabolic syndrome (MetS) was defined according to the International Diabetes Federation consensus definition. Obesity was defined as body mass index ≥30 kg/m2.
Results
MUO was associated with higher odds of SC‐MI compared with MHNO (odds ratio [OR], 1.53; 95% confidence interval [CI], 1.22‐1.92, P = 0.0005). This association was stronger in men vs women (OR [95% CI]: 2.20 [1.58‐2.07] vs 1.08 [0.79‐1.48]), respectively; interaction P‐value = 0.002) but was consistent in subgroups stratified by age and race. There was no significant association of MHO or MUNO with SC‐MI compared with MHNO, but there was a trend toward higher odds of SC‐MI in the MUNO group (P‐value for trend across MHNO, MUNO, and MUO = 0.0002).
Conclusions
Our findings suggest that a combination of obesity and MetS confers worse prognosis and early preventive strategies aimed at weight loss and management of MetS components may decrease the risk of future poor outcomes.
Keywords: metabolic syndrome, metabolically healthy obesity, NHANES III, subclinical myocardial injury
1. INTRODUCTION
Although metabolic abnormalities and obesity are known risk factors for cardiovascular disease (CVD), a subcategory of obesity without metabolic syndrome (MetS), referred to as metabolically healthy obesity (MHO) has yielded contradictory estimates of association with CVD.1, 2 MHO was associated with increased CVD risk in previous studies including four meta‐analyses.3, 4, 5, 6, 7 However, several individual studies did not find any association between MHO and CVD.8, 9, 10
As this controversy about MHO and CVD continues, an examination of MHO and other obesity phenotypes with cardiovascular markers of poor outcomes may provide further evidence of more proximal risks associated with these phenotypes. One such marker is the cardiac infarction/injury score (CIIS), an electrocardiographic‐based scoring system used to define subclinical myocardial injury (SC‐MI).11 CIIS has been associated with future adverse events such as coronary heart disease (CHD), cardiovascular and all‐cause mortality.12, 13, 14 An examination of the association of obesity phenotypes with a marker of SC‐MI using 12‐lead electrocardiogram is a simple and cost‐effective way to stratify the CVD risk associated with these phenotypes. Therefore, we sought to examine the cross‐sectional association between obesity phenotypes and SC‐MI in a sample from the third National Health and Nutrition Examination Survey (NHANES III) free of clinically diagnosed CVD. We hypothesized that obesity phenotypes (MUNO, MHO, and MUO) would be associated with prevalent SC‐MI independent of potential confounders.
2. METHODS
2.1. Study population
NHANES is a periodic survey of a representative sample of the civilian noninstitutionalized US population. Its principal aim is to determine estimates of disease prevalence and health status of the US population. The National Center for Health Statistics of the Center for Disease Control and Prevention Institutional Review Board approved the protocol for NHANES‐III. All participants gave written informed consent. Baseline data were collected during an in‐home interview and a subsequent visit to a mobile examination center between 1988 and 1994. The following characteristics were self‐reported: age, sex, race/ethnicity, income, prevalent CVD, cancer history, smoking status, and leisure time physical activity. Medication history, including the use of antihypertensive agents and lipid‐lowering therapies, also were self‐reported. A physical examination was performed to obtain body mass index (BMI). Blood pressure readings were taken during the in‐home evaluation and again during the mobile examination center visit and averaged for each participant. Fasting blood samples were collected to measure total cholesterol, high‐density lipoprotein cholesterol, triglycerides, and glucose, using laboratory procedures as reported by NCHS.15
For this analysis, we only included NHANES‐III participants who underwent an ECG recording (n = 8561). We excluded participants with a history of CVD (myocardial infarction, heart failure, or stroke), any major electrocardiographic abnormalities based on Minnesota Code classification,16 taking anti‐arrhythmic drugs, with implanted pacemakers, with cancer on chemotherapy, or missing key covariates. Finally, to be eligible for this analysis, participants must have provided a blood sample after fasting for at least 8 hours. After all exclusions (n = 5138), 3423 participants were included in the final analysis.
Obesity was defined as BMI ≥ 30 kg/m2. Obese and nonobese participants were subsequently divided into two subgroups based on the presence of MetS. MetS was defined according to the International Diabetes Federation consensus definition as having three or more metabolic abnormalities17 (Supporting information Table S1).
2.2. Electrocardiogram
Resting 12‐lead electrocardiograms were obtained with a Marquette MAC 12 system (Marquette Medical Systems, Milwaukee, Wisconsin) during the mobile examination visits by trained technicians. Analysis of electrocardiograms was achieved through a computerized automated process and visual inspection by a trained technician located in a centralized core laboratory. The derivation of the CIIS and methodology have been described previously.11 The SC‐MI defined by CIIS is based on a weighted scoring system taking several objective electrocardiographic waveform components related to myocardial injury and ischemia, both discrete and continuous, and generating a risk‐stratified scoring system. This system was designed to improve on previous models that relied on more subjective criteria and decision trees which were vulnerable to the erroneous application at each branch along the decision tree. The score is defined by a combination of 11 discrete and four continuous features and provides a simple scoring scheme suitable for both visual and computer classification of a standard 12‐lead ECG. SC‐MI was defined as CIIS values ≥10 points.11, 12
2.3. Statistical analysis
Baseline characteristics were compared across nonobesity (MHNO and MUNO) and obesity (MHO and MUO). Continuous variables were reported as mean ± SD, while categorical variables were reported as frequency and percentage. Student t‐test was used to compare the continuous variables while χ 2 was used to compare the categorical variables. Multivariable logistic regression analysis was used to compute odds ratios (ORs) and 95% confidence interval (CI) for the cross‐sectional association between each obesity phenotype (MHNO [reference], MUNO, MHO, and MUO) and SC‐MI. We calculated P for trend across obesity phenotypes using a multivariable logistic regression model. Two incremental models were constructed: model 1 adjusted for age, sex, race (non‐whites), and socioeconomic status. Model 2 was further adjusted for smoking, physical activity and low‐density lipoprotein (LDL‐C).
We also conducted a subgroup analysis of the association between obesity phenotypes and SC‐MI stratified by age (dichotomized at 65), sex and race (white vs non‐white). The models were adjusted similarly to model 2 as mentioned above.
Additional analyses were performed as follows: First, we performed multivariable linear regression analysis with each obesity phenotype (MHNO [reference] MUNO, MHO, and MUO) as the independent variable and CIIS as the continuous outcome variable to calculate the adjusted mean ± SE. Models were adjusted as mentioned above. Secondly, to assess whether increasing BMI is associated with higher CIIS, we examined the association of BMI categories (18.5‐24.9 (reference), 25‐29.9, 30‐34.9, 35‐39.9, and ≥ 40) with CIIS using linear regression models. Model 1 was adjusted for age, sex, non‐whites, socioeconomic status and model 2 adjusted for model 1 plus smoking, physical activity, LDL‐C, and all MetS components.
All statistical analyses were performed using with SAS version 9.4 (SAS Institute Inc, Cary, NC), and P‐values were considered significant if less than 0.05.
3. RESULTS
This analysis included 3423 participants (57.85 ± 13.06 years, 53.3% women, 49.3% non‐Hispanic whites) of whom 49.6%, 21.8%, 9.6%, and 18.8% had MHNO, MUNO, MHO, and MUO, respectively. SC‐MI was present in 21.7% (n = 744) of participants. The prevalence of SC‐MI was 19.3% in MHNO, 24.3% in MUNO, 16.9% in MHO and 27.4% in MUO participants. Table 1 shows baseline characteristics of participants stratified by nonobesity and obesity.
Table 1.
Baseline Characteristics of the study participants
| Characteristics, mean ± SD or n (%) | Nonobesity | P‐valuea | Obesity | P‐valuea | ||
|---|---|---|---|---|---|---|
| Metabolically healthy n = 1700 | Metabolically unhealthy n = 748 | Metabolically healthy n = 331 | Metabolically unhealthy n = 644 | |||
| Age (years) | 57.2 ± 13.3 | 62.0 ± 13.1 | <0.0001 | 52.8 ± 11.2 | 57.1 ± 11.6 | <0.0001 |
| Male (%) | 853 (50.1%) | 362 (48.4%) | 0.41 | 110 (33.2%) | 271 (42.0%) | 0.007 |
| Non‐Hispanic white | 883 (51.9%) | 408 (54.5%) | 0.23 | 114 (34.4%) | 285 (44.2%) | 0.003 |
| Total annual family income <20 000 | 678 (40.3%) | 346 (47.2%) | 0.001 | 146 (44.9%) | 330 (52.4%) | 0.02 |
| Systolic blood pressure (mm Hg) | 125.51 ± 18.20 | 137.38 ± 18.54 | <0.0001 | 125.20 ± 16.49 | 135.72 ± 16.93 | <0.0001 |
| Diastolic blood pressure (mm Hg) | 74.14 ± 9.56 | 77.93 ± 10.25 | <0.0001 | 76.69 ± 8.90 | 79.24 ± 9.93 | <0.0001 |
| Insulin resistance (%) | 438 (25.8%) | 543 (72.6%) | <0.0001 | 61 (18.6%) | 458 (71.1%) | <0.0001 |
| Triglycerides (mg/dL) | 111.0 ± 64.1 | 203.6 ± 117.2 | <0.0001 | 113.9 ± 74.1 | 207.8 ± 192.5 | <0.0001 |
| HDL cholesterol (mg/dL) | 57.3 ± 16.8 | 44.1 ± 13.3 | <0.0001 | 54.1 ± 12.8 | 43.9 ± 12.0 | <0.0001 |
| LDL‐C (mg/dL) | 132.4 ± 38.1 | 142.0 ± 37.1 | <0.0001 | 136.4 ± 35.8 | 142.3 ± 41.1 | 0.02 |
| Waist circumference (cm) | 88.0 ± 9.1 | 97.2 ± 12.8 | <0.0001 | 106.3 ± 10.4 | 110.7 ± 10.0 | <0.0001 |
| Body mass index (Kg/m2) | 24.2 ± 2.9 | 26.6 ± 2.4 | <0.0001 | 33.5 ± 3.6 | 34.3 ± 4.4 | 0.005 |
| Smoking (%) | ||||||
| Current smoker | 457 (26.8%) | 172 (22.9%) | 0.04 | 54 (16.3%) | 120 (18.6%) | 0.37 |
| Former smoker | 534 (31.4%) | 246 (32.8%) | 0.47 | 82 (24.7%) | 223 (34.6%) | 0.001 |
| Never smoker | 709 (41.7%) | 330 (44.1%) | 0.26 | 195 (58.9%) | 301 (46.7%) | 0.0003 |
| Physical activity (METs per week)b | 13.6 (2.3‐34.8) | 10.1 (1.0‐31.3) | 0.001 | 7.0 (0.8‐24.4) | 5.8 (0‐24.4) | 0.36 |
| SC‐MI (%) | 329 (19.3%) | 182 (24.3%) | 0.005 | 56 (16.9%) | 177 (27.4%) | 0.0002 |
| Cardiac infarction/injury score | 4.8 ± 6.4 | 5.6 ± 7.1 | 0.009 | 4.2 ± 5.7 | 6.1 ± 7.4 | <0.0001 |
Abbreviations: LDL‐C, low‐density lipoprotein cholesterol; HDL, high‐density cholesterol; SC‐MI, subclinical myocardial injury; METs, metabolic equivalent.
P‐value by student t test for continuous and χ 2 for categorical variables.
METs reported as median and IQR.
Among obesity group, MUO were more likely to be old, men, white, smokers, and to have low annual income and physical activity levels compared to MHO. Among the nonobesity group, MUNO were more likely to be old, women, white, nonsmokers, and to have low annual income and physical activity levels compared to MHNO.
In multivariable models adjusted for potential confounders, MUO was associated with higher odds of SC‐MI (OR 1.53; 95% CI, 1.22‐1.92, P = 0.0005). There was no statistically significant association between MHO or MUNO with SC‐MI; However, a trend of higher odds of SC‐MI was observed in MUNO (P‐value for trend across MHNO, MUNO, and MUO = 0.0002) (Table 2). A similar pattern of association was observed when using CIIS as a continuous variable, as shown in Table 3, there was a pattern of higher mean values in MUO followed by MUNO in multivariable linear regression models.
Table 2.
Multivariable odds ratio and 95% CI of association between obesity phenotypes and subclinical myocardial injury
| Obesity phenotypes | Model 1a | Model 2b | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P‐value | Odds ratio (95% CI) | P‐value | |
| Healthy nonobese | Reference | Reference | ||
| Unhealthy nonobese | 1.20 (0.97, 1.49) | 0.72 | 1.14 (0.92, 1.42) | 0.95 |
| Healthy obese | 0.97 (0.70, 1.34) | 0.11 | 0.96 (0.69, 1.33) | 0.16 |
| Unhealthy obese | 1.60 (1.29, 1.99) | 0.0001 | 1.53 (1.22, 1.92) | 0.0005 |
Abbreviations: LDL‐C, low density‐lipoprotein cholesterol.
Model 1 adjusted for age, sex, non‐whites and socioeconomic status.
Model 2 adjusted for model 1 plus smoking and physical activity and LDL‐C.
Table 3.
Least mean square and SE of cardiac infarction/injury score across obesity phenotypes
| Obesity phenotypes | Model 1a | Model 2b |
|---|---|---|
| Mean ± SE | Mean ± SE | |
| Healthy nonobese | 4.92 ± 0.16 | 4.98 ± 0.16 |
| Unhealthy nonobese | 5.27 ± 0.24 | 5.11 ± 0.25 |
| Healthy obese | 4.78 ± 0.37 | 4.76 ± 0.38 |
| Unhealthy obese | 6.28 ± 0.26 | 6.20 ± 0.27 |
Abbreviation: LDL‐C, low‐density lipoprotein cholesterol.
Least square mean and SE calculated from multivariable linear regression.
Model 1 adjusted for age, sex, non‐whites and socioeconomic status.
Model 2 adjusted for model 1 plus smoking and physical activity and LDL‐C.
In subgroup analysis, heterogeneity in the association between obesity phenotypes and SC‐MI was observed by sex. With MHNO as a reference, all obesity phenotypes and particularly MUO had a stronger association with SC‐MI in men compared to women (OR [95% CI]: 2.20 [1.58‐2.07] vs 1.08 [0.79‐1.48] respectively; interaction P‐value = 0.002). There was no statistically significant interaction by age or race (Table 4).
Table 4.
Multivariable odds ratios and 95% CI for the association between obesity phenotypes and subclinical myocardial injury in subgroups
| Subgroups | SC‐MI, n (%) | Obesity phenotype | Odds ratio (95% CI) | Interaction P‐value |
|---|---|---|---|---|
| Male | 99/362 (27.3%) | Unhealthy nonobese | 1.52 (1.12‐2.07) | 0.002 |
| 24/110 (21.8%) | Healthy obese | 1.36 (0.82‐2.25) | ||
| 89/271 (32%) | Unhealthy obese | 2.20 (1.58‐3.07) | ||
| Female | 83/386 (21.5%) | Unhealthy nonobese | 0.83 (0.60‐1.14) | |
| 32/221 (14.4%) | Healthy obese | 0.71 (0.46‐1.10) | ||
| 88/373 (23.5%) | Unhealthy obese | 1.08 (0.79‐1.48) | ||
| White | 109/408 (26.7%) | Unhealthy nonobese | 1.11 (0.83‐1.48) | 0.69 |
| 17/114 (14.9%) | Healthy obese | 0.74 (0.42‐1.29) | ||
| 83/285 (29.1%) | Unhealthy obese | 1.46 (1.06‐2.01) | ||
| Non‐white | 73/340 (21.4%) | Unhealthy nonobese | 1.19 (0.85‐1.67) | |
| 39/217 (17.9%) | Healthy obese | 1.13 (0.75‐1.72) | ||
| 94/359 (26.1%) | Unhealthy obese | 1.62 (1.17‐2.24) | ||
| Age > 65 y | 86/295 (29.1%) | Unhealthy nonobese | 1.05 (0.75‐1.47) | 0.59 |
| 12/53 (22.6%) | Healthy obese | 0.83 (0.42‐1.65) | ||
| 48/159 (30.1%) | Unhealthy obese | 1.20 (0.79‐1.81) | ||
| Age ≤ 65 y | 96/453 (21.1%) | Unhealthy nonobese | 1.26 (0.94‐1.68) | |
| 44/278 (15.8%) | Healthy obese | 0.96 (0.66‐1.40) | ||
| 129/485 (26.6%) | Unhealthy obese | 1.70 (1.30‐2.23) |
Abbreviation: LDL‐C, low‐density lipoprotein cholesterol.
Reference group = metabolically healthy nonobese.
Model adjusted for Age, sex, non‐whites, socioeconomic status, smoking and physical activity and LDL‐C.
Table S2 and Figure 1 show the results of linear association of BMI with CIIS. Higher values of CIIS score were observed with increasing BMI independent of socio‐demographic and CVD risk factors (trend P‐value 0.22).
Figure 1.
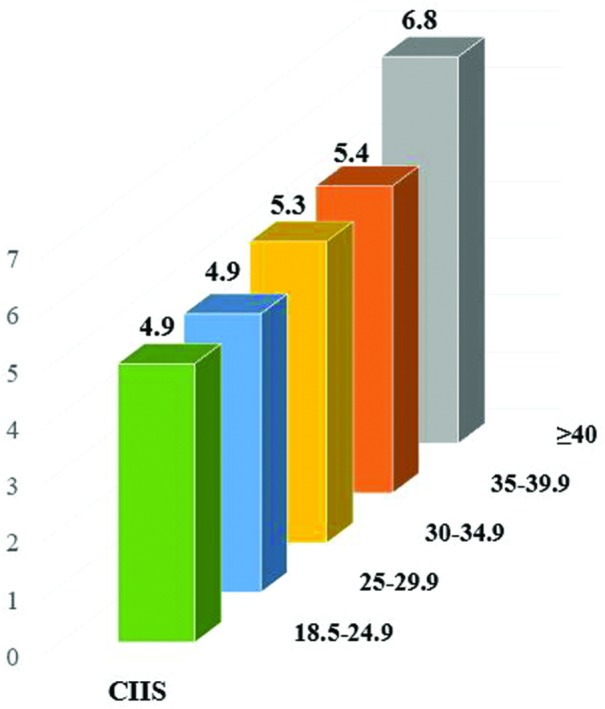
Mean cardiac infarction/injury score (CIIS) across body mass index (BMI) categories. Mean CIIS across BMI categories in a model adjusted for age, sex, race, socioeconomic status, smoking, physical activity, low‐density lipoprotein cholesterol (LDL‐C), insulin resistance, hypertension, elevated triglyceride (TG), Low high‐density lipoprotein (HDL), and high waist circumference (WC)
4. DISCUSSION
We examined the cross‐sectional association between obesity phenotypes and SC‐MI using data from NHANES III. The key findings of our study are as follows1: The prevalence of SC‐MI was highest in MUO followed by MUNO, MHO, and then MHNO2; Among obesity phenotypes, MUO was significantly associated with higher odds of SC‐MI, and there was trend towards more abnormalities in MUNO following MUO3; A similar pattern of higher mean values of CIIS in MUO followed by MUNO was observed4; heterogeneity in association between obesity phenotypes and SC‐MI by sex was observed where the association was stronger in men vs women5; there was an incremental increase in CIIS with higher values among those participants with higher BMI independent of MetS.
These results taken together indicate that the combination of obesity and MetS is associated with higher odds of SC‐MI and mean values of CIIS. BMI and other measures of adiposity have been consistently associated with CVD, and the major impact of obesity on cardiovascular health is mediated by accompanying metabolic abnormalities.18 Observation of higher CIIS with increasing BMI also suggests that excess weight is not without consequences and thus challenges the notion that obesity can be healthy.
MHO is considered a transient state; the duration and severity of obesity leads to an unhealthy state with the passage of time, thus increasing the risk for CVD events.6, 19 Therefore, a cross‐sectional examination may limit the adequate assessment of potential risks associated with MHO. This may explain the weaker association between MHO with SC‐MI which did not reach statistical significance when compared to MHNO.
We observed a strong association of obesity phenotypes with SC‐MI among men. Men generally have a higher incidence of CHD and higher age‐adjusted CHD mortality rate compared to women.20, 21, 22 Our findings of gender differences in the association of SC‐MI add to accumulating evidence of sex/gender differences in the prevalence and outcomes of different CVD. Future investigation should assess whether genetic background, emerging risk factors, access to health care, awareness, and adherence of medications contribute to sex differences.
In previous studies, MUNO had similar CVD risks as those with MUO.3, 4 In support of these findings, we observed higher odds of SC‐MI with MUNO, especially in men, suggesting that maintaining metabolic health remains important even in the absence of obesity. Finally, the MUO group has consistently exhibited an unfavorable prognosis in terms of CVD events and mortality across all studies,3, 4 likely due to the cumulative effect of obesity and MetS. A strong association of MUO with SC‐MI in our study supports these findings.
Our study has certain limitations. First, we are unable to establish a temporal relationship between obesity phenotypes and SC‐MI due to the cross‐sectional design of the study; However, it is unlikely that SC‐MI leads to obesity and Mets, but the opposite is more plausible. Some of the measurements such as smoking and physical activity are self‐reported and thus subject to recall bias. Finally, we adjusted for several confounders, but residual confounding remains a possibility. Strengths of the study include a large sample size and a community‐living multiracial population with generalizability to the US population, as well as the fact that the key variables were ascertained using standard protocols.
5. CONCLUSION
Our results in NHANES‐III provide evidence that MetS may contribute to myocardial injury especially in those with obesity. Also, the higher the obesity class based on BMI, the higher is the risk of myocardial injury independent of MetS. With the increasing prevalence of obesity and MetS, it is important to identify high‐risk populations so that finite resources can be allocated to appropriate groups.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
Supporting information
Table S1. Definition of metabolic syndrome and obesity phenotypes.
Table S2. Association of cardiac infarction/injury score (CIIS) with body mass index (BMI) categories.
Vasim I, Ahmad MI, Mongraw‐Chaffin M, Soliman EZ. Association of obesity phenotypes with electrocardiographic subclinical myocardial injury in the general population. Clin Cardiol. 2019;42:373–378. 10.1002/clc.23155
Izzah Vasim and Muhammad I. Ahmad contributed equally to this study.
REFERENCES
- 1. Stefan N, Haring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1(2):152‐162. [DOI] [PubMed] [Google Scholar]
- 2. Bluher M. Are metabolically healthy obese individuals really healthy? Eur J Endocrinol. 2014;171(6):R209‐R219. [DOI] [PubMed] [Google Scholar]
- 3. Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: a systematic review and meta‐analysis. Ann Intern Med. 2013;159(11):758‐769. [DOI] [PubMed] [Google Scholar]
- 4. Eckel N, Meidtner K, Kalle‐Uhlmann T, Stefan N, Schulze MB. Metabolically healthy obesity and cardiovascular events: a systematic review and meta‐analysis. Eur J Prev Cardiol. 2016;23(9):956‐966. [DOI] [PubMed] [Google Scholar]
- 5. Zheng R, Zhou D, Zhu Y. The long‐term prognosis of cardiovascular disease and all‐cause mortality for metabolically healthy obesity: a systematic review and meta‐analysis. J Epidemiol Community Health. 2016;70(10):1024‐1031. [DOI] [PubMed] [Google Scholar]
- 6. Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses' Health Study): 30 year follow‐up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6:714‐724. [DOI] [PubMed] [Google Scholar]
- 7. Fan J, Song Y, Chen Y, Hui R, Zhang W. Combined effect of obesity and cardio‐metabolic abnormality on the risk of cardiovascular disease: a meta‐analysis of prospective cohort studies. Int J Cardiol. 2013;168(5):4761‐4768. [DOI] [PubMed] [Google Scholar]
- 8. Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all‐cause and cardiovascular disease mortality. J Clin Endocrinol Metab. 2012;97(7):2482‐2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mirzaei B, Abdi H, Serahati S, et al. Cardiovascular risk in different obesity phenotypes over a decade follow‐up: Tehran Lipid and Glucose Study. Atherosclerosis. 2017;258:65‐71. [DOI] [PubMed] [Google Scholar]
- 10. Calori G, Lattuada G, Piemonti L, et al. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care. 2011;34(1):210‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rautaharju PM, Warren JW, Jain U, Wolf HK, Nielsen CL. Cardiac infarction injury score: an electrocardiographic coding scheme for ischemic heart disease. Circulation. 1981;64(2):249‐256. [DOI] [PubMed] [Google Scholar]
- 12. O'Neal WT, Shah AJ, Efird JT, Rautaharju PM, Soliman EZ. Subclinical myocardial injury identified by cardiac infarction/injury score and the risk of mortality in men and women free of cardiovascular disease. Am J Cardiol. 2014;114(7):1018‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Richardson K, Engel G, Yamazaki T, Chun S, Froelicher VF. Electrocardiographic damage scores and cardiovascular mortality. Am Heart J. 2005;149(3):458‐463. [DOI] [PubMed] [Google Scholar]
- 14. Dekker JM, Schouten EG, Kromhout D, Klootwijk P, Pool J. The Cardiac Infarction Injury Score and coronary heart disease in middle‐aged and elderly men: the Zutphen Study. J Clin Epidemiol. 1995;48(6):833‐840. [DOI] [PubMed] [Google Scholar]
- 15. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital and health statistics Ser 1, Programs and collection procedures. 1994;32:1‐407. [PubMed] [Google Scholar]
- 16. Prineas RJ, Crow RS, Zhang Z‐M. The Minnesota Code Manual of Electrocardiographic Findings: Including Measurement and Comparison with the Novacode: Standards And Procedures For ECG Measurement in Epidemiologic and Clinical Trails. 2nd ed. London, England: Springer; 2010. [Google Scholar]
- 17. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640‐1645. [DOI] [PubMed] [Google Scholar]
- 18. Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body‐mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet (London, England). 2014;383(9921):970‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mongraw‐Chaffin M, Foster MC, Anderson CAM, et al. Metabolically healthy obesity, transition to metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2018;71(17):1857‐1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang ZM, Rautaharju PM, Prineas RJ, et al. Race and sex differences in the incidence and prognostic significance of silent myocardial infarction in the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2016;133(22):2141‐2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bots SH, Peters SAE, Woodward M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Global Health. 2017;2(2):e000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mosca L, Barrett‐Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124(19):2145‐2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definition of metabolic syndrome and obesity phenotypes.
Table S2. Association of cardiac infarction/injury score (CIIS) with body mass index (BMI) categories.


