Abstract
Background
Guidelines recommend using risk stratification tools in acute myocardial infarction (AMI) to assist decision‐making. The Thrombolysis in Myocardial Infarction Risk Score for Secondary Prevention (TRS‐2P) has been recently developed to characterize long‐term risk in patients with MI.
Hypothesis
We aimed to assess the TRS‐2P in the French Registry of Acute ST Elevation or non‐ST elevation MI registries.
Methods
We used data from three 1‐month French registries, conducted 5 years apart, from 2005 to 2015, including 13 130 patients with AMI (52% ST‐elevation myocardial infarction [STEMI]). Atherothrombotic risk stratification was performed using the TRS‐2P score. Patients were divided in to three categories: G1 (low‐risk, TRS‐2P = 0/1); G2 (intermediate‐risk, TRS‐2P = 2); and G3 (high‐risk, TRS‐2P ≥ 3). Baseline characteristics and outcomes were analyzed according to TRS‐2P categories.
Results
A total of 12 715 patients (in whom TRS‐2P was available) were included. Prevalence of G1, G2, and G3 was 43%, 24%, and 33% respectively. Clinical characteristics and management significantly differed according to TRS‐2P categories. TRS‐2P successfully defined residual risk of death at 1 year (C‐statistic 0.78): 1‐year survival was 98% in G1, 94% in G2, and 78.5% in G3 (P < 0.001). Using Cox multivariate analysis, G3 was independently associated with higher risk of death at 1 year (hazard ratio [HR] 4.61; 95% confidence interval [CI]: 3.61‐5.89), as G2 (HR 2.08; 95% CI: 1.62‐2.65) compared with G1. The score appeared robust and correlated well with mortality in STEMI and NSTEMI populations, as well as in each cohort separately.
Conclusions
The TRS‐2P appears to be a robust risk score, identifying patients at high risk after AMI irrespective of the type of MI and historical period.
Keywords: acute myocardial infarction, mortality, prevention, score
1. INTRODUCTION
Risk stratification tools enable personalized risk assessment and may help guide therapeutic decision‐making. Guidelines recommend their use in acute myocardial infarction (AMI) to identify high‐risk patients and to assist with short‐term prognostication and therapeutic decision‐making (eg, early invasive strategy).1, 2, 3, 4, 5, 6, 7, 8 Several scores have been developed, especially in patients at the acute stage of MI; however, they remain underutilized in clinical practice in part they require specific tools as well as a perception that the impact on treatment decisions is limited, or both. The Thrombolysis in Myocardial Infarction (TIMI) Risk Score for Secondary Prevention (TRS‐2P) is a simple nine‐point risk stratification tool, derived in patients with previous MI to predict recurrent cardiovascular (CV) events.9, 10, 11 This score has the advantage of being very simple to use and may assist with decisions on long‐term response to treatment. Recently, the TRS‐2P was validated in a clinical trial of acute coronary syndrome (ACS) patients followed for ~7 years.12 To our knowledge, the TRS‐2P score has never been evaluated in a routine‐practice population, focusing on patients who are discharged after an AMI. The aim of the present study was to test its robustness in several historical cohorts of patients after AMI, using the French Registry of Acute ST Elevation or non‐ST elevation Myocardial Infarction (FAST‐MI) registries.
2. METHODS
2.1. Patient population
Three nationwide French registries were conducted 5 years apart over a 10‐year period (2005‐2015): FAST‐MI 2005 (NCT00673036),13 FAST‐MI 2010 (NCT01237418),14 and FAST‐MI 2015 (NCT02566200)15 (Supporting Information Methods S1). The methods used for these registries have been detailed previously.13, 14, 15 Briefly, their primary objectives were to evaluate the characteristics, management, and outcomes of AMI patients, as seen in routine clinical practice, on a country‐wide scale.
All registries consecutively included patients with ST‐elevation myocardial infarction (STEMI) or non‐ST‐elevation myocardial infarction (NSTEMI) admitted to cardiac intensive care units (ICUs) within 48 hours of symptom onset, during a specified 1‐month period (October‐December 2005, 2010, and 2015). AMI was defined by increased levels of cardiac biomarkers (troponins, creatine kinase (CK), or creatine kinase‐MB (CK‐MB)) together with either compatible symptoms or electrocardiography (ECG) changes. Patients who died soon after admission and for whom cardiac markers were not measured were included if they had signs or symptoms associated with typical ST‐segment changes. Exclusion criteria were as follows: (a) refusal to participate, (b) iatrogenic MIs, defined as occurring within 48 hours of any therapeutic procedure, and (c) AMI diagnosis invalidated in favor of another diagnosis. STEMI was diagnosed when ST‐elevation ≥1 mm was seen in at least two contiguous leads in any location on the index or qualifying ECG, or when presumed new left bundle branch block or documented new Q waves were observed. In the absence of ST‐segment elevation, patients meeting the inclusion criteria were considered to have NSTEMI. A total of 13 130 patients (52% STEMI) were included in the three surveys.
Participation in the study was offered to all institutions, including university teaching hospitals, general and regional hospitals, and private clinics that received AMI emergencies. Physicians were instructed that the study should not affect clinical care or management. The study was conducted in accordance with the guidelines on good clinical practice and French law. The study protocols for the 2005 registry was reviewed by the CPP in Biomedical Research of Saint Antoine University Hospital; the 2010 registry was reviewed and approved by the CPP of Saint Louis University Hospital, Paris; and the protocol of 2015 registry was reviewed and approved by the CPP of Saint Louis University Hospital Paris Ile de France IV. Data file collection and storage were approved by the Commission Nationale Informatique et Liberté. All patients were informed of the nature and aims of the surveys and could request to be excluded; in addition, written consent was obtained for all three surveys.
2.2. Data collection
Data on baseline characteristics, including demographics (age, sex, body mass index), risk factors (hypertension, diabetes, current smoking, hypercholesterolemia, family history of coronary artery disease), and medical history (MI, previous myocardial revascularization, stroke, heart failure, peripheral artery disease [PAD], chronic renal failure), were collected as previously described.13, 14, 15 Information on the use of cardiac procedures, including use of percutaneous coronary intervention (PCI), use of medications (anticoagulants, antiplatelet agents, diuretics, beta‐blockers, angiotensin‐converting enzyme inhibitors (ACE‐I) or angiotensin receptor blockers (ARB), and lipid lowering agents) in the first 48 hours and at‐hospital discharge was collected.
Bleeding was classified as major or minor according to the TIMI criteria.16 Regarding bleeding complications, four end points of interest were used: in‐hospital major bleeding (defined as a fall in hemoglobin ≥5 g, fall in hematocrit ≥15%, intracranial hemorrhage, retroperitoneal bleeding), minor bleeding (defined as a fall in hemoglobin between 3 and 5 g/dL, fall in hematocrit between 10% and 15%), use of any transfusion during the hospital stay, and 1‐year survival.
For all surveys, follow‐up was centralized at the French Society of Cardiology.
2.3. Statistical analysis
Each patient was assessed for the presence of any of the nine previously described risk indicators in the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events [TRA‐2P]—TIMI 50 trial at baseline9, 10, 11: age ≥ 75 years, diabetes mellitus, hypertension, PAD, previous stroke, previous coronary artery bypass grafting, history of heart failure, active smoking, and renal dysfunction (defined by an estimated glomerular filtration rate < 60 mL/min/1.73 m2 (using the Modification of Diet in Renal Disease equation). All variables, with the exception of age and renal dysfunction, were determined on the basis of clinical history. As described, each atherothrombotic risk indicator was weighted evenly to define total risk for each patient as the arithmetic sum of risk indicators. Simple risk categories were defined to parallel the annualized risk of death observed in the derivation population from patients in TRA2P, thus translating to a low‐risk category with 0 to 1 risk indicators (Group 1), an intermediate‐risk category with 2 risk indicators (Group 2), and a high‐risk category with ≥3 risk indicators (Group 3). The discriminatory capacity of the risk indicators was assessed by the area under the receiver operating characteristics curve (c‐statistic) as a measure of model performance.
Continuous variables are reported as means (SDs) or medians and interquartile ranges, when appropriate. Discrete variables are described as counts and percentages. Groups were compared by analysis of variance for continuous variables and χ2 (or Fisher exact tests) for discrete variables. Temporal trends were tested using linear‐by‐linear association tests for binary and Jonckheere‐Terpstra tests for continuous variables. Odds ratios and hazard ratios (HRs) are presented with their 95% confidence intervals (CIs).
Multivariable analyses of correlates of 1‐year mortality were performed using Cox backward stepwise multiple logistic regression, using a threshold of 0.10 for variable elimination. Beside time period, variables included in the final models were selected ad hoc, based on their physiological relevance and potential to be associated with outcomes; they comprised age, gender, risk factors, comorbidities, type of MI, TRS‐2P categories, year, and management. Sensitivity analyses were performed focused on patients discharged alive in the main analysis, inpatients with STEMI or NSTEMI separately, and in each of the three historical cohorts. Analyses were repeated using forward stepwise analysis to check the consistency of the results. Statistical analyses were performed using IBM SPSS 23.0 (IBM SPSS Inc., Chicago, IL). For all analyses, two‐sided P values <0.05 were considered significant.
3. RESULTS
3.1. Study population
A total of 12 715 patients (97%) had all nine variables included in the TRS‐2P score available at discharge and were included in the main analysis. Prevalence of Groups 1, 2, and 3 was 43%, 24%, and 33%, respectively. Over the 10‐year period, the overall risk of patients admitted for AMI decreased, with the proportion in Group 3 declining from 43% to 29% (P < 0.001; Figure S1). The distribution of the nine variables according to the TRS‐2P categories is presented in Figure S2. TRS‐2P successfully defined patients with high‐, intermediate‐, and low‐CV risk profile (Table 1). GRACE score was 168 ± 36 in Group 3, 139 ± 31 in Group 2, and 127 ± 27 in Group 1 (P < 0.001); simple risk index (SRI) was 35 ± 17, 26 ± 13, and 20 ± 10 in Groups 3, 2, and 1, respectively. In addition, the risk for major bleeding defined by the CRUSADE score decreased from Group 3 to Group 1.
Table 1.
Baselines characteristics and clinical presentation
| Overall (n = 12 715) | Low (0–1) (n = 5446) | Intermediate (2) (n = 3108) | High (≥3) (n = 4161) | P value | |
|---|---|---|---|---|---|
| Age (y) | 65.9 ± 14.1 | 58.7 ± 11.8 | 66.0 ± 13.4 | 75.4 ± 11.5 | <0.001 |
| Female | 3612 (28) | 1110 (20) | 916 (29.5) | 1586 (38) | <0.001 |
| BMI (kg/m2) | 27.0 ± 4.7 | 26.6 ± 4.3 | 27.3 ± 4.9 | 27.4 ± 5.1 | <0.001 |
| Risk factors | |||||
| Hypertension | 7016 (55) | 1096 (20) | 2252 (72.5) | 3668 (88) | <0.001 |
| Diabetes | 3168 (25) | 188 (3.5) | 745 (24) | 2235 (54) | <0.001 |
| Hypercholesterolemia | 5718 (45) | 1939 (36) | 1482 (48) | 2297 (55) | <0.001 |
| Current smoking | 4234 (33) | 1988 (36.5) | 1229 (39.5) | 1017 (24) | <0.001 |
| Family history | 3041 (24) | 1671 (31) | 754 (24) | 616 (15) | <0.001 |
| Medical history | |||||
| Prior MI | 2214 (17) | 498 (9) | 505 (16) | 1211 (29) | <0.001 |
| Prior PCI | 2041 (16) | 513 (9) | 491 (16) | 1037 (25) | <0.001 |
| Prior CABG | 651 (5) | 43 (0.8) | 101 (3) | 507 (12) | <0.001 |
| History of heart failure | 634 (5) | 14 (0.3) | 55 (2) | 565 (14) | <0.001 |
| History of stroke | 785 (6) | 56 (1) | 132 (4) | 597 (14) | <0.001 |
| Peripheral artery disease | 1064 (8) | 28 (0.5) | 127 (4) | 909 (22) | <0.001 |
| Chronic renal failure | 638 (5) | 23 (0.4) | 65 (2) | 550 (13) | <0.001 |
| Prior medications | |||||
| Aspirin | 3085 (24) | 653 (12) | 712 (23) | 1720 (41) | <0.001 |
| Clopidogrel | 1394 (11) | 214 (4) | 272 (9) | 908 (22) | <0.001 |
| Beta‐blockers | 3207 (25) | 683 (12.5) | 874 (28) | 1650 (40) | <0.001 |
| Statins | 3648 (29) | 954 (17.5) | 924 (30) | 1770 (42.5) | <0.001 |
| ACE‐inhibitors or ARB | 4305 (34) | 848 (16) | 1228 (39.5) | 2229 (53) | <0.001 |
| Clinical presentation | |||||
| STEMI | 6650 (52) | 3365 (62) | 1670 (54) | 1615 (39) | <0.001 |
| Killip class | <0.001 | ||||
| I | 10 544 (83) | 5331 (98) | 2770 (89) | 2443 (59) | |
| II | 1227 (10) | 81 (1.5) | 224 (7) | 922 (22) | |
| III | 714 (6) | 14 (0.3) | 71 (2) | 629 (15) | |
| IV | 180 (1) | 10 (0.2) | 28 (0.9) | 142 (3.4) | |
| LV function | 51.7 ± 11.7 | 53.8 ± 10.3 | 52.4 ± 11.4 | 48.3 ± 12.9 | <0.001 |
| GRACE score | 143.3 ± 36.0 | 126.5 ± 26.7 | 139.2 ± 31.1 | 167.9 ± 36.2 | <0.001 |
| SRI score | <0.001 | ||||
| Median (IQR) | 18.3 (13.3‐24.7) | 22.8 (16.3‐31.7) | 32.6 (24.0‐43.0) | ||
| n | 5283 | 3025 | 4065 | ||
| CRUSADE | <0.001 | ||||
| Median (IQR) | 18.0 (9.0‐26.0) | 26.0 (16.0‐36.0) | 44.1 (33.0‐53.0) | ||
| n | 5041 | 2868 | 3764 | ||
| CRP (mg/L) | <0.001 | ||||
| Median (IQR) | 4.0 (2.0‐8.9) | 5.0 (2.9‐11.4) | 8.8 (4.0‐33.0) | ||
| n | 4025 | 2307 | 3153 | ||
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blockers; BMI, body mass index; CABG, coronary artery bypass grafting; CRP, C‐reactive protein; LV, left ventricular; MI, myocardial infarction; NSTEMI, Non‐ST‐elevation myocardial infarction; PCI, percutaneous coronary intervention; SRI, simple risk index; STEMI, ST‐elevation myocardial infarction.
Values are expressed as mean (± SD) or number (percentage).
The rate of STEMI patients was higher in Group 1, while the rate of patients with heart failure at admission (Killip class ≥ 2) was higher in Group 3. Interestingly, biomarkers of inflammation (eg, C‐reactive protein) increased from Group 1 to Group 3.
3.2. Early management
Early management including medications and myocardial revascularization were significantly different according to TRS‐2P categories (Table 2). Overall, Group 3 patients received less antiplatelet agents, statin, beta‐blocker, ACE‐I, or ARB during the first 48 hours after admission as at discharge compared with both Groups 1 and 2 (P < 0.001 for all). In Group 3 patients, the use of appropriate secondary prevention treatment (dual antiplatelet therapy and statins for all; ACE‐I/ARB and beta‐blockers as indicated) was lower especially in patients with renal dysfunction (42% vs 55%, P < 0.001) and older patients (<60 years: 60%; 60‐74 years: 53%; ≥75 years: 45%; P = 0.001). In addition, the use of invasive strategy (coronary angiography with or without PCI) was lower in Group 3, in which the rate of multivessel disease was higher. Radial access was preferentially used in low‐ or intermediate‐risk patients. Finally, a full myocardial revascularization strategy during hospitalization was more frequently used in both Groups 1 and 2 (P < 0.001).
Table 2.
In‐hospital management
| Overall (n = 12 715) | Low (0‐1) (n = 5446) | Intermediate (2) (n = 3108) | High (≥3) (n = 4161) | P value | |
|---|---|---|---|---|---|
| Medications | |||||
| Aspirin | 11 767 (92.5) | 5126 (94) | 2905 (93.5) | 3736 (90) | <0.001 |
| Clopidogrel | 7627 (60) | 2891 (53) | 1909 (61) | 2827 (68) | <0.001 |
| Ticagrelor | 2483 (19.5) | 1365 (25) | 613 (20) | 505 (12) | <0.001 |
| Prasugrel | 1683 (13) | 1113 (20) | 384 (12) | 186 (4.5) | <0.001 |
| GPIIb/IIIa | 198 (2) | 112 (2) | 49 (2) | 37 (0.9) | <0.001 |
| UFH | 5266 (41) | 2098 (38.5) | 1226 (39) | 1942 (47) | <0.001 |
| LMWH | 6844 (54) | 3223 (59) | 1741 (56) | 1880 (45) | <0.001 |
| Bivalirudine | 275 (2) | 152 (3) | 74 (2) | 49 (1) | <0.001 |
| Fondaparinux | 1934 (15) | 879 (16) | 466 (15) | 589 (14) | 0.03 |
| Statins | 9820 (77) | 4486 (82) | 2429 (78) | 2905 (70) | <0.001 |
| Beta‐blockers | 9390 (74) | 4305 (79) | 2361 (76) | 2724 (65.5) | <0.001 |
| ACE‐inhibitors or ARB | 7610 (60) | 3195 (59) | 1958 (63) | 2457 (59) | <0.001 |
| Procedures | |||||
| CAG | 11 800 (93) | 5381 (99) | 2975 (96) | 3444 (83) | <0.001 |
| CAG results | <0.001 | ||||
| No significant lesions (<50%) | 718 (6) | 384 (7) | 175 (6) | 159 (4) | |
| One‐VD | 4830 (38) | 2642 (49) | 1212 (39) | 976 (24) | |
| Two‐VD | 3436 (27) | 1522 (28) | 898 (29) | 1016 (24) | |
| Three‐VD | 2156 (17) | 755 (14) | 575 (18.5) | 826 (20) | |
| CABG | 603 (5) | 42 (1) | 99 (3) | 462 (11) | |
| PCI | 7819 (66) | 3976 (74) | 2009 (68) | 1834 (53) | <0.001 |
| Radial access | 7134 (78) | 3577 (84) | 1795 (80) | 1762 (67) | <0.001 |
| Drug‐eluting stent | 5084 (48) | 2494 (51) | 1293 (48.5) | 1297 (43) | <0.001 |
| Complete myocardial revascularization | 5588 (61) | 2879 (66) | 1428 (61) | 1273 (52) | <0.001 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blockers; CABG, coronary artery bypass grafting; CAG, coronary angiography; UFH, unfractionned heparin; LMWH, low‐molecular‐weight heparin; NSTEMI, non‐ST‐elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction; VD, vessel disease.
Values are expressed as mean (± SD) or number (percentage).
3.3. Outcomes
In‐hospital complications are described in (Table 3). The rate of re‐MI, atrial fibrillation, stroke, and major and minor bleedings were higher in Group 3 patients. Mortality at 30 days was 9% in Group 3, 3% in Group 2, and 1% in Group 1 (P < 0.001).
Table 3.
In‐hospital complications and clinical outcomes
| Overall (n = 12 715) | Low (0‐1) (n = 5446) | Intermediate (2) (n = 3108) | High (≥3) (n = 4161) | P value | |
|---|---|---|---|---|---|
| Re‐MI | 130 (1.0) | 32 (0.6) | 26 (0.8) | 72 (2) | <0.001 |
| Intrastent thrombosis | 53 (0.6) | 25 (0.6) | 12 (0.5) | 16 (0.6) | <0.001 |
| Atrial fibrillation | 770 (6) | 174 (3) | 167 (5) | 429 (10) | <0.001 |
| Ventricular fibrillation | 245 (2) | 110 (2) | 54 (2) | 81 (2) | 0.66 |
| Stroke | 75 (0.6) | 16 (0.3) | 16 (0.5) | 43 (1.0) | <0.001 |
| Major bleeding | 259 (2) | 77 (1) | 53 (2) | 129 (3) | <0.001 |
| Minor bleeding | 360 (3) | 124 (2) | 90 (3) | 146 (3.5) | 0.001 |
| Transfusions | 436 (3) | 69 (1) | 87 (3) | 280 (7) | <0.001 |
| Death at 30 days | 502 (3.9) | 49 (0.9) | 83 (2.7) | 370 (8.9) | <0.001 |
| Death at 1 year | 1197 (9) | 127 (2.3) | 177 (5.7) | 893 (21.5) | <0.001 |
Abbreviations: MI, myocardial infarction; NSTEMI, non‐ST‐elevation myocardial infarction; STEMI, ST‐elevation myocardial infarction.
Values are expressed as mean (± SD) or number (percentage).
Distribution of patients across the full range of risk indicators is provided in (Figure 1). TRS 2P score successfully defined residual risk of death at 1 year (C‐statistic 0.78): 1‐year survival was 98% in Group 1, 94% in Group 2, and 78.5% in Group 3 (P < 0.001). Using Cox multivariate analysis, Group 3 and Group 2 were associated with a higher risk of death at 1‐year (HR = 4.61; 95% CI: 3.61‐5.89, P < 0.001, and HR = 2.08; 95% CI: 1.62‐2.65, P < 0.001, respectively) compared with Group 1 (Figure 2). Similar trends were found after censoring patients dying during hospitalization.
Figure 1.
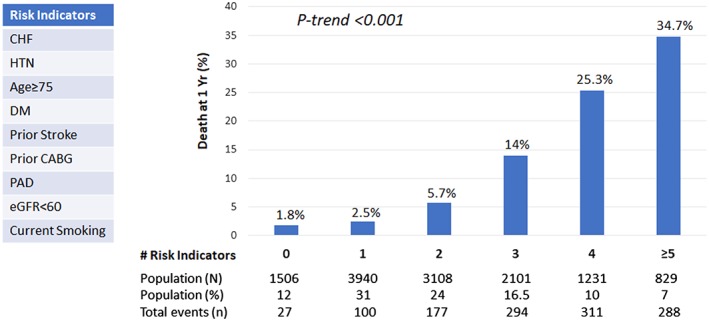
Risk stratification of death at 1 year. One‐year Kaplan‐Meier estimates are shown. The P value is based on the χ 2 test for trend. CABG, coronary artery bypass graft; CHF, congestive heart failure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HTN, hypertension; PAD, peripherical artery disease
Figure 2.
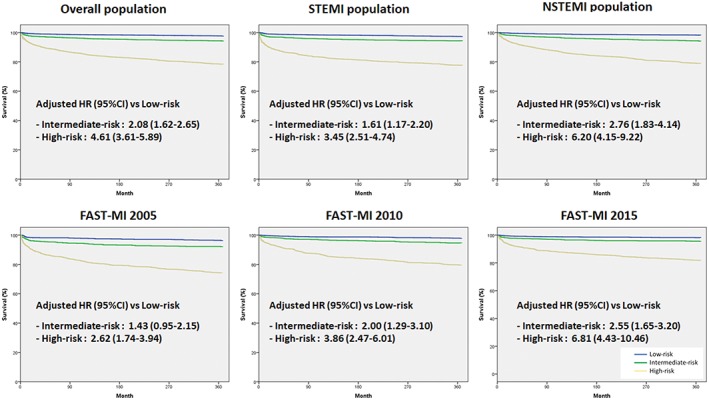
One‐year mortality according to Thrombolysis in Myocardial Infarction Risk Score for Secondary Prevention (TRS2P) categories. The survival curves are unadjusted, and the adjusted hazard ratios (HRs) are provided with their 95% confidence intervals (CIs). NSTEMI, non‐ST‐elevation myocardial infarction; STEMI, ST‐elevation myocardial infarction
3.4. Subgroup analyses
Similar trends were found using TRS‐2P score according to type of MI and year of survey (Tables S1‐S6; Figure S2). The score appeared robust and correlated well with mortality in STEMI (C‐statistic 0.77) and NSTEMI (c‐statistic 0.78) populations, as well as in each of the historical cohorts separately: 2005 (c‐statistic 0.76), 2010 (c‐statistic 0.78) and 2015 (c‐statistic 0.78).
4. DISCUSSION
The main findings of this study are that the easily calculated TRS‐2P appears to be a robust risk score, identifying patients at high‐risk after AMI, irrespective of the type of MI and historical period. In addition, we showed that the rate of high‐risk patients among those hospitalized for AMI decreased over the 10‐year period from 2005 to 2015.
4.1. Change in risk‐profile
Several sources including registries specific to AMI and large databases, have shown a decrease in mortality over the past 20 years.17, 18, 19, 20, 21 Using the FAST MI program, we previously reported that this decrease was correlated partially with a substantial change in patient risk profile, and not only with changes in management. Specifically, the absolute 6‐month mortality decrease from 1995 to 2015 was 11.9% (observed) vs 10.1% (standardized), attesting a 15% reduction related to the changes in patient risk profile in STEMI patients (17% in NSTEMI patients).21 In the present analysis, the rate of high‐risk patients in TRS2P score decreased by 32% over the 10‐year period.
4.2. Atherothrombotic risk assessment
Patients after AMI demonstrate a range of residual risk for recurrent CV events. Therefore, risk stratification tools have been developed, usually derived from clinical trials or specific registries to identify high‐risk patients and to assist with prognostication and therapeutic decision making. A limited number of risk scores, however, are available for patients in secondary prevention after AMI. The TRS‐2P score has been proposed by the TIMI group for stable ischemic heart disease (IHD) using data from the TRA2P‐TIMI 50 trial.9, 10, 11 Nine independent risk predictors were identified in this cohort. These variables are highly consistent with those used for other risk scores. Diabetes mellitus, hypertension, and current smoking are established risk factors for disease progression recognized by stable IHD practice guidelines as high‐risk comorbid conditions warranting particular focus for medical therapy. Moreover, the presence of atherosclerosis outside the coronary bed, heart failure and renal dysfunction are also now well recognized as potent risk indicators across the spectrum of IHD. The trial showed a strong graded relationship with the rate of CV death/MI/ischemic stroke and the individual components. The TRS‐2P score was, however, defined in a selected population in which for example, women and minorities made up a small proportion of the study population.9, 10, 11 Recently, the TRS‐2P score has been validated in routine practice using two large, independent integrated healthcare delivery systems in United States between 2008 and 2013 (ie, Cleveland Clinic and Geisinger Health System).22 However, to our knowledge, this stratification tool has never been evaluated in a routine‐practice population, focusing on patients who are discharged alive after an AMI. In our analysis, the TRS‐2P score appears to be a robust risk score to identify high risk patients irrespective of the type of MI and historical period. Moreover, this score appears relevant in patients hospitalized for an acute MI (eg, not only in stable CAD or patients having sustained an infarct in the previous year).
The TRS‐2P has never been compared with Global Registry of Acute Coronary Events (GRACE), SRI or Can Rapid risk stratification of Unstable angina patients Suppress Adverse outcomes with Early implementation of the ACC/AHA Guidelines (CRUSADE) scores. Our data show that TRS‐2P score is consistent with these validated scores to define a high‐risk population (ie, Group 3) in terms of bleeding risk and ischemic risk.
4.3. Risk assessment and therapeutic intensification
Atherothrombotic risk assessment may be useful to identify high‐risk patients who have the greatest potential to benefit from more intensive secondary prevention therapy such as antithrombotic or lipid‐lowering. In the TRA2P‐TIMI 50 trial, the risk stratification tool identified a gradient of risk for recurrent events and distinguished a pattern of increasing absolute benefit with vorapaxar.9, 10, 11 Similarly, using data from the IMPROVE IT trial, the TRS‐2P score identified a strong gradient of risk for recurrent CV events; and an increasingly favorable relative and absolute benefit from the addition of ezetimibe to simvastatin therapy with increasing risk‐profile.12 Finally, this score could be evaluated to identify high‐risk patients for new strategies as PCSK9‐inhibitors or prolonged double antiplatelet therapy in ACS patients.23, 24 Yet, in clinical practice, the highest risk patients are paradoxically often the least intensively treated.
4.4. Limitations
The TRS‐2P score was designed to be a simple tool, using readily available clinical data. There are other previously identified risk indicators and other yet to be identified parameters that may provide additional refinement for stratification. However, the ability of this simple scoring system to identify differential treatment benefit for different classes of secondary prevent therapy supports its clinically utility. Our data are derived from an observational study of AMI patients admitted in ICUs while TRS‐2P was defined in a population of stable patients with previous MI. In addition, our analyses were focused on the mortality at one‐year while this risk stratification tool was developed for all CV‐events at 3‐year. The rate of CV death was not available. Finally, we cannot exclude that other factor than those collected in the surveys could also explain the evolution observed according to TRS2P categories.
5. CONCLUSION
Atherothrombotic risk assessment may be useful to identify high‐risk patients who have the greatest potential to benefit from more intensive secondary preventive therapy. Using a routine‐practice population, TRS‐2P appears to be a robust risk score, identifying patients at high‐risk after AMI irrespective of the type of MI and historical period.
Supporting information
Appendix S1.
ACKNOWLEDGMENTS
The French Society of Cardiology received grants for supporting the FAST‐MI program from Amgen, AstraZeneca, Bayer, BMS, Boehringer‐Ingelheim, Daiichi Sankyo, Eli Lilly, MSD, Pfizer, and Sanofi. The authors are deeply indebted to all patients who accepted to participate in the surveys and to the physicians who took care of the patients at the participating institutions.
Puymirat E, Bonaca M, Fumery M, et al. Atherothrombotic risk stratification after acute myocardial infarction: The Thrombolysis in Myocardial Infarction Risk Score for Secondary Prevention in the light of the French Registry of Acute ST Elevation or non‐ST Elevation Myocardial Infarction registries. Clin Cardiol. 2019;42:227–234. 10.1002/clc.23131
Funding information Amgen Foundation; AstraZeneca; Bayer; Boehringer Ingelheim; Daiichi Sankyo Company; Eli Lilly and Company; Merck Sharp and Dohme; Pfizer; Sanofi
REFERENCES
- 1. Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non‐ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835‐842. [DOI] [PubMed] [Google Scholar]
- 2. Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST‐elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102:2031‐2037. [DOI] [PubMed] [Google Scholar]
- 3. Granger CB, Goldberg RJ, Dabbous O, et al. Global Registry of Acute Coronary Events Investigators. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345‐2353. [DOI] [PubMed] [Google Scholar]
- 4. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354‐2394. [DOI] [PubMed] [Google Scholar]
- 5. American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions , O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78‐e140. [DOI] [PubMed] [Google Scholar]
- 6. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119‐177. [DOI] [PubMed] [Google Scholar]
- 7. Huynh T, Kouz S, Yan AT, et al. Canada Acute Coronary Syndrome Risk Score: a new risk score for early prognostication in acute coronary syndromes. Am Heart J. 2013;166:58‐63. [DOI] [PubMed] [Google Scholar]
- 8. Fox KA, Fitzgerald G, Puymirat E, et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open. 2014;4(2):e004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bohula EA, Bonaca MP, Braunwald E, et al. Atherothrombotic risk stratification and the efficacy and safety of vorapaxar in patients with stable ischemic heart disease and previous myocardial infarction. Circulation. 2016;134:304‐313. [DOI] [PubMed] [Google Scholar]
- 10. Morrow DA, Braunwald E, Bonaca MP, et al. TRA 2P‐TIMI 50 Steering Committee and Investigators. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404‐1413. [DOI] [PubMed] [Google Scholar]
- 11. Scirica BM, Bonaca MP, Braunwald E, et al. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2°P‐TIMI 50 trial. Lancet. 2012;380:1317‐1324. [DOI] [PubMed] [Google Scholar]
- 12. Bohula EA, Morrow DA, Giugliano RP, et al. Atherothrombotic risk stratification and ezetimibe for secondary prevention. J Am Coll Cardiol. 2017;69:911‐921. [DOI] [PubMed] [Google Scholar]
- 13. Cambou J‐P, Simon T, Mulak G, et al. The French registry of Acute ST elevation or non‐ST‐elevation Myocardial Infarction (FAST‐MI): study design and baseline characteristics. Arch Mal Coeur Vaiss. 2007;100:524‐534. [PubMed] [Google Scholar]
- 14. Hanssen M, Cottin Y, Khalife K, et al. FAST‐MI 2010 Investigators. French Registry on Acute ST‐elevation and non ST‐elevation Myocardial Infarction 2010. FAST‐MI 2010. Heart Br Card Soc. 2012;98:699‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Belle L, Cayla G, Cottin Y, et al. FAST‐MI 2015 investigators. French Registry on Acute ST‐elevation and non‐ST‐elevation Myocardial Infarction 2015 (FAST‐MI 2015). Design and baseline data. Arch Cardiovasc Dis. 2017;110:366‐378. [DOI] [PubMed] [Google Scholar]
- 16. Rao AK, Pratt C, Berke A, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988;11:1‐11. [DOI] [PubMed] [Google Scholar]
- 17. Rosamond WD, Chambless LE, Heiss G, et al. Twenty‐two‐year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987‐2008. Circulation. 2012;125:1848‐1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rogers WJ, Frederick PD, Stoehr E, et al. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non‐ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156:1026‐1034. [DOI] [PubMed] [Google Scholar]
- 19. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155‐2165. [DOI] [PubMed] [Google Scholar]
- 20. Fox KA, Steg PG, Eagle KA, et al. GRACE Investigators. Decline in rates of death and heart failure in acute coronary syndromes, 1999‐2006. JAMA. 2007;297:1892‐1900. [DOI] [PubMed] [Google Scholar]
- 21. Puymirat E, Simon T, Cayla G, et al. Acute Myocardial Infarction: Changes in Patient Characteristics, Management, and 6‐Month Outcomes Over a Period of 20 Years in the FAST‐MI Program (French Registry of Acute ST‐Elevation or Non‐ST‐Elevation Myocardial Infarction) 1995 to 2015. Circulation. 2017;136:1908‐1919. [DOI] [PubMed] [Google Scholar]
- 22. Williams BA, Chagin KM, Bash LD, et al. External validation of the TIMI risk score for secondary cardiovascular events among patients with recent myocardial infarction. Atherosclerosis. 2018;272:80‐86. [DOI] [PubMed] [Google Scholar]
- 23. Bonaca MP, Bhatt DL, Cohen M, et al. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791‐1800. [DOI] [PubMed] [Google Scholar]
- 24. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713‐1722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


