Abstract
Thrombo‐embolism is one of the serious complications of takotsubo syndrome (TS) in addition to heart failure, pulmonary edema, cardiogenic shock, cardiac arrest, life‐threatening arrhythmias, left ventricular outlet tract obstruction, mitral regurgitation, cardiac rupture, and death. The most common cardio‐embolic events in TS are cerebral, renal, and peripheral embolism. Approximately, one‐third of patients with left ventricular thrombus (LVT) in TS develop embolic complications. Cardio‐embolism in TS may occur with or without the presence of detectable LVT. In the present report, the thrombo‐embolic complications in TS with the emphasis on the association of TS to both acute coronary syndrome (ACS) including coronary embolism and ischemic stroke including cerebral embolism are reviewed. This serious complication is elucidated by demonstration of the case of a 67‐year‐woman with mid‐apical TS complicated by LVT, left anterior descending artery (LAD) and left middle cerebral artery (segment M2) thrombo‐embolic occlusions. The cerebral artery thrombotic occlusion was treated successfully with endovascular thrombectomy with complete resolution of the neurological deficits. There was spontaneous recanalization of the apical LAD occlusion verified by cardiac computed tomography angiography.
Keywords: broken heart syndrome, cerebral infarction, myocardial infarction, myocardial stunning, stroke, takotsubo, thrombo‐embolism
1. INTRODUCTION
Takotsubo syndrome (TS) is an increasingly recognized acute cardiac syndrome with a clinical presentation resembling that of an acute coronary syndrome (ACS).1 TS is characterized by a reversible left ventricular wall motion abnormality (LVWMA) with a unique circumferential pattern resulting in a conspicuous ballooning of the left ventricle during systole.2 The term takotsubo was introduced in 1990 and 1991 to describe the left ventricular silhouette during systole in five patients with a clinical picture of myocardial infarction without obstructive coronary artery disease.3, 4 The disease is characterized by relatively high complication rates during the acute and sub‐acute stages.5, 6, 7, 8 One‐fifth to two‐thirds of patients with TS may develop complications depending on the type of trigger factor, emotional or physical, presence of chronic comorbidities, gender, and age of the patients.5, 6, 7, 8, 9 One of the serious complications of TS is thrombo‐embolism in addition to heart failure, pulmonary edema, cardiogenic shock, cardiac arrest, life‐threatening arrhythmias, left ventricular outlet tract obstruction, mitral regurgitation, cardiac rupture, and death.5, 6, 7, 8, 10 In this report, the thrombo‐embolic complications in TS with the emphasis on the association of TS to both ACS including coronary embolism and ischemic stroke including cerebral embolism are reviewed. This complication is exemplified with a typical case of mid‐apical TS complicated by left ventricular thrombus (LVT) and both coronary and cerebral embolism.
2. DEMONSTRATION OF A CASE
A 67‐year‐old woman presented with dyspnea and chest pain. The patient had a history of depression medicated regularly with Sertraline. She had no history of smoking, diabetes mellitus, hypertension, or obesity and had not a family history of ischemic heart disease. Three days prior to admission, the patient had been told that her son had been subjected to robbery and abuse. Minutes after this intense emotional stress, the patient experienced shortness of breath associated with nausea and vomiting. The dyspnea continued and 3 days later, the patient started to have chest pain, which resulted in admission of the patient. Electrocardiogram (ECG) revealed sinus rhythm with ST‐elevations in the inferior leads and in V3 through V5 with anterior T‐wave inversions. There was modest elevation of troponin T with maximum value 528 ng/L on the third day of admission. Bedside trans‐thoracic echocardiography showed akinesia in the mid and apical segments of the left ventricle with normal contractions at the basal segments, findings consistent with TS. Immediate invasive coronary angiography (CAG) showed coronary arteries, which initially were deemed to be normal (Figure 1A,B). Left ventriculography confirmed the left ventricular mid‐apical ballooning in two projections with a circumferential pattern with normal contractility at the basal segments (Figure 1C–F). Careful review of the CAG revealed occlusion of left anterior descending artery (LAD) in the apical segment (Figure 1G black arrow, even seen in Figure 1A). Signs of LVT could not be seen with certainty in reviewing left ventriculography and echocardiography; however, contrast echocardiography was not performed. Half an hour after CAG, the patient developed expressive aphasia, right‐sided hemiparesis, and paresthesia. Emergency brain computed tomography and angiography showed thrombotic occlusion of the left middle cerebral artery M2 segment (Figure 2A with magnification in Figure 2B). Owing to initiation with ticagrelor medication, thrombolytic treatment was deemed to be inappropriate. Emergency neuro‐intervention in the form of thrombectomy in the M2 segment of the left middle cerebral artery was performed using a Solitaire 4 x 20 stentriever (Covidien‐Medtronic, Minneapolis, MN, USA) together with a balloon guide 8F catheter in the left internal carotid artery and with simultaneous manual aspiration. Full revascularization (mTICI score 3) was achieved after the first thrombectomy attempt (Figure 2C before thrombectomy and 2D after). There was complete resolution of the neurological signs and symptoms. Repeated echocardiography on day 2 showed mid‐apical ballooning but no detectable signs of LVT. Cardiac magnetic resonance (CMR) imaging on day 5 of admission confirmed circumferential mid‐apical ballooning with normal contractility in the basal segments (Figure 3A‐H). On native T1 using Modified Look‐Locker Inversion recovery (MOLLI) imaging, increased myocardial T1 was present in the mid‐apical segments suggesting the presence of edema (Figure 3I‐L, arrows). There were signs of myocardial infarction in the apical inferior segment corresponding to the apical LAD occlusion but no late gadolinium enhancement (LGE) in the remainder of the mid‐apical hypo−/akinetic segments of the left ventricle (Figure 3M‐P). There was a small filling defect in the apical region of the left ventricle consistent with LVT (Figure 3A,B white arrow). The patient was put on anticoagulation, beta blockers, and angiotensin‐converting enzyme inhibitors. Follow‐up echocardiography 3 weeks later showed normalization of the left ventricular dysfunction apart from mild hypokinesia in a limited infarcted infero‐apical segment. There was no sign of detectable LVT. Seven weeks later, cardiac computed tomography angiography showed spontaneous recanalization of the apical LAD segment (Figure 4A at admission, black arrow, and Figure 4B and C at follow up, white arrows).
Figure 1.
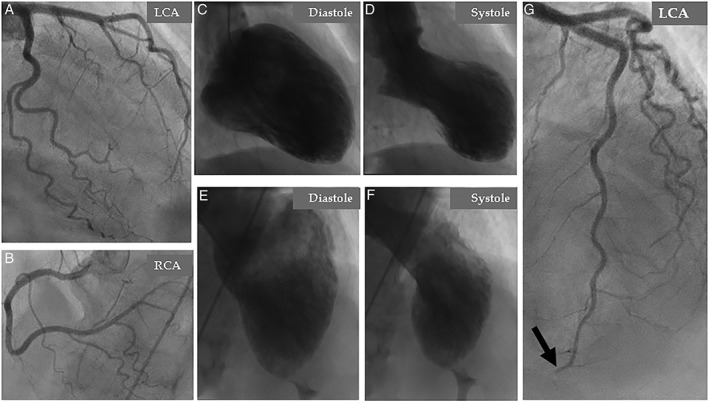
Coronary angiography: (A), left coronary artery (LCA), and (B), right coronary artery (RCA) deemed initially to be normal. Contrast left ventriculography in two projections; right anterior oblique (C and D) and left cranial anterior oblique (E and F) shows circumferential mid‐apical ballooning and normal contractility of the basal segments during systole. Careful review of coronary angiography reveals an abrupt occlusion of the apical segment of the left anterior descending artery (LAD) (G, black arrow)
Figure 2.
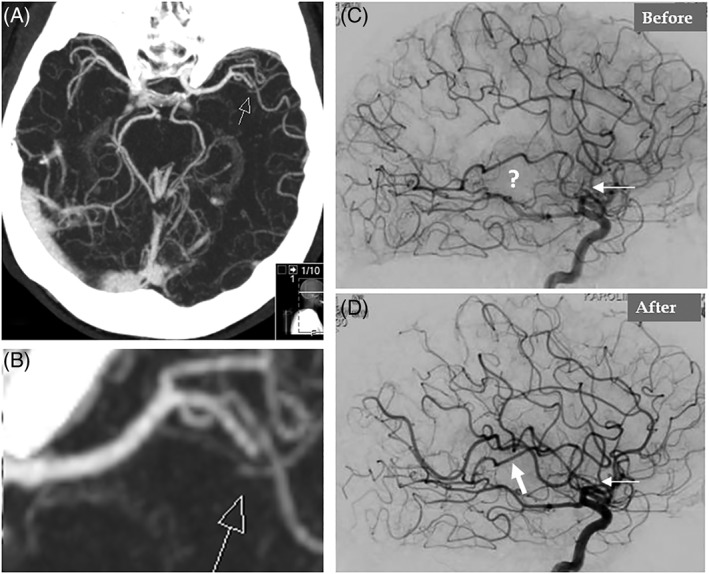
Cerebral angiography shows occlusion in the left middle cerebral artery at segment M2 (A, arrow) with amplification in (B). Occlusion of the left middle cerebral artery at segment M2 is seen (C, thin white arrow and question mark before thrombectomy). Complete recanalization of the left middle cerebral artery after endovascular thrombectomy (D, white arrows)
Figure 3.
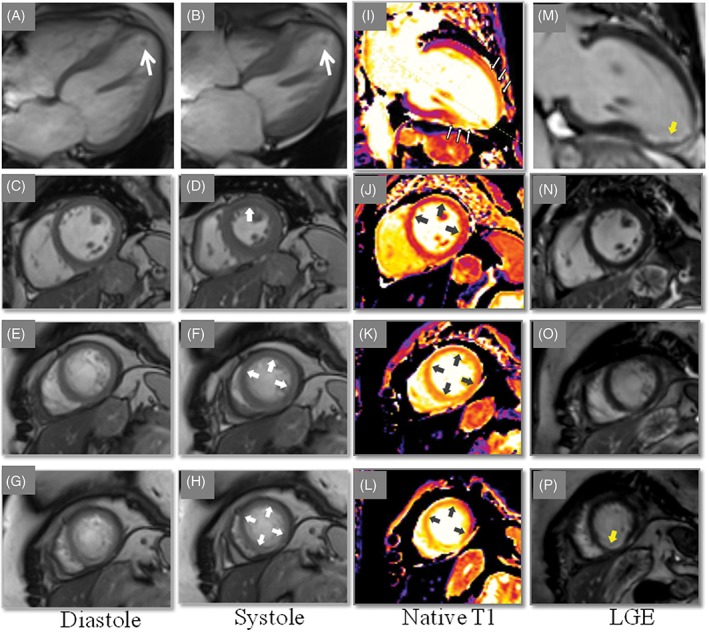
Cardiac magnetic resonance (CMR) imaging reveals mid‐apical ballooning in four chamber view (A, diastole and B, systole. A thrombus is seen in the apical part of the left ventricle (A and B, white arrows). Mid‐ and apical short‐axis end diastolic‐ and systolic images show regional hypokinesia (C, D, E, F, G, and H, white arrows). Native T1, Modified Look‐Locker Inversion recovery (MOLLI), T1 map demonstrated diffusely increased T1 values (1422‐1560 ms) in the mid‐ and apical region of left ventricular (LV) wall (gray arrows) indicating edema in these segments (I, J, K, and L). on late gadolinium enhancement (LGE) imaging, no evidence of focal LGE in the hypokinetic segments (M, N, and O) apart from the sub‐endocardial infarction seen in the apical inferior wall (P, yellow arrow)
Figure 4.
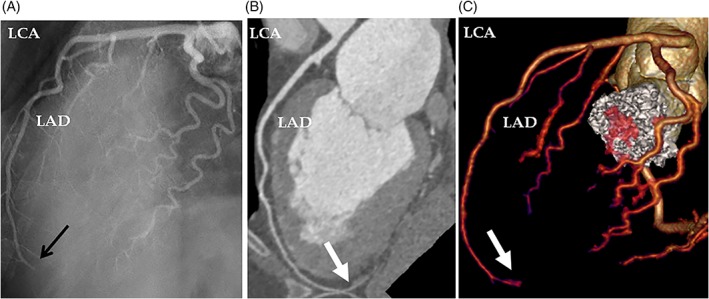
The LAD occlusion, which was seen at admission coronary angiography (A, black arrow) is recanalized on cardiac computed tomography angiography during follow up 7 weeks later (B, white arrow). The recanalization is also seen on volume rendering image of cardiac computed tomography (C, white arrow). LAD, left anterior descending artery; LCA, left coronary artery
3. DISCUSSION
LVT formation and cardio‐embolic events are among the serious complications of TS.11, 12, 13, 14, 15 The case demonstrated in this review has typical emotional‐triggered TS with dyspnea as the main symptom. On admission day, the patient experienced chest pain that most probably was because of abrupt occlusion of the distal apical segment of LAD causing acute myocardial infarction confirmed by CMR imaging. The relatively small distal apical LAD occlusion could not explain the extensive mid‐apical LVWMA. Embolization from the LVT, detected by CMR imaging (Figure 3), was the most likely cause of the LAD apical occlusion. A further embolic event occurred in the left middle cerebral artery, which was successfully treated with neuro‐interventional thrombectomy. Other possible causes of ischemic stroke, such as atrial fibrillation or stenoses of the brain supplying arteries were not identified.
4. PREVALENCE OF LVT, THROMBO‐EMBOLISM, AND CARDIO‐EMBOLIC COMPLICATIONS IN TS
The prevalence of thrombo‐emblism including LVT and the cardio‐embolic complications are summarized in Table 1. LVT has been reported in 1% to 8% in patients with TS.5, 12, 13 Thrombo‐embolism has been reported in 2% to 14% of patients with TS in general.6, 11, 13, 14, 15, 16 Cardio‐embolic events have occurred in 17% to 33% in those with LVT in TS.12, 13, 16 Cases of cardio‐embolic events in the absence of detectable LVT have also been reported.14, 17, 18 The most common reported sites of cardio‐embolic complications are cerebral, renal, and peripheral limb arteries.12, 13, 15, 18
Table 1.
The table reveals the prevalence of thrombo‐embolism, LVT, and cardio‐embolic events in patients with TS
| Authors | No of patients with TS | No of patients with thrombo‐embolism (both LVT and cardio‐embolism) (%) | No of patients with LVT (%) | No of patients with cardio‐embolism | Localization of the cardiac embolus | Others |
|---|---|---|---|---|---|---|
| Haghi et al13 | 52 | 4 (8%) | 4 (8%) | 0 (0%) | — | One patient had thrombosis of the abdominal aorta, renal infarction, and iliac artery occlusion before the onset of TS |
| Mitsuma et al14 | 21 | 3 (14%) | 1 (4.8%) | 2 (9.5%) | Stroke in 2 patients | — |
| Sharkey et al15 | 136 | 5 (3.7%) | 5 (3.7%) | 2 (1.5%) | Cerebral in 1 patient, and both cerebral and pulmonary in 1 patient | LVT in 4 patients and both LVT and RV thrombus in 1 patient. |
| Kurisu et al11 | 95 | 5 (5.3%) | 5 (5.3%) | 1 (1.1%) | Cerebral infarction in 1 patient | Mural thrombus and immobile in 2 patients, and protruding and mobile in 3 patients |
| Templin et al5 | 1750 | NA | (1.3%) | NA | NA | — |
| Y‐Hassan6 | 80 (pheochromocytoma‐triggered TS) | 7 (8.75%) | NA | NA | NA | 6 of 7 patients with thrombo‐embolism had mid‐apical pattern of TS |
| Santoro et al16 | 541 | 12 (2.2%) | 12 (2.2%) | 2 (0.4%) | Stroke in 2 patients | Mural thrombus in 5 patients and protruding in 7 patients. All patients with LVT had apical TS |
| De Gregorio et al12 review of single casesa | Review of 14 studies (13 single case studies and 2 cases), total 15 patients with TS and thrombo‐embolism | 15 | 14 | 5 | Stroke i 3 patients, renal infarction in 1, and popliteal artery in 1 | All patients with LVT and apical or mid‐apical pattern of TS |
| Haghi et al13 review of single casesa | Review of 14 literature cases with TS and LVT | 14 | 14 | 3 | Stroke in 1, TIA in 1 and renal infarction in 1 | — |
Abbreviations: LVT, left ventricular thrombus; No, number; RV, right ventricle; TIA, transient ischemic attack; TS, takotsubo syndrome.
The localization of LVT and the site cardiac emboli if available are also seen.
Authors reviewed only patients with TS complicated by thrombo‐embolism and not all TS patients.
In most of the cases, the LVT occurs in the apical or mid‐apical patterns of TS, and at the apical region of the left ventricle where low blood flow is a contributing factor for LVT formation.6, 12, 16 All 15 patients with LVT reported by de Gregorio et al12 had apical or mid‐apical ballooning pattern of TS. Similarly, all 12 patients with LVT reported by Santoro et al16 had apical ballooning pattern. LVT has also been reported (but rarely) in apical sparing patterns of TS13, 18 where the thrombus may occur adjacent to the papillary cardiac muscles.13 Right ventricular thrombus has also been reported in cases of biventricular TS with15 or without pulmonary embolic event.19 The thrombus in the left ventricle has been classified as mural or protruding.11 A mural thrombus is flat and parallel to the endocardial surface of the left ventricle. A protruding thrombus is projected into the left ventricular cavity; it is usually spherical and may be mobile where some parts of the thrombus moves independently of the underlying myocardium. The thrombus is mural in about 40% and protruding in about 60% of patients with LVT in TS.11, 16
5. ACS INCLUDING CORONARY EMBOLISM AND TS: TRIGGER AND/OR CONSEQUENCE
ACS and TS have almost identical clinical and electrocardiographic presentation. Most of the patients with TS have normal coronary arteries. However, obstructive chronic coronary artery disease and TS may coexist.20, 21, 22 It is important to recognize that ACS, irrespective of the cause of the acute coronary lesion, may trigger TS.23, 24, 25, 26, 27, 28 Moreover, acute myocardial infarction due to acute coronary occlusion from a coronary embolus may be a complication of TS. Recently, Y‐Hassan29 reported a case of ACS due to severe three‐vessel disease triggering typical mid‐apical TS. The myocardial stunning in the same patient caused systo‐diastolic compression of a segment of LAD with myocardial bridging during the acute and sub‐acute stages of the disease with relief of compression during recovery of the LVWMA few weeks later. Consequently, tight coronary stenosis and TS may trigger each other. The most likely cause of the apical LAD occlusion in the case demonstrated is coronary embolism secondary to the apical LVT. The small apical LAD occlusion could not explain the extensive LVWMA seen in the mid‐apical region.
To the best of our knowledge, the demonstrated patient is the first report on TS complicated by LVT, with both coronary and cerebral embolization. Two cases of only coronary embolus in TS have been reported.30, 31 The first one was a case of right coronary artery thrombo‐embolic occlusion occurring in the early in‐hospital course of a patient with mid‐apical TS without detectable LVT.30 The second case was a coronary embolus in the distal branch of right coronary artery deemed to be secondary to TS also without observed LVT.31 Furthermore, a case of myocardial infarction secondary to TS was described by Kato et al.32 That case was a 71‐year‐old woman with mid‐ventricular TS associated with occlusion in a septal branch emanated from the distal LAD causing myocardial infarction limited to the septal branch territory. This was verified by invasive coronary angiography and CMR imaging. The history and clinical findings in that patient indicated that the septal occlusion was secondary to TS. Another case of subacute stent thrombosis in the right coronary artery associated with mid‐apical TS complicated by LVT and peripheral right leg embolization has been reported.33 In that case, whether subacute stent thrombosis triggered TS or it was a consequence of TS, can be discussed. The coronary occlusion in the demonstrated patient was treated conservatively because the occlusion was deemed too peripheral to be treated by percutaneous coronary intervention. There was spontaneous recanalization of the coronary artery (Figure 4B,C). Although the evidence for the embolic cause of coronary occlusion in the current case is adequate, the possibility of spontaneous coronary artery dissection (SCAD) in LAD cannot be completely excluded. Spontaneous resolution and recanalization of the dissected vessel is also a characteristic feature of SCAD. During the last few years, many cases with findings of TS and SCAD in the same patient have been reported.28
6. ISCHEMIC STROKE INCLUDING CEREBRAL EMBOLISM AND TS: TRIGGER OR CONSEQUENCE
In patients with both ischemic stroke and TS, it is essential to determine whether the cardiac dysfunction is triggered by stroke, is the underlying cause of stroke, or if it is an unrelated complication. Ischemic stroke is one among many other central nervous system diseases, such as subarachnoid hemorrhage, epilepsy, intra‐cerebral bleeding, Guillain‐Barre' syndrome, limbic encephalitis, traumatic brain injury, reversible posterior leukoencephalopathy syndrome, and amyotrophic lateral sclerosis, which may trigger TS.34, 35 Patients with ischemic stroke usually have no history of chest pain preceding the TS onset. TS, especially those with mid‐apical ballooning pattern with or without detectable LVT, may be complicated by cardio‐embolic stroke,12, 13 which usually occurs within the first week of the disease onset. Other patients may present with both stroke and TS simultaneously. Patients presenting with ischemic stroke showed to have TS and LVT have also been reported.36 In such cases, it will be extremely difficult to determine whether TS is the cause or consequence of ischemic stroke.37
Cerebral embolism is a serious and the most common site of embolic events in TS12, 38; other sites are kidneys18 and lower limbs.39, 40 The thrombotic occlusion of the left middle cerebral artery in the demonstrated case was clearly an embolism secondary to TS because it occurred after the onset of TS. However, an iatrogenic complication to the invasive coronary and left ventricular angiography cannot be completely excluded as it occurred half an hour after this investigation. The cerebral embolus was treated successfully with endovascular thrombectomy with complete resolution of the neurological deficits. To the best of our knowledge, the case is the first one with cerebral embolism secondary to TS to be treated with endovascular thrombectomy. Two other similar cases with TS complicated by embolic occlusion of the middle cerebral artery at segment M2 have been reported.17, 38 The first one was a case with extensive left ventricular apical ballooning with no detectable LVT that developed acute thrombotic occlusion of the middle cerebral artery segment M2.38 That case was treated successfully with intra‐arterial rt‐PA thrombolysis without any complications. Thrombolysis was avoided in our case because of ongoing treatment with ticagrelor. The second case was a 44‐year‐old woman with mid‐apical TS complicated by embolic occlusion of the right middle cerebral artery at segment M2, detected by MR angiography, causing acute infarction in the right insular cortex. Because of spontaneous resolution of the neurological deficits, endovascular intervention was not performed in that patient. She was treated with anticoagulation and there was complete recanalization of the right middle cerebral artery 8 days later.17
7. DETECTION OF LVT AND TREATMENT OF THROMBO‐EMBOLISM IN TS
LVT may develop early in the course of TS before admission and some cases may present with cardio‐embolic complications such as stroke. The current case presented with both TS and peripheral coronary embolus. LVT may also develop during days after admission and even late in the course of TS.13, 41, 42 In three of the four patients with LVT reported by Haghi et al,13 the LVT was present at the time of diagnosis. In one patient, the thrombus was absent initially and developed later. Nerella et al42 reported a case with apical TS without detectable LVT during the first day. Repeated echocardiography on day five revealed LVT. Another case,41 left ventriculography revealed apical TS without detectable LVT. On follow‐up 2 weeks later, echocardiography showed a large mobile LVT. Seven weeks later, follow‐up echocardiography showed complete resolution of the LVT without any cardio‐embolic event.41 LVT with renal infarction as cardio‐embolic complication has occurred 4 weeks after the initial TS.43 Cardio‐embolic events may occur in TS with overt LVT as in the current case or TS without evident LVT.14, 17 Otani et al17 presented a case of a cerebral embolus as a complication of TS and reviewed 19 other similar cases. They found no evidence of LVT in 10 (50%) of those 20 patients. To prevent the serious thrombo‐embolic complications in TS, LVT should be looked for and detected as early as possible and treated appropriately with anticoagulation. Accordingly, cardiac imaging should be done as early as possible during admission days and should be repeated. Echocardiography is the most accessible and feasible image modality to achieve this task. It can be done at bedside and can be repeated without any risk to the patient. Contrast echocardiography should be considered to detect intra‐ventricular thrombus. Focused cardiac ultrasonography (FCU) should also be considered as an important adjunct to formal echocardiography.44 FCU has revealed severe left ventricular dysfunction with a large mobile LVT.44 The skill and the awareness of echocardiographers for the disease condition are very important. However, echocardiography may have a limitation to clearly visualize the left ventricular apex where the LVT usually occurs. In such cases, CMR imaging or contrast computed tomography may be needed to disclose LVT. In the case demonstrated, repeated echocardiography did not reveal LVT; contrast echocardiography was not performed. CMR imaging 5 days after admission detected the LVT. Contrast computed tomography but not echocardiography has clearly depicted a LVT in a case report.45
LVT and cardio‐embolic complications should be treated with anticoagulation as soon as possible. It is sensible to treat even cases with extensive mid‐apical ballooning, where the risk of thrombo‐embolism is substantial, as a prophylactic measure. In a recent study,16 increased troponin levels (troponin I level >10 ng/mL) and apical ballooning were strongly associated with the occurrence of LVT and anticoagulation should be considered in such cases as a prophylactic treatment. The anticoagulation should be continued for 2 to 3 months or at least until the LVWMA and LVT have resolved. The LVT usually resolves when the LVWMA improves or resolves. Worth to mention, the cardiac thrombo‐embolism may occur even during treatment with anticoagulation. Despite anticoagulation and therapeutic INR, Nerella et al42 reported a case with apical TS and LVT detected by echocardiography on day 5 of admission. This patient developed renal infarction on day 8. Surgical resection of large LVT in TS have been performed through transapical extirpation46 and through trans‐mitral thrombectomy.47
Highlights:
Thrombo‐embolic complications in TS are reviewed.
The complex cause or consequence relations between TS and myocardial infarction is discussed.
The complex cause and consequence relations between TS and cerebral infarction are discussed.
The review is illustrated with a case with the combination of left ventricular thrombus, and coronary and cerebral embolism in TS with enlightening figures.
The case presented will be the first cerebral embolism in TS to be treated with successful endovascular thrombectomy.
8. CONCLUSION
Thrombo‐embolic complications in TS are reviewed. The causal link (trigger or consequence) between TS and both ACS including coronary embolism and acute ischemic stroke including cerebral embolism are discussed. This TS complication and its association to ACS and ischemic stroke are elucidated with a case of mid‐apical TS complicated by LVT and both coronary and cerebral embolism. The cerebral embolism was successfully treated with endovascular thrombectomy and there was spontaneous recanalization of the apical LAD occlusion.
CONFLICTS OF INTEREST
The authors declare no potential conflict of interests.
Y‐Hassan S, Holmin S, Abdula G, Böhm F. Thrombo‐embolic complications in takotsubo syndrome: Review and demonstration of an illustrative case. Clin Cardiol. 2019;42:312–319. 10.1002/clc.23137
REFERENCES
- 1. Y‐Hassan S, De Palma R. Contemporary review on the pathogenesis of takotsubo syndrome: the heart shedding tears: norepinephrine churn and foam at the cardiac sympathetic nerve terminals. Int J Cardiol. 2016;228:528‐536. [DOI] [PubMed] [Google Scholar]
- 2. Ghadri JR, Wittstein IS, Prasad A, et al. International expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032‐2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Y‐Hassan S, Yamasaki K. History of takotsubo syndrome: is the syndrome really described as a disease entity first in 1990? Some inaccuracies. Int J Cardiol. 2013;166(3):736‐737. [DOI] [PubMed] [Google Scholar]
- 4. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases. J Cardiol. 1991;21(2):203‐214. [PubMed] [Google Scholar]
- 5. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929‐938. [DOI] [PubMed] [Google Scholar]
- 6. Y‐Hassan S. Clinical features and outcome of Pheochromocytoma‐induced Takotsubo syndrome: analysis of 80 published cases. Am J Cardiol. 2016;117(11):1836‐1844. [DOI] [PubMed] [Google Scholar]
- 7. Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on Takotsubo syndrome: a position statement from the taskforce on Takotsubo syndrome of the heart failure Association of the European Society of cardiology. Eur J Heart Fail. 2016;18(1):8‐27. [DOI] [PubMed] [Google Scholar]
- 8. Y‐Hassan S. Clinical features and outcome of epinephrine‐induced takotsubo syndrome: analysis of 33 published cases. Cardiovasc Revasc Med. 2016;17(7):450‐455. [DOI] [PubMed] [Google Scholar]
- 9. Y‐Hassan S, Tornvall P. Epidemiology, pathogenesis, and management of takotsubo syndrome. Clin Auton Res. 2018;28(1):53‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghadri JR, Wittstein IS, Prasad A, et al. International expert consensus document on Takotsubo syndrome (part II): diagnostic workup, outcome, and management. Eur Heart J. 2018;39(22):2047‐2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kurisu S, Inoue I, Kawagoe T, et al. Incidence and treatment of left ventricular apical thrombosis in Tako‐tsubo cardiomyopathy. Int J Cardiol. 2011;146(3):e58‐e60. [DOI] [PubMed] [Google Scholar]
- 12. de Gregorio C, Grimaldi P, Lentini C. Left ventricular thrombus formation and cardioembolic complications in patients with Takotsubo‐like syndrome: a systematic review. Int J Cardiol. 2008;131(1):18‐24. [DOI] [PubMed] [Google Scholar]
- 13. Haghi D, Papavassiliu T, Heggemann F, Kaden JJ, Borggrefe M, Suselbeck T. Incidence and clinical significance of left ventricular thrombus in tako‐tsubo cardiomyopathy assessed with echocardiography. QJM. 2008;101(5):381‐386. [DOI] [PubMed] [Google Scholar]
- 14. Mitsuma W, Kodama M, Ito M, et al. Thromboembolism in Takotsubo cardiomyopathy. Int J Cardiol. 2010;139(1):98‐100. [DOI] [PubMed] [Google Scholar]
- 15. Sharkey SW, Windenburg DC, Lesser JR, et al. Natural history and expansive clinical profile of stress (tako‐tsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55(4):333‐341. [DOI] [PubMed] [Google Scholar]
- 16. Santoro F, Stiermaier T, Tarantino N, De Gennaro L, Moeller C, et al. Left ventricular thrombi in Takotsubo syndrome: incidence, predictors, and management: results from the GEIST (German Italian stress cardiomyopathy) registry. J Am Heart Assoc. 2017;6:e006990. 10.1161/JAHA.117.006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Otani Y, Tokunaga K, Kawauchi S, et al. Cerebral infarction arising from Takotsubo cardiomyopathy: case report and literature review. NMC Case Rep J. 2016;3(4):119‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Y‐Hassan S, Shahgaldi K. Thrombo‐embolic renal infarction in a case of mid‐ventricular takotsubo syndrome. Intern Med. 2011;50(19):2175‐2178. [DOI] [PubMed] [Google Scholar]
- 19. De Gennaro L, Ruggiero M, Musci S, Tota F, De Laura D, et al. Biventricular thrombosis in biventricular stress(takotsubo)‐cardiomyopathy. J Thromb Thrombolysis. 2017;44(2):234‐237. [DOI] [PubMed] [Google Scholar]
- 20. Winchester DE, Ragosta M, Taylor AM. Concurrence of angiographic coronary artery disease in patients with apical ballooning syndrome (tako‐tsubo cardiomyopathy). Catheter Cardiovasc Interv. 2008;72(5):612‐616. [DOI] [PubMed] [Google Scholar]
- 21. Gaibazzi N, Ugo F, Vignali L, Zoni A, Reverberi C, Gherli T. Tako‐Tsubo cardiomyopathy with coronary artery stenosis: a case‐series challenging the original definition. Int J Cardiol. 2009;133(2):205‐212. [DOI] [PubMed] [Google Scholar]
- 22. Y‐Hassan S, Jernberg T. Bromocriptine‐induced coronary spasm caused acute coronary syndrome, which triggered its own clinical twin‐‐Takotsubo syndrome. Cardiology. 2011;119(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 23. Gurlek C, van Es J, van der Burgh PH, Galjee MA, von Birgelen C. Full pattern of transient apical ballooning of the left ventricle triggered by minor myocardial infarction. Neth Heart J. 2007;15(9):310‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. S Y‐H. Takotsubo syndrome triggered by acute coronary syndrome in a cohort of 20 patients: an often missed diagnosis. Int J Cardiol Res. 2015;02(2):28‐33. [Google Scholar]
- 25. Messas N, Blondet C, Jesel L, et al. Diagnostic relevance of optical coherence tomography imaging in aborted acute myocardial infarction with a Takotsubo component. Int J Cardiol. 2015;195:123‐125. [DOI] [PubMed] [Google Scholar]
- 26. Y‐Hassan S, Themudo R, Maret E. Spontaneous coronary artery dissection and takotsubo syndrome: the chicken or the egg causality dilemma. Catheter Cardiovasc Interv. 2017;89:1215‐1218. [DOI] [PubMed] [Google Scholar]
- 27. Y‐Hassan S, Bohm F. The causal link between spontaneous coronary artery dissection and takotsubo syndrome: a case presented with both conditions. Int J Cardiol. 2016;203:828‐831. [DOI] [PubMed] [Google Scholar]
- 28. Y‐Hassan S. Spontaneous coronary artery dissection and takotsubo syndrome: an often overlooked association; review. Cardiovasc Revasc Med. 2018;19:717‐723. [DOI] [PubMed] [Google Scholar]
- 29. Y‐Hassan S. Tight coronary artery stenosis and takotsubo syndrome triggered each other: well‐illustrated in a case. Cardiovasc Revasc Med. 2018;19:2‐4. [DOI] [PubMed] [Google Scholar]
- 30. Angulo‐Llanos R, Sanz‐Ruiz R, Solis J, Fernandez‐Aviles F. Acute myocardial infarction: an uncommon complication of takotsubo cardiomyopathy. Catheter Cardiovasc Interv. 2013;82(6):909‐913. [DOI] [PubMed] [Google Scholar]
- 31. Heseltine TD, Karthikeyan VJ, Morris J. Takotsubo cardiomyopathy with secondary coronary embolus. BMJ Case Rep. 2014. Apr 25;2014. pii: bcr2013203145. 10.1136/bcr-2013-203145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kato K, Sakai Y, Ishibashi I, Kobayashi Y. Mid‐ventricular takotsubo cardiomyopathy preceding acute myocardial infarction. Int J Cardiovasc Imaging. 2015;31(4):821‐822. [DOI] [PubMed] [Google Scholar]
- 33. Tota F, Ruggiero M, Sassara M, et al. Subacute stent thrombosis and stress‐induced cardiomyopathy: trigger or consequence? Am J Cardiovasc Dis. 2013;3(3):175‐179. [PMC free article] [PubMed] [Google Scholar]
- 34. Finsterer J, Wahbi K. CNS disease triggering Takotsubo stress cardiomyopathy. Int J Cardiol. 2014;177(2):322‐329. [DOI] [PubMed] [Google Scholar]
- 35. Dande AS, Pandit AS. Broken heart syndrome, neurogenic stunned myocardium and stroke. Curr Treat Options Cardiovasc Med. 2013;15(3):265‐275. [DOI] [PubMed] [Google Scholar]
- 36. Grabowski A, Kilian J, Strank C, Cieslinski G, Meyding‐Lamade U. Takotsubo cardiomyopathy‐‐a rare cause of cardioembolic stroke. Cerebrovasc Dis. 2007;24(1):146‐148. [DOI] [PubMed] [Google Scholar]
- 37. Bersano A, Melchiorre P, Moschwitis G, et al. Tako‐tsubo syndrome as a consequence and cause of stroke. Funct Neurol. 2014;29(2):135‐137. [PMC free article] [PubMed] [Google Scholar]
- 38. Jabiri MZ, Mazighi M, Meimoun P, Amarenco P. Tako‐tsubo syndrome: a cardioembolic cause of brain infarction. Cerebrovasc Dis. 2010;29(3):309‐310. [DOI] [PubMed] [Google Scholar]
- 39. Battimelli A, Polito MV, Di Maio M, Poto S, Pierro L, et al. Stress‐related cardiomyopathy, ventricular dysfunction, artery thrombosis: a hidden pheochromocytoma. Am J Emerg Med. 2014;32(3):286 e285‐286 e289. [DOI] [PubMed] [Google Scholar]
- 40. Figueredo VM, Gupta S. Embolic complication of Tako‐Tsubo cardiomyopathy. QJM. 2009;102(11):820‐822. [DOI] [PubMed] [Google Scholar]
- 41. Singh V, Mayer T, Salanitri J, Salinger MH. Cardiac MRI documented left ventricular thrombus complicating acute Takotsubo syndrome: an uncommon dilemma. Int J Cardiovasc Imaging. 2007;23(5):591‐593. [DOI] [PubMed] [Google Scholar]
- 42. Nerella N, Lodha A, Tiu CT, Chandra PA, Rose M. Thromboembolism in takotsubo syndrome: a case report. Int J Cardiol. 2008;124(2):e37‐e38. [DOI] [PubMed] [Google Scholar]
- 43. Sasaki N, Kinugawa T, Yamawaki M, et al. Transient left ventricular apical ballooning in a patient with bicuspid aortic valve created a left ventricular thrombus leading to acute renal infarction. Circ J. 2004;68(11):1081‐1083. [DOI] [PubMed] [Google Scholar]
- 44. Hrymak C, Liu S, Koulack J, Funk DJ, Schaffer SA, Tam JW. Embolus from probable Takotsubo cardiomyopathy: a bedside diagnosis. Can J Cardiol. 2014;30(12):1732 e1739‐1732 e1711. [DOI] [PubMed] [Google Scholar]
- 45. Ouchi K, Nakamura F, Ikutomi M, et al. Usefulness of contrast computed tomography to detect left ventricular apical thrombus associated with takotsubo cardiomyopathy. Heart Vessels. 2016;31(5):822‐827. [DOI] [PubMed] [Google Scholar]
- 46. Suzuki R, Kudo T, Kurazumi H, et al. Transapical extirpation of a left ventricular thrombus in Takotsubo cardiomyopathy. J Cardiothorac Surg. 2013;8:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zaikokuji K, Sawazaki M, Tomari S, Uemura T. Transmitral thrombectomy to treat a patient with Takotsubo cardiomyopathy. Asian Cardiovasc Thorac Ann. 2018;26(3):236‐238. [DOI] [PubMed] [Google Scholar]


