Abstract
Although the exact mechanism and most involved region of the vestibular system have not yet been fully clarified, vestibular dysfunction has been demonstrated in patients with diabetes mellitus (DM). Vestibular evoked myogenic potential (VEMP) is a short latency electromyographic response to sound or vibration stimuli that may reflect otolith organ or related reflex functions. Since its first description in 1992, VEMP has become a significant part of the vestibular test battery as an objective measurement tool. In diabetic patients, VEMP responses have been studied in order to determine any otolith organ or related reflex dysfunctions. Here, we review the literature with regard to VEMP findings representing any peripheral vestibular end-organ dysfunction in patients with DM. Distinctive vestibular end-organ impairments seem to be demonstrated in patients with DM either with or without DNP via objective vestibular testing tools including VEMP recordings according to relevant studies. However, further studies with larger sample sizes are required to reveal the more definitive findings of VEMP recordings regarding the vestibular pathologies in patients with DM.
Keywords: Diabetes mellitus, Vestibular diseases, Cervical vestibular evoked myogenic potentials, Ocular vestibular evoked myogenic potentials
Diabetes mellitus (DM) is a chronic metabolic disease characterized by insulin insufficiency causing chronic degenerative complications involving almost every organ of the human body (American Diabetes Association, 2012). In patients with DM, peripheral neuropathy and retinopathy are well-established pathologies that induce balance impairment and falls (Simoneau et al., 1994; Schwartz et al., 2008). However, in recent studies, vestibular dysfunction due to peripheral end-organ pathologies was considered as a distinctive complication of DM contributing to vestibular system disorders (Agrawal et al., 2009). As an objective tool to determine otolith organ or related reflex dysfunction, vestibular evoked myogenic potential (VEMP) responses have been studied in diabetic patients in order to recognize peripheral end-organ pathologies (Bektaş et al., 2008; Kamali et al., 2013; Konukseven et al., 2015; Kalkan et al., 2018). Here, we review the literature with regard to VEMP findings representing any peripheral vestibular end-organ pathology in diabetic patients.
1. Vestibular evoked myogenic potentials
VEMP is a short-latency electromyographic response to sound or vibration stimuli that is considered to demonstrate ipsilateral saccular and inferior vestibular nerve functions (cervical VEMP), as well as contralateral utricular and superior vestibular nerve functions (ocular VEMP) (Rosengren and Kingma, 2013; Colebatch et al., 2016). Since its first description by Colebatch and Halmagyi in 1992, VEMP has become a significant part of the vestibular test battery as an objective measurement tool. Although VEMP testing is mainly used for assessing otolith functions in clinical settings, it has also been used to evaluate the effects of sound stimuli on vestibular reflexes and otolithic projections to various muscular groups and the brain (Rosengren and Colebatch, 2018).
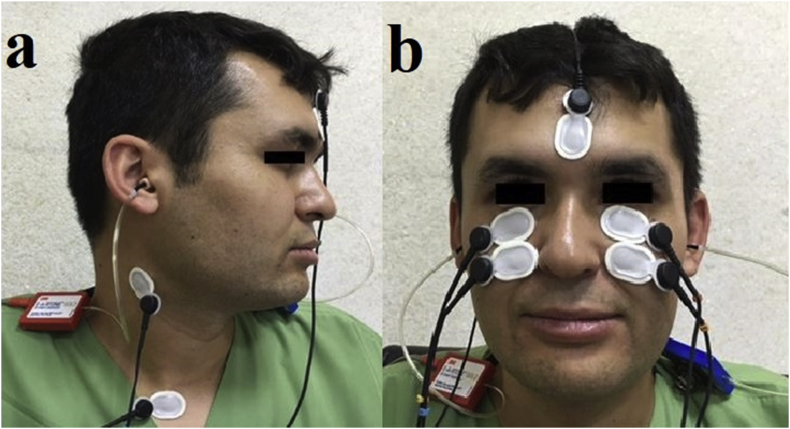
During VEMP testing, surface electrodes are placed on the patient's skin for recording myogenic potentials in response to sound or vibration to provide quick, safe and reliable otolith function measurement (Fig. 1). Cervical VEMP (cVEMP) measures the inhibitory myogenic potentials of the ipsilateral tensed sternocleidomastoid muscle (sacculo-collic reflex) and is considered to evaluate saccular vestibular signals conducted via the vestibulospinal tract (Rosengren et al., 2010; Rosengren and Kingma, 2013; Rosengren and Colebatch, 2018). Ocular VEMP (oVEMP) obtains the excitatory contralateral inferior oblique muscle potentials (utriculo-ocular reflex) and is considered to measure vestibular functions from the utricle to the oculomotor nucleus, including the superior vestibular nerve and the contralateral medial longitudinal fasciculus (Rosengren et al., 2010; Rosengren and Kingma, 2013; Rosengren and Colebatch, 2018; Bayram et al., 2018).
Fig. 1.
Electrode placement for cVEMP (a) and oVEMP (b).
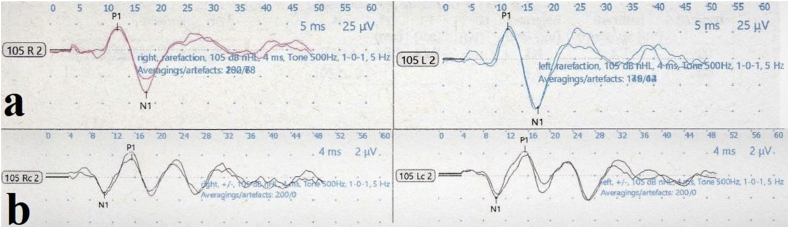
The normal electromyographic responses of cVEMP recordings display a biphasic waveform with an initial positivity (p13) and subsequent negativity (n23), whereas normal oVEMP waves are represented by an initial negativity (n10) and subsequent positivity (p15) (Rosengren and Colebatch, 2018) (Fig. 2). Latency and threshold of the peaks and peak-to-peak amplitudes are calculated for both reflexes. The threshold value describes the minimum sound stimulus required to elicit a biphasic VEMP waveform. Since VEMP amplitudes are significantly influenced by the force of muscular contraction or stimulus intensity, the amplitude asymmetry ratio (AR) is usually used as the main measurement of interest for amplitude values. The AR value is calculated by the Jongkees formula: (larger-smaller)/(larger + smaller) × 100 (Colebatch et al., 2016).
Fig. 2.
Examples of normal cVEMP (a) and oVEMP (b) traces (Neuro-Audio Version, 2010; Neurosoft, Ivanovo, Russia).
VEMP responses have been shown to be influenced by pathological or physiological conditions. Conductive hearing loss may cause the absence of VEMP responses due to the low access of sound intensity at the oval window (Han et al., 2016) whereas VEMP responses are not influenced by sensorineural hearing (Murofushi, 2014). In people older than 60 years, VEMP responses usually show attenuation (Fife et al., 2017). Stimulus characteristics such as intensity, frequency, shape, duration and rise time can significantly affect the normal range of VEMP parameters, hence establishing their own normative data is recommended for the clinics.
2. Role of VEMP testing in the diagnosis of common vestibular diseases
Abnormal cVEMP or oVEMP findings including latency, amplitude and threshold may be a sign of pathological conditions along the vestibulo-collic or vestibulo-ocular reflex pathways. Unilateral absence of both cVEMP and oVEMP responses may indicate a lesion localized at the vestibular end organs, otolith projections and nerve root entry, whereas central disorders or demyelinating pathologies of the vestibular nerve may present with delayed latencies of both reflexes (Colebatch et al., 2016). Nowadays, VEMP testing constitutes an important part of the vestibular test battery and provides either diagnostic or assistive contributions in the clinical evaluation of common vestibular diseases such as superior canal dehiscence syndrome (SCDS), Ménière's disease (MD) and vestibular neuritis (VN) (Murofushi, 2016).
SCDS was first described by Minor et al., in 1998 and the disease is characterized by a defect nearly always located in the bone overlying the superior semicircular canal (SCC). In the diagnosis of SCDS, recognition of the defect with temporal bone computed tomography scans is essential. Nevertheless, VEMP findings can also provide significant data regarding diagnosis and defect functionality. Significantly lower cVEMP thresholds were demonstrated in patients with SCDS (Welgampola et al., 2008; Niesten et al., 2013). Corrected cVEMP amplitude values have 100% sensitivity and 93% specificity in SCDS diagnosis (Fife et al., 2017). In oVEMP testing, higher amplitudes with pathologically lower thresholds for air-conducted sound stimuli were demonstrated in patients with SCDS (Minor et al., 1998; Rosengren et al., 2008; Fife et al., 2017). In the diagnosis of SCDS, the sensitivity and specificity of oVEMP thresholds were 77% and 93%, respectively, while the sensitivity and specificity range of oVEMP amplitudes were 77%–100% and 98%–100%, respectively (Fife et al., 2017). Welgampola et al. (2008) reported that successful canal plugging resulted in normalization of reflex thresholds, therefore VEMP testing may also be useful in monitoring the effectiveness of plugging surgery in SCDS. However, VEMP recordings are particularly amenable for unilateral SCDS by means of interaural comparison rather than bilateral vestibulopathy.
Although MD is usually diagnosed by clinical criteria, laboratory tests such as caloric test are also performed to support the diagnosis in some cases (Ervin, 2004). de Waele et al. (1999) published the first report of cVEMP in MD with the absence of cVEMP responses in 54% of patients’ affected ears. Reduction of ipsilateral cVEMP amplitudes has been reported in different studies with a prevalence of around 50% in MD (Huang et al., 2011; Taylor et al., 2011), while oVEMP abnormalities were demonstrated with a prevalence of 45–54% (Taylor et al., 2011; Murofushi et al., 2011; Winters et al., 2011). VEMP testing enables demonstration of a vestibular loss in MD but there is not sufficient evidence regarding the usefulness of VEMP in diagnosing MD. However, it can be used for monitoring the status of vestibular dysfunction during the disease process (Fife et al., 2017).
Vestibular neuritis is an acute vestibular pathology that is mostly diagnosed by the typical clinical signs of acute unilateral loss of vestibular function (Jeong et al., 2013). VEMP testing is suggested to indicate the pattern of vestibular nerve involvement in VN whether the disease affects the superior, inferior division of the nerve or pan-neuritis (Colebatch et al., 2016). However, sufficient data does not exist in the literature concerning VEMP findings that would frankly determine which vestibular structures are influenced in VN (Fife et al., 2017). Data are also insufficient regarding the role of VEMP in the diagnosis of other vestibular diseases such as in the literature, hence VEMP testing may not be useful in the diagnosis of these pathological conditions (Fife et al., 2017). However, specific VEMP abnormalities related to common vestibular diseases are a significant research interest among clinicians and promise to contribute data to the diagnosis.
3. Diabetes mellitus and vestibular dysfunction
Although the exact mechanism has not yet been fully clarified, vestibular dysfunction has been demonstrated in diabetic patients (Agrawal et al., 2009; Agrawal et al., 2010; Ward et al., 2015). The ratio of vestibular dysfunction was shown 70% higher among diabetic patients than healthy people (Agrawal et al., 2009). The falling risk is significantly increased in DM and diabetic neuropathy (DNP) (Agrawal et al., 2010). Besides the role of DNP, peripheral vestibular end-organ pathologies may also promote balance impairments in these patients. Agrawal et al. (2010) reported that vestibular dysfunction may independently induce balance disorders even after adjusting for DNP in patients with DM. Moreover, vestibular dysfunction may occur without prominent vestibular symptoms in patients with DM, as was recently introduced to the literature as ‘subclinical vestibular neuropathy’ (Konukseven et al., 2015). In this scenario, it is suggested that vestibular pathology can be determined via objective vestibular diagnostic testing tools although patients with DM were free of vestibular symptoms.
A number of morphological studies demonstrated vestibular end-organ disease in experimental diabetic animals (Myers and Ross, 1987; Myers, 1998). In experimentally-induced DM, type 1 hair cell loss in the saccule (Myers and Ross, 1987) and vestibulocochlear nerve myelin degeneration (Myers, 1998) have been shown due to hyperglycemia-induced metabolic stress. As a consequence of metabolic stress, extracellular matrix overproduction and accumulation of lysosomes and lipid droplets were observed in the utricle and saccule (Myers and Ross, 1987). Due to excessive accumulation of extracellular matrix, the diffusion of oxygen and waste products was impaired and the presence of hair cell degeneration mostly occurs in the saccule. Also, vestibulocochlear nerve damage was demonstrated, including disruption of the myelin sheath lamellae and myelin sheath thinning in diabetic rats (Myers, 1998).
Recent developments in the field of the vestibular test battery have enabled investigators to evaluate each vestibular end organ separately (Curthoys, 2012), and the studies demonstrated that each vestibular end-organ function can be affected in DM (Kamali et al., 2013; Ward et al., 2015; Konukseven et al., 2015). In the study of Ward et al. (2015), the authors determined the relative sparing of posterior SCC in diabetic patients. Kocdor et al. (2016) found a neuroepithelial pathology in the saccules of diabetic patients, manifested as a lower density of type I vestibular hair cells and concluded that selective and deleterious effects on human vestibular sensory epithelia may be present in DM. Jáuregui-Renaud et al. (2017) demonstrated utricular dysfunction via decreased response to utricular stimulation by unilateral centrifugation in patients with type 2 DM. These findings promoted studies investigating VEMP findings to reflect any otolith organ or related reflex dysfunction in DM.
4. Diabetes mellitus and VEMP
In 2001, Perez et al. (2001) evaluated vestibular evoked potentials in experimentally-induced DM type 2 DM and showed significantly prolonged first wave latency with decreased amplitude in diabetic animals. According to these findings, the authors stated that vestibular impairment of the inner ear has been unveiled by using an objective test in DM. However, since 2001, the number of studies regarding VEMP responses in DM is still limited in the literature. In these studies, VEMP testing was mostly performed to evaluate peripheral vestibular end organ pathologies and also to demonstrate the effect of DNP on vestibular function in patients with DM. The prevalence of DNP is about 8% in newly diagnosed disease, while the ratio of DNP is greater than 50% in longstanding DM (Deli et al., 2013). Agrawal et al. (2010) found that diabetic patients with severe DNP had a more frequent vestibular impairment than diabetic patients without DNP (76% versus 49%). In their study, DNP also increased the risk of vestibular dysfunction in age-adjusted analyses. In order to evaluate the effect of DM on the VEMP pathway, Bektaş et al. (2008) studied cVEMP recordings in 25 non-insulin-dependent DM patients with DNP, 13 non-insulin-dependent DM patients without DNP, and 21 healthy subjects, and showed that VEMP responses were normal in non-insulin-dependent DM patients with or without DNP. Kamali et al. (2013) demonstrated that cVEMP latencies were significantly prolonged in type 1 diabetic patients either with or without DNP, whereas amplitudes values were similar in the study groups. In their study, prolongation of cVEMP latencies was more frequent in the DNP group than the diabetic patients without DNP and they postulated that this result may indicate lesions in the retrolabyrinthine area, particularly in the vestibulospinal tract. The authors attributed their postulation to the study of Morufushi et al. (2001) conducted with patients with MD, VN, acoustic neuroma (AN) and multiple sclerosis (MS) in 2001. In the study of Morufushi et al. (2001), patients with MS and large AN showed a latency prolongation in cVEMP responses and the authors suggested that prolonged p13 latencies indicate vestibulospinal tract lesions.
In later studies, oVEMP recordings were also used to evaluate vestibular pathologies in DM. Ward et al. (2015) aimed to functionally localize vestibular impairments in adult type 2 DM and demonstrated that both cVEMP peak-to-peak amplitude and oVEMP n1 amplitude were decreased in the DM group. They concluded that in adult type 2 DM, vestibular impairments related to both SCC and otolith organs were higher than age-matched healthy subjects according to VEMP and the head thrust dynamic visual acuity test. Konukseven et al. (2015) evaluated air-conducted oVEMP and cVEMP findings in 30 diabetic and 30 prediabetic patients and 31 healthy controls without any central or peripheral vestibular pathologies. In their study, oVEMP and cVEMP latencies were significantly longer in diabetic patients than in prediabetic patients and healthy controls, however, amplitude values were similar among the groups. The authors indicated that prolonged latencies might be present due to a retrolabyrinthine demyelinating lesion or sensorimotor polyneuropathies, which are common in type 2 DM. However, Kalkan et al. (2018) showed no significant difference between type 2 diabetic patients with DNP (33 patients), without DNP and age- and sex-matched control subjects in terms of cVEMP and oVEMP latencies, whereas peak-to-peak amplitudes were significantly higher in diabetic patients with or without DNP. The ratio of diabetic patients without vestibular symptoms in the studies of Konukseven et al. (2015) and Kalkan et al. (2018) was 70% and 100%, respectively. The similar patient characteristics in these two studies supported a new term called ‘subclinical vestibular neuropathy’ introduced by Konukseven et al. (2015), describing the presence of a vestibular dysfunction in diabetic patients without any vestibular symptoms. This pathological condition was also previously described by Rigon et al. (2007) without a specific term. In the study of Kalkan et al. (2018), the authors also aimed to evaluate SCC functions via video head impulse test (vHIT), but no significant difference was demonstrated in terms of vHIT results among the groups. Given the fact that it is well tolerated by patients, vHIT is recommended in the first line to objectively assess each SCC function as a part of the vestibular battery (Çınar et al., 2018).
Recent studies have revealed that the prevalence of BPPV was higher in DM (Cohen et al., 2004; Yoda et al., 2011; D'Silva et al., 2017). Yoda et al. (2011) showed a significantly higher prevalence of cupular and free-floating deposits in lateral and posterior SCCs in type 1 DM and suggested that dislodged otoconia related to DM originates from the utricle and induces disease symptoms. D'Silva et al. (2017) evaluated cVEMP and oVEMP in four groups of participants including healthy controls (n = 20), patients with type 2 DM without vestibular symptoms (n = 19), patients with BPPV (n = 18) and patients with both DM and BPPV (n = 14). They hypothesized that the comorbidity of DM and BPPV causes a higher frequency of abnormal VEMP responses than in the other study groups. However, according to the cVEMP and oVEMP findings, the authors concluded that BPPV and DM did not seem to have a distinct cumulative effect (D'Silva et al., 2017). Also in their study, contrary to previous reports (Kamali et al., 2013; Konukseven et al., 2015; Kalkan et al., 2018), diabetic patients did not demonstrate any VEMP abnormalities including latency and amplitude values compared to healthy controls.
As summarized above, VEMP findings in DM show conflicting results in the literature that could be attributed to several factors (Table 1). First, the number of studies evaluating vestibular end organ pathologies with objective vestibular testing tools in diabetic patients is still insufficient in the literature. Secondly, heterogeneous patient groups, particularly in terms of disease duration and HbA1c levels, may affect the VEMP responses. DM has the capability of generating multi-organ disease, including the central and peripheral vestibular system. Therefore, results obtained from objective vestibular diagnostic tools can be affected by the degree and localization of DM-induced pathology involving vestibular structures.
Table 1.
A summary of published articles investigating the association of diabetes mellitus and vestibular evoked myogenic potentials.
| Author | Year Published | Study Type | Sample (n) | Test | Findings |
|---|---|---|---|---|---|
| Peres et al. | 2001 | Experimental | 14 sand rat | VsEPs | Prolonged first wave latency decreased amplitude |
| Bektaş et al. | 2008 | Clinical | 25 NIDDM + PNP, 13 NIDDM, 21 controls | cVEMP | No difference |
| Kamali et al. | 2013 | Clinical | 14 type Ι DM + PNP, 10 type Ι DM, 24 controls | cVEMP | Prolonged latency in type Ι DM + PNP |
| Ward et al. | 2015 | Clinical | 25 type II DM, 25 controls | cVEMP oVEMP | Decreased cVEMP peak-to-peak amplitude and oVEMP n1 amplitude |
| Konukseven et al. | 2015 | Clinical | 30 type II DM, 30 prediabetes, 31 controls | cVEMP oVEMP | Prolonged VEMP latencies in type II DM |
| Kalkan et al. | 2018 | Clinical | 33 type ΙI DM + PNP, 33 type ΙI DM, 35 controls | cVEMP oVEMP vHIT | Lower peak-to-peak VEMP amplitudes in the DM + DPN and DM groups |
cVEMP: cervical vestibular evoked myogenic potentials, DM: diabetes mellitus, NIDDM: non-insulin-dependent diabetes mellitus, PNP: polyneuropathy, oVEMP: ocular vestibular evoked myogenic potentials, VsEPs: vestibular evoked potentials.
VEMP, as an objective way to assess the vestibular system, promises researchers future advancements in understanding one of the most unique and sophisticated parts of the human body. Distinctive vestibular end-organ impairments seem to be demonstrated in patients with DM either with or without DNP via objective vestibular testing tools including VEMP recordings according to relevant studies. However, further studies with larger sample sizes are required to reveal the more definitive findings of VEMP recordings regarding the vestibular pathologies in patients with DM.
Funding
None.
Acknowledgements
None.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.joto.2019.05.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Agrawal Y., Carey J.P., Della-Santina C.C., Schubert M.C., Minor L.B. Disorders of balance and vestibular function in US adults: data from the national health and nutrition examination survey, 2001-2004. Arch. Intern. Med. 2009;169:938–944. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- Agrawal Y., Carey J.P., Della-Santina C.C., Schubert M.C., Minor L.B. Diabetes, vestibular dysfunction, and falls: analyses from the national health and nutrition examination survey. Otol. Neurotol. 2010;31:1445–1450. doi: 10.1097/MAO.0b013e3181f2f035. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Standards of medical care in diabetes. 2012. Diabetes Care. 2012;35:11–63. [Google Scholar]
- Bayram A., Kalkan M., Ünsal N., Kale A., Küçük B., Mutlu C. Does blindness affect ocular vestibular evoked myogenic potentials? Am. J. Otolaryngol. 2018;39:290–292. doi: 10.1016/j.amjoto.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Bektas D., Gazioglu S., Arslan S., Cobanoglu B., Boz C., Caylan R. VEMP responses are not affected in non-insulin-dependent diabetes mellitus patients with or without polyneuropathy. Acta Otolaryngol. 2008;128:768–771. doi: 10.1080/00016480701714251. [DOI] [PubMed] [Google Scholar]
- Cohen H.S., Kimball K.T., Stewart M.G. Benign paroxysmal positional vertigo and comorbid conditions. ORL. J. Otorhinolaryngol. Relat. Spec. 2004;66:11–15. doi: 10.1159/000077227. [DOI] [PubMed] [Google Scholar]
- Colebatch J.G., Halmagyi G.M. Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology. 1992;42:1635–1636. doi: 10.1212/wnl.42.8.1635. [DOI] [PubMed] [Google Scholar]
- Colebatch J.G., Rosengren S.M., Welgampola M.S. Vestibular-evoked myogenic potentials. Handb. Clin. Neurol. 2016;137:133–155. doi: 10.1016/B978-0-444-63437-5.00010-8. [DOI] [PubMed] [Google Scholar]
- Curthoys I.S. The interpretation of clinical tests of peripheral vestibular function. The Laryngoscope. 2012;122:1342–1352. doi: 10.1002/lary.23258. [DOI] [PubMed] [Google Scholar]
- Çınar Y., Bayram A., Culfa R., Mutlu C. Analyses with the video head impulse test during the canalith repositioning maneuver in patients with isolated posterior semicircular canal benign paroxysmal positional vertigo. Turk. Arch. Otolaryngol. 2018;56:81–84. doi: 10.5152/tao.2018.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DʼSilva L.J., Staecker H., Lin J., Maddux C., Ferraro J., Dai H., Kluding P.M. Otolith dysfunction in persons with both diabetes and benign paroxysmal positional vertigo. Otol. Neurotol. 2017;38:379–385. doi: 10.1097/MAO.0000000000001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waele C., Huy P.T., Diard J.P., Freyss G., Vidal P.P. Saccular dysfunction in Ménière's disease. Am. J. Otol. 1999;20:223–232. [PubMed] [Google Scholar]
- Deli G., Bosnyak E., Pusch G., Komoly S., Feher G. Diabetic neuropathies: diagnosis and management. Neuroendocrinology. 2013;98:267–280. doi: 10.1159/000358728. [DOI] [PubMed] [Google Scholar]
- Ervin S.E. Ménière's disease: identifying classic symptoms and current treatments. AAOHN J. 2004;52:156–158. [PubMed] [Google Scholar]
- Fife T.D., Colebatch J.G., Kerber K.A., Brantberg K., Strupp M., Lee H., Walker M.F., Ashman E., Fletcher J., Callaghan B., Gloss D.S. 2nd. Practice guideline: cervical and ocular vestibular evoked myogenic potential testing: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology. Neurology. 2017;89:2288–2296. doi: 10.1212/WNL.0000000000004690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P., Zhang R., Chen Z., Gao Y., Cheng Y., Zhang Q., Xu M. Evaluation of ocular and cervical vestibular evoked myogenic potentials in a conductive hearing loss model. J. Otolaryngol. 2016;11:192–197. doi: 10.1016/j.joto.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.H., Wang S.J., Young Y.H. Localization and prevalence of hydrops formation in Ménière's disease using a test battery. Audiol. Neuro. Otol. 2011;16:41–48. doi: 10.1159/000312199. [DOI] [PubMed] [Google Scholar]
- Jáuregui-Renaud K., Aranda-Moreno C., Herrera-Rangel A. Utricular hypofunction in patients with type 2 diabetes mellitus. Acta Otorhinolaryngol. Ital. 2017;37:430–435. doi: 10.14639/0392-100X-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S.H., Kim H.J., Kim J.S. Vestibular neuritis. Semin. Neurol. 2013;33:185–194. doi: 10.1055/s-0033-1354598. [DOI] [PubMed] [Google Scholar]
- Kalkan M., Bayram A., Gökay F., Cura H.S., Mutlu C. Assessment of vestibular-evoked myogenic potentials and video head impulse test in type 2 diabetes mellitus patients with or without polyneuropathy. Eur. Arch. Oto-Rhino-Laryngol. 2018;27:719–724. doi: 10.1007/s00405-018-4873-z. [DOI] [PubMed] [Google Scholar]
- Kamali B., Hajiabolhassan F., Fatahi J., Nasli-Esfahani E., Sarrafzadeh J., Faghihzadeh S. Effects of diabetes mellitus type Ι with or without neuropathy on vestibular evoked myogenic potentials. Acta Med. Iran. 2013;51:107–112. [PubMed] [Google Scholar]
- Kocdor P., Kaya S., Erdil M., Cureoglu S., Paparella M.M., Adams M.E. Vascular and neuroepithelial histopathology of the saccule in humans with diabetes mellitus. Otol. Neurotol. 2016;37:553–557. doi: 10.1097/MAO.0000000000001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konukseven O., Polat S.B., Karahan S., Konukseven E., Ersoy R., Cakir B., Kutluhan A., Aksoy S. Electrophysiologic vestibular evaluation in type 2 diabetic and prediabetic patients: air conduction ocular and cervical vestibular evoked myogenic potentials. Int. J. Audiol. 2015;54:536–543. doi: 10.3109/14992027.2014.971887. [DOI] [PubMed] [Google Scholar]
- Minor L.B., Solomon D., Zinreich J.S., Zee D.S. Sound- and/or pressure induced vertigo due to bone dehiscence of the superior semicircular canal. Arch. Otolaryngol. Head Neck Surg. 1998;124:249–258. doi: 10.1001/archotol.124.3.249. [DOI] [PubMed] [Google Scholar]
- Murofushi T. Vestibular evoked myogenic potential. World J. Otorhinolaryngol. 2014;4:6–11. [Google Scholar]
- Murofushi T. Clinical application of vestibular evoked myogenic potential (VEMP) Auris Nasus Larynx. 2016;43:367–376. doi: 10.1016/j.anl.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Murofushi T., Shimizu K., Takegoshi H., Cheng P.W. Diagnostic value of prolonged latencies in the vestibular evoked myogenic potential. Arch. Otolaryngol. Head Neck Surg. 2001;127:1069–1072. doi: 10.1001/archotol.127.9.1069. [DOI] [PubMed] [Google Scholar]
- Murofushi T., Nakahara H., Yoshimura E., Tsuda Y. Association of air-conducted sound oVEMP findings with cVEMP and caloric test findings in patients with unilateral peripheral vestibular disorders. Acta Otolaryngol. 2011;131:945–950. doi: 10.3109/00016489.2011.580003. [DOI] [PubMed] [Google Scholar]
- Myers S.F. Myelin-sheath abnormalities in the vestibular nerves of chronically diabetic rats. Otolaryngol. Head Neck Surg. 1998;119:432–438. doi: 10.1016/s0194-5998(98)70098-1. [DOI] [PubMed] [Google Scholar]
- Myers S.F., Ross M.D. Morphological evidence of vestibular pathology in long-term experimental diabetes mellitus. II. Connective tissue and neuroepithelial pathology. Acta Otolaryngol. 1987;104:40–49. doi: 10.3109/00016488709109045. [DOI] [PubMed] [Google Scholar]
- Niesten M.E., McKenna M.J., Herrmann B.S., Grolman W., Lee D.J. Utility of cVEMPs in bilateral superior canal dehiscence syndrome. The Laryngoscope. 2013;123:226–232. doi: 10.1002/lary.23550. [DOI] [PubMed] [Google Scholar]
- Perez R., Ziv E., Freeman S., Sichel J.Y., Sohmer H. Vestibular end-organ impairment in an animal model of type 2 diabetes mellitus. The Laryngoscope. 2001;111:110–113. doi: 10.1097/00005537-200101000-00019. [DOI] [PubMed] [Google Scholar]
- Rigon R., Rossi A.G., Coser P.L. Otoneurologic findings in type 1 diabetes mellitus patients. Rev. Bras. Otorinolaringol. 2007;73:106–111. doi: 10.1016/S1808-8694(15)31130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren S.M., Colebatch J.G. The contributions of vestibular evoked myogenic potentials and acoustic vestibular stimulation to our understanding of the vestibular system. Front. Neurol. 2018;9:481. doi: 10.3389/fneur.2018.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren S.M., Kingma H. New perspectives on vestibular evoked myogenic potentials. Curr. Opin. Neurol. 2013;26:74–80. doi: 10.1097/WCO.0b013e32835c5ef3. [DOI] [PubMed] [Google Scholar]
- Rosengren S.M., Aw S.T., Halmagyi G.M. Ocular vestibular evoked myogenic potentials in superior canal dehiscence. J. Neurol. Neurosurg. Psychiatry. 2008;79:559–568. doi: 10.1136/jnnp.2007.126730. [DOI] [PubMed] [Google Scholar]
- Rosengren S.M., Welgampola M.S., Colebatch J.G. Vestibular evoked myogenic potentials: past, present and future. Clin Neurophysiol. 2010;121:36–51. doi: 10.1016/j.clinph.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Schwartz A.V., Vittinghoff E., Sellmeyer D.E., Feingold K.R., de Rekeneire N., Strotmeyer E.S., Shorr R.I., Vinik A.I., Odden M.C., Park S.W., Faulkner K.A., Harris T.B., Health, Aging, and Body Composition Study Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31:391–396. doi: 10.2337/dc07-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneau G.G., Ulbrecht J.S., Derr J.A., Becker M.B., Cavanagh P.R. Postural instability in patients with diabetic sensory neuropathy. Diabetes Care. 1994;17:1411–1421. doi: 10.2337/diacare.17.12.1411. [DOI] [PubMed] [Google Scholar]
- Taylor R.L., Wijewardene A.A., Gibson W.P., Black D.A., Halmagyi G.M., Welgampola M.S. The vestibular evoked-potential profile of Ménière's disease. Clin. Neurophysiol. 2011;122:1256–1263. doi: 10.1016/j.clinph.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Ward B.K., Wenzel A., Kalyani R.R., Agrawal Y., Feng A.L., Polydefkis M., Ying H.S., Schubert M.C., Zuniga M.G., Della-Santina C.C., Carey J.P. Characterization of vestibulopathy in individuals with type 2 diabetes mellitus. Otolaryngol. Head Neck Surg. 2015;153:112–118. doi: 10.1177/0194599815576717. [DOI] [PubMed] [Google Scholar]
- Welgampola M.S., Myrie O.A., Minor L.B., Carey J.P. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology. 2008;70:464–472. doi: 10.1212/01.wnl.0000299084.76250.4a. [DOI] [PubMed] [Google Scholar]
- Winters S.M., Campschroer T., Grolman W., Klis S.F. Ocular vestibular evoked myogenic potentials in response to air-conducted sound in Ménière's disease. Otol. Neurotol. 2011;32:1273–1280. doi: 10.1097/MAO.0b013e31822e5ac9. [DOI] [PubMed] [Google Scholar]
- Yoda S., Cureoglu S., Yildirim-Baylan M., Morita N., Fukushima H., Harada T., Paparella M.M. Association between type 1 diabetes mellitus and deposits in the semicircular canals. Otolaryngol. Head Neck Surg. 2011;145:458–462. doi: 10.1177/0194599811407610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.