Abstract
Peripheral neuropathy is a common side effect of chemotherapeutic agents that frequently necessitates dose-reduction, truncation of, or change in therapy. HDAC6 inhibition has demonstrated preclinical efficacy in preventing and/or reversing chemotherapy-induced peripheral neuropathy and furthermore has demonstrated synergistic antitumor activity with various chemotherapies. Here, we report the abbreviated results of a Phase Ib trial of ricolinostat, an HDAC6-specific inhibitor, in combination with paclitaxel, in the treatment of recurrent ovarian, fallopian tube, or primary peritoneal cancer.
Keywords: HDAC inhibitor, HDAC6, Ovarian cancer, Peripheral neuropathy, Paclitaxel, Taxane
Highlights
-
•
Combination ricolinostat (HDAC6 inhibitor) and paclitaxel was well tolerated at the starting dose level.
-
•
One patient developed grade 1 peripheral neuropathy with combination therapy.
-
•
Two patients responded to combination ricolinostat + paclitaxel, with DOR 23.4 and 37.3 weeks respectively.
1. Introduction
Histone deacetylase-6 (HDAC6) is a class IIb histone deacetylase with a broad range of transcriptional and non-transcriptional targets and effects. HDAC6 facilitates the degradation of damaged or misfolded proteins by trafficking them to aggresomes and promoting fusion of autophagosomes and lysosomes. Therefore, HDAC6 inhibition in a tumor cell already predisposed to producing large amounts of abnormal proteins may amplify cellular stress and induce cell death (Boyault et al., 2007). HDAC6 additionally impairs the chaperone activity of HSP90 and alters the function of p53, further suggesting a role in oncogenesis and a therapeutic role for HDAC6 inhibition (Seidel et al., 2016; Bali et al., 2005). HDAC6 also modulates microtubule dynamics via deacetylation of α-tubulin (Hubbert et al., 2002).
HDAC inhibitors have potential as an oncologic therapy, with largest effect in synergistic combinations, such as with proteasome inhibitors in lymphoma (Amengual et al., 2015) and multiple myeloma (Qi et al., 2017) and various chemotherapeutic agents in preclinical evaluations of solid tumors, including ovarian and uterine cancer (Singh et al., 2011; Chobanian et al., 2004; Budman et al., 2011). In ovarian cancer cell lines, HDAC inhibition led to G1 or G2 cell cycle arrest and apoptosis. In unreported internal data, ricolinostat (ACY1215, ACY63, ACY161-63), an orally active, small molecule, selective inhibitor of HDAC6 was tested in combination with paclitaxel in preclinical ovarian cancer xenotransplant models and showed synergy in tumor suppression compared to either agent alone.
The effect of HDAC6 on α-tubulin led to the hypothesis that HDAC6 inhibition may reverse axonal transport defects, such as those seen in chemotherapy-induced peripheral neuropathy, and potentially ameliorate this dose-limiting aspect of treatment. HDAC6-specific inhibition by ricolinostat and another experimental agent has prevented and reversed cisplatin-mediated allodynia, pain, and numbness in murine models (Krukowski et al., 2017). HDAC6 inhibition in a vincristine-based murine model both prevented peripheral neuropathy and reduced tumor progression (Van Helleputte et al., 2018). Therefore, the rationale behind and potential benefit of a combination of ricolinostat and paclitaxel in women with ovarian cancer was two-fold: first, to capture synergistic anti-tumor effects, and second, to utilize ricolinostat's microtubule effects to attenuate taxane-induced neurotoxicity.
2. Patients & methods
We designed and conducted an open-label Phase Ib study of weekly paclitaxel and daily oral ricolinostat for patients with recurrent ovarian, peritoneal, or fallopian tube cancer at Dana-Farber Cancer Institute from March 2016 to January 2017. The primary objective of this study was to establish a maximally tolerated dose (MTD) of oral daily ricolinostat in combination with paclitaxel. Secondary endpoints included safety and tolerability, objective response rate (ORR), duration of response (DOR), and progression free survival (PFS). Institutional review board approval was obtained. Each patient provided signed informed consent before study enrollment.
2.1. Patient population
Participants were required to have histologically confirmed recurrent or persistent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma, and had to have received at least one prior platinum-based chemotherapy regimen for management of primary disease. Additional eligibility included recurrence within 12 months of the last platinum-containing regimen, ECOG performance status of 0 or 1, and measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. There was no limitation on number of prior therapies. Prior paclitaxel was allowed, unless recurrence or progression occurred on or within 8 weeks of the last dose of paclitaxel. Prior use of an HDAC inhibitor was exclusionary.
Participants were required to recover from prior treatment-related toxicities to grade 1 or better, and needed to demonstrate adequate bone marrow and organ function, including leukocyte count ≥3000/mcL, ANC ≥1500/mcL, and platelets ≥100,000/mcL. Exclusion criteria included the use of chemotherapy, radiation, or small molecule kinase inhibitors within four weeks of study entry, hormonal therapy within one week of study treatment initiation, or radiation to >25% of the marrow. Additionally, patients were excluded if they had severe or uncontrolled comorbidities or evidence of other malignancies within the preceding three years, excepting non-melanoma skin cancers, carcinoma-in-situ of the breast or cervix, or primary endometrial cancer with stage not greater than IA, grade 1 or 2, no lymphovascular invasion, and no more than superficial myometrial invasion.
2.2. Treatment plan & safety assessment
At the starting dose (dose level 1), ricolinostat was given orally at 80 mg daily on days 1–21 of a 28 day cycle. Paclitaxel was given weekly on days 1, 8, and 15 on a 28 day cycle, at a dose of 80 mg/m2. Additional dose escalation levels included ricolinostat given orally at 120 mg daily, 180 mg daily, or 240 mg daily on days 1–21 of a 28-day cycle, in combination with paclitaxel as above. Patients were treated on an outpatient basis and remained on study until disease progression, voluntary withdrawal, or drug-related toxicity. All patients followed for up to 30 days after removal from protocol therapy or until death. The primary endpoint was determination of the MTD. Tumor assessment by CT or MRI was repeated every 2 cycles.
Toxicity was assessed using the National Cancer Institute Common Terminal Criteria for Adverse Events version 4.0. Neuropathy was assessed using the TNSn© (Total Neuropathy Score-nurse) in expansion cohorts. Radiographic response was assessed using RECIST v1.1.
2.3. Statistical design
Dose escalation was designed with a standard 3 + 3 enrollment scheme; at least 6 patients needed to be treated at MTD, with no >1 patient experiencing a DLT at the MTD level of ricolinostat with paclitaxel. Once the MTD/RP2D was established, three expansion cohorts of 10 patients each were planned: (1) patients without significant peripheral neuropathy (grade 1 or less) would receive ricolinostat/paclitaxel at the established MTD; (2) patients with grade 2 peripheral neuropathy would receive ricolinostat at the combination MTD together with weekly paclitaxel dosed at 70 mg/m2; and (3) a cohort of patients would receive ricolinostat/paclitaxel at the established MTD with the addition of bevacizumab. An anticipated 15–24 patients were expected to enroll to the dose escalation portion of the study, with up to 30 patients enrolled in the expansion groups.
3. Results
3.1. Patients
A total of six patients were enrolled between March 28, 2016 and January 5, 2017, all of whom received treatment at dose level 1 with ricolinostat 80 mg daily Days 1–21 and paclitaxel 80 mg/m2 Days 1,8,15. Patient baseline and disease characteristics are detailed in Table 1. Median age was 60.5 years, patients were predominantly Caucasian (83%), and histology was predominantly adenocarcinoma (67%). All six patients were evaluated for toxicity and efficacy. The study was terminated early after development of ricolinostat in ovarian cancer was halted following the acquisition of Acetylon by Celgene.
Table 1.
Baseline characteristics.
| Characteristic | N (%) |
|---|---|
| Age (in years), Median (Range) | 60.5 (42, 80) |
| Race | |
| White | 5 (83%) |
| Asian | 1 (17%) |
| Ethnicity | |
| Non-Hispanic | 6 (100%) |
| Stage at diagnosis | |
| III | 4 (67%) |
| IV | 2 (33%) |
| Histology | |
| Adenocarcinoma | 4 (67%) |
| Other - high grade serous carcinoma | 1 (17%) |
| Other - Serous | 1 (17%) |
| Differentiation grade | |
| Poorly differentiated | 6 (100%) |
3.2. Safety
Therapy was well tolerated, and most toxicities were grade 1–2 in severity. There were no grade 4 toxicities. The most commonly experienced treatment related toxicity was grade 1 or 2 nausea, occurring in 4 patients (67%). Two patients (33%) experienced grade 3 toxicities, one of which was a grade 3 anemia and the other a grade 3 neutropenia. One patient (17%) experienced peripheral sensory neuropathy, which was grade 1 in severity. Treatment-related AEs are further detailed in Table 2.
Table 2.
Treatment-related AEs.
| Maximum grade |
Total |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
|||||||
| N | % | N | % | N | % | N | % | ||
| Category of toxicity | Toxicity | – | – | 1 | 16.7 | 1 | 16.7 | 2 | 33.3 |
| Blood and lymphatic system disorders | Anemia | ||||||||
| Cardiac disorders | Chest pain - cardiac | 1 | 16.7 | – | – | – | – | 1 | 16.7 |
| Gastrointestinal disorders | Constipation | 1 | 16.7 | – | – | – | – | 1 | 16.7 |
| Nausea | 3 | 50.0 | 1 | 16.7 | – | – | 4 | 66.7 | |
| Vomiting | 2 | 33.3 | – | – | – | – | 2 | 33.3 | |
| General disorders and admin site conditions | Fatigue | 2 | 33.3 | – | – | – | – | 2 | 33.3 |
| Localized edema | 1 | 16.7 | – | – | – | – | 1 | 16.7 | |
| Investigations | Neutrophil count decreased | – | – | 1 | 16.7 | 1 | 16.7 | 2 | 33.3 |
| Metabolism and nutrition disorders | Anorexia | – | – | 1 | 16.7 | – | – | 1 | 16.7 |
| Dehydration | – | – | 1 | 16.7 | – | – | 1 | 16.7 | |
| Hypocalcemia | 1 | 16.7 | – | – | – | – | 1 | 16.7 | |
| Hypomagnesemia | 2 | 33.3 | – | – | – | – | 2 | 33.3 | |
| Musculoskeletal and connective tissue disorders | Back pain | – | – | 1 | 16.7 | – | – | 1 | 16.7 |
| Bone pain | – | – | 1 | 16.7 | – | – | 1 | 16.7 | |
| Generalized muscle weakness | – | – | 1 | 16.7 | – | – | 1 | 16.7 | |
| Nervous system disorders | Dysgeusia | 1 | 16.7 | – | – | – | – | 1 | 16.7 |
| Peripheral sensory neuropathy | 1 | 16.7 | – | – | – | – | 1 | 16.7 | |
| Psychiatric disorders | Anxiety | 1 | 16.7 | – | – | – | – | 1 | 16.7 |
| Skin and subcutaneous tissue disorders | Alopecia | – | – | 2 | 33.3 | – | – | 2 | 33.3 |
| Nail discoloration | 1 | 16.7 | – | – | – | – | 1 | 16.7 | |
| Rash maculo-papular | 1 | 16.7 | – | – | – | – | 1 | 16.7 | |
3.3. Clinical activity
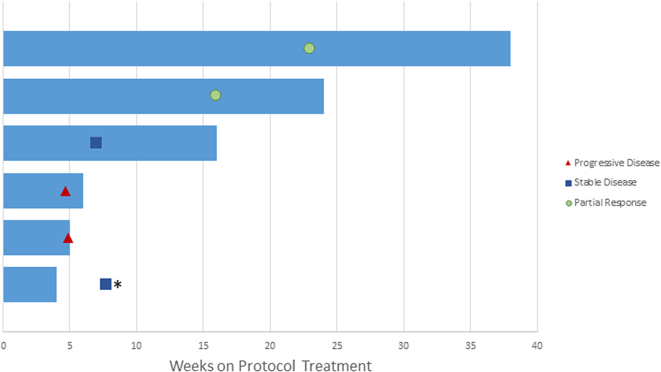
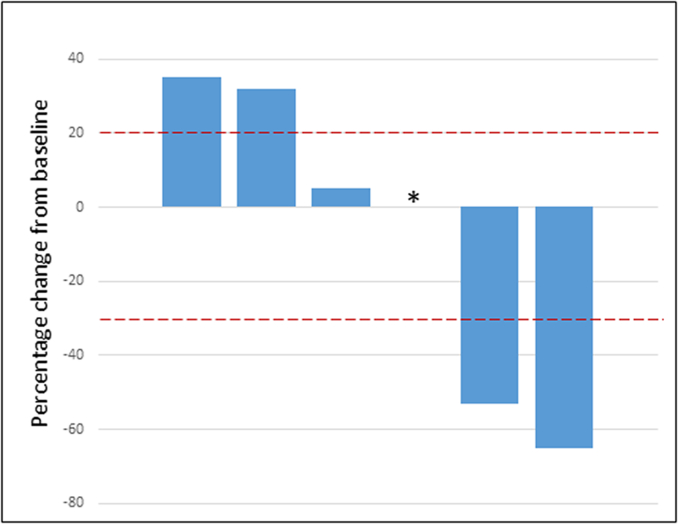
Patients received a median of 2.5 cycles of treatment. One patient voluntarily withdrew from the study after one cycle of treatment and had radiographically stable disease at time of withdrawal. Two patients experienced a confirmed PR, one of whom had a 65% decrease in target lesions and a DOR of 37.3 weeks, and the second of whom had a 53% decrease in target lesions and a DOR 23.4 weeks. Best responses in the additional three patients included stable disease (1 patient) and progressive disease (2 patients). One patient whose best response was PD was removed from study treatment and subsequently died during the follow up period. Clinical activity is detailed in Fig. 1, Fig. 2 and supplemental Table S1 and Fig. S1.
Fig. 1.
Swimmer's plot. Best overall responses (PD, SD, PR) are notated. ⁎One patient who withdrew from the study after receiving 1 cycle of treatment had stable disease at time of their follow-up assessment.
Fig. 2.
Waterfall plot. ⁎One patient who withdrew from the study after receiving 1 cycle of treatment had a percent difference of 0% in target lesions at time of their follow-up assessment.
4. Discussion
We report the results of six patients enrolled in a phase 1b trial of combination paclitaxel and ricolinostat in women with recurrent epithelial ovarian cancer. The primary objective, determination of the MTD, was not met due to premature termination of the study. Combination therapy at the dose level tested, dose level 1, was generally well tolerated, without any grade 4 AEs.
Due to the low accrual of the study, statistically significant conclusions cannot be drawn. We did observe evidence of activity, with two responders with DORs of 23.4 and 37.3 weeks. In the context of this study, it is not possible to determine whether this activity was driven by the weekly paclitaxel alone, or whether the addition of ricolinostat to weekly paclitaxel could have enhanced anti-tumor activity. Recent data suggest there may be a molecular basis for certain tumors to be more sensitive to HDAC6 inhibition. For example, tumors carrying ARID1A mutations, such as in clear cell carcinomas of the ovary, are reliant on HDAC6 for tumorigenesis and have enhanced susceptibility to HDAC6 inhibition (Bitler et al., 2017). In contrast, mutations in other components of the SWI/SNF complex did not seem to impart this enhanced susceptibility. Work done in esophageal squamous cell carcinoma suggests ricolinostat downregulates the PI3K/AKT/mTOR pathway, and in this setting, treatment of cancer cells with ricolinostat and a pan-AKT inhibitor led to greater cell death than ricolinostat alone. Theoretically, this effect may be abrogated by activating mutations in the PI3K/AKT/mTOR pathway (Cao et al., 2018). Thus, future trials evaluating HDAC6 inhibitors in solid tumors, with or without paclitaxel, remain of potential therapeutic interest, but ideally should consider the tumor's molecular characteristics to investigate whether specific molecular alterations predict for increased benefit.
The potential neuropathy-attenuating effect of ricolinostat could not be fully assessed. Regardless, the potential benefit of an HDAC inhibitor to mitigate peripheral neuropathy related to anti-microtubule agents is tantalizing, given the wide use of these agents across solid malignancies and the current inability to reliably predict who may be most susceptible to developing neuropathy. The mechanism of taxane-induced neurotoxicity is thought to be multifaceted, causing die-back of peripheral neurons, impaired function of ganglions (including dorsal root ganglia), microtubule accumulation in Schwann cells, development of a segmental demyelination, and activation of macrophages and microglia in the peripheral nervous system. Paclitaxel appears to impart the most severe effect on nerve conduction (Wozniak et al., 2018). This is particularly noteworthy, given its common use across all stages of ovarian cancer, as part of combination regimens and as monotherapy, and in up-front, platinum sensitive, and platinum resistant settings. It is considered one of the most active chemotherapeutic agents in ovarian cancer. Unfortunately, the high rates of paclitaxel-associated neuropathy often necessitate dose reduction, treatment delays, truncation of therapy, or transition to a different agent. Taxane-induced neuropathy can be significant and long-lasting, negatively impacting patients' quality of life and serving as a risk factor for falls and greater debility(Kober et al., 2018). Neuropathy may consequently lead to avoidance of later-line chemotherapies that may otherwise be effective. In this context, preventing or attenuating taxane-induced peripheral neuropathy with HDAC6 inhibition could have meaningful clinical impact.
Overall, this Phase1b study was prematurely terminated due to external factors, but was able to demonstrate that the combination of ricolinostat and weekly paclitaxel at the tested dose level was well-tolerated and demonstrated activity in a cohort of 6 patients. The observations made in this cohort of patients support future investigation of HDAC6 inhibitors in ovarian cancer.
Author contributions
EKL and JFL contributed to the conception and writing of this manuscript. JFL and UAM contributed to trial conception and development. ZTW provided statistical analysis. MJB, AAW, NH, and PAK contributed to trial recruitment. JC provided coordination of trial conduct.
Disclosures
JFL reports advisory board participation for AstraZeneca, Tesaro, Inc., Mersana Therapeutics, Clovis Oncology, Merck, and Genentech, outside the submitted work; and Institutional PI on industry-sponsored trials from Genentech/Roche, AstraZeneca, Boston Biomedical, Atara Biotherapeutics, Bristol-Myers Squibb, Agenus, CytomX Therapeutics, Regeneron Pharmaceuticals, Tesaro, Clovis Oncology, Aravive Biologics, Vigeo Therapeutics, and Arch Oncology. UAM reports personal fees from Astrazeneca, Myriad Genetics, Clovis, Merck, Eli Lilly, Mersana, Geneos, Fuji Film, 2X Oncology, Cerulean, and Immunogen, outside the submitted work. PAK reports grants from National Comprehensive Cancer Network during the conduct of the study; and personal fees from Astrazeneca, Merck, Pfizer, and Tesaro, outside the submitted work. EKL, ZTW, MJB, AAW, NH, and JC have no conflicts or disclosures.
Acknowledgements
Acetylon Pharmaceuticals, now Celgene (Summit, NJ).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2019.07.010.
Appendix A. Supplementary data
Supplementary material
References
- Amengual J.E., Johannet P., Lombardo M., Zullo K., Hoehn D., Bhagat G., Scotto L., Jirau-serrano X., Radeski D., Heinen J., Jiang H., Cremers S., Zhang Y., Jones S., Connor O.A.O. Dual targeting of protein degradation pathways with the selective HDAC6 inhibitor ACY-1215 and bortezomib is synergistic in lymphoma. Clin Cancer Res. 2015:4663–4676. doi: 10.1158/1078-0432.CCR-14-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali P., Pranpat M., Bradner J., Balasis M., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Seto E., Bhalla K. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 2005;280:26729–26734. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- Bitler B.G., Wu S., Park P.H., Hai Y., Aird K.M., Wang Y., Zhai Y., Kossenkov A.V., Vara-Ailor A., Rauscher F.J., Zou W., Speicher D.W., Huntsman D.G., Conejo-Garcia J.R., Cho K.R., Christianson D.W., Zhang R. ARID1A-mutated ovarian cancers depend on HDAC6 activity. Nat. Cell Biol. 2017;19:962–973. doi: 10.1038/ncb3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyault C., Zhang Y., Fritah S., Caron C., Gilquin B., Kwon S.H., Garrido C., Yao T.-P., Vourc’h C., Matthias P., Khochbin S. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev. 2007;21:2172–2181. doi: 10.1101/gad.436407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budman D.R., Tai J., Calabro A., John V. The histone deacetylase inhibitor panobinostat demonstrates marked synergy with conventional chemotherapeutic agents in human ovarian cancer cell lines. Investig. New Drugs. 2011;29:1224–1229. doi: 10.1007/s10637-010-9467-6. [DOI] [PubMed] [Google Scholar]
- Cao J., Lv W., Wang L., Xu J., Yuan P., Huang S., He Z., Hu J. Ricolinostat (ACY-1215) suppresses proliferation and promotes apoptosis in esophageal squamous cell carcinoma via miR-30d/PI3K/AKT/mTOR and ERK pathways. Cell Death Dis. 2018;9 doi: 10.1038/s41419-018-0788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian N.H., Greenberg V.L., Gass J.M., Desimone C.P., Van Nagell J.R., Zimmer S.G. Histone deacetylase inhibitors enhance paclitaxel-induced cell death in ovarian cancer cell lines independent of p53 status. Anticancer Res. 2004;24:539–545. [PubMed] [Google Scholar]
- Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X.-F., Yao T.-P. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- Kober K.M., Mazor M., Abrams G., Olshen A., Conley Y.P., Hammer M., Schumacher M., Chesney M., Smoot B., Mastick J., Paul S.M., Levine J.D., Miaskowski C. Phenotypic characterization of paclitaxel-induced peripheral neuropathy in cancer survivors. J. Pain Symptom Manag. 2018;56:908–919.e3. doi: 10.1016/j.jpainsymman.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukowski K., Ma J., Golonzhka O., Laumet G.O., Gutti T., Van Duzer J.H., Mazitschek R., Jarpe M.B., Heijnen C.J., Kavelaars A. HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain. 2017;158:1126–1137. doi: 10.1097/j.pain.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi R., Wang Y., Bruno P.M., Xiao H., Yingjie Y., Li T., Lauffer S., Wei W., Chen Q., Kang X., Song H., Yang X., Huang X., Detappe A., Matulonis U., Pepin D., Hemann M.T., Birrer M.J., Ghoroghchian P.P. Nanoparticle conjugates of a highly potent toxin enhance safety and circumvent platinum resistance in ovarian cancer. Nat. Commun. 2017;8:1–12. doi: 10.1038/s41467-017-02390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel C., Schnekenburger M., Mazumder A., Teiten M.-H., Kirsch G., Dicato M., Diederich M. 4-Hydroxybenzoic acid derivatives as HDAC6-specific inhibitors modulating microtubular structure and HSP90α chaperone activity against prostate cancer. Biochem. Pharmacol. 2016;99:31–52. doi: 10.1016/j.bcp.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Singh B.N., Zhou H., Li J., Tipton T., Wang B., Shao G., Gilbert E.N., Li Q., Jiang S.-W. Preclinical studies on histone deacetylase inhibitors as therapeutic reagents for endometrial and ovarian cancers. Future Oncol. 2011;7:1415–1428. doi: 10.2217/fon.11.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Helleputte L., Kater M., Cook D.P., Eykens C., Rossaert E., Haeck W., Jaspers T., Geens N., Vanden Berghe P., Gysemans C., Mathieu C., Robberecht W., Van Damme P., Cavaletti G., Jarpe M., Van Den Bosch L. Inhibition of histone deacetylase 6 (HDAC6) protects against vincristine-induced peripheral neuropathies and inhibits tumor growth. Neurobiol. Dis. 2018;111:59–69. doi: 10.1016/j.nbd.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Wozniak K.M., Vornov J.J., Wu Y., Liu Y., Carozzi V.A., Rodriguez-Menendez V., Ballarini E., Alberti P., Pozzi E., Semperboni S., Cook B.M., Littlefield B.A., Nomoto K., Condon K., Eckley S., DesJardins C., Wilson L., Jordan M.A., Feinstein S.C., Cavaletti G., Polydefkis M., Slusher B.S. Peripheral neuropathy induced by microtubule-targeted chemotherapies: insights into acute injury and long-term recovery. Cancer Res. 2018;78:817–829. doi: 10.1158/0008-5472.CAN-17-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material