Abstract
Background
Ovarian cancer has the highest mortality rate of all gynaecological malignancies with an overall five‐year survival rate of 30% to 40%. In the past two decades it has become apparent and more commonly accepted that a majority of ovarian cancers originate in the fallopian tube epithelium and not from the ovary itself. This paradigm shift introduced new possibilities for ovarian cancer prevention. Salpingectomy during a hysterectomy for benign gynaecological indications (also known as opportunistic salpingectomy) might reduce the overall incidence of ovarian cancer. Aside from efficacy, safety is of utmost importance, especially due to the preventive nature of opportunistic salpingectomy. Most important are safety in the form of surgical adverse events and postoperative hormonal status. Therefore, we compared the benefits and risks of hysterectomy with opportunistic salpingectomy to hysterectomy without opportunistic salpingectomy.
Objectives
To assess the effect and safety of hysterectomy with opportunistic salpingectomy versus hysterectomy without salpingectomy for ovarian cancer prevention in women undergoing hysterectomy for benign gynaecological indications; outcomes of interest include the incidence of epithelial ovarian cancer, surgery‐related adverse events and postoperative ovarian reserve.
Search methods
The Cochrane Gynaecology and Fertility (CGF) Group trials register, CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL and two clinical trial registers were searched in January 2019 together with reference checking and contact with study authors.
Selection criteria
We intended to include both randomised controlled trials (RCTs) and non‐RCTs that compared ovarian cancer incidence after hysterectomy with opportunistic salpingectomy to hysterectomy without opportunistic salpingectomy in women undergoing hysterectomy for benign gynaecological indications. For assessment of surgical and hormonal safety, we included RCTs that compared hysterectomy with opportunistic salpingectomy to hysterectomy without opportunistic salpingectomy in women undergoing hysterectomy for benign gynaecological indications.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. The primary review outcomes were ovarian cancer incidence, intraoperative and short‐term postoperative complication rate and postoperative hormonal status. Secondary outcomes were total surgical time, estimated blood loss, conversion rate to open surgery (applicable only to laparoscopic and vaginal approaches), duration of hospital admission, menopause‐related symptoms and quality of life.
Main results
We included seven RCTs (350 women analysed). The evidence was of very low to low quality: the main limitations being a low number of included women and surgery‐related adverse events, substantial loss to follow‐up and a large variety in outcome measures and timing of measurements.
No studies reported ovarian cancer incidence after hysterectomy with opportunistic salpingectomy compared to hysterectomy without opportunistic salpingectomy in women undergoing hysterectomy for benign gynaecological indications. For surgery‐related adverse events, there were insufficient data to assess whether there was any difference in both intraoperative (odds ratio (OR) 0.66, 95% confidence interval (CI) 0.11 to 3.94; 5 studies, 286 participants; very low‐quality evidence) and short‐term postoperative (OR 0.13, 95% CI 0.01 to 2.14; 3 studies, 152 participants; very low‐quality evidence) complication rates between hysterectomy with opportunistic salpingectomy and hysterectomy without opportunistic salpingectomy because the number of surgery‐related adverse events was very low. For postoperative hormonal status, the results were compatible with no difference, or with a reduction in anti‐Müllerian hormone (AMH) that would not be clinically relevant (mean difference (MD) ‐0.94, 95% CI ‐1.89 to 0.01; I2 = 0%; 5 studies, 283 participants; low‐quality evidence). A reduction in AMH would be unfavourable, but due to wide CIs, the postoperative change in AMH can still vary from a substantial decrease to even a slight increase.
Authors' conclusions
There were no eligible studies reporting on one of our primary outcomes ‐ the incidence of ovarian cancer specifically after hysterectomy with or without opportunistic salpingectomy. In our meta‐analyses we found insufficient data to assess whether there was any difference in surgical adverse events, with a very low number of events in women undergoing hysterectomy with and without opportunistic salpingectomy. For postoperative hormonal status we found no evidence of a difference between the groups. The maximum difference in time to menopause, calculated from the lower limit of the 95% CI and the natural average AMH decline, would be approximately 20 months, which we consider to be not clinically relevant. However, the results should be interpreted with caution and even more so in very young women for whom a difference in postoperative hormonal status is potentially more clinically relevant. Therefore, there is a need for research on the long‐term effects of opportunistic salpingectomy during hysterectomy, particularly in younger women, as results are currently limited to six months postoperatively. This limit is especially important as AMH, the most frequently used marker for ovarian reserve, recovers over the course of several months following an initial sharp decline after surgery. In light of the available evidence, addition of opportunistic salpingectomy should be discussed with each woman undergoing a hysterectomy for benign indication, with provision of a clear overview of benefits and risks.
Plain language summary
Surgical removal of the womb and fallopian tubes compared to surgical removal of the womb without fallopian tubes for ovarian cancer prevention
Review question
Cochrane researchers reviewed the evidence for the effect of surgical removal of the womb (hysterectomy) together with the fallopian tubes (salpingectomy) versus hysterectomy without salpingectomy for ovarian cancer prevention.
Background
Ovarian cancer is the deadliest form of cancer of the female reproductive system. Screening for ovarian cancer is not effective, so preventive measures are needed. From previous studies, we learned that most types of ovarian cancer arise in the fallopian tubes. For that reason, the removal of the fallopian tubes (salpingectomy) during hysterectomy could lower the risk of ovarian cancer. The fallopian tubes have no function after completion of childbearing and salpingectomy is simple to perform.
Because salpingectomy is a preventive measure, it should not have serious side effects or risks. When considering possible risks of salpingectomy, it might lead to a higher complication rate because an extra surgical step has to be performed. Another possible risk could be an earlier onset of menopause. The ovaries and fallopian tubes lie close together and, in part, share their blood supply. Surgery to the fallopian tube could thus damage part of the blood supply to the ovaries. This damage could result in an earlier age of menopause. Ovarian reserve can be measured with the concentration of Anti‐Müllerian hormone (AMH) in the blood. As women get older and come closer to menopause, the AMH concentration decreases.
To investigate the effectiveness and safety of salpingectomy for prevention of ovarian cancer, we compared the risks and benefits of hysterectomy with salpingectomy to hysterectomy without salpingectomy.
Study characteristics
We found seven randomised controlled trials comparing hysterectomy with salpingectomy to hysterectomy without salpingectomy. They included a total of 350 women undergoing a hysterectomy for benign conditions of the female reproductive tract. The evidence is current to January 2019.
Key results
We found no studies that reported ovarian cancer incidence after hysterectomy with salpingectomy to hysterectomy without salpingectomy.
The number of complications that occur after hysterectomy is generally very low. This means that only a few complications occurred in the trials included in this review and we were unable to make a good comparison of complication rates.
We found no evidence for any difference in onset of menopause after hysterectomy with salpingectomy. Our results suggest that the AMH concentrations after hysterectomy with salpingectomy would be between 1.89 pmol/L lower and 0.01 pmol/L higher than after hysterectomy without salpingectomy. The minimum difference in AMH concentration (0.01 pmol/L) represents no difference in the onset of menopause. The maximum difference in AMH concentration (1.89 pmol/L) shows that menopause could occur up to 20 months earlier after hysterectomy with salpingectomy compared to hysterectomy without salpingectomy. This result is calculated from the average decline of AMH per year.
Quality of the evidence
The evidence was of very low to low quality. The main limitations in the evidence were a low number of complications, meaning no comparison could be made, and differences in outcome measures of the included studies. Also, the total numbers of included studies and included women were low.
Summary of findings
Summary of findings for the main comparison. Hysterectomy with opportunistic salpingectomy compared with hysterectomy without opportunistic salpingectomy for ovarian cancer prevention.
| Hysterectomy with opportunistic salpingectomy compared with hysterectomy without opportunistic salpingectomy for ovarian cancer prevention | ||||||
|
Patient or population: premenopausal women undergoing hysterectomy for benign gynaecological indications Settings: secondary and tertiary care Intervention: hysterectomy with opportunistic salpingectomy Comparison: hysterectomy without opportunistic salpingectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Hysterectomy without opportunistic salpingectomy | Hysterectomy with opportunistic salpingectomy | |||||
| Incidence of epithelial ovarian cancer | ‐ | ‐ | ‐ | 0 (0 studies) | ‐ | No studies reported on cancer incidence after hysterectomy with opportunistic salpingectomy compared to hysterectomy without opportunistic salpingectomy. |
| Surgery‐related adverse events: intraoperative complications | 21 per 1000 | 7 fewer per 1000 (19 fewer to 29 more) | OR 0.66 (0.11 to 3.94) | 104 (1 RCT) |
⊕⊝⊝⊝ VERY LOWa,b,c | Five studies reported on this outcome, but due to the low complication rate of hysterectomy in general, four studies reported no adverse events. |
| Surgery‐related adverse events: short‐term postoperative complications | 27 per 1000 | 23 fewer per 1000 (27 fewer to 29 more) | OR 0.13 (0.01 to 2.14) | 68 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | Three studies reported on this outcome, but due to the low complication rate of hysterectomy in general, two studies reported no adverse events. |
|
Postoperative hormonal status (AMH) pmol/L |
3.59 to 13.00 pmol/L | The mean postoperative AMH value in the intervention groups was 0.94 lower (1.89 lower to 0.01 higher) | ‐ | 283 (5 RCTs) | ⊕⊕⊝⊝ LOWd,e | The maximum possible decline (the lower limit of the 95% CI) corresponds to the natural decline of AMH concentration of approximately 6 to 20 months depending on age. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided under the heading 'Hysterectomy without opportunistic salpingectomy' and is based on results from included studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AMH: anti‐Müllerian hormone; CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded 1 level for imprecision; total number of observed events was very low. bDowngraded 1 level for limitations of study design; unclear definitions of adverse events. cDowngraded 1 level for risk of bias; although multiple RCTs reported on this outcome, all events occurred in 1 study. dDowngraded 1 level for risk of bias; incomplete outcome data in a majority of the trials. eDowngraded 1 level for inconsistency; postoperative AMH concentration measured between 3 to 6 months postoperatively.
Background
Description of the condition
Epithelial ovarian cancer has the highest mortality rate of all gynaecological malignancies, with an overall five‐year survival rate of 30% to 40% (Bolton 2012; Siegel 2017). This dismal prognosis is mainly the result of non‐specific symptoms, leading to detection at an advanced stage of disease. Despite progress over the past decades in the field of cancer treatment in general, only limited improvements have been made in ovarian cancer. Studies aimed at the detection of ovarian cancer at an early stage of disease failed to show substantial survival benefit. Hence, preventive measures that are both safe and effective are needed. Currently, the only option for prevention of ovarian carcinoma is bilateral salpingo‐oophorectomy (BSO; the removal of both ovaries and fallopian tubes). However, BSO is not suitable for all women as it results in immediate menopause, which in turn leads to elevated risks of, for example, cardiovascular disease and all‐cause mortality (Mytton 2017; Parker 2009; Rocca 2006).
Description of the intervention
A bilateral salpingectomy is defined as the surgical excision of both fallopian tubes, up to the tubal corner of the uterus. The procedure can be implemented in several ways, for example during a hysterectomy (the removal of the uterus), a common treatment for both benign and malignant gynaecological conditions. It is then called an opportunistic salpingectomy. The surgical approach taken during hysterectomy can be vaginal, per laparotomy or per laparoscopy. Possible additional complications of the salpingectomy procedure include an increased chance of excessive blood loss, infection or damage to adjacent visceral organs.
How the intervention might work
Over the past two decades, it has become apparent and more commonly accepted that serous epithelial ovarian cancer, the most common histological subtype of ovarian cancer, probably arises from the epithelium of the fallopian tube rather than from the ovary itself (Chen 2017; Kindelberger 2007; Perez‐Lopez 2017; Piek 2001a; Piek 2003). This insight has given rise to the hypothesis that salpingectomy, after the completion of childbearing, may reduce the risk of ovarian cancer (Chen 2017; Kindelberger 2007; Long 2017; Perez‐Lopez 2017). One suggestion has been to combine salpingectomy with hysterectomy for benign gynaecological conditions, but there is concern that this could lead to an increase in surgical complications. Additionally, salpingectomy could affect the ovarian reserve since the ovaries and the fallopian tubes (partially) share the same blood supply. Thus, excision of the fallopian tubes could harm part of the ovarian blood supply and affect ovarian reserve.
Why it is important to do this review
Since 2001, accumulating evidence points towards the epithelium of the fallopian tubes as a precursor site for epithelial ovarian cancer (Chen 2017; Kindelberger 2007; Long 2017; Perez‐Lopez 2017; Piek 2001a; Piek 2001b). In some countries, this insight has resulted in the implementation of opportunistic salpingectomies in women undergoing hysterectomy for benign gynaecological conditions. The Royal College of Obstetricians and Gynaecologists, the American College of Obstetricians and Gynecologists, and the European Menopause and Andropause Society each recently published statements on the importance of discussing the possibility of opportunistic salpingectomy with women undergoing hysterectomy for benign gynaecological conditions. However, they also stated that more research on the topic is needed, since it remains to be elucidated whether opportunistic salpingectomy will really result in a decreased incidence of ovarian cancer and whether opportunistic salpingectomy is safe (primum non nocere) (ACOG 2015; Ntoumanoglou‐Schuiki 2018; Perez‐Lopez 2017; RCOG 2014).
In this review, we aimed to summarise and analyse the current literature on both prevention of ovarian cancer and possible additional risks of carrying out opportunistic salpingectomy during hysterectomy for benign gynaecological conditions.
Objectives
To assess the effect and safety of hysterectomy with opportunistic salpingectomy versus hysterectomy without salpingectomy for ovarian cancer prevention in women undergoing hysterectomy for benign gynaecological indications; outcomes of interest include the incidence of epithelial ovarian cancer, surgery‐related adverse events and postoperative ovarian reserve.
Methods
Criteria for considering studies for this review
Types of studies
Because of the relatively low incidence of ovarian cancer and the necessity of a follow‐up spanning several decades, our first listed objective, the effect of opportunistic salpingectomy on the incidence of epithelial ovarian cancer, is not a particularly suitable outcome for a randomised controlled trial (RCT). Therefore, we considered both RCTs and non‐RCTs to be eligible for this objective. Since the risk of bias is larger in non‐RCTs than in RCTs, we limited non‐RCTs to cohort studies (both retrospective and prospective) and case‐control studies.
Our second and third objectives, the effect of opportunistic salpingectomy on the incidence of surgery‐related adverse events and on postoperative ovarian reserve, were suitable outcomes for RCTs. Therefore, we considered only RCTs to be eligible for inclusion in this review for these objectives.
Types of participants
Participants included in this review were individuals with a population‐based risk of ovarian cancer undergoing surgery for benign gynaecological conditions. We excluded trials that included:
women with a history of ovarian cancer;
women with an elevated risk of ovarian cancer based on a proven gene germline mutation such as BRCA1/2 mutation carriers;
women who have undergone previous bilateral oophorectomy;
women who have undergone previous bilateral salpingectomy.
The exclusion of women with a proven BRCA1/2 gene germline mutation is important since there are limited data available to suggest that mutation carriers may undergo an earlier menopause than the general population (Finch 2013). Moreover, this review focusses on the effect of an opportunistic intervention. The lifetime risk of BRCA1/2 gene germline mutation carriers is of such magnitude that it warrants prophylactic surgery rather than opportunistic surgery.
Types of interventions
We considered both RCTs and non‐RCTs that compared hysterectomy with opportunistic salpingectomy to hysterectomy without opportunistic salpingectomy to be eligible for inclusion.
Types of outcome measures
Primary outcomes
For RCTs and non‐RCTs
-
Incidence of epithelial ovarian cancer
Epithelial ovarian cancer is defined as a pathologically confirmed diagnosis derived from the ovary or fallopian tube
For RCTs
-
Surgery‐related adverse event
Intraoperative complications (including injuries to the bladder, ureters, intestines, blood vessels, nerves and excessive blood loss)
Short‐term postoperative complications (including vascular, wound, gastrointestinal, neurological, respiratory and urinary tract complications)
Ovarian reserve, measured by postoperative hormonal status
Preferably by assessment of the difference between pre‐ and postoperative Anti‐Müllerian Hormone (∆AMH) concentrations (Depmann 2016; van Rooij 2005), or where possible, of the postoperative value statistically adjusted for the preoperative value. If ∆AMH was not available, we used the difference in postoperative AMH value between intervention and control.
Secondary outcomes
For RCTs
Total surgical time
Estimated blood loss
Conversion rate to open surgery (applicable only to laparoscopic and vaginal approaches)
Duration of hospital admission
Menopause‐related symptoms
Quality of life
Search methods for identification of studies
We searched for all published and unpublished studies investigating opportunistic salpingectomy during hysterectomy for benign disease in consultation with On Ying Chan (Radboud University Information Specialist) and Marian Showell (Cochrane Gynaecology and Fertility (CGF) Group Information Specialist).
Electronic searches
We searched for papers published in all languages and, where necessary, obtained translations. We searched the following databases, from their inception until 8 January 2019.
Cochrane Gynaecology and Fertility Group (CGF) Specialised Register of Controlled Trials, searched 8 January 2019, PROCITE platform (Appendix 1).
Cochrane Central Register of Controlled Trials via CENTRAL Register of Studies Online (CRSO), searched 8 January 2019, Web platform (Appendix 2).
MEDLINE (Epub Ahead of Print, In‐Process & other Non‐indexed Citations), searched from 1946 to 8 January 2019, Ovid platform (Appendix 3).
Embase, searched from 1980 to 8 January 2019, Ovid platform (Appendix 4).
PsycINFO, searched from 1806 to 8 January 2019, Ovid platform (Appendix 5).
CINAHL, searched from 1961 to 8 January 2019, Ebsco platform (Appendix 6).
Additionally, we searched trial registries for ongoing and registered trials in January 2019; web platform (Appendix 7):
clinicaltrials.gov (a service of the US National Institutes of Health);
who.int/trialsearch/default.aspx (the World Health Organization International Trials Registry Platform search portal).
Searching other resources
We handsearched the reports of conferences from the following sources: ESGO (European Society of Gynaecological Oncology), SGO (Society of Gynecological Oncology), ESHRE (European Society of Human Reproduction and Embryology), EMAS (European Menopause and Andropause Society) and IMS (International Menopause Society). To identify additional trials, we handsearched the reference lists of all relevant trials obtained by the initial search to identify additional trials. We limited the search to articles and reports published since 1997, as the fallopian tube has been considered as the origin of epithelial ovarian cancer only since 2001 (Piek 2001a).
Data collection and analysis
Selection of studies
We imported titles and abstracts retrieved by the search into the reference manager database Covidence (Covidence). Two review authors (LL, MS) independently screened the references and checked them for duplicates. The same two review authors (LL, MS) obtained full text versions of potentially relevant studies and independently assessed them for eligibility. Disagreements were resolved by discussion and, where necessary, by consultation with a third review author (JW). We documented the selection process, including reasons for exclusion, in a PRISMA flow chart (Moher 2009).
Where the judgement of a review author could be biased due to a conflict of interest, one of the other review authors assessed that particular study. In this case, LL and JP were authors of one of the eligible studies. Therefore, MS and JW assessed this trial for eligibility.
Data extraction and management
We used a predesigned data extraction form based on the Cochrane Handbook for Systematic Reviews of Interventionsfor the extraction of relevant data from included trials (Higgins 2011b). Prior to data extraction, three review authors (LL, MS, JW) performed an independent trial run of the data extraction form on a sample of studies. Three review authors (LL, MS, JW) independently extracted data on the number of participants, characteristics of participants, characteristics of the intervention with and without opportunistic salpingectomy, study quality, duration of follow‐up and outcomes. Any disagreements were resolved by discussion. We attempted to retrieve missing data by contacting the study authors. For studies with multiple publications, we collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We assigned these studies a single study identifier.
Assessment of risk of bias in included studies
Three review authors (JW, MS, LL) independently assessed the methodological quality of the included studies; disagreements were resolved by discussion.
For non‐randomised studies, we planned to assess the likelihood of bias according to the ROBINS‐I (a tool for assessing the risk of bias in non‐randomised studies of interventions) (Sterne 2016). We defined the hypothetical 'target' trial necessary for the use of the ROBINS‐I as a large RCT in which women would be allocated to the intervention group (i.e. hysterectomy with opportunistic salpingectomy) or the control group (i.e. hysterectomy without opportunistic salpingectomy). Baseline information on both groups should have included age, parity, the use of oral contraceptives and surgical history. During a follow‐up period of at least 40 years, family history (breast and ovarian cancer), age at menopause, use of oral contraceptives, abdominal surgery and the occurrence of epithelial ovarian cancer should have been documented. If a participant was diagnosed with epithelial ovarian cancer, data on age at diagnosis, tumour stage and histology of the primary tumour should have been collected.
We planned to assess eligible studies for bias due to confounding, selection of participants, classification of interventions, missing data, measurement of outcomes and selection of the reported result. The following domains were identified as potential confounders, which therefore should preferably be similar among study groups, or else suitably controlled using statistical methods: age, parity, family history of ovarian or breast cancer, use of oral contraception and history of tubal ligation. We identified no cointerventions that could potentially confound the results. Confounding might result in considerable heterogeneity between studies and requires adequate methods to control for it, such as stratification of regression modelling with propensity scores or covariates. We planned to assess the appropriateness and quality of these methods critically. We planned to compare non‐RCTs to their published protocol, where available, to assess selective or incomplete reporting.
We assessed the risk of bias in randomised studies with Cochrane's ‘Risk of bias’ assessment tool (Higgins 2011a), and included the following domains: random sequence generation, allocation concealment, incomplete outcome data and selective reporting. Where available, we compared the published protocols of selected studies to the reported outcomes so as to assess selective or incomplete reporting bias.
Measures of treatment effect
For non‐RCTs, we planned to extract and report both unadjusted and adjusted effect estimates. For cohort studies, we planned to calculate a hazard ratio (HR). We expected there to be a long duration of follow‐up for the epithelial ovarian cancer outcome, which could have resulted in selection bias over time. Therefore, we planned to calculate HRs for different time points. For case‐control studies, we planned to calculate odds ratios (ORs) with 95% confidence intervals (CIs) by extracting the number of participants in each treatment arm that experienced the outcome of interest, and the number of participants assessed per outcome. For dichotomous data extracted from RCTs (i.e. adverse surgical events), we calculated ORs with 95% CIs. For continuous data (i.e. postoperative hormonal status), we estimated mean differences (MDs) with 95% CIs for variables with a normal distribution where the same measure was used to assess the outcome. Where the included studies used different measures to assess the same outcome, we used the standardised mean difference (SMD). For skewed continuous variables, we extracted mean values and standard deviations. As a sensitivity analysis, we transformed skewed data prior to meta‐analysis according to method 1 as presented by Higgins 2008a, which does not assume a common standard deviation in the two groups.
If the data necessary to calculate ORs or MDs were not available, we made use of the most detailed numerical data available that facilitated similar analyses of included studies. In addition, we attempted to retrieve missing data by contacting the study authors.
Unit of analysis issues
The primary analysis was performed per woman included in the studies.
Dealing with missing data
For non‐RCTs, we planned to conduct sensitivity analyses to assess how robust our conclusions were to assumptions about missing data (Higgins 2008b).
For RCTs, we analysed the retrieved data according to the intention‐to‐treat principle as far as possible. For analyses of adverse events, we defined women dropping out postrandomisation but prior to surgery as not having the event. For the outcome 'postoperative hormonal status', it is not straightforward to perform an intention‐to‐treat analysis in the presence of dropouts, without access to the individual participant data from the trial. In case of missing data, we contacted the original researchers in an attempt to obtain the missing data. Where these attempts did not provide us with extra data, we only made use of the available data.
Assessment of heterogeneity
For non‐RCTs, we expected heterogeneity, and thus we planned to base our assessment of heterogeneity on consideration of the different study designs and analysis details.
To examine whether meta‐analysis was possible for RCTs, we assessed the statistical heterogeneity of the included studies using the I2 statistic. An I2 value of 50% or higher was considered as indicating substantial heterogeneity. We also considered the similarity of the protocols, since meta‐analysis is not a sensible option when the trial characteristics are disparate.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact through the performance of an extensive search for eligible studies and by being alert for the duplication of data. If there were 10 or more studies in an analysis, we planned to use a funnel plot to explore the possibility of small study effects (the tendency for estimates of the intervention effect to be more beneficial in smaller studies). To prevent language bias, we did not exclude any studies based on language. If the studies proved to be exceptionally difficult to translate, we asked the authors to provide a summary of their methods and results. We compared the studies, authors and their affiliations so as to avoid multiple publication bias.
Data synthesis
When we considered the selected studies to be similar enough for meta‐analysis, we combined the data using a fixed‐effect model. We made the following comparisons.
Incidence of epithelial ovarian cancer after hysterectomy with opportunistic salpingectomy versus incidence of epithelial ovarian cancer after hysterectomy without opportunistic salpingectomy.
Surgical outcomes of hysterectomy with opportunistic salpingectomy versus hysterectomy without opportunistic salpingectomy.
Ovarian reserve after hysterectomy with opportunistic salpingectomy versus hysterectomy without opportunistic salpingectomy.
Subgroup analysis and investigation of heterogeneity
Where possible, depending on the availability of the data, we planned to perform the following subgroup analyses.
-
Effect of opportunistic salpingectomy on the incidence of epithelial ovarian cancer in the following subgroups:
premenopausal versus postmenopausal women
-
Effect of opportunistic salpingectomy on the incidence of epithelial ovarian cancer in the following subgroups:
nulliparous versus parous women
-
Incidence of epithelial ovarian cancer in:
women who have a history of tubal ligation versus women who have no history of tubal ligation
-
Incidence of surgery‐related adverse events depending on surgical approach:
abdominal approach versus laparoscopic approach
vaginal approach versus laparoscopic approach
abdominal approach versus vaginal approach
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes to determine whether the review conclusion would remain the same if:
eligibility had been restricted to studies without high risk of bias (which we defined as those with no high risk of bias in any domain);
a random‐effects model had been adopted;
non‐RCTs had been excluded (only applicable if RCTs have been included).
Where individual participant data were available, we used multiple imputation so that all randomised women were included in the estimate for that specific study (according to Sterne 2009), to assess whether imputation of missing data made a difference in our outcome. We included age, preoperative value, the surgeon performing the procedure, treatment allocation, and treatment received in the imputation model, and used a chained equations approach as implemented in the mi package (Su 2011) in R (R Core Team 2017).
In addition, we made the posthoc decision to conduct a complier analysis for the postoperative hormonal status outcome, using available individual participant data. This analysis estimates the effect of undergoing, rather than of simply being allocated to, hysterectomy with opportunistic salpingectomy compared to hysterectomy without opportunistic salpingectomy. This seemed appropriate in light of nontrivial rates of noncompliance in the studies. We used an instrumental variable approach as implemented in the ivregress command implemented in Stata (StataCorp 2013).
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro and Cochrane methods (GRADEpro GDT 2015). This table evaluates the overall quality of the body of evidence for the main review outcomes (incidence of epithelial ovarian cancer, surgery‐related adverse events and postoperative hormonal status) for the main review comparison (hysterectomy with opportunistic salpingectomy versus hysterectomy without opportunistic salpingectomy). If appropriate, we planned to prepare additional 'Summary of findings' tables for the main review outcomes of other important comparisons (premenopausal versus postmenopausal women, nulliparous women versus parous women, women with a history of tubal ligation versus women with no history of tubal ligation, abdominal approach versus laparoscopic approach, vaginal approach versus laparoscopic approach and abdominal approach versus vaginal approach). We assessed the quality of the evidence using the GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness and publication bias. Two review authors (LL, JW) working independently made judgements about the evidence quality (high, moderate, low or very low); disagreements were resolved by discussion and the consultation of a third review author (MS). We justified, documented and incorporated all judgements into the report of results for each outcome.
We extracted study data, formatted our comparisons in data tables and prepared a 'Summary of findings' table before writing the results and conclusions of our review.
Results
Description of studies
Results of the search
In total, we identified 3866 records for screening after the removal of duplicate studies. After screening the title/abstract and full text, 10 RCTs were eligible for inclusion. We found no suitable non‐RCTs. Three of these eligible studies were still recruiting women and therefore not available for analysis (Characteristics of ongoing studies). The selection process is summarised in a PRISMA flow chart (Figure 1).
1.
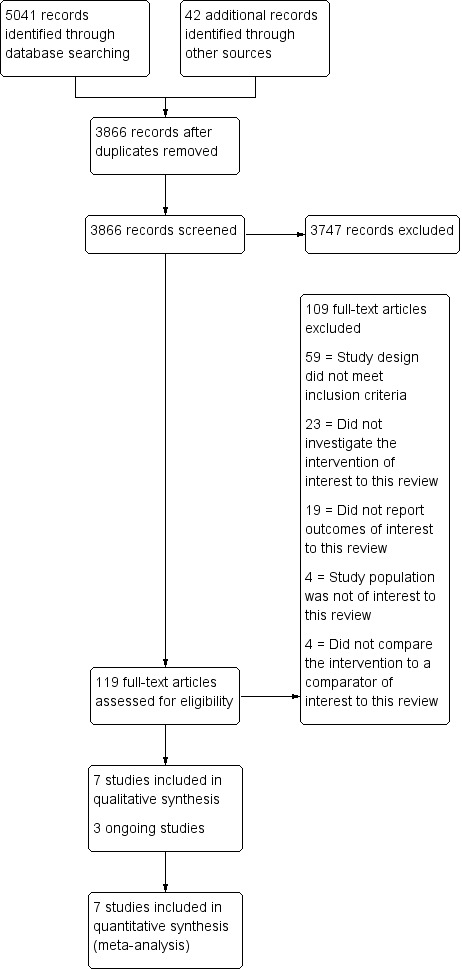
Study flow diagram.
Included studies
Study design and setting
We included a total of seven studies in this review (Behnamfar 2017; Findley 2013; Popov 2015; Sezik 2007; Song 2016; Tehranian 2017; van Lieshout 2018). Three ongoing studies were still recruiting women and therefore results were not yet available (NCT03045965; NCT02086344; NCT01628432). Six studies were published in English and one study was published in Russian (Popov 2015). An overview of the included studies is presented in the Characteristics of included studies table. We attempted to contact the authors of six studies for additional information (Behnamfar 2017; Chen 2018; Popov 2015; Sezik 2007; Song 2016; Tehranian 2017) which yielded a response and additional data from two (Popov 2015; Sezik 2007), and no response from four authors (Behnamfar 2017; Chen 2018; Song 2016; Tehranian 2017). Individual participant data were available for van Lieshout 2018 as two trial authors also took part in the writing of this review.
Participants
In all included studies only premenopausal participants were eligible for participation.
Behnamfar 2017 included 40 women planning to undergo a hysterectomy for benign reasons. Eighteen women with a mean age of 48.5 (standard deviation (SD) 2.03) years were randomly allocated to the intervention group (with opportunistic salpingectomy) and 22 women with a mean age of 47.7 years (SD 3.03) were randomly allocated to the control group (without opportunistic salpingectomy).
Findley 2013 included 30 women who were undergoing elective laparoscopic hysterectomy with planned preservation of the ovaries for benign indications. The mean age of participants was 37.2 (SD 4.7) years and 15 women were allocated to each group (i.e. intervention and control).
Popov 2015 included 54 women planning to undergo a laparoscopic hysterectomy. Twenty‐nine women with a mean age of 44 years were allocated to the intervention group and 25 women with a mean age of 45 years were allocated to the control group.
Sezik 2007 included 24 women scheduled for hysterectomy without oophorectomy. In each group 12 women were included with a mean age of 41.6 (SD 1.7) years in the intervention group and 41.1 (SD 1.4) years in the control group.
Song 2016 included 68 women planning to undergo laparoscopic hysterectomy for benign uterine diseases. In each group, 34 women were included with a median age of 43 (interquartile range (IQR) 41 to 47) years in the intervention group and 44 (IQR 41 to 46) years in the control group.
Tehranian 2017 included 30 premenopausal women undergoing abdominal hysterectomy for non‐malignant gynaecologic disease with preservation of the ovaries. In each group 15 women were included with a median age of 39.8 (SD 3.72) years in the intervention group and 40.5 (SD 3.02) in the control group.
van Lieshout 2018 included 104 women with an indication for either laparoscopic or abdominal hysterectomy for benign indications (such as fibroids or bleeding disorders). In each group 52 women were included with a median age of 44.5 (IQR 41.3 to 46.8) years in the intervention group and 44.0 (IQR 42.3 to 48.0) years in the control group.
A detailed description of participants per study is provided in the Characteristics of included studies table.
Interventions
In all studies, hysterectomies were performed with or without opportunistic salpingectomy. One study did not specify which approach for hysterectomy was used (Behnamfar 2017). In three studies only laparoscopic hysterectomies (Findley 2013; Popov 2015; Song 2016), in two studies only abdominal hysterectomies (Sezik 2007; Tehranian 2017), and in one study (van Lieshout 2018), both laparoscopic and abdominal hysterectomies were performed.
Outcomes
Primary outcomes
-
Incidence of epithelial ovarian cancer
None of the included studies assessed this outcome measure
Surgery‐related adverse events
Intraoperative complications: five studies described the occurrence of salpingectomy‐related intraoperative complications (Findley 2013; Popov 2015; Song 2016; Tehranian 2017; van Lieshout 2018), such as excessive blood loss.
Short‐term postoperative complications: three studies described the occurrence of short‐term postoperative complications (Findley 2013; Popov 2015; Song 2016), such as vaginal vault bleeding.
-
Postoperative hormonal status
Seven studies investigated postoperative hormonal status (Behnamfar 2017; Findley 2013; Popov 2015; Sezik 2007; Song 2016; Tehranian 2017; van Lieshout 2018), of which five studies were by the preferred method; AMH (Findley 2013; Popov 2015; Song 2016; Tehranian 2017; van Lieshout 2018). Four studies included (additional) measurements (Behnamfar 2017; Popov 2015; Sezik 2007; Tehranian 2017), for example follicle stimulating hormone (FSH), luteinising hormone (LH) and estradiol.
Secondary outcomes
Total surgical time
Five studies assessed total surgical time (Findley 2013; Popov 2015; Song 2016; Tehranian 2017; van Lieshout 2018).
Estimated blood loss
Five studies assessed estimated blood loss (Findley 2013; Popov 2015; Song 2016; Tehranian 2017; van Lieshout 2018).
Conversion rate to open surgery (applicable only to laparoscopic and vaginal approaches)
Two studies assessed conversion rate to open surgery (Song 2016; van Lieshout 2018).
Duration of hospital admission
Three studies assessed duration of hospital admission (Popov 2015; Song 2016; van Lieshout 2018).
Menopause‐related symptoms
None of the included studies assessed menopause‐related symptoms.
Quality of life
One study assessed quality of life (Popov 2015), measured by the use of the 36‐Item Short Form Survey (SF‐36).
Excluded studies
We excluded 109 studies from the review, for the following reasons.
Fifty‐nine out of 109 studies were not RCTs or eligible non‐RCTs (study design did not meet the inclusion criteria).
Four out of 109 studies had a study population that was not of interest to this review.
Twenty‐three out of 109 studies did not compare hysterectomy with salpingectomy to hysterectomy without opportunistic salpingectomy (did not investigate the intervention of interest to this review).
Four out of 109 studies did not compare hysterectomy with salpingectomy to hysterectomy without opportunistic salpingectomy (did not compare the intervention to a comparator of interest to this review).
Nineteen out of 109 studies did not report outcomes of interest to this review.
We excluded four large cohort studies which investigated the incidence of ovarian cancer after opportunistic salpingectomy. All trials investigated opportunistic salpingectomy either during a variety of surgeries or as a sterilisation method. However, none had specific data available on opportunistic salpingectomy in combination with hysterectomy (Chen 2018; Falconer 2015; Lessard‐Anderson 2014; Madsen 2015). Two other trials appeared suitable but we excluded them after contact with the author (Wierrani 1993), or the translation revealed them to be non‐randomised (Yi 2012), and they did not report on the incidence of ovarian cancer.
Risk of bias in included studies
Allocation
Sequence generation
We rated four studies at low risk of selection bias related to sequence generation as they used computer randomisation or a random numbers table (Findley 2013; Song 2016; Tehranian 2017; van Lieshout 2018). The other three studies did not describe the method used (Behnamfar 2017; Popov 2015; Sezik 2007), and thus we rated them at unclear risk of bias.
Allocation concealment
Four studies described methods of allocation concealment and we rated them at low risk of selection bias related to allocation concealment (Findley 2013; Song 2016; Tehranian 2017; van Lieshout 2018). The other three studies did not, or not sufficiently, describe their methods and thus we rated them at unclear risk of bias (Behnamfar 2017; Popov 2015; Sezik 2007).
Incomplete outcome data
We considered two studies to be at low risk of attrition bias as they analysed all, or most, women randomised (Popov 2015; Sezik 2007). We rated Song 2016 at unclear risk of attrition bias as the sample size was retrospectively amended in the protocol and four studies at high risk of attrition bias due to substantial loss to follow‐up (> 10%) (Behnamfar 2017; Findley 2013; Tehranian 2017; van Lieshout 2018) .
Selective reporting
Two studies reported the outcomes according to protocol and thus we judged them at low risk of reporting bias (Findley 2013; van Lieshout 2018). We judged three studies at unclear risk of reporting bias as two studies were not registered in a clinical trial registry (Popov 2015; Sezik 2007), and one study was not registered until after completion of the trial (Tehranian 2017). Two studies were rated at high risk of reporting bias: one study listed AMH as an outcome in the protocol but did not mention it in the report (Behnamfar 2017), and one study changed the primary outcome retrospectively in the trial register from AMH to change in AMH (Song 2016).
Other potential sources of bias
Unequal distribution of (experienced) surgeons among the study groups could result in bias. None of the studies reported on possible surgeon effects. Due to the availability of individual participant data from the van Lieshout 2018 trial, we performed additional analysis adjusting for a possible surgeon effect in this study. For other studies this was not possible, therefore we rated these studies at unclear risk of other potential sources of bias.
A general overview of risk of bias of included studies is presented in the 'Risk of bias' summary (Figure 2), a more detailed overview per study is given in the 'Risk of bias' graph (Figure 3).
2.
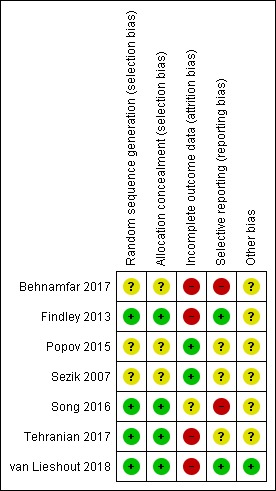
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
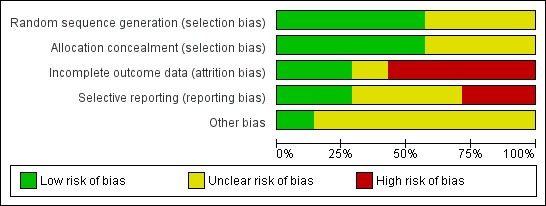
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
See: Table 1
1 Hysterectomy with bilateral salpingectomy versus hysterectomy without bilateral salpingectomy
Primary outcomes
1.1 Incidence of epithelial ovarian cancer
No studies reported on the incidence of epithelial ovarian cancer after hysterectomy with opportunistic salpingectomy compared to hysterectomy without opportunistic salpingectomy.
1.2. Surgery‐related adverse events
1.2.1 Intraoperative complications
Due to the small number of observed events (5 events in total), we found insufficient evidence to determine if there was a difference in risk of intraoperative complications when comparing hysterectomy with opportunistic salpingectomy to hysterectomy without opportunistic salpingectomy (odds ratio (OR) 0.66, 95% confidence interval (CI) 0.11 to 3.94; I2 = 0%; 5 studies, 286 participants; very low‐quality evidence). This means that, if 55 out of 1000 women having hysterectomy without opportunistic salpingectomy have intraoperative complications, then between 6 and 177 out of 1000 women having hysterectomy with opportunistic salpingectomy would be expected to have intraoperative complications (Analysis 1.1, Figure 4).
1.1. Analysis.
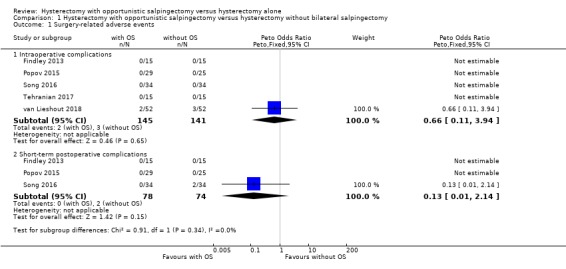
Comparison 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without bilateral salpingectomy, Outcome 1 Surgery‐related adverse events.
4.
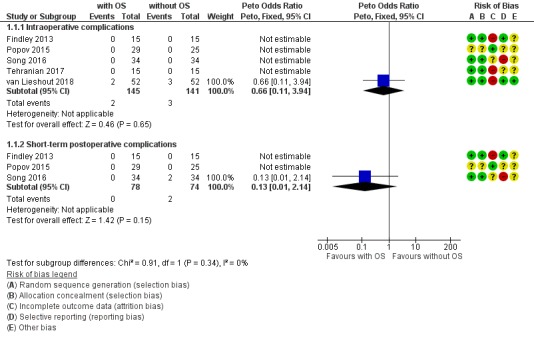
Forest plot of comparison: 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without opportunistic salpingectomy, outcome: 1.1 Surgery‐related adverse events. With OS: hysterectomy with opportunistic salpingectomy; without OS: hysterectomy without opportunistic salpingectomy
Only one RCT of five in the analysis found any adverse events. However, this demonstrates that intraoperative adverse events are rare, both in women undergoing hysterectomy with opportunistic salpingectomy (where 2 out of 145 had an adverse event) and in women undergoing hysterectomy without opportunistic salpingectomy (where 3 out of 141 had an adverse event).
1.2.2 Short‐term postoperative complications
Due to the small number of observed events (2 events), there was insufficient evidence to determine if there was a difference in risk of short‐term postoperative complications when comparing hysterectomy with opportunistic salpingectomy to hysterectomy without opportunistic salpingectomy (OR 0.13, 95% CI 0.01 to 2.14; I2 = 0%; 3 studies, 152 participants; very low‐quality evidence). This means that, if 59 out of 1000 women having hysterectomy without opportunistic salpingectomy have short‐term postoperative complications, then between one and 118 out of 1000 women having hysterectomy with opportunistic salpingectomy would be expected to have short‐term postoperative complications (Analysis 1.1, Figure 4).
Only one RCT of three in the analysis found any adverse events. However, this demonstrates that short‐term postoperative adverse events are rare, both in women undergoing hysterectomy with opportunistic salpingectomy (where none out of 78 had an adverse event) and in women undergoing hysterectomy without opportunistic salpingectomy (where 2 out of 74 had an adverse event).
1.3. Postoperative hormonal status
1.3.1 Anti‐Müllerian hormone (AMH)
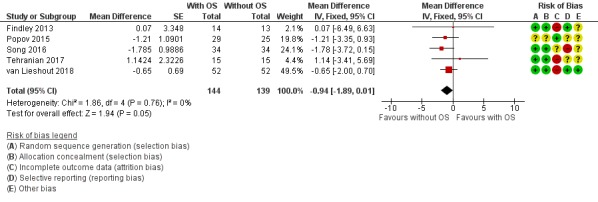
The results were compatible with no difference, or with a reduction in AMH that would not be clinically significant (mean difference (MD) ‐0.94, 95% CI ‐1.89 to 0.01; I2 = 0%; 5 studies, 283 participants; low‐quality evidence). A reduction in AMH would be unfavourable, but due to wide CIs, the postoperative change in AMH can still vary from a substantial decrease to even a slight increase (Analysis 1.2, Figure 5).
1.2. Analysis.
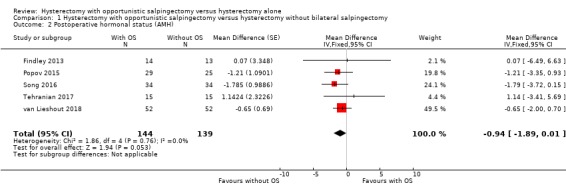
Comparison 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without bilateral salpingectomy, Outcome 2 Postoperative hormonal status (AMH).
5.
Forest plot of comparison: 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without opportunistic salpingectomy, outcome: 1.2 Postoperative hormonal status (AMH). With OS: hysterectomy with opportunistic salpingectomy; without OS: hysterectomy without opportunistic salpingectomy
As our protocol did not account for a difference in duration of follow‐up for hormone measurements, we performed a posthoc analysis per reported time point; one study reported AMH four to six weeks after surgery (MD ‐1.57, 95% CI ‐11.09 to 7.95; 1 study, 23 participants; Findley 2013), four studies reported AMH three months after surgery (MD ‐1.16, 95% CI ‐2.89 to 0.56; 4 studies, 179 participants; Findley 2013; Popov 2015; Song 2016; Tehranian 2017), and one study reported AMH six months after surgery (MD ‐0.65, 95% CI ‐2.00 to 0.70; 1 study, 104 participants; van Lieshout 2018; Analysis 1.3).
1.3. Analysis.
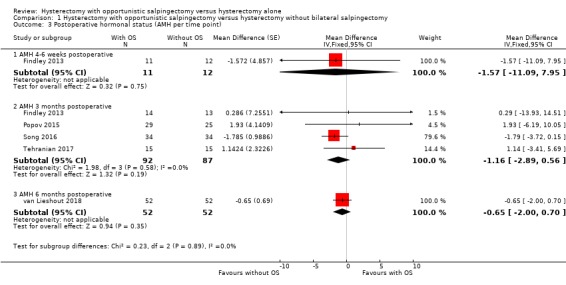
Comparison 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without bilateral salpingectomy, Outcome 3 Postoperative hormonal status (AMH per time point).
1.3.2 Follicle stimulating hormone (FSH)
We found no evidence for a difference in postoperative FSH values; we are uncertain if the addition of opportunistic salpingectomy to hysterectomy may affect FSH (MD ‐0.59, 95% CI ‐1.58 to 0.40; I2 = 29%; 4 studies, 145 participants; low‐quality evidence). This means there could be a reduction as large as 1.58 IU/L or an increase as large as 0.40 IU/L (Analysis 1.4).
1.4. Analysis.
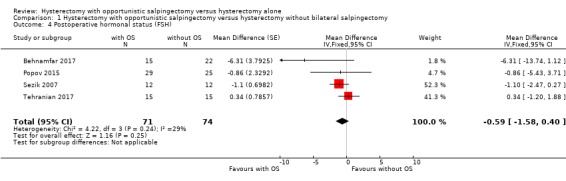
Comparison 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without bilateral salpingectomy, Outcome 4 Postoperative hormonal status (FSH).
We performed a posthoc analysis per reported time point; one study reported FSH one month after surgery (MD ‐1.00, 95% CI ‐2.28 to 0.28; 1 study, 24 participants; Sezik 2007), two studies reported FSH three months after surgery (MD 0.24, 95% CI ‐1.18 to 1.66; two studies, 84 participants; Popov 2015; Sezik 2007; Tehranian 2017), and two studies reported FSH six months after surgery (MD ‐1.27, 95% CI ‐2.62 to 0.08; 2 studies, 61 participants; Behnamfar 2017; Sezik 2007; Analysis 1.5). These results are in line with the possibility of a slight change in FSH concentration after hysterectomy with opportunistic salpingectomy compared to hysterectomy without opportunistic salpingectomy, although considerable uncertainty remains at each time point.
1.5. Analysis.
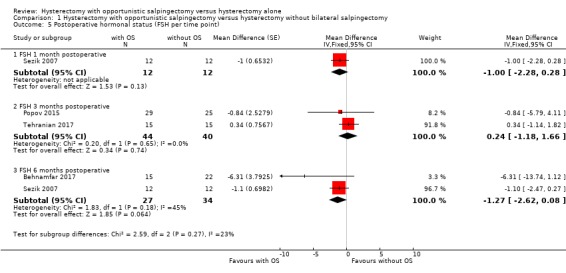
Comparison 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without bilateral salpingectomy, Outcome 5 Postoperative hormonal status (FSH per time point).
1.3.3 Luteinising hormone (LH)
There was no evidence for a difference in postoperative LH values; the addition of opportunistic salpingectomy to a hysterectomy may result in an indeterminate change in LH (MD ‐0.73, 95% CI ‐2.14 to 0.68; I2 = 27%; 3 studies, 115 participants; low‐quality evidence). This means that there could be a reduction as large as 2.14 IU/L or an increase as large as 0.68 IU/L (Analysis 1.6).
1.6. Analysis.
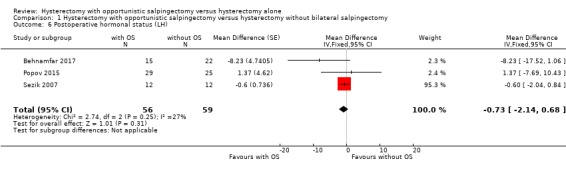
Comparison 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without bilateral salpingectomy, Outcome 6 Postoperative hormonal status (LH).
We performed a posthoc analysis per reported time point; one study reported LH one month after surgery (MD ‐0.40, 95% CI ‐1.84 to 1.04; 1 study, 24 participants; Sezik 2007), one study reported LH three months after surgery (MD 1.37, 95% CI ‐7.69 to 10.43; 1 study, 54 participants; Popov 2015), and two studies reported LH six months after surgery (MD ‐0.78, 95% CI ‐2.21 to 0.65; 2 studies, 61 participants; Behnamfar 2017; Sezik 2007; Analysis 1.7). These results are in line with the possibility of a slight change in LH concentration after hysterectomy with opportunistic salpingectomy compared to hysterectomy without opportunistic salpingectomy, although considerable uncertainty remains at each time point.
1.7. Analysis.
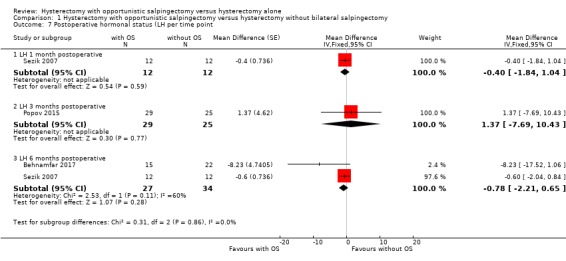
Comparison 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without bilateral salpingectomy, Outcome 7 Postoperative hormonal status (LH per time point.
1.3.4 Estradiol
There was no evidence for a difference in postoperative estradiol values, but the addition of opportunistic salpingectomy to a hysterectomy may result in an indeterminate change in estradiol (MD 4.51, 95% CI ‐28.96 to 37.38; I2 = 0%; 2 studies, 78 participants; very low‐quality evidence). This means that there could be a reduction as large as 8.08 IU/L or an increase as large as 10.16 IU/L (Analysis 1.8).
1.8. Analysis.
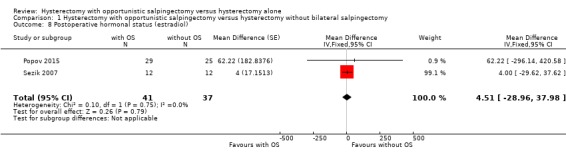
Comparison 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without bilateral salpingectomy, Outcome 8 Postoperative hormonal status (estradiol).
We performed a posthoc analysis per reported time point: one study reported estradiol one month after surgery (MD ‐5.00, 95% CI ‐41.46 to 31.46; 1 study, 24 participants; Sezik 2007), one study reported estradiol three months after surgery (MD 62.22, 95% CI ‐296.14 to 420.58; 1 study, 54 participants; Popov 2015) and one study reported estradiol six months after surgery (MD 4.00, 95% CI ‐29.62 to 37.62; 1 study, 24 participants; Sezik 2007; Analysis 1.9). These results are in line with the possibility of an indeterminate change in Estradiol concentration after hysterectomy with opportunistic salpingectomy compared to hysterectomy without opportunistic salpingectomy, although considerable uncertainty remains at each time point.
1.9. Analysis.
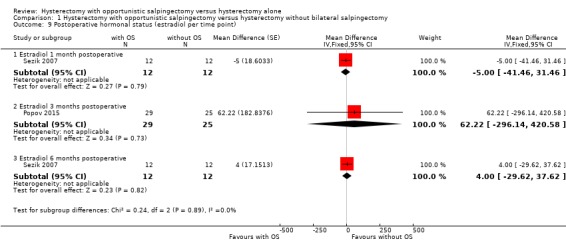
Comparison 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without bilateral salpingectomy, Outcome 9 Postoperative hormonal status (estradiol per time point).
A summary of important primary outcomes is presented in Table 1.
Secondary outcomes
4. Total surgical time
We found no evidence for a difference in total surgical time between women undergoing hysterectomy with opportunistic salpingectomy and women undergoing hysterectomy without opportunistic salpingectomy (MD 0.35 min, 95% CI ‐6.64 to 7.33, I2 = 64%; 5 studies, 286 participants; low‐quality evidence; Analysis 1.10).
1.10. Analysis.
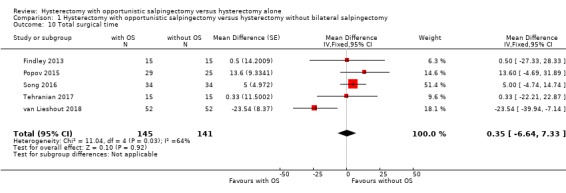
Comparison 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without bilateral salpingectomy, Outcome 10 Total surgical time.
5. Estimated blood loss
For estimated blood loss, we found no evidence for a difference between women undergoing hysterectomy with opportunistic salpingectomy and women undergoing hysterectomy without opportunistic salpingectomy (MD ‐3.25 mL, 95% CI ‐16.09 to 9.59, I2 = 13%; 5 studies, 286 participants; moderate‐quality evidence; Analysis 1.11).
1.11. Analysis.
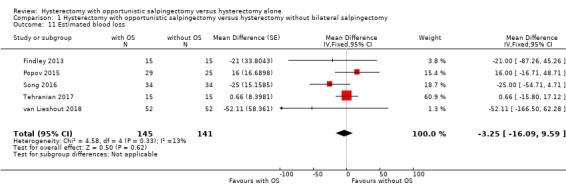
Comparison 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without bilateral salpingectomy, Outcome 11 Estimated blood loss.
6. Conversion rate to open surgery
We found no evidence for a difference in conversion rate to open surgery between women undergoing hysterectomy with opportunistic salpingectomy and women undergoing hysterectomy without opportunistic salpingectomy (OR 0.66, 95% CI 0.11 to 3.94; 2 studies, 172 participants; low‐quality evidence; Analysis 1.12).
1.12. Analysis.
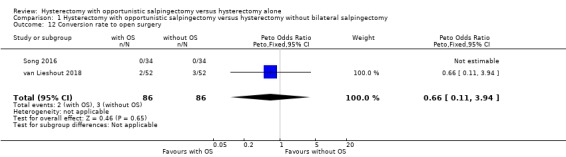
Comparison 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without bilateral salpingectomy, Outcome 12 Conversion rate to open surgery.
7. Duration of hospital admission
For the duration of hospital admission, we found no evidence for a difference between women undergoing hysterectomy with opportunistic salpingectomy compared to women undergoing hysterectomy without opportunistic salpingectomy (MD ‐0.02 days, 95% CI ‐0.22 to 0.17; I2 = 10%; 3 studies, 226 participants; moderate‐quality evidence; Analysis 1.13).
1.13. Analysis.
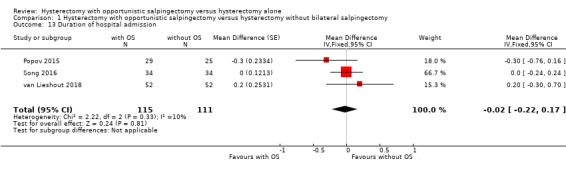
Comparison 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without bilateral salpingectomy, Outcome 13 Duration of hospital admission.
8. Menopause‐related symptoms
No studies reported on the incidence of objectified menopause‐related symptoms.
9. Quality of life
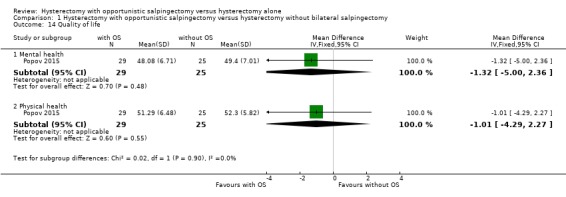
9.1 Mental health
One study reported mental health with use of the SF‐36 (MD ‐1.32, 95% CI ‐5.00 to 2.36; 1 study, 54 participants; very low‐quality evidence; Popov 2015). Hysterectomy with opportunistic salpingectomy might result in a minor decrease in mental health as measured by the SF‐36, compared to hysterectomy without opportunistic salpingectomy. This means the SF‐36 score could decrease by as much as 5.00 points or increase by as much as 2.36 points out of a maximum of 100 points after hysterectomy with opportunistic salpingectomy compared to hysterectomy without opportunistic salpingectomy (Analysis 1.14).
1.14. Analysis.
Comparison 1 Hysterectomy with opportunistic salpingectomy versus hysterectomy without bilateral salpingectomy, Outcome 14 Quality of life.
9.2 Physical health
One study reported physical health with use of the SF‐36 (MD ‐1.01, 95% CI ‐4.29 to 2.27; 1 study, 54 participants; very low‐quality evidence; Popov 2015). Hysterectomy with opportunistic salpingectomy might result in a minor decrease in physical health as measured by the SF‐36, compared to hysterectomy without opportunistic salpingectomy. This means the SF‐36 score could decrease by as much as 4.29 points or increase by as much as 2.27 points (out of a maximum of 100 points) if salpingectomy was added to hysterectomy (Analysis 1.14).
Subgroup analysis
1. Effect of opportunistic salpingectomy on the incidence of epithelial ovarian cancer in the following subgroups
Premenopausal versus postmenopausal women
No studies reported on the incidence of epithelial ovarian cancer.
2. Effect of opportunistic salpingectomy on the incidence of epithelial ovarian cancer in the following subgroups
Nulliparous versus parous women
No studies reported on the incidence of epithelial ovarian cancer.
3. Incidence of epithelial ovarian cancer
Women who have a history of tubal ligation versus women who have no history of tubal ligation
No studies reported on the incidence of epithelial ovarian cancer.
4. Incidence of surgery‐related adverse events depending on surgical approach
We originally planned to make three comparisons: abdominal versus laparoscopic approach; vaginal versus laparoscopic approach; and abdominal versus vaginal approach. However, none of the studies reported on the incidence of adverse events after vaginal approach as most studies focused either on abdominal (Tehranian 2017), or laparoscopic (Findley 2013; Popov 2015; Song 2016), or included both abdominal and laparoscopic hysterectomies (van Lieshout 2018), limiting our subgroup analysis to abdominal versus laparoscopic hysterectomy.
For intraoperative adverse events, four trials reported on laparoscopic hysterectomy (Findley 2013; Popov 2015; Song 2016; van Lieshout 2018) and two trials reported on abdominal hysterectomy (Tehranian 2017; van Lieshout 2018). One single study (van Lieshout 2018), reported any adverse events. We found no evidence for a difference in incidence of intraoperative adverse events between the abdominal (OR 0.38, 95% CI 0.05 to 2.82; 2 studies, 109 participants; very low‐quality evidence) and laparoscopic (OR 6.80, 95% CI 0.13 to 343.88; 4 studies, 175 participants; very low‐quality evidence) approach. Four out of five adverse events were reported after abdominal hysterectomies and one adverse event was reported after laparoscopic hysterectomy (Analysis 2.1).
2.1. Analysis.
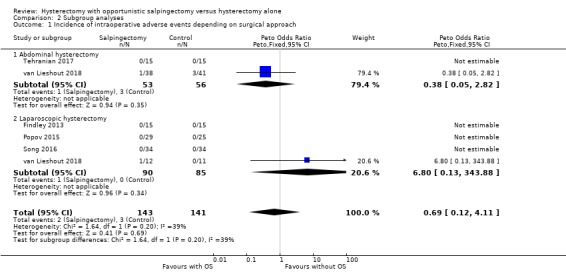
Comparison 2 Subgroup analyses, Outcome 1 Incidence of intraoperative adverse events depending on surgical approach.
Three trials reported on short‐term postoperative adverse events (Findley 2013; Popov 2015; Song 2016). As all three studies focused on the laparoscopic approach, subgroup analysis was not possible.
Sensitivity analysis
1. Eligibility restricted to studies without high risk of bias (which we define as those with no high risk of bias in any domain)
We classified two trials as not being at 'high risk of bias' in any domain (Popov 2015; Sezik 2007). However, even though these trials did not classify as 'high risk of bias' in any domain, we judged them at unclear risk of bias for four domains each. In addition, only one trial reported on surgery‐related adverse events and only one trial used AMH as a measure for postoperative hormonal status. Due to unclear risk of bias and an insufficient number of included studies per outcome, we refrained from undertaking this sensitivity analysis.
2. Adoption of a random‐effects model
As the number of surgery‐related adverse events was low and treatment effects were expected to be small, we opted to use the Peto odds ratio rather than Mantel‐Haenszel odds ratio. However, as the Peto odds ratio is a fixed‐effect method, we could not adopt a random‐effects model for comparison.
For postoperative AMH status, adoption of a random‐effects model did not result in a different outcome from adoption of a fixed‐effect model. (Analysis 3.1)
3.1. Analysis.
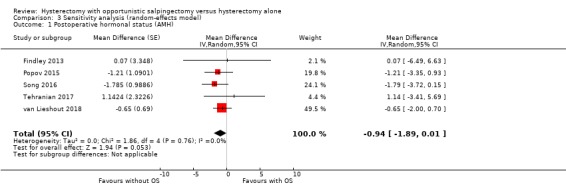
Comparison 3 Sensitivity analysis (random‐effects model), Outcome 1 Postoperative hormonal status (AMH).
3. Exclusion of non‐RCTs
As we did not include any non‐RCTs in this review, we could not conduct the planned sensitivity analysis to assess the impact of excluding non‐RCTs.
4. Skewed data in AMH analysis
We conducted a sensitivity analysis for skewed data in the analysis of AMH levels (MD ‐0.25, 95% CI ‐0.43 to ‐0.06; I2 = 68%; 5 studies, 283 participants; Analysis 4.1). The results remained compatible with a reduction in AMH that would not be clinically significant (see Analysis 1.2).
4.1. Analysis.
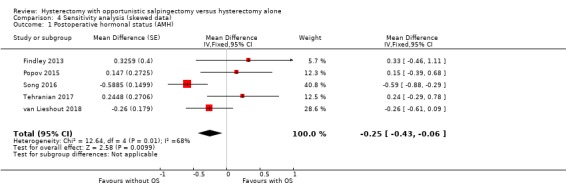
Comparison 4 Sensitivity analysis (skewed data), Outcome 1 Postoperative hormonal status (AMH).
Other analysis
As individual participant data was available for the study of van Lieshout 2018, an additional complier analysis was performed for this study only. We performed this analysis once without accounting for skewness of data (MD ‐0.83, 95% ‐2.44 to 0.79; 1 study, 104 participants) and once accounting for skewness of data (MD ‐0.34, 95% CI ‐0.75 to 0.08; 1 study, 104 participants). This is in line with our conclusions that results were compatible with no difference, or with a reduction in AMH that would not be clinically significant. Using multiple imputation due to the missing data in van Lieshout 2018 made no substantive difference to the results.
Discussion
Summary of main results
We found no eligible studies reporting one of our primary outcomes; the incidence of epithelial ovarian cancer after hysterectomy with or without opportunistic salpingectomy.
The number of surgery‐related adverse events was very low. As complications are generally rare for this type of surgery, large numbers of studies and participants are needed to determine if there is a difference in incidence of surgery‐related adverse events between hysterectomy with opportunistic salpingectomy or hysterectomy without opportunistic salpingectomy. With the limited number of studies and participants, we were unable to detect possible differences.
For postoperative hormonal status, we compared anti‐Müllerian hormone (AMH), follicle stimulating hormone (FSH), luteinising hormone (LH) and estradiol values. There was a large variety in duration of follow‐up, and the number of available studies per outcome varied between two and five. For AMH, the results were compatible with no difference, or with a reduction in AMH that would not be clinically significant. A reduction in AMH would be unfavourable, but due to wide confidence intervals (CIs), the postoperative change in AMH can still vary from a substantial decrease to even a slight increase. For FSH, LH and estradiol, there might be an indeterminate difference, meaning the true difference can either be a decrease or increase of the individual values. An increase of FSH and LH would be unfavourable, and an increase of estradiol would be favourable.
For the secondary outcomes of this review, we found no evidence of a difference between hysterectomy with opportunistic salpingectomy compared to hysterectomy without opportunistic salpingectomy for total surgical time, estimated blood loss, conversion rate to open surgery, duration of hospital admission or quality of life. No studies reported on the incidence of menopause‐related symptoms.
Overall completeness and applicability of evidence
All seven studies included in this review provided a direct answer to a part of the review question, either for surgery‐related adverse events or postoperative hormonal status; none of the trials reported epithelial ovarian cancer incidence.
None of the studies included postmenopausal women, limiting the applicability of the evidence from this review to the premenopausal population. Furthermore, the results of this review are not applicable for vaginal hysterectomy. The included studies limited surgical approach to abdominal or laparoscopic hysterectomies. In one study (van Lieshout 2018), two participants did have a vaginal hysterectomy. However, these women were excluded from the trial and no salpingectomies were performed.
We found a lack of clear definitions of surgery‐related adverse events in most of the studies. In combination with unclear and varying durations of follow‐up, the relevance of the identified data is hard to determine.
The majority of the studies used AMH values for the postoperative hormonal status outcome, which has the strongest correlation with time to menopause (van Rooij 2005). The other studies used a combination of several other (hormonal) measurements, resulting in a low number of studies per outcome. In addition to several outcome measures, the change in hormonal status was reported in several ways. Some studies reported pre‐ and postoperative values, while others reported the difference between pre‐ and postoperative values or a decline rate, expressed as a percentage. These differences in outcome measure and reporting complicate the interpretation of results. Another complicating factor in the meta‐analysis of hormone‐related outcomes was the skewness of outcome data, which resulted in several studies reporting outcomes in median with interquartile range rather than mean values with standard deviations. Attempts to contact the study authors resulted in additional outcome data, but yielded no response for one of the studies. For this study, we transformed the data to mean and standard deviation which might introduce imprecision in the reported results (Song 2016).
Quality of the evidence
The findings of this review are based on a limited number of seven studies, which included a total of 350 women. Most of the studies are small, and a few did not meet the desired sample size or retrospectively altered the desired sample size. In addition, loss to follow‐up was substantial in a majority of the studies, possibly resulting in attrition bias. The limitations of the included studies and the small number of included women have resulted in assessment of the available evidence as of very low to low quality.
As mentioned previously, the number of both intraoperative and postoperative adverse events were low and thus we were unable to determine if there may be a small difference in incidence of these events between hysterectomy with or without opportunistic salpingectomy. The low complication rate also resulted in many studies without reported events which means that both intra‐ and postoperative results are each based on a single randomised controlled trial (RCT). Additionally, none of the studies accounted for possible surgeon effects, meaning that outcomes could have been influenced by an unequal distribution of experienced surgeons among study groups. As experience is an important factor in aspects such as intra‐ or postoperative complications and surgical time, the role of this performance effect in the overall result is uncertain. Individual participant data were available for one study (van Lieshout 2018), allowing for additional analysis which revealed no substantial impact of surgeon effect on the outcomes. However, for the other studies we could not estimate surgeon effects and thus this could possibly introduce bias.
Measurement of postoperative hormonal status varied widely among the studies. For example, the postoperative hormonal status was determined at different time points, varying from three to six months after surgery. AMH values drop sharply after hysterectomy, only to recover over the course of approximately six months (Hehenkamp 2007). This time frame implies that most of the included trials have measured transient postoperative hormonal values which might not be representative of the real effect on time to menopause.
While AMH has the strongest correlation with time to menopause, several studies determined postoperative hormonal status based on FSH, LH or estradiol concentrations. In case of a diminished ovarian reserve, one would expect AMH and estradiol values to decrease, and FSH and LH values to increase. While the slight decrease in AMH concentration after hysterectomy with opportunistic salpingectomy compared to hysterectomy without opportunistic salpingectomy corresponds to what would be expected if opportunistic salpingectomy were to affect postoperative hormonal status, this is different for some of the other outcomes. FSH values seemed to decrease slightly while there appeared to be a minor increase in estradiol values. However, we found no evidence for any difference, due to the wide CIs, and so the true effect could go either way. A complicating factor in the use of these hormonal outcomes, which might account for our results, is the variation over the course of the menstrual cycle. Some studies specified at what time in the menstrual cycle blood samples were drawn and elaborated on how they estimated time in the menstrual cycle after hysterectomy (Popov 2015; Sezik 2007), others did not or not fully (Behnamfar 2017; Tehranian 2017). As crucial information is possibly lacking, the results should be interpreted with caution.
Potential biases in the review process
We carried out an extensive electronic search, which we completed by a manual search of reference lists. Two review authors independently assessed studies regarding eligibility, all decisions were reasoned and were made in an attempt to be conservative. In case of questions or incomplete data, we attempted to contact the authors of individual studies. We sent out requests for additional data to nine authors, of which four responded. We received additional data from two authors (Popov 2015; Sezik 2007). As mentioned previously, transformation of median and interquartile range (IQR) values to mean and standard deviation for one of the major studies in this review might have affected our results (Song 2016).
Agreements and disagreements with other studies or reviews
Prior to the realisation of this Cochrane Review, several (systematic) reviews, meta‐analyses and large cohort trials have been published on the effect of opportunistic salpingectomy on ovarian cancer incidence (Falconer 2015; Madsen 2015; Yoon 2016). Madsen et al performed a nationwide case‐control study and found an ovarian cancer risk reduction of 43% after opportunistic salpingectomy. Furthermore, Falconer et al found a hazard ratio of 0.35 for ovarian cancer risk after opportunistic salpingectomy in a nationwide population‐based study. Yoon et al performed a meta‐analysis with the previously described studies and observed an overall risk reduction of 49% in ovarian cancer risk after opportunistic salpingectomy. As none of these studies assessed the effect of hysterectomy with opportunistic salpingectomy compared to hysterectomy without opportunistic salpingectomy they are not fully comparable to this specific review. However, as the risk reduction is most likely achieved through the opportunistic salpingectomy itself, it is highly likely that opportunistic salpingectomy during hysterectomy will result in a similar protective effect.
In this review, the effect of opportunistic salpingectomy during hysterectomy on surgery‐related adverse events could not be estimated with certainty due to a very low number of events in the included studies. In our results, the difference in complication rate varied from an odds ratio (OR) of 0.09 to 1.86 in favour of hysterectomy with opportunistic salpingectomy. As stated above, no studies regarding vaginal hysterectomy are included in this review and thus information about safety is not available from this review. Several feasibility studies regarding this subject have been published, demonstrating a feasibility rate of 74% to 88% (Antosh 2017; Lamblin 2018; Robert 2015).
Our findings regarding hormonal status of women after hysterectomy with or without opportunistic salpingectomy are in accordance with previous literature; no clinically relevant differences were found (Mohamed 2017). A Canadian observational study measured ovarian reserve in 79 women, three to five years after hysterectomy with opportunistic salpingectomy. They found no evidence for differences compared to control women (Venturella 2016). Additionally, a meta‐analysis of studies among women opting for assisted reproductive technologies investigated the effect of salpingectomy on ovarian reserve and (for reasons other than ectopic pregnancy) found no evidence of differences (Kotlyar 2017). In this review, the maximum follow‐up time of included studies is six months, which can represent an overestimation in AMH decline because of a temporary decline in AMH concentration after surgery (Hehenkamp 2007). Although there is no evidence for a difference in observed postoperative AMH concentration, the 95% CI ranged from ‐1.89 to 0.01 pmol/L, which means that the true difference in AMH concentration most likely lies between ‐1.89 and 0.01 pmol/L. According to Marca et al, the median AMH concentration in 40 year‐old women is 16.52 pmol/L with an IQR of 9.42 to 27.57 pmol/L (La Marca 2012). Van Rooij et al investigated the natural decline in AMH concentration per year among women in different age groups (van Rooij 2005). The maximum decline of 1.89 pmol/L equals the natural decline in AMH concentration of approximately six months for women above the age of 40. In women between 36 and 40 years of age, a decrease of 1.89 pmol/L equals the natural AMH decline of approximately 16 months, and below the age of 36 it equals approximately 20 months (van Rooij 2005). Based on these assumptions, we consider a range with a maximum mean difference (MD) of ‐1.89 pmol/L to a minimum MD of 0.01 pmol/L as not to be clinically relevant, in addition to not having evidence of effect.
Authors' conclusions
Implications for practice.
We found no eligible studies reporting on the incidence of epithelial ovarian cancer specifically after hysterectomy with or without opportunistic salpingectomy in women undergoing surgery for benign gynaecological indications. For premenopausal women undergoing abdominal or laparoscopic hysterectomy for benign indications, we found insufficient data to assess whether there was any difference in surgery‐related adverse events between hysterectomy with opportunistic salpingectomy and hysterectomy without opportunistic salpingectomy. In addition, due to the low number and characteristics of included studies we judged the results as to be of low to very low quality, further complicating a clear practical translation of results. On the other hand, the low number of events is an important finding in itself, as it questions the clinical relevance of a small increase in adverse events when opportunistic salpingectomy is performed. For postoperative hormonal status, we found no evidence of a clinically relevant effect of hysterectomy with opportunistic salpingectomy compared to hysterectomy without opportunistic salpingectomy. However, results should be interpreted with caution. Furthermore, we found no available evidence on the safety and feasibility of opportunistic salpingectomy in the long term or during vaginal hysterectomy, even though this remains the preferential surgical approach to hysterectomy for benign indications (Aarts 2015).
Outside the scope of this review there is a growing body of evidence from non‐randomised and observational studies for the effectiveness of opportunistic salpingectomy, during other interventions or as a method of sterilisation, on the incidence of ovarian cancer. Most of this evidence is indirect but as ovarian cancer can seldom be cured, the likely benefits seem to outweigh the potential hazards of this preventive intervention. Therefore, in women undergoing a hysterectomy for benign indications, the addition of opportunistic salpingectomy can be discussed with the provision of a clear overview of current evidence of benefits and risks. However, as a measure of uncertainty remains on both surgical and hormonal safety, caution is needed for very young women and research is needed to establish a lower age limit for opportunistic salpingectomy during hysterectomy for benign indications.
Implications for research.
Most studies on the effect of opportunistic salpingectomy on ovarian cancer incidence are of suboptimal design or have a limited study population, restricting applicability for the general population. In addition, a limited number of studies focus on the comparison of hysterectomy with opportunistic salpingectomy compared to hysterectomy without opportunistic salpingectomy. High quality non‐randomised trials with large study populations and long‐term follow‐up are needed as the incidence of ovarian cancer is low and peak incidence is around 70 years of age. For surgery‐related adverse events, large randomised controlled trials (RCTs) are needed, as the occurrence of adverse events is rare. Moreover, future studies should elaborate on their definition of surgery‐related adverse events to enable pooling of data. Future research should also focus on long‐term effects on ovarian reserve and the safety of opportunistic salpingectomy during vaginal hysterectomy. Although none of the studies were aimed at the vaginal approach, it remains the preferential approach of hysterectomy (Aarts 2015). Besides establishing the safety of opportunistic salpingectomy during vaginal hysterectomy, it should also clarify whether risk and benefits justify a strategic switch from a vaginal to laparoscopic approach, if necessary. Furthermore, as time to menopause is the gold standard for hormonal status assessment, studies with long‐term follow‐up on hormonal status are needed. There are three large ongoing RCTs at the time of publication of this review, the largest of which is the HOPPSA trial conducted in Sweden (NCT03045965). The HOPPSA trial aims to include 4400 women and follow‐up will continue to 2050. The large number of participants and long duration of follow‐up will allow for critical evaluation and a firm establishment of the intervention effect.
Additional research is needed to establish the optimal lower age limit to undergo opportunistic salpingectomy and to evaluate effectiveness and safety in the postmenopausal population, especially to determine the optimal age to opt for salpingo‐oophorectomy instead of salpingectomy, as with increasing age the benefits of salpingo‐oophorectomy will outweigh the negative effects. As opportunistic salpingectomy is expected to be a preventive measure for a rare yet severe event, high quality RCTs should also be conducted on feasibility during other surgical interventions such as a laparoscopic sterilisation, instead of a tubal ligation.
What's new
| Date | Event | Description |
|---|---|---|
| 13 September 2019 | Amended | Minor edit in Abstract |
Acknowledgements
We thank Marian Showell (Cochrane Gynaecology and Fertility (CGF) Group Information Specialist), On Ying Chan (Radboud University Information Specialist) and Godelieve Engbersen (Elisabeth‐Tweesteden Hospital Information Specialist) for conducting the electronic search.
Thanks to all co‐operating authors of trials included in this review, especially professor dr. M. Sezik and professor dr. A. Popov for kindly providing us with additional study data. We also thank Cholpon Tashtanbekova and Yu‐Tian Xiao for providing us with translations of articles written in Russian and Chinese, respectively.
Furthermore, we thank Selma Mourad, Rik van Eekelen and Sarah Lensen for their time and effort in the reviewing of our manuscript.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility specialised register search strategy
Searched 8 January 2019
PROCITE platform
Keywords CONTAINS "salpingectomy" or "salpingo‐oophorectomy" or Title CONTAINS "salpingectomy" or "salpingo‐oophorectomy" (64 hits)
Appendix 2. CENTRAL via The CENTRAL Register of Studies Online (CRSO) search strategy
Searched 8 January 2019
Web platform
#1 MESH DESCRIPTOR Salpingectomy EXPLODE ALL TREES 38 #2 salpingectom*:TI,AB,KY 179 #3 ((tubal adj3 excision*) or tubectom*):TI,AB,KY 7 #4 #1 OR #2 OR #3 184 #5 MESH DESCRIPTOR Prophylactic Surgical Procedures EXPLODE ALL TREES 9 #6 Prophyla*:TI,AB,KY 27184 #7 Opportunistic:TI,AB,KY 2369 #8 MESH DESCRIPTOR Hysterectomy EXPLODE ALL TREES 1676 #9 hysterectom*:TI,AB,KY 4384 #10 #5 OR #6 OR #7 OR #8 OR #9 33183 #11 #4 AND #10 55
Appendix 3. MEDLINE search strategy
Searched from 1946 to 8 January 2019
OVID Platform
1 Salpingectomy/ (1066) 2 salpingectom*.tw. (1814) 3 salpingectom*.kf. (197) 4 ((tubal adj3 excision*) or tubectom*).tw. (212) 5 ((tubal adj3 excision*) or tubectom*).kf. (41) 6 or/1‐5 (2716) 7 Ovarian Neoplasms/ (75583) 8 (ovar* adj3 (serou* or cancer* or carcinom* or neoplas* or malignanc* or malignant)).tw. (69647) 9 (ovar* adj3 (serou* or cancer* or carcinom* or neoplas* or malignanc* or malignant)).kf. (11190) 10 or/7‐9 (97468) 11 Postoperative Complications/ (341206) 12 (Post?operative adj2 Complication*).tw. (63167) 13 Post?operative complication*.kf. (6981) 14 Intraoperative Complications/ (30460) 15 (Intra?operative adj2 Complication*).tw. (8424) 16 (Intra?operative adj2 Complication*).kf. (210) 17 (peri?operative adj2 complication*).tw. (9727) 18 perioperative complication*.kf. (155) 19 surgical injur*.tw. (897) 20 Blood loss, surgical/ (16280) 21 blood loss.tw. (46567) 22 h?emorrhage*.tw. (174588) 23 infection/ (36969) 24 infectio*.tw. (1380789) 25 or/11‐24 (1943525) 26 Ovarian reserve/ (693) 27 ovarian reserve*.tw. (2614) 28 Follicle Stimulating Hormone/ (35194) 29 Follicle Stimulating Hormone*.tw. (18425) 30 fsh.tw. (33119) 31 follitropin.tw. (624) 32 primary ovarian insufficiency/ (2352) 33 ovarian insufficiency.tw. (1081) 34 Anti‐mullerian hormone/ (2700) 35 (anti?mullerian or mullerian?inhibiting or mullerian regression or AMH).tw. (3407) 36 ovarian failure.tw. (3574) 37 or/26‐36 (58918) 38 10 or 25 or 37 (2095299) 39 Prophylactic Surgical Procedures/ and (fallopian or tubal or tubes or tube).tw. (27) 40 Prophyla*.tw. (152189) 41 Opportunistic.tw. (34444) 42 or/39‐41 (184781) 43 38 or 42 (2202041) 44 6 and 43 (1054)
Appendix 4. Embase search strategy
Searched from 1980 to 8 January 2019
OVID Platform
1 salpingectomy/ (4155) 2 salpingectom*.tw. (2885) 3 ((tubal adj3 excision*) or tubectom*).tw. (186) 4 (tubal adj3 remov*).tw. (59) 5 (tube* adj3 remov*).tw. (5253) 6 or/1‐5 (9991) 7 ovary tumor/ (24311) 8 (ovar* adj3 (serou* or cancer* or carcinom* or neoplas* or malignanc* or malignant)).tw. (95864) 9 or/7‐8 (108104) 10 postoperative complication/ (291673) 11 (Post?operative adj2 Complication*).tw. (86713) 12 peroperative complication/ (38226) 13 (Intra?operative adj2 Complication*).tw. (13318) 14 (peri?operative adj2 complication*).tw. (14851) 15 (peroperative adj2 complication*).tw. (280) 16 surgical injur*.tw. (1092) 17 operative blood loss/ (17703) 18 blood loss.tw. (72255) 19 h?emorrhage*.tw. (218438) 20 infection/ (288994) 21 infectio*.tw. (1667269) 22 or/10‐21 (2278821) 23 ovarian reserve/ (5064) 24 ovarian reserve*.tw. (5458) 25 follitropin/ (50783) 26 Follicle Stimulating Hormone*.tw. (19308) 27 fsh.tw. (40767) 28 follitropin.tw. (760) 29 premature ovarian failure/ (4105) 30 ovarian insufficiency.tw. (1615) 31 ovarian failure.tw. (5208) 32 Muellerian inhibiting factor/ (6014) 33 (anti?mullerian or mullerian?inhibiting or mullerian regression or AMH).tw. (6637) 34 or/23‐33 (77327) 35 prophylactic surgical procedure/ (442) 36 Prophyla*.tw. (205019) 37 Opportunistic.tw. (43092) 38 or/35‐37 (245559) 39 9 or 22 or 34 or 38 (2598824) 40 6 and 39 (3200)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 8 January 2019
OVID Platform
1 salpingectom*.tw. (18) 2 ((tubal adj3 excision*) or tubectom*).tw. (14) 3 or/1‐2 (32)
Appendix 6. CINAHL search strategy
Searched from 1961 to 8 January 2019
EBSCO Platform
S11 S5 AND S10 223 S10 S6 OR S7 OR S8 OR S9 45,050 S9 TX Opportunistic 7,012 S8 TX Prophyla* 29,414 S7 TX hysterectom* 9,505 S6 (MM "Hysterectomy+") 3,582 S5 S1 OR S2 OR S3 OR S4 579 S4 TX (tubes N2 excision*) 3 S3 TX ((tubal N2 excision*) or tubectom*) 24 S2 TX salpingectom* 559 S1 (MM "Salpingectomy") 199
Appendix 7. Clinical trial registries search strategy
Searched May 2018
Web platform
clinicaltrials.gov/ (a service of the US National Institutes of Health);
who.int/trialsearch/default.aspx (the World Health Organization International Trials Registry Platform search portal).
'Salpingectomy AND hysterectomy'
Data and analyses
Comparison 1. Hysterectomy with opportunistic salpingectomy versus hysterectomy without bilateral salpingectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgery‐related adverse events | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Intraoperative complications | 5 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.11, 3.94] |
| 1.2 Short‐term postoperative complications | 3 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.14] |
| 2 Postoperative hormonal status (AMH) | 5 | 283 | Mean Difference (Fixed, 95% CI) | ‐0.94 [‐1.89, 0.01] |
| 3 Postoperative hormonal status (AMH per time point) | 5 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 AMH 4‐6 weeks postoperative | 1 | 23 | Mean Difference (Fixed, 95% CI) | ‐1.57 [‐11.09, 7.95] |
| 3.2 AMH 3 months postoperative | 4 | 179 | Mean Difference (Fixed, 95% CI) | ‐1.16 [‐2.89, 0.56] |
| 3.3 AMH 6 months postoperative | 1 | 104 | Mean Difference (Fixed, 95% CI) | ‐0.65 [‐2.00, 0.70] |
| 4 Postoperative hormonal status (FSH) | 4 | 145 | Mean Difference (Fixed, 95% CI) | ‐0.59 [‐1.58, 0.40] |
| 5 Postoperative hormonal status (FSH per time point) | 4 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 5.1 FSH 1 month postoperative | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐1.0 [‐2.28, 0.28] |
| 5.2 FSH 3 months postoperative | 2 | 84 | Mean Difference (Fixed, 95% CI) | 0.24 [‐1.18, 1.66] |
| 5.3 FSH 6 months postoperative | 2 | 61 | Mean Difference (Fixed, 95% CI) | ‐1.27 [‐2.62, 0.08] |
| 6 Postoperative hormonal status (LH) | 3 | 115 | Mean Difference (Fixed, 95% CI) | ‐0.73 [‐2.14, 0.68] |
| 7 Postoperative hormonal status (LH per time point | 3 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 7.1 LH 1 month postoperative | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐0.4 [‐1.84, 1.04] |
| 7.2 LH 3 months postoperative | 1 | 54 | Mean Difference (Fixed, 95% CI) | 1.37 [‐7.69, 10.43] |
| 7.3 LH 6 months postoperative | 2 | 61 | Mean Difference (Fixed, 95% CI) | ‐0.78 [‐2.21, 0.65] |
| 8 Postoperative hormonal status (estradiol) | 2 | 78 | Mean Difference (Fixed, 95% CI) | 4.51 [‐28.96, 37.98] |
| 9 Postoperative hormonal status (estradiol per time point) | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 9.1 Estradiol 1 month postoperative | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐5.0 [‐41.46, 31.46] |
| 9.2 Estradiol 3 months postoperative | 1 | 54 | Mean Difference (Fixed, 95% CI) | 62.22 [‐296.14, 420.58] |
| 9.3 Estradiol 6 months postoperative | 1 | 24 | Mean Difference (Fixed, 95% CI) | 4.0 [‐29.62, 37.62] |
| 10 Total surgical time | 5 | 286 | Mean Difference (Fixed, 95% CI) | 0.35 [‐6.64, 7.33] |
| 11 Estimated blood loss | 5 | 286 | Mean Difference (Fixed, 95% CI) | ‐3.25 [‐16.09, 9.59] |
| 12 Conversion rate to open surgery | 2 | 172 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.11, 3.94] |
| 13 Duration of hospital admission | 3 | 226 | Mean Difference (Fixed, 95% CI) | ‐0.02 [‐0.22, 0.17] |
| 14 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 Mental health | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐1.32 [‐3.00, 2.36] |
| 14.2 Physical health | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐1.01 [‐4.29, 2.27] |
Comparison 2. Subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of intraoperative adverse events depending on surgical approach | 5 | 284 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.12, 4.11] |
| 1.1 Abdominal hysterectomy | 2 | 109 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.05, 2.82] |
| 1.2 Laparoscopic hysterectomy | 4 | 175 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.80 [0.13, 343.88] |
Comparison 3. Sensitivity analysis (random‐effects model).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative hormonal status (AMH) | 5 | Mean Difference (Random, 95% CI) | ‐0.94 [‐1.89, 0.01] |
Comparison 4. Sensitivity analysis (skewed data).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative hormonal status (AMH) | 5 | Mean Difference (Fixed, 95% CI) | ‐0.25 [‐0.43, ‐0.06] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Behnamfar 2017.
| Methods | Design: single‐centre, two‐arm parallel group trial Randomisation: simple sampling, details unclear Total number randomised: n = 40 Withdrawals and exclusions: n = 3 (participants from intervention group did not return for 6 months follow‐up) Funding: none reported |
|
| Participants | Participants undergoing hysterectomy for benign reasons. Mean age: 48.5 (SD 2.03) years in the intervention group and 47.7 (SD 3.03) in the control group Inclusion criteria: regular menstruation cycle, no history of malignancy, not postmenopausal, underlying reasons of myxomatosis uterus or menorrhagia Exclusion criteria: operation cancelling, no accessibility of hormone measurement before or after operation due to any reason and postsurgical pathology of malignancy |
|
| Interventions |
Intervention: hysterectomy with bilateral salpingectomy Control: hysterectomy with preservation of the fallopian tubes |
|
| Outcomes |
|
|
| Notes | Posthoc registration of protocol in trial registry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Simple sampling, further details unclear |
| Allocation concealment (selection bias) | Unclear risk | Participants were enrolled in a list, further details unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial loss to follow‐up in intervention group; 17% |
| Selective reporting (reporting bias) | High risk | AMH mentioned as outcome in posthoc registered protocol, not reported in the results of the study |
| Other bias | Unclear risk | Clustering effect among surgeons unclear |
Findley 2013.
| Methods | Design: single‐centre, two‐arm parallel group trial Randomisation: computerised random number generation Total number randomised: n = 30 Withdrawals and exclusions: after 4 to 6 weeks 4 lost to follow‐up in the intervention group and 3 in the control group. After 3 months 1 lost to follow‐up in the intervention group and 2 in the control group Funding: training grant from the National Institutes of Health at the University of North Carolina |
|
| Participants | Premenopausal women planning to undergo elective laparoscopic hysterectomy for benign indications with planned preservation of the ovaries. Mean age: 37.2 (SD 4.7) years Inclusion criteria: premenopausal, age 18 to 45 years Exclusion criteria: personal history of gynaecologic malignancy, known BRCA1/2 carriers or non‐English speaking participants |
|
| Interventions |
Intervention: laparoscopic hysterectomy with ovarian preservation with salpingectomy Control: laparoscopic hysterectomy with ovarian preservation without salpingectomy |
|
| Outcomes |
|
|
| Notes | Pilot trial In protocol AMH measurement at 1 month, in article at 4 to 6 weeks postoperatively |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number generator, allocation sequence of random, permuted blocks of 4 and 6 |
| Allocation concealment (selection bias) | Low risk | Procedure indicator cards inside sequentially numbered, opaque, sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial loss to follow‐up; data of 23% of the original population missing at 4 to 6 weeks and data of 10% of the original population missing at 3 months postoperatively |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as per protocol in clinical trial registry |
| Other bias | Unclear risk | Clustering effect among surgeons unclear |
Popov 2015.
| Methods | Design: Single‐centre, two‐arm parallel group trial Randomisation: method not given Total number randomised: n = 54 Withdrawals and exclusions: 0 Funding: budget from the Moscow Regional Research Institute of Obstetrics and Gynaecology |
|
| Participants | Women planning to undergo a laparoscopic hysterectomy. Mean age: 44 years in the intervention group and 45 years in the control group Inclusion criteria: age 18 to 50 years, menstrual cycle Exclusion criteria: malignancy, pregnancy, infection of clinical significance and high risk of morbidity (American Society of Anaesthesiologists (ASA) classification IV or V) |
|
| Interventions |
Intervention: hysterectomy with bilateral salpingectomy Control: hysterectomy without bilateral salpingectomy |
|
| Outcomes |
|
|
| Notes | Not registered in clinical trial registry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not given |
| Allocation concealment (selection bias) | Unclear risk | Method not given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not registered; no protocol available for comparison |
| Other bias | Unclear risk | Clustering effect among surgeons unclear |
Sezik 2007.
| Methods | Design: Two‐arm, parallel group trial Randomisation: method not given Total number randomised: n = 24 Withdrawals and exclusions: 0 Funding: none reported |
|
| Participants | Women scheduled for hysterectomy without oophorectomy. Mean age: 41.6 (SD 1.7) years in the intervention group and 41.1 (SD 1.4) years in the control group Inclusion criteria: age under 43 years, absence of menopausal symptoms, regular (every 22 to 34 days without any breakthrough bleeding) menstrual cycles, baseline FSH value of < 10 IU/mL and mean ovarian volume > 5m3 Exclusion criteria: present or past smoking history, hormone replacement treatment and/or hormonal contraception for the last 6 months, history of pelvic surgery, cardiovascular disease and cystic (> 10 mm) or any solid ovarian mass in transvaginal ultrasound |
|
| Interventions |
Intervention: total abdominal hysterectomy and complete excision of the fallopian tubes bilaterally Control: total abdominal hysterectomy with conservation of the paraovarian fallopian tube |
|
| Outcomes |
|
|
| Notes | Not registered in trial registry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not given |
| Allocation concealment (selection bias) | Unclear risk | Method not given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not registered; no protocol available for comparison |
| Other bias | Unclear risk | Clustering effect among surgeons unclear |
Song 2016.
| Methods | Design: multicentre, two‐arm parallel group trial Randomisation: random allocation on a 1:1 basis with stratification by institution Total number randomised: n = 68 Withdrawals and exclusions: 0 Funding: none reported |
|
| Participants | Participants who were planning to undergo laparoscopic hysterectomy for benign uterine diseases. Mean age: 42.9 (SD 4.2) years Inclusion criteria: age 19 to 52 years, regular menstruation (defined as duration of menstruation cycle between 21 and 45 days), appropriate medical status for laparoscopic surgery Exclusion criteria: any ovarian cysts requiring ovarian surgery, any suspicious finding of malignant gynaecologic diseases, history of prior salpingectomy or salpingo‐oophorectomy, pregnant or menopausal status, preoperative AMH under 0.30 ng/mL, use of hormonal treatments within 3 months before surgery, any other endocrine disease or inability to understand and provide written informed consent |
|
| Interventions |
Intervention: laparoscopic hysterectomy with opportunistic salpingectomy Control: laparoscopic hysterectomy without opportunistic salpingectomy |
|
| Outcomes |
|
|
| Notes | Posthoc changes in sample size reported in protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence was generated prior to initiation of the study using an interactive internet‐based response system. Random allocation on a 1:1 basis with stratification by institution |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes. After participants signed consent, the clinician called the trial office who opened the envelope and informed the clinician. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sample size retrospectively amended in trial registry |
| Selective reporting (reporting bias) | High risk | Primary outcome changed from AMH concentration to change in AMH concentration retrospectively in trial registry |
| Other bias | Unclear risk | Clustering effect among surgeons unclear |
Tehranian 2017.
| Methods | Design: single‐centre, two‐arm parallel group trial Randomisation: sequence generation with proprietary computer application according to randomised block design Total number randomised: n = 30 Withdrawals and exclusions: 0 Funding: none reported |
|
| Participants | Premenopausal women who were undergoing abdominal hysterectomy for non‐malignant gynaecologic disease with preservation of the ovaries. Mean age: 40.13 (95% CI 38.88 to 41.38) years Inclusion criteria: age under 45 years, elective hysterectomy without oophorectomy, absence of menopausal symptoms, baseline FSH value of < 10 IU/mL Exclusion criteria: history of pelvic surgery, cystic (< 10 mm) or any solid ovarian mass in transvaginal ultrasound, hormone replacement treatment and/or hormonal contraception for the last 6 months, present or past smoking history |
|
| Interventions |
Intervention: hysterectomy with bilateral salpingectomy Control: hysterectomy without bilateral salpingectomy |
|
| Outcomes |
|
|
| Notes | In protocol, sample size was set at 40 participants in total | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number sequence, generated with a proprietary computer application, according to randomised block design |
| Allocation concealment (selection bias) | Low risk | Procedure indication cards inside a set of numbered, opaque, sealed envelops. None of the staff had access to the codes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Posthoc registration of protocol in clinical trial registry; sample size in registered protocol set at 40 participants |
| Selective reporting (reporting bias) | Unclear risk | Posthoc registration of protocol |
| Other bias | Unclear risk | Clustering effect among surgeons unclear |
van Lieshout 2018.
| Methods | Design: multicentre, two‐arm parallel group trial Randomisation: online randomisation tool Total number randomised: n = 104 Withdrawals and exclusions: 6 in intervention group (1 dropped out and 5 lost to follow‐up) and 9 in control group (1 dropped out and 7 lost to follow‐up) Funding: grant from the Elisabeth‐Tweesteden Hospital Research Fund |
|
| Participants | Women with an indication for either laparoscopic or abdominal hysterectomy for benign indications (such as fibroids or bleeding disorders). Mean age: 44.0 (SD 0.5) years in the intervention group and 44.6 (SD 0.7) years in the control group Inclusion criteria: premenopausal women, age 30 to 55 years, indication for laparoscopic or abdominal hysterectomy Exclusion criteria: a history of gynaecological malignancy or salpingitis, a known germline BRCA1/2 mutation, use of hormones in the three weeks prior to surgery, a form of hereditary cancer in the family history |
|
| Interventions |
Intervention: hysterectomy with bilateral salpingectomy Control: hysterectomy with preservation of the fallopian tubes |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generations with online randomisation tool |
| Allocation concealment (selection bias) | Low risk | Allocation with use of online randomisation tool |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High risk for AMH outcome due to substantial loss to follow‐up; 12% |
| Selective reporting (reporting bias) | Low risk | Reporting according to protocol in clinical trial registry |
| Other bias | Low risk | Clustering effect among surgeons assessed in additional analysis |
AMH: anti‐Müllerian hormone BMI: body mass index CI: confidence interval FSH: follicle stimulating hormone LH: luteinising hormone SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelkerim 2015 | Different intervention |
| Abernethy 2016 | Did not report outcomes of interest to this review |
| ACOG 2015 | Study design did not meet inclusion criteria |
| Addar 2005 | Did not report outcomes of interest to this review |
| Adelman 2018 | Study design did not meet inclusion criteria |
| Aggarwal 2017 | Study design did not meet inclusion criteria |
| Al‐Niaimi 2012 | Study design did not meet inclusion criteria |
| Almeida 2016 | Did not investigate the intervention of interest to this review |
| Anderson 2013 | Study design did not meet inclusion criteria |
| Andrade 2015 | Did not report outcomes of interest to this review |
| Angulo 2014 | Did not report outcomes of interest to this review |
| Antosh 2017 | Study design did not meet inclusion criteria |
| Arden 2012 | Did not investigate the intervention of interest to this review |
| Arvizo 2017 | Study design did not meet inclusion criteria |
| Atalay 2016 | Did not compare the intervention to a comparator of interest to this review |
| Backes 2014 | Study design did not meet inclusion criteria |
| Bakkum‐Gamez 2018 | Study design did not meet inclusion criteria |
| Balsarkar 2017 | Study design did not meet inclusion criteria |
| Batista 2012 | Did not investigate the intervention of interest to this review |
| Belaisch‐Allart 2006 | Study design did not meet inclusion criteria |
| Bell 2017 | Did not report outcomes of interest to this review |
| Berlit 2013 | Study design did not meet inclusion criteria |
| Bradley 2015 | Study design did not meet inclusion criteria |
| Brawley 2015 | Study design did not meet inclusion criteria |
| Brewer 2018 | Study design did not meet inclusion criteria |
| Cadish 2017a | Study design did not meet inclusion criteria |
| Cadish 2017b | Study design did not meet inclusion criteria |
| Carlin 2014 | Did not investigate the intervention of interest to this review |
| Chen 2018 | No data available for opportunistic salpingectomy specificically in adition to hysterectomy |
| Chene 2016 | Study design did not meet inclusion criteria |
| Cho 2012 | Did not investigate the intervention of interest to this review |
| Cooney 2015a | Did not report outcomes of interest to this review |
| Cooney 2015b | Study design did not meet inclusion criteria |
| Crum 2016 | Study design did not meet inclusion criteria |
| Daly 2015 | Study design did not meet inclusion criteria |
| Danilyants 2017 | Did not report outcomes of interest to this review |
| Davydov 1972 | Study design did not meet inclusion criteria |
| Dawle 1979 | Did not investigate the intervention of interest to this review |
| Desquesne 1996 | Did not investigate the intervention of interest to this review |
| Dietl 2011 | Study design did not meet inclusion criteria |
| Dietl 2014a | Study design did not meet inclusion criteria |
| Dietl 2014b | Study design did not meet inclusion criteria |
| Dwyer 2012 | Study design did not meet inclusion criteria |
| Ebeid 2013 | Did not report outcomes of interest to this review |
| ESGE 2015 | Study design did not meet inclusion criteria |
| Falconer 2015 | No data available for salpingectomy specifically in addition to hysterectomy |
| Fathalla 2017 | Study design did not meet inclusion criteria |
| Foulkes 2013 | Study design did not meet inclusion criteria |
| Frishman 2013 | Study design did not meet inclusion criteria |
| Garcia 2016 | Did not report outcomes of interest to this review |
| Ghezzi 2009 | Study design did not meet inclusion criteria |
| Giannakeas 2015 | Did not investigate the intervention of interest to this review |
| Gierach 2014 | Did not investigate the intervention of interest to this review |
| Gilks 2013 | Study design did not meet inclusion criteria |
| Hanley 2015 | Study design did not meet inclusion criteria |
| Hanley 2017 | Did not report outcomes of interest to this review |
| Harmsen 2015 | Study population was not of interest to this review |
| Harris 2015 | Study design did not meet inclusion criteria |
| He 2017 | Did not report outcomes of interest to this review |
| Herzog 2013 | Study design did not meet inclusion criteria |
| Joshi 1979 | Did not investigate the intervention of interest to this review |
| Kaplan 2015 | Study design did not meet inclusion criteria |
| Kershenovich 2016 | Study design did not meet inclusion criteria |
| Kolmorgen 1993 | Did not investigate the intervention of interest to this review |
| Kotsopoulos 2013 | Study design did not meet inclusion criteria |
| Kwon 2015 | Study design did not meet inclusion criteria |
| Lamblin 2018 | Did not report outcomes of interest to this review |
| Leblanc 2014 | Study population was not of interest to this review |
| Lessard‐Anderson 2014 | No data available for salpingectomy specifically in addition to hysterectomy |
| Madsen 2015 | No data available for salpingectomy specifically in addition to hysterectomy |
| Manchandra 2017 | Study design did not meet inclusion criteria |
| Maroni 1980 | Did not investigate the intervention of interest to this review |
| Maseela 1980 | Study design did not meet inclusion criteria |
| Matthews 2016 | Study design did not meet inclusion criteria |
| McAlpine 2014 | Study design did not meet inclusion criteria |
| McAlpine 2016 | Study design did not meet inclusion criteria |
| Mettler 2016 | Did not compare the intervention to a comparator of interest to this review |
| Mikhail 2016 | Did not report outcomes of interest to this review |
| Minig 2015 | Did not report outcomes of interest to this review |
| Morelli 2013a | Study design did not meet inclusion criteria |
| Morelli 2013b | Study design did not meet inclusion criteria |
| Naaman 2017 | Study design did not meet inclusion criteria |
| Nair 1991 | Did not investigate the intervention of interest to this review |
| Nandakumar 1995 | Did not investigate the intervention of interest to this review |
| Narod 2013 | Study design did not meet inclusion criteria |
| NCT02284711 | Did not compare the intervention to a comparator of interest to this review |
| Nezhat 2019 | Study design did not meet inclusion criteria |
| Paul 2018 | Study population was not of interest to this review |
| Perez‐Lopez 2016 | Study design did not meet inclusion criteria |
| Petrov 2016 | Study population was not of interest to this review |
| Philipp 1980 | Did not compare the intervention to a comparator of interest to this review |
| Poole 2015 | Study design did not meet inclusion criteria |
| Pursell 2016 | Study design did not meet inclusion criteria |
| Ranney 1978 | Did not investigate the intervention of interest to this review |
| Rodriguez‐Triana 2013 | Did not report outcomes of interest to this review |
| Ruiz 2016 | Did not investigate the intervention of interest to this review |
| Saunders 2017 | Study design did not meet inclusion criteria |
| Setubal 2017 | Study design did not meet inclusion criteria |
| Siedhoff 2012 | Did not report outcomes of interest to this review |
| Singh 1971 | Did not investigate the intervention of interest to this review |
| Singh 2017 | Study design did not meet inclusion criteria |
| Skorupska 2016 | Did not report outcomes of interest to this review |
| Szender 2015 | Study design did not meet inclusion criteria |
| Tanner 2013 | Study design did not meet inclusion criteria |
| Tellawi 2013 | Study design did not meet inclusion criteria |
| Terada 2016 | Did not investigate the intervention of interest to this review |
| Walsh 2018 | Study design did not meet inclusion criteria |
| Wierrani 1993 | Study design did not meet inclusion criteria (non‐randomised) |
| Yi 2012 | Study design did not meet inclusion criteria (non‐randomised) |
Characteristics of ongoing studies [ordered by study ID]
NCT01628432.
| Trial name or title | Effect of total salpingectomy during conservative hysterectomy for benign disease on ovarian function: non inferiority randomized controlled trial |
| Methods | Design: two‐arm, parallel group trial Planned total number randomised: n = 350 |
| Participants | Women having hysterectomies for benign disease with failure of conservative treatment Inclusion criteria: age between 18 and 52 years, signed informed consent, nonmenopausal women (AMH > 0.21 ng/mL) Exclusion criteria: pregnancy, desire of future pregnancy, participant unable to give informed consent, any physical or psychiatric condition that could impair the participants ability to co‐operate with postoperative data collection, previous salpingo an/or oophorectomy (unilateral or bilateral), genital cancer disease or atypical endometrial hyperplasia, hyperandrogenism, any ovarian mass that needs surgical exploration, any immunotherapy that could interfere with immunological tests |
| Interventions |
Intervention: bilateral salpingectomy during conservative hysterectomy with conservation of the ovaries Control: standard conservative hysterectomy with conservation of both ovaries and tubes |
| Outcomes |
|
| Starting date | July 2012 |
| Contact information | Unknown |
| Notes | Estimated primary completion date: August 2017 Estimated study completion date: October 2018 |
NCT02086344.
| Trial name or title | Ovarian reserve modification after laparoscopic hysterectomy with bilateral salpingectomy |
| Methods | Design: two‐arm, parallel group trial Planned total number randomised: n = 167 |
| Participants | Women planning hysterectomy for benign reasons Inclusion criteria: indication for laparoscopic hysterectomy, accomplished reproductive desire Exclusion criteria: family history of ovarian cancer or a known BRCA1/2 mutation, current or past history of cancer, no consent for opportunistic salpingectomy, previous adnexal surgery, polycystic ovary syndrome, oestrogen‐progestin therapy in the two months prior to enrolment, acute or chronic pelvic inflammatory disorders, malignant gynaecological neoplasms, prior chemotherapy or radiotherapy, autoimmune diseases, chronic, metabolic and systemic disorders, including hyperandrogenism, hyperprolactinaemia, diabetes mellitus and thyroid disease, hypogonadotropic hypogonadism, taking medication that can cause menstrual irregularities, other clinical conditions |
| Interventions |
Intervention: standard total laparoscopic hysterectomy with prophylactic bilateral salpingectomy Control: standard total laparoscopic hysterectomy without prophylactic bilateral salpingectomy |
| Outcomes |
|
| Starting date | February 2014 |
| Contact information | Prof. F. Zullo Department of Obstetrics and Gynaecology, university division UMG, Catanzaro, Italy |
| Notes | Estimated primary completion date: December 2016 Estimated study completion date: December 2016, however according to clinicaltrials.gov still recruiting |
NCT03045965.
| Trial name or title | Hysterectomy and OPPortunistic Salpingectomy |
| Methods | Design: two‐arm, parallel group trial Planned total number randomised: n = 4400 |
| Participants | Women planning hysterectomy for benign reasons Inclusion criteria: age between 20 and 54 years, willingness to be randomised, vaginal route may be included if the surgeon is confident with performing vaginal salpingectomy Exclusion criteria: previous bilateral oophorectomy and/or salpingectomy, planned oophorectomy and/or salpingectomy (for reasons such as known carriers of BRCA1/2 gene mutations or Lynch syndrome), non‐understanding of written study information |
| Interventions |
Intervention: hysterectomy (laparoscopic, laparotomic or vaginal) with bilateral salpingectomy Control: hysterectomy (laparoscopic, laparotomic, or vaginal) without bilateral salpingectomy |
| Outcomes |
|
| Starting date | June 2017 |
| Contact information | Dr. A Strandell Department of Obstetrics and Gynecology, University of Gothenburg, Sweden |
| Notes | Estimated primary completion date: June 2021 Estimated study completion date: December 2050 |
AFC: antral follicle count AMH: anti‐Müllerian hormone ∆AMH: difference in pre‐ and postoperative AMH concentration FI: flow index FSH: follicle stimulating hormone OV: ovarian volume VFI: vacularisation flow index VI: vascularisation index
Differences between protocol and review
For surgery‐related adverse events, some studies reported all adverse events while others stated no adverse events were related to the salpingectomy. To enable pooling of data we decided only to include complications attributed to salpingectomy. As the event rate was very low, we decided to used Peto odds ratio instead of Mantel Haenszel odds ratio.
In the protocol, we indicated a preference for postoperative anti‐Müllerian hormone (AMH) concentrations as a measure for postoperative hormonal status. However, due to the strong correlation of pre‐ and postoperative AMH concentrations in the individual participant data analysis we decided to use the difference in AMH concentration (∆AMH), or the postoperative value adjusted for baseline measures, instead of postoperative values, where possible.
Although many studies did use AMH, some had chosen otherwise, or reported several other measures as well. We did not expect the wide range of outcome measures, including some outcome measures without evidence of a correlation with time to menopause. We decided to make a selection of outcome measures rather than extracting data of all used measures. As other hormonal values also have a correlation with time to menopause, and they were used in several studies at a time, we decided to include follicle stimulating hormone (FSH), luteinising hormone (LH) and estradiol. In addition, the duration of follow‐up varied between studies. We made a posthoc decision to report hormonal outcomes per time point, in addition to reporting the outcomes at the end of follow‐up.
The decision to perform a complier analysis, estimating the effect of undergoing (rather than being allocated to) the procedure was also determined posthoc. Similarly, the sensitivity analysis performed in light of the skewed AMH data was also not prespecified.
Contributions of authors
JP conceived the idea for this study. The objectives and methods were further clarified by JP, JdH, MCV, JW, SH, LvL and MS. JW, LvL, MS drafted the manuscript. JP, JdH MCV and SH reviewed the manuscript and provided critical feedback.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
JP, JdH, MCV, JW, SH and MS have no interests to declare.
LvL is first author of the included study, van Lieshout 2018. She took no part in selecting the study for inclusion, or in extracting and entering data from it.
Dual first author
Dual first author
Edited (no change to conclusions)
References
References to studies included in this review
Behnamfar 2017 {published data only}
- Behnamfar F, Jabbari H. Evaluation of ovarian function after hysterectomy with or without salpingectomy: A feasible study. Journal of Research in Medical Sciences 2017;22:68. [DOI: 10.4103/jrms.JRMS_81_17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Findley 2013 {published data only}
- Findley AD, Siedhoff MT, Hobbs KA, Steege JF, Carey ET, McCall CA, et al. Short‐term effects of salpingectomy during laparoscopic hysterectomy on ovarian reserve: a pilot randomized controlled trial. Fertility and Sterility 2013;100:1704‐08. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Popov 2015 {published data only}
- Popov AA, Slobodyanyuk BA, Koval AA, Barto RA, Mironenko KV. The effect of laparoscopic hysterectomy with salpingectomy on ovarian function: the results of randomized clinical trial [Влияние лапароскопической гистерэктомии с маточными трубами на функциональное состояние яичников: результаты рандомизированного клинического исследования]. Коллектив авторов 2015;4:35‐48. [DOI: 10.17116/repro201521435-42] [DOI] [Google Scholar]
Sezik 2007 {published data only}
- Sezik M, Ozkaya O, Demir F, Sezik HT, Kaya H. Total salpingectomy during abdominal hysterectomy: Effects on ovarian reserve and ovarian stromal blood flow. Journal of Obstetrics and Gynaecology Research 2007;33:863‐9. [DOI: 10.1111/j.1447-0756.2007.00669.x] [DOI] [PubMed] [Google Scholar]
Song 2016 {published data only}
- Song T, Kim MK, Kim M‐L, Jung YW, Yun BS, Seong SJ, et al. Impact of opportunistic salpingectomy on anti‐Müllerian hormone in patients undergoing laparoscopic hysterectomy: a multicentre randomised controlled trial. British Journal of Obstetrics and Gynaecology 2017;124:314‐20. [DOI] [PubMed] [Google Scholar]
Tehranian 2017 {published data only}
- Tehranian A, Zangbar RH, Aghajani F, Sepidarkish M, Rafiei S, Esfidani T. Effects of salpingectomy during abdominal hysterectomy on ovarian reserve: a randomized controlled trial. Gynecological Surgery 2017;14:17. [DOI: 10.1186/s10397-017-1019-z; IRCT2014123118866N4] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Lieshout 2018 {published and unpublished data}
- Lieshout LA, Pijlman B, Vos MC, Groot MJM, Houterman S, Coppus SF, et al. Opportunistic salpingectomy in women undergoing hysterectomy: Results from the HYSTUB randomised controlled trial. Maturitas 2018;107:1‐6. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdelkerim 2015 {published data only}
- Abdelkerim A. Gynaecological laparoscopic surgery in regional hospitals Noth Kordofan State, Sudan, from 2008 to 2014. International Journal of Gynecology and Obstetrics 2015;131:E277‐8. [Google Scholar]
Abernethy 2016 {published data only}
- Abernethy M, Vieira BL, Kim JY, Drury KE, Kenton K. The low risk of concomitant adnexal surgery. American Journal of Obstetrics and Gynecology 2016;214:S469. [Google Scholar]
ACOG 2015 {published data only}
- ACOG. Salpingectomy for ovarian cancer prevention. Obstetrics and Gynecology 2015;125:279‐81. [DOI] [PubMed] [Google Scholar]
Addar 2005 {published data only}
- Addar MH, Al‐Motawa JA. Factors associated with complications in gynecological surgeries. Saudi Medical Journal 2005;26:1420‐3. [PubMed] [Google Scholar]
Adelman 2018 {published data only}
- Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. American Journal of øbstetrics and Gynecology 2018;218:269‐79. [DOI] [PubMed] [Google Scholar]
Aggarwal 2017 {published data only}
- Aggarwal N, Sharma S. Opportunistic salpingectomy: Remove the tubes and save the ovaries. Journal of mid‐life health 2016;7:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Almeida 2016 {published data only}
- Almeida A, Brandao P, Ramoa P. Transvaginal NOTES in adnexal surgery ‐ Case series. Gynecological Surgery 2016;13:S116. [Google Scholar]
Al‐Niaimi 2012 {published data only}
- Al‐Niaimi AN, Ahmed M, Petersen CB. Epithelial ovarian cancer. Obstetrics and Gynecology Clinics of North America 2012;39:269‐83. [DOI] [PubMed] [Google Scholar]
Anderson 2013 {published data only}
- Anderson CK, Wallace S, Guiahi M, Sheeder J, Behbakht K, Spillman MA. Risk‐reducing salpingectomy as preventive strategy for pelvic serous cancer. International Journal of Gynecological Cancer 2013;23:417‐21. [DOI] [PubMed] [Google Scholar]
Andrade 2015 {published data only}
- Andrade A, Nogueira B, Reis J, Faustino F. Total laparoscopic hysterectomy ‐ starting from zero. Gynecological Surgery 2015;12:S473. [Google Scholar]
Angulo 2014 {published data only}
- Angulo AC, Rivero JA, Bosque VA, Araujo M, Barriola V. Incidence of tubal precancerous lesions in patients undergoing benign gynecological procedures. Journal of minimally invasive gynecology 2014;21:S153. [Google Scholar]
Antosh 2017 {published data only}
- Antosh D, High R, Brown HW, Oliphant SS, Abed H, Grimes C. Prophylactic salpingectomy during vaginal hysterectomy: A feasibility study. American Journal of Obstetrics and Gynecology 2017;216:S568‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arden 2012 {published data only}
- Arden D, Seifert E, Donnellan N, Mansuria S, Ted L, Guido R. Intra‐peritoneal instillation of bupivacaine for reduction of post‐operative pain after laparoscopic hysterectomy: A double‐blind randomized controlled trial. Journal of Minimally Invasive Gynecology 2012;19:S52. [DOI] [PubMed] [Google Scholar]
Arvizo 2017 {published data only}
- Arvizo C, Uy‐Kroh MJ. Bilateral salpingectomy using percutaneous minilaparoscopy. Journal of Minimally Invasive Gynecology 2017;24:194‐5. [DOI] [PubMed] [Google Scholar]
Atalay 2016 {published data only}
- Atalay MA, Cetinkaya Demir B, Ozerkan K. Change in the ovarian environment after hysterectomy with bilateral salpingectomy: is it the technique or surgery itself?. European Journal of Obstetrics, Gynecology and Reproductive Biology 2016;204:57‐61. [DOI] [PubMed] [Google Scholar]
Backes 2014 {published data only}
- Backes FJ. Salpingectomy, why not?. American Journal of Obstetrics and Gynecology 2014;210:385‐6. [DOI] [PubMed] [Google Scholar]
Bakkum‐Gamez 2018 {published data only}
- Bakkum‐Gamez JN, Cliby WA. Opportunistic salpingectomy to decrease the mortality from ovarian cancer: Can we expand the pool of eligible patients?. Surgery 2018;164:935‐6. [DOI] [PubMed] [Google Scholar]
Balsarkar 2017 {published data only}
- Balsarkar G. Opportunistic salpingectomy as an ovarian cancer primary prevention strategy. Journal of Obstetrics and Gynaecology of India 2017;67:243‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Batista 2012 {published data only}
- Batista CS, Osako T, Clemente EM, Batista FC, Osako MT. Observational evaluation of preoperative, intraoperative, and postoperative characteristics in 117 Brazilian women without uterine prolapse undergoing vaginal hysterectomy. International Journal of Women's Health 2012;4:505‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Belaisch‐Allart 2006 {published data only}
- Belaisch‐Allart J, Mayenga JM, Castaing N, Allart JP. Is tubal and uterine surgery deleterious to ovarian reserve?. Gynecologie Obstetrique et Fertilite 2006;34:1111‐7. [DOI] [PubMed] [Google Scholar]
Bell 2017 {published data only}
- Bell K. Retrospective review of prophylactic bilateral salpingectomy: Evaluation of rates & perioperative complications. Obstetrics and Gynecology 2017;129:85S‐6S. [Google Scholar]
Berlit 2013 {published data only}
- Berlit S, Tuschy B, Kehl S, Brade J, Sutterlin M, Hornemann A. Laparoscopic supracervical hysterectomy with concomitant bilateral salpingectomy ‐ why not?. Anticancer Research 2013;33:2771‐4. [PubMed] [Google Scholar]
Bradley 2015 {published data only}
- Bradley MS, Visco AG. Role of salpingectomy at the time of urogynecologic surgery. Current Opinion in Obstetrics & Gynecology 2015;23:385‐9. [DOI] [PubMed] [Google Scholar]
Brawley 2015 {published data only}
- Brawley OW. Ovarian cancer prevention: Time for primetime?. Cancer 2015;121:2121‐3. [DOI] [PubMed] [Google Scholar]
Brewer 2018 {published data only}
- Brewer MA. The debate çontinues on salpingectomy. www.reliasmedia.com/articles/142078‐the‐debate‐continues‐on‐salpingectomy 2018.
Cadish 2017a {published data only}
- Cadish LA, Shepherd JP, Barber EL, Ridgeway B. Risks and benefits of opportunistic salpingectomy during vaginal hysterectomy: A cost‐effectiveness analysis. American Journal of Obstetrics and Gynecology 2017;216:S568. [DOI] [PubMed] [Google Scholar]
Cadish 2017b {published data only}
- Cadish LA, Shepherd JP, Barber E, Ridgeway B. Risks and benefits of opportunistic salpingectomy during vaginal hysterectomy: a decision analysis. American Journal of Obstetrics and Gynecology 2017;217:603.e1‐.e6. [DOI] [PubMed] [Google Scholar]
Carlin 2014 {published data only}
- Carlin R, Berlanda V, Dalpra F, Perin E, Mereu L, Tateo S. Outcomes of total laparoscopic hysterectomy: Impact of uterine size. Gynecological Surgery 2014;11:250‐1. [Google Scholar]
Chen 2018 {published data only (unpublished sought but not used)}
- Chen Y, Du H, Bao L, Liu W. Opportunistic salpingectomy at benign gynecological surgery for reducing ovarian cancer risk: a 10‐year single centre experience from China and a literature review. Journal of Cancer 2018;9:141‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chene 2016 {published data only}
- Chene G, Meysonnier C, Buenerd A, Moret S, Nadaud B, Beaufils E, et al. Feasibility of opportunistic salpingectomy at the time of vaginal hysterectomy for benign pathology and evaluation of occult tubal lesions prevalence: Preliminary study. Journal de gynecologie, obstetrique et biologie de la reproduction 2016;45:549‐58. [DOI] [PubMed] [Google Scholar]
Cho 2012 {published data only}
- Cho HY, Kang SW, Kim HB, Park SH, Park ST. Prophylactic adnexectomy along with vaginal hysterectomy for benign pathology. Archives of Gynecology and Obstetrics 2012;286:1221‐5. [DOI] [PubMed] [Google Scholar]
Cooney 2015a {published data only}
- Cooney EJ, Hoffman MK. Current trends associated with prophylactic salpingectomy with ovarian preservation at a large tertiary hospital. Journal of Minimally Invasive Gynecology 2015;22:S44‐5. [DOI] [PubMed] [Google Scholar]
Cooney 2015b {published data only}
- Cooney EJ, Hoffman M, Thompson D. Increase in prophylactic salpingectomy across all approaches following an educational initiative. Journal of Minimally Invasive Gynecology 2015;22:S19. [DOI] [PubMed] [Google Scholar]
Crum 2016 {published data only}
- Crum CP. Preventing ovarian cancer. Journal of Clinical Oncology 2016;34:198. [DOI] [PubMed] [Google Scholar]
Daly 2015 {published data only}
- Daly MB, Dresher CW, Yates MS, Jeter JM, Karlan BY, Alberts DS, et al. Salpingectomy as a means to reduce ovarian cancer risk. Cancer Prevention Research 2015;8:342‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Danilyants 2017 {published data only}
- Danilyants N, MacKoul PJ, Baxi RP, Does L, Haworth L. Comparison of clinical outcomes of laparoscopic hysterectomy in an ambulatory surgery center versus outpatient hospital setting. Journal of Minimally Invasive Gynecology 2017;24:S171. [Google Scholar]
Davydov 1972 {published data only}
- Davydov SN, Lipis SM. The sequelae of tubectomy. Akusherstvo i ginekologiia 1972;48:36‐40. [PubMed] [Google Scholar]
Dawle 1979 {published data only}
- Dawle AV, Vartak MM, Chatterjee C. Report on tubectomy cases done at Rural Medical College Ambajogai (during 2 years). Journal of Obstetrics and Gynaecology of India 1979;29:655‐8. [PubMed] [Google Scholar]
Desquesne 1996 {published data only}
- Dequesne. Total and subtotal laparoscopic hysterectomy. The Journal of the American Association of Gynecologic Laparoscopists 1996;3:S9‐S10. [DOI] [PubMed] [Google Scholar]
Dietl 2011 {published data only}
- Dietl J, Wischhusen J. The forgotten fallopian tube. Nature Reviews, Cancer 2011;11:227. [DOI] [PubMed] [Google Scholar]
Dietl 2014a {published data only}
- Dietl J. Bilateral prophylactic salpingectomy for the prevention of ovarian carcinoma. Gynakologische Praxis 2014;38:455‐6. [Google Scholar]
Dietl 2014b {published data only}
- Dietl J, Wischhusen J. The postreproductive salpingectomy. Fertility and Sterility 2014;101:e20. [DOI] [PubMed] [Google Scholar]
Dwyer 2012 {published data only}
- Dwyer PL. Ovarian cancer and the pelvic floor surgeon: the case for prophylactic bilateral salpingectomy during POP surgery. International Urogynecology Journal 2012;23:655‐6. [DOI] [PubMed] [Google Scholar]
Ebeid 2013 {published data only}
- Ebeid E, Dominic B. Prophylactic bilateral salpingectomy at total laparoscopic hysterectomy for benign gynaecological diseases. Gynecological Surgery 2013;10:S24. [Google Scholar]
ESGE 2015 {published data only}
- European Society for Gynaecological Endoscopy. Abstracts of the 24th Annual Congress of the European Society for Gynaecological Endoscopy. Gynecological Surgery 2015;12:Suppl 1. [Google Scholar]
Falconer 2015 {published data only (unpublished sought but not used)}
- Falconer H, Yin L, Grönberg H, Altman D. Ovarian cancer risk after salpingectomy: a nationwide population‐based study. Journal of the National Cancer Institute 2015;107:1‐6. [DOI] [PubMed] [Google Scholar]
Fathalla 2017 {published data only}
- Fathalla MF. A paradigm shift in the origin of ovarian cancer: the ovary is no longer to blame: Is the ovary to blame for tubal carcinoma?. British Journal of Obstetrics and Gynaecology 2017;124:1796‐7. [DOI] [PubMed] [Google Scholar]
Foulkes 2013 {published data only}
- Foulkes WD. Preventing ovarian cancer by salpingectomy. Current Oncology 2013;20:139‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frishman 2013 {published data only}
- Frishman GN. Prophylactic salpingectomy: does it make the cut?. Journal of Minimally Invasive Gynecology 2013;125:404‐5. [DOI] [PubMed] [Google Scholar]
Garcia 2016 {published data only}
- Garcia C, Martin M, Tucker LY, Lyon L, Armstrong MA, McBride‐Allen S, et al. Experience with opportunistic salpingectomy in a large, community‐based health system in the United States. Obstetrics and Gynecology 2016;128:277‐83. [DOI] [PubMed] [Google Scholar]
Ghezzi 2009 {published data only}
- Ghezzi F, Cromi A, Siesto G, Bergamini V, Zefiro F, Bolis P. Infectious morbidity after total laparoscopic hysterectomy: does concomitant salpingectomy make a difference?. British Journal of Obstetrics and Gynaecology 2009;116:589‐93. [DOI] [PubMed] [Google Scholar]
Giannakeas 2015 {published data only}
- Giannakeas V, Sopik V, Shestopaloff K, Iqbal J, Rosen B, Akbari M, et al. A model for estimating ovarian cancer risk: application for preventive oophorectomy. Gynecologic Oncology 2015;139:242‐7. [DOI] [PubMed] [Google Scholar]
Gierach 2014 {published data only}
- Gierach GL, Pfeiffer RM, Patel DA, Black A, Schairer C, Gill A, et al. Long‐term overall and disease‐specific mortality associated with benign gynecologic surgery performed at different ages. Menopause 2014;43:592‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilks 2013 {published data only}
- Gilks CB, Miller D. Opportunistic salpingectomy for women at low risk for development of ovarian carcinoma: the time has come. Gynecologic Oncology 2013;129:443‐4. [DOI] [PubMed] [Google Scholar]
Hanley 2015 {published data only}
- Hanley GE, McAlpine JN, Kwon JS, Mitchell G. Opportunistic salpingectomy for ovarian cancer prevention. Gynecologic Oncology Research and Practice 2015;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanley 2017 {published data only}
- Hanley GE, McAlpine JN, Pearce CL, Miller D. The performance and safety of bilateral salpingectomy for ovarian cancer prevention in the United States. American Journal of Obstetrics and Gynecology 2017;216:270e1‐9. [DOI] [PubMed] [Google Scholar]
Harmsen 2015 {published data only}
- Harmsen MG, Arts‐de Jong M, Hoogerbrugge N, Maas AH, Prins JB, Bulten J, et al. Early salpingectomy (TUbectomy) with delayed oophorectomy to improve quality of life as alternative for risk‐reducing salpingo‐oophorectomy in BRCA1/2 mutation carriers (TUBA study): a prospective non‐randomised multicentre study. BMC Cancer 2015;15:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2015 {published data only}
- Harris AL. Salpingectomy and ovarian cancer prevention. Nursing for Women's Health 2015;21:543‐9. [DOI] [PubMed] [Google Scholar]
He 2017 {published data only}
- He D, Wang L, Sheng M. Influence of prophylactic bilateral salpingectomy in ovarian function and its preventive effect on pelvic disease. Journal of Jilin University Medicine Edition 2017;43:635‐9. [Google Scholar]
Herzog 2013 {published data only}
- Herzog TJ, Dinkelspiel HE. Fallopian tube removal: "STIC‐ing" it to ovarian cancer: What is the utility of prophylactic tubal removal?. Current Oncology 2013;20:148‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joshi 1979 {published data only}
- Joshi CK, Maheshwari S, Soangara MR. A follow‐up study of tubectomy acceptors in Bikaner. Journal of the Indian Medical Association 1979;73:1‐4. [PubMed] [Google Scholar]
Kaplan 2015 {published data only}
- Kaplan AI, Ghezzi F, Cromi A, Siesto G, Bergamini V, Zefiro F, et al. Infectious morbidity after total laparoscopic hysterectomy: does concomitant salpingectomy make a difference?. British Journal of Obstetrics and Gynaecology 2015;60:46‐56. [DOI] [PubMed] [Google Scholar]
Kershenovich 2016 {published data only}
- Kershenovich J, Diaz BP, Dickter C, Alfaro JA. Total laparoscopic hysterectomy with bilateral salpingectomy using ThunderBeat energy. Journal of Minimally Invasive Gynecology 2016;215:S158. [Google Scholar]
Kolmorgen 1993 {published data only}
- Kolmorgen K, Seidenschnur G, Rother D. Diagnostic and surgical laparoscopy in patients in the peri‐ and postmenopauses. Archives of Gynecology and Obstetric 1993;254:293‐4. [Google Scholar]
Kotsopoulos 2013 {published data only}
- Kotsopoulos IC, Tsapanos VS. Patient groups that fimbriectomy could reduce high grade serous ovarian cancer incidence. Gynecologic Oncology 2013;128:151‐2. [DOI] [PubMed] [Google Scholar]
Kwon 2015 {published data only}
- Kwon JS. Ovarian cancer risk reduction through opportunistic salpingectomy. Journal of Gynecologic Oncology 2015;26:83‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamblin 2018 {published data only}
- Lamblin G, Meysonnier C, Moret S, Nadaud B, Mellier G, Chene G. Opportunistic salpingectomy during vaginal hysterectomy for a benign pathological condition. International Urogynecology Journal 2018;29:715‐21. [DOI] [PubMed] [Google Scholar]
Leblanc 2014 {published data only}
- Leblanc E, Vennin P, Narducci F, Merlot B, Bresson L, Farre I, et al. Prophylactic adnexectomy in women at hereditary risk for ovarian carcinomas: towards new tracks?. Oncologie 2014;16:438‐44. [Google Scholar]
Lessard‐Anderson 2014 {published data only}
- Lessard‐Anderson CR, Handlogten KS, Molitor RJ, Dowdy SC, Cliby WA, Weaver AL, et al. Effect of tubal sterilization technique on risk of serous epithelial ovarian and primary peritoneal carcinoma. Gynecologic Oncology 2014;135:423‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madsen 2015 {published data only}
- Madsen C, Baandrup L, Dehlendorff C, Kjær SK. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nation‐wide case‐control study. ACTA Obstetricia et Gynecologica 2015;94:86‐94. [DOI] [PubMed] [Google Scholar]
Manchandra 2017 {published data only}
- Manchanda R. Opportunistic salpingectomy for prevention of ovarian cancer. British Journal of Obstetrics and Gynaecology 2017;124:890. [DOI] [PubMed] [Google Scholar]
Maroni 1980 {published data only}
- Maroni E, Keller‐Fluckinger B, Schreiner WE. The incidence of diseases of the adnexa following hysterectomy (residual ovary syndrome), which requires laparotomy. Geburtshilfe und Frauenheilkunde 1980;40:25‐31. [DOI] [PubMed] [Google Scholar]
Maseela 1980 {published data only}
- Maseela T, Scheele R. Hysterosalpingectomy in the prophylaxis of primary carcinoma of the uterine tube. Fortschritte der Medizin 1980;98:818‐22. [PubMed] [Google Scholar]
Matthews 2016 {published data only}
- Matthews CA. Management strategies for the ovaries at the time of hysterectomy for benign disease. Obstetrics and Gynecology Clinics of North America 2016;43:539‐49. [DOI] [PubMed] [Google Scholar]
McAlpine 2014 {published data only}
- McAlpine JN, Tone AE, Rozenberg N, Hanley G, Heywood M, Stuart G, et al. Opportunistic salpingectomy: Impact of a regional program for ovarian cancer prevention. International Journal of Gynecological Cancer 2014;210:471.e1‐11. [Google Scholar]
McAlpine 2016 {published data only}
- McAlpine JN, Tone, AA, Hanley GE. Opportunistic salpingectomy: we chose to act, not wait. Journal of Obstetrics and Gynaecology Canada 2016;38:425‐7. [DOI] [PubMed] [Google Scholar]
Mettler 2016 {published data only}
- Mettler L. When is tubectomy and when ovarectomy indicated in benign gynecological surgery. Gynecological Endocrinology 2016;32:33. [Google Scholar]
Mikhail 2016 {published data only}
- Mikhail E, Salemi JL, Wyman A, Salihu HM, Imudia AN, Hart S. National trends of bilateral salpingectomy during vaginal hysterectomy with and without laparoscopic assistance, United States 1998‐2011. Journal of Minimally Invasive Gynecology 2016;23:1063‐9. [DOI] [PubMed] [Google Scholar]
Minig 2015 {published data only}
- Minig L, Chuang L, Patrono MG, Cardenas‐Rebollo JM, Garcia‐Donas J. Surgical outcomes and complications of prophylactic salpingectomy at the time of benign hysterectomy in premenopausal women. Journal of Minimally Invasive Gynecology 2015;22:653‐7. [DOI] [PubMed] [Google Scholar]
Morelli 2013a {published data only}
- Morelli M, Venturella R, Mocciaro R, Cello A, Rania E, Lico D, et al. It sounded like a good idea at the time. Journal of Obstetrics and Gynaecology Canada 2013;35:505‐6. [DOI] [PubMed] [Google Scholar]
Morelli 2013b {published data only}
- Morelli M, Venturella R, Zullo F. Risk‐reducing salpingectomy as a new and safe strategy to prevent ovarian cancer. American Journal of Obstetrics and Gynecology 2013;209:395‐6. [DOI] [PubMed] [Google Scholar]
Naaman 2017 {published data only}
- Naaman Y, Hazan Y, Gillor M, Marciano G, Bardenstein R, Shoham Z, et al. Does the addition of salpingectomy or fimbriectomy to hysterectomy in premenopausal patients compromise ovarian reserve? A prospective study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2017;210:270‐4. [DOI] [PubMed] [Google Scholar]
Nair 1991 {published data only}
- Nair TN. A follow up study of women who have undergone tubectomy in a rural community. Indian Journal of Public Health 1991;35:126‐7. [PubMed] [Google Scholar]
Nandakumar 1995 {published data only}
- Nandakumar A, Anantha N, Dhar M, Ahuja V, Kumar R, Reddy S, et al. A case‐control investigation on cancer of the ovary in Bangalore, India. International Journal of Cancer 1995;63:361‐5. [DOI] [PubMed] [Google Scholar]
Narod 2013 {published data only}
- Narod SA. Salpingectomy to prevent ovarian cancer. Current Oncology 2013;20:145‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02284711 {published data only}
- NCT02284711. Impact of salpingectomy on ovarian reserve (SALPOVA). clinicaltrials.gov/ct2/show/NCT02284711 (first received 6 November 2014).
Nezhat 2019 {published data only}
- Nezhat FR, Martinelli VT. Opportunistic salpingectomy: an appropriate procedure during all pelvic surgeries. American Journal of Obstetrics and Gynecology 2019;220(1):10‐11. [DOI] [PubMed] [Google Scholar]
Paul 2018 {published data only}
- Paul PG, Mannur S, Shintre H, Paul G, Gulati G, Mehta S. Thirteen years of experience with opportunistic bilateral salpingectomy during TLH in low‐risk premenopausal women. The Journal of Obstetrics and Gynecology of India 2018;68:314‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez‐Lopez 2016 {published data only}
- Perez‐Lopez FR, Chedraui P. Surgical prevention of epithelial ovary cancer without oophorectomy: changing the future. Climacteric 2016;19:417‐8. [DOI] [PubMed] [Google Scholar]
Petrov 2016 {published data only}
- Petrov IA, Tikhonovskaya OA, Okorokov AO, Kupriyanova II, Petrova MS, Logvinov SV. Preventive salpingectomy and ovarian reserve: experimental study. Bulletin of Experimental Biology and Medicine 2016;162:255‐9. [DOI] [PubMed] [Google Scholar]
Philipp 1980 {published data only}
- Philipp K. Results of routine removal of ovaries and/or tubes as part of vaginal hysterectomy. Geburtshilfe und Frauenheilkunde 1980;40:159‐63. [DOI] [PubMed] [Google Scholar]
Poole 2015 {published data only}
- Poole EM, Rice MS Crum CP, Tworoger SS. Salpingectomy as a potential ovarian cancer risk‐reducing procedure. Journal of the National Cancer Institute 2015;107(2):dju490. [DOI] [PubMed] [Google Scholar]
Pursell 2016 {published data only}
- Pursell N, ElSahwi K. Robotic total laparoscopic hysterectomy and salpingectomy. Journal of Minimally Invasive Gynecology 2016;22:S128. [Google Scholar]
Ranney 1978 {published data only}
- Ranney BS. Sequelae of incomplete gynecologic operations: I. Uterine tubes. South Dakota Journal of Medicine 1978;31:17. [PubMed] [Google Scholar]
Rodriguez‐Triana 2013 {published data only}
- Rodriguez‐Triana V, Park S, Amneus M, Holschneider C. Routine bilateral salpingectomy at the time of hysterectomy with ovarian preservation. Gynecologic Oncology 2013;130:e95‐6. [Google Scholar]
Ruiz 2016 {published data only}
- Ruiz MP, Morales‐Ramirez PB, Dziadek OL, Algren SD. Epithelial ovarian cancer and type of peritoneal insult: a case‐control study. European Journal of Obstetrics Gynecology and Reproductive Biology 2016;205:170‐3. [DOI] [PubMed] [Google Scholar]
Saunders 2017 {published data only}
- Saunders A, Anderson L, McGovern PG. Bilateral salpingectomy. Contemporary OB/GYN 2017;55:16‐21. [Google Scholar]
Setubal 2017 {published data only}
- Setubal AG, Alves JS, Lavado O, Faria J. Mini‐laparoscopy for removal (partial) of adnexae at the time of hysterectomy. Journal of Minimally Invasive Gynecology 2017;24:201‐2. [DOI] [PubMed] [Google Scholar]
Siedhoff 2012 {published data only}
- Siedhoff MT, Findley AD, McCall CA, Carey ET, Steege JF. Appendectomy and salpingectomy during laparoscopic hysterectomy. Journal of Minimally Invasive Gynecology 2012;19:S145‐6. [DOI] [PubMed] [Google Scholar]
Singh 1971 {published data only}
- Singh S, Lucas W. Tubectomy camp: Dhariwal. The Journal of the Christian Medical Association of India 1971;46:539‐43. [PubMed] [Google Scholar]
Singh 2017 {published data only}
- Singh SS, Bougie O, Suen MW, Flaxman T, Arendas K. The practical side of opportunistic salpingectomy. Journal of Obstetrics and Gynaecology Canada 2017;39:1124‐5. [DOI] [PubMed] [Google Scholar]
Skorupska 2016 {published data only}
- Skorupska KA, Miotla P, Kubik‐Komar A, Rechberger E, Adamiak‐Godlewska A, Rechberger T. Are there any differences in quality of life and sexual functions after various types of hysterectomy ‐ does prophylactic salpingectomy matter?. Ginekologia Polska 2016;87:26‐31. [DOI] [PubMed] [Google Scholar]
Szender 2015 {published data only}
- Szender JB, Shashikant BL. Fallopian tube ligation or salpingectomy as means for reducing risk of ovarian cancer. American Medical Association Journal of Ethics 2015;17(9):843‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanner 2013 {published data only}
- Tanner EJ, Long KC, Visvanathan K, Fader AN. Prophylactic salpingectomy in premenopausal women at low risk for ovarian cancer: risk‐reducing or risky?. Fertility and Sterility 2013;100:1530‐1. [DOI] [PubMed] [Google Scholar]
Tellawi 2013 {published data only}
- Tellawi AR, Morozov VV. Prophylactic salpingectomy. The future of ovarian cancer prevention?. Contemporary OB/GYN 2013;58:46‐66. [Google Scholar]
Terada 2016 {published data only}
- Terada KY, Ahn HJ, Kessel B. Differences in risk for type 1 and type 2 ovarian cancer in a large cancer screening trial. Journal of Gynecologic Oncology 2016;27:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2018 {published data only}
- Walsh CS. Who is at risk after risk reduction?. Cancer 2018;124:884‐7. [DOI] [PubMed] [Google Scholar]
Wierrani 1993 {published data only (unpublished sought but not used)}
- Wierrani F, Huber M, Schramm W, Grin W, Grunberger W. Effect of salpingectomy in hysterectomy on female sex hormones [Der einfluß von salpingektomie bei der hysterektomie auf die weiblichen sexualhormone]. Gynäkologisch‐geburtshilfliche Rundschau 1993;33:54‐6. [DOI] [PubMed] [Google Scholar]
Yi 2012 {published data only}
- Yi Q, Ling S, Chen K, He W, Li L, Yi C. Evaluation of the clinical value of simultaneous hysterectomy and bilateral salpingectomy in perimenopausal women. Zhonghua gu chan ke za zhi 2012;47:110‐4. [PubMed] [Google Scholar]
References to ongoing studies
NCT01628432 {published data only}
- NCT01628432. Effect of Salpingectomy During Conservative Hysterectomy (SALPINGOVA) [Effect of Total Salpingectomy During Conservative Hysterectomy for Benign Disease on Ovarian Function: Non Inferiority Randomized Controlled Trial]. clinicaltrials.gov/ct2/show/NCT01628432 (first received 26 June 2012).
NCT02086344 {published data only}
- NCT02086344. Ovarian Reserve Modification After Lps Hysterectomy With Bilateral Salpingectomy [The Effect in Term of Ovarian Reserve Modification of Adding Prophylactic Bilateral Salpingectomy (PBS) to TLH for Preventing Ovarian Cancer]. clinicaltrials.gov/ct2/show/NCT02086344 (first received 13 March 2014).
NCT03045965 {published data only}
- NCT03045965. Hysterectomy and OPPortunistic SAlpingectomy (HOPPSA) [Hysterectomy and Opportunistic Salpingectomy]. clinicaltrials.gov/ct2/show/NCT03045965 (first received 8 February 2017).
Additional references
Aarts 2015
- Aarts JW, Nieboer TE, Johnson N, Tavender E, Garry R, Mol BW, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD003677.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolton 2012
- Bolton KL, Chenevix‐Trench G, Goh C, Sadetzki S, Ramus SJ, Karlan BY, et al. Assosciation between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. Journal of the American Medical Society 2012;307(4):382‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2017
- Chen F, Gaitskell K, Garcia MJ, Albukhari A, Tsaltas J, Ahmed AA. Serous tubal intraepithelial carcinomas associated with high‐grade serous ovarian carcinomas: a systematic review. Britisch Journal of Obstetrics and Gynaecology 2017;124(6):872‐8. [PUBMED: 28218502] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence. Melbourne, Australia: Veritas Health Innovation, Accessed 2018.
Depmann 2016
- Depmann M, Eijkemans MJ, Broer SL, Scheffer GJ, Rooij IA, Laven JS, et al. Does anti‐Müllerian hormone predict menopause in the general population? Results of a prospective ongoing cohort study. Human Reproduction 2016;31:1579‐87. [DOI] [PubMed] [Google Scholar]
Finch 2013
- Finch A, Valentini A, Greenblatt E, Lynch HT, Ghadirian P, Armel S, et al. Frequency of premature menopause in women who carry a BRCA1 or BRCA2 mutation. Fertily and Sterility 2013;99(6):1724‐8. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 18 May 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Hehenkamp 2007
- Hehenkamp WJ, Volkers NA, Broekmans FJ, Jong FH, Themmen AP, Birnie E, et al. Loss of ovarian reserve after uterine artery embolization: a randomized comparison with hysterectomy. Human Reproduction 2007;22(7):1996‐2005. [DOI] [PubMed] [Google Scholar]
Higgins 2008a
- Higgins JP, White IR, Anzures‐Cabrera J. Meta‐analysis of skewed data: combining results reported on log‐transformed or raw scales. Statistics in Medicine 2008;27(29):6072‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008b
- Higgins JP, White IR, Wood AM. Imputation methods for missing outcome data in meta‐analysis of clinical trials. Clinical Trials 2008;5(3):225‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kindelberger 2007
- Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. The American Journal of Surgical Pathology 2007;31(2):161‐9. [PUBMED: 17255760] [DOI] [PubMed] [Google Scholar]
Kotlyar 2017
- Kotlyar A, Gingold J, Shue S, Falcone T. The effect of salpingectomy on ovarian function. Journal of Minimally Invasive Gynecology 2017;24:563‐78. [DOI] [PubMed] [Google Scholar]
La Marca 2012
- Marca A, Spada E, Grisendi V, Argento C, Papaleo E, Milani S, et al. Normal serum anti‐Müllerian hormone levels in the general female population and the relationship with reproductive history. European Journal of Obstetrics & Gynecology and Reproductive Biology 2012;163:180‐4. [DOI: ] [DOI] [PubMed] [Google Scholar]
Long 2017
- Long Roche KC, Abu‐Rustum NR, Nourmoussavi M, Zivanovic O. Risk‐reducing salpingectomy: Let us be opportunistic. Cancer 2017;123(10):1714‐20. [PUBMED: 28334425] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohamed 2017
- Mohamed AA, Yosef AH, James C, Al‐Hussaini TK, Bedaiwy MA, Amer SAKS. Ovarian reserve after salpingectomy: a systematic review and meta‐analysis. Acta Obstetricia et Gynecologica Scandinavica 2017;96:795‐803. [DOI: 10.1111/aogs.13133] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA group. Preferred reporting items for systematic reviews and meta‐analysis: the PRISMA statement. BMJ 2009;338:b2535. [DOI: 10.1136/bmj.b2535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mytton 2017
- Mytton J, Evison F, Chilton PJ, Lilford RJ. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ (Clinical research edition) 2017;356:j372. [PUBMED: 28167486] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ntoumanoglou‐Schuiki 2018
- Ntoumanoglou‐Schuiki A, Tomasch G, Laky R, Taumberger N, Bjelic‐Radisic V, Tamussino K. Opportunistic prophylactic salpingectomy for prevention of ovarian cancer: What do national societies advise?. European Journal of Obstetrics and Gynecology and Reproductive Biology 2018;225:110‐12. [DOI] [PubMed] [Google Scholar]
Parker 2009
- Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, et al. Ovarian conservation at the time of hysterectomy and long‐term health outcomes in the nurses' health study. Obstetrics and Gynecology 2009;113(5):1027‐37. [PUBMED: 19384117] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez‐Lopez 2017
- Perez‐Lopez FR, Ceausu I, Depypere H, Kehoe S, Lambrinoudaki I, Mueck A, et al. Interventions to reduce the risk of ovarian and fallopian tube cancer: a European Menopause and Andropause Society Postition Statement. Maturitas 2017;100:86‐91. [PUBMED: 28389043] [DOI] [PubMed] [Google Scholar]
Piek 2001a
- Piek JM, Diest PJ, Zweemer RP, Jansen JW, Poort‐Keesom RJ, Menko FH, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. Journal of Pathology 2001;195:451‐6. [DOI] [PubMed] [Google Scholar]
Piek 2001b
- Piek JM, Diest PJ, Zweemer RP, Kenemans P, Verheijen RH. Tubal ligation and risk of ovarian cancer. Lancet 2001;358:844. [DOI] [PubMed] [Google Scholar]
Piek 2003
- Piek JM, Verheijen RH, Kenemans P, Massuger LF, Bulten H, Diest PJ. BRCA1/2‐related ovarian cancers are of tubal origin: a hypothesis. Gynecologic Oncology 2003;90(2):491. [DOI] [PubMed] [Google Scholar]
R Core Team 2017 [Computer program]
- R Core Team. R: A Language and Environment for Statistical Computing. Version 3.4.2. Vienna, Austria: R Foundation for Statistical Computing, 2017.
RCOG 2014
- Royal College of Obstetricians and Gynaecologists. The distal fallopian tube as the origin of non‐uterine pelvic high‐grade serous carcinomas. Scientific impact paper no. 44. November 2014. www.rcog.org.uk/globalassets/documents/guidelines/scientific‐impact‐papers/sip44hgscs.pdf (accessed 25 September 2017).
Robert 2015
- Robert M, Cenaiko D, Sepandj J, Iwanicki S. Success and complications of salpingectomy at the time of vaginal hysterectomy. Journal of Minimally Invasive Gynecology 2015;22(5):864‐9. [DOI] [PubMed] [Google Scholar]
Rocca 2006
- Rocca WA, Grossardt BR, Andrade M, Malkasian GD, Melton LJ 3rd. Survival patterns after oophorectomy in premenopausal women: a population‐based cohort study. The Lancet. Oncology 2006;7(10):821‐8. [PUBMED: 17012044] [DOI] [PubMed] [Google Scholar]
Siegel 2017
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: A Cancer Journal for Clinicians January/February 2017;67(1):7‐30. [DOI] [PubMed] [Google Scholar]
StataCorp 2013 [Computer program]
- StataCorp. Stata. Version 13. College Station, TX, USA: StataCorp, 2013.
Sterne 2009
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ (Clinical research edition) 2016;355:i4919. [PUBMED: 27733354] [DOI] [PMC free article] [PubMed] [Google Scholar]
Su 2011
- Su YS, Gelman A, Hill J, Yajima M. Multiple imputation with diagnostics (mi) in R: opening windows into the black box. Journal of Statistical Software 2011;45(2):1‐31. [Google Scholar]
van Rooij 2005
- Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, Jong FH, et al. Serum antimüllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertility and Sterility 2005;83(4):979‐87. [DOI] [PubMed] [Google Scholar]
Venturella 2016
- Venturella R, Lico D, Borelli M, Imbrogno MG, Cevenini G, Zupi E, et al. 3 to 5 years later: long‐term effects of prophylactic bilateral salpingectomy on ovarian function. Journal of Minimally Invasive Gynecology 2016;24:145‐50. [DOI] [PubMed] [Google Scholar]
Yoon 2016
- Yoon SH, Kim SN, Shim SH, Kang SB, Lee SJ. Bilateral salpingectomy can reduce the risk of ovarian cancer in the general population: A meta‐analysis. European Journal of Cancer 2016;55:38‐46. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
van Lieshout 2017
- Lieshout LA M, Steenbeek MP, Hullu JA, Vos MC, Houterman S, Wilkinson J, et al. Hysterectomy with salpingectomy versus hysterectomy alone. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD012858] [DOI] [PMC free article] [PubMed] [Google Scholar]


