Abstract
Objectives
To evaluate survival and functional outcomes in surgically‐treated spinal metastasis patients and to identify the prognostic value of the remaining options for systemic treatment.
Methods
The current study reviewed 100 consecutive patients who received surgery for spinal metastasis in a single center from March 2012 to June 2016. The decision for surgery had been made in a weekly multidisciplinary tumor board after considering multiple factors. Among these factors, those associated with the functional outcome were identified using crosstab and logistic regression analyses. Survival analysis applying the Kaplan–Meier curve and the Cox proportional hazards model was used to identify factors associated with improved survival.
Results
Of the 100 patients, there were 62 men and 38 women, with a mean age of 60.4 years at the time of surgery. The median postoperative survival of the whole cohort was 16.2 months (95% confidence interval: 10.1–22.3). When patients were stratified by the functional outcome, a significantly large proportion of patients with good functional outcome (Eastern Cooperative Oncology Group performance status better than 3) had an available option for systemic treatment at the time of surgery (P < 0.001, Pearson χ2‐test). Logistic regression analysis found that the presence of remaining options for systemic treatment at the time of decision‐making for surgery was associated with improved postoperative functional performance status (P = 0.004, odds ratio = 7.59). Survival analysis also found that the availability of remaining options for systemic treatment was associated with improved survival (P = 0.001, hazard ratio = 0.22). This finding was statistically more significant in a group of patients with a low revised Tokuhashi score of 0 to 8 (P < 0.001) when compared to the group of patients with a high revised Tokuhashi score of 9 to 15 (P = 0.082).
Conclusions
Availability of remaining options for systemic treatment is an important factor to consider when deciding on surgical treatment for spinal metastasis.
Keywords: Chemoradiotherapy, Neoplasm metastasis, Performance status, Proportional hazards model, Survival
Introduction
The spine is the most common site for malignant skeletal metastasis, and the incidence of spinal metastasis is increasing as a result of aging populations and improved survival rates in cancer patients1. The purpose of surgical treatment in patients with spinal metastasis is to maintain the quality of life during the remaining survival period, by preserving ambulation and reducing pain2, 3. To achieve this goal, surgeons and oncologists try to consider every factor that affects clinical outcomes when deciding whether to perform a surgical treatment. However, in some cases, it is not clear whether the benefits from surgery will outweigh the inherent morbidity and risks of the operation.
Various “classification‐based” decision‐making systems have been used to aid this decision‐making process4, 5, 6. Although these scoring systems have been found to be useful in predicting patient survival, recent studies have shown that the accuracy of these systems is reduced when they are applied to malignancies associated with poor prognoses, such as lung cancer7. A similar study also found that the accuracy of these systems has been declining over time8. These findings are likely due to the inability of classification‐based scoring systems to reflect recent advancements in systemic treatment and improvements in survival9, 10, 11.
As an alternative, several authors have proposed “principle‐based” decision‐making systems that reflect currently available treatments, including chemotherapy and radiotherapy12, 13, 14. In these systems, previous responsiveness to adjuvant therapy is included as a factor to consider when deciding on the appropriate treatment for a spinal metastasis patient. However, based on recent clinical experience, the authors of the present study postulated that the availability of remaining options for systemic treatment after surgery, rather than the response to previous treatment, was a strong prognostic factor affecting the clinical outcomes. If this could be proven, it would be a useful indicator for predicting the effectiveness of surgical treatment in patients with spinal metastasis. Therefore, the purpose of this study was to evaluate survival and functional outcomes in surgically‐treated spinal metastasis patients and to identify the prognostic value of available options for systemic treatment.
Materials and Methods
Study Design
This study is a retrospective review of prospectively collected data from patients who received palliative surgical treatment for spinal metastasis between March 2012 and June 2016 in the author's center. Consecutive series of patients were retrieved from databases within the electronic medical records system, using formulated queries comprised of relevant keywords. Patients who received curative resection of solitary metastatic lesion of malignancies with a favorable prognosis, such as breast and thyroid cancer, were excluded from the current study. Patients with follow‐up periods of less than 12 months were also excluded. The study obtained ethical approval and waiver of consent by the institutional review board of the author's center.
Diagnosis of Spinal Metastasis
In cases where spinal metastasis was the first manifestation of the malignancy, the diagnosis of spinal metastasis was confirmed by an image‐guided biopsy of the spinal lesion. However, in patients who were treated and followed up after tissue‐based diagnosis of malignancy at the non‐spinal primary site, spinal metastasis was diagnosed based on the presence of typical radiologic findings by an independent radiologist using MRI.
The Decision for Surgical Treatment
The decision to perform surgery was made using a multidisciplinary approach through a weekly tumor board consisting of a medical oncologist, a radiation oncologist, a radiologist, a pathologist, an orthopaedic oncologist, and an orthopaedic spine surgeon. Information on the estimated life expectancy and availability of remaining systemic treatment or radiotherapy was provided by the medical oncologists or the radiation oncologists who had been treating the particular patient. Pathologists and radiologists also provided relevant information on tumor pathology and radiologic findings. After thorough discussion, the decision as to whether to operate on a particular patient was made unanimously by all specialists, and details on the purpose and method of surgery were also decided based on the abovementioned multiple factors. For patients in emergency status, the decision to operate was made based on interdisciplinary real‐time consultation. Regarding the clinical condition of the patient, surgery was considered in patients experiencing severe pain that precluded ambulation or daily activities, or in patients with signs of spinal cord dysfunction, such as upper motor neuron signs. Radiological findings suggesting mechanical spinal instability were also considered as a surgical indication.
Data Collection
Demographic data, including age, sex and information on the status of the patient's primary malignancy, were collected retrospectively, based on electronic medical records. The revised Tokuhashi scoring system5 and the Tomita scoring system4, including the stratification criteria presented in the original articles, was used to assess the preoperative status of these patients. The preoperative functional performance was evaluated using the Karnofsky performance status (KPS) and the Eastern Cooperative Oncology Group performance status (ECOG PS) scales15. Whether a patient had remaining options for postoperative systemic treatment and radiotherapy at the time of decision‐making for surgical treatment was evaluated and recorded. Finally, information regarding surgical treatment, including the purpose, the surgical approach, and the method of operation, was also collected.
Outcome Measurement
Postoperative Survival Using the Kaplan–Meier Survivorship Curve
Postoperative survival was defined as the time interval between the dates of operation and death or the last follow‐up, if a patient was alive, which were retrieved from the medical records. Median postoperative survival derived from Kaplan–Meier survivorship curve analysis was used for outcome assessment. Because a sufficient survival period is required to warrant surgery for spinal metastasis, the postoperative survival is the most important outcome measure in this study.
Eastern Cooperative Oncology Group Performance Status Scale and Functional Outcome
The functional outcome was assessed using the ECOG PS scale15, which was routinely recorded at every outpatient visit and hospital admission. The cohort was divided into two groups according to their postoperative function. The good outcome group was defined as a group of patients who maintained a functional status better than ECOG PS 3 (= ECOG PS 0–2) for more than 3 months postoperatively, and the poor outcome group as a group of patients who were unable to do so. Patients who died within 3 months after the operation were included in the poor outcome group by definition. Functional status is also an essential parameter because the goal of surgery in spinal metastasis is to preserve function, especially ambulation, during the remaining life.
Statistical Analysis
Variables of patients within the two functional groups (good and poor) were compared using Pearson's χ2‐test and Fisher's exact test. Factors associated with functional outcomes were further evaluated using logistic regression analysis. Survival analysis was performed, using Kaplan–Meier curves, on the actual survival period of the patients and the period of postoperative functional maintenance, which was defined as the period with functional status better than the ECOG PS 3. To identify factors associated with outcomes, a Cox proportional hazards model was also generated. Factors with a P‐value <0.10 in the univariate analysis entered the multivariate analysis, and a P‐value of <0.05 was considered statistically significant. SPSS Statistics version 25.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis.
Results
General Information and Survival of the Study Cohort
In total, 104 patients received palliative surgical treatment for spinal metastasis in the authors’ center from March 2012 to June 2016. Four patients were excluded because their follow‐up period was less than 12 months and survival was not identified, leaving 100 patients who were included in this retrospective study. The cohort consisted of 62 male and 38 female patients, with a mean age of 60.4 years at the time of surgery. Metastatic lesions in the spinal column involved the cervical spine in 21 cases, the thoracic region in 60, the lumbar region in 34, and the sacrum in 2. The lung was the most common site for primary malignancy (n = 29, 29%), followed by breast, liver, kidney, and prostate (Table 1).
Table 1.
Survival according to primary cancer and chronicity of metastatic lesion
| Primary cancer | Mean (months) | Median (months) | ||||
|---|---|---|---|---|---|---|
| Estimate | SE | 95% CI | Estimate | SE | 95% CI | |
| Lung (n = 29) | 11.8 | 2.1 | 7.7–15.8 | 7.8 | 1.9 | 3.9–11.6 |
| Breast (n = 9) | 34.3 | 5.9 | 22.6–46.0 | NA | NA | NA |
| Liver (n = 9) | 18.0 | 5.0 | 8.1–28.0 | 14.1 | 6.8 | 0.7–27.4 |
| Renal (n = 8) | 28.4 | 6.6 | 15.4–41.5 | 20.2 | 11.2 | 0.0–42.1 |
| Prostate (n = 8) | 37.1 | 9.2 | 19.2–53.1 | 49.8 | 17.4 | 15.6–83.9 |
| Multiple myeloma (n = 6) | 30.8 | 8.0 | 15.2–46.5 | 23.2 | 3.2 | 16.8–29.5 |
| Thyroid (n = 6) | 33.6 | 5.2 | 23.2–43.9 | NA | NA | NA |
| Colon (n = 4) | 10.1 | 4.4 | 1.4–18.8 | 5.3 | 2.4 | 0.5–10.0 |
| Rectal (n = 4) | 8.8 | 2.7 | 3.5–14.1 | 7.7 | 5.6 | 0.0–18.7 |
| Stomach (n = 4) | 2.1 | 0.8 | 0.5–3.8 | 2.0 | 1.3 | 0.0–4.4 |
| Others (n = 13) | 31.0 | 5.9 | 19.3–42.6 | 26.4 | 5.9 | 14.8–37.9 |
| Synchronous (n = 10) | 33.5 | 6.4 | 20.8–46.1 | 23.2 | 4.2 | 14.9–31.5 |
| Metachronous (n = 90) | 22.4 | 2.4 | 17.6–27.2 | 13.5 | 2.4 | 8.7–18.2 |
| Total (n = 100) | 23.8 | 2.4 | 19.1–28.5 | 16.2 | 3.1 | 10.1–22.3 |
CI, confidence interval; SE, standard errors; NA, not applicable.
Twenty‐eight patients were alive at the last follow‐up, with a minimum follow‐up period of 12 months. The median postoperative survival of the whole cohort, retrieved from Kaplan–Meier curve analysis, was 16.2 months (95% confidence interval [CI]: 10.1–22.3) (Table 1). The shortest median postoperative survival occurred in patients with stomach cancer (2.0 months; 95% CI: 0.1–4.4) and the longest in patients with prostate cancer (49.8 months; 95% CI: 15.6–83.9). Patients who had metastatic lesions in the spine at the time of initial diagnosis of the primary malignancy showed a median survival of 23.2 months (95% CI: 14.9–31.5) after operation, which is longer than that of the patients diagnosed with spinal metastasis later in the disease course who had a median survival of 13.5 months (95% CI: 8.7–18.2), although statistically not significant (P = 0.067, log‐rank test).
Functional Outcome
Over the entire follow‐up period, 74 patients lost their ambulatory function (ECOG PS 3 and 4), of which 21 lost the function within 3 months post‐surgery; these patients comprised the poor functional group. The most common cause for loss of ambulation was deterioration of general condition due to the progression of the malignancy at the primary site or metastasis to an organ other than the spine, in both the total patient cohort (73.0%) and the poor outcome group (71.4%) (Table 2). These patients had no evidence of other possible causes for loss of ambulation, such as spinal cord compression or extraspinal bone metastasis. Epidural spinal cord compression due to the progression of the metastatic spinal lesion was the cause of loss of ambulation in 11/74 (14.9%) patients for the total cohort and 3/21 (14.3%) patients for the poor outcome group.
Table 2.
Causes of loss of ambulation
| Cause | During total follow‐up period | Within 3 months after operation |
|---|---|---|
| Progression of primary cancer | 54 (73.0%) | 15 (71.4%) |
| Progression of epidural cord compression | 11 (14.9%) | 3 (14.3%) |
| Brain metastasis | 2 (2.7%) | 0 (0%) |
| Extraspinal bone metastasis | 2 (2.7%) | 0 (0%) |
| Others | 5 (6.8%) | 3 (14.3%) |
| Total | 74 (100%) | 21 (100%) |
Patients within each of the functional groups were stratified according to their primary malignancy (Table 3). Relatively high proportions of patients with stomach (4/4, 100%), lung (11/29, 37.9%) and colorectal (3/8, 37.5%) cancers were classified into the poor functional group. Among the patients with other malignancies, including breast, prostate, renal, liver, myeloma, and thyroid cancers, only 3 of 59 (5.1%) showed poor functional outcome. While 21 of 90 (23.3%) patients with metachronous metastatic lesions showed poor functional outcome, none of the patients with synchronous metastatic lesions lost their ambulatory function within 3 months after the operation, although the difference was not statistically significant (P = 0.115, Fisher's exact test). (Table 3).
Table 3.
Functional outcome according to primary tumor and chronicity of metastatic lesion
| Good* (n = 79) | Poor (n = 21) | Total (n = 100) | ||
|---|---|---|---|---|
| Primary tumor | Lung | 18 (62.1%) | 11 (37.9%) | 29 (100%) |
| Stomach | 0 (0%) | 4 (100%) | 4 (100%) | |
| Colorectal | 5 (62.5%) | 3 (37.5%) | 8 (100%) | |
| Others | 56 (94.9%) | 3 (5.1%) | 59 (100%) | |
| Diagnosis of metastasis | Synchronous | 10 | 0 | 10 |
| Metachronous | 69 | 21 | 90 | |
Good functional outcome group is defined as a group of patients who maintained a functional status better than Eastern Cooperative Oncology Group performance status 3 (= ECOG PS 0–2) for more than 3 months postoperatively.
Factors Associated with Functional Outcomes
Among the evaluated variables, the presence of remaining options for systemic treatment and radiotherapy, as well as the Tomita and revised Tokuhashi scores, showed statistically significant differences between the two functional groups (Table 4). Logistic regression analysis also revealed that the remaining options for systemic treatment (P = 0.004; odds ratio [OR], 7.59), along with the palsy score in the revised Tokuhashi system (P = 0.002; OR, 7.15), were significantly associated with the functional outcome. Even though preoperative and immediate postoperative function did not differ significantly between patient groups with or without remaining options for systemic treatment, patients with a remaining option for systemic treatment had a better ECOS PS at 6 months postoperatively (Fig. 1).
Table 4.
Analysis of factors associated with functional outcome and survival
| Functional group comparison | Cox proportional hazards analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 100) | Good (n = 79) | Poor (n = 21) | P‐value | Median survival (95% CI) | Hazard ratio‡ (95% CI) | P‐value | ||
| Tokuhashi score | 0–8 | 68 | 47 | 21 | <0.001* | 9.4 (4.0–14.8) | 1.00 | 0.007 |
| 9–11 | 22 | 22 | 0 | 26.4 (15.2–37.6) | 0.37 (0.19–0.70) | |||
| 12–15 | 10 | 10 | 0 | 49.8 (0.0–101.6) | 0.34 (0.08–1.44) | |||
| Tomita score | 2–3 | 22 | 22 | 0 | 0.003* | 49.8 (38.8–60.7) | 1.00 | 0.037 |
| 4–5 | 14 | 12 | 2 | 20.0 (NA) | 0.84 (0.28–2.57) | |||
| 6–7 | 25 | 18 | 7 | 11.3 (4.6–17.9) | 1.74 (0.66–4.58) | |||
| 8–10 | 37 | 25 | 12 | 8.5 (0.0–17.7) | 2.51 (1.01–0.37) | |||
| Systemic option | No | 28 | 14 | 14 | <0.001† | 3.7 (2.5–4.8) | 1.00 | 0.001 |
| Yes | 72 | 65 | 7 | 21.6 (17.9–25.4) | 0.22 (0.13–0.37) | |||
| Radiotherapy option | No | 44 | 30 | 14 | 0.019† | 12.9 (4.2–21.6) | 1.00 | 0.778 |
| Yes | 56 | 49 | 7 | 18.6 (11.8–25.5) | 0.93 (0.56–1.53) | |||
CI, confidence interval; NA, not applicable
P‐value derived from χ2‐test for trend.
P‐value derived from Pearson's χ2‐test.
Adjusted hazard ratio and P‐value are derived from multivariate analysis of the Cox proportional hazards model.
Figure 1.
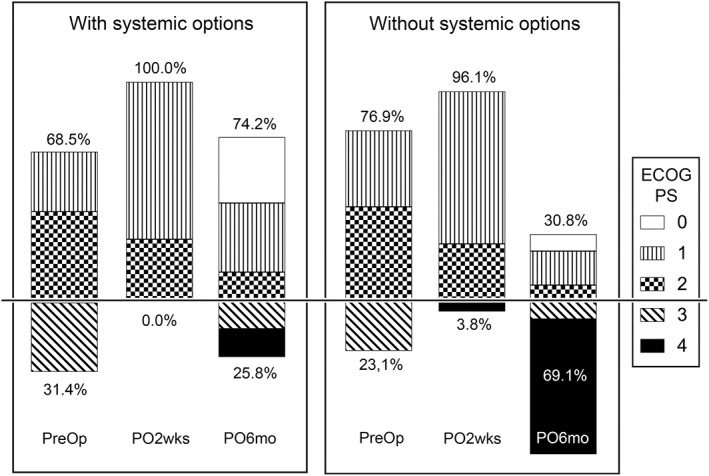
Effect of remaining systemic treatment options on functional outcomes. The proportion of patients with poor functional performance status (ECOG PS 3 and 4) is similar between two groups, with and without systemic options, at preoperative and immediate postoperative periods, but distinguishable at 6 months postoperatively. ECOG PS, Eastern Cooperative Oncology Group performance status; PO2wks, 2 weeks postoperative; PO6mo, 6 months postoperative; PreOp, preoperative.
Factors Associated with Survival
Survival analysis using Kaplan–Meier curves and a Cox proportional hazards model showed that stratification using the revised Tokuhashi and Tomita scoring systems and remaining options for systemic treatment were significantly associated with the actual survival duration of patients in this cohort (Table 4, Fig. 2A). The presence of remaining options for systemic treatment was also associated with the “functional survival duration,” defined as the time interval between the date of operation and the date of loss of ambulation ability (ECOG PS 3, 4) or last follow‐up (Fig. 2B). When patients were stratified by the revised Tokuhashi score (0–8, 9–11, 12–15), as in the original article, the availability of systemic treatment options showed a statistically significant association with longer actual survival duration in a group of patients with a Tokuhashi score of 0 to 8 (Fig. 2C), but not in the groups of patients with a score of 9–11 and 12–15. (Fig. 2D).
Figure 2.
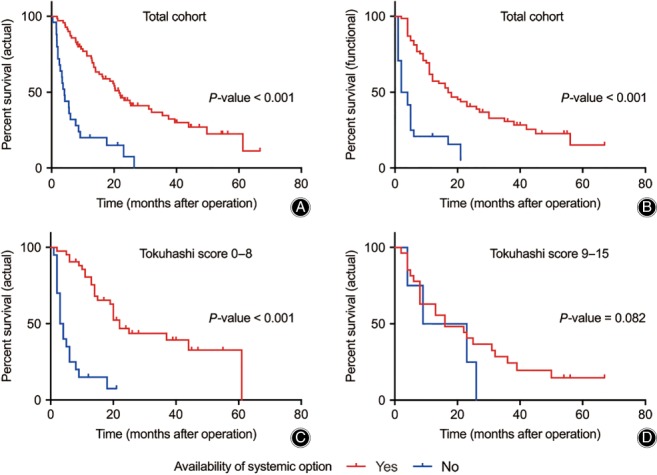
Survival curves for actual and functional survival stratified by remaining systemic treatment options. (A) Curve for actual survival of total cohort. (B) Curve for functional survival for total cohort. (C) Curve for actual survival of the patients with Tokuhashi score of 0–8. (D) Curve for actual survival of the patients with Tokuhashi score of 9–11 and 12–15 combined. (All P‐values for the Kaplan–Meier curves are derived from log‐rank test).
Discussion
The current study examined the prognostic value of the remaining options for systemic treatment in patients with spinal metastasis. Several other studies have examined the prognostic value of previous systemic treatment applied prior to the diagnosis of spinal metastasis, rather than the remaining options for postoperative systemic treatment, and found conflicting results2, 14, 16. However, a history of previous systemic therapy is distinctly different from the availability of remaining systemic treatment options. Because the occurrence of spinal metastasis itself despite previous systemic treatment can be considered a treatment failure, the prognosis of a patient is likely to be associated with the remaining options for systemic treatment, as shown in the results of the current study.
The generally accepted length of remaining life expectancy to warrant surgical treatment for spinal metastasis is 3 months postoperatively17, 18, 19, and ideally, patients should remain ambulatory as long as possible during this period. Therefore, preservation of ambulatory function for more than 3 months postoperatively was chosen as the criteria for the good outcome group in the current study. Because the patient with ECOG PS 2 is ambulatory by definition, ECOG PS better than 3 (ECOG PS 0–2) was adopted as a threshold for the preservation of ambulatory function. According to the criteria, 79% of all patients in our cohort had ECOG PS better than 3 for more than 3 months after surgery and was designated as a good outcome group. This result is comparable to the study by Majeed et al., in which 45/55 (81.8%) patients who received surgery for spinal metastasis were ambulatory (independently or with aids) at 6 weeks postoperatively20.
In the current study, among the 21 patients who lost ambulatory function (ECOG PS 3–4) within 3 months postoperatively, a deterioration in the general condition due to the progression of the primary malignancy was the most frequent reason for the loss of ambulation, rather than the progression of the metastatic spinal lesion (Table 2). Furthermore, among patients who had an available option for systemic treatment, nearly 70% had preserved ambulatory function until 6 months postoperatively (Fig. 1). These results further emphasize the importance of systemic control of the primary malignancy to improve the outcome of surgical treatment in patients with spinal metastasis. To the best of our knowledge, the current study is the first to describe the specific causes of loss of ambulation in patients with spinal metastasis.
Regarding the survival time as an outcome measure, the median survival after surgery of our cohort was longer than those in previously reported studies2, 21, 22, 23, 24, 25. This result may be due to the patient selection criteria because the current study only included patients who had a predicted life expectancy that was long enough to warrant surgical treatment. It could also be a result of efforts to identify patients who could benefit the most from surgical treatment, using a multidisciplinary approach. When examined according to their primary tumors, which are considered as the most important prognostic factor in various scoring systems4, 5, 6, patients with prostate, breast, and thyroid cancer showed longer median survival after operation, whereas the survival period in patients with lung, colorectal, and stomach cancer was shorter, as in previous reports21, 23, 26. Tumor biology and the timing of spinal metastasis occurrence during the disease course are considered as the underlying causes for such differences in survival.
In this study, patients with synchronous metastatic spinal lesions had longer survival times (Table 1) and good functional outcomes (Table 3), compared to the patients with metachronous metastatic lesions. Similar results have been reported in previous studies in certain types of cancer. In advanced non‐small cell lung cancer, patients who had adverse events requiring palliative procedures, including metastatic spinal cord compression, at the time of cancer diagnosis showed more favorable prognosis (median survival, 14.6 months) than patients with later adverse events during their disease course (median survival, 2.7 months)27. Differences in tumor biology between the two distinctive clinical groups may have caused the difference in survival and functional outcome. Furthermore, patients with synchronous metastatic lesions generally have more applicable systemic treatment options remaining, because they have not received any previous treatment for their malignancy; this was true for the 10 patients in our cohort with synchronous lesions. These findings suggested that a more aggressive approach for these patients would be suitable in the management of spinal metastasis.
In 2005, Tokuhashi suggested conservative treatment or palliative surgery for patients with a revised Tokuhashi score of 0 to 8, because their predicted life expectancies were less than 6 months5. However, in the current study, the availability of remaining options for systemic treatment showed a significant association with longer survival period in a group of patients with lower Tokuhashi scores (0–8), but not in the groups with higher scores (9–11 and 12–15) (Fig. 2). This finding seems to be related to recent advances in systemic treatment and suggests that the patients that have low Tokuhashi scores and tumors with poor prognosis such as lung cancer, who were previously considered as candidates for only conservative treatment, can also benefit from the surgical treatment, if the patient has available systemic options remaining after surgery (Fig. 3). However, for patients who have a low Tokuhashi score and no options for systemic treatment, surgical treatment should be considered cautiously because poor prognosis is expected (Fig. 4).
Figure 3.
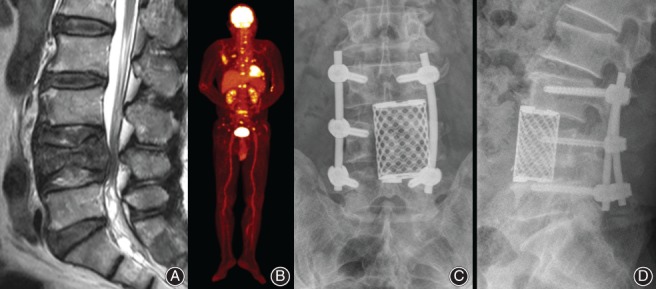
Non‐small cell lung cancer in a 61‐year‐old man. (A) T2‐weighted sagittal MRI shows spinal metastasis and pathologic fracture at the L4 level. (B). The patient had multiple metastases in brain and multiple bones. Although his preoperative revised Tokuhashi score was 5, the patient had various options for systemic treatment because the spinal metastasis was his first clinical manifestation of the malignancy. (C, D) The patient underwent partial anterior corpectomy of L4 combined with posterior stabilization and survived 15 months after the operation.
Figure 4.
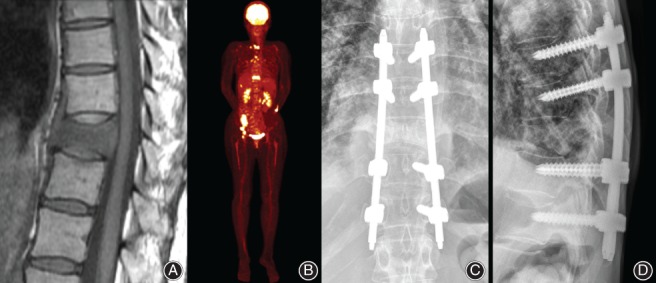
Non‐small cell lung cancer in a 55‐year‐old female. (A) T2‐weighted sagittal MRI shows spinal metastasis and pathologic fracture at the T10 level. (B) The patient had multiple metastases in the brain, ovary and multiple bones. Her preoperative revised Tokuhashi score was 5, but she had no available options for systemic treatment at the time of operation for spinal metastasis. (C, D) The patient underwent posterior percutaneous pedicle screw fixation and died 4 months after surgery due to the progression of brain metastasis.
The current study has several limitations. First, because it is a retrospective study, there may be a selection bias and confounding factors that have not been considered. Second, a variety of systemic treatments were applied to this cohort, including hormonal and targeted therapies such as monoclonal antibodies. Third, multiple myeloma patients were included in this study, which, by definition, is a systemic hematological disorder rather than a metastatic disease, and usually shows good prognosis and treatment response. Fourth, the small sample size for individual malignancies might have lowered the statistical significance of this study. Fifth, the difference between preoperative and postoperative pain scales, which is an important factor to evaluate in surgically treated spinal metastasis patients, was not assessed. Finally, in this study, preservation of ambulatory function was defined as an ECOG PS better than 3. However, a certain stage of ECOG PS can include a broad spectrum of patients, and those with the same ECOG PS may differ in their actual functionality. Heterogeneity of the study cohort mentioned above might have a confounding effect on the results and interpretation of the current study.
Despite the limitations mentioned above, the current study provided significant and detailed information about the causes of loss of ambulation and the factors associated with survival and functional outcome in patients with spinal metastasis, including the origin and chronicity of the malignancies, and the presence of remaining options for systemic treatment. The results of this study advocate more aggressive surgical management not only for patients who have primary tumors with favorable overall prognosis, but also metastatic spinal lesions diagnosed at the time of initial presentation, and remaining options for systemic treatment, even with a low Tokuhashi score.
In conclusion, when deciding on surgical treatment for spinal metastasis, a tailored decision‐making process based on up‐to‐date knowledge is required, and availability of remaining options for systemic treatment is an essential factor to consider in this process.
Disclosure: The authors have no financial conflicts of interest.
References
- 1. Laufer I, Sciubba DM, Madera M, et al Surgical management of metastatic spinal tumors. Cancer Control, 2012, 19: 122–128. [DOI] [PubMed] [Google Scholar]
- 2. Nathan SS, Healey JH, Mellano D, et al Survival in patients operated on for pathologic fracture: implications for end‐of‐life orthopedic care. J Clin Oncol, 2005, 23: 6072–6082. [DOI] [PubMed] [Google Scholar]
- 3. Patchell RA, Tibbs PA, Regine WF, et al Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet, 2005, 366: 643–648. [DOI] [PubMed] [Google Scholar]
- 4. Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine, 2001, 26: 298–306. [DOI] [PubMed] [Google Scholar]
- 5. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine, 2005, 30: 2186–2191. [DOI] [PubMed] [Google Scholar]
- 6. Bauer HC, Wedin R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop Scand, 1995, 66: 143–146. [DOI] [PubMed] [Google Scholar]
- 7. Hessler C, Vettorazzi E, Madert J, Bokemeyer C, Panse J. Actual and predicted survival time of patients with spinal metastases of lung cancer: evaluation of the robustness of the Tokuhashi score. Spine, 2011, 36: 983–989. [DOI] [PubMed] [Google Scholar]
- 8. Zoccali C, Skoch J, Walter CM, Torabi M, Borgstrom M, Baaj AA. The Tokuhashi score: effectiveness and pitfalls. Eur Spine J, 2016, 25: 673–678. [DOI] [PubMed] [Google Scholar]
- 9. Leithner A, Radl R, Gruber G, et al Predictive value of seven preoperative prognostic scoring systems for spinal metastases. Eur Spine J, 2008, 17: 1488–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen H, Xiao J, Yang X, Zhang F, Yuan W. Preoperative scoring systems and prognostic factors for patients with spinal metastases from hepatocellular carcinoma. Spine, 2010, 35: E1339–E1346. [DOI] [PubMed] [Google Scholar]
- 11. Morgen SS, Lund‐Andersen C, Larsen CF, Engelholm SA, Dahl B. Prognosis in patients with symptomatic metastatic spinal cord compression: survival in different cancer diagnosis in a cohort of 2321 patients. Spine, 2013, 38: 1362–1367. [DOI] [PubMed] [Google Scholar]
- 12. Paton GR, Frangou E, Fourney DR. Contemporary treatment strategy for spinal metastasis: the “LMNOP” system. Can J Neurol Sci, 2011, 38: 396–403. [DOI] [PubMed] [Google Scholar]
- 13. Laufer I, Rubin DG, Lis E, et al The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist, 2013, 18: 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paulino Pereira NR, Janssen SJ, van Dijk E, et al Development of a prognostic survival algorithm for patients with metastatic spine disease. J Bone Joint Surg Am, 2016, 98: 1767–1776. [DOI] [PubMed] [Google Scholar]
- 15. Oken MM, Creech RH, Tormey DC, et al Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol, 1982, 5: 649–655. [PubMed] [Google Scholar]
- 16. Kataoka M, Kunisada T, Tanaka M, et al Statistical analysis of prognostic factors for survival in patients with spinal metastasis. Acta Med Okayama, 2012, 66: 213–219. [DOI] [PubMed] [Google Scholar]
- 17. Walker MP, Yaszemski MJ, Kim CW, Talac R, Currier BL. Metastatic disease of the spine: evaluation and treatment. Clin Orthop, 2003, 415: S165–S175. [DOI] [PubMed] [Google Scholar]
- 18. White BD, Stirling AJ, Paterson E, Asquith‐Coe K, Melder A. Diagnosis and management of patients at risk of or with metastatic spinal cord compression: summary of NICE guidance. BMJ, 2008, 337: a2538. [DOI] [PubMed] [Google Scholar]
- 19. Choi D, Crockard A, Bunger C, et al Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J, 2010, 19: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Majeed H, Kumar S, Bommireddy R, Klezl Z, Calthorpe D. Accuracy of prognostic scores in decision making and predicting outcomes in metastatic spine disease. Ann R Coll Surg Engl, 2012, 94: 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arrigo RT, Kalanithi P, Cheng I, et al Predictors of survival after surgical treatment of spinal metastasis. Neurosurgery, 2011, 68: 674–681. [DOI] [PubMed] [Google Scholar]
- 22. Wise JJ, Fischgrund JS, Herkowitz HN, Montgomery D, Kurz LT. Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine, 1999, 24: 1943–1951. [DOI] [PubMed] [Google Scholar]
- 23. van der Linden YM, Dijkstra SP, Vonk EJ, Marijnen CA, Leer JW. Prediction of survival in patients with metastases in the spinal column: results based on a randomized trial of radiotherapy. Cancer, 2005, 103: 320–328. [DOI] [PubMed] [Google Scholar]
- 24. Yao A, Sarkiss CA, Ladner TR, Jenkins AL 3rd. Contemporary spinal oncology treatment paradigms and outcomes for metastatic tumors to the spine: a systematic review of breast, prostate, renal, and lung metastases. J Clin Neurosci, 2017, 41: 11–23. [DOI] [PubMed] [Google Scholar]
- 25. Tatsui CE, Suki D, Rao G, et al Factors affecting survival in 267 consecutive patients undergoing surgery for spinal metastasis from renal cell carcinoma. J Neurosurg Spine, 2014, 20: 108–116. [DOI] [PubMed] [Google Scholar]
- 26. Sioutos PJ, Arbit E, Meshulam CF, Galicich JH. Spinal metastases from solid tumors. Analysis of factors affecting survival. Cancer, 1995, 76: 1453–1459. [DOI] [PubMed] [Google Scholar]
- 27. Kim HJ, Kim YJ, Seo MD, et al Patterns of palliative procedures and clinical outcomes in patients with advanced non‐small cell lung cancer. Lung Cancer, 2009, 65: 242–246. [DOI] [PubMed] [Google Scholar]


