Abstract
Objective
To investigate the effect of tumor necrosis factor alpha (TNF‐α) on the proliferation of fibroblast‐like synoviocytes (FLS) and the expression of programmed cell death factor 5 (PDCD5) in an inflammatory microenvironment, for the further understanding of the mechanism of action of TNF‐α in promoting the proliferation of synovial cells and the apoptosis of the chondrocytes.
Methods
Articular carriage specimens were obtained from 21 cases with osteoarthritis and 12 cases with femoral neck fractures as healthy controls during arthroplasties. The expression of PDCD5 was evaluated by immunofluorescence analyzed by mean option density (MOD) detected using the software ImagePro Plus. Real‐time PCR was performed to evaluate the transcriptions of PDCD5 and TNF‐α in synovium. FLS cells derived from rheumatoid arthritis patients were cultured in vitro and incubated with different concentrations of TNF‐α. The effects of TNF‐α at different concentrations on the proliferation of FLS cells were detected by Cell Counting Kit‐8 (CCK‐8) assay to evaluate the cell proliferation rate. After incubation with the absence or presence of recombinant human TNF‐α at different concentrations, the FLS cells were isolated for detection of PDCD5 protein and PDCD5 gene. The expression of PDCD5 protein was detected by western‐blot and the transcription of PDCD5 gene from the cells was detected by real‐time quantitative PCR.
Results
The MOD of PDCD5 as well as TNF‐α of osteoarthritis cartilage sections were significantly increased compared with those of the controls, and in synovium there was a positive correlation between transcriptions of their mRNA. When the concentration of TNF‐α was 1 ng/mL, the cell proliferation rate was not significantly different from that of the control group (P = 0.592), while the proliferation of FLS cells was significantly promoted when the concentration of TNF‐α was 5, 10, 15, or 20 ng/mL, and the proliferation‐promoting rates were 35.64% ± 6.96%, 48.72% ± 7.69%, 45.60% ± 8.85%, and 39.32% ± 6.18%, respectively (P < 0.01). The transcription of PDCD5 gene was significantly downregulated, which was 80.44% ± 4.07% and 84.30% ± 5.48%, respectively (P < 0.05), in the FLS cells incubated with TNF‐α at the concentration of 10 and 15 ng/mL for 24 h. When the concentration of TNF‐α was 1, 5, or 20 ng/mL, the transcription of PDCD5 mRNA in FLS cells was not significantly different from that in the control group (P > 0.05). The expression of PDCD5 protein was only significantly downregulated when the concentration of TNF‐α was 10 ng/mL (P < 0.01), while the expression of PDCD5 protein in FLS cells was not significantly different from that in the control group (P > 0.05).
Conclusion
The expression of PDCD5 as well as TNF‐α in osteoarthritis cartilage and synovium was significantly higher than in healthy tissues, and TNF‐α can promote the proliferation of FLS cells in patients with rheumatoid arthritis, and inhibit the expression of PDCD5. PDCD5 may be involved in the abnormal proliferation of synoviocytes and the degeneration of chondrocytes stimulated by TNF‐α.
Keywords: Fibroblast‐likes synoviocytes, Inflammatory microenvironment, Programmed cell death 5, Tumor necrosis factor α
Introduction
According to the standardization of osteoarthritis definitions by osteoarthritis research society international (OARSI), osteoarthritis (OA) is a disorder involving movable joints characterized by cell stress and extracellular matrix degradation initiated by micro‐ and macro‐injuries that activates maladaptive repair responses, including pro‐inflammatory pathways of innate immunity1. OA has long been considered a non‐inflammatory arthropathy resulting from aging or physical deterioration compared with “inflammatory arthritis,” such as rheumatoid arthritis and mandatory spondylitis. Thus, suppression of inflammatory responses via modulation of inflammatory cytokines, which are responsive for inflammatory responses, is a promising strategy for OA therapy.
However, recent studies have proved that the role of inflammatory processes in the mechanism of OA is non‐negligible. Synovial inflammation of OA resulting from cartilage debris released by trauma or other stress activates synoviocytes to release matrix metalloproteinase (MMP) and various inflammatory cytokines, such as tumor necrosis factor α, thereby further promoting matrix degradation and cartilage degradation. By inhibiting the expression of inflammatory factors such as MMP3, the inflammation of osteoarthritis could be alleviate2. The relationship between synovial inflammation and structural damage of the cartilage during the progression of arthritis has been proved by several previous studies, as well as the fact that inflammatory cytokines can affect the prognosis of patients diagnosed with OA3.
Programmed cell death 5 (PDCD5) is a gene located on chromosome 19q12‐q13.1, with a full‐length cDNA of 559 bp and an AATAAA tail signal and a poly A tail, involving 6 exons, encoding 125 amino acids, whose expression is increased during the apoptotic process of TF‐1 cells induced by cytokine withdrawal4. PDCD5 can promote apoptosis induced by different apoptosis‐inducing factors and is widely expressed in various tissues5. Previous studies have found that PDCD5 expression is upregulated in osteoarthritic chondrocytes, suggesting that PDCD5 may be involved in the destruction process of osteoarthritic chondrocytes6. In rheumatoid arthritis, it was found that excessive expression of PDCD5 can enhance the apoptosis of rheumatoid arthritis (RA) FLS induced by triptolide, and the promoting effect of riptolide for the apoptosis of FLS grows stronger with the upregulation of PDCD5 expression, suggesting that PDCD5 may play a role in the process of FLS apoptosis7, 8. At the same time, it was found that TNF‐α can continuously activate FLS, resulting in changes in the microenvironment, such as upregulation of decoy receptors in apoptosis inhibitory molecules9, NF‐κb10 and upstream signaling molecule protein kinase Akt activation11, and P53 mutation12, ultimately enhancing the anti‐apoptotic ability of fibroblast‐like synoviocytes (FLS) and continuing to survive.
The tumor necrosis factor (TNF) family is a cluster of cytokines that may play an important role in the inflammatory response of arthritis. Studies have illustrated the relationship between TNF‐α and inflammatory arthropathy in patients with rheumatoid arthritis and OA. TNF‐α is thus considered a potential target for the treatment of rheumatoid arthritis and OA13. Currently, anti‐TNF‐α therapies are being applied to treat patients with OA and have achieved satisfactory results by reducing inflammation14, 15. However, the mechanism by which TNF‐α and PDCD5 affects the cartilage and synovial fibroblasts in inflammatory environments in patients with arthritis remains unclear.
In our former studies, we illustrated that PDCD5 concentrations in plasma and synovial fluid were significantly elevated and meanwhile inversely correlated with TNF‐α in RA patients compared with controls16.
This study was designed to evaluate the correlation between TNF‐α and PDCD5 in osteoarthritis, and to study its mechanism. The purpose of the present study is to explore: the effect of TNF‐α on the proliferation of FLS in vitro; the effects of TNF‐α on the transcription of PDCD5 gene and the expression of PDCD5 protein in vitro; and the relationship between PDCD5 and TNF‐α in tissues from cartilage or synovium.
Materials and Methods
Obtaining of Cartilage and Synovium Specimens
The cartilage and synovium specimens were obtained from OA patients who underwent total knee arthroplasty at Peking University People's Hospital from November 2016 to January 2017. Thirty‐six cases in total, involving 26 cases clinically diagnosed with OA and 10 individuals without OA in sites of sampling, were included in the study. All cases with OA were clinically diagnosed with OA in compliance with the 1987 American College of Rheumatology (ACR) criteria17. Individuals with rheumatic inflammatory arthritis or other autoimmune diseases that may affect the cartilage were excluded. Considering potential confounders such as age or gender, the study included cases with similar age and gender distribution. The entire research work is in line with universal medical ethic principles and had been approved by the Ethics Committee of Peking University People's Hospital.
Immunohistochemistry
The cartilage and synovium were trimmed to blocks with a side length of approximately 1 cm. The samples were fixed in 4% paraformaldehyde for 48 h, with cartilage decalcified in 10% EDTA solution for 4 weeks, embedded in paraffin, and sectioned (3 μm) after dehydrated with gradient alcohol. The sections were dewaxed, hydrated, and incubated with 0.3% (volume fraction) H2O2/methanol for 30 min at room temperature, blocked by normal sheep serum for 20 min.
After incubation with antibodies at 4°C overnight, the sections were incubated with secondary antibody at 37°C for 30 min. The sections were washed three times with PBS for 5 min each time and colored with DAB‐H2O2. After the reaction, the sections were dehydrated with alcohol, hyalinized with the xylene, and sealed with the neutral resin.
Recovery of Fibroblast‐like Synoviocytes and Cell Cultures
The cryogenic vials containing FLS provided by the Arthritis Clinic & Research Center of Peking University People's Hospital were removed from liquid nitrogen and heated in a 37°C water bath to completely dissolve the cell suspension in 3 min. The fully dissolved cell suspension was quickly transferred onto a sterile clean bench, and the cell suspension in the vials was pipetted into a centrifuge tube containing the culture solution, centrifuged at 250 g for 5 min, and the supernate was discarded. The supernatant was resuspended by adding 2–3 mL of pre‐warmed complete medium.
The new cell suspension was inoculated into the culture flask. After the appropriate amount of the culture solution was added, the cells were incubated in an incubator, and the medium was changed the next day to remove dead cells. Thereafter, the liquid was changed every 3 days. FLS cells from 3–7 passages were selected as cases for the research.
Fibroblast‐like Synoviocytes Proliferation Assays
To assess the effect of TNF‐α on the proliferation of FLS, FLS from passages 3–5 were incubated with TNF‐α in different concentrations, and the proliferation of FLS was measured by CCK‐8 assay. The FLS cells were inoculated into 96‐well plates at a density of 1 × 105/mL 24 h before the tests. The cells were divided into three groups: the pores from the blank controller were incubated without any cells; cells from the treatment group were incubated with recombinant human TNF‐α at several concentrations (0, 1, 5, 10, 15, and 20 ng/mL); and cells from the control group were incubated with nothing but medium. Then the cells were incubated in growth medium with Cell Counting Kit‐8 (CCK‐8) Reagent (Dojindo, Tabaru, Japan).
After incubation for 4 h, the OD value (absorbance) of each sample was measured at 450 nm with a spectrophotometer.
Real‐Time PCR
The fragment of synovium was placed in an RNase‐free EP tube. It was left at room temperature for 15 min after Trizol was added. After centrifugation at 4°C, the centrifuge was transferred to another RNase‐free EP tube, and left to stand for 5 min at room temperature. With chloroform added, the mixture was shacked gently for 15 s, left to stand for 3 min at room temperature, and centrifuged at 4°C.
The upper aqueous phase was then transferred to the third RNase‐free EP tube; 280 μl of absolute ethanol was added. After gentle mixing, the mixture was transferred to RNeasy Mini Column, and centrifuged twice. The sedimentation was washed with 75% alcohol mixed with diethyl decarbonate (DEPC) water added into the EP tube. The RNA was precipitated and centrifuged at 6300 × g for 5 min at 4°C. After dissolving with by DEPC water, 1 μL of the mixture was taken for the measurement of the OD value and concentration of stock solution.
Similarly, FLS were incubated in six culture flasks with the absence or presence of recombinant human TNF‐α for 24 h. Then the RNA was isolated with the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions, and the total RNA samples were transferred at −80°C.
The cDNA was prepared for real‐time PCR, with β2‐microglobulin as an internal control. Details of primers and probes used are illustrated in Table 1. The conditions for each of 40 PCR cycles were as follows: denaturation at 94°C for 40 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. The reaction was preceded with an initial denaturation step of 94°C for 10 min and ended with a final extension step of 72°C for 5 min. PCR were checked for amplification using the ABI PRISM 7500 PCR instrument, and data were analyzed according to the comparative Ct method and were normalized by β2‐microglobulin expression in each sample.
Table 1.
Primers and probes used for target amplification
| Gene | Primer |
|---|---|
| PDCD5 | Forward:GTG ATG CGG CCC AAC AG |
| Reverse:ATC CAG AAC TTG GGC TAA GAT ACT G | |
| TNF‐α | Forward:CAT CCA ACC TTC CCA AAC GC |
| Reverse:CGA AGT GGT GGT CTT GTT GC | |
| β2‐microglobulin | Forward:GAG TAT GCC TGC CGT GTG |
| Reverse:AAT CCA AAT GCG GCA TCT |
PDCD5, programmed cell death factor 5; TNF‐α, tumor necrosis factor alpha.
Western Blot
The cells were trypsinized and divided into six 75‐cm2 culture flasks. After the cells were cultured to logarithmic growth phase, 1% of TNF‐α at 0, 1, 5, 10, 15, and 20 ng/mL concentration was re‐added. The culture medium of FCS‐DMEM was further cultured for 48 h.
Total protein from cells was extracted by incubation in RIPA buffer. After reaction with the 5× loading buffer at 95°C for 5 min, it was immediately transferred to an ice‐cold environment, and finally stored at −80°C. The protein samples were electroblotted onto polyvinylidene difluoride membranes.
Membranes were blocked for 1 h at 37°C and incubated overnight at 4°C with rabbit polyclonal antibody to human PDCD5 (with a final concentration of 1 μg/mL) as a primary antibody and mouse monoclonal antibody to human β‐actin as a loading control.
The membrane was washed three times with TBS‐T for 10 min each time and incubated with the goat anti‐rabbit and goat anti‐mouse IgG conjugate with HRP as a secondary antibody for 40 min at room temperature. Protein bands were detected with ECL. We then analyzed the band intensity.
Parameters and Statistical Analysis
Data analysis was performed using ImagePro Plus (Version 6.0, Rockville, MD, USA) and SPSS (Version 22.0, Chicago, IL, USA).
The quantification of IHC was formalized into a parameter called mean optical density (MOD) detected by ImagePro Plus, which was obtained by dividing the optical density cumulative value of each point on the picture by the area of the target distribution.
The results of PCR were evaluated by the relative quantity (RQ) calculated by ΔΔCt methods.
Each group of data was tested for normality by Kolmogorov–Smirnov test (K‐S test), and the homogeneity test of variance was performed by F‐test. Data with normal distributions and homogeneity of variance were analyzed with independent sample t‐tests and one‐way ANOVA; data with normal distributions but without homogeneity were analyzed with t‐tests; data without normal distributions were analyzed with the Mann–Whitney rank sum test (MW test). All data were presented in the format of mean value ± standard deviation (±SD); P < 0.05 is considered statistically significant. The experiment was repeated at least three times.
Results
Expression of Programmed Cell Death 5 and Tumor Necrosis Factor α in Cartilage and Synovium
The MOD of PDCD5 in OA cartilage was (23.45 ± 5.49) × 10−3, while the MOD of the control cartilage was (15.79 ± 10.76) × 10−3. The calculated P‐value was 0.096 for the K‐S test, which means a normal distribution. An independent sample t‐test showed that P = 0.025.
The MOD of TNF‐α in the OA cartilage was (25.72 ± 6.47) × 10−3, while for the control group it was (15.24 ± 9.47) × 10−3. P = 0.004 in the K‐S test, indicating skewed distribution of the data. For MW test results, we obtained P < 0.01.
That is, the MOD value of PDCD5 and TNF‐α was higher in the osteoarthritic cartilage than in the control group, and the difference was statistically significant (Fig. 1).
Figure 1.
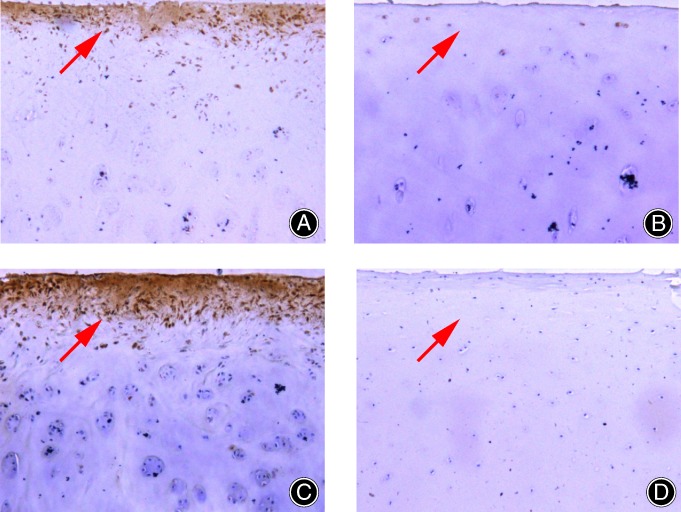
The expression of programmed cell death factor 5 (PDCD5) and tumor necrosis factor alpha (TNF‐α) in the sections: (A) The expression of PDCD5 in osteoarthritis (OA) cartilage; (B) the expression of PDCD5 in the control group; (C) the expression of TNF‐α in OA cartilage; and (D) the expression of TNF‐α in the control group. The expression of both PDCD5 and TNF‐α were increased in OA cartilage compared with the control group.
In synovium, the transcription of both PDCD5 and TNF‐α were increased in OA synovium compared with that of the control group. The difference for TNF‐α was not statistically significant (Table 2). However, when the transcription level of PDCD5 was considered as a variable and the RQ value of TNF‐α was another, Pearson correlation analysis showed that the Pearson correlation coefficient was 0.64 (P < 0.01). Thus, there is a positive correlation between them.
Table 2.
Different expression of PDCD5 and TNF‐α in synovium of OA and control group
| Gene | Groups | RQ | P‐value |
|---|---|---|---|
| PDCD5 | Control | 1.00 | 0.004 |
| OA | 1.47 | ||
| TNF‐α | Control | 2.23 | 0.226 |
| OA | 462.61 |
The transcription of both PDCD5 and TNF‐α were increased in OA synovium compared with that of the control group, yet just PDCD5 with statistical significance. OA, osteoarthritis; PDCD5, programmed cell death factor 5; TNF‐α, tumor necrosis factor alpha.
Effect of Tumor Necrosis Factor α on the Proliferation of Fibroblast‐like Synoviocytes
We found that when the concentration of TNF‐α was 1 ng/mL, the cell proliferation rate was not significantly different from that of the control group (P = 0.592), while when the concentration of TNF‐α was 5, 10, 15, and 20 ng/mL, TNF‐α could promote the proliferation of FLS. The rates of promoting FLS proliferation are illustrated in Table 3 and Fig. 2.
Table 3.
Proportion to promote FLS proliferation with different concentrations of TNF‐α
| Concentration of TNF‐α (ng/mL) | Mean value of proportion to promote FLS proliferation (%) | Standard deviation (%) | P‐value |
|---|---|---|---|
| 1 | 6.51 | 4.26 | 0.59 |
| 5 | 35.64 | 6.96 | <0.01 |
| 10 | 48.72 | 7.69 | <0.01 |
| 15 | 45.60 | 8.85 | <0.01 |
| 20 | 39.32 | 6.18 | <0.01 |
When the concentrations of TNF‐α were 5, 10, 15, and 20 ng/mL, TNF‐α could promote the proliferation of FLS, with the most evident effect at the concentration of 10 ng/mL. FLS, fibroblast‐like synoviocytes; TNF‐α, tumor necrosis factor alpha.
Figure 2.
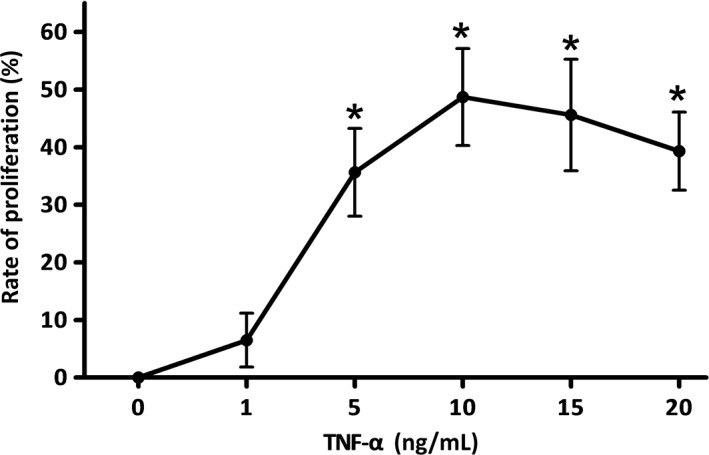
The promotion effect on fibroblast‐like synoviocytes (FLS) proliferation of tumor necrosis factor alpha (TNF‐α) at different concentrations. When the concentrations of TNF‐α were 5, 10, 15, and 20 ng/mL, TNF‐α could promote the proliferation of FLS (P < 0.01). The promotion effect on FLS proliferation peaked when the concentration of TNF‐α was 10 ng/mL, within the range the research had tested.
As the data indicated, only when the concentration of TNF‐α reaches a certain value can the proliferation of FLS be promoted, but this effect is not continuously enhanced as the concentration of TNF‐α increases. At least within the range we tested, the promotion effect on FLS proliferation was most significant when the concentration of TNF‐α was 10 ng/mL.
Inhibitory Effect of Tumor Necrosis Factor α on Programmed Cell Death 5 mRNA Transcription and Protein Expression
The transcription of PDCD5 gene was quantified by real‐time PCR after exposure to different TNF‐α concentrations. RQ values for different groups are illustrated in Table 4.
Table 4.
RQ values in RT‐PCR with different concentrations of TNF‐α
| Concentration of TNF‐α (ng/mL) | RQ | P‐value |
|---|---|---|
| 1 | 0.9369 | 0.284 |
| 5 | 0.8840 | 0.153 |
| 10 | 0.8049 | 0.014 |
| 15 | 0.8430 | 0.038 |
| 20 | 0.9169 | 0.19 |
When the concentration of TNF‐α was 10 or 15 ng/mL, the transcription of PDCD5 mRNA in FLS cells was significantly lower than that in the control group. FLS, fibroblast‐like synoviocytes; PDCD5, programmed cell death factor 5; RQ, relative quantity; TNF‐α, tumor necrosis factor alpha.
We found when TNF‐α was at concentrations of 10 and 15 ng/mL in FLS for 48 h, the mRNA levels of PDCD5 were significantly decreased, and when the concentration of TNF‐α was 1, 5, or 20 ng/mL, the transcription of PDCD5 mRNA in FLS cells was not significantly different from that in the control group (P < 0.05), as indicated by Fig. 3.
Figure 3.
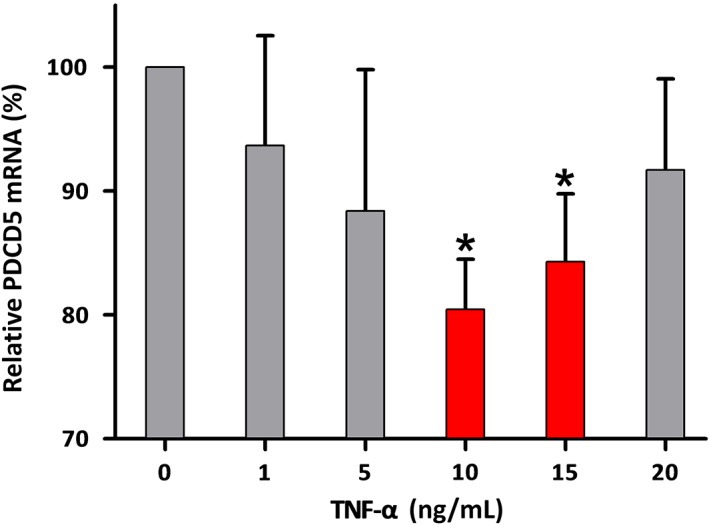
Transcription of programmed cell death factor 5 (PDCD5) mRNA in FLS after incubated with different dose of tumor necrosis factor alpha (TNF‐α) for 48 h. Within the tested range, different concentrations of TNF‐α showed a common inhibitory effect on the transcription of PDCD5 mRNA, but this effect was only statistically significant when the concentrations were 10 and 15 ng/mL (P < 0.05).
As for the expression of PDCD5 protein, when TNF‐α was at a concentration of 10 ng/mL incubated with FLS for 48 h, the level of PDCD5 protein was decreased with statistical significance (P < 0.01), while its expression was not significantly different from that in the control group (P > 0.05), as illustrated in Fig. 4 and Table 5.
Figure 4.
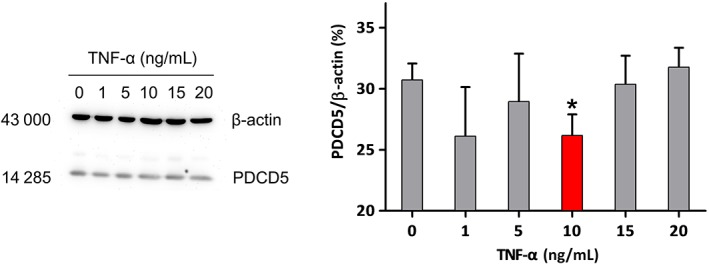
The bands of western blot for the expression of programmed cell death factor 5 (PDCD5) protein in fibroblast‐like synoviocytes (FLS) after incubation with different doses of tumor necrosis factor alpha (TNF‐α) for 48 h and the corresponding mean option density. In the tested range, different concentrations of TNF‐α showed different effects on the expression of PDCD5 protein, but the effect was statistically significant (P < 0.01) only when the concentration of TNF‐α was 10 ng/mL, which was inhibitory.
Table 5.
Grey value of bands in western blot with different concentrations of TNF‐α
| Concentration of TNF‐α (ng/mL) | Relative protein expression | P‐value |
|---|---|---|
| 0 | 30.86 | 0.85 |
| 1 | 26.80 | 0.58 |
| 5 | 30.39 | 0.41 |
| 10 | 27.04 | <0.01 |
| 15 | 30.39 | 0.06 |
| 20 | 32.00 | 0.56 |
When the concentration of TNF‐α was 10 ng/mL, the expression of PDCD5 protein in FLS cells was significantly decreased compared with the control group. FLS, fibroblast‐like synoviocytes; PDCD5, programmed cell death factor 5; TNF‐α, tumor necrosis factor alpha.
Discussion
General Review
Osteoarthritis first manifests as molecular disorders, including abnormalities in joint tissue metabolism, anatomical and/or physiological disorders, of which the main pathological features are cartilage degradation, bone remodeling, osteophyte formation, joint inflammation, and loss of normal joint functions1. Synovitis is an early manifestation of OA, which may alter cartilage function, promote vascular proliferation, and even cause abnormal bone turnover rate, thereby aggravating the joint structure destruction of OA. Thus, exploring the proliferative characteristics of FLS during the pathogenesis of OA is important for a better understanding of OA synovitis and its pathogenesis18.
In turn, abnormal hyperplasia of FLS contributes to OA progression and may be also etiologically regulated by cytokines such as TNF‐α, which is produced primarily by activated macrophages and is capable of inducing FLS proliferation, as well as the production of collagenase, stromelysin, and other enzymes that promote invasion of cartilage and bone19, 20. It has been confirmed that miR‐126 or miR‐155 negatively regulate the expression of TNF‐α in synovitis and, thus, show protective effects21, 22. Obviously, the regulation of FLS plays a vital role in synovitis.
As a pro‐apoptotic gene, the increased expression of PDCD5 has been demonstrated in FLS of synovitis and could enhance triptolide‐induced FLS apoptosis7, 16. The increased expression of PDCD5 may be related with the strengthened apoptosis of chondrocytes and the degeneration of the cartilage.
Therefore, whether there is any relationship between PDCD5 and TNF‐α, and whether TNF‐α could regulate the transcription and expression of some apoptosis gene, to affect the apoptosis of FLS and chondrocytes, remains to be seen.
Effect of Tumor Necrosis Factor α on programmed Cell Death 5 and Fibroblast‐like Synoviocytes
To validate this hypothesis, we explored the effect of TNF‐α on the proliferation of FLS, and its regulated effect on the procedures of PDCD5 translation and expression in FLS and in cartilage. First, our results indicated that the expressions of both PDCD5 and TNF‐α were higher in OA cartilage and synovium. Meanwhile, TNF‐α could promote the proliferation of FLS, which manifested a dose‐dependent pattern. This is consistent with some previous research23. Moreover, our in vitro experiment determined an inhibitory effect of TNF‐α on PDCD5 transcription in FLS by PCR technology. That is, the increase of TNF‐α expression could induce a reduced expression of PDCD5 in synovial membrane through some unknown signaling pathways, which results in the complete inability of FLS to respond to apoptosis signals in synoviocytes.
Yet as we can see, the similar trend of the increased expression of PDCD5 and TNF‐α seemed to be somehow contradictory, with the correlation analysis leaving the issue even more confusing. We hypothesized that there is a negative feedback mechanism between PDCD5 and TNF‐α, inducing different effects in vitro and in vivo. The effect of TNF‐α on different cells may vary. The increase in the inflammatory reaction leads to the increase of PDCD5 expression in cartilage, which promotes the degeneration of cartilage and causes a vicious circle.
To demystify this issue, future studies will focus on which signal pathway has participated in this process. Previous study has proved that TNF‐α derived from pristane primed T cells could activate p38 MAPK and NF‐kappaB pathways in FLS24, and TNF‐α‐induced ASK1‐p38/JNK pathway is an important mediator in FLS with potential therapeutic value25. Besides, various long noncoding RNAs may be associated with the pathogenesis of OA interacting with other cytokines such as matrix metalloproteinase (MMP)‐9, MMP‐13, bone morphogenetic protein (BMP)‐2, COL2A1 and ADAMTS5.26 This may be instructive for further studies.
Limitations of the Study
There are several limitations of the study: the FLS in the cell experiments were obtained from preserved cell lines, rather than tissue specimens corresponding with IHC; the research is mainly focused on correlation analysis, lacking studies on causality or other further mechanisms; and there is an absence of animal experiments, so we could not confirm whether the conclusion is also tenable in‐vivo, which may explain the contradiction we discussed.
Conclusion
In conclusion, TNF‐α could promote the proliferation of FLS and inhibit the expression of PDCD5 by inhibiting its transcription in synovium, while in the OA cartilage and synovium the expressions of both PDCD5 and TNF‐α are increased.
Grant Sources: This work was funded by the National Natural Science Foundation of China (Grant Number: 81371925 and 81802233) and a Peking University International Hospital Research Grant (No. YN2018QN02).
References
- 1. OARSI . Definition of osteoarthritis by Osteoarthritis Research Society International 2015. Available from: https://www.oarsi.org/research/standardization-osteoarthritis-definitions (accessed 17 January 2019).
- 2. Zeng J, Wang H, Shen Z, Yao X, Wu C, Pan T. Curcumin Inhibits Proliferation of Synovial Cells by Downregulating Expression of Matrix Metalloproteinase‐3 in Osteoarthritis. Orthopaedic Surgery, 2018, 11: 117–125. 10.1111/os.12412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mancarella L, Addimanda O, Cavallari C, Meliconi R. Synovial inflammation drives structural damage in hand osteoarthritis: a narrative literature review. Curr Rheumatol Rev, 2017, 13: 43–50. [DOI] [PubMed] [Google Scholar]
- 4. Liu H, Wang Y, Zhang Y, et al TFAR19, a novel apoptosis‐related gene cloned from human leukemia cell line TF‐1, could enhance apoptosis of some tumor cells induced by growth factor withdrawal. Biochem Biophys Res Commun, 1999, 254: 203–210. [DOI] [PubMed] [Google Scholar]
- 5. Yingyu C, Yingmei Z, Ronghua S, Quansheng S, Chunhui D, Dalong M. Preparation and identification of monoclonal antibodies against human apoptosis‐related protein TFAR19. Acta Acad Med Sin, 2000, 22: 502–504. [PubMed] [Google Scholar]
- 6. Aixin C, Ying W, Dalong M, Haowei Z, Siquan L. Characterization of programmed cell death 5 (PDCD5) gene in human cartilage and its possible significance. J Peking Univ (Health Sci), 2003, 35: 481–484. [PubMed] [Google Scholar]
- 7. Jiang J, Wang N, Guan Z, Houshan LV. Programmed cell death 5 factor enhances triptolide‐induced fibroblast‐like synoviocyte apoptosis of rheumatoid arthritis. Artif Cells Blood Substit Immobil Biotechnol, 2010, 38: 38–42. [DOI] [PubMed] [Google Scholar]
- 8. Wang N, Lu HS, Guan ZP, et al Involvement of PDCD5 in the regulation of apoptosis in fibroblast‐like synoviocytes of rheumatoid arthritis. Apoptosis, 2007, 12: 1433–1441. [DOI] [PubMed] [Google Scholar]
- 9. Park YW, Ji JD, Lee JS, Ryang DW, Yoo DH. Actinomycin D renders cultured synovial fibroblasts susceptible to tumour necrosis factor related apoptosis‐inducing ligand (TRAIL)‐induced apoptosis. Scand J Rheumatol, 2003, 32: 356–363. [DOI] [PubMed] [Google Scholar]
- 10. Zhang HG, Huang N, Liu D, et al Gene therapy that inhibits nuclear translocation of nuclear factor kappaB results in tumor necrosis factor alpha‐induced apoptosis of human synovial fibroblasts. Arthritis Rheum, 2000, 43: 1094–1095. [DOI] [PubMed] [Google Scholar]
- 11. Karin M, Lin A. NF‐kappaB at the crossroads of life and death. Nat Immunol, 2002, 3: 221–227. [DOI] [PubMed] [Google Scholar]
- 12. Xu L, Chen Y, Song Q, Xu D, Wang Y, Ma D. PDCD5 interacts with Tip60 and functions as a cooperator in acetyltransferase activity and DNA damage‐induced apoptosis. Neoplasia, 2009, 11: 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaneko K, Sugitani M, Goto M, Murashima A. Tocilizumab and pregnancy: four cases of pregnancy in young women with rheumatoid arthritis refractory to anti‐TNF biologics with exposure to tocilizumab. Mod Rheumatol, 2016, 26: 672–675. [DOI] [PubMed] [Google Scholar]
- 14. Yuan PW, Liu DY, Chu XD, Hao YQ, Zhu C, Qu Q. Effects of preventive administration of juanbi capsules on TNF‐alpha, IL‐1 and IL‐6 contents of joint fluid in the rabbit with knee osteoarthritis. J Tradit Chin Med, 2010, 30: 254–258. [DOI] [PubMed] [Google Scholar]
- 15. Stannus O, Jones G, Cicuttini F, et al Circulating levels of IL‐6 and TNF‐alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr Cartil, 2010, 18: 1441–1447. [DOI] [PubMed] [Google Scholar]
- 16. Wang J, Guan Z, Ge Z. Plasma and synovial fluid programmed cell death 5 (PDCD5) levels are inversely associated with TNF‐alpha and disease activity in patients with rheumatoid arthritis. Biomarkers, 2013, 18: 155–159. [DOI] [PubMed] [Google Scholar]
- 17. Altman RD. Criteria for the classification of osteoarthritis of the knee and hip. Scand J Rheumatol Suppl, 1987, 65: 31–39. [DOI] [PubMed] [Google Scholar]
- 18. Qiang S, Xingfu L, Dong L, Huaishui H, Guofeng D, Huaxiang L. Effects of total glucosides of paeony on the proliferation of fibroblast‐like synovial cells in osteoarthritis. Chin J Rheumatol, 2007, 11: 473–476. [Google Scholar]
- 19. Kim KN, Watanabe S, Ma Y, Thornton S, Giannini EH, Hirsch R. Viral IL‐10 and soluble TNF receptor act synergistically to inhibit collagen‐induced arthritis following adenovirus‐mediated gene transfer. J Immunol, 2000, 164: 1576–1581. [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi T, Okamoto K, Kobata T, Hasunuma T, Sumida T, Nishioka K. Tumor necrosis factor alpha regulation of the FAS‐mediated apoptosis‐signaling pathway in synovial cells. Arthritis Rheum, 1999, 42: 519–526. [DOI] [PubMed] [Google Scholar]
- 21. Gao J, Kong R, Zhou X, Ji L, Zhang J, Zhao D. MiRNA‐126 expression inhibits IL‐23R mediated TNF‐alpha or IFN‐gamma production in fibroblast‐like synoviocytes in a mice model of collagen‐induced rheumatoid arthritis. Apoptosis, 2018, 23: 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu N, Feng X, Wang W, Zhao X, Li X. Paeonol protects against TNF‐alpha‐induced proliferation and cytokine release of rheumatoid arthritis fibroblast‐like synoviocytes by upregulating FOXO3 through inhibition of miR‐155 expression. Inflamm Res, 2017, 66: 603–610. [DOI] [PubMed] [Google Scholar]
- 23. Chen Q, Casali B, Pattacini L, Boiardi L, Salvarani C. Tumor necrosis factor‐alpha protects synovial cells from nitric oxide induced apoptosis through phosphoinositide 3‐kinase Akt signal transduction. J Rheumatol, 2006, 33: 1061–1068. [PubMed] [Google Scholar]
- 24. Zhu W, Jiang C, Xu J, et al Pristane primed rat T cells enhance TLR3 expression of fibroblast‐like synoviocytes via TNF‐alpha initiated p38 MAPK and NF‐kappaB pathways. Clin Immunol Immunopathol, 2015, 156: 141–153. [DOI] [PubMed] [Google Scholar]
- 25. Umar S, Hedaya O, Singh AK, Ahmed S. Thymoquinone inhibits TNF‐alpha‐induced inflammation and cell adhesion in rheumatoid arthritis synovial fibroblasts by ASK1 regulation. Toxicol Appl Pharmacol, 2015, 287: 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xing D, Liang J, Li Y, et al Identification of Long Noncoding RNA Associated with Osteoarthritis in Humans. Orthopaedic Surgery, 2014, 6: 288–293. 10.1111/os.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]


