Abstract
Pulmonary vascular resistance (PVR) and PVR index (PVRI) are key variables in a broad range of contexts, including prediction of outcomes in heart and liver transplantation, determining candidacy for closure of atrial or ventricular septal defects, and guiding treatment of pulmonary hypertension. Significant variability exists among the units used to report PVRI in current literature, making the interpretation of data and translation into clinical practice difficult. Here, we will review the measurement and derivation of PVR and PVRI and demonstrate the extent of confusion in the literature. We conducted a literature search of all published articles in PubMed using the term “PVRI.” This yielded 218 sources with defined units for PVRI, including 33 unique variants. Among all reviewed literature, 45.4% of sources reported PVRI with units ending in m2 (meters squared), which we defined as correct, whereas 54.6% reported PVRI with units not ending in m2, which we defined as incorrect. This lack of uniformity has led to considerable confusion among researchers and clinicians, with potentially life‐altering consequences.
1. BACKGROUND
1.1. Pulmonary vascular resistance
Pulmonary vascular resistance (PVR) describes the resistance that blood must overcome to pass through the pulmonary vasculature. PVR index (PVRI) relates the absolute value of PVR to the patient's body surface area to account for the effect of body size on blood flow.
Although imperfect, the hydraulic equivalent of Ohms law (where resistance = voltage/current) is the most practical formula for determining PVR.
PVR = pressure gradient/pulmonary blood flow.
where pressure gradient = mean pulmonary artery pressure (mPAP) − mean pulmonary artery wedge pressure (PAWP). The accurate measurement of mPAP and PAWP is essential to achieving valid results. It is worth noting that considerable debate exists regarding the timing of PAWP measurement within the respiratory cycle.1 Current consensus documents recommend that PAOP be measured end‐expiration.2 However, some authors suggest that this approach may not represent the most physiological approach as variation across respiratory phases impacts pressure and resistance.1, 3
If PAWP is not obtainable (eg, as may occur with pulmonary artery aneurysm), and assuming the absence of mitral stenosis, then mean left atrial pressure (LAP) may be substituted. Left ventricular end‐diastolic pressure (LVEDP) may also be used as a surrogate for PAOP in this scenario, but with 2 to 3 mm Hg higher values as end‐diastolic pressure is greater than mean diastolic pressure.
In the absence of intracardiac shunting, pulmonary blood flow (Qp) and cardiac output (CO) can be accurately measured using the thermodilution method.1 However, in the presence of a right‐to‐left shunt, the thermodilution method may be confounded by recirculation of the indicator (most often cold saline injectate) through the shunt or tricuspid regurgitation.4 In such cases, the Fick method can be used to determine Qp.
Fick equation:
It should be noted that recent studies have called into question the interchangeability of the thermodilution and Fick methods for the determination of cardiac output.5 Moreover, the accuracy of the Fick method is dependent on the proper assessment of oxygen consumption (VO2). VO2 can be determined through direct measurement or derived using empirical formulae. While both methods of VO2 determination are commonly used, most authors agree that measured VO2 is superior to estimated VO2 for the calculation of pulmonary blood flow.6, 7, 8 When comparing these methods directly, Chase et al found significant discrepancies between measured and estimated VO2 in the setting of heart failure with reduced ejection fraction.9 Therefore, measured VO2 should be used whenever possible.
Next, oxygen saturations are obtained from the pulmonary veins (or from the left atrium or ventricle or assumed if necessary) and from the pulmonary arteries. The following formula is used to determine the oxygen content at each location:
The difference between the pulmonary veins and arteries can then be determined through simple subtraction. All of the above equations can then be used to determine PVR.
1.2. PVR index
Clinicians often index units to express them in terms relative to the wide range of body surface area (BSA) among patients. Pediatric cardiologists have traditionally indexed flows based on the assumption that Qp changes proportionately with body surface area (BSA), while the trans‐pulmonary pressure gradient does not.10
Because indexing of PVR occurs relative to flow and because flow is in the denominator of the equation for PVR, the units of m−2 change to m2 when they are brought up to the numerator in the final result for PVRI:
In adult cardiology, the cardiac index (CI) is the most ubiquitous measurement reported, and its units are L min−1 m−2. PVRI, on the other hand, is correctly reported in units ending in m2. We speculate that many authors incorrectly assume that PVRI is indexed as per m2 or m−2 because of their familiarity with CI.
2. METHODS
A literature search for the term “PVRI” was conducted for all journal articles published between 1 January, 1980 and 3 April, 2018 using PubMed. Search results were screened for duplicates. The clinical context and units used to define PVRI were recorded for each source. Text, figures, and tables were checked for consistency. Units were recorded as published and then standardized to a common format for ease of classification.
Presumed typographical errors were checked against the remainder of text and figures. The units “U,” “IU,” and “UW” were presumed to imply Wood Units or Hybrid Resistance Units. Division signs and dashes implying “per unit” were converted to exponent format for consistency. Correct units for PVRI were defined as the following: WU m2, mm Hg L−1 min m2, and or 80 dynes sec cm−5 m2. Incorrect units were those with m2 in the denominator or its equivalent (m−2), those that did not include m2 in any form, and those that were mathematically nonequivalent to the correct unit definitions. Sources in which units for PVRI were ambiguous or undefined were recorded but not considered correct or incorrect.
Articles for which complete text or figures were unavailable (and did not state units used for PVRI within the abstract) were not included in the study. When no measurable units or ambiguous units were used to define PVRI, the entries were recorded as “ambiguous.” When different units were used to describe PVRI within a single article, each variant was recorded. Sources were classified as either pediatric or nonpediatric. Pediatric sources were defined as those published in pediatric journals or in which the study subjects were children (age < 18), or in which the primary citations were in pediatric journals. When present, the method used for measuring VO2 or calculating PVRI was also recorded.
The initial literature search yielded 326 unique articles in which PVRI was a key variable. Of these results, 94 were excluded because of lack of availability of the full article or text, and 14 were excluded because of ambiguous or undefined units. This yielded 218 sources with defined units for PVRI and full‐text availability. Forty‐five articles were determined to be pediatric in nature; 173 were deemed nonpediatric (Figure 1).
Figure 1.

Flowchart demonstrating selection of journal articles for inclusion in this review
3. RESULTS
Among sources with defined units and full‐text availability, there were 33 unique variations of units used to define PVRI. Four literature sources used two or more units to define PVRI. Overall, the four most common units used to define PVRI were WU m2 (24%), dynes sec cm−5 m2 (22%), dynes sec cm−5 m−2 (18%), and WU m−2 (8%). The remaining 29 variants together made up 29% of all units used.
Among all literature, WU m2 (n = 52) and dynes sec cm−5 m2 (n = 48) were the most commonly used units to define PVRI, followed by dynes sec cm−5 m−2 (n = 26) and WU m−2 (n = 12) (Figure 2).
Figure 2.

Incidence of units used to define pulmonary vascular resistance index (PVRI) in all literature. WU m2 (n = 52) and dynes sec cm−5 m2 (n = 48) were the most commonly used units overall in defining PVRI. The next most common units were dynes sec cm‐5 m−2 (n = 39) and WU m−2 (n = 18). WU, wood unit
Overall, there were more incorrect than correct units used to define PVRI. Among reviewed nonpediatric literature, 41.0% of articles correctly defined PVRI, while 59.0% were incorrect. Among reviewed pediatric literature, 62.2% of articles correctly defined PVRI, while 37.8% were incorrect. Among all reviewed literature, 45.4% of articles correctly defined PVRI, while 54.6% were incorrect (Figure 3).
Figure 3.
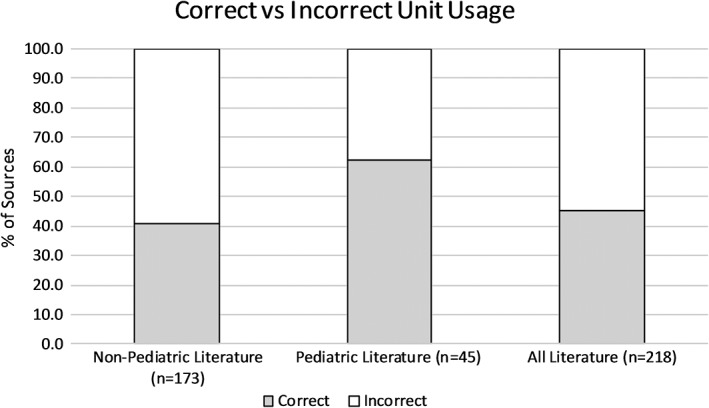
Correct vs incorrect pulmonary vascular resistance index (PVRI) Units. Among nonpediatric literature, 41.0% of articles correctly defined PVRI, while 59.0% were incorrect. Among pediatric literature, 62.2% of articles correctly defined PVRI, while 37.8% were incorrect. Among all literature, 45.4% of articles correctly defined PVRI, while 54.6% were incorrect
4. CONCLUSIONS
Given that PVRI is incorrectly or ambiguously defined in the majority of reviewed literature, it is evident that significant confusion exists among the medical and scientific community. Compounding this issue further is the high degree of variability in the measurement of components used to determine PVRI, including VO2, pressure, and flow. The use of incorrect units or inconsistent measurements creates the potential for errors in clinical decision‐making and adverse patient outcomes.
One such example is the diagnosis of pulmonary arterial hypertension (PAH) and the decision of whether to recommend closure of a ventricular septal defect (VSD) or atrial septal defect (ASD) in the adult patient. Closure of a VSD or ASD in the setting of PAH (defined by the American Heart Association (AHA)/American Thoracic Society (ATS) 2015 Guidelines for Pediatric Pulmonary Hypertension as PVRI >3 WU m2) may lead to unacceptable mortality and morbidity, with complications including acute pulmonary hypertensive crisis, right ventricular failure, and respiratory failure.11, 12, 13 Conversely, failure to close a defect in an adult with nonprohibitive PAH may lead to decreased quality of life and shortened life expectancy.14 Using incorrect units for PVRI could lead to underdiagnosis of PAH and poor outcomes following closure of an ASD or VSD.
For example, a patient with a BSA of 2 m2 and a measured PVR of 2 WU would have a PVRI of 4 WU m2. However, if units ending in m−2 are used to define PVRI (as we have seen is commonplace), then PVRI could be incorrectly reported as 1 WU m−2.
Another example of clinical decision‐making involving PVRI is the assessment of candidacy for cardiac transplantation. Both the International Society for Heart and Lung Transplantation (ISHLT) and American Society of Transplant Physicians (ASTP) utilize PVR and PVRI in their listing criteria for cardiac transplantation as pulmonary hypertension has been associated with poor outcomes following cardiac transplantation.15, 16 Per ISHLT 2006 Guidelines for the Care of Cardiac Transplant Candidates and ASTP 1998 Listing Criteria for Cardiac Transplantation, a PVR of >5 WU and a PVRI of >6 WU are relative contraindications to cardiac transplantation.17, 18 The use of WU to define both PVR and PVRI is clearly erroneous. Recent literature citing the aforementioned sources correctly use WU m2 to define PVRI, presumably expressing the original authors' intent.19
Other clinical areas where PVRI plays an important role include: choice of pharmacotherapy in the medical management of pulmonary hypertension, vasopressor selection in critically ill patients, and hemodynamic assessment in the perioperative period.20, 21, 22, 23
Thus, we contend that a concerted effort should be made by the editorial staff of academic journals and texts to encourage the use of consistent units to define PVRI. We believe that doing so will greatly facilitate the communication and comprehension of scientific literature and help to prevent erroneous diagnosis or clinical decisions.
CONFLICTS OF INTEREST
The authors declare no potential conflict of interests.
REFERENCES
- 1. BL LV, Pomerantsev E, Channick RN. Reliance on end‐expiratory wedge pressure leads to misclassification of pulmonary hypertension. Eur Respir J. 2014;44:425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. VV ML, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573‐1619. [DOI] [PubMed] [Google Scholar]
- 3. Ryan JJ, Rich JD, Thiruvoipati T, Swamy R, Kim GH, Rich S. Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in the classification of patients with pulmonary hypertension. Am Heart J. 2012;163:589‐594. [DOI] [PubMed] [Google Scholar]
- 4. Weyland A, Wietasch G, Hoeft A, et al. The effect of an intracardiac left‐right shunt on thermodilution measurements of cardiac output. An extracorporeal circulation model. Anesthesiology. 1995;44:13‐23. [DOI] [PubMed] [Google Scholar]
- 5. Opotowsky AR, Hess E, Maron BA, et al. Thermodilution vs estimated fick cardiac output measurement in clinical practice: an analysis of mortality From the Veterans Affairs Clinical Assessment, Reporting, and Tracking (VA CART) Program and Vanderbilt University . JAMA Cardiol. 2017;2:1090‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolf A, Pollman MJ, Trindade PT, Fowler MB, Alderman EL. Use of assumed versus measured oxygen consumption for the determination of cardiac output using the Fick principle. Cathet Cardiovasc Diagn. 1998;43(4):372‐380. [DOI] [PubMed] [Google Scholar]
- 7. Kendrick AH, West J, Papouchado M, Rozkovec A. Direct Fick cardiac output: are assumed values of oxygen consumption acceptable? Eur Heart J. 1988;9:337‐342. [DOI] [PubMed] [Google Scholar]
- 8. Fakler U, Pauli C, Hennig M, Sebening W, Hess J. Assumed oxygen consumption frequently results in large errors in the determination of cardiac output. J Thorac Cardiovasc Surg. 2005;130:272‐276. [DOI] [PubMed] [Google Scholar]
- 9. Chase PJ, Davis PG, Wideman L, Starnes JW, Schulz MR, Bensimhon DR. Comparison of estimations versus measured oxygen consumption at rest in patients with heart failure and reduced ejection fraction who underwent right‐sided heart catheterization. Am J Cardiol. 2015;116:1724‐1730. [DOI] [PubMed] [Google Scholar]
- 10. Garson A. The Science and Practice of Pediatric Cardiology. Philadelphia, PA: Lea and Febiger; 1990. [Google Scholar]
- 11. Talwar S, Keshri VK, Choudhary SK, et al. Unidirectional valved patch closure of ventricular septal defects with severe pulmonary arterial hypertension: hemodynamic outcomes. J Thorac Cardiovasc Surg. 2014;148:2570‐2575. [DOI] [PubMed] [Google Scholar]
- 12. Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903‐975. [DOI] [PubMed] [Google Scholar]
- 13. Abman S, Hansmann G, Archer S, et al. 2015 AHA/ATS Guidelines for Pediatric Pulmonary Hypertension . Circulation. 2015;132:2037‐2099. [DOI] [PubMed] [Google Scholar]
- 14. Supomo S, Hartopo AB, Anggrahini DW, Darmawan H, Dinarti LK. Large atrial septal defect closure in a patient with severe pulmonary arterial hypertension. Korean J Thorac Cardiovasc Surg. 2017;50:378‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kula S. Wood units · m2 or Wood units/m2: does it matter? Anatolian J Cardiol. 2016;16:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Butler J, Stankewicz MA, Wu J, et al. Pre‐transplant reversible pulmonary hypertension predicts higher risk for mortality after cardiac transplantation. J Heart and Lung Transplant. 2005;24:170‐177. [DOI] [PubMed] [Google Scholar]
- 17. Goland S, Czer LSC, Kass RM, et al. Pre‐existing pulmonary hypertension in patients with end‐stage heart failure: impact on clinical outcome and hemodynamic follow‐up after orthotopic heart transplantation. J Heart and Lung Transplant. 2007;26:312‐318. [DOI] [PubMed] [Google Scholar]
- 18. Mehra MR, Kobashigawa J, Starling R, et al. Listing Criteria for Heart Transplantation: International Society for Heart and Lung Transplantation Guidelines for the Care of Cardiac Transplant Candidates. J Heart Lung Transplant. 2006;25:1024‐1042. [DOI] [PubMed] [Google Scholar]
- 19. Miller L. Listing criteria for cardiac transplantation: results of an American Society of Transplant Physicians‐National Institutes of Health Conference. Transplantation. 1998;66:947‐951. [DOI] [PubMed] [Google Scholar]
- 20. De Jonge N, Kirkels JH, Klöpping C, et al. Guidelines for heart transplantation. Neth Heart J. 2008;16:79‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beghetti M, Rudzinsk A, Zhang M. Efficacy and safety of oral sildenafil in children with Down syndrome and pulmonary hypertension. BMC Cardiovasc Disord. 2017;17:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shoemaker WC, Appel PL, Kram HB, Duarte D, Harrier HD, Ocampo HA. Comparison of hemodynamic and oxygen transport effects of dopamine and dobutamine in critically ill surgical patients. Chest. 1989;96:120‐126. [DOI] [PubMed] [Google Scholar]
- 23. Al‐Hamoudi WK, Alqahtani S, Tandon P, Ma M, Lee SS. Hemodynamics in the immediate post‐transplantation period in alcoholic and viral cirrhosis . World J Gastroenterol. 2010;16:608‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]


