Abstract
Radiotherapy is one of the main modalities of cancer treatment. However, tumor recurrence following radiotherapy occurs in many cancer patients. A key to solving this problem is the optimization of radiosensitivity. In recent years, long non-coding RNAs (lncRNAs), which affect the occurrence and development of tumors through a variety of mechanisms, have become a popular research topic. LncRNAs have been found to influence radiosensitivity by regulating various mechanisms, including DNA damage repair, cell cycle arrest, apoptosis, cancer stem cells regulation, epithelial–mesenchymal transition, and autophagy. LncRNAs are expected to become a potential therapeutic target for radiotherapy in the future. This article reviews recent advances in the role and mechanism of lncRNAs in tumor radiosensitivity.
Keywords: apoptosis, cancer, epithelial-mesenchymal transition, lncRNA, radioresistance, radiotherapy
Among various RNA species, non-coding RNAs are RNA molecules that transcribe but do not encode proteins. LncRNAs are a group of RNAs, ranging in length from 200 to 100,000 nucleotides, which participate in diverse cellular processes and are involved in disease progression [1]. LncRNAs are often classified into five categories: antisense lncRNA, intronic transcript lncRNA, large intergenic non-coding RNA (lincRNA), promoter-associated lncRNA, and untranslated region-associated lncRNA [2]. LncRNAs are mainly localized to the cell nucleus or cytoplasm [3]. Nuclear lncRNAs can regulate the chromatin architecture of cells by interacting with chromatin remodeling complexes. They also regulate gene expression on the same chromosome [4]. The most common mechanism cytoplasmic lncRNAs use to regulate genes is to act as a competitive endogenous RNA (ceRNA). CeRNAs are reported to function as microRNA (miRNA) sponges by binding specific complementary sequences and protecting target miRNA from being repressed by miRNA [5]. LncRNAs affect biological pathways in various cancers through different mechanisms and influence the expression of target genes at both the transcriptional and post-transcriptional levels.
Radiotherapy is an effective treatment method for many cancers, and approximately half to two-thirds of all patients with cancer receive this type of therapy [6,7]. However, radioresistance is a primary factor that leads to poor prognosis. Sensitivity to radiotherapy is the key to its therapeutic effect on malignant tumors and is a complex process associated with multiple genes, factors, and mechanisms. During radiotherapy, ionizing radiation first induces water radiolysis to produce reactive oxygen species (ROS). Oxygen then provides unpaired electrons for free radicals in DNA molecules, thereby stabilizing infrared-induced DNA damage. Damaged DNA or excessive ROS activate apoptotic signaling pathways in cancer cells, leading to cell death [8]. The effect of radiotherapy is determined by following the 4’Rs of radiobiology: repair of DNA damage, redistribution of the cell cycle, repopulation of tumors, and reoxygenation of hypoxic tumor areas [9]. Radioresistance can be overcome by reducing DNA repair and decreasing DNA damage tolerance through the activation of intracellular pro-survival and antiapoptotic signaling pathways. Previous studies have indicated that lncRNAs influence radioresistance through mechanisms (Table 1 and Figure 1) that include repair of DNA damage, cell cycle arrest, apoptosis, CSCs regulation, epithelial–mesenchymal transition (EMT), and autophagy. In this review, we summarize research on several representative mechanisms of lncRNAs in radiation therapy.
Table 1. Summary of lncRNAs that regulate radiosensitivity in radiation therapy.
| LncRNA | Radiosensitivity | Types of tumor | Mechanism | Molecular target | Reference |
|---|---|---|---|---|---|
| LINP1 | Down | Cervical cancer | DNA damage repair | N/A | [13] |
| MALAT1 | Down | Cervical cancer | DNA damage repair | miR-145 | [15] |
| Down | ESCC | DNA damage repair | CKS1 | [16] | |
| Down | NPC | CSC | miR-1/slug | [54] | |
| HOTAIR | Down | Cervical caner | DNA damage repair | p21 protein | [19] |
| Down | Pancreatic ductal cancer | Wnt/β-catenin pathway | WIF-1 | [25] | |
| Down | Pancreatic cancer | Autophagy | Atg7 | [69] | |
| CRNDE | Down | Lung cancer | DNA damage repair | p21 protein | [20] |
| BOKAS | Down | ESCC | Wnt/β-catenin pathway | WISP1 | [28] |
| LincRNA-p21 | Up | Colorectal cancer | Wnt/β-catenin pathway | N/A | [31] |
| Up | Gastric cancer | Wnt/β-catenin pathway | N/A | [32] | |
| Down | Hepatoma and glioma cancer | Autophagy | N/A | [71] | |
| UCA1 | Down | Prostate cancer | P13k/Akt pathway | N/A | [35] |
| ANCR | Down | NPC | P13k/Akt pathway | N/A | [36] |
| LincRNA-ROR | Down | Colorectal cancer | Apoptosis | p53/miR-145 | [39] |
| ANRIL | Down | NPC | Apoptosis | miR-125a | [40] |
| CCAT1 | Down | Breast cancer | Apoptosis | miR-148b | [41] |
| OIP5-AS1 | Up | Colorectal cancer | Apoptosis | DYRK1Y/miR-369-3p | [43] |
| PVT1 | Down | Lung cancer | Apoptosis | miR-195 | [44] |
| DGCR5 | Down | Laryngeal cancer | Apoptosis | miR-195 | [45] |
| CYTOR | Down | Lung cancer | Apoptosis | miR-195 | [46] |
| NEAT1 | UP | NPC | Apoptosis | miR-101-3b | [47] |
| Down | NPC | EMT | miR-204/ZEB1 | [61] | |
| GAS5 | Up | Cervical cancer | Apoptosis | miR-106b | [48] |
| TALNEC2 | Down | Glioma | CSC | N/A | [56] |
| TUG1 | Down | Bladder cancer | EMT | miR-145/ZEB2 | [60] |
| Down | Bladder cancer | N/A | HMGB | [74] | |
| HULC | Down | Prostate cancer | Autophagy | N/A | [71] |
| SNHG18 | Down | Glioma | N/A | Semaphorin 5A | [73] |
| SUMO1P3 | Down | Hepatocellular cancer | N/A | N/A | [75] |
| GACAT3 | Up | Lung cancer | N/A | TIMP2 | [76] |
N/A: The corresponding mechanisms and targets are not yet clear, and need further exploration.
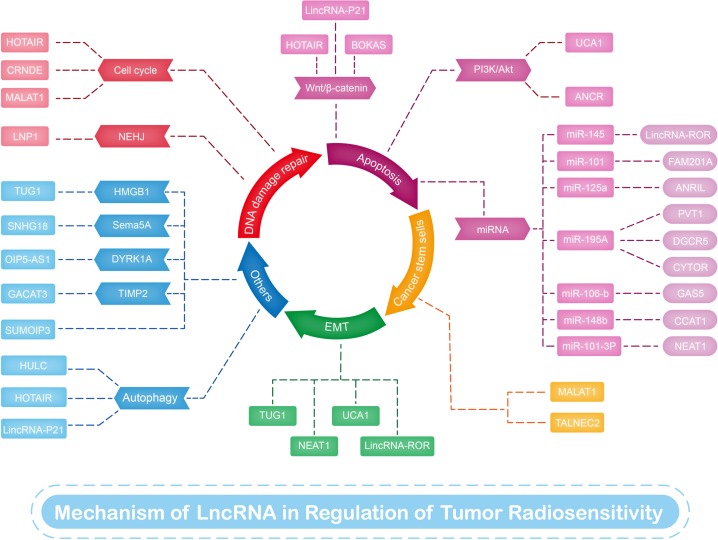
Figure 1. LncRNAs involved in radiosensitivity of various cancers.
This figure summarizes the mechanisms and targets of lncRNAs in regulating radiosensitivity of radiotherapy.
DNA damage repair
Ionizing radiation can generate high ROS known as free radicals and cause DNA double-strand breaks that ultimately lead to extensive genomic instability and cell death. After DNA damage, cells initiate a repair system to fix DNA damage, in turn reducing sensitivity to radiotherapy [10]. DNA damage can be repaired either by homologous recombination or through non-homologous end joining (NHEJ). The latter is a major pathway for the repair of damaged DNA and is a key determinant of infrared (IR) resistance in cancer cells [11]. Zhang et al. [12] found that lncRNA in the NHEJ pathway 1 (LINP1) accelerated NHEJ repair and decreased the sensitivity of tumors to ionizing radiation by staining for γ-H2AX to assess the level of double-strand breaks. Wang et al. [13] also revealed that in cervical cancer, LINP1 enhanced the efficiency of DNA damage repair via the NHEJ pathway and decreased radiosensitivity.
Following IR-induced DNA damage, molecules at cell cycle checkpoints begin to regulate and arrest cell cycle progression, subsequently repairing damaged DNA or initiating apoptosis if this repair is unsuccessful. Cell cycle arrest is closely related to radiosensitivity, and when cancer cells are arrested in the G2/M phase, their radiosensitivity increases [14]. More researchers have found that lncRNAs regulate radiosensitivity by affecting DNA damage repair via cell cycle arrest. Lu et al. [15] found that knockdown of metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) negatively regulated miR-145 levels and increased radiosensitivity. In another study, the authors reported that the downregulation of MALAT1 affected DNA repair by inducing G2/M arrest, which is vital in radiotherapy. Moreover, Li et al. [16] showed that MALAT1 induced the radioresistance of esophageal squamous cell carcinoma (ESCC) cells by down-regulating the expression of cdc kinase subunit 1 (CKS1), which is a regulatory protein that induces G2/M arrest by inhibiting p27 [17]. Other studies showed that p53/p21 activation induced G1 arrest [18] and that the suppression of p53 activation promoted G2/M phase arrest. Jing et al. [19] demonstrated that hox transcript antisense intergenic lncRNA (HOTAIR) increased radioresistance by inhibiting the expression of p21 and inducing cell cycle arrest at the S phase. Likewise, Zhang et al. [20] found that the colorectal neoplasia differentially expressed (CRNDE) gene contributed to radioresistance in lung cancer cells by suppressing p21 expression and affecting the G1/S transition. The aforementioned studies illustrated that lncRNAs could regulate the radiosensitivity of various cancers such as cervical cancer, ESCC, and lung cancer by affecting DNA repair via accelerating NHEJ repair, regulating the expression of p53/p21 and affecting cell cycle arrest. However, the mechanism underlying lncRNA-mediated regulation of DNA damage repair is complicated and the related studies have only been conducted in vitro. More in vivo models therefore need to be established to further study these mechanisms.
Apoptosis
Apoptosis, also known as programmed cell death, is the main mode of cell death after irradiation [21], and is regulated by both intrinsic and extrinsic pathways. The intrinsic pathway responds to signals such as ultraviolet radiation or DNA damage and activates ‘executioner’ caspases through a mitochondria-dependent pathway [22]. Radiation can trigger apoptosis intrinsically or extrinsically by causing DNA damage or other types of severe cellular injury such as reactive oxidative stress, thus leading to downstream gene cascade reactions involving Bcl-2, caspases, Wnt/β-catenin, and other molecular signals [23]. The Wnt/β-catenin signaling pathway is a pro-survival pathway that facilitates extensive crosstalk with other signaling pathways such as phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) and signal transducers and activators of transcription (STAT). Accumulating evidence indicates that the Wnt/β-catenin pathway regulates radiosensitivity by participating in proliferation and apoptosis [24]. Jiang et al. [25] found that HOTAIR affected the radiosensitivity of pancreatic ductal adenocarcinoma cells via Wnt inhibitory factor 1, which was demonstrated to be a primary inhibitor of the Wnt/β-catenin pathway [26]. In lung cancer radiotherapy, radiosensitivity can be reduced by inactivating β-catenin, mediated by HOTAIR upregulation [27]. The gene encoding Wnt1-inducible signaling pathway protein 1 (WISP1) is expressed in response to Wnt1 and β-catenin and mediates radioresistance both in vitro and in xenograft tumor models. LncRNA BOKAS was found as a natural antisense transcript of BOK, Zhang et al. [28] found that BOKAS promoted WISP1 upregulation and induced radioresistance. Moreover, lincRNA-p21, a downstream agent of p53 [29], downregulated the expression of β-catenin at the post-transcriptional level [30], and increased the sensitivity of colorectal cancer [31] and gastric cancer cells [32] to radiotherapy via the Wnt/β-catenin signaling pathway.
PI3K/Akt is another critical signal pathway influencing cellular proliferation, apoptosis, and progression [33]. Its activity induces the resistance of human cancer cells to radiation therapy via three main mechanisms: intrinsic radioresistance, tumor cell proliferation, and hypoxia [34]. Ghiam et al. [35] observed that knockdown of urothelial carcinoma associated 1 (UCA1) in prostate cancer cells could induce radiosensitivity by reducing Akt activation. Recently, Ma et al. [37] eported that anti-differentiation non-coding RNA (ANCR) promoted radioresistance by inhibiting phosphatase and tensin homolog (PTEN) [36], which was confirmed to regulate the PI3K/Akt pathway.
The majority of miRNAs play vital roles in regulating biological processes such as cell differentiation and apoptosis [38]. In recent studies, lncRNAs were reported to function as miRNA sponges to regulate radiosensitivity in cancer cells. Yang et al. [39] discovered that lincRNA–ROR reduced the radiosensitivity of colorectal cancer cells by suppressing cell viability and promoting apoptosis via activation of p53/miR-145. Similarly, antisense non-coding RNA in the INK4 locus (ANRIL) enhanced the radioresistance of nasopharyngeal carcinoma (NPC) cells by targeting miR-125a [40]. LncRNA colon-cancer-associated transcript-1 (CCAT1) also improved the radioresistance of breast cancer cells by negatively regulating miR-148b expression [41]. Family with sequence similarity 201-member A (FAM201A) was reported to mediate the radiosensitivity of ESCC by regulating ataxia telangiectasia mutation and mammalian target of rapamycin (mTOR) expression via miR-101 [42]. The dual-specificity tyrosine phosphorylation-regulated kinase-1A (DYRK1A) functions as both a tumor-suppressing and oncogenic factor in various cancers. LncRNA opa-interacting protein 5 antisense RNA 1 (OIP5-AS1) was found to increase the radiosensitivity of colorectal cancer cells by regulating the expression of DYRK1A and miR-369-3p [43]. Coincidentally, the lncRNAs plasmacytoma variant translocation 1 (PVT1), diGeorge syndrome critical region gene 5 (DGCR5), and cytoskeleton regulator RNA (CYTOR) were confirmed to decrease the radiosensitivity of non-small cell lung cancer cells by sponging miR-195 [44–46]. On the other hand, other lncRNAs increase radiosensitivity via miRNA targeting. For instance, Wang et al. [47] found that nuclear enriched abundant transcript 1 (NEAT1) enhanced the radiosensitivity of NPC cells and promoted their apoptosis by targeting miR101-3p. Moreover, growth arrest special 5 (GAS5) increased the radiosensitivity of cervical cancer cells by inhibiting miR-106b [48]. Collectively, these findings indicated that lncRNAs could affect the proliferation and apoptosis of cancer cells by regulating related signaling pathways, such as the Wnt/β-catenin pathway and PI3K/Akt pathway, or by acting as miRNAs sponges, thereby affecting the radiosensitivity of cancer cells. The discovery of more signaling pathways and miRNAs involved in this mechanism is needed to potentially improve the radiosensitivity of cancer cells.
CSCs
CSCs are small subpopulations of cancer cells that can renew themselves and have the ability to generate heterogeneous cell lineages that make up the tumor [49]. During malignant transformation, CSCs display specific molecular characteristics and properties, which may be related to tumor invasiveness, resistance to treatment, and the tendency toward metastasis and diffusion [50]. CSCs are strongly related to tumor aggressiveness and treatment response, and eliminating CSCs can cure cancer [51]. CSCs are reported to promote radioresistance via various pathways that include activating the DNA damage response, scavenging intracellular ROS, inducing hypoxia, and modulating the microenvironment. Increasing evidence indicated that CSCs contributed to radioresistance, which was associated with both intrinsic and extrinsic determinants and could result in the failure of radiation treatment [52]. The intrinsic determinants reportedly include ROS levels, DNA repair capability, cell cycle status, apoptosis, autophagy, and regulation of survival pathways, whereas extrinsic determinants involve hypoxic microenvironments [53]. Thus, CSCs have been investigated in basic cancer research and these studies are rapidly expanding into many related aspects including radiosensitization. The results of recent investigations suggested the possibility that lncRNAs may influence cancer radiotherapy by regulating CSCs. Jin et al. [54] examined the radiosensitivity of NPC cells and demonstrated that MALAT1 expression was increased in NPC cell lines. They observed that MALAT1 decreased the sensitivity of NPC cells to IR by modulating CSC activity and regulating the miR-1/slug axis. Glioma stem cells (GSCs) are a small subpopulation of CSCs that have been shown to be involved in tumor infiltration, resistance to cancer therapy, and tumor recurrence. Previous investigations have shown that CSCs promote glioma radioresistance via preferential activation of the DNA damage checkpoint response and upregulation of DNA repair capacity [55]. Recently, Brodie et al. [56] demonstrated that tumor-associated long non-coding RNA expressed on chromosome 2 (TALNEC2) promoted the tumorigenic potential and mesenchymal transformation of GSCs and increased their resistance to radiation. All of the above-mentioned findings showed that multiple mechanisms contributed to the role of CSCs in radioresistance. LncRNAs such as MALAT1 and TALNEC2 can increase the radiation resistance of NPC cell lines and glioma cells by regulating the activity of CSCs. However, very few studies on the effect of lncRNAs on cancer radiotherapy through CSCs have been conducted, and these models are still in the experimental stages in vitro. A better understanding of CSCs and associated lncRNAs that regulate radioresistance could potentially lead to new treatment strategies for cancer radiotherapy.
EMT
EMT is an important biological process through which epithelial cells lose their polarity and are converted into the mesenchymal phenotype. EMT is an embryonic procedure that induces the loss of cell–cell contact and invasion, and is associated with resistance to chemotherapeutic drugs and radiation [57]. Cells undergoing EMT acquire mesenchymal traits with the upregulation of N-cadherin, vimentin, and transcription factors including snail, slug, zinc finger E-box-binding homeobox 1 (ZEB1), and zinc finger E-box-binding homeobox 2 (ZEB2) [58]. Snail and slug inhibit p53-mediated apoptosis in response to IR, whereas ZEB1 promotes IR-induced DNA damage repair. EMT contributes to radioresistance by inducing hypoxia, increasing DNA repair ability and activating growth factor pathways [59]. LncRNA taurine up-regulated gene 1 (TUG1) has been shown to induce radioresistance by promoting EMT and targeting the miR-145/ZEB2 axis [60]. Likewise, Lu et al. [61] reported that NEAT1 knockdown reversed the EMT phenotype by targeting miR-204/ZEB1 in NPC cells, indicating that NEAT1 serves as an EMT inducer and can lead to the radioresistance of NPC cells. Yang et al. [62] demonstrated that downregulating UCA1 reduced the expression of EMT markers such as matrix metalloproteinase (MMP)-2, MMP-9, ZEB1, and vimentin. The authors concluded that the downregulation of UCA1 could induce radiosensitivity in colorectal cancer cells by suppressing EMT. Another study showed that lincRNA–ROR induced EMT [63], but whether it promoted radioresistance by regulating EMT has not been studied. As mentioned above, lncRNAs can regulate the radiosensitivity of cancer cells by inducing or inhibiting EMT through different mechanisms. This suggests that EMT is involved in radioresistance and that specifically targeting EMT can provide a novel solution to improve the therapeutic effectiveness of radiation against cancer.
Other mechanisms
Autophagy has been identified as a key catabolic process that contributes to the maintenance of cellular homeostasis via the decay of damaged or unwanted proteins and dysfunctional cytoplasmic organelles [64]. Autophagy is a procedure of cellular self-consumption that begins with Unc-51-like kinase (ULK) activity in the ULK/ATG13/ATG101/FIP-200 (RB1CC1) complex. At various stages, autophagy is regulated by multiple pathways such as PI3K/Akt/mTOR complex 1 and AMP-activated protein kinase. Autophagy has two opposite functions associated with radiation stress in cancer cells. One of these is cytoprotection, inhibition of which renders cancer cells sensitive to treatment, and the other is cytotoxicity, which promotes cancer cell death [65]. Several investigations have demonstrated that inhibiting autophagy in cancer cells, such as in ESCC and non-small cell lung cancer (NSCLC), contributes to the radiosensitivity of tumor cells [66,67]. Autophagy is reported to contribute to radioresistance through factors such as the degree of tumor hypoxia, the presence of CSCs, and the ability to repair DNA damage [68]. LncRNAs reportedly regulate cancer cell radiosensitivity by modulating different autophagic effects. HOTAIR, mentioned in the previous section, was found to decrease radiosensitivity by promoting autophagy via the upregulation of Atg7 expression in pancreatic cancer cells [69]. Moreover, Chen et al. [70] demonstrated that lncRNA highly upregulated in liver cancer (HULC) reduced autophagy by interacting with Beclin-1 and inhibiting mTOR, thereby decreasing the sensitivity of prostate cancer cells to irradiation. In addition, Shen et al. [71] observed that lincRNA-p21 decreased radiosensitivity in hypoxic hepatoma and glioma cells by downregulating autophagy through the hypoxia-inducible factor-1/Akt/mTOR/P70S6K pathway in hypoxic tumor cells. Collectively, these data show the complex crosstalk between autophagy and lncRNAs that is relevant in radiotherapy. However, more complex experimental studies are required to further elucidate the various mechanisms.
Other lncRNAs have been reported to regulate radiosensitivity by targeting specific molecules that are claimed to be involved in radiotherapy. Small nucleolar RNA host gene 18 (SNHG18) is significantly upregulated in clinical glioma tissues and is negatively associated with semaphorin 5A (Sema5A) expression. A recent study showed that inhibiting SNHG18 reduced the radioresistance of glioma cells via Sema5A [72]. Another study found that knockdown of high mobility group box 1 (HMGB1) led to greater DNA damage and enhanced radiosensitivity [73]. LncRNA TUG1 knockdown enhanced the radiosensitivity of bladder cancer cells by reducing the expression of HMGB1 [74]. Zhou et al. reported that small ubiquitin-like modifier 1 pseudogene 3 (SUMO1P3) reduced radiosensitivity in hepatocellular carcinoma cells [75], whereas the lncRNA gastric cancer-associated transcript 3 (GACAT3) enhanced radiosensitivity by targeting tissue inhibitor of metalloproteinases 2 (TIMP2) [76]. However, the mechanisms of these lncRNAs need to be further investigated to identify their specific association with radiotherapy.
Discussion and perspectives
Radiotherapy is an important cancer treatment, but its effectiveness is limited by radioresistance. The underlying mechanisms behind radioresistance have not been fully elucidated. Overcoming radioresistance is one of the main challenges in cancer research. This review has summarized the lncRNAs that are associated with radiosensitivity via phenomena that include DNA damage repair, apoptosis, CSC regulation, EMT and autophagy.
LncRNAs have been demonstrated to be involved in a variety of mechanisms, such as transcriptional regulation, protein modification, translation and formation of RNA-protein or protein-protein complexes. Many researchers believe that lncRNAs alone might not be sufficient to drive cell signaling, and that in turn, cell signaling might not be triggered without lncRNAs [77]. Studies on lncRNAs are of great significance in exploring the molecular signaling underlying complex radioresistant processes. Recently, advances of lncRNAs expressions in cancers have highlighted the potential roles as biomarkers in diagnosis and prognosis of the patients [78,79]. At present, there has been no study on the relationship between radiotherapy efficacy and lncRNAs as biomarkers in radiotherapy patients. Therefore, investigations of clinical radiotherapy sensitivity and lncRNAs are necessary to directly prove the relationship between lncRNAs and radiotherapy sensitivity of cancer cells. In addition, in clinical application, lncRNAs have also been recognized as oncogenes or tumor suppressor genes that can regulate the sensitivity of cancer cells to anticancer regimens [80]. LncRNAs present a potential molecular biomarker in radiotherapy and targeted molecular agents against lncRNAs would be a promising new way to treat radioresistance of cancers from current, as evidenced by both in vitro and in vivo studies. However, more basic and clinical studies are needed to investigate how lncRNAs and signaling molecules work together to influence various aspects of radiosensitivity as well as to study the application value of lncRNAs in radiotherapy.
Abbreviations
- ANCR
anti-differentiation non-coding RNA
- ANRIL
antisense non-coding RNA in the INK4 locus
- CCAT1
colon-cancer-associated transcript-1
- CRNDE
colorectal neoplasia differentially expressed
- DGCR5
DiGeorge syndrome critical region gene 5
- HOTAIR
hox transcript antisense intergenic lncRNA
- MALAT1
metastasis-associated lung adenocarcinoma transcript 1
- NEAT1
nuclear enriched abundant transcript 1
- NHEJ
non-homologous end joining
- PTEN
phosphatase and tensin homolog
- PVT1
plasmacytoma variant translocation 1
- TIMP2
tissue inhibitor of metalloproteinases 2
- UCA1
urothelial carcinoma associated 1
- WISP1
Wnt1 inducible signaling pathway protein 1
Funding
This study was sponsored by grants from the National Natural Science Foundation of China [81703024].
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Author Contribution
Xi Yang and Jing Cai designed the review. Jiamin Zhu, Shusen Chen, and Baixia Yang wrote this paper. Weidong Mao revised the manuscript.
References
- 1.Mercer T.R., Dinger M.E. and Mattick J.S. (2009) Long non-coding RNAs: insights into functions. Nat. Rev. Genet. 10, 155–159 10.1038/nrg2521 [DOI] [PubMed] [Google Scholar]
- 2.Ponting C.P., Oliver P.L. and Reik W. (2009) Evolution and functions of long noncoding RNAs. Cell 136, 629–641 10.1016/j.cell.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 3.Dinger M.E.et al. (2008) Differentiating protein-coding and noncoding RNA: challenges and ambiguities. PLoS Comput. Biol. 4, e1000176 10.1371/journal.pcbi.1000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W.et al. (2018) Epigenetic modifications in cardiovascular aging and diseases. Circ. Res. 123, 773–786 10.1161/CIRCRESAHA.118.312497 [DOI] [PubMed] [Google Scholar]
- 5.Thomson D.W. and Dinger M.E. (2016) Endogenous microRNA sponges: evidence and controversy. Nat. Rev. Genet. 17, 272–283 10.1038/nrg.2016.20 [DOI] [PubMed] [Google Scholar]
- 6.Delaney G.et al. (2005) The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 104, 1129–1137 10.1002/cncr.21324 [DOI] [PubMed] [Google Scholar]
- 7.Smith L.et al. (2009) Proteomic identification of putative biomarkers of radiotherapy resistance: a possible role for the 26S proteasome? Neoplasia 11, 1194–1207 10.1593/neo.09902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rey S.et al. (2017) Molecular targeting of hypoxia in radiotherapy. Adv. Drug. Deliv. Rev. 109, 45–62 10.1016/j.addr.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 9.Good J.S. and Harrington K.J. (2013) The hallmarks of cancer and the radiation oncologist: updating the 5Rs of radiobiology. Clin. Oncol. (R. Coll. Radiol.) 25, 569–577 10.1016/j.clon.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 10.Shimura T.et al. (2010) Acquired radioresistance of human tumor cells by DNA-PK/AKT/GSK3beta-mediated cyclin D1 overexpression. Oncogene 29, 4826–4837 10.1038/onc.2010.238 [DOI] [PubMed] [Google Scholar]
- 11.Goldstein M. and Kastan M.B. (2015) The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu. Rev. Med. 66, 129–143 10.1146/annurev-med-081313-121208 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y.et al. (2016) Long noncoding RNA LINP1 regulates repair of DNA double-strand breaks in triple-negative breast cancer. Nat. Struct. Mol. Biol. 23, 522–530 10.1038/nsmb.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X.et al. (2018) LINP1 facilitates DNA damage repair through non-homologous end joining (NHEJ) pathway and subsequently decreases the sensitivity of cervical cancer cells to ionizing radiation. Cell Cycle 17, 439–447 10.1080/15384101.2018.1442625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y.et al. (2017) 14-3-3sigma Contributes to Radioresistance By Regulating DNA Repair and Cell Cycle via PARP1 and CHK2. Mol. Cancer Res. 15, 418–428 10.1158/1541-7786.MCR-16-0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu H.et al. (2016) Long non-coding RNA MALAT1 modulates radiosensitivity of HR-HPV+ cervical cancer via sponging miR-145. Tumour Biol. 37, 1683–1691 10.1007/s13277-015-3946-5 [DOI] [PubMed] [Google Scholar]
- 16.Li Z.et al. (2017) Long noncoding RNA MALAT1 affects the efficacy of radiotherapy for esophageal squamous cell carcinoma by regulating Cks1 expression. J. Oral Pathol. Med. 46, 583–590 10.1111/jop.12538 [DOI] [PubMed] [Google Scholar]
- 17.Ganoth D.et al. (2001) The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat. Cell Biol. 3, 321–324 10.1038/35060126 [DOI] [PubMed] [Google Scholar]
- 18.Kramer D.L.et al. (1999) Polyamine analogue induction of the p53-p21WAF1/CIP1-Rb pathway and G1 arrest in human melanoma cells. Cancer Res. 59, 1278–1286 [PubMed] [Google Scholar]
- 19.Jing L.et al. (2015) HOTAIR enhanced aggressive biological behaviors and induced radio-resistance via inhibiting p21 in cervical cancer. Tumour Biol. 36, 3611–3619 10.1007/s13277-014-2998-2 [DOI] [PubMed] [Google Scholar]
- 20.Zhang M.et al. (2018) Long noncoding RNA CRNDE/PRC2 participated in the radiotherapy resistance of human lung adenocarcinoma through targeting p21 expression. Oncol. Res. 26, 1245–1255 10.3727/096504017X14944585873668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaman S., Wang R. and Gandhi V. (2014) Targeting the apoptosis pathway in hematologic malignancies. Leuk. Lymphoma 55, 1980–1992 10.3109/10428194.2013.855307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gondhowiardjo S. (2004) Apoptosis, angiogenesis and radiation treatment. Acta. Med. Indones 36, 100–108 [PubMed] [Google Scholar]
- 23.Zaman S., Wang R. and Gandhi V. (2014) Targeting the apoptosis pathway in hematologic malignancies. Leuk. Lymphoma 55, 1980–1992 10.3109/10428194.2013.855307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart D.J. (2014) Wnt signaling pathway in non-small cell lung cancer. J. Natl. Cancer Inst. 106, djt356 10.1093/jnci/djt356 [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y.et al. (2016) The long non-coding RNA HOTAIR affects the radiosensitivity of pancreatic ductal adenocarcinoma by regulating the expression of Wnt inhibitory factor 1. Tumour Biol. 37, 3957–3967 10.1007/s13277-015-4234-0 [DOI] [PubMed] [Google Scholar]
- 26.Xue J.et al. (2017) Activation of PPARalpha by clofibrate sensitizes pancreatic cancer cells to radiation through the Wnt/beta-catenin pathway. Oncogene. 37, 953–962 10.1038/onc.2017.401 [DOI] [PubMed] [Google Scholar]
- 27.Chen J.et al. (2015) Radiotherapy induced Lewis lung cancer cell apoptosis via inactivating beta-catenin mediated by upregulated HOTAIR. Int. J. Clin. Exp. Pathol. 8, 7878–7886 [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H.et al. (2015) Targeting WISP1 to sensitize esophageal squamous cell carcinoma to irradiation. Oncotarget 6, 6218–6234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huarte M.et al. (2010) A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142, 409–419 10.1016/j.cell.2010.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon J.H.et al. (2012) LincRNA-p21 suppresses target mRNA translation. Mol. Cell 47, 648–655 10.1016/j.molcel.2012.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G.et al. (2014) LincRNA-p21 enhances the sensitivity of radiotherapy for human colorectal cancer by targeting the Wnt/beta-catenin signaling pathway. Oncol. Rep. 31, 1839–1845 10.3892/or.2014.3047 [DOI] [PubMed] [Google Scholar]
- 32.Chen L.et al. (2018) LincRNA-p21 enhances the sensitivity of radiotherapy for gastric cancer by targeting the beta-catenin signaling pathway. J. Cell. Biochem. 120, 6178–6187 10.1002/jcb.27905 [DOI] [PubMed] [Google Scholar]
- 33.Gupta A.K.et al. (2003) Radiation sensitization of human cancer cells in vivo by inhibiting the activity of PI3K using LY294002. Int. J. Radiat. Oncol. Biol. Phys. 56, 846–853 10.1016/S0360-3016(03)00214-1 [DOI] [PubMed] [Google Scholar]
- 34.Bussink J., van der Kogel A.J. and Kaanders J.H. (2008) Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 9, 288–296 10.1016/S1470-2045(08)70073-1 [DOI] [PubMed] [Google Scholar]
- 35.Fotouhi Ghiam A.et al. (2017) Long non-coding RNA urothelial carcinoma associated 1 (UCA1) mediates radiation response in prostate cancer. Oncotarget 8, 4668–4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma X.et al. (2018) LncRNA ANCR promotes proliferation and radiation resistance of nasopharyngeal carcinoma by inhibiting PTEN expression. Onco. Targets Ther. 11, 8399–8408 10.2147/OTT.S182573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Georgescu M.M. (2010) PTEN tumor suppressor network in PI3K-Akt pathway control. Genes. Cancer 1, 1170–1177 10.1177/1947601911407325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J.et al. (2015) Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics 13, 17–24 10.1016/j.gpb.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang P.et al. (2017) The long noncoding RNA-ROR promotes the resistance of radiotherapy for human colorectal cancer cells by targeting the p53/miR-145 pathway. J. Gastroenterol. Hepatol. 32, 837–845 10.1111/jgh.13606 [DOI] [PubMed] [Google Scholar]
- 40.Hu X., Jiang H. and Jiang X. (2017) Downregulation of lncRNA ANRIL inhibits proliferation, induces apoptosis, and enhances radiosensitivity in nasopharyngeal carcinoma cells through regulating miR-125a. Cancer Biol. Ther. 18, 331–338 10.1080/15384047.2017.1310348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai Y.et al. (2018) Down-regulation of LncRNA CCAT1 enhances radiosensitivity via regulating miR-148b in breast cancer. Cell Biol. Int. 42, 227–236 10.1002/cbin.10890 [DOI] [PubMed] [Google Scholar]
- 42.Chen M.et al. (2018) Long noncoding RNA FAM201A mediates the radiosensitivity of esophageal squamous cell cancer by regulating ATM and mTOR expression via miR-101. Front. Genet. 9, 611 10.3389/fgene.2018.00611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou Y.et al. (2018) LncRNA OIP5-AS1 regulates radioresistance by targeting DYRK1A through miR-369-3p in colorectal cancer cells. Eur. J. Cell Biol. 97, 369–378 10.1016/j.ejcb.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 44.Wu D.et al. (2017) Knockdown of Lncrna PVT1 enhances radiosensitivity in non-small cell lung cancer by sponging Mir-195. Cell. Physiol. Biochem. 42, 2453–2466 10.1159/000480209 [DOI] [PubMed] [Google Scholar]
- 45.Tang T., Shan G. and Zeng F. (2018) Knockdown of DGCR5 enhances the radiosensitivity of human laryngeal carcinoma cells via inducing miR-195. J. Cell. Physiol. 234, 12918–12925 10.1002/jcp.27958 [DOI] [PubMed] [Google Scholar]
- 46.Zhang J. and Li W. (2018) Long noncoding RNA CYTOR sponges miR-195 to modulate proliferation, migration, invasion and radiosensitivity in nonsmall cell lung cancer cells. Biosci. Rep. 38, BSR20181599 10.1042/BSR20181599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y.et al. (2017) Long non-coding RNA NEAT1 regulates epithelial membrane protein 2 expression to repress nasopharyngeal carcinoma migration and irradiation-resistance through miR-101-3p as a competing endogenous RNA mechanism. Oncotarget 8, 70156–70171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao J.et al. (2018) LncRNA GAS5 confers the radio sensitivity of cervical cancer cells via regulating miR-106b/IER3 axis. Int. J. Biol. Macromol. 126, 994–1001 10.1016/j.ijbiomac.2018.12.176 [DOI] [PubMed] [Google Scholar]
- 49.Clarke M.F.et al. (2006) Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 66, 9339–9344 10.1158/0008-5472.CAN-06-3126 [DOI] [PubMed] [Google Scholar]
- 50.Frank N.Y., Schatton T. and Frank M.H. (2010) The therapeutic promise of the cancer stem cell concept. J. Clin. Invest. 120, 41–50 10.1172/JCI41004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skvortsova I.et al. (2015) Radiation resistance: Cancer stem cells (CSCs) and their enigmatic pro-survival signaling. Semin. Cancer Biol. 35, 39–44 10.1016/j.semcancer.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 52.Butof R., Dubrovska A. and Baumann M. (2013) Clinical perspectives of cancer stem cell research in radiation oncology. Radiother. Oncol. 108, 388–396 10.1016/j.radonc.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 53.Chang L.et al. (2016) Cancer stem cells and signaling pathways in radioresistance. Oncotarget 7, 11002–11017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin C.et al. (2016) The role of MALAT1/miR-1/slug axis on radioresistance in nasopharyngeal carcinoma. Tumour Biol. 37, 4025–4033 10.1007/s13277-015-4227-z [DOI] [PubMed] [Google Scholar]
- 55.Bao S.et al. (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 10.1038/nature05236 [DOI] [PubMed] [Google Scholar]
- 56.Brodie S.et al. (2017) The novel long non-coding RNA TALNEC2, regulates tumor cell growth and the stemness and radiation response of glioma stem cells. Oncotarget 8, 31785–31801 10.18632/oncotarget.15991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foroni C.et al. (2012) Epithelial-mesenchymal transition and breast cancer: role, molecular mechanisms and clinical impact. Cancer Treat. Rev. 38, 689–697 10.1016/j.ctrv.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 58.Scheel C.et al. (2011) Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 145, 926–940 10.1016/j.cell.2011.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Creighton C.J., Chang J.C. and Rosen J.M. (2010) Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J. Mammary Gland Biol. Neoplasia 15, 253–260 10.1007/s10911-010-9173-1 [DOI] [PubMed] [Google Scholar]
- 60.Tan J.et al. (2015) Double-negative feedback loop between long non-coding RNA TUG1 and miR-145 promotes epithelial to mesenchymal transition and radioresistance in human bladder cancer cells. FEBS Lett. 589, 3175–3181 10.1016/j.febslet.2015.08.020 [DOI] [PubMed] [Google Scholar]
- 61.Lu Y.et al. (2016) The long non-coding RNA NEAT1 regulates epithelial to mesenchymal transition and radioresistance in through miR-204/ZEB1 axis in nasopharyngeal carcinoma. Tumour Biol. 37, 11733–11741 10.1007/s13277-015-4773-4 [DOI] [PubMed] [Google Scholar]
- 62.Yang X.et al. (2018) Downregulation of long noncoding RNA UCA1 enhances the radiosensitivity and inhibits migration via suppression of epithelialmesenchymal transition in colorectal cancer cells. Oncol. Rep. 40, 1554–1564 [DOI] [PubMed] [Google Scholar]
- 63.Hou P.et al. (2014) LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis. 5, e1287 10.1038/cddis.2014.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xin Y.et al. (2017) Role of autophagy in regulating the radiosensitivity of tumor cells. J. Cancer Res. Clin. Oncol. 143, 2147–2157 10.1007/s00432-017-2487-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Y.et al. (2015) Autophagy and its function in radiosensitivity. Tumour Biol. 36, 4079–4087 10.1007/s13277-015-3496-x [DOI] [PubMed] [Google Scholar]
- 66.Chen Y.et al. (2015) Combining radiation with autophagy inhibition enhances suppression of tumor growth and angiogenesis in esophageal cancer. Mol. Med. Rep. 12, 1645–1652 10.3892/mmr.2015.3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu G.et al. (2017) Role of autophagy and apoptosis in non-small-cell lung cancer. Int. J. Mol. Sci. 18, 367 10.3390/ijms18020367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li H.et al. (2018) Autophagy-regulating microRNAs: potential targets for improving radiotherapy. J. Cancer Res. Clin. Oncol. 144, 1623–1634 10.1007/s00432-018-2675-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu C.et al. (2018) Inhibition of long non-coding RNA HOTAIR enhances radiosensitivity via regulating autophagy in pancreatic cancer. Cancer Manag. Res. 10, 5261–5271 10.2147/CMAR.S174066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen C.et al. (2018) LncRNA HULC mediates radioresistance via autophagy in prostate cancer cells. Braz. J. Med. Biol. Res. 51, e7080 10.1590/1414-431x20187080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen Y.et al. (2017) LincRNA-p21 knockdown enhances radiosensitivity of hypoxic tumor cells by reducing autophagy through HIF-1/Akt/mTOR/P70S6K pathway. Exp. Cell Res. 358, 188–198 10.1016/j.yexcr.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 72.Zheng R.et al. (2016) Upregulation of long noncoding RNA small nucleolar RNA host gene 18 promotes radioresistance of glioma by repressing semaphorin 5A. Int. J. Radiat. Oncol. Biol. Phys. 96, 877–887 10.1016/j.ijrobp.2016.07.036 [DOI] [PubMed] [Google Scholar]
- 73.Ke S.et al. (2015) Downregulation of high mobility group box 1 modulates telomere homeostasis and increases the radiosensitivity of human breast cancer cells. Int. J. Oncol. 46, 1051–1058 10.3892/ijo.2014.2793 [DOI] [PubMed] [Google Scholar]
- 74.Jiang H.et al. (2017) Down-regulation of LncRNA TUG1 enhances radiosensitivity in bladder cancer via suppressing HMGB1 expression. Radiat. Oncol. 12, 65 10.1186/s13014-017-0802-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y.et al. (2018) Knockdown of SUMO1P3 represses tumor growth and invasion and enhances radiosensitivity in hepatocellular carcinoma. Mol. Cell. Biochem. 450, 125–134 10.1007/s11010-018-3379-8 [DOI] [PubMed] [Google Scholar]
- 76.Yang X.et al. (2018) High expression of lncRNA GACAT3 inhibits invasion and metastasis of non-small cell lung cancer to enhance the effect of radiotherapy. Eur. Rev. Med. Pharmacol. Sci. 22, 1315–1322 [DOI] [PubMed] [Google Scholar]
- 77.Peng W.X.et al. (2017) LncRNA-mediated regulation of cell signaling in cancer. Oncogene 36, 5661–5667 10.1038/onc.2017.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sarfi M., Abbastabar M. and Khalili E. (2019) Long noncoding RNAs biomarker-based cancer assessment. J. Cell. Physiol. 234, 16971–16986 10.1002/jcp.28417 [DOI] [PubMed] [Google Scholar]
- 79.Crea F.et al. (2014) Identification of a long non-coding RNA as a novel biomarker and potential therapeutic target for metastatic prostate cancer. Oncotarget 5, 764–774 10.18632/oncotarget.1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Q.N.et al. (2017) Long non-coding RNAs in anti-cancer drug resistance. Oncotarget 8, 1925–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]