Abstract
Introduction
Questions remain whether HIV pre‐exposure prophylaxis (PrEP) can be translated into a successful public health intervention, leading to a decrease in population‐level HIV incidence. We use examples from HIV treatment and contraceptives to discuss expectations for PrEP uptake, adherence, and persistence and their combined impact on the epidemic.
Discussion
Targets for PrEP uptake must be based on the local HIV epidemic and will depend on appropriate estimates of the key populations at risk for HIV. However, there is evidence that targets, once established, can successfully be met and that uptake may increase with awareness. Messaging around adherence should include that daily adherence is the goal (except for those MSM for whom event‐driven dosing is a good fit), but perfect adherence should not be a barrier. Ideally, clients persist on PrEP for as long as they are at risk for HIV. While PrEP will be most effective when coverage is focused on high‐risk populations, normalizing rather than stigmatizing PrEP will be highly beneficial.
Conclusions
While many challenges to PrEP implementation exist, we focused on the three key steps of uptake, adherence and persistence as measurable processes that can lead to improved coverage and decreased HIV incidence.
Keywords: HIV prophylaxis, Implementation, Adherence, Uptake, Retention, Prevention
1. Introduction
Randomized controlled trials demonstrated that antiretroviral pre‐exposure prophylaxis (PrEP), in pill and vaginal ring forms, is protective against HIV 1, 2, 3, 4. PrEP was first approved for use in 2012 as daily pills and in 2015 global guidelines recommended PrEP as part of effective combination HIV prevention, paving the way for implementation projects in a variety of settings and populations. Nevertheless, because of adherence challenges, including uptake and persistence (i.e. continued PrEP use), in some populations, there have been questions whether PrEP is achieving implementation success and whether it can contribute to reductions in new HIV infections 5. Global use is currently far short of the UNAIDS goal of 3 PrEP million users by 2020, while 1.8 million individuals were infected with HIV in 2017 6 Strong trial results, regulatory approval and clinical guidelines, all of which PrEP has, do not always translate into successful public health interventions.
PrEP is a novel HIV prevention approach – a biomedical intervention for otherwise‐healthy persons requiring at least some continued contact with the healthcare system – with no clear prior model to develop expectations for success. PrEP draws important, but distinct, parallels with ART (another use of antiretrovirals) and contraception (another prevention strategy) that may help inform parameters for success in PrEP implementation. In light of data from PrEP clinical trials and early implementation projects, we consider expectations across key domains – specifically, uptake, adherence and persistence 7 – that can lead to an impact in HIV incidence (Figure 1).
Figure 1.
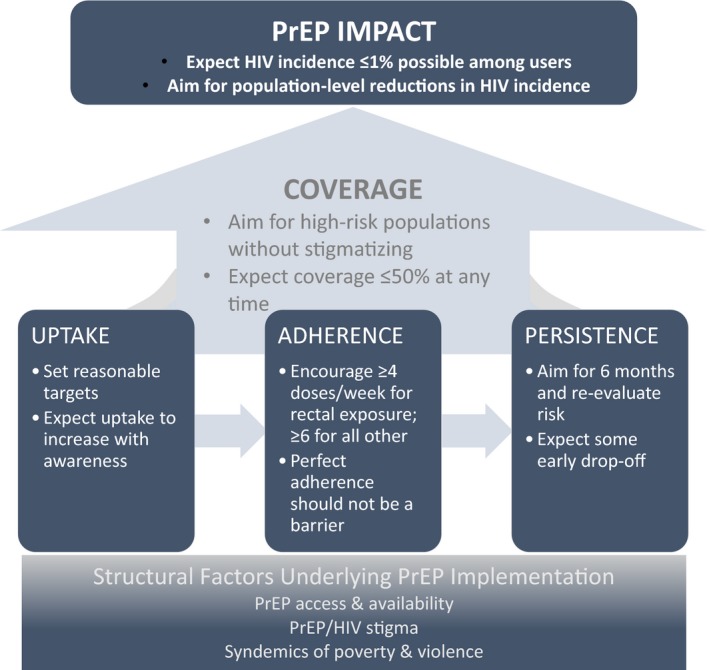
Conceptual framework for PrEP impact.
2. Discussion
2.1. Expectations for uptake
For an individual, PrEP uptake is the first step in achieving protection against HIV. For a population, the number of persons initiating PrEP is the foundation of achieving impact. Of the targeted three million PrEP users, 2018 estimates suggest 300,000 unique PrEP users exist globally, mostly in the United States 8. With antiretroviral therapy (ART), the UNAIDS initiative aims for 90% uptake by 2020. This target uses a fairly straightforward denominator – all persons living with HIV. In contrast, the PrEP denominator is not as easily defined, as HIV risk is hard to assess outside of clinical trials or may not even been known by the participant; for instance, even the CDC's multiplier approach is limited by missing data for risk groups other than MSM 9. PrEP use is not lifelong and depends on risk for HIV, which fluctuates over time. In contraception, a common metric is unmet need, which is determined by the prevalence of several factors, such as pregnancy risk and fertility intention 10, 11 and may be more relevant to developing a PrEP denominator. However, calculating unmet need is a difficult undertaking 12 and there may be more accessible metrics for PrEP, such as the PrEP‐to‐need ratio 13. It is important to note that there is no threshold or goal for PrEP‐to‐need ratios, but it has been used to compare PrEP uptake between key populations 14.
Determining unmet need and uptake targets for PrEP is very much a work in progress. Countries and municipalities have used a variety of approaches to set meaningful uptake targets. In the United States, the CDC estimated the denominator population for PrEP, calculating that 1.1 million individuals meet basic eligibility criteria 9, with only a minority (currently around 220,000) having started PrEP to date 8 and a lively debate exists around improving these criteria 15. Seattle, on a smaller scale, set a target of 50% of high‐risk men who have sex with men (MSM) for PrEP initiation and have reached 36% 16. New South Wales, Australia set a target of 3700 high‐risk users for its PrEP rollout programme, calculated as 9% of high‐risk persons; demand rapidly exceeded expectations and more than 9000 initiated PrEP 17, with an observed 31.5% decrease in new HIV infections in the general MSM population over the following year 18. Uptake was also high in multiple open‐label extension projects 19, 20, 21, 22, 23, suggesting high acceptance among individuals with increased awareness and motivation to use PrEP 16, 24, 25, 26. Most of these results come from MSM, a well‐defined key population in high resource settings; identifying the population at need for women (and other key populations) may face other challenges, including limited resources for research, stigma, gender expectations and the relatively nascent rollout in these populations.
2.2. Expectations for adherence
Adherence is key to PrEP's HIV protective effect and was admittedly the Achilles’ heel of some PrEP clinical trials 27. Data from pharmacokinetic studies have helped define potential benchmarks for PrEP use, correlating HIV protection with four or more doses per week for rectal exposure and six or more doses per week for other exposures 19, 28, 29. HIV protection has been nearly complete for those with PrEP detected in their body across a range of settings 19, 23, 30, 31. These data allow for some flexibility regarding event‐driven dosing for MSM, which has demonstrated protection and is recommended in many situations 20, 32, 33.
Setting programmatic benchmarks for adherence is essential for identifying shortfalls for further evaluation and intervention. Daily adherence will be the goal for most individuals 34, 35, but perfection need not be a barrier to potential users 27; event‐driven and seasonal use of PrEP present additional challenges for assessing adherence that require consideration. Given programmatic constraints, adherence should be measured through the most reasonably accessible and preferably objective measure 14, 27, 36. For instance, PrEP adherence (assessed by pharmacy refills) in a large U.S. healthcare system was 92% on average, and no HIV seroconversions were seen 31. In Sydney's rollout programme, adherence was 83% on average as measured by pharmacy refill, with an HIV incidence rate of 0.05 per 100 person‐years (and zero among those actively on PrEP) 37. A range of interventions to improve ART adherence exist, including motivational interviewing, support groups (both real world and online), and text messaging and may be adapted to support PrEP 38, 39.
For ART, 100% adherence is the ideal and an objective measure of adequate adherence is viral suppression. The UNAIDS target is for 90% of those taking ART to be virally suppressed, with current global estimates of 82% 40. For contraception, user‐dependent methods, including condoms and injectables, can prevent upwards of 98% of pregnancies with perfect use, although with typical adherence prevention can be as low as 82% 41. Notably, methods depending less on users, such as implants, tend to have the smallest gap between typical and perfect use 41. As long‐acting injectable PrEP is currently under study, this experience has potential relevance for future PrEP options. For now, contraceptives and ART demonstrate average real‐world adherence resulting in effectiveness on the order of 80% to 90% and this may be a reasonable expectation for PrEP as well.
2.3. Expectations for persistence
Once an individual starts PrEP, persistence reflects sustained use; a related concept that prevention programmes may also consider is retention, meaning continued involvement in HIV prevention, such as returning for clinic visits. For ART, indefinite persistence is absolutely essential – individuals benefit only when taking ART. In contrast, for contraception, life‐long, uninterrupted persistence is rarely desired. PrEP falls somewhere between the two, arguably much closer to contraception than to ART.
In theory, the target for PrEP persistence is simple: individuals should take PrEP as long as they are at risk for HIV, a concept known as prevention‐effective adherence 36. However, this concept can be challenging to put in practice for individuals (as mapping risk may be difficult for both PrEP users and providers) and for programmes (since measuring use and risk periods may be difficult to measure and track at scale); financial and systematic barriers may also impede patients from achieving their desired persistence 42, 43, 44. For individuals, risk should be considered in terms of periods or seasons, not days or weeks 45; thus, we suggest an expectation of six months of use, while not a perfect fit for all PrEP users, might be useful for programme evaluation and good habit development 46. A discrete period of planned PrEP use aligns with CDC recommendations that PrEP needs be reevaluated every 12 months 47; a six‐month period is also in line with early implementation projects 30, 32, 48. For instance, the median time on PrEP was 6.3 months for U.S. MSM 49 and in South Africa, 52% of participants had high TFV‐DP concentrations at 30 weeks in the ADAPT study 34. Rates were higher for the dapivirine ring, with 86% using it after six months in one open‐label study 21. However, retention has been lower in some PrEP demonstration projects among some key populations, suggesting an important area for future work 50, 51. Similar to adherence, actually assessing persistence can be challenging, in particular when event‐driven PrEP is used and the time between expected refills is unclear; measures of visit retention may be important to understand persistence as well. Further work understanding event‐driven and seasonal PrEP use is needed.
Additionally, there are data to suggest that persistence has been improving since PrEP was first approved. Data from Gilead Science (Figure 2) found only 10% of users remained on PrEP by six months when first introduced, increasing to 50% two years later and >60% by 2017. This change over time suggests that persistence may increase with community awareness of and support for PrEP 52. Whether persistence will show a similar pattern over time in other populations and countries is yet to be seen.
Figure 2.
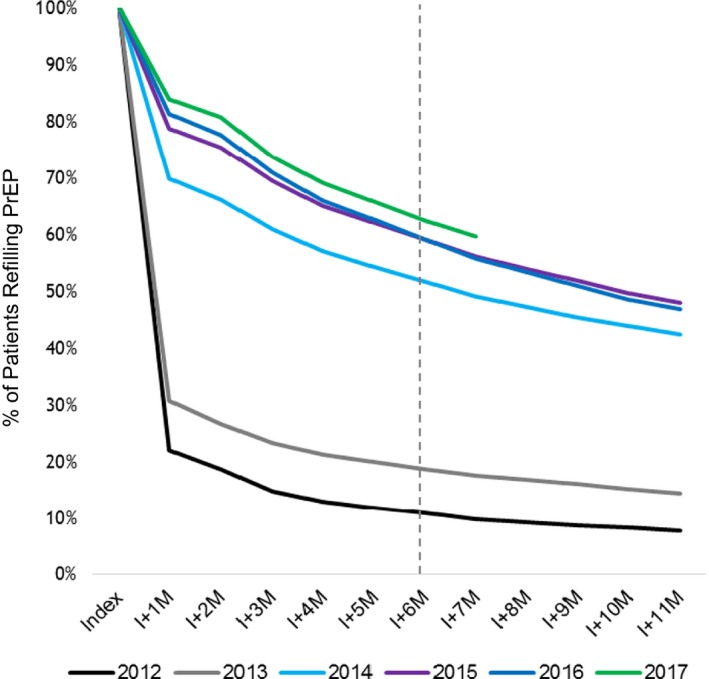
Rapid increase in PrEP persistence among U.S. users, by pharmacy refill Data from 2012 to 2017, a greater proportion of PrEP users were refilling their prescriptions at six months (dotted line). Data courtesy of Gilead Science Inc.
Among individuals living with HIV, the current expectation is lifelong ART persistence, but data suggest only 72% to 89% of those on therapy continue to 12 months 40. Contraceptive continuation rates are even lower: in the United States, 44% of women continued oral contraceptives after six months (by pharmacy refill) dropping to 29% after 12 months; only 40% of women continued with vaginal contraceptive rings by six months 53. Altogether, this data suggests an ambitious aim would be for half or slightly more of PrEP users to continue for six months. However, like contraception, it should not be considered a failure for some individuals to try out a method, discontinue it, try it again, or find another method 54, 55, 56. Importantly, as noted above, PrEP persistence should align with risk for HIV acquisition and some individuals may have shorter, seasonal periods of risk, while still benefitting from PrEP during those periods.
2.4. Expectations for coverage leading to impact
The impact of PrEP should be assessed by the overall reduction in HIV incidence not just among users but in the broader population. We define coverage as the composite of uptake and persistence with sufficient adherence on PrEP within the target population, functioning then as the link to impact 57; critically, high uptake without persistence will not lead to impactful coverage. Like prevalence, coverage can be measured at a given point or over a period of time, though this time period should be reported clearly 14, 57. Mathematical models suggest focusing on coverage of high‐risk groups, rather than the general population, is more cost‐effective and in some settings prevents more total infections 58, 59, 60. Even among highest risk groups, complete coverage is not necessarily the goal and is likely not feasible 61, 62, 63, 64. While adherence and drug costs are important questions in modelling, several papers show that even 50% coverage of high‐risk groups can be cost‐effective 61, 64. However, there are benefits to widespread access to PrEP, including normalization of PrEP and better clinical conversations of sexual behaviour 65, and we do not suggest tight restrictions on who can access PrEP.
As noted above, the current UNAIDS goal for ART coverage is 90%, with a recent global estimate of 77% 40. Recent modern contraceptive coverage estimates were 46% among LMIC 66 and 57% globally 67. While the target population for PrEP is smaller than for either contraceptives or ART, it seems reasonable, over time, to expect average PrEP coverage among the at‐risk denominator to be up to 50%, with large variation among countries and populations.
A notable and unexpected finding of PrEP has been that effectiveness (i.e. HIV protection in open‐label settings) has been higher than efficacy (i.e. protection seen in clinical trials) (Figure 3). A drop in benefit is usually seen with implementation of an intervention as it moves from controlled clinical trials to more open delivery (known as the efficacy‐effectiveness gap) 68, 69. A potential explanation is that once individuals know PrEP works (and are not taking a placebo 70), they are willing to use PrEP and use it well.
Figure 3.
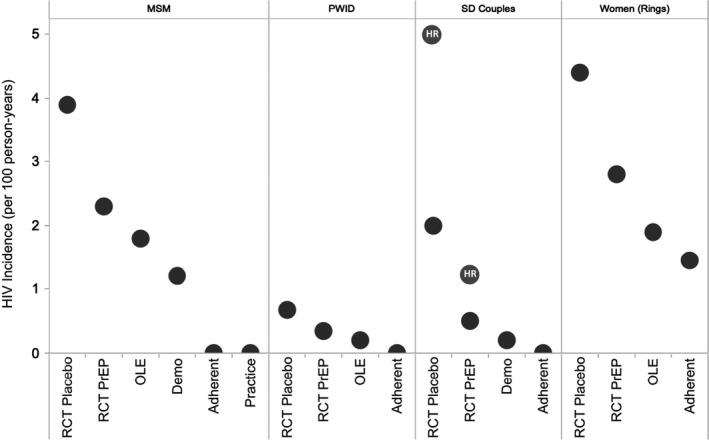
PrEP effectiveness, by study type and population. Compared to placebo populations, HIV incidence (y‐axis) decreases among those taking PrEP whether in clinical studies or open‐label settings, across various at‐risk groups: MSM, PWID, sero‐discordant couples, and women. HIV incidence is lowest when PrEP adherence is high. HR =high risk sero‐discordant couples 71; Demo, demonstration project; OLE, open‐label extension; Practice, Real‐world clinical practice; RCT, randomized controlled trial. Data sources: MSM 4, 19, 72, 73, PWID 23, 74, Serodiscordant Couples 3, 30, Vaginal Rings 1, 21, 75.
Moreover, PrEP benefits extend not only the individual but the overall population, providing something akin to herd protection by reducing secondary infections 76. Rapid population‐level reductions in HIV incidence on the order of 25% to 40% attributable in part to PrEP may already be happening in London, Sydney and San Francisco, based on preliminary, although observational, data 18, 26, 77. With strong uptake, adherence and persistence, data from various open‐label settings (Figure 3) suggest HIV incidence among users can be reduced to around 1% per year or less; among those who are highly adherent, it can approach 0% (with true breakthrough infections carefully investigated to inform future practice).
For ART, the current 90‐90‐90 initiative was derived from models suggesting these benchmarks would lead to an 80% decrease in AIDS‐related mortality and 90% reduction in HIV infections by 2030 78. The impact of contraception on maternal health has been an estimated 44% reduction in maternal deaths globally 79. However, ART and contraception provide secondary benefits as well, such as reducing the number of orphans and improving the economic and educational status of women and their families respectively 80, 81. Secondary benefits for PrEP can be expected as well, such as increased STI testing 82 and early detection of other conditions 83.
3. Conclusions
Defining success in PrEP implementation requires appropriate expectations and goals for each step along the path to achieving impact – and the patience to meet these goals. While we have focused on three specific aspects of adherence that are measurable and proximal to population‐level impact, we recognize that many factors – from national and local polices to social influences and the daily challenges facing those most at‐risk for HIV – underlie PrEP implementation and must be addressed (Figure 1). The rollout of PrEP has generally been slow and in some cases challenging, as was true with contraceptives 84 and other HIV prevention methods 85 – although successes in both low‐ and high‐resources settings should not be ignored 37, 86, 87. Through the use of informed, tailored targets around PrEP uptake, adherence, persistence and coverage, as presented in this paper, we can improve PrEP rollout, maximize HIV prevention efforts, and work towards population‐level reductions in HIV incidence.
Competing interests
JMB, NRM, MNP and JEH have been part of studies with study drug donated by Gilead Sciences.
Author's contributions
MNP and JMB developed the concept and drafted the initial manuscript. JEH, NH, JR and NRM assisted with finalizing the manuscript.
Acknowledgements
Funding
This study was supported by NIH Clinical Center R01 MH095507 and P30 AI027757.
Pyra, M. N. , Haberer, J. E. , Hasen, N. , Reed, J. , Mugo, N. R. and Baeten, J. M. Global implementation of PrEP for HIV prevention: setting expectations for impact. J Int AIDS Soc. 2019; 22:e25370
References
- 1. Baeten JM, Palanee‐Phillips T, Brown ER, Schwartz K, Soto‐Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV‐1 prevention in women. N Engl J Med [Internet]. 2016. Feb 22 [cited 2016 Nov 3];375(222). Available from: 10.1056/nejmoa1506110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nel A, van Niekerk N, Kapiga S, Bekker L‐G, Gama C, Gill K, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med [Internet]. 2016. Nov 30 [cited 2018 Apr 18];375(22). Available from: http://www.nejm.org/doi/10.1056/NEJMoa1602046?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dwww.ncbi.nlm.nih.gov [DOI] [PubMed] [Google Scholar]
- 3. Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen J. Concern as HIV prevention strategy languishes. Science. 2018;359(6381):1205. [DOI] [PubMed] [Google Scholar]
- 6. Global HIV & AIDS statistics — 2018 fact sheet [Internet]. UNAIDS; [cited 2019 May 30]. Available from: https://www.unaids.org/en/resources/fact-sheet [Google Scholar]
- 7. Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Country Updates[Internet]. PrEPWatch. [cited 2018 May 1]. Available from: https://www.prepwatch.org/country-updates/
- 9. Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre‐exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28(12):850–857.e9. [DOI] [PubMed] [Google Scholar]
- 10. Bradley SEK, Casterline JB. Understanding unmet need: history, theory, and measurement. Stud Fam Plann. 2014;45(2):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cleland J, Machiyama K. Unmet need for family planning: past achievements and remaining challenges. Semin Reprod Med. 2015;33(1):11–16. [DOI] [PubMed] [Google Scholar]
- 12. Brown W, Druce N, Bunting J, Radloff S, Koroma D, Gupta S, et al. Developing the “120 by 20” Goal for the Global FP2020 Initiative. Stud Fam Plann. 2014;45(1):73–84. [DOI] [PubMed] [Google Scholar]
- 13. Siegler AJ, Mouhanna F, Giler RM, Weiss K, Pembleton E, Guest J, et al. The prevalence of PrEP use and the PrEP‐to‐need ratio in the fourth quarter of 2017, United States. Ann Epidemiol [Internet]. 2018. Jun 14 [cited 2018 Jun 18]. Available from: https://www.annalsofepidemiology.org/article/S1047-2797(18)30107-8/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenberg ES, Marcus JL. Progress and pitfalls in measuring HIV preexposure prophylaxis coverage in the United States. Ann Epidemiol. 2018;28(12):830–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calabrese SK, Krakower DS, Willie TC, Kershaw TS, Mayer KH. US guideline criteria for HIV Pre‐exposure prophylaxis: clinical considerations and caveats. Clin Infect Dis[Internet]. [cited 2019 Feb 5]; Available from: http://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciz046/5303779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. HIV/AIDS Epidemiology Unit, Public Health . HIV/AIDS Epidemiology Report 2017, Vol 86. Seattle WA: Washington State Department of Health; 2017. [Google Scholar]
- 17. Zablotska IB, Selvey C, Guy R, Price K, Holden J, Schmidt H‐M, et al. Expanded HIV pre‐exposure prophylaxis (PrEP) implementation in communities in New South Wales, Australia (EPIC‐NSW): design of an open label, single arm implementation trial. BMC Public Health [Internet]. 2018. Feb 2;18. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5797394/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grulich AE, Guy R, Amin J, Jin F, Selvey C, Holden J, et al. Population‐level effectiveness of rapid, targeted, high‐coverage roll‐out of HIV pre‐exposure prophylaxis in men who have sex with men: the EPIC‐NSW prospective cohort study. Lancet HIV. 2018;5(11):e629–e637. [DOI] [PubMed] [Google Scholar]
- 19. Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre‐exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Molina J‐M, Charreau I, Spire B, Cotte L, Chas J, Capitant C, et al. Efficacy, safety, and effect on sexual behaviour of on‐demand pre‐exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV. 2017;4(9):e402–e410. [DOI] [PubMed] [Google Scholar]
- 21. Baeten JM, Palanee‐Phillips T, Mgodi N, Mayo A, Nel A, Rosenberg Z, et al. High uptake and reduced HIV‐1 incidence in an open‐label trial of the dapivirine ring. In: Conference on Retroviruses and Opportunistic Infections. Boston MA; 2018.
- 22. Nel A, Van Niekerk N, Van Baelen B, Rosenberg Z. HIV Incidence and Adherence in DREAM: An Open‐Label Trial of Dapivirine Vaginal Ring. In: Conference on Retroviruses and Opportunistic Infections. Boston MA; 2018.
- 23. Martin M, Vanichseni S, Suntharasamai P, Sangkum U, Mock PA, Chaipung B, et al. Factors associated with the uptake of and adherence to HIV pre‐exposure prophylaxis in people who have injected drugs: an observational, open‐label extension of the Bangkok Tenofovir Study. Lancet HIV. 2017;4(2):e59–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mugo N. PrEP Works, Now What? [Internet]. CROI; 2018. [cited 2018 Mar 9]; Boston MA. Available from: http://www.croiwebcasts.org/console/player/37265?mediaType=audio&&crd_fl=1&ssmsrq=1520619920109&ctms=5000&csmsrq=1151
- 25. Mayer K, Grasso C, Krakower D, Powell V, Boswell S, Marcus J. Increasing PrEP Uptake, Persistent Disparities in At‐risk Patients in a Boston Community Health Center. In: Conference on Retroviruses and Opportunistic Infections [Internet]. Boston MA; 2018. [cited 2018 Mar 9]. Available from: http://www.croiconference.org/sessions/increasing-prep-uptake-persistent-disparities-risk-patients-boston-center
- 26. Wilson C. Massive drop in London HIV rates may be due to internet drugs [Internet]. New Scientist. 2017[cited 2018 May 30]. Available from: https://www.newscientist.com/article/2117426-massive-drop-in-london-hiv-rates-may-be-due-to-internet-drugs/
- 27. Haberer JE. Current concepts for PrEP adherence in the PrEP revolution: from clinical trials to routine practice. Curr Opin HIV AIDS. 2016;11(1):10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cottrell ML, Yang KH, Prince HMA, Sykes C, White N, Malone S, et al. A translational pharmacology approach to predicting HIV pre‐exposure prophylaxis outcomes in men and women using tenofovir disoproxil fumarate±emtricitabine. J Infect Dis. 2016;214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine‐tenofovir exposure and pre‐exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baeten JM, Heffron R, Kidoguchi L, Mugo NR, Katabira E, Bukusi EA, et al. Integrated delivery of antiretroviral treatment and pre‐exposure prophylaxis to HIV‐1‐serodiscordant couples: a prospective implementation study in Kenya and Uganda. PLoS Med. 2016;13(8):e1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marcus JL, Hurley LB, Hare CB, Nguyen DP, Phengrasamy T, Silverberg MJ, et al. Preexposure prophylaxis for HIV prevention in a large integrated health care system: adherence, renal safety, and discontinuation. J Acquir Immune Defic Syndr. 2016;73(5):540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Molina J‐M, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On‐demand preexposure prophylaxis in men at high risk for HIV‐1 infection. N Engl J Med. 2015;373(23):2237–2246. [DOI] [PubMed] [Google Scholar]
- 33. Saag MS, Benson CA, Gandhi RT, Hoy JF, Landovitz RJ, Mugavero MJ, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the international antiviral society‐USA panel. JAMA. 2018;320(4):379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bekker L‐G, Roux S, Sebastien E, Yola N, Amico KR, Hughes JP, et al. Daily and non‐daily pre‐exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomised, open‐label, phase 2 trial. Lancet HIV [Internet]. 2017. Available from: http://www.sciencedirect.com/science/article/pii/S235230181730156X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grant RM, Mannheimer S, Hughes JP, Hirsch‐Moverman Y, Loquere A, Chitwarakorn A, et al. Daily and Nondaily Oral Preexposure Prophylaxis in Men and Transgender Women Who Have Sex With Men: The Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT Study. Clin Infect Dis [Internet]. [cited 2018 May 1]; Available from: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/cix1086/4840078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haberer JE, Bangsberg DR, Baeten JM, Curran K, Koechlin F, Amico KR, et al. Defining success with HIV pre‐exposure prophylaxis: a prevention‐effective adherence paradigm. AIDS. 2015;29(11):1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grulich AE, Guy RJ, Amin J, Schmidt H‐M, Selvey C, Holden J, et al. Rapid reduction in HIV Diagnoses after Targeted PrEP implementation in NWS, Australia. In: Conference on Retroviruses and Opportunistic Infections [Internet]. Boston MA; 2018. [cited 2018 May 14]. Available from: http://www.croiconference.org/sessions/rapid-reduction-hiv-diagnoses-after-targeted-prep-implementation-nsw-australia
- 38. Haberer JE, Sabin L, Amico KR, Orrell C, Galárraga O, Tsai AC, et al. Improving antiretroviral therapy adherence in resource‐limited settings at scale: a discussion of interventions and recommendations. J Int AIDS Soc [Internet]. 2017;20(1). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5467606/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pellowski JA, Price DM, Harrison AD, Tuthill EL, Myer L, Operario D, et al. A systematic review and meta‐analysis of antiretroviral therapy (ART) adherence interventions for women living with HIV. AIDS Behav. 2019;23(8):1998–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Global_AIDS_update_2017_en.pdf[Internet]. [cited 2018 Apr 9]. Available from: http://www.unaids.org/sites/default/files/media_asset/Global_AIDS_update_2017_en.pdf
- 41. Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wood BR, McMahan VM, Naismith K, Stockton JB, Delaney LA, Stekler JD. Knowledge, practices, and barriers to HIV preexposure prophylaxis prescribing among washington state medical providers. Sex Transm Dis. 2018;45(7):452–458. [DOI] [PubMed] [Google Scholar]
- 43. Calabrese SK, Magnus M, Mayer KH, Krakower DS, Eldahan AI, Gaston Hawkins LA, et al. Putting PrEP into practice: lessons learned from early‐adopting U.S. providers’ firsthand experiences providing HIV pre‐exposure prophylaxis and associated care. PLoS One [Internet]. 2016. [cited 2019 Feb 11];11(6). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4909282/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Whitfield THF, John SA, Rendina HJ, Grov C, Parsons JT. Why I quit pre‐exposure prophylaxis (PrEP)? A mixed‐method study exploring reasons for PrEP discontinuation and potential re‐initiation among gay and bisexual Men. AIDS Behav. 2018;22(11):3566–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mugo NR, Ngure K, Kiragu M, Irungu E, Kilonzo N. The preexposure prophylaxis revolution; from clinical trials to programmatic implementation. Curr Opin HIV AIDS. 2016;11(1):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gardner B, Lally P, Wardle J. Making health habitual: the psychology of ‘habit‐formation’ and general practice. Br J Gen Pract. 2012;62(605):664–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Preexposure prophylaxis for the prevention of HIV infection in the United State‐ 2017 Update: a clinical practice guideline [Internet]. Centers for Disease Control and Prevention, US Public Health Service; 2017. [cited 2018 Jun 13]. Available from: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf
- 48. Pyra M, Haberer JE, Heffron R, Kidoguchi L, Brown ER, Bukusi EA, et al. Brief report: PrEP use during periods of HIV risk among east african women in serodiscordant relationships. J Acquir Immune Defic Syndr. 2018;77(1):41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spinelli MA, Scott H, Vittinghoff E, Liu AY, Morehead‐Gee A, Gonzalez R, et al. Factors impacting appropriate HIV/STI screening and PrEP persistence in primary care. In: Conference on Retroviruses and Opportunistic Infections. Boston MA; 2018.
- 50. Kyongo J. How long will they take it? Oral pre‐exposure prophylaxis (PrEP) retention for female sex workers, men who have sex with men and young women in a demonstration project in Kenya. AIDS 2018; Amsterdam, The Netherlands: 2018.
- 51. Eakle R, Gomez GB, Naicker N, Bothma R, Mbogua J, Cabrera Escobar MA, et al. HIV pre‐exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: results from a prospective observational demonstration project. PLoS Med [Internet]. 2017. [cited 2018 May 15];14(11). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5697804/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Delaney KP, Sanchez T, Bowles K, Oraka E, DiNenno EA, Sullivan PS. Awareness and use of PrEP appear to be increasing among internet samples of US MSM. In: Conference on Retroviruses and Opportunistic Infections. Boston, MA; 2018.
- 53. Nelson AL, Westhoff C, Schnare SM. Real‐world patterns of prescription refills for branded hormonal contraceptives: a reflection of contraceptive discontinuation. Obstet Gynecol. 2008;112(4):782–787. [DOI] [PubMed] [Google Scholar]
- 54. Smit JA, Beksinska ME. Hormonal contraceptive continuation and switching in South Africa: implications for evaluating the association of injectable hormonal contraceptive use and HIV. J Acquir Immune Defic Syndr. 2013;62(3):363–365. [DOI] [PubMed] [Google Scholar]
- 55. Greene GJ, Swann G, Fought AJ, Carballo‐Diéguez A, Hope TJ, Kiser PF, et al. Preferences for long‐acting pre‐exposure prophylaxis (PrEP), daily oral PrEP, or condoms for HIV Prevention among U.S. men who have sex with men. AIDS Behav. 2017;21(5):1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Luecke EH, Cheng H, Woeber K, Nakyanzi T, Mudekunye‐Mahaka IC, van der Straten A. Stated product formulation preferences for HIV pre‐exposure prophylaxis among women in the VOICE‐D (MTN‐003D) study. J Int AIDS Soc [Internet]. 2016. [cited 2016 Nov 3];19(1). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4887458/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dunbar MS, Kripke K, Haberer J, Castor D, Dalal S, Mukoma W, et al. Understanding and measuring uptake and coverage of oral pre‐exposure prophylaxis delivery among adolescent girls and young women in sub‐Saharan Africa. Sex Health. 2018;15(6):513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nichols BE, Boucher CAB, van Dijk JH, Thuma PE, Nouwen JL, Baltussen R, et al. Cost‐effectiveness of pre‐exposure prophylaxis (PrEP) in preventing HIV‐1 infections in Rural Zambia: a modeling study. PLoS One [Internet]. 2013. Mar 18 [cited 2016 Nov 9];8(3). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3601101/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Verguet S, Stalcup M, Walsh JA. Where to deploy pre‐exposure prophylaxis (PrEP) in sub‐Saharan Africa? Sex Transm Infect. 2013;89(8):628–634. [DOI] [PubMed] [Google Scholar]
- 60. Alistar SS, Grant PM, Bendavid E. Comparative effectiveness and cost‐effectiveness of antiretroviral therapy and pre‐exposure prophylaxis for HIV prevention in South Africa. BMC Med. 2014;17(12):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kessler J, Myers JE, Nucifora KA, Mensah N, Toohey C, Khademi A, et al. Evaluating the impact of prioritization of antiretroviral pre‐exposure prophylaxis (PrEP) in New York City. AIDS Lond Engl [Internet]. 2014[cited 2018 May 3]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4556593/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Juusola JL, Brandeau ML, Owens DK, Bendavid E. The cost‐effectiveness of preexposure prophylaxis for HIV prevention in men who have sex with men in the United States. Ann Intern Med. 2012;156(8):541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Abbas UL, Anderson RM, Mellors JW. Potential impact of antiretroviral chemoprophylaxis on HIV‐1 transmission in resource‐limited settings. PLoS One [Internet]. 2007. [cited 2016 Nov 10];2(9). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1975470/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gomez GB, Borquez A, Case KK, Wheelock A, Vassall A, Hankins C. The cost and impact of scaling up pre‐exposure prophylaxis for HIV prevention: a systematic review of cost‐effectiveness modelling studies. PLoS Med. 2013;10(3):e1001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Calabrese SK, Krakower DS, Mayer KH. Integrating HIV preexposure prophylaxis (PrEP) into routine preventive health care to avoid exacerbating disparities. Am J Public Health. 2017;107(12):1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cahill N, Sonneveldt E, Stover J, Weinberger M, Williamson J, Wei C, et al. Modern contraceptive use, unmet need, and demand satisfied among women of reproductive age who are married or in a union in the focus countries of the Family Planning 2020 initiative: a systematic analysis using the Family Planning Estimation Tool. Lancet Lond Engl. 2018;391(10123):870–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Alkema L, Kantorova V, Menozzi C, Biddlecom A. National, regional, and global rates and trends in contraceptive prevalence and unmet need for family planning between 1990 and 2015: a systematic and comprehensive analysis. Lancet. 2013;381(9878):1642–1652. [DOI] [PubMed] [Google Scholar]
- 68. Nordon C, Karcher H, Groenwold RHH, Ankarfeldt MZ, Pichler F, Chevrou‐Severac H, et al. The “Efficacy‐Effectiveness Gap”: historical background and current conceptualization. Value Health. 2016;19(1):75–81. [DOI] [PubMed] [Google Scholar]
- 69. Eichler H‐G, Abadie E, Breckenridge A, Flamion B, Gustafsson LL, Leufkens H, et al. Bridging the efficacy–effectiveness gap: a regulator's perspective on addressing variability of drug response. Nat Rev Drug Discov. 2011;10(7):495–506. [DOI] [PubMed] [Google Scholar]
- 70. Corneli A, Perry B, McKenna K, Agot K, Ahmed KM, Taylor J, et al. Participants’ explanations for non‐adherence in the FEM‐PrEP clinical trial. J Acquir Immune Defic Syndr. 2016;71(4):452–461. [DOI] [PubMed] [Google Scholar]
- 71. Murnane PM, Celum C, Mugo N, Campbell JD, Donnell D, Bukusi E, et al. Efficacy of pre‐exposure prophylaxis for HIV‐1 prevention among high risk heterosexuals: subgroup analyses from the Partners PrEP Study. AIDS Lond Engl [Internet]. 2013. [cited 2015 Apr 17];27(13). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3882910/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre‐exposure prophylaxis to prevent the acquisition of HIV‐1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open‐label randomised trial. Lancet [Internet]. [cited 2015 Oct 26]; Available from: http://www.sciencedirect.com/science/article/pii/S0140673615000562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Marcus JL, Volk JE, Pinder J, Liu AY, Bacon O, Hare CB, et al. Successful implementation of HIV preexposure prophylaxis: lessons learned from three clinical settings. Curr HIV/AIDS Rep. 2016;13(2):116–124. [DOI] [PubMed] [Google Scholar]
- 74. Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethachawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2013;15:381. [DOI] [PubMed] [Google Scholar]
- 75. Brown E, Palanee‐Phillips T, Marzinke M, Hendrix CW, Dezutti C, Soto‐Torres L, et al. Residual dapivirine ring levels indicate higher adherence to vaginal ring is associated with HIV‐1 protection. In: 21st International AIDS Conference. Durban, South Africa; 2016.
- 76. Volz EM, Le Vu S, Ratmann O, Tostevin A, Dunn D, Orkin C, et al. Molecular epidemiology of HIV‐1 subtype B reveals heterogeneous transmission risk: implications for intervention and control. J Infect Dis. 2018;217(10):1522–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Land E. 51% reduction in San Francisco HIV infections since 2012. BETA Blog [Internet]. 2017 Sep 15 [cited 2018 May 30]; Available from: https://betablog.org/2016-hiv-epidemiology-report-sf/
- 78. UNAIDS . 90‐90‐90: an ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: UNAIDS; 2014. p. 40. [Google Scholar]
- 79. Ahmed S, Li Q, Liu L, Tsui AO. Maternal deaths averted by contraceptive use: an analysis of 172 countries. Lancet. 2012;380(9837):111–125. [DOI] [PubMed] [Google Scholar]
- 80. Anema A, Au‐Yeung CG, Joffres M, Kaida A, Vasarhelyi K, Kanters S, et al. Estimating the impact of expanded access to antiretroviral therapy on maternal, paternal and double orphans in sub‐Saharan Africa, 2009‐2020. AIDS Res Ther. 2011;7(8):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sonfield A, Hasstedt K, Kavanaugh ML, Anderson R. The social and economic benefits of women's ability to determine whether and when to have children. The Guttmacher Institute; 2013. p. 48.
- 82. Jenness SM, Weiss KM, Goodreau SM, Gift T, Chesson H, Hoover KW, et al. Incidence of gonorrhea and chlamydia following human immunodeficiency virus preexposure prophylaxis among men who have sex with men: a modeling study. Clin Infect Dis. 2017;65(5):712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Marcus JL, Levine K, Grasso C, Krakower DS, Powell V, Bernstein KT, et al. HIV preexposure prophylaxis as a gateway to primary care. Am J Public Health. 2018;108(10):1418–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Myers JE, Sepkowitz KA. A pill for HIV prevention: Déjà Vu all over again? Clin Infect Dis. 2013;56(11):1604–1612. [DOI] [PubMed] [Google Scholar]
- 85. Reed JB, Patel RR, Baggaley R. Lessons from a decade of voluntary medical male circumcision implementation and their application to HIV pre‐exposure prophylaxis scale up. Int J STD AIDS. 2018;29(14):1432–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kenya Country Updates [Internet]. PrEPWatch. [cited 2019 Jun 17]. Available from: https://www.prepwatch.org/country/kenya/
- 87. Masyuko S, Mukui I, Njathi O, Kimani M, Oluoch P, Wamicwe J, et al. Pre‐exposure prophylaxis rollout in a national public sector program: the Kenyan case study. Sex Health. 2018;15(6):578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]


