Abstract
Introduction
It remains unclear whether marital status could affect the breast cancer‐caused special survival (BCSS) of patients with breast cancer. Therefore, we sought to explore the influence of demographic and pathological factors on prognosis of patients with breast cancer.
Materials and methods
We selected patients meeting the eligibility criteria from the Surveillance, Epidemiology, and End Results (SEER) cancer registry program. We assessed the effect of marital status on overall survival (OS) and BCSS using Kaplan‐Meier curve and multivariate Cox proportional hazards regression.
Results
Compared with divorced/separated/widowed (DSW) patients, the married (AHR 0.7483, 95% CI: 0.729‐0.7682, P < 0.001) and single patients had better BCSS (AHR 0.9096, 95% CI: 0.8796‐0.9406, P < 0.001). Married patients kept better prognosis among all age subgroups, while the better BCSS of single patients occurred only in groups older than 35 years. As for race and hormone receptor status (HRs), the better BCSS of single patients was only observed in white race (AHR 0.881, 95% CI: 0.8457‐0.9177, P < 0.001) and patients with ER+/PR + status (AHR 0.8844, 95% CI: 0.8393‐0.932, P < 0.001).
Conclusion
Our findings demonstrated that married and single patients with breast cancer had better prognosis than their DSW counterparts. Age, race, and HRs could affect the correlation between marital status and BCSS.
Keywords: breast cancer, marital status, prognosis, SEER
1. INTRODUCTION
Psychosocial factors are associated with the outcome of cancer patients. It can be said with certainty that cancer patients could obtain better prognosis from some psychosocial factors like coping strategies, emotional support, and social integration.1, 2 Marriage is one of the most important forms of social relations influencing on cancer patients. Previous studies have demonstrated that marital status could affect the survival outcome of several kinds of cancers, and it might act as an independent prognostic factor for overall survival (OS) in patients with breast cancer.3, 4, 5 Married patients were considered to have more emotional and financial support, which helps them to be diagnosed at earlier stage and receive proper treatments with better adherence, then finally prolong their overall survival.6, 7, 8, 9 However, further analysis of marital subgroups was neglected, which might reveal the potential mechanism generating the influence of marital status on prognosis.
Breast cancer is the most frequently diagnosed cancer for women worldwide and is also the leading cause of cancer death for female patients in over 100 countries.10 The incidence of breast cancer in the US population is expected to reach 30%‐40% by 2020.11 As a systemic disease, the formation of breast cancer is due to complex interaction of psychosocial and physiological factors.12 Recently, the association between marital status and OS has been studied for some cancers. These studies proved that marital status acts as an independent prognostic factor for survival in patients with several cancers including breast cancer, and married patients gain a significant survival benefit compared with unmarried patients.13, 14, 15, 16, 17, 18, 19, 20 These conclusions suggest us a general relationship between marital status and the survival of breast cancer patients. However, some specific problems still need to be solved. First, most of these studies only took OS into consideration and neglected to investigate cancer‐specific survival, including breast cancer‐caused special survival (BCSS). Second, majority of their data were based on small samples, restricted by population diversity or limited follow‐up time. Moreover, previous studies neglected to explore the difference among the unmarried groups and whether the effect of marital status on prognosis differs across patients' demographic and pathological subgroups.15, 17 Among these, age and hormone receptor status (HRs) were reported as two key independent prognostic factors for breast cancer survival.21
In view of present research station, we conducted this analysis to explore the correlation between marital status and BCSS and whether the association varied by age, race, and HRs. Our study was based on the data provided by Surveillance, Epidemiology, and End Results (SEER) cancer registry program. The SEER database includes population‐based data from 18 cancer registries in population‐based catchment areas related to cancer diagnoses, treatment and survival in approximately 30% of the population in the United States from 1973 to 2014.22
2. MATERIALS AND METHODS
2.1. Patient selection and data extraction
To estimate the correlations between marital status and BCSS in patients, we used SEER*Stat 8.3.4 software to extract eligible patients included in the database. We enrolled a total of 476 028 patients diagnosed with breast cancer, who were aged 18 years or above at diagnosis, between 2004 and 2012. After excluding cases diagnosed using autopsy or death certificates only, we selected 298 434 patients according to the following criteria: (a) only one primary malignancy in their lifetime; (b) limited to the following histological types, according to the International Classification of Diseases for Oncology, 3rd Edition (ICD‐O‐3): 8500, 8501, 8510, 8512, 8513, 8514, 8520, 8521, 8522, 8523, 8524, 8525, 8530, and 8541; (c) known race; (d) known marital status; (e) known grade; (f) known stage (Breast Cancer Adjusted AJCC Cancer Staging Manual 6th Edition); (g) known surgery situation; and (h) known survival months after diagnosis. We excluded patients for whom the aforementioned data were missing. Marital status at diagnosis was the primary variable of interest, and participants were classified as married, single, and divorced/separated/widowed (DSW), which were considered as unfavorable marital status. The access to and use of SEER data did not require informed patient consent, and all procedures were performed in accordance with approved guidelines. The Ethics Committee of the Second Affiliated Hospital of Xi'an Jiaotong University approved this study.
2.2. Statistical analysis
Descriptive statistics were performed to investigate baseline characteristics of the patient population. Patients' clinical characteristics were compared between different categories of marital status using the Chi‐squared test. We evaluated the OS and BCSS rates among different categories of marital status using Kaplan‐Meier curves and the log‐rank tests. For multivariate analysis, multivariate Cox proportional analyses were used to calculate hazard ratios (HR) and their corresponding 95% confidence intervals (CI), to assess the influence of marital status on overall and cancer‐specific survival. We also explored the effect of marital status according to age, race, and HRs using Cox regression analyses and calculated the adjusted hazard ratio (AHR) to assess the survival difference among marital subgroups in special demographic or pathological subgroups. A two‐sided P value of less than 0.05 was determined to be statistically significant. All statistical analyses were performed and figures were created using R 3.5.3 software (R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1. Patients' baseline characteristics
In view of the inclusion criteria, this study included 298 434 patients diagnosed with breast cancer between 2004 and 2012. A flowchart of the study participant selection process was shown in Figure 1. The 10‐year survival rate for the married, single, and DSW participants was 79.9%, 71.1% and 59%, respectively. As shown in Table 1, there were many more female participants (296 500; 99.4%) than the males (1934; 0.6%), so it was inappropriate to compare clinicopathological characteristics by sex. Among eligible patients, 176 109 (59.0%) were married at diagnosis, 43 262 (14.5%) were single, and 79 063 (26.5%) were DSW. The rate of being married was higher among patients aged 35‐70 years, as well as among Asian or Pacific Islanders (API), and this rate decreased with higher tumor stage. Black patients, as well as patients younger than 35 years, showed the highest rate of being single. And not surprisingly, the rate of being DSW was highest among patients aged 70 years or older. Compared with the single or DSW, tumors in the married were more inclined to be at stage 0 or I. There was a less proportion of being dead attributable to breast cancer in the married subgroup as well. In addition, married patients received more surgeries either in the females or males, and the females received more breast‐conserving surgery (BCS) (Table 2).
Figure 1.
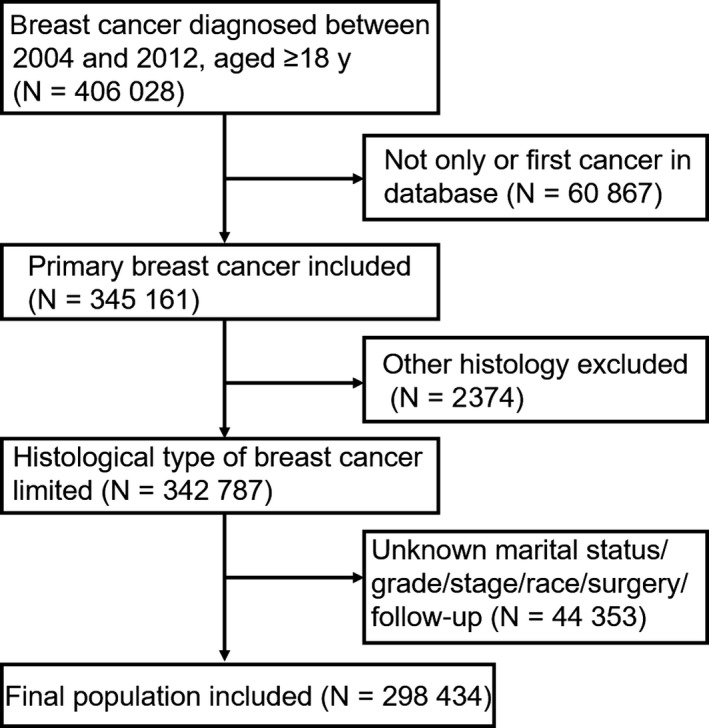
Flowchart for included patients from the SEER
Table 1.
Baseline clinicopathological characteristics of patients with breast cancer in SEER database
| Characteristics | Total | Married | Single | DSW | P‐value |
|---|---|---|---|---|---|
| Overall | 298 434 | 176 109 (59%) | 43 262 (14.5%) | 79 063 (26.5%) | |
| Age (years) | <0.001 | ||||
| <35 | 6813 | 3807 (55.9%) | 2551 (37.4%) | 455 (6.7%) | |
| 35‐70 | 219 252 | 142 260 (64.9%) | 34 869 (15.9%) | 42 123 (19.2%) | |
| ≥70 | 72 369 | 30 042 (41.5%) | 5842 (8.1%) | 36 485 (50.4%) | |
| Sex | <0.001 | ||||
| Female | 296 500 | 174 763 (58.9%) | 42 984 (14.5%) | 78 753 (26.6%) | |
| Male | 1934 | 1346 (69.6%) | 278 (14.4%) | 310 (16%) | |
| Race | <0.001 | ||||
| White | 241 019 | 146 707 (60.9%) | 30 110 (12.5%) | 64 202 (26.6%) | |
| Black | 31 729 | 11 973 (37.7%) | 9720 (30.6%) | 10 036 (31.6%) | |
| AN/AI | 1542 | 794 (51.5%) | 307 (19.9%) | 441 (28.6%) | |
| API | 24 144 | 16 635 (68.9%) | 3125 (12.9%) | 4384 (18.2%) | |
| Stage | <0.001 | ||||
| Stage 0/I | 138 499 | 85 193 (61.5%) | 16 938 (12.2%) | 36 368 (26.3%) | |
| Stage II | 108 087 | 63 388 (58.6%) | 16 666 (15.4%) | 28 033 (25.9%) | |
| Stage III | 39 362 | 21 795 (55.4%) | 6955 (17.7%) | 10 612 (27%) | |
| Stage IV | 12 486 | 5733 (45.9%) | 2703 (21.6%) | 4050 (32.4%) | |
| Grade | <0.001 | ||||
| Grade I | 59 689 | 36 007 (60.3%) | 7 424 (12.4%) | 16 258 (27.2%) | |
| Grade II | 128 227 | 75 435 (58.8%) | 17 766 (13.9%) | 35 026 (27.3%) | |
| Grade III | 107 872 | 63 108 (58.5%) | 17 604 (16.3%) | 27 160 (25.2%) | |
| Grade IV | 2646 | 1559 (58.9%) | 468 (17.7%) | 619 (23.4%) | |
| Surgery | <0.001 | ||||
| BCS | 163 932 | 98 869 (60.3%) | 22 091 (13.5%) | 42 972 (26.2%) | |
| Other | 121 334 | 71 667 (59.1%) | 18 162 (15%) | 31 505 (26%) | |
| No surgery | 13 168 | 5573 (42.3%) | 3 009 (22.9%) | 4 586 (34.8%) | |
| HRs | <0.001 | ||||
| ER+/PR+ | 192 975 | 115 270 (59.7%) | 27 030 (14%) | 50 675 (26.3%) | |
| ER+/PR‐ | 34 393 | 19 488 (56.7%) | 5008 (14.6%) | 9897 (28.8%) | |
| ER‐/PR+ | 3223 | 1951 (60.5%) | 531 (16.5%) | 741 (23%) | |
| ER‐/PR‐ | 54 669 | 32 027 (58.6%) | 8727 (16%) | 13 915 (25.5%) | |
| Other | 13 174 | 7373 (56%) | 1966 (14.9%) | 3835 (29.1%) | |
| Cause of death | <0.001 | ||||
| Alive or dead of other cause | 266 598 | 160 869 (60.3%) | 37 418 (14%) | 68 311 (25.6%) | |
| Dead (attributable to this cancer) | 31 836 | 15 240 (47.9%) | 5844 (18.4%) | 10 752 (33.8%) | |
Abbreviations: AN/AI, American Indian/Alaska Native; API, Asian or Pacific Islander; BCS, breast‐conserving surgery; DSW, divorced/separated/widowed; ER, estrogen receptor; HRs, hormone receptor status; PR, progesterone receptor.
Table 2.
Baseline demographic characteristics of patients stratified by marital status (%)
| Characteristics | Female, N = 296 500 | Male, N = 1934 | ||||
|---|---|---|---|---|---|---|
| Married | Single | DSW | Married | Single | DSW | |
| N = 174 763 | N = 42 984 | N = 78 753 | N = 1346 | N = 278 | N = 310 | |
| Age (years) | ||||||
| <35 | 2.2 | 5.9 | 0.6 | 0.6 | 1.4 | 0.3 |
| 35‐70 | 80.9 | 80.6 | 53.3 | 62.6 | 75.9 | 46.8 |
| ≥70 | 16.9 | 13.4 | 46.1 | 36.8 | 22.7 | 52.9 |
| Race | ||||||
| White | 83.3 | 69.6 | 81.2 | 83 | 69.8 | 82.6 |
| Black | 6.8 | 22.4 | 12.7 | 10.3 | 26.6 | 15.2 |
| AN/AI | 0.5 | 0.7 | 0.6 | 0.4 | 0 | 0 |
| API | 9.5 | 7.2 | 5.6 | 6.4 | 3.6 | 2.3 |
| Stage | ||||||
| Stage 0/I | 48.5 | 39.3 | 46.1 | 33.6 | 21.9 | 21.6 |
| Stage II | 35.9 | 38.5 | 35.4 | 42.9 | 44.6 | 45.2 |
| Stage III | 12.3 | 16 | 13.4 | 17.5 | 20.5 | 23.2 |
| Stage IV | 3.2 | 6.2 | 5.1 | 6 | 12.9 | 10 |
| Grade | ||||||
| Grade I | 20.5 | 17.2 | 20.6 | 11.4 | 13.3 | 9.7 |
| Grade II | 42.8 | 41 | 44.3 | 51.6 | 51.4 | 50.3 |
| Grade III | 35.8 | 40.7 | 34.3 | 36.4 | 34.9 | 38.7 |
| Grade IV | 0.9 | 1.1 | 0.8 | 0.5 | 0.4 | 1.3 |
| Surgery | ||||||
| BCS | 56.5 | 51.3 | 54.5 | 9.8 | 11.9 | 8.7 |
| Other | 40.3 | 41.8 | 39.7 | 86.5 | 77.3 | 85.2 |
| No surgery | 3.2 | 6.9 | 5.8 | 3.7 | 10.8 | 6.1 |
| HRs | ||||||
| ER+/PR+ | 65.3 | 62.4 | 64 | 81.8 | 79.9 | 81.6 |
| ER+/PR‐ | 11.1 | 11.6 | 12.5 | 9.4 | 10.4 | 8.4 |
| ER‐/PR+ | 1.1 | 1.2 | 0.9 | 0.4 | 0.7 | 1 |
| ER‐/PR‐ | 18.3 | 20.3 | 17.7 | 2.2 | 3.6 | 1.9 |
| Other | 4.2 | 4.5 | 4.8 | 6.2 | 5.4 | 7.1 |
| Cause of death | ||||||
| Alive or dead of other cause | 91.4 | 86.5 | 86.4 | 86.3 | 77.7 | 80.3 |
| Dead (attributable to this cancer) | 8.6 | 13.5 | 13.6 | 13.7 | 22.3 | 19.7 |
Abbreviations: DSW, divorced/separated/widowed; AN/AI, American Indian/Alaska Native; API, Asian or Pacific Islander; BCS, breast‐conserving surgery; HRs, hormone receptor status; ER, estrogen receptor; PR, progesterone receptor.
3.2. Effects of marital status on overall survival and cancer‐specific survival
Kaplan‐Meier curves were generated by marital status to estimate overall and cancer‐specific survival of patients with breast cancer. In further log‐rank tests, the married subgroup showed significantly (P < 0.001) better OS and BCSS than the unmarried groups, while there was no significant difference of BCSS between the single and the DSW (Figure 2). Considering the possible interaction among variables, we then conducted multivariate Cox regression analysis. It turned out that compared with the DSW, being married and being single were both related to better OS (married: HR 0.6444, 95% CI: 0.6318‐0.6573, P < 0.001; single: HR 0.8712, 95% CI: 0.8484‐0.8947, P < 0.001) and BCSS (married: HR 0.7483, 95% CI: 0.729‐0.7682, P < 0.001; single: HR 0.9096, 95% CI: 0.8796‐0.9406, P < 0.001). As for age subgroups, patients aged 70 years or older suffered worse OS (HR 3.3121, 95% CI: 3.2506‐3.3748, P < 0.001) and BCSS (HR 1.8478, 95% CI: 1.8005‐1.8963, P < 0.001) than patients aged 35‐70 years or younger than 35 years, between which there was no remarkable difference of survival observed. Compared with female patients, male experienced worse OS (HR 1.5298, 95% CI: 1.4112‐1.6584, P < 0.001) and BCSS (HR 1.2934, 95% CI: 1.1553‐1.448, P < 0.001). In different race subgroups, API patients showed better prognosis, while the black showed worse BCSS (using the white as reference; API: BCSS‐HR 0.7619, 95% CI: 0.7262‐0.7993, P < 0.001; black: BCSS‐HR 1.2984, 95% CI: 1.2602‐1.3379, P < 0.001). Compared with patients without surgery, better BCSS was observed across those who received BCS (HR 0.3349, 95% CI: 0.322‐0.3483, P < 0.001) or other operations (HR 0.4234, 95% CI: 0.4087‐0.4386, P < 0.001). In addition, the higher the grade, the worse the prognosis. And similar trends could be found across the stage. Compared with other HRs subgroups, the subgroup of estrogen receptor positive and progesterone receptor positive (ER+/PR+) was associated with better OS and BCSS (Table 3).
Figure 2.
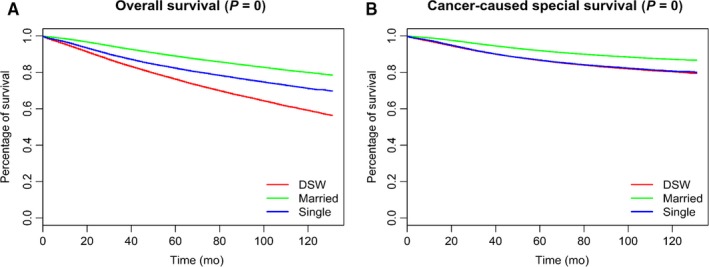
Kaplan‐Meier Survival curves: The overall (A) and cancer‐caused special survival (B) of patients with breast cancer according to marital status
Table 3.
Multivariate analyses of overall and cancer‐caused special survival of patients with breast cancer
| Variable | Multivariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| OS HR | 95% CI | P‐Value | BCSS HR | 95% CI | P‐Value | ||
| Age (years) | Age (years) | ||||||
| 35‐70 | Baseline | 35‐70 | Baseline | ||||
| <35 | 0.9767 | 0.9213‐1.0354 | 0.4290 | <35 | 0.9914 | 0.9326‐1.0538 | 0.7814 |
| ≥70 | 3.3121 | 3.2506‐3.3748 | <0.001 | ≥70 | 1.8478 | 1.8005‐1.8963 | <0.001 |
| Sex | Sex | ||||||
| Female | Baseline | Female | Baseline | ||||
| Male | 1.5298 | 1.4112‐1.6584 | <0.001 | Male | 1.2934 | 1.1553‐1.448 | <0.001 |
| Race | Race | ||||||
| White | Baseline | White | Baseline | ||||
| Black | 1.2781 | 1.247‐1.3099 | <0.001 | Black | 1.2984 | 1.2602‐1.3379 | <0.001 |
| AN/AI | 1.2703 | 1.1369‐1.4193 | <0.001 | AN/AI | 1.1988 | 1.0434‐1.3775 | 0.0105 |
| API | 0.7354 | 0.7073‐0.7647 | <0.001 | API | 0.7619 | 0.7262‐0.7993 | <0.001 |
| Marital | Marital | ||||||
| DSW | Baseline | DSW | Baseline | ||||
| Married | 0.6444 | 0.6318‐0.6573 | <0.001 | Married | 0.7483 | 0.729‐0.7682 | <0.001 |
| Single | 0.8712 | 0.8484‐0.8947 | <0.001 | Single | 0.9096 | 0.8796‐0.9406 | <0.001 |
| Stage | Stage | ||||||
| Stage 0/I | Baseline | Stage 0/I | Baseline | ||||
| Stage II | 1.6699 | 1.6305‐1.7103 | <0.001 | Stage II | 2.9902 | 2.8713‐3.114 | <0.001 |
| Stage III | 3.7727 | 3.673‐3.8751 | <0.001 | Stage III | 9.0426 | 8.673‐9.428 | <0.001 |
| Stage IV | 10.8121 | 10.4493‐11.1874 | <0.001 | Stage IV | 30.9787 | 29.5487‐32.478 | <0.001 |
| Grade | Grade | ||||||
| Grade I | Baseline | Grade I | Baseline | ||||
| Grade II | 1.1837 | 1.1494‐1.2189 | <0.001 | Grade II | 1.6902 | 1.6046‐1.7803 | <0.001 |
| Grade III | 1.5688 | 1.5215‐1.6177 | <0.001 | Grade III | 2.5914 | 2.4598‐2.7299 | <0.001 |
| Grade IV | 1.5395 | 1.4257‐1.6624 | <0.001 | Grade IV | 2.5379 | 2.3036‐2.7959 | <0.001 |
| Surgery | Surgery | ||||||
| No surgery | Baseline | No surgery | Baseline | ||||
| BCS | 0.3433 | 0.3321‐0.3548 | <0.001 | BCS | 0.3349 | 0.322‐0.3483 | <0.001 |
| Other | 0.425 | 0.4121‐0.4384 | <0.001 | Other | 0.4234 | 0.4087‐0.4386 | <0.001 |
| HRs | HRs | ||||||
| ER+/PR+ | Baseline | ER+/PR+ | Baseline | ||||
| ER+/PR‐ | 1.3344 | 1.3003‐1.3693 | <0.001 | ER+/PR‐ | 1.5644 | 1.5132‐1.6175 | <0.001 |
| ER‐/PR+ | 1.5926 | 1.4786‐1.7155 | <0.001 | ER‐/PR+ | 1.9623 | 1.8015‐2.1375 | <0.001 |
| ER‐/PR‐ | 1.7221 | 1.6837‐1.7614 | <0.001 | ER‐/PR‐ | 2.1679 | 2.1089‐2.2285 | <0.001 |
| Other | 1.4388 | 1.3878‐1.4917 | <0.001 | Other | 1.6497 | 1.5719‐1.7313 | <0.001 |
Abbreviations: AN/AI, American Indian/Alaska Native; API, Asian or Pacific Islander; BCS, breast‐conserving surgery; BCSS, breast cancer‐caused special survival; CI, confidence interval; DSW, divorced/separated/widowed; ER, estrogen receptor; HR, hazard ratio; HRs, hormone receptor status; OS, overall survival; PR, progesterone receptor.
3.3. Effects of marital status stratified by demographic and pathological subgroups
To further investigate the prognostic effect of marital status on prognosis by different demographic and pathological subgroups, we stratified all cases according to age, sex, race, and HRs and performed multivariate analyses. Compared with the DSW and the single, the better OS of married patients was consistent among most subgroups, although this effect vanished in American Indian/Alaska Native (AN/AI) patients. Compared with the DSW, the significant OS benefit of the single occurred only in patients aged 35 years or older (35‐70 years: AHR 0.8848, 95% CI: 0.8542‐0.9164, P < 0.001; ≥70 years: AHR 0.8469, 95% CI: 0.8087‐0.8868, P < 0.001) according to age. And the better survival of single patients over the DSW was consistent in the race of white and black, as well as the HRs of ER+/PR+, ER+/PR‐, or ER‐/PR+ (Table 4). As for BCSS, there was no significant difference observed in AN/AI patients among three marital subgroups, while married patients showed better BCSS than DSW patients in most subgroups except for the male. Compared with the DSW, better BCSS of the single was observed only in patients aged 35 years or older (35‐70 years: AHR 0.9313, 95% CI: 0.8944‐0.9697, P = 0.001; ≥70 years: AHR 0.8752, 95% CI: 0.8148‐0.94, P < 0.001), in race of white (AHR 0.881, 95% CI: 0.8457‐0.9177, P < 0.001), or in HRs of ER+/PR+ (AHR 0.8844, 95% CI: 0.8393‐0.932, P < 0.001) or ER‐/PR‐ (AHR 0.9202, 95% CI: 0.8693‐0.9741, P = 0.004) (Table 5).
Table 4.
Adjusted hazard ratio for overall survival associated with marital status in different clinicopathological subgroups
| OS | Married vs DSW | Single vs DSW | Married vs Single | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | AHR | 95% CI | P‐value | AHR | 95% CI | P‐value | AHR | 95% CI | P‐value |
| All | 0.6444 | 0.6318‐0.6573 | <0.001 | 0.8712 | 0.8484‐0.8947 | <0.001 | 0.7396 | 0.7208‐0.759 | <0.001 |
| <35 | 0.683 | 0.5484‐0.8507 | <0.001 | 0.8625 | 0.6911‐1.0765 | 0.191 | 0.7919 | 0.6994‐0.8966 | <0.001 |
| 35‐70 | 0.6872 | 0.6677‐0.7074 | <0.001 | 0.8848 | 0.8542‐0.9164 | <0.001 | 0.7767 | 0.7526‐0.8016 | <0.001 |
| ≥70 | 0.6076 | 0.5908‐0.6249 | <0.001 | 0.8469 | 0.8087‐0.8868 | <0.001 | 0.7175 | 0.6831‐0.7535 | <0.001 |
| Sex | |||||||||
| Female | 0.6451 | 0.6324‐0.6581 | <0.001 | 0.8686 | 0.8456‐0.8921 | <0.001 | 0.7427 | 0.7237‐0.7622 | <0.001 |
| Male | 0.6308 | 0.51735‐0.7691 | <0.001 | 1.1371 | 0.87716‐1.4742 | 0.332 | 0.5547 | 0.44385‐0.6933 | <0.001 |
| Race | |||||||||
| White | 0.6289 | 0.6154‐0.6428 | <0.001 | 0.8533 | 0.8266‐0.8809 | <0.001 | 0.737 | 0.7148‐0.76 | <0.001 |
| Black | 0.7601 | 0.7189‐0.8036 | <0.001 | 0.9129 | 0.8633‐0.9652 | 0.001 | 0.8326 | 0.7871‐0.8808 | <0.001 |
| AN/AI | 0.872 | 0.6688‐1.1369 | 0.311 | 0.9635 | 0.693‐1.3395 | 0.825 | 0.905 | 0.6653‐1.231 | 0.525 |
| API | 0.6216 | 0.5676‐0.6808 | <0.001 | 0.9107 | 0.8045‐1.0308 | 0.139 | 0.6826 | 0.6112‐0.7624 | <0.001 |
| HRs | |||||||||
| ER+/PR+ | 0.611 | 0.5943‐0.6281 | <0.001 | 0.8618 | 0.8294‐0.8954 | <0.001 | 0.709 | 0.6828‐0.7361 | <0.001 |
| ER+/PR‐ | 0.6389 | 0.6063‐0.6734 | <0.001 | 0.8673 | 0.8092‐0.9297 | <0.001 | 0.7367 | 0.6879‐0.7889 | <0.001 |
| ER‐/PR+ | 0.6483 | 0.5449‐0.7713 | <0.001 | 0.8364 | 0.6663‐1.0499 | 0.123 | 0.7751 | 0.629‐0.9552 | 0.017 |
| ER‐/PR‐ | 0.7176 | 0.6908‐0.7455 | <0.001 | 0.8954 | 0.8516‐0.9414 | <0.001 | 0.8015 | 0.7648‐0.84 | <0.001 |
| Other | 0.6301 | 0.5817‐0.6825 | <0.001 | 0.924 | 0.836‐1.0213 | 0.122 | 0.6819 | 0.617‐0.7536 | <0.001 |
Abbreviations: AHR: adjusted hazard ratio; AN/AI: American Indian/Alaska Native; API: Asian or Pacific Islander; CI: confidence interval; DSW: divorced/separated/widowed; ER: estrogen receptor; HRs: hormone receptor status; OS: overall survival; PR: progesterone receptor.
Table 5.
Adjusted HR for cancer‐caused special survival associated with marital status in different clinicopathological subgroups
| BCSS | Married vs DSW | Single vs DSW | Married vs Single | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | AHR | 95% CI | P‐value | AHR | 95% CI | P‐value | AHR | 95% CI | P‐value |
| All | 0.7483 | 0.729‐0.7682 | <0.001 | 0.9096 | 0.8796‐0.9406 | <0.001 | 0.8227 | 0.7976‐0.8487 | <0.001 |
| Age (years) | |||||||||
| <35 | 0.6856 | 0.545‐0.8624 | 0.001 | 0.8676 | 0.6883‐1.0937 | 0.229 | 0.7902 | 0.69431‐0.8993 | <0.001 |
| 35‐70 | 0.7774 | 0.752‐0.8037 | <0.001 | 0.9313 | 0.8944‐0.9697 | 0.001 | 0.8348 | 0.8057‐0.8649 | <0.001 |
| ≥70 | 0.711 | 0.68‐0.7434 | <0.001 | 0.8752 | 0.8148‐0.94 | <0.001 | 0.8124 | 0.753‐0.8764 | <0.001 |
| Sex | |||||||||
| Female | 0.7488 | 0.7294‐0.7688 | <0.001 | 0.9071 | 0.8771‐0.9382 | <0.001 | 0.8255 | 0.8001‐0.8517 | <0.001 |
| Male | 7.53E‐01 | 0.56039‐1.0109 | 0.059 | 1.2325 | 0.8539‐1.779 | 0.264 | 0.6277 | 0.4639‐0.8494 | 0.001 |
| Race | |||||||||
| White | 0.7321 | 0.7109‐0.7539 | <0.001 | 0.881 | 0.8457‐0.9177 | <0.001 | 0.831 | 0.8003‐0.8629 | <0.001 |
| Black | 0.8455 | 0.7907‐0.9042 | <0.001 | 0.9888 | 0.925‐1.057 | 0.741 | 0.8551 | 0.8019‐0.9119 | <0.001 |
| AN/AI | 1.0282 | 0.72678‐1.4545 | 0.875 | 1.123 | 0.743‐1.6974 | 0.582 | 0.9196 | 0.6369‐1.3278 | 0.655 |
| API | 0.7073 | 0.6297‐0.7945 | <0.001 | 0.9508 | 0.8156‐1.1084 | 0.519 | 0.7417 | 0.6509‐0.8451 | <0.001 |
| HRs | |||||||||
| ER+/PR+ | 0.7175 | 0.6892‐0.7469 | <0.001 | 0.8844 | 0.8393‐0.932 | <0.001 | 0.8113 | 0.7726‐0.8518 | <0.001 |
| ER+/PR‐ | 0.7479 | 0.6992‐0.7999 | <0.001 | 0.9489 | 0.8719‐1.0326 | 0.224 | 0.7882 | 0.7277‐0.8537 | <0.001 |
| ER‐/PR+ | 0.6542 | 0.5355‐0.7991 | <0.001 | 0.8251 | 0.6382‐1.0667 | 0.142 | 0.7928 | 0.6284‐1.0003 | 0.05 |
| ER‐/PR‐ | 0.7916 | 0.7574‐0.8273 | <0.001 | 0.9202 | 0.8693‐0.9741 | 0.004 | 0.8602 | 0.8167‐0.906 | <0.001 |
| Other | 0.731 | 0.6556‐0.8149 | <0.001 | 0.9839 | 0.8648‐1.1193 | 0.805 | 0.7429 | 0.6572‐0.8399 | <0.001 |
Abbreviations: AHR, adjusted hazard ratio; AN/AI, American Indian/Alaska Native; API, Asian or Pacific Islander; BCSS, breast cancer‐caused special survival; CI, confidence interval; DSW, divorced/separated/widowed; ER, estrogen receptor; HRs, hormone receptor status; PR, progesterone receptor.
Given that the benefit of being single over being DSW varied across some age, race, and HRs subgroups, we conducted analysis to explore whether there were differences of the stage, grade or surgery condition of single patients among these subgroups. As shown in Table 6 and Figure 3, the proportion of stage 0/I, grade 1 and BCS were obviously lower in the <35 years subgroup, in which the protective effect of being single did not occur. What's more, the white race or patients at the ER+/PR + status showed better stage and grade situation, with more BCS accepted than their counterparts.
Table 6.
Stage, grade, and surgery conditions of patients single at diagnosis in different clinicopathological subgroups (%)
| Variable | Stage | Grade | Surgery | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage 0/I | Stage II | Stage III | Stage IV | Grade 1 | Grade 2 | Grade 3 | Grade 4 | BCS | Other | No | |||
| Age (years) | Age (years) | Age (years) | |||||||||||
| <35 | 23.25 | 47.59 | 22.07 | 7.1 | <35 | 5.53 | 31.09 | 61.98 | 1.41 | <35 | 35.44 | 57.04 | 7.53 |
| 35‐70 | 38.99 | 38.59 | 16.26 | 6.16 | 35‐70 | 17.08 | 40.77 | 41.06 | 1.09 | 35‐70 | 51.94 | 41.4 | 6.66 |
| ≥70 | 47.04 | 34.17 | 12.38 | 6.42 | ≥70 | 22.7 | 47.19 | 29.2 | 0.91 | ≥70 | 52.65 | 38.87 | 8.47 |
| Race | Race | Race | |||||||||||
| White | 42.08 | 37.37 | 14.88 | 5.67 | White | 19.31 | 43.38 | 36.35 | 0.96 | White | 52.73 | 41.16 | 6.11 |
| Black | 29.92 | 41.43 | 20.29 | 8.36 | Black | 10.65 | 32.97 | 54.95 | 1.43 | Black | 46.74 | 43.26 | 10 |
| AN/AI | 32.9 | 40.39 | 20.2 | 6.51 | AN/AI | 16.94 | 40.07 | 42.35 | 0.65 | AN/AI | 50.81 | 45.6 | 3.58 |
| API | 40.32 | 40.38 | 14.11 | 5.18 | API | 16.7 | 44.03 | 38.02 | 1.25 | API | 48.48 | 45.6 | 5.92 |
| HRs | HRs | HRs | |||||||||||
| ER+/PR+ | 44.27 | 37.03 | 13.56 | 5.15 | ER+/PR+ | 23.05 | 49.89 | 26.48 | 0.58 | ER+/PR+ | 54.79 | 39.53 | 5.68 |
| ER+/PR‐ | 35.34 | 37.02 | 18.87 | 8.77 | ER+/PR‐ | 13.56 | 39.42 | 46.07 | 0.96 | ER+/PR‐ | 46.85 | 44.43 | 8.73 |
| ER‐/PR+ | 26.74 | 46.14 | 20.15 | 6.97 | ER‐/PR+ | 3.77 | 19.96 | 73.82 | 2.45 | ER‐/PR+ | 47.65 | 41.62 | 10.73 |
| ER‐/PR‐ | 26.19 | 44.29 | 22.21 | 7.31 | ER‐/PR‐ | 1.62 | 16.12 | 80 | 2.26 | ER‐/PR‐ | 43.85 | 47.54 | 8.61 |
| Other | 39.37 | 35.25 | 15.31 | 10.07 | Other | 17.96 | 40.44 | 38.91 | 2.7 | Other | 43.49 | 44.81 | 11.7 |
Abbreviations: AN/AI, American Indian/Alaska Native; API, Asian or Pacific Islander; BCS, breast‐conserving surgery; ER, estrogen receptor; HRs, hormone receptor status; PR, progesterone receptor.
Figure 3.
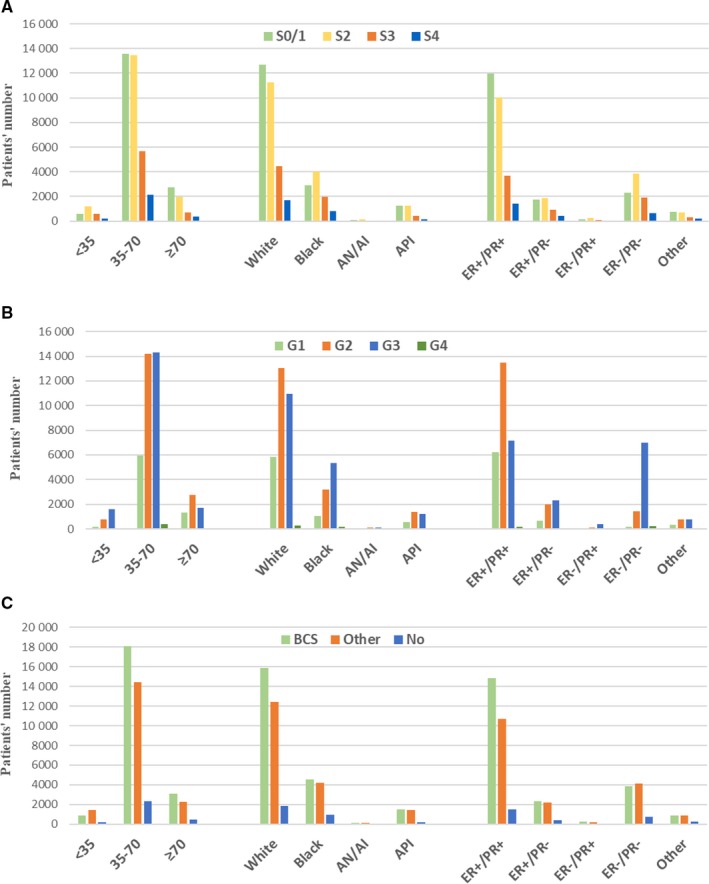
Number of Single patients in different clinicopathological subgroups stratified by stage (A), grade (B), and surgery (C) conditions
4. DISCUSSION
In this study, we confirmed what previous studies had shown that marital status impacted on OS of breast cancer patients and we further studied long term cancer‐specific survival of patients. Based on analysis of a large cohort including 298 434 patients and enrolling an integrated range of factors into the multivariate Cox analysis, we found that the benefit of BCSS for being married or single compared with being DSW was consistent with OS, and the association between marital status and BCSS varied by age, race, and HRs. To our knowledge, this is the first study to determine that the effect of marital status on prognosis varies by these demographic and pathological factors, which might reveal the potential mechanism generating this relationship. In further exploration, we found that the variation among these subgroups might be associated with the stage, the grade, and the surgery situation of patients.
A significant difference of OS among three marital subgroups could be observed from the results of 10‐year survival rate and Kaplan‐Meier curves, while the remarkable benefit of BCSS only occurred between the married and other two subgroups. After eliminating the possible interaction among variables with multivariate analysis, we found that single patients showed better BCSS than their DSW counterparts. This result was contrary to our expectation, since single patients were considered lacking for possible social and emotional support from marriage, as well as lower proportion of being at early stage or grade (Table 2). We then explored the effect of marital status on prognosis by different demographic and pathological subgroups, such as age, sex, race, and HRs. Difference of effect of marital status was observed among variable subgroups, and the survival benefit of being single occurred only in several subgroups. Meanwhile, the BCSS benefit of being married over being DSW vanished in AN/AI and male patients. These results hinted that the relationship between marital status and prognosis of patients with breast cancer might be associated with some demographic and pathological factors including age, sex, race, and HRs. Given that there were many more female participants than male, we paid our attention to other three factors. In further analysis of the proportion of stage, grade, and surgery situation in different demographic and pathological subgroups, we found that the BCSS benefit mostly occurred in subgroups showing more stage 0/I, lower grade, and better operation situation.
The first possible underlying reason why married patients with breast cancer had better prognosis is that married patients generally have greater financial resources, which might support them to undergo earlier physical examination, obtain better insurance coverage, and receive more adjuvant therapy.23, 24, 25 Compared with unfavorable marital status and possible financial distress of DSW patients, single patients might also benefit from better economic conditions. Second, undertreatment might also contribute to worse prognosis of the DSW. In this study, we observed that married patients with breast cancer received more surgeries than their counterparts, which indicated that worse prognosis in DSW patients can partly be attributed to undertreatment. Third, psychosocial support may contribute to a better prognosis among married patients with breast cancer. It has been well reported that decreased psychosocial support and greater psychological stress were associated with tumor progression and immune dysfunction. It has also been documented that married patients experienced less depression after diagnosis. This might partly be attributable to the fact that married patients can share the burden of negative emotions and receive psychological support from their spouses.26, 27, 28, 29, 30, 31, 32, 33 In previous studies of breast cancer, it has been confirmed that social support acted as a predictor of natural killer cell activation.34 Moreover, sex hormone disorder induced by psychological factors is closely related to tumor occurrence and development,27 which might partly explain why single patients showed better survival than DSW patients, as well as why the BCSS benefit of the single varied across different HRs.
We have suspected that single patients with no experience of marriage and childbearing history might suffer worse survival outcome compared with DSW patients, as what was reported that single patients showed an increased risk of breast cancer.35 However, our results of detailed difference among subgroups of marital status indicated that the protective effect of marriage was time‐bound and might be strongly affected by psychological factors. Compared with other counterparts, the worse prognosis of DSW patients might be resulted from negative emotions and worse economic situation due to their unfavorable marital status. In addition, the variation of relationship between marital status and prognosis of patients with breast cancer across race, grade, and HRs indicated that there might be a complicated and comprehensive mechanism underlying, such as genetic factors, hormone levels, function of immune system, psychological factors, social support, and treatment situation. The specific mechanism and its potential clinical practice remain to further research.
Subject to restrictions on our own knowledge and research methods, this study has several limitations. The first is the inherent biases present in any retrospective study. Second, some information relating to both marital status and prognosis of patients with breast cancer was unavailable in the SEER database, such as reproductive history, levels of hormone, and subsequent therapy. Therefore, we were unable to clarify the detailed mechanism of the relationship between marital status and prognosis of patients. Third, marital status was only recorded at diagnosis; details about the duration or quality of the marriage, or any changes in marital status, were not tracked, which might affect the BCSS of patients. Fourth, data of ER and PR status in the SEER database were collected from different local pathology laboratories, which might increase the possibility of bias. Finally, the results of our study are restricted to the United States, as some baseline characteristics such as race, marital status, and surgery history might be different in other countries.
5. CONCLUSIONS
Our study demonstrated that married and single patients with breast cancer had better prognosis than their DSW counterparts, which affected by the age, race and HRs. Meanwhile, married patients obtained better survival outcome than single patients. There might be a complicated and comprehensive mechanism underlying the relationship between marital status and prognosis of patients with breast cancer. Further studies should be conducted to clarify the specific influence and mechanism of these factors. According to our research, greater social and psychological support should be provided to DSW patients.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
AUTHOR CONTRIBUTIONS
The authors' responsibilities were as follows: Zhen Zhai and Yi Zheng designed the study; Zhijun Dai managed the study; Zhen Zhai, Linghui Zhou and Peng Xu extracted the data; Tian Tian, Pengtao Yang and Shuai Lin performed the analyses; Yujiao Deng, Cong Dai, Qian Hao and Na Li interpreted the evidence and wrote the manuscript; Hongtao Li and Zhijun Dai revised the article. All authors agreed to be accountable for the work.
ACKNOWLEDGMENTS
We acknowledge the efforts of Surveillance, Epidemiology, and End Results program.
Zhai Z, Zhang F, Zheng Y, et al. Effects of marital status on breast cancer survival by age, race, and hormone receptor status: A population‐based Study. Cancer Med. 2019;8:4906–4917. 10.1002/cam4.2352
ZZ, FZ and YZ contributed equally to this work.
Funding information
This work was supported by the National Natural Science Foundation of China (No.81471670) and the Key research and development plan, Shaanxi Province, China (2017ZDXM‐SF‐066).
Contributor Information
Hongtao Li, Email: lht4656@163.com.
Zhijun Dai, Email: dzj0911@126.com.
REFERENCES
- 1. Baum A, Posluszny DM. Health psychology: mapping biobehavioral contributions to health and illness. Annu Rev Psychol. 1999;50:137‐163. [DOI] [PubMed] [Google Scholar]
- 2. Soler‐Vila H, Kasl SV, Jones BA. Prognostic significance of psychosocial factors in African‐American and white breast cancer patients: a population‐based study. Cancer. 2003;98:1299‐1308. [DOI] [PubMed] [Google Scholar]
- 3. Johnson NJ, Backlund E, Sorlie PD, Loveless CA. Marital status and mortality: the national longitudinal mortality study. Ann Epidemiol. 2000;10:224‐238. [DOI] [PubMed] [Google Scholar]
- 4. Aizer AA, Chen M‐H, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31:3869‐3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hinyard L, Wirth LS, Clancy JM, Schwartz T. The effect of marital status on breast cancer‐related outcomes in women under 65: a SEER database analysis. Breast (Edinburgh, Scotland). 2017;32:13‐17. [DOI] [PubMed] [Google Scholar]
- 6. Chang SM, Barker FG 2nd. Marital status, treatment, and survival in patients with glioblastoma multiforme: a population based study. Cancer. 2005;104:1975‐1984. [DOI] [PubMed] [Google Scholar]
- 7. Reyes Ortiz CA, Freeman JL, Kuo YF, Goodwin JS. The influence of marital status on stage at diagnosis and survival of older persons with melanoma. J Gerontol Series A. 2007;62:892‐898. [DOI] [PubMed] [Google Scholar]
- 8. Goodwin JS, Hunt WC, Key CR, Samet JM. The effect of marital status on stage, treatment, and survival of cancer patients. JAMA. 1987;258:3125‐3130. [PubMed] [Google Scholar]
- 9. Cohen SD, Sharma T, Acquaviva K, Peterson RA, Patel SS, Kimmel PL. Social support and chronic kidney disease: an update. Adv Chronic Kidney Dis. 2007;14:335‐344. [DOI] [PubMed] [Google Scholar]
- 10. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 11. Weir HK, Thompson TD, Soman A, Moller B, Leadbetter S. The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer. 2015;121:1827‐1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer. 2008;8:56‐61. [DOI] [PubMed] [Google Scholar]
- 13. Abdollah F, Sun M, Thuret R, Abdo Al'a, Morgan M, Jeldres C, et al. The effect of marital status on stage and survival of prostate cancer patients treated with radical prostatectomy: a population‐based study. Cancer Causes Control. 2011;22:1085‐1095. [DOI] [PubMed] [Google Scholar]
- 14. Baine M, Sahak F, Lin C, Chakraborty S, Lyden E, Batra SK. Marital status and survival in pancreatic cancer patients: a SEER based analysis. PLoS ONE. 2011;6:e21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen D‐N, Song C‐G, Ouyang Q‐W, Jiang Y‐Z, Ye F‐G, Ma F‐J, et al. Differences in breast cancer characteristics and outcomes between Caucasian and Chinese women in the US. Oncotarget. 2015;6:12774‐12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Q, Gan L, Liang L, Li X, Cai S. The influence of marital status on stage at diagnosis and survival of patients with colorectal cancer. Oncotarget. 2015;6:7339‐7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93:41‐47. [DOI] [PubMed] [Google Scholar]
- 18. Qiu M, Yang D, Xu R. Impact of marital status on survival of gastric adenocarcinoma patients: results from the Surveillance Epidemiology and End Results (SEER) Database. Sci Rep. 2016;6:21098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi R‐L, Chen Q, Yang Z, Pan G, Zhang Z, Wang WeiHua, et al. Marital status independently predicts gastric cancer survival after surgical resection–an analysis of the SEER database. Oncotarget. 2016;7:13228‐13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiao W‐J, Zhu Y, Dai BO, Zhang H‐L, Shi G‐H, Shen Y‐J, et al. Conditional survival among patients with adrenal cortical carcinoma determined using a national population‐based surveillance, epidemiology, and end results registry. Oncotarget. 2015;6:44955‐44962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johansson A, Trewin CB, Hjerkind KV, Ellingjord‐Dale M, Johannesen TB, Ursin G. Breast cancer‐specific survival by clinical subtype after 7 years follow‐up of young and elderly women in a nationwide cohort. Int J Cancer. 2019;144:1251‐1261. [DOI] [PubMed] [Google Scholar]
- 22. Duggan MA, Anderson WF, Altekruse S, Penberthy L, Sherman ME. The surveillance, epidemiology, and end results (SEER) program and pathology: toward strengthening the critical relationship. Am J Surg Pathol. 2016;40:e94‐e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ali AA, Xiao H, Kiros GE. Health insurance and breast‐conserving surgery with radiation treatment. Am J Managed Care. 2014;20:502‐516. [PubMed] [Google Scholar]
- 24. Elmore L, Deshpande A, Daly M, Margenthaler JA. Postmastectomy radiation therapy in T3 node‐negative breast cancer. J Surg Res. 2015;199:90‐96. [DOI] [PubMed] [Google Scholar]
- 25. Patel K, Kanu M, Liu J, Bond B, Brown E, Williams E, et al. Factors influencing breast cancer screening in low‐income African Americans in Tennessee. J Commun Health. 2014;39:943‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cairney J, Boyle M, Offord DR, Racine Y. Stress, social support and depression in single and married mothers. Soc Psychiatry Psychiatr Epidemiol. 2003;38:442‐449. [DOI] [PubMed] [Google Scholar]
- 28. Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58:193‐210. [DOI] [PubMed] [Google Scholar]
- 29. Garssen B, Goodkin K. On the role of immunological factors as mediators between psychosocial factors and cancer progression. Psychiatry Res. 1999;85:51‐61. [DOI] [PubMed] [Google Scholar]
- 30. Moreno‐Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6:1863‐1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Powell ND, Tarr AJ, Sheridan JF. Psychosocial stress and inflammation in cancer. Brain Behav Immun. 2013;30(Suppl):S41‐S47. [DOI] [PubMed] [Google Scholar]
- 32. Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617‐625. [DOI] [PubMed] [Google Scholar]
- 33. Tong G, Geng Q, Cheng J, Chai J, Xia YI, Feng R, et al. Effects of psycho‐behavioral interventions on immune functioning in cancer patients: a systematic review. J Cancer Res Clin Oncol. 2014;140:15‐33. [DOI] [PubMed] [Google Scholar]
- 34. Levy SM, Herberman RB, Whiteside T, Sanzo K, Lee J, Kirkwood J. Perceived social support and tumor estrogen/progesterone receptor status as predictors of natural killer cell activity in breast cancer patients. Psychosom Med. 1990;52:73‐85. [DOI] [PubMed] [Google Scholar]
- 35. Kato I, Tominaga S, Terao C. An epidemiological study on marital status and cancer incidence. Jpn J Cancer Res. 1989;80:306‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]


