Abstract
Background
Infections with human papillomavirus (HPV) types 16 and 18 account for ~70% of invasive cervical cancers but the degree of protection from naturally acquired anti‐HPV antibodies is uncertain. We examined the risk of HPV infections as defined by HPV DNA detection and cervical abnormalities among women >25 years in the Human Papilloma VIrus Vaccine Immunogenicity ANd Efficacy trial's (VIVIANE, NCT00294047) control arm.
Methods
Serum anti‐HPV‐16/18 antibodies were determined at baseline and every 12 months in baseline DNA‐negative women (N = 2687 for HPV‐16 and 2705 for HPV‐18) by enzyme‐linked immunosorbent assay (ELISA) from blood samples. HPV infections were identified by polymerase chain reaction (PCR) every 6‐months, and cervical abnormalities were confirmed by cytology every 12 months. Data were collected over a 7‐year period. The association between the risk of type‐specific infection and cervical abnormalities and serostatus was assessed using Cox proportional hazard models.
Results
Risk of newly detected HPV‐16‐associated 6‐month persistent infections (PI) (hazard ratio [HR] = 0.56 [95%CI:0.32; 0.99]) and atypical squamous cells of undetermined significance (ASC‐US+) (HR = 0.28 [0.12; 0.67]) were significantly lower in baseline seropositive vs baseline seronegative women. HPV‐16‐associated incident infections (HR = 0.81 [0.56; 1.16]) and 12‐month PI (HR = 0.53 [0.24; 1.16]) showed the same trend. A similar trend of lower risk was observed in HPV‐18‐seropositive vs ‐seronegative women (HR = 0.95 [0.59; 1.51] for IIs, HR = 0.43 [0.16; 1.13] for 6‐month PIs, HR = 0.31 [0.07; 1.36] for 12‐month PIs, and HR = 0.61 [0.23; 1.61] for ASC‐US+).
Conclusions
Naturally acquired anti‐HPV‐16 antibodies were associated with a decreased risk of subsequent infection and cervical abnormalities in women >25 years. This possible protection was lower than that previously reported in 15‐ to 25‐year‐old women.
Keywords: human papillomavirus infection, naturally acquired antibodies, redetection or reactivation of HPV infection, cervical abnormality, risk reduction
1. BACKGROUND
Infections with human papillomavirus (HPV) types 16 and 18 are responsible for approximately 70% of invasive cervical cancers.1 While most infections clear on their own, some develop into precancerous lesions and cervical cancer.
Previous studies have shown that many women with incident HPV‐16 or HPV‐18 infections develop serum antibodies of the corresponding type of HPV.2, 3, 4, 5, 6, 7, 8 These naturally acquired antibodies can remain detectable for at least 4‐5 years after the initial infection.9 Whether or not these naturally acquired antibodies protect against future infection remains debatable.10, 11, 12, 13, 14, 15, 16, 17, 18
Risk of incident HPV infections in adult women is positively associated with new sexual partners and with the lifetime number of sexual partners.19, 20 In older women, both new viral acquisition and intermittent detections of HPV from past HPV exposures are likely to account for what has been classified as apparent new HPV infections. In women 30‐50 years of age, factors associated with repeat HPV detection have been shown to be comparable in short‐term and longer‐term studies, suggesting association between short‐term repeat detection and long‐term persistence.21 As incident HPV detection is negatively associated with viral load as well as with repeat detection, this suggests that actual new acquisition of HPV is less common than reactivation or intermittent persistence.
The role of naturally acquired antibodies in the prevention of new infections and cervical abnormalities can be explored in the control arms of large HPV vaccine trials. A correlation between naturally acquired antibodies to HPV‐16 (and to a lesser extent HPV‐18) and reduced risk of newly detected infection was demonstrated in younger women (15‐25 years) in the control arm of the PApilloma TRIal against Cancer In young Adults (PATRICIA; NCT00122681).12 Here, we examined the risk of “newly” detected HPV infections and cervical abnormalities among women >25 years in relation to naturally acquired HPV‐16/18 antibodies in the control arm of the VIVIANE during a 7‐year follow‐up period.22, 23
Our aim was to assess whether the risk factors for HPV infection differed between seropositive and seronegative women. We also analyzed risk factors stratified by baseline serostatus to mitigate the limitations in differentiating between new and reactivated infections.
2. METHODS
2.1. Study participants and procedures
Women aged >25 years were included in the control arm of the multinational, VIVIANE trial and were followed up for seven years. VIVIANE is the Human Papilloma Virus: Vaccine Immunogenicity and Efficacy trial. This is a phase 3 double‐blind, controlled vaccine trial based on age, cytology, region, and serostatus.23 The methodology of VIVIANE has been presented in detail elsewhere.24
Our analysis included women DNA‐negative for HPV‐16 and −18 at Month 0, with normal or low‐grade cytology (ie, negative or atypical squamous cells of undetermined significance [ASC‐US] or low‐grade squamous intraepithelial lesion [LSIL]) at Month 0, who had received at least one control vaccine dose (Al[OH]3) and who had sexual intercourse before or during the follow‐up (Figure 1).
Figure 1.
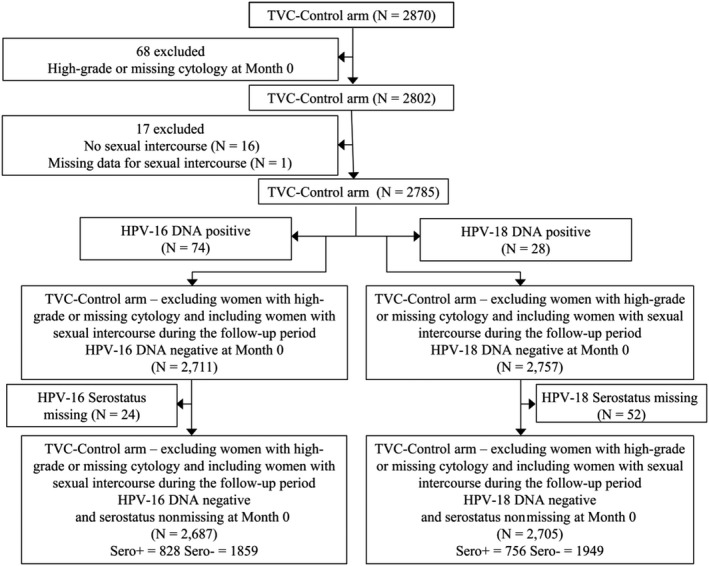
Flowcharts. HPV, human papillomavirus; TVC, total vaccinated cohort; N, number of women; Sero+, women seropositive for HPV‐16/18; Sero −, women seronegative for HPV‐16/18
Serum anti‐HPV‐16/18 antibodies were determined by enzyme‐linked immunosorbent assay (ELISA) from blood samples collected at baseline and every 12 months thereafter. Seropositivity was defined as an antibody level greater than or equal to the assay cutoff which was 8 ELISA units (EU)/mL for HPV‐16 and 7 EU/mL for HPV‐18.25
Liquid‐based cytology samples were tested for HPV using DNA typing PCR‐based assays every six months and cytopathological examinations every12 months.25 Information on known risk factors that predispose women to HPV cervical infection or recognized cofactors for cervical carcinogenesis was also collected through questionnaires. These data were collected at study entry and included demographic information, smoking habits, past and current sexual history, and reproductive status. In addition, data on participants' sexual behavior and use of contraception were collected every six months up to month 48.
Written informed consent was obtained from each woman before any study‐specific procedures were implemented. The protocol and other materials were approved by a national, regional, or investigational center Independent Ethics Committee or Institutional Review Board. The trial was conducted based on the Code of Ethics of the World Medical Association (Declaration of Helsinki).
The endpoints included in these analyses were (a) newly detected HPV‐16 and HPV‐18 incident infections, (b) 6‐ and 12‐month persistent infection (PI), ASC‐US+, and (c) histopathologically confirmed cervical intraepithelial neoplasia grade 1 or greater (CIN1+ and CIN2+). HPV‐16 and HPV‐18 serostatus were the main exposure variables.
2.2. Statistics
The analyses were performed on the total vaccinated cohort (TVC) of the control arm of the VIVIANE trial and included all women who received at least one control vaccine dose, who were DNA‐negative for HPV‐16 and HPV‐18 at Month 0, and who also had a normal or low‐grade cytology (ie, negative or ASC‐US or LSIL) at Month 0. All analyses were performed on women who had ever had sexual intercourse before study entry or during the follow‐up period.
Analyses were performed using SAS version 9.2. The incidence rate (IR) was calculated as the number of incident events divided by the total person‐time. Person‐years were calculated as the sum of the follow‐up for each participant expressed in years. The follow‐up period started on the day after first vaccination (control vaccine) and ended on the first occurrence of the endpoint or the last visit (whichever occurred first). The relationship between the exposure variables and the risk of newly detected infections or cervical abnormalities was assessed using Cox proportional hazard models. Univariate analyses were done to obtain unadjusted hazard ratios of the determinants of interest (not shown). For each endpoint, the following multivariable Cox models were performed including:
the type‐specific serostatus at baseline as a binary variable;
the type‐specific serostatus as a binary time‐dependent variable;
the antibody level as a time‐dependent continuous variable;
log‐transformed antibody level as a time‐dependent continuous variable.
For each endpoint, we included nine covariates in these models: region, age at inclusion, age at first sexual intercourse, marital status, smoking status at baseline, number of sexual partners during the past year, previous pregnancy, history of Chlamydia trachomatis infection, history of HPV infection/treatment or nonintact cervix. HPV‐associated infection or treatment was defined as two or more abnormal smears in sequence, an abnormal colposcopy or biopsy, or treatment of the cervix after abnormal smear or colposcopy findings. The histories of HPV infection/treatment were collected at baseline using medical history.
For ASC‐US+ only, previous type‐specific HPV infection was included as a time‐dependent variable since the presence of these cells indicates an active infection at a specific point in time. For CIN1+ and CIN2+ endpoints, no inferential analyses were performed due to the low number of cases. Also, analyses of determinants of interest were performed separately for the baseline seronegative and seropositive subjects to help determine whether newly detected infections were new or had been reactivated. The analysis is based on two assumptions: (a) An association between a latent reactivated infection and a known risk factor should be weaker than an association between a new infection and a known risk factor. (b) The reactivation of a PI should be more frequent in the baseline seropositive (representing presumed prior HPV infection exposure) subjects than in the baseline seronegative (representing presumed naïve, absent prior HPV infection exposure) subjects.
3. RESULTS
3.1. Study population
In total, 2687 and 2705 participants were included in the analysis of HPV‐16 and HPV‐18 endpoints, respectively (Figure 1). There was a difference of 3% between HPV‐16/18 by serostatus at baseline. Seroprevalence at enrollment was 31% (828/2687 seropositive women) for HPV‐16 and 28% (756/2705 seropositive women) for HPV‐18 (Table 1). This difference is entirely in agreement with the well‐known higher prevalence of 16 than 18 in HPV infections.
Table 1.
Frequency distributions of exposure variables and risk factors at study entry ‐ TVC‐Control arm‐excluding high grade or missing cytology at Month 0 – Ever had sexual intercourse
| Overall (N = 2785) | Baseline HPV‐16 serostatus (N = 2687) | Baseline HPV‐18 serostatus (N = 2705) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sero− (N = 1859) | Sero+ (N = 828) | Sero− (N = 1949) | Sero+ (N = 756) | ||||||||
| Exposure variables and Risk factors | Category | n | % | n | % | n | % | n | % | n | % |
| Marital status | Living or Lived with partner | 2354 | 84.52 | 1629 | 87.63 | 659 | 79.59 | 1677 | 86.04 | 618 | 81.75 |
| Single | 430 | 15.44 | 230 | 12.37 | 169 | 20.41 | 271 | 13.90 | 138 | 18.25 | |
| Missing | 1 | 0.04 | 1 | 0.05 | |||||||
| Number of pack years | [0; 0.5] | 2016 | 72.39 | 1415 | 76.12 | 549 | 66.30 | 1451 | 74.45 | 514 | 67.99 |
| ≥0.5 | 757 | 27.18 | 439 | 23.61 | 273 | 32.97 | 493 | 25.30 | 237 | 31.35 | |
| Missing | 12 | 0.43 | 5 | 0.27 | 6 | 0.72 | 5 | 0.26 | 5 | 0.66 | |
| Smoking status at baseline | No | 2398 | 86.10 | 1639 | 88.17 | 688 | 83.09 | 1702 | 87.33 | 631 | 83.47 |
| Yes | 386 | 13.86 | 220 | 11.83 | 140 | 16.91 | 246 | 12.62 | 125 | 16.53 | |
| Missing | 1 | 0.04 | . | . | 1 | 0.05 | . | ||||
| Sexual history at study entry | No | 5 | 0.18 | 4 | 0.22 | 1 | 0.12 | 4 | 0.21 | 1 | 0.13 |
| Yes | 2779 | 99.78 | 1855 | 99.78 | 827 | 99.88 | 1944 | 99.74 | 755 | 99.87 | |
| Missing | 1 | 0.04 | . | . | 1 | 0.05 | . | ||||
| History of HPV – Infection/treatment or not intact cervix | No | 2431 | 87.29 | 1674 | 90.05 | 685 | 82.73 | 1725 | 88.51 | 636 | 84.13 |
| Yes | 354 | 12.71 | 185 | 9.95 | 143 | 17.27 | 224 | 11.49 | 120 | 15.87 | |
| Age at first sexual intercourse (years) | <15 | 141 | 5.06 | 66 | 3.55 | 67 | 8.09 | 73 | 3.75 | 59 | 7.80 |
| 15–17 | 899 | 32.28 | 507 | 27.27 | 354 | 42.75 | 557 | 28.58 | 309 | 40.87 | |
| 18–25 | 1561 | 56.05 | 1137 | 61.16 | 377 | 45.53 | 1173 | 60.18 | 354 | 46.83 | |
| >26 | 177 | 6.36 | 146 | 7.85 | 27 | 3.26 | 141 | 7.23 | 32 | 4.23 | |
| Missing | 7 | 0.25 | 3 | 0.16 | 3 | 0.36 | 5 | 0.26 | 2 | 0.26 | |
| Number of lifetime sexual partners | 0 | 5 | 0.18 | 4 | 0.22 | 1 | 0.12 | 4 | 0.21 | 1 | 0.13 |
| 1 | 1068 | 38.35 | 876 | 47.12 | 174 | 21.01 | 864 | 44.33 | 183 | 24.21 | |
| 2–5 | 1017 | 36.52 | 666 | 35.83 | 310 | 37.44 | 703 | 36.07 | 286 | 37.83 | |
| 6–10 | 361 | 12.96 | 177 | 9.52 | 167 | 20.17 | 214 | 10.98 | 130 | 17.20 | |
| 11–15 | 146 | 5.24 | 68 | 3.66 | 69 | 8.33 | 79 | 4.05 | 60 | 7.94 | |
| 16–20 | 67 | 2.41 | 23 | 1.24 | 41 | 4.95 | 30 | 1.54 | 35 | 4.63 | |
| >20 | 120 | 4.31 | 45 | 2.42 | 66 | 7.97 | 54 | 2.77 | 61 | 8.07 | |
| Missing | 1 | 0.04 | . | . | 1 | 0.05 | . | ||||
| Number of sexual partners during the last year | 0 | 301 | 10.81 | 195 | 10.49 | 96 | 11.59 | 214 | 10.98 | 85 | 11.24 |
| 1 | 2219 | 79.68 | 1533 | 82.46 | 623 | 75.24 | 1585 | 81.32 | 574 | 75.93 | |
| 2‐3 | 230 | 8.26 | 115 | 6.19 | 95 | 11.47 | 132 | 6.77 | 83 | 10.98 | |
| ≥4 | 34 | 1.22 | 16 | 0.86 | 14 | 1.69 | 17 | 0.87 | 14 | 1.85 | |
| Missing | 1 | 0.04 | 1 | 0.05 | |||||||
| At least one previous pregnancy | No | 439 | 15.76 | 277 | 14.90 | 138 | 16.67 | 299 | 15.34 | 119 | 15.74 |
| Yes | 2345 | 84.20 | 1582 | 85.10 | 690 | 83.33 | 1649 | 84.61 | 637 | 84.26 | |
| Missing | 1 | 0.04 | 1 | 0.05 | |||||||
| Chlamydia trachomatis | No | 2626 | 94.29 | 1794 | 96.50 | 741 | 89.49 | 1862 | 95.54 | 693 | 91.67 |
| Yes | 133 | 4.78 | 56 | 3.01 | 72 | 8.70 | 70 | 3.59 | 55 | 7.28 | |
| Missing | 26 | 0.93 | 9 | 0.48 | 15 | 1.81 | 17 | 0.87 | 8 | 1.06 | |
| Contraception during lifetimea | No contraception | 413 | 14.83 | 298 | 16.03 | 102 | 12.32 | 297 | 15.24 | 110 | 14.55 |
| Hormonal use for contraception or another indication | 1802 | 24.85 | 1139 | 61.27 | 596 | 71.98 | 1220 | 62.60 | 515 | 68.12 | |
| Intra‐Uterine Device | 692 | 25.96 | 477 | 25.66 | 193 | 23.31 | 501 | 25.71 | 172 | 22.75 | |
| Sterilized | 723 | 35.30 | 498 | 26.79 | 204 | 24.64 | 522 | 26.78 | 183 | 24.21 | |
| Menopausal Status | Premenopausal | 2448 | 87.90 | 1636 | 88.00 | 721 | 87.08 | 1717 | 88.10 | 657 | 86.90 |
| Perimenopausal | 180 | 6.46 | 116 | 6.24 | 60 | 7.25 | 117 | 6.00 | 59 | 7.80 | |
| Postmenopausal | 142 | 5.10 | 93 | 5.00 | 46 | 5.56 | 101 | 5.18 | 39 | 5.16 | |
| Missing | 15 | 0.54 | 14 | 0.75 | 1 | 0.12 | 14 | 0.72 | 1 | 0.13 | |
N = total Number of subjects with a given group.
A subject can be included in more than one category.
Among those seropositive at enrollment, the geometric mean antibody concentration was 38.3 EU/mL (range: 8‐2527) and 23.3 EU/mL (range: 7‐725) for HPV‐16 and HPV‐18, respectively.
At enrollment, 45% of women were 26‐35 years old, 44% were 36‐45 years old, and 11% were ≥46 years old. Nearly all participants had been previously sexually active at the start of the study, except five who had their first sexual intercourse during the follow‐up. 56% had started sexual activity between 18 and 25 years (32% between 15 and 17), 80% had had one sexual partner during the previous year, and 84% had had a previous pregnancy. Moreover, 14% of women were current smokers, 5% were C trachomatis‐positive, and 87.8% were classified as pre‐menopausal, 6.5% as peri‐menopausal, 5.1% as post‐menopausal, while the status for the remaining 0.5% was missing.
3.2. Incidence rates of the endpoints
The IR per 100 person‐years of newly detected infections was 1.07 (95% confidence interval [CI]: 0.91‐1.25) for HPV‐16 and 0.64 (0.52‐0.78) for HPV‐18. For 6‐month PI, the IRs were 0.56 (0.44‐0.69) for HPV‐16 and 0.23 (0.16‐0.32) for HPV‐18. For 12‐month PI, these were 0.30 (0.22‐0.40) for HPV‐16 and 0.13 (0.08‐0.20) for HPV‐18.
The IRs for ASC‐US+ were 0.34 (0.26‐0.45) for HPV‐16 and 0.21 (0.14‐0.30) for HPV‐18.
During the seven years of follow‐up, 13 new HPV‐16 CIN1+ cases, 14 HPV‐18 CIN1+ cases, 8 HPV‐16 CIN2+ cases, and 9 HPV‐18 CIN2+ cases were detected.
3.3. Multivariable models
The multivariable Cox proportional hazard model, including the serostatus at baseline as a binary variable, showed that the risk of newly detected HPV‐16, 6‐month PI and ASC‐US+ was statistically significantly lower in seropositive vs seronegative women (hazard ratio [HR] = 0.56 [0.32‐0.99; P = 0.04] and 0.28 [0.12‐0.67; P = 0.004], respectively; Table 2). Analysis for HPV‐16 incident infections and 12‐month PI also showed a somewhat lower risk in seropositive than seronegative women although the difference was not statistically significant (HR = 0.81 [0.56‐1.16; P = 0.26] and 0.53 [0.24‐1.16; P = 0.11], respectively). With regard to HPV‐18, we found the risk of newly detected infections and cervical abnormalities was lower in seropositive vs seronegative women, but not statistically significant (HR = 0.95 [0.59‐1.51; P = 0.82] for incident infections, 0.43 [0.16‐1.13; P = 0.09] for 6‐month PI, 0.31 [0.07‐1.36; P = 0.12] for 12‐month PI, and 0.61 [0.23‐1.61; P = 0.32] for ASC‐US+; Table 3). Other determinants (Tables 2 and 3, and Supplementary Tables) associated with a higher risk of new infections were ≥2 sexual partners during the past year (for incident HPV‐16 and HPV‐18 infections, and 6‐month and 12‐month HPV‐16 and HPV‐18 PI), being single (for incident HPV‐18 infections), a history of HPV infection/treatment, or having a nonintact cervix (for incident HPV‐16 and HPV‐18 infections). Women older than 35 years at enrollment had a lower risk of incident HPV‐16 infections as well as HPV‐18 incident infections, 6‐month PI, and ASC‐US+. The risk of infections varied significantly among geographical regions. The risk factors associated with ASC‐US+ were a history of HPV infection/treatment, a nonintact cervix (for HPV‐18), and a previous type‐specific HPV infection (for HPV‐16 and HPV‐18).
Table 2.
Multivariable Cox model for HPV‐16 newly detected infections and cervical abnormalities including serostatus at baseline
| Risk factor | Category | Enrollment serostatus (binary) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incident infection | 6‐mo PI | 12‐mo PI | ASC‐US+ | ||||||||||||||
| N | n | Hazard ratio (95% CI) | P‐value | N | n | Hazard ratio (95% CI) | P‐value | N | n | Hazard ratio (95% CI) | P‐value | N | n | Hazard ratio (95% CI) | P‐value | ||
| HPV‐16 serostatus | Negative | 1814 | 114 | 1 | ‐ | 1779 | 67 | 1 | ‐ | 1755 | 38 | 1 | ‐ | 1787 | 43 | 1 | ‐ |
| Positive | 790 | 45 | 0.81 (0.56‐1.16) | 0.2559 | 767 | 17 | 0.56 (0.32‐0.99) | 0.0446 | 753 | 8 | 0.53 (0.24‐1.16) | 0.1123 | 774 | 9 | 0.28 (0.12‐0.67) | 0.0043 | |
| Age at inclusion | 26‐35 | 1157 | 94 | 1 | ‐ | 1134 | 45 | 1 | ‐ | 1109 | 21 | 1 | ‐ | 1139 | 33 | 1 | ‐ |
| ≥36 | 1157 | 65 | 0.58 (0.42‐0.82) | 0.0016 | 1412 | 39 | 0.78 (0.50‐1.24) | 0.2946 | 1399 | 25 | 0.99 (0.54‐1.82) | 0.9817 | 1423 | 19 | 0.57 (0.31‐1.03) | 0.0640 | |
| Region | Europe | 505 | 22 | 1 | ‐ | 495 | 6 | 1 | ‐ | 491 | 5 | 1 | ‐ | 500 | 8 | 1 | ‐ |
| Asia Pacific | 779 | 38 | 1.19 (0.68‐2.08) | 0.5445 | 772 | 23 | 2.57 (1.01‐6.52) | 0.0476 | 765 | 15 | 1.75 (0.61‐5.05) | 0.3004 | 772 | 10 | 0.77 (0.28‐2.10) | 0.6065 | |
| Latin America | 679 | 43 | 1.56 (0.90‐2.71) | 0.1142 | 663 | 29 | 3.86 (1.54‐9.70) | 0.0040 | 658 | 16 | 2.26 (0.79‐6.48) | 0.1308 | 666 | 18 | 1.66 (0.65‐4.21) | 0.2862 | |
| North America | 641 | 56 | 2.38 (1.42‐3.97) | 0.0009 | 616 | 26 | 4.28 (1.74‐10.54) | 0.0015 | 594 | 10 | 1.89 (0.63‐5.66) | 0.2575 | 623 | 16 | 1.32 (0.50‐3.46) | 0.5716 | |
| Age at first sexual intercourse grouped | ≥18 | 1654 | 98 | 1 | ‐ | 1621 | 57 | 1 | ‐ | 1600 | 33 | 1 | ‐ | 1630 | 32 | 1 | ‐ |
| 15‐17 | 817 | 50 | 0.85 (0.58‐1.23) | 0.3775 | 799 | 57 | 0.62 (0.36‐1.09) | 0.0967 | 784 | 9 | 0.56 (0.26‐1.22) | 0.1473 | 804 | 14 | 1.05 (0.53‐2.08) | 0.8832 | |
| <15 | 127 | 11 | 1.08 (0.56‐2.07) | 0.8271 | 120 | 8 | 1.48 (0.67‐3.27) | 0.3329 | 118 | 4 | 1.30 (0.43‐3.95) | 0.6433 | 121 | 6 | 2.20 (0.79‐6.16) | 0.1322 | |
| Marital status at baseline | Living or lived with partner | 2227 | 129 | 1 | ‐ | 2177 | 69 | 1 | ‐ | 2151 | 41 | 1 | ‐ | 2190 | 44 | 1 | ‐ |
| Single | 377 | 30 | 0.79 (0.49‐1.29) | 0.3493 | 369 | 15 | 0.89 (0.46‐1.75) | 0.7434 | 357 | 5 | 0.70 (0.24‐2.04) | 0.5116 | 371 | 8 | 0.67 (0.27‐1.67) | 0.3878 | |
| Smoking status at baseline | No | 2264 | 131 | 1 | ‐ | 2221 | 72 | 1 | ‐ | 2192 | 40 | 1 | ‐ | 2229 | 40 | 1 | ‐ |
| Yes | 340 | 28 | 1.35 (0.88‐2.06) | 0.1701 | 325 | 12 | 1.15 (0.61‐2.17) | 0.6678 | 316 | 6 | 1.10 (0.45‐2.70) | 0.8278 | 332 | 12 | 1.78 (0.89‐3.59) | 0.1049 | |
| Number of sexual partners during the last year | 0 | 283 | 16 | 1 | ‐ | 277 | 8 | 1 | ‐ | 272 | 2 | 1 | ‐ | 279 | 5 | 1 | ‐ |
| 1 | 2096 | 112 | 0.88 (0.52‐1.52) | 0.6563 | 2052 | 59 | 1.02 (0.48‐2.18) | 0.9634 | 2026 | 35 | 2.26 (0.54‐9.57) | 0.2663 | 2064 | 36 | 0.89 (0.33‐2.35) | 0.8105 | |
| ≥2 | 225 | 31 | 2.36 (1.26‐4.44) | 0.0074 | 217 | 17 | 3.53 (1.47‐8.48) | 0.0048 | 210 | 9 | 8.20 (1.70‐39.49) | 0.0087 | 218 | 11 | 1.72 (0.56‐5.28) | 0.3399 | |
| Pregnancy | No | 401 | 35 | 1 | ‐ | 393 | 19 | 1 | ‐ | 384 | 7 | 1 | ‐ | 394 | 11 | 1 | ‐ |
| Yes | 2203 | 124 | 0.74 (0.47‐1.14) | 0.1732 | 2153 | 65 | 0.57 (0.31‐1.05) | 0.0696 | 2124 | 39 | 0.82 (0.33‐2.06) | 0.6793 | 2167 | 41 | 0.91 (0.40‐2.06) | 0.8211 | |
| Chlamydia infection at baseline | No | 2458 | 150 | 1 | ‐ | 2407 | 81 | 1 | ‐ | 2371 | 44 | 1 | ‐ | 2420 | 48 | 1 | ‐ |
| Yes | 122 | 7 | 0.59 (0.27‐1.29) | 0.1848 | 115 | 2 | 0.36 (0.08‐1.51) | 0.1618 | 113 | 2 | 0.95 (0.21‐4.32) | 0.9486 | 117 | 3 | 2.57 (0.71‐9.27) | 0.1483 | |
| History of HPV infection/treatment or not intact cervix | No | 2285 | 128 | 1 | ‐ | 2234 | 72 | 1 | ‐ | 2202 | 39 | 1 | ‐ | 2246 | 3 | 1 | ‐ |
| Yes | 319 | 31 | 1.56 (1.03‐2.35) | 0.0348 | 312 | 12 | 1.17 (0.62‐2.18) | 0.6316 | 306 | 7 | 1.31 (0.57‐3.02) | 0.5240 | 315 | 8 | 1.03 (0.45‐2.36) | 0.9416 | |
| Previous HPV‐16 infection | No | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 2561 | 24 | 1 | ‐ | ||||||
| Yes | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 128 | 28 | 122.89 (67.91‐222.37) | <.0001 | |||||||
HPV = human papillomavirus; PI = persistent infection; CI = confidence interval; ACS‐US+ = atypical squamous cell of undetermined significance or greater; N = total number of subjects; n = number of cases reported. Bold: P‐values <0.05
Table 3.
Multivariable Cox model for HPV‐18 newly detected infections and cervical abnormalities including serostatus at baseline
| Risk factor | Category | Enrollment serostatus (binary) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incident infection | 6‐mo PI | 12‐mo PI | ASC‐US+ | ||||||||||||||
| N | n | Hazard ratio (95% CI) | P‐value | N | n | Hazard ratio (95% CI) | P‐value | N | n | Hazard ratio (95% CI) | P‐value | N | n | Hazard ratio (95% CI) | P‐value | ||
| HPV‐18 serostatus | Negative | 1907 | 97 | 1 | ‐ | 1874 | 31 | 1 | ‐ | 1840 | 18 | 1 | ‐ | 1882 | 26 | 1 | ‐ |
| Positive | 713 | 25 | 0.95 (0.59‐1.51) | 0.8165 | 689 | 5 | 0.43 (0.16‐1.13) | 0.0883 | 681 | 2 | 0.31 (0.07‐1.36) | 0.1198 | 696 | 6 | 0.61 (0.23‐1.61) | 0.3176 | |
| Age at inclusion | 26‐35 | 1156 | 70 | 1 | ‐ | 1133 | 28 | 1 | ‐ | 1108 | 15 | 1 | ‐ | 1137 | 25 | 1 | ‐ |
| ≥36 | 1464 | 27 | 0.35 (0.22‐0.56) | <.0001 | 1430 | 8 | 0.29 (0.13‐0.65) | 0.0029 | 1413 | 5 | 0.39 (0.13‐1.15) | 0.0863 | 1441 | 7 | 0.32 (0.13‐0.77) | 0.0115 | |
| Region | Europe | 518 | 19 | 1 | ‐ | 508 | 6 | 1 | ‐ | 502 | 7 | 1 | ‐ | 513 | 5 | 1 | ‐ |
| Asia Pacific | 775 | 28 | 1.22 (0.65‐2.32) | 0.5361 | 768 | 9 | 1.32 (0.41‐4.24) | 0.6364 | 761 | 6 | 1.70 (0.38‐7.60) | 0.4907 | 768 | 7 | 1.16 (0.31‐4.36) | 0.8207 | |
| Latin America | 688 | 23 | 1.14 (0.58‐2.21) | 0.7049 | 672 | 8 | 1.35 (0.41‐4.45) | 0.6178 | 666 | 3 | 0.98 (0.18‐5.43) | 0.9834 | 675 | 11 | 3.04 (0.82‐11.25) | 0.0964 | |
| North America | 639 | 27 | 1.12 (0.60‐2.09) | 0.7281 | 615 | 13 | 2.06 (0.71‐5.98) | 0.1837 | 592 | 7 | 1.99 (0.49‐8.13) | 0.3378 | 622 | 9 | 2.70 (0.73‐9.97) | 0.1371 | |
| Age at first sexual intercourse grouped | ≥18 | 1666 | 67 | 1 | ‐ | 1633 | 23 | 1 | ‐ | 1610 | 14 | 1 | ‐ | 1642 | 23 | 1 | ‐ |
| 15‐17 | 821 | 31 | 0.96 (0.59‐1.55) | 0.8591 | 804 | 12 | 0.81 (0.37‐1.78) | 0.6062 | 787 | 6 | 0.71 (0.24‐2.09) | 0.5349 | 809 | 6 | 0.41 (0.15‐1.12) | 0.0818 | |
| <15 | 126 | 9 | 1.57 (0.74‐3.34) | 0.2430 | 119 | 1 | 0.37 (0.05‐2.91) | 0.3439 | 117 | 0 | Not estimated | ‐ | 120 | 3 | 1.82 (0.46‐7.24) | 0.3945 | |
| Marital status at baseline | Living or lived with partner | 2234 | 67 | 1 | ‐ | 2185 | 24 | 1 | ‐ | 2156 | 12 | 1 | ‐ | 2198 | 22 | 1 | ‐ |
| Single | 385 | 30 | 2.13 (1.22‐3.71) | 0.0076 | 377 | 12 | 1.96 (0.79‐4.86) | 0.1452 | 364 | 8 | 2.79 (0.82‐9.48) | 0.1010 | 379 | 10 | 1.66 (0.58‐4.71) | 0.3426 | |
| Smoking status at baseline | No | 2270 | 82 | 1 | ‐ | 2228 | 29 | 1 | ‐ | 2196 | 16 | 1 | ‐ | 2236 | 28 | 1 | ‐ |
| Yes | 349 | 15 | 0.88 (0.49‐1.58) | 0.6754 | 334 | 7 | 1.33 (0.56‐3.14) | 0.5190 | 324 | 4 | 1.43 (0.45‐4.51) | 0.5416 | 341 | 4 | 0.78 (0.26‐2.35) | 0.6547 | |
| Number of sexual partners during the last year | 0 | 291 | 7 | 1 | ‐ | 285 | 2 | 1 | ‐ | 280 | 2 | 1 | ‐ | 287 | 3 | 1 | ‐ |
| 1 | 2097 | 68 | 1.46 (0.65‐3.26) | 0.3565 | 2054 | 25 | 1.85 (0.42‐8.16) | 0.4151 | 2026 | 13 | 1.09 (0.22‐5.27) | 0.9169 | 2066 | 23 | 1.23 (0.34‐4.36) | 0.7530 | |
| ≥2 | 231 | 22 | 3.28 (1.36‐7.88) | 0.0080 | 223 | 9 | 4.69 (0.97‐22.56) | 0.0540 | 214 | 5 | 2.92 (0.53‐16.23) | 0.2197 | 224 | 6 | 1.57 (0.35‐7.15) | 0.5589 | |
| Pregnancy | No | 404 | 21 | 1 | ‐ | 396 | 9 | 1 | ‐ | 386 | 6 | 1 | ‐ | 397 | 8 | 1 | ‐ |
| Yes | 2215 | 76 | 1.17 (0.66‐2.06) | 0.5931 | 2166 | 27 | 1.14 (0.46‐2.83) | 0.7715 | 2134 | 14 | 1.03 (0.32‐3.35) | 0.9621 | 2180 | 24 | 0.96 (0.32‐2.87) | 0.9462 | |
| Chlamydia infection at baseline | No | 2477 | 89 | 1 | ‐ | 2427 | 33 | 1 | ‐ | 2388 | 18 | 1 | ‐ | 2440 | 30 | 1 | ‐ |
| Yes | 118 | 6 | 0.82 (0.34‐1.96) | 0.6579 | 111 | 2 | 0.80 (0.18‐3.52) | 0.7643 | 109 | 1 | 0.73 (0.09‐5.82) | 0.7666 | 113 | 1 | 0.27 (0.03‐2.53) | 0.2534 | |
| History of HPV infection/treatment or not intact cervix | No | 2285 | 75 | 1 | ‐ | 2236 | 27 | 1 | ‐ | 2203 | 1 | 1 | ‐ | 2248 | 22 | 1 | ‐ |
| Yes | 335 | 22 | 1.72 (1.03‐2.86) | 0.0373 | 327 | 9 | 1.79 (0.79‐4.09) | 0.1658 | 318 | 5 | 1.64 (0.52‐5.20) | 0.3980 | 330 | 10 | 2.57 (1.10‐6.01) | 0.0288 | |
| Previous cervical HPV‐18 infection | No | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 2578 | 20 | 1 | ‐ | ||||||
| Yes | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 68 | 12 | 122.93 (54.69‐276.33) | <.0001 | |||||||
HPV = human papillomavirus; PI = persistent infection; CI = confidence interval; ACS‐US+ = atypical squamous cell of undetermined significance or greater; N = total number of subjects; n = number of cases reported. Bold: P‐values <0.05
The other multivariable Cox proportional hazard models (including the serostatus as a time‐dependent variable, antibody level as a time‐dependent variable, and log‐transformed level as a time‐dependent variable) showed similar results (Figure 2 and Supplementary Tables).
Figure 2.
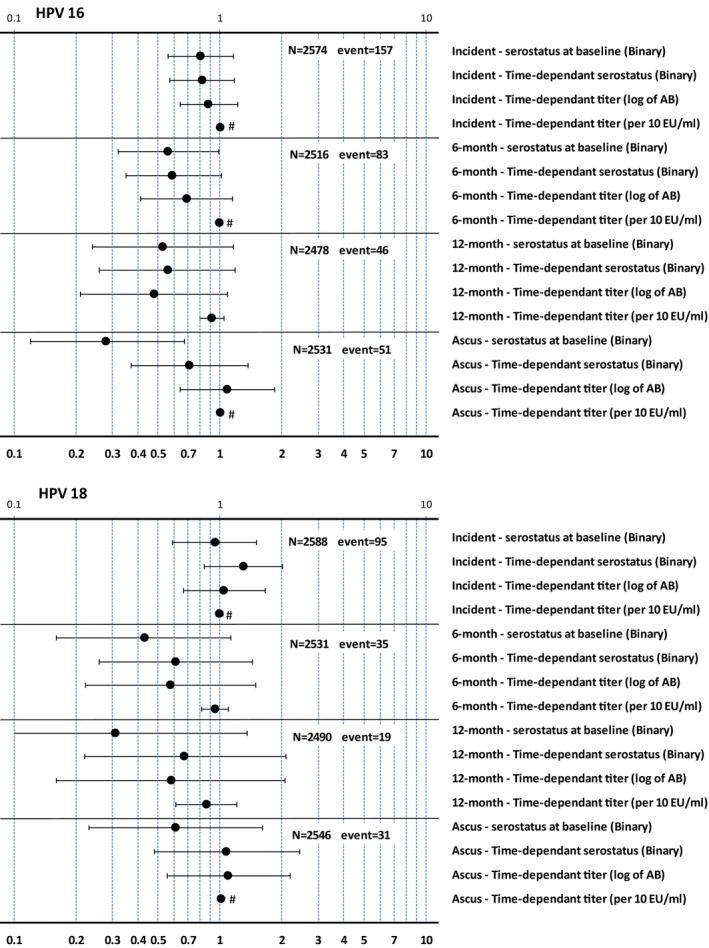
Risk ratio of incident, 6‐mo persistent, and 12‐mo persistent infection and atypical squamous cell of undetermined significance or greater in HPV‐16/HPV‐18 type‐specific seropositive vs seronegative women. Error bars represent 95% confidence intervals; #95% confidence intervals are narrow and not visible; HPV, human papillomavirus; PI, persistent infection; bin, binary; ab, antibody; ACS‐US+, atypical squamous cell of undetermined significance or greater
The analyses stratified by baseline serostatus showed that these risk factors (number of sexual partners in the last 12 months, living single and smoking) were more marked in seronegative than in seropositive women (Table 4).
Table 4.
Multivariable Cox model for HPV‐16 and HPV‐18 6‐mo persistent infection and incident infection according to different serostatus at baseline
| Risk factor | HPV‐16 6‐mo PI | HPV‐18 6‐mo PI | HPV‐16 incident infection | HPV‐18 incident infection | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seronegative | Seropositive | Seronegative | Seropositive | Seronegative | Seropositive | Seronegative | Seropositive | |||||||||
| N = 1767 event=67 | N = 749 event=16 | N = 1852 event=30 | N = 679 event=5 | N = 1802 event=114 | N = 772 event=43 | N = 1885 event =70 | N = 703 event=25 | |||||||||
| Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | |
| Age at inclusion | ||||||||||||||||
| 26‐35 | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ |
| ≥36 | 0.73 (0.44‐1.22) | 0.2306 | 1.27 (0.43‐3.74) | 0.6625 | 0.33 (0.14‐0.77) | 0.0103 | Not estimated | ‐ | 0.62 (0.42‐0.92) | 0.0177 | 0.51 (0.27‐0.97) | 0.0409 | 0.33 (0.19‐0.57) | <0.001 | 0.41 (0.17‐1.01) | 0.0527 |
| Region | ||||||||||||||||
| Europe | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ |
| Asia Pacific | 2.70 (0.88‐8.29) | 0.0825 | 2.79 (0.50‐15.71) | 0.2447 | 1.16 (0.35‐3.81) | 0.8069 | Not estimated | ‐ | 1.13 (0.59‐2.13) | 0.7172 | 1.25 (0.37‐4.21) | 0.7169 | 1.06 (0.48‐2.31) | 0.8893 | 1.85 (0.57‐5.95) | 0.3033 |
| Latin America | 5.00 (1.66‐15.02) | 0.0042 | 1.33 (0.18‐10.04) | 0.7804 | 0.83 (0.23‐2.95) | 0.7750 | Not estimated | ‐ | 1.46 (0.77‐2.76) | 0.2433 | 2.03 (0.65‐6.36) | 0.2225 | 1.07 (0.49‐2.33) | 0.8717 | 1.41 (0.38‐5.25) | 0.6131 |
| North America | 5.27 (1.77‐15.73) | 0.0029 | 2.80 (0.58‐13.52) | 0.2002 | 1.74 (0.57‐5.28) | 0.3319 | Not estimated | ‐ | 2.27 (1.24‐4.17) | 0.0081 | 2.76 (1.03‐7.43) | 0.0445 | 1.44 (0.69‐3.00) | 0.3268 | 0.59 (0.18‐2.01) | 0.4022 |
| Age at first sexual intercourse grouped | ||||||||||||||||
| ≥18 | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ |
| 15‐17 | 0.58 (0.30‐1.09) | 0.0915 | 0.67 (0.20‐2.29) | 0.5274 | 0.67 (0.28‐1.60) | 0.3669 | 5.52 (0.37‐81.42) | 0.2133 | 0.81 (0.52‐1.26) | 0.3457 | 0.88 (0.43‐1.81) | 0.7343 | 0.97 (0.56‐1.68) | 0.9072 | 0.79 (0.29‐2.15) | 0.6378 |
| <15 | 0.86 (0.29‐2.55) | 0.7829 | 4.04 (1.06‐15.47) | 0.0413 | Not estimated | ‐ | 29.71 (0.70‐1263.78) | 0.0763 | 0.58 (0.20‐1.64) | 0.3042 | 1.77 (0.69‐4.54) | 0.2372 | 0.78 (0.23‐2.63) | 0.6868 | 3.58 (1.19‐10.83) | 0.0237 |
| Marital status at baseline | ||||||||||||||||
| Living or lived with partner | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ |
| Single | 0.97 (0.45‐2.11) | 0.9381 | 0.87 (0.22‐3.46) | 0.8395 | 1.93 (0.70‐5.36) | 0.2039 | 2.73 (0.20‐36.42) | 0.4484 | 0.75 (0.42‐1.37) | 0.3537 | 1.02 (0.45‐2.30) | 0.9587 | 1.93 (1.00‐3.73) | 0.0514 | 2.64 (0.93‐7.44) | 0.0672 |
| Smoking status at baseline | ||||||||||||||||
| No | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ |
| Yes | 1.36 (0.68‐2.74) | 0.3825 | 0.68 (0.15‐3.17) | 0.6283 | 1.40 (0.55‐3.58) | 0.4801 | 0.84 (0.07‐9.42) | 0.8874 | 1.75 (1.07‐2.86) | 0.0247 | 0.72 (0.29‐1.74) | 0.4633 | 1.03 (0.59‐3.92) | 0.9385 | 0.57 (0.16‐1.98) | 0.3747 |
| Number of sexual partners during the last year | ||||||||||||||||
| 0 | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ |
| 1 | 1.08 (0.45‐2.59) | 0.8652 | 0.72 (0.15‐3.52) | 0.6853 | 1.58 (0.35‐7.15) | 0.5522 | Not estimated | ‐ | 0.84 (0.45‐1.57) | 0.5839 | 0.99 (0.34‐2.92) | 0.9919 | 1.52 (0.59‐3.92) | 0.3916 | 1.33 (0.28‐6.22) | 0.7199 |
| ≥2 | 4.49 (1.62‐12.49) | 0.0039 | 2.04 (0.33‐12.59) | 0.4428 | 4.51 (0.90‐22.75) | 0.0679 | Not estimated | ‐ | 2.60 (1.23‐5.50) | 0.0127 | 1.98 (0.59‐6.69) | 0.2704 | 3.36 (1.18‐9.63) | 0.0238 | 3.36 (0.67‐16.91) | 0.1419 |
| Pregnancy | ||||||||||||||||
| No | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ |
| Yes | 0.59 (0.30‐1.19) | 0.1396 | 0.57 (0.15‐2.22) | 0.4207 | 1.74 (0.60‐5.01) | 0.3044 | 0.10 (0.01‐1.72) | 0.1119 | 0.60 (0.36‐1.01) | 0.0127 | 1.44 (0.57‐3.63) | 0.4343 | 1.40 (0.70‐2.79) | 0.3415 | 0.70 (0.25‐1.97) | 0.4954 |
| Chlamydia infection at baseline | ||||||||||||||||
| No | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ |
| Yes | Not estimated | ‐ | 0.85 (0.18‐4.06) | 0.8404 | 0.59 (0.08‐4.55) | 0.6120 | 1.97 (0.15‐26.31) | 0.6071 | 0.17 (0.02‐1.22) | 0.0777 | 1.09 (0.44‐2.72) | 0.8547 | 0.73 (0.22‐2.45) | 0.6094 | 1.03 (0.26‐4.12) | 0.9658 |
| History of HPV infection/treatment or not intact cervix | ||||||||||||||||
| No | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ | 1 | ‐ |
| Yes | 0.87 (0.39‐1.94) | 0.7329 | 1.74 (0.58‐5.22) | 0.3202 | 2.10 (0.86‐5.13) | 0.1024 | 0.76 (0.07‐8.56) | 0.8229 | 1.36 (0.80‐2.32) | 0.2582 | 1.89 (0.96‐3.71) | 0.0648 | 1.82 (0.99‐3.35) | 0.0530 | 1.19 (0.43‐3.30) | 0.7309 |
HPV = human papillomavirus N = number of subjects used in the model; event = number of HPV‐type‐specific 6‐mo persistent cervical infection; PI = persistent infection; CI = confidence interval. Bold: P‐values <0.05
4. DISCUSSION
In this study, HPV‐16‐seropositive women of 25 years and older had a moderate decrease in risk of developing a new type‐specific HPV, PI, and ASC‐US+ compared to seronegative women. This result agrees with the hypothesis that naturally acquired HPV antibodies probably provide only partial protection against subsequent infection with the same HPV type. However, HPV‐18‐seropositive women had deficient levels of protection. Any naturally acquired protection afforded by either antibody is unlikely to be better than the benefits acquired by vaccination. Another study has found that women aged between 30 and 50 who were seropositive for high risk (HR) HPV at baseline had a higher incidence of new type‐specific HPV infection than women who were seronegative.26
The association between seropositivity and the reduced risk of new infection was less in our study of 26+‐year‐old women than demonstrated in our study of younger women aged 15‐25 years in PATRICIA and in the Costa Rica Vaccine Trial.12, 15 This low protective effect or even absence of protective effect in >25‐year‐old women could suggest waning of the natural immunity but it could also reflect reactivation of prior infection.26
In the present study, we were not able to determine an accurate antibody threshold value for a defined reduction rate in infection. In the PATRICIA trial, HPV‐16 antibody levels comprised between 200 and 500 EU/mL were associated with a 90% reduction of incident infection, of 6‐month PI and of ASC‐US+.12 For HPV‐18, seropositivity was associated with a lower risk of ASC‐US+ and CIN1+ but no association was found between naturally acquired antibodies and new infection.12
The current study also attempted to consider the change in serostatus during the follow‐up period. Including the serostatus as a time‐dependent variable and as a continuous variable in the Cox models is original. In a recent meta‐analysis, assessing the naturally acquired immunity against HPV infection, none of the 14 included studies considered the possible change of serostatus during the follow‐up period.27 Overall, our various models gave consistent results. However, the interpretation of the time‐dependent serostatus models can be challenging because of the interaction between the change in antibody titers and the incidence of new HPV infections. Because the serology was collected every 12 months and the cervical sample every six months, new, but undetected, infection could have boosted the antibody titer.
In another analysis of the control cohort of the VIVIANE trial, the risk of detecting CIN after natural HPV infection in women aged >25 years was similar to that observed in women aged 15‐25 years from the PATRICIA trial.24 This observation suggests that there are little to no age‐related differences in the detection of natural HPV infection and their associated CIN lesions.
Our analysis of determinants when considered separately for the baseline seronegative and seropositive subjects partially supports the hypothesis suggested by other studies that most of the newly detected HPV infections in seropositive women would be a reactivation of prior HPV infections.19, 20
The strengths of this study included the large cohort size of approximately 2700 women, and the relatively extended follow‐up period of seven years, which allowed for a thorough evaluation of an unvaccinated cohort. This study also had several limitations. A cervical sample test was performed only every six months, which could have meant that some incident HPV infections were not detected. In addition, it was not possible to determine whether an infection was quiescent, persistent at undetectable levels or was a new infection. Evidence exists that type‐specific HPV infection can present after a period of nondetection.28 Based on this assumption, some infections considered as new could indeed be a PI. This scenario could also bias the assessment of the relationship between natural antibodies and risk of new infection. Furthermore, the number of CIN1+ and CIN2+ cases was too low to allow for inferential analyses. Since we were unable to define which HPV type caused the abnormal cytology, ASC‐US+ lesions could ensue from non‐HPV‐16/18 types.
Further research is needed to better understand the natural history of HPV infection and the link between seropositivity and subsequent protection in women of different age groups.
In conclusion, multivariable Cox analyses showed evidence of lower risk of newly detected incident and persistent HPV infections and ASC‐US+ in women with naturally acquired antibodies against HPV‐16. The results for HPV‐18 are not conclusive since only a limited and nonsignificant decrease in risk was observed. These findings are consistent with a partial protective role of naturally acquired HPV antibodies against future infection with the corresponding HPV type. However, no threshold of antibody levels necessary for protection could be defined.
CONFLICT OF INTEREST
D Rosillon and F Struyf are employed by the GSK group of companies and received GSK shares. L Baril was employed by the GSK group of companies at the time of the study and received GSK shares. G Dubin is currently a full‐time employee of Takeda Pharmaceuticals, Deerfield, Illinois, and receives salary and stock shares. MR Del Rosario‐Raymundo reports payment of honorarium as principal investigator and support for travel to meetings for the study from the GSK group of companies during the conduct of the study; payment for lectures including service on speakers' bureaus from the GSK group of companies. M Martens reports grants from the GSK group of companies, during the conduct of the study. C Bouchard reports grants from the GSK group of companies, during the conduct of the study. She reports grants and honorarium from Merck. KL Fong reports grant from the GSK group of companies via her institution for the conduct of the study. MC Bozonnat is a consultant outsourced from 4Clinics to the GSK group of companies. A Chatterjee received grant funding for clinical trials, and served on the speakers' bureau and advisory boards for the GSK group of companies and Merck. SM Garland has received advisory board fees and grants from CSL and the GSK group of companies, and lectures fees from Merck, the GSK group of companies, and Sanofi Pasteur. In addition, she received funding through her institution to conduct HPV vaccines studies for MSD and the GSK group of companies. She is a member of the Merck Global Advisory Board as well as the Merck Scientific Advisory Committee for HPV. E Lazcano‐Ponce received fees to conduct HPV vaccines studies from the GSK group of companies and Merck. SA McNeil has received research grants from the GSK group of companies and Sanofi Pasteur and speaker honoraria from Merck. B Romanowski received research grants, travel support, and speaker honoraria from the GSK group of companies. SR Skinner received funds through her institution from the GSK group of companies to cover expenses involved in the collection of data for this study. The GSK group of companies provided funds to reimburse expenses incurred with travel to conference to present data from other studies and paid honoraria to her institution for work conducted in the context of Advisory Board and educational meetings. CM Wheeler's institution received a contract from the GSK group of companies to act as a clinical trial site for this study, and reimbursements for travel related to publication activities and for HPV vaccine studies. Her institution also received funding from Merck to conduct HPV vaccine trials, and from Roche Molecular Systems equipment and reagents for HPV genotyping studies, outside the submitted work. X Castellsagué received research funding through his institution (ICO) from Merck & Co, SPMSD, the GSK group of companies, and Genticel. He also received honoraria for conferences from Vianex and SPMSD. G Minkina, as an investigator at a study clinical site, received fees from the GSK group of companies through her institution. She also received funding from Merck Sharp & Dohme to participate as principal investigator in efficacy trials. She received travel support to attend scientific meetings, honoraria for speaking engagements and participation in advisory board meetings, and consulting fees from the GSK group of companies and Merck Sharp & Dohme. T Stoney received honoraria from the GSK group of companies for study committee membership (Asia Pacific study follow‐up committee for Zoster studies), for conference attendance, and travel support. Her institution also received additional funding from a bioCSL grant for a project in which she is an investigator, funded by National Health and Medical Research Council. She also received travel support for participation in study investigator meetings from Novartis Vaccine and Diagnostics, Sanofi Pasteur, Alios BioPharma, and Pfizer. SC Quek received honoraria and travel expenses from the GSK group of companies for speaking at various symposia. A Savicheva received grants and fees from the GSK group of companies to participate in an epidemiological study (HERACLES). J Salmeron received grants from the GSK group of companies, Qiagen, and Merck Inc. D Money has received grants from Merck, the GSK group of companies, Novartis, and Sanofi for studies conduct. CS Vallejos, TYK Lim, B ter Harmsel, M Cruickshank, A Fiander, and A Ilancheran have nothing to disclose.
Supporting information
ACKNOWLEDGMENTS
The authors thank all study participants and their families, all clinical study site personnel who contributed to the conduct of this trial, and Dr. N Chakhtoura and Dr. L Myron as investigators. Writing support services were provided by John Bean (Bean Medical Writing), Kristel Vercauteren, and Claire Verbelen (XPE Pharma & Science, Belgium) on behalf of GSK, Wavre, Belgium. The authors would also like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Thibaud André coordinated manuscript development and editorial support.
Rosillon D, Baril L, Del Rosario‐Raymundo MR, et al. Risk of newly detected infections and cervical abnormalities in adult women seropositive or seronegative for naturally acquired HPV‐16/18 antibodies. Cancer Med. 2019;8:4938–4953. 10.1002/cam4.1879
†Deceased 12 June 2016.
Trademark: Cervarix is a trademark of the GSK group of companies.
Funding information
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also funded costs associated with the development and the publishing of the present manuscript.
REFERENCES
- 1. de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross‐sectional worldwide study. Lancet Oncol. 2010;11(11):1048‐1056. [DOI] [PubMed] [Google Scholar]
- 2. Faust H, Jelen MM, Poljak M, Klavs I, Učakar V, Dillner J. Serum antibodies to human papillomavirus (HPV) pseudovirions correlate with natural infection for 13 genital HPV types. J Clin Virol. 2013;56(4):336‐341. [DOI] [PubMed] [Google Scholar]
- 3. Kirnbauer R, Hubbert NL, Wheeler CM, Becker TM, Lowy DR, Schiller JT. A virus‐like particle enzyme‐linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J Natl Cancer Inst. 1994;86(7):494‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kjellberg L, Wang Z, Wiklund F, et al. Sexual behaviour and papillomavirus exposure in cervical intraepithelial neoplasia: a population‐based case‐control study. J Gen Virol. 1999;80(Pt 2):391‐398. [DOI] [PubMed] [Google Scholar]
- 5. Porras C, Bennett C, Safaeian M, et al. Determinants of seropositivity among HPV‐16/18 DNA positive young women. BMC Infect Dis. 2010;10:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tong Y, Ermel A, Tu W, Shew M, Brown DR. Association of HPV types 6, 11, 16, and 18 DNA detection and serological response in unvaccinated adolescent women. J Med Virol. 2013;85(10):1786‐1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Viscidi RP, Kotloff KL, Clayman B, Russ K, Shapiro S, Shah KV. Prevalence of antibodies to human papillomavirus (HPV) type 16 virus‐like particles in relation to cervical HPV infection among college women. Clin Diagn Lab Immunol. 1997;4(2):122‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carter JJ, Koutsky LA, Hughes JP, et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181(6):1911‐1919. [DOI] [PubMed] [Google Scholar]
- 9. Geijersstam V, Kibur M, Wang Z, et al. Stability over time of serum antibody levels to human papillomavirus type 16. J Infect Dis. 1998;177(6):1710‐1714. [DOI] [PubMed] [Google Scholar]
- 10. Wentzensen N, Rodriguez AC, Viscidi R, et al. A competitive serological assay shows naturally acquired immunity to human papillomavirus infections in the Guanacaste Natural History Study. J Infect Dis. 2011;204(1):94‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palmroth J, Namujju P, Simen‐Kapeu A, et al. Natural seroconversion to high‐risk human papillomaviruses (hrHPVs) is not protective against related HPV genotypes. Scand J Infect Dis. 2010;42(5):379‐384. [DOI] [PubMed] [Google Scholar]
- 12. Castellsagué X, Naud P, Chow S‐N, et al. Risk of newly detected infections and cervical abnormalities in women seropositive for naturally acquired human papillomavirus type 16/18 antibodies: analysis of the control arm of PATRICIA. J Infect Dis. 2014;210(4):517‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ho G, Studentsov Y, Hall CB, et al. Risk factors for subsequent cervicovaginal human papillomavirus (HPV) infection and the protective role of antibodies to HPV‐16 virus‐like particles. J Infect Dis. 2002;186(6):737‐742. [DOI] [PubMed] [Google Scholar]
- 14. Olsson S‐E, Kjaer SK, Sigurdsson K, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5(10):696‐704. [DOI] [PubMed] [Google Scholar]
- 15. Safaeian M, Porras C, Schiffman M, et al. Epidemiological study of anti‐HPV16/18 seropositivity and subsequent risk of HPV16 and ‐18 infections. J Natl Cancer Inst. 2010;102(21):1653‐1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Viscidi RP, Schiffman M, Hildesheim A, et al. Seroreactivity to human papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: results from a population‐based study in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13(2):324‐327. [DOI] [PubMed] [Google Scholar]
- 17. Viscidi RP, Snyder B, Cu‐Uvin S, et al. Human papillomavirus capsid antibody response to natural infection and risk of subsequent HPV infection in HIV‐positive and HIV‐negative women. Cancer Epidemiol Biomarkers Prev. 2005;14(1):283‐288. [PubMed] [Google Scholar]
- 18. Wilson L, Pawlita M, Castle PE, et al. Seroprevalence of 8 oncogenic human papillomavirus genotypes and acquired immunity against reinfection. J Infect Dis. 2014;210(3):448‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trottier H, Ferreira S, Thomann P, et al. Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer Res. 2010;70(21):8569‐8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rositch AF, Burke AE, Viscidi RP, Silver MI, Chang K, Gravitt PE. Contributions of recent and past sexual partnerships on incident human papillomavirus detection: acquisition and reactivation in older women. Cancer Res. 2012;72(23):6183‐6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fu TJ, Fu Xi L, Hulbert A, et al. Short‐term natural history of high‐risk human papillomavirus infection in mid‐adult women sampled monthly. Int J Cancer. 2015;137(10):2432‐2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wheeler CM, Skinner SR, Del Rosario‐Raymundo MR, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04‐adjuvanted vaccine in women older than 25 years: 7‐year follow‐up of the phase 3, double‐blind, randomised controlled VIVIANE study. Lancet Infect Dis. 2016;16(10):1154‐1168. [DOI] [PubMed] [Google Scholar]
- 23. Skinner SR, Szarewski A, Romanowski B, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04‐adjuvanted vaccine in women older than 25 years: 4‐year interim follow‐up of the phase 3, double‐blind, randomised controlled VIVIANE study. Lancet. 2014;384(9961):2213‐2227. [DOI] [PubMed] [Google Scholar]
- 24. Skinner RS, Wheeler CM, Romanowski B, et al. Progression of HPV infection to detectable cervical lesions or clearance in adult women: Analysis of the control arm of the VIVIANE study. Int J Cancer. 2016;138(10):2428‐2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dessy FJ, Giannini SL, Bougelet CA, et al. Correlation between direct ELISA, single epitope‐based inhibition ELISA and pseudovirion‐based neutralization assay for measuring anti‐HPV‐16 and anti‐HPV‐18 antibody response after vaccination with the AS04‐adjuvanted HPV‐16/18 cervical cancer vaccine. Hum Vaccin. 2008;4(6):425‐434. [DOI] [PubMed] [Google Scholar]
- 26. Fu T‐CJ, Carter JJ, Hughes JP, et al. Re‐detection vs. new acquisition of high‐risk human papillomavirus in mid‐adult women. Int J Cancer. 2016;139(10):2201‐2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beachler DC, Jenkins G, Safaeian M, Kreimer AR, Wentzensen N. Natural Acquired Immunity Against Subsequent Genital Human Papillomavirus Infection: A Systematic Review and Meta‐analysis. J Infect Dis. 2016;213(9):1444‐1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Insinga RP, Perez G, Wheeler CM, et al. Incidence, duration, and reappearance of type‐specific cervical human papillomavirus infections in young women. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1585‐1594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


