Abstract
Background
Diffusion tensor imaging (DTI) is widely used; however, most of the prior studies have resulted in presurgical decreased fractional anisotropy (FA) values in patients with cervical spondylotic myelopathy (CSM). We used ZOOM DTI and could acquire highly accurate FA values during perioperative periods, which indicated different insights than preceding studies. The objective of this study was to assess the perioperative FA change in patients with CSM and determine the prognostic factor.
Methods
Twenty-eight patients with CSM and healthy control subjects were enrolled in this study. Twenty patients (71%) had intracordal high intensity before surgery. All patients underwent decompressive surgery. ZOOM DTI and the Japanese Orthopaedic Association (JOA) assessment were performed before and after surgery. The region of interest was manually contoured to omit the surrounding cerebrospinal fluid. The axial plane of the most stenotic cervical level was assessed.
Results
FA values before surgery and at 1 week after surgery, and FA values at 1 week after surgery and at 6 months after surgery differed significantly as determined. The FA values of patients with intracordal high intensity significantly decreased after surgery and significantly increased from 1 week to 6 months, whereas those of patients without intracordal high intensity did not significantly change. JOA scores at 6 months after surgery (13.1) improved significantly compared with JOA scores before surgery (10.8). Only FA values at 1 week after surgery had a significant positive relationship with JOA scores presurgery and at 6 months after surgery.
Conclusions
The presurgical FA value in patients with CSM did not differ from that of normal control subjects, but significantly decreased after surgery, and significantly increased 6 months after surgery. We concluded that the postsurgical FA value approximates the proper state of the damaged cord and the presurgical FA value includes a masked effect as an aligned fiber effect because of compression by degenerative construction. Only the FA value at 1 week had a significant positive relationship with the JOA score presugery and at 6 months, which established that the postsurgical FA value may be a more accurate prognostic factor than the presurgical FA value.
Key words: Cervical degenerative spondylosis, Diffusion tensor imaging, Fractional anisotropy, Prognostic factor, Tractography
Abbreviations and Acronyms: CSM, Cervical spondylotic myelopathy; DTI, Diffusion tensor imaging; FA, Fractional anisotropy; FOV, Field-of-view; JOA, Japanese Orthopaedic Association; MRI, Magnetic resonance imaging; ROI, Region of interest; SCI, Spinal cord injury
Introduction
Cervical spondylotic myelopathy (CSM) is the most common cause of spinal cord dysfunction. Conventional magnetic resonance imaging (MRI) reveals compression of the spinal cord or root at the level of the lesion only. Intracordal high intensity on T2-weighted imaging may be a prognostic factor; however, the signal does not always indicate the irreversible state of the cervical spinal cord lesion nor correlate with clinical outcomes.1, 2
Tractography using diffusion tensor imaging (DTI) is frequently performed to assess the localization of motor fibers in patients with a space-occupying lesion, such as a brain tumor or hematoma, which can compress or damage the neural fibers. Visualization of the winding corticospinal tract helps surgeons to avoid the loss of such fibers during surgery.
To date, similar trials for the spinal cord using 1.5-Tesla MRI have been performed; however, the smaller cross-sectional area of the cord and the environmental disadvantage of being surrounded by tissues that have a variety of magnetic susceptibilities can lead to questionable study results, particularly regarding the accuracy of the acquired values. High-resolution DTI of the cervical spinal cord prolongs the scan time, which is not preferable in the clinical setting, but lower-resolution scans reduce the accuracy of fractional anisotropy (FA) value measurement. Given this information, novel techniques such as ZOOM DTI in 3-Tesla MRI (Philips, Amsterdam, The Netherlands) have been developed. The advantages of cervical spinal DTI with ZOOM DTI are as follows. First, small field-of-view (FOV) imaging reduces the readout scan time; therefore, the artifacts caused by off resonance are also reduced. Second, the phase encode step is reduced and no phase wrap is required because of the limited FOV excited with noncoplanar spin echo and outer volume suppression. In our previous study, we demonstrated that ZOOM DTI yielded better visibility than conventional DTI.3 We also found that the FA value of the cervical spinal cord was approximately 0.6, which decreased at lower levels. In another study, we observed no significant difference between elderly and young healthy volunteers regarding cervical FA values at each spinal cord level despite mild cervical degenerative changes in the elderly persons.4
In terms of spinal cord injury (SCI) or motor neuron diseases such as amyotrophic lateral sclerosis, apparent destruction or degeneration of fibers results in a marked decrease in the FA value.5, 6 In addition, most of the prior research on CSM concluded that the FA value of patients before surgery was significantly lower than that of healthy control subjects.7, 8, 9, 10 We yielded highly accurate perioperative FA values in patients with CSM using ZOOM DTI and therefore examined the perioperative changes of acquired values.
Methods
Informed consent was obtained from all study participants for this research.
Subjects
Twenty-eight patients with CSM were enrolled in this study. The group consisted of 15 men (54%) and 13 women (46%), with ages ranging from 42 to 92 years (mean, 71.3 ± 12.7 years). Twenty patients (71%) had intracordal high intensity before surgery. All patients underwent decompressive surgery, including 16 anterior cervical diskectomies/corpectomies and fusions (57%) using 11 polyether ether ketone, 3 titanium-coated polyether ether ketone, or 2 titanium mesh cages, and 12 laminoplasties (43%). We used the Japanese Orthopaedic Association (JOA) assessment as the patient's functional evaluation instrument, which includes upper and/or lower extremity function, sensory function, and bladder function. ZOOM DTI and JOA assessment were performed before surgery and at 1 week, 6 months, and 1 year after surgery. Twelve normal control subjects were also included in this study for comparison with the patients with CSM.
Imaging Parameters and Quantitative Analysis
On 3-Telsa ZOOM DTI, the imaging parameters were as follows: repetition time 4500 milliseconds, echo time 81 milliseconds, FOV 70 × 47 mm2, matrix 70 × 47, number of sample average 10, and scan time 10 minutes and 35 seconds. Suppression slabs were implemented by successive quadratic phase radiofrequency pulses11 followed by crusher gradients.
The FA map was calculated on ZOOM DTI and conventional DTI. The FA value and apparent diffusion coefficient of the spinal cord were measured. The setting of the region of interest (ROI) is provided in Figure 1. The ROI was manually contoured around the whole spinal cord without distinction of gray and/or white matter and was made to be as large as possible; however, the surrounding cerebrospinal fluid was omitted by checking a 3-dimensional ROI cube. All operations were performed using Fiber Track (Philips, Andover, Massachusetts, USA), which is the software in the MRI scanner. This procedure was repeated from the C3-4 level to the C6-7 level in all patients and control subjects.
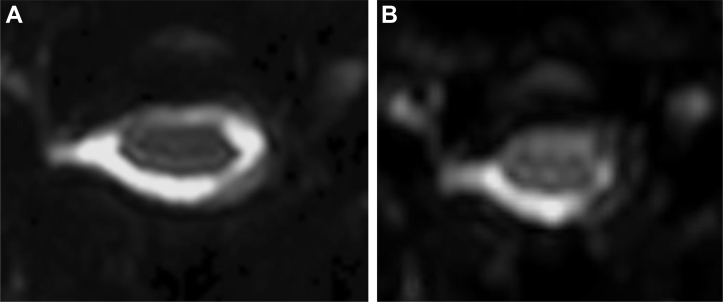
Figure 1.
Setting of the region of interest −1: diffusion-weighted b = 0 image of ZOOM diffusion tensor imaging (DTI) (A) has better visibility than that of conventional DTI (B).
The axial plane of the most stenotic (compressed) cervical level, which was the area of the target lesion in all patients, was used for assessment.
For statistical analysis, a repeated-measures design and the Tukey-Kramer method were used for comparison between each time point (P < 0.05) with JMP 13 software (SAS Institute Inc., Cary, North Carolina, USA).
This study was approved by the institutional review board (30–009).
Results
The patients' demographic data are provided in Table 1.
Table 1.
Summary of Patient Characteristics
| Characteristic | Normal Control Subjects | Patients |
|---|---|---|
| Mean age at surgery ±SD (years) | 73.8 ± 11.5 | 71.3 ± 12.7 |
| Female/male | 7/5 | 13/15 |
| Intracordal high signal | 0 | 20 |
| Level of responsible lesion | ||
| C3-4 | 5 | |
| C4-5 | 9 | |
| C5-6 | 11 | |
| C6-7 | 3 | |
| Total | 28 |
Values are number or as otherwise indicated.
Perioperative Change in FA Values
Because of the small number of patients with CSM (n = 3) in whom the target lesion occurred at the C6-7 level, we excluded this level. The titanium cages used for anterior cervical diskectomies/corpectomies and fusions did not disturb the ROI contouring via the presence of metal artifacts.
The mean FA values of patients and control subjects at each level are provided in Table 2, and there was no significant difference between patients and normal control subjects. The mean FA values at presurgery, 1 week after surgery, and 6 months after surgery of all patients were 0.569 ± 0.114, 0.485 ± 0.096, and 0.556 ± 0.103, respectively. There were significant differences between FA values at presurgery and 1 week (P = 0.0076) and between FA values at 1 week and 6 months (P = 0.0442) in the patient group as determined by the Tukey-Kramer method. The perioperative FA changes at each cervical disk level are also provided in Table 2. The chronological FA change in patients with intracordal T2-weighted imaging high signal was significantly different compared with that of patients without intracordal high signal throughout the follow-up period (repeated-measures design, P = 0.0031); however, the FA value presurgery in patients with intracordal high intensity did not differ from that in patients without intracordal high intensity (P = 0.3728). The FA value of the intracordal high-intensity group significantly decreased after surgery (P = 0.0049) and significantly increased from 1 week to 6 months after surgery (P = 0.0390), whereas the FA value of patients without intracordal high intensity did not undergo any significant changes (Table 3).
Table 2.
Fractional Anisotropy Value at Each Level
| Level | FA at Presurgery: Normal Control Subjects | FA at Presurgery: Patients | FA at 1 Week: Patients | FA at 6 Months: Patients |
|---|---|---|---|---|
| C3-4 | 0.646 ± 0.078 | 0.625 ± 0.138 | 0.480 ± 0.078 | 0.613 ± 0.032 |
| C4-5 | 0.630 ± 0.088 | 0.566 ± 0.100 | 0.487 ± 0.064 | 0.553 ± 0.096 |
| C5-6 | 0.563 ± 0.081 | 0.571 ± 0.090 | 0.505 ± 0.116 | 0.531 ± 0.091 |
| Mean value of all 3 levels | 0.569 ± 0.114 | 0.485 ± 0.096∗ | 0.556 ± 0.103∗ |
Values are mean ± SD.
FA, fractional anisotropy.
Significant difference by repeated-measures design.
Table 3.
Fractional Anisotropy Change of Patients with and without Intracordal High Signal
| T2-Weighted Image | Number | FA Presurgery | FA at 1 Week | FA at 6 Months |
|---|---|---|---|---|
| With intracordal high signal | 20 | 0.558 ± 0.116 | 0.459 ± 0.071∗ | 0.537 ± 0.093∗ |
| Without intracordal high signal | 7 | 0.601 ± 0.088 | 0.560 ± 0.110 | 0.633 ± 0.022 |
Values are mean ± SD or as otherwise indicated.
FA, fractional anisotropy.
Significant difference by repeated-measures design.
Prognostic Assessment
The JOA score at 1 year (14.3) and 6 months (13.1) improved significantly compared with the JOA score presurgery (10.8) (P < 0.0001 and P = 0.0002, respectively).
Only the FA value at 1 week after surgery had a significant positive relationship with the JOA score at presurgery and 6 months (R2 = 0.339 and R2 = 0.258, P = 0.0049 and P = 0.0263, respectively) (Table 4). The JOA score at 1 year also had a tendency for a positive relationship with the FA value at 1 week; however, it did not have significance (P = 0.0593).
Table 4.
Prognostic Assessment by the Japanese Orthopaedic Association Assessment
| JOA Score | Mean ± SD | Relationship with Each FA Value (P Value) |
||
|---|---|---|---|---|
| FA Presurgery | FA at 1 Week | FA at 6 Months | ||
| JOA score presurgery | 10.8 ± 3.34 | 0.5646 | 0.0049∗ | 0.6946 |
| JOA score at 6 months | 13.1 ± 2.75 | 0.4085 | 0.0263∗ | 0.0650 |
| JOA score at 1 year | 14.3 ± 2.24 | 0.1721 | 0.0593 | 0.3938 |
FA, fractional anisotropy; JOA, Japanese Orthopaedic Association.
Significant difference by repeated-measures design.
Discussion
In the brain and spinal cord white matter, cellular membranes and myelin sheaths act as natural barriers to molecular diffusion, including the movement of water molecules, which affects isotropy.12 There are fewer reports on spinal DTI than on brain DTI, and most used conventional 1.5-Telsa MRI. ZOOM DTI is a recently developed method for the estimation of fiber tracts.13 In particular, in small organs, such as the spinal cord, finer spatial resolution that can clearly distinguish the boundary between the target and the surrounding tissue is preferable. ZOOM DTI has previously been demonstrated to yield better visibility than conventional DTI.3
Of note, fluctuation of the FA and apparent diffusion coefficient values was strongly influenced by cerebrospinal fluid. To acquire accurate values, drawing and setting the 3-dimensional ROI contour along the cervical curvature should be performed with care to omit the surrounding cerebrospinal fluid14 to avoid a decreased FA value (Figures 1 and 2).9, 11 The decrement of the FA values at the C5-6 level in normal control subjects illustrates the difficulty associated with excluding the cerebrospinal fluid influence when setting the ROI around the apex of the cervical cord sagittal curvature. Not only cerebrospinal fluid involvement, but also susceptibility and motion artifacts at lower levels, particularly C5-6 and C6-7, cannot be ignored even in ZOOM DTI. Therefore, in our previous study, the cervical spinal cord FA values gradually decreased and the apparent diffusion coefficient values increased toward the lower levels.3 Furthermore, there was no significant difference between elderly and young volunteers despite the mild cervical degenerative changes in elderly persons.4
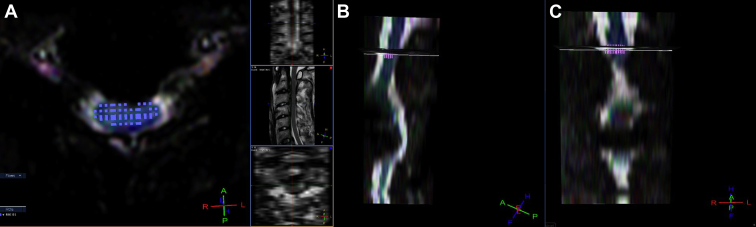
Figure 2.
Setting of the region of interest (ROI) −2: the setting of the ROI is manually contoured around the entire spinal cord as large as possible to omit the surrounding cerebrospinal fluid (A: axial) by checking the 3-dimensional ROI square (B: sagittal, C: coronal).
With respect to chronic traumatic SCI, Rao et al.5 reported that all patients with SCI had significantly decreased FA values in both residual and remote normal fibers using 3.0-Telsa MRI. Wang et al.6 also reported that patients with amyotrophic lateral sclerosis had significantly lower FA values than the control group. There is no doubt that the injured cord with edema has a decreased FA value. However, in patients with CSM, the neural fiber condition varies in its severity because of damage to the spinal cord; therefore, it is possible that most of the fibers in patients with CSM avoid severe damage and most are only compressed, which can differ from the pathology of SCI or neurodegenerative disease without compression. Most of the previous studies reported that the preoperative FA value in patients with cervical spondylosis was low and that this was a prognostic factor.9, 10, 15, 16 Bhosale et al.16 reported the reduction in FA at the stenotic level significantly improved after surgery; however, this study is performed by 1.5-Telsa MRI and there are no concrete numerical data of pre- and postsurgical FA values. Banaszek et al.17 also reported a significant decrease in the FA value even in the presence of only subarachnoid space obliteration without any compression or T2-weighted imaging signal change in the spinal cord in 132 symptomatic patients with cervical spondylosis compared with control groups. However, this study used 1.5-Telsa MRI and includes radiculopathy, which can contradict itself. Although a few studies have used 3-Telsa MRI,7, 18 the signal-to-noise ratio and resolution of spinal cord DTI are still limited. In the systematic review of DTI in patients with CSM reported by Rindler et al., even though there were 4 reports using 3-Telsa MRI, most of FOV values were larger (approximately 200 × 200 mm2) than our study of the ZOOM DTI method (70 × 47 mm2). We reported that ZOOM DTI was superior to conventional DTI for inter- and intrarater reliability.3 The visibility and distortion ratio were better, and the SD was smaller in ZOOM DTI; however, they were estimated in the same 3-Telsa MRI instrument. Regarding the size of the FOV, ZOOM DTI is the smallest in past reports (e.g., 53 × 140 mm2 by Ellingson et al.19). They also assessed cervical stenosis and the average FA value at C1-2 was 0.61, whereas the average FA at the site of compression was 0.50, which was significantly lower. However, we have reported that the FA value at the lower cervical level of normal control subjects is gradually decreased because of artifacts3; therefore, lower FA values in the same spinal cord can be a regular phenomenon.
Given these previous reports and our results, we concluded that the preoperative FA value in patients with CSM is not necessarily lower and in line with that of patients with a damaged cord without any compression. In our hypothesis, the vectors of the molecular movement in compressed and aligned neural fibers lead to a uniform direction that causes a slight increase in the FA value, which may be a masked effect by offsetting the dispersion of vectors in the damaged edematous spinal cord. The significant decrease in the FA value after decompression surgery of the intracordal high signal group indicates that the postoperative FA value approximates the proper state of the cord by omitting the preoperative aligned fiber effect (Figure 3). The essential FA values without any compression in the intracordal high signal group should be lower because of its edema and fiber damage. That is, the preoperative FA value is determined by the balance between injury with edema and compression of the spinal cord. Therefore, the postoperative FA value can be a prognostic factor for patients with CSM. In our study, only the FA value at 1 week postsurgery had a significant positive relationship with the JOA score at presurgery and at 6 months after surgery, which confirms our hypothesis. In addition, because spinal cord edema disappears in the chronic stage, the FA value must be improved. The FA value at 6 months after surgery was higher than the FA value at 1 week after surgery in our results, which relates to the clinical time course. The JOA score at 1 year after surgery did not have a significant positive relationship with the FA value at 1 week after surgery; however, most patients were older than 70 years old in our study. They had other complications, which might have affected the JOA score.
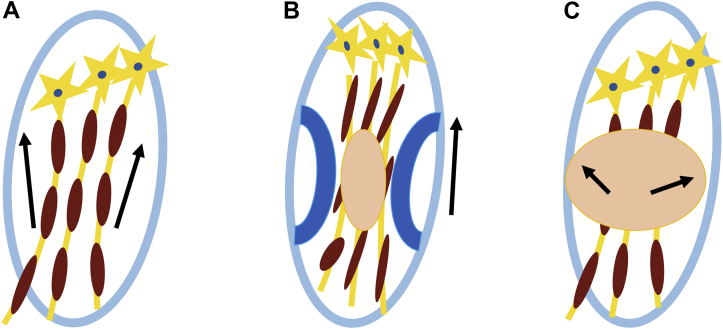
Figure 3.
Aligned fiber effect. (A) Water molecule movement is constricted to neural fibers (normal state). (B) Compressed and aligned fibers by surrounding degenerative construction leads to uniform vectors of molecular movement, which can lead to an increased fractional anisotropy (FA) value (arrow). This effect can offset the decreased FA value derived from injured fibers. (C) Decompressed fibers with edema and injured fibers permit more varied direction of molecular movement (decreased FA value).
Conclusions
The presurgical FA value in patients with CSM did not differ from the FA value of normal control subjects, but significantly decreased after cervical decompression surgery, and significantly increased 6 months after surgery, which was remarkable in intracordal high signal intensity patients. We concluded that the postsurgical FA value approximates the proper state of the damaged cord and the presurgical FA value includes a masked effect as aligned fiber effect because of compression by degenerative construction. Only the FA value at 1 week after surgery had a significant positive relationship with the JOA score at presurgery and at 6 months after surgery, which established that the postsurgical FA value may be a more accurate prognostic factor than the presurgical FA value.
Declaration of Competing Interest
This study was supported by Philips Healthcare.
References
- 1.Lin E., Long H., Li G., Lei W. Does diffusion tensor data reflect pathological changes in the spinal cord with chronic injury. Neural Regen Res. 2013;8:3382–3390. doi: 10.3969/j.issn.1673-5374.2013.36.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellingson B.M., Salamon N., Hardy A.J., Holly L.T. Prediction of neurological impairment in cervical spondylotic myelopathy using a combination of diffusion MRI and proton MR spectroscopy. PLoS One. 2015;10:e0139451. doi: 10.1371/journal.pone.0139451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokohama T., Iwasaki M., Oura D., Furuya S., Okuaki T. The reliability of reduced field-of-view DTI for highly accurate quantitative assessment of cervical spinal cord tracts. Magn Reson Med Sci. 2019;18:36–43. doi: 10.2463/mrms.mp.2017-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furuya S., Iwasaki M., Yokohama T., Ohura D., Okuaki T. Highly accurate analysis of the cervical neural tract of the elderly using ZOOM DTI. Neurospine. 2018;15:169–174. doi: 10.14245/ns.1836116.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao J.S., Zhao C., Yang Z.Y. Diffusion tensor tractography of residual fibers in traumatic spinal cord injury: a pilot study. J Neuroradiol. 2013;40:181–186. doi: 10.1016/j.neurad.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y., Liu L., Ma L. Preliminary study on cervical spinal cord in patients with amyotrophic lateral sclerosis using MR diffusion tensor imaging. Acad Radiol. 2014;21:590–596. doi: 10.1016/j.acra.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Cui J.L., Li X., Chan T.Y., Mak K.C., Luk K.D., Hu Y. Quantitative assessment of column-specific degeneration in cervical spondylotic myelopathy based on diffusion tensor tractography. Eur Spine J. 2015;24:41–47. doi: 10.1007/s00586-014-3522-5. [DOI] [PubMed] [Google Scholar]
- 8.Demir A., Ries M., Moonen C.T. Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology. 2003;229:37–43. doi: 10.1148/radiol.2291020658. [DOI] [PubMed] [Google Scholar]
- 9.Facon D., Ozanne A., Fillard P., Lepeintre J.F., Tournoux-Facon C., Ducreux D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol. 2005;26:1587–1594. [PMC free article] [PubMed] [Google Scholar]
- 10.Song T., Chen W.J., Yang B. Diffusion tensor imaging in the cervical spinal cord. Eur Spine J. 2011;20:422–428. doi: 10.1007/s00586-010-1587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ries M., Jones R.A., Dousset V., Moonen C.T. Diffusion tensor MRI of the spinal cord. Magn Reson Med. 2000;44:884–892. doi: 10.1002/1522-2594(200012)44:6<884::aid-mrm9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Budrewicz S., Szewczyk P., Bladowska J. The possible meaning of fractional anisotropy measurement of the cervical spinal cord in correct diagnosis of amyotrophic lateral sclerosis. Neurol Sci. 2016;37:417–421. doi: 10.1007/s10072-015-2418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilm B.J., Svensson J., Henning A., Pruessmann K.P., Boesiger P., Kollias S.S. Reduced field-of-view MRI using outer volume suppression for spinal cord diffusion imaging. Magn Reson Med. 2007;57:625–630. doi: 10.1002/mrm.21167. [DOI] [PubMed] [Google Scholar]
- 14.Roine T., Jeurissen B., Perrone D. Isotropic non-white matter partial volume effects in constrained spherical deconvolution. Front Neuroinform. 2014;8:28. doi: 10.3389/fninf.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajasekaran S., Yerramshetty J.S., Chittode V.S., Kanna R.M., Balamurali G., Shetty A.P. The assessment of neuronal status in normal and cervical spondylotic myelopathy using diffusion tensor imaging. Spine. 2014;39:1183–1189. doi: 10.1097/BRS.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 16.Bhosale S., Ingale P., Srivastava S., Marathe N., Bhide P. Diffusion tensor imaging as an additional postoperative prognostic predictor factor in cervical myelopathy patients: an observational study. J Craniovertebr Junction Spine. 2019;10:10–13. doi: 10.4103/jcvjs.JCVJS_77_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banaszek A., Bladowska J., Szewczyk P., Podgorski P., Sasiadek M. Usefulness of diffusion tensor MR imaging in the assessment of intramedullary changes of the cervical spinal cord in different stages of degenerative spine disease. Eur Spine J. 2014;23:1523–1530. doi: 10.1007/s00586-014-3323-x. [DOI] [PubMed] [Google Scholar]
- 18.Rindler R.S., Chokshi F.H., Malcolm J.G. Spinal diffusion tensor imaging in evaluation of preoperative and postoperative severity of cervical spondylotic myelopathy: systematic review of literature. World Neurosurg. 2017;99:150–158. doi: 10.1016/j.wneu.2016.11.141. [DOI] [PubMed] [Google Scholar]
- 19.Ellingson B.M., Salamon N., Woodworth D.C., Yokota H., Holly L.T. Reproducibility, temporal stability, and functional correlation of diffusion MR measurements within the spinal cord in patients with asymptomatic cervical stenosis or cervical myelopathy. J Neurosurg Spine. 2018;28:472–480. doi: 10.3171/2017.7.SPINE176. [DOI] [PMC free article] [PubMed] [Google Scholar]