Abstract
Background
Glioma‐related epilepsy (GRE) is defined as symptomatic epileptic seizures secondary to gliomas, it brings both heavy financial and psychosocial burdens to patients with diffuse glioma and significantly decreases their quality of life. To date, there have been no clinical guidelines that provide recommendations for the optimal diagnostic and therapeutic procedures for GRE patients.
Methods
In March 2017, the Joint Task Force for GRE of China Association Against Epilepsy and Society for Neuro‐Oncology of China launched the guideline committee for the diagnosis and treatment of GRE. The guideline committee conducted a comprehensive review of relevant domestic and international literatures that were evaluated and graded based on the Oxford Centre for Evidence‐Based Medicine Levels of Evidence, and then held three consensus meetings to discuss relevant recommendations. The recommendations were eventually given according to those relevant literatures, together with the experiences in the diagnosis and treatment of over 3000 GRE cases from 24 tertiary level hospitals that specialize in clinical research of epilepsy, glioma, and GRE in China.
Results
The manuscript presented the current standard recommendations for the diagnostic and therapeutic procedures of GRE.
Conclusions
The current work will provide a framework and assurance for the diagnosis and treatment strategy of GRE to reduce complications and costs caused by unnecessary treatment. Additionally, it can serve as a reference for all professionals involved in the management of patients with GRE.
Keywords: adult diffuse glioma, diagnosis and treatment, epilepsy, guideline
1. INTRODUCTION
Glioma‐related epilepsy (GRE) is defined as symptomatic epileptic seizures secondary to gliomas. The epileptogenesis of GRE involves various factors including tumor location, tumor histology, microenvironment, and specific genetic alterations.1, 2, 3, 4, 5 GRE is volatile, unpredictable, closely related to the progression/recurrence of gliomas,6 and accordingly places heavy financial and psychosocial burdens on patients and their families.7 Moreover, the effect of current conventional treatment strategy for GRE, which consists of antiepileptic drugs (AEDs) and anti‐tumor therapies, is unsatisfactory, despite the above treatments, seizures cannot be effectively controlled in 20%‐40% of patients.1, 8
Low‐grade gliomas (LGG) are highly epileptogenic and epilepsy is the most common initial symptom occurring in 65%‐90% of cases.9, 10, 11 Isocitrate dehydrogenase 1 (IDH1) mutation, younger age (<38 years), and cortex involvement have been proposed to be associated with a higher frequency of preoperative GRE in LGGs.9, 12 Over 50% of GRE are drug‐resistant preoperatively, and postoperative seizure freedom rates range from 43 to 87 percent depending on the extent of resection.13 In addition to gross‐total resection, older age, generalized seizures, shorter history of seizures and low‐expression of Ki‐67 have been identified as predictors of favorable postoperative seizure control.14, 15 As for high‐grade gliomas (HGG), the incidence rate of GRE is approximately 40%‐64%.16, 17 Over 70% of glioblastoma (GBM) patients with GRE preoperatively can become seizure‐free in the early stage after tumor resection, and total resection is still a positive predictor for postoperative seizure control.16, 18 Additionally, postoperative GRE relapse in HGG is typically correlated with tumor recurrence/progression.6 It is also worth noting that no matter in LGG or GBM patients, preoperative GRE is usually correlated with prolonged overall survival.19
According to the 2016 World Health Organization (WHO) classification,20 the diffuse gliomas include WHO grade II and III astrocytic tumors, grade II and III oligodendrogliomas, and grade IV GBMs. In the current guideline, LGGs are referred to WHO grade II adult diffuse gliomas, while HGGs are referred to WHO grade III‐IV adult diffuse gliomas.
2. THE DIAGNOSTIC PROCESS FOR GRE
It is important to recognize that the diagnostic process of GRE should include the diagnoses of both glioma and epilepsy, and the identification of the correlation between them.
The diagnosis of glioma necessitates localization and pathological diagnosis. A patient should receive appropriate history‐taking and physical clinical evaluation at the initial visit. Magnetic resonance imaging (MRI) is essential for preoperative diagnosis, and the conventional scanning sequences should include T1‐weighted, T2‐weighted and contrast‐enhanced T1‐weighted imaging, diffusion‐weighted imaging (DWI), perfusion‐weighted imaging (PWI) and fluid‐attenuated inversion recovery imaging (FLAIR). Moreover, magnetic resonance spectroscopy (MRS), computed tomography (CT), and positron emission tomography (PET) are also helpful supporting methods for the evaluation of glioma. If the tumor involves the eloquent cortex, diffusion tensor imaging and functional MRI should be utilized for the localization of cortical functional areas and fiber tracking.21, 22 For making a definitive diagnosis of glioma, a surgery/biopsy with subsequent pathological evaluation is necessary. A comprehensive pathological evaluation for glioma should include both histopathological and molecular pathological examinations according to the 2016 WHO classification.20 Special attention should be paid to IDH1 mutation status, which is closely associated with GRE, especially for LGG patients.12, 23
As for the diagnosis of epilepsy, seizure history and clinical signs of epileptic seizures should be conventionally documented for patients with glioma.2 Moreover, a 2‐hour video electroencephalogram (EEG), including a non‐rapid eye movement stage I‐II sleep EEG, should be performed for patients with definite or possible epileptic seizures.2 Seizure type should be classified according to the 2017 International League Against Epilepsy (ILAE) guidelines.24
Overall, a preoperative diagnosis of GRE can be made based on clinical signs, EEG and imaging findings. Ictal EEG can be used for the differential diagnosis of non‐epileptic attacks in patients without typical clinical seizures or interictal epileptiform discharges; furthermore, it should also be performed when the clinical signs and interictal epileptiform discharges are contradictory to the localization of tumor on MRI.25 PET, ictal single‐photon emission CT (SPECT), and magnetoencephalography (MEG) can also be used to localize the epileptogenic zone, if necessary.26, 27 If various diagnostic reports show no significant correlation between tumor and epilepsy, intracranial EEG can be used to determine the relationship between them.2 Figure 1 shows the diagnostic flowchart for GRE.
Figure 1.
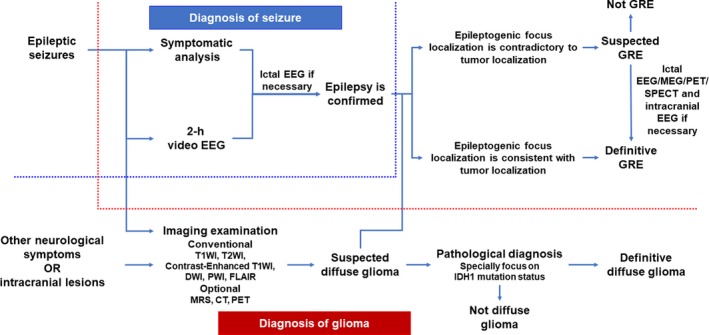
Diagnostic flowchart for glioma‐related epilepsy
3. TREATMENT OF GRE
3.1. Antiepileptic drugs
The administration of AEDs for glioma patients should be initiated as soon as possible after a definite epileptic seizure. The selection of AEDs mainly depends on the seizure type and should follow an individualized treatment plan with adequate drug dosage and duration.
A fundamental principle for AED use in GRE patients is that hepatic enzyme‐inducing AEDs should be avoided for patients receiving chemotherapeutic agents.28, 29 Among various AEDs, levetiracetam (LEV) and valproic acid (VPA) can be administrated conveniently with various dosage forms to improve seizure control, survival and quality of life for GRE patients, and are accordingly recommended for the monotherapy of GRE patients.8, 28, 30, 31 If seizure control cannot be achieved by LEV or VPA alone, polytherapy with LEV and VPA is recommended.8, 32 It must be noted that as the limitations of available clinical studies, particularly the heterogeneity in terms of dose and duration of drug administration, it is not recommended to use LEV or VPA for reasons other than seizure control in glioma patients.33 Additionally, lacosamide has a greater curative effect and fewer side effects in GRE patients resistant or intolerant to other AEDs.34
The prophylactic application of AEDs has been a controversial issue for a long time.1, 29, 35, 36 For patients without preoperative GRE, the vast majority of clinical evidences supported that the prophylactic application of AEDs during the perioperative period had no benefit.37, 38 Even for patients with preoperative GRE, there were also plenty of clinical trials suggesting that prophylactic AED use following surgery did not influence the rate of perioperative seizures.36 And in 2000, the American Academy of Neurology recommended against prophylactic AED use for patients with brain tumor.39 However, according to a survey published in 2017, AED prophylaxis was still routinely used for patients with brain tumor in actual practice.40 Such disconnection between the recommendation and clinical practice may be due to the lack of well‐designed contemporary clinical trials.35, 40 For now, we should be cautious in dealing with this issue; using prophylactic AEDs in certain high‐risk subgroups would be more appropriate. In the current guideline, we recommend that for patients with preoperative GRE, early postoperative AED application is generally acquired; for patients without preoperative GRE, perioperative prophylactic AEDs should be applied in the presence of the following high‐risk factors: (a) frontal or temporal lobe gliomas41; (b) chemotherapeutic drug polymer implants during surgery42; (c) cortical gliomas or severe cortical injury during tumor resection; (d) gliomas with an oligodendroglial component43; (e) recurrent or progressive malignant gliomas; (f) an extended surgical procedure (cortex exposed for over four hours) or anticipated postoperative brain edema/cerebral cortex infarction.
AED withdrawal is also a complex issue for glioma patients. Unlike patients with idiopathic epilepsy, for GRE patients, seizure risk is highly influenced by tumor status and anti‐tumor treatment, and therefore makes predicting the precise seizure risk very difficult. In addition, the side effects of AEDs, the financial, and psychosocial reasons should also be considered. A newly published prospective study suggested that AED withdrawal should only be considered in carefully selected patients with a low risk of tumor progression, even the patients had experienced long‐term seizure freedom.44 As for the timing of AED withdrawal, we recommend that for patients without preoperative and postoperative seizures, withdrawal of prophylactic AEDs is feasible at 2 weeks after surgery. Patients without preoperative GRE can gradually withdraw AEDs after a 3‐month administration period in those with a single early‐stage postoperative seizure, however, for patients with repeated postoperative seizures, AED withdrawal should be delayed after a minimum seizure freedom period of 1 year. For patients with preoperative GRE, patients can withdraw AEDs after a minimum of 1 year of seizure freedom when their seizure histories are shorter than 6 months and tumors are completely removed, however, for those with a long seizure history, incomplete tumor resection, distant epileptiform EEG discharges, preoperative drug‐resistant seizures or focal seizures without a loss of consciousness, we recommend that the optimal timing of AED withdrawal should be at least 2 years without seizures after the surgery and needs to be considered carefully.44, 45 AED withdrawal is not recommended for two subgroups in any case: (a) all GBM patients46; (b) other HGG patients (patients with anaplastic glioma) with incomplete tumor resection or intractable postoperative seizures.
3.2. Surgery
It is important to be clear that up to now, the primary purpose of surgery in patients with glioma has been oncologic tumor control but not seizure control. However, neurosurgeons have realized the importance of seizure control for patients with glioma and regarded it as a second goal of surgery. In terms of seizure control, gross‐total resection is better than sub‐total resection.9, 13, 15, 47 A recent study showed that for LGG patients with preoperative epilepsy, the postoperative seizure control was more likely when the extent of resection was over 91%.48 Additionally, “supratotal” resection can achieve better seizure control than even gross‐total resection.49 Accordingly, for patients with GRE, the maximal safe resection is helpful to improve not only local tumor control and survival but also postoperative seizure control. For patients with tumors involving the eloquent cortex, gross‐total resection is not feasible, the most advanced technologies should be used to achieve removing the tumor maximally to reduce postoperative seizures while protecting brain function, for instance, “engraving surgery” can be effective. Additionally, intraoperative electrocorticography is recommended for LGG patients with preoperative refractory GRE to guide the resection of the epileptogenic area and to improve the postoperative seizure outcome.50
Epilepsy relapse or aggravation could be related to tumor recurrence or progression in patients with GRE.16 Postoperative MRI (combined with contrast‐enhancement) should be performed within 24‐72 hours after surgery to evaluate the extent of resection, as this influences the incidence and timepoint of postoperative seizures greatly. Relapse of epilepsy following a long‐term postoperative seizure‐free period may suggest tumor recurrence.6 If tumor recurrence is accompanied by frequent drug‐resistant seizures, an operation is feasible after a comprehensive assessment of the patient's condition. If the patient suffers from postoperative seizures without evidence of tumor recurrence, an evaluation following the principle of refractory epilepsy should be performed. In cases of drug‐resistant GRE, surgery should be considered when the quality of life of patient is significantly decreased due to frequent seizures.2
3.3. Management of intraoperative and early postoperative epilepsy
Direct electrical stimulation for functional cortical or subcortical mapping may lead to epileptic seizures during awake craniotomy of gliomas, intraoperative seizures are usually partial seizures and the incidence is approximately 3.2%‐15.5%.51, 52, 53 Patients with the following risk factors are more likely to experience intraoperative seizures: younger age, frontal lobe (mainly supplementary motor area) involvement, preoperative seizure history, treatment with multiple AEDs preoperatively, and IDH1 mutation.51, 54, 55 Intravenous injection of LEV or VPA can be prophylactically used for these high‐risk patients. Once intraoperative seizures occur, the surgeons should stop the stimulation and irrigate the cortex with ice‐cold Ringer's solution or saline immediately.56 In general, the intraoperative seizures can be quickly resolved by the above procedure, in case of seizure persistence, benzodiazepines should be injected in a timely manner to stop the seizure. Additionally, intraoperative electromyographic monitoring could be used for early detection of potential seizure onset.
In the case of immediate or early postoperative seizures, electrocardiography and routine blood, urinalysis, blood glucose, hepatic and renal function, and electrolyte tests should be performed to exclude non‐epileptic attacks caused by cardiac incident, hypoglycemia, or electrolyte disturbance. Subsequently, CT or MRI should be performed to exclude intracranial hemorrhage and infarction after the initial postoperative seizure is controlled. If the patient's condition allows, 2‐hour EEG monitoring should be used to observe the correlation between abnormal epileptiform discharges and brain edema/residual tumor. There is no need to change the therapeutic strategy for patients receiving AEDs, while monotherapeutic AEDs should be applied to those who do not receive prophylactic AEDs perioperatively.16 The blood concentration of AEDs should be monitored when multiple episodes are observed and add‐on treatment with another type of AED must be considered when seizures are poorly controlled.
Figures 2 and 3 show the process flowcharts for patients with preoperative GRE and those without, respectively.
Figure 2.
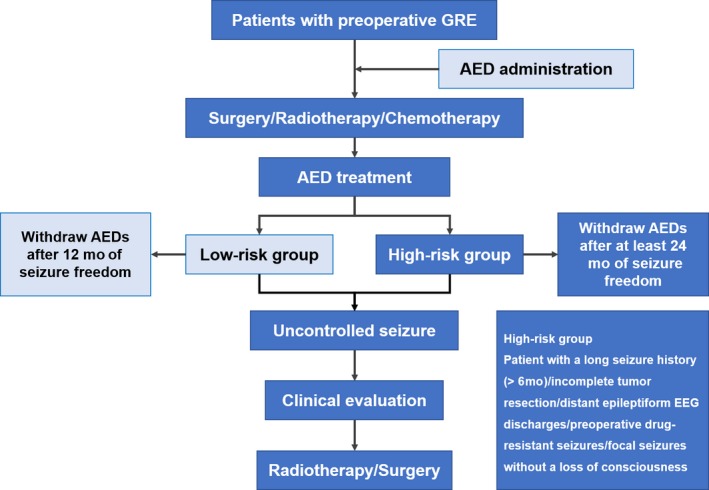
Process flowchart for patients with preoperative glioma‐related epilepsy. For glioblastoma patients and other high‐grade gliomas patients with incomplete tumor resection or intractable postoperative seizures, antiepileptic drug withdrawal is not recommended
Figure 3.
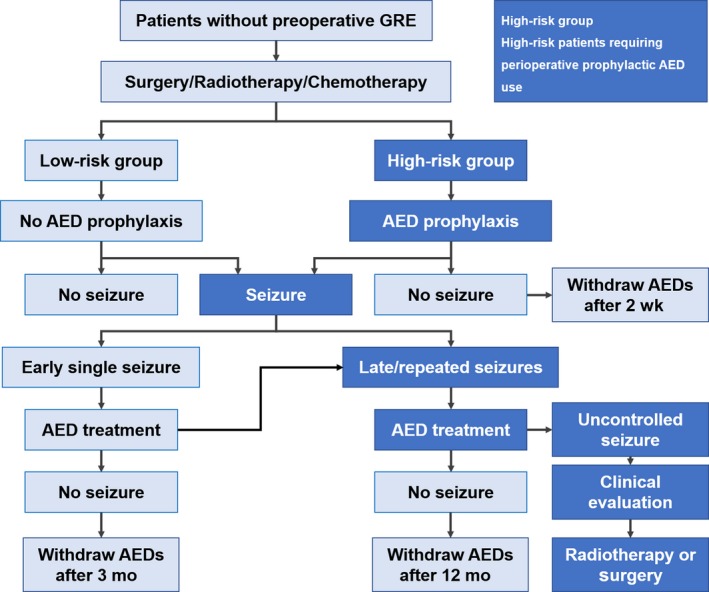
Process flowchart for patients without preoperative glioma‐related epilepsy. For glioblastoma patients and other high‐grade gliomas patients with incomplete tumor resection or intractable postoperative seizures, antiepileptic drug withdrawal is not recommended. Early seizures, seizures appear within 2 wks after surgery; Late seizures, seizures appear over 2 wks after surgery
3.4. Radiotherapy
All relevant clinical evidences showed that radiotherapy had a significant effect on inhibiting GRE.57 For patients with GRE, the radiotherapy strategy is the same as those without GRE and postoperative radiotherapy at the early stage is recommended.58, 59 It is noteworthy that seizure control is more often achieved in patients with a long seizure duration before the start of radiotherapy and is not strictly associated with tumor shrinkage on MRI.60 Additionally, glioma patients with frequent refractory epilepsy and intolerance to surgery can receive radiotherapy, irrespective of tumor relapse.
3.5. Chemotherapy and other treatments
Similar to radiotherapy, chemotherapy (procarbazine‐lomustine‐vincristine or temozolomide) is also correlated with improved seizure control in 30%‐100% of patients with GRE, regardless of surgical resection.57
Moreover, an adjuvant ketogenic diet may be useful to suppress glioma cell proliferation, as well as reduce seizure frequency and severity.
3.6. Note
Relevant recommendations are summarized in Table 1. The levels of evidence and grades of recommendation were evaluated and given according to the Oxford Centre for Evidence‐based Medicine Levels of Evidence and Grades of Recommendation (https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/). For some controversial issues, we also proposed Chinese expert consensus as references for clinical practice.
Table 1.
Conclusion and recommendations
| Recommendations | Level of evidences | Grade of recommendation |
|---|---|---|
| Diagnosis | ||
| MRI is essential to obtain a definite preoperative diagnosis of glioma | Ⅱb | B |
| Pathological evaluation for glioma should be performed according to 2016 WHO classification | Ⅰa | A |
| Special attention should be paid to the IDH1 mutation status | Ⅱa | B |
| Seizure type should be classified according to the 2017 ILAE guidelines | Ⅰa | A |
| AEDs | ||
| The administration of AEDs should be initiated as soon as possible after a definite seizure | Expert consensus | For reference |
| Hepatic enzyme‐inducing AEDs should be avoided for patients undergoing chemotherapy | Ⅰb | A |
| LEV and VPA are recommended for the monotherapy of GRE patients | Ⅰb | A |
| Polytherapy with VPA and LEV can be more effective when monotherapy is unsatisfactory | Ⅱb | B |
| For patients with preoperative GRE, early postoperative AED application is generally acquired | Expert consensus | For reference |
| For patients without preoperative GRE, prophylactic AEDs is acquired for high‐risk subgroups | Expert consensus | For reference |
| The timing of AED withdrawal should be carefully considered (see 2.1, paragraph 4) | Expert consensus | For reference |
| Surgery and management of intraoperative and early postoperative epilepsy | ||
| Maximal safe resection is helpful to improve postoperative seizure control | Ⅱa | B |
| Intraoperative electrocorticography is recommended for LGG patients with refractory GRE | Ⅱb | B |
| Irrigating the cortex with ice‐cold Ringer's solution or saline is useful to control intraoperative seizures | Ⅳ | C |
| Radiotherapy, chemotherapy, and other treatments | ||
| Radiotherapy has a significant effect on inhibiting GRE | Ⅱa | B |
| Chemotherapy is also effective for the control of GRE | Ⅱa | B |
AEDs, antiepileptic drugs; GRE, glioma‐related epilepsy; ILAE, International league against epilepsy; LEV, levetiracetam; LGG, low‐grade gliomas; MRI, magnetic resonance imaging; VPA, valproic acid.
ACKNOWLEDGMENTS
The Expert Panel wishes to express its gratitude to all members of the Joint Task Force for GRE of China Association Against Epilepsy and Society for Neuro‐Oncology of China for their help.
Liang S, Fan X, Zhao M, et al. Clinical practice guidelines for the diagnosis and treatment of adult diffuse glioma‐related epilepsy. Cancer Med. 2019;8:4527–4535. 10.1002/cam4.2362
Shuli Liang and Xing Fan contributed equally to this study.
Contributor Information
Shichuo Li, Email: shichuoli@163.com.
Tao Jiang, Email: taojiang1964@163.com.
REFERENCES
- 1. van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6(5):421‐430. [DOI] [PubMed] [Google Scholar]
- 2. CAAE . Chinese guideline of diagnosis and treatment for epilepsy. Beijing: People's Medical Publishing House; 2015. [Google Scholar]
- 3. Wang Y, Qian T, You G, et al. Localizing seizure‐susceptible brain regions associated with low‐grade gliomas using voxel‐based lesion‐symptom mapping. Neuro Oncol. 2015;17(2):282‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fan X, Wang Y‐Y, Zhang C‐B, et al. Expression of RINT1 predicts seizure occurrence and outcomes in patients with low‐grade gliomas. J Cancer Res Clin Oncol. 2015;141(4):729‐734. [DOI] [PubMed] [Google Scholar]
- 5. Zhou X‐W, Wang X, Yang Y, et al. Biomarkers related with seizure risk in glioma patients: A systematic review. Clin Neurol Neurosurg. 2016;151:113‐119. [DOI] [PubMed] [Google Scholar]
- 6. Di Bonaventura C, Albini M, D'Elia A, et al. Epileptic seizures heralding a relapse in high grade gliomas. Seizure. 2017;51:157‐162. [DOI] [PubMed] [Google Scholar]
- 7. Shin JY, Kizilbash SH, Robinson SI, et al. Seizures in patients with primary brain tumors: what is their psychosocial impact? J Neurooncol. 2016;128(2):285‐291. [DOI] [PubMed] [Google Scholar]
- 8. Kerkhof M, Dielemans J, van Breemen MS, et al. Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro Oncol. 2013;15(7):961‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. You G, Sha Z‐Y, Yan W, et al. Seizure characteristics and outcomes in 508 Chinese adult patients undergoing primary resection of low‐grade gliomas: a clinicopathological study. Neuro Oncol. 2012;14(2):230‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kerkhof M, Vecht CJ. Seizure characteristics and prognostic factors of gliomas. Epilepsia. 2013;54(Suppl 9):12‐17. [DOI] [PubMed] [Google Scholar]
- 11. Pallud J, Audureau E, Blonski M, et al. Epileptic seizures in diffuse low‐grade gliomas in adults. Brain. 2014;137(Pt 2):449‐462. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Shan X, Wu Z, Wang Y, Ling M, Fan X. IDH1 mutation is associated with a higher preoperative seizure incidence in low‐grade glioma: A systematic review and meta‐analysis. Seizure. 2018;55:76‐82. [DOI] [PubMed] [Google Scholar]
- 13. Englot DJ, Han SJ, Berger MS, Barbaro NM, Chang EF. Extent of surgical resection predicts seizure freedom in low‐grade temporal lobe brain tumors. Neurosurgery. 2012;70(4):921‐928; discussion 8. [DOI] [PubMed] [Google Scholar]
- 14. Yuan Y, Xiang W, Yanhui L, et al. Ki‐67 overexpression in WHO grade II gliomas is associated with poor postoperative seizure control. Seizure. 2013;22(10):877‐881. [DOI] [PubMed] [Google Scholar]
- 15. Shan X, Fan X, Liu X, Zhao Z, Wang Y, Jiang T. Clinical characteristics associated with postoperative seizure control in adult low‐grade gliomas: a systematic review and meta‐analysis. Neuro Oncol. 2018;20(3):324‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vecht CJ, Kerkhof M, Duran‐Pena A. Seizure prognosis in brain tumors: new insights and evidence‐based management. Oncologist. 2014;19(7):751‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang P, Liang T, Zhang C, et al. Clinicopathological factors predictive of postoperative seizures in patients with gliomas. Seizure. 2016;35:93‐99. [DOI] [PubMed] [Google Scholar]
- 18. Liang S, Zhang J, Zhang S, Fu X. Epilepsy in Adults with Supratentorial Glioblastoma: Incidence and Influence Factors and Prophylaxis in 184 Patients. PLoS ONE. 2016;11(7):e0158206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan X, Li Y, Shan X, et al. Seizures at presentation are correlated with better survival outcomes in adult diffuse glioma: A systematic review and meta‐analysis. Seizure. 2018;59:16‐23. [DOI] [PubMed] [Google Scholar]
- 20. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803‐820. [DOI] [PubMed] [Google Scholar]
- 21. Jiang T, Mao Y, Ma W, et al. CGCG clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett. 2016;375(2):263‐273. [DOI] [PubMed] [Google Scholar]
- 22. Thust SC, Heiland S, Falini A, et al. Glioma imaging in Europe: A survey of 220 centres and recommendations for best clinical practice. Eur Radiol. 2018;28(8):3306‐3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Y‐C, Zhang L, Li E‐N, et al. Comparison of Posterolateral Fusion and Posterior Lumbar Interbody Fusion in the Treatment of Lumbar Spondylolithesis: A Meta‐Analysis. J Invest Surg. 2018;1‐8. [DOI] [PubMed] [Google Scholar]
- 24. Fisher RS, Cross JH, D'Souza C, et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58(4):531‐542. [DOI] [PubMed] [Google Scholar]
- 25. Cavanna AE, Seri S. Neurophysiological investigations for the diagnosis of non‐epileptic attack disorder in neuropsychiatry services: from safety standards to improved effectiveness. Acta neuropsychiatrica. 2016;28(4):185‐194. [DOI] [PubMed] [Google Scholar]
- 26. van Dellen E, Douw L, Hillebrand A, et al. MEG network differences between low‐ and high‐grade glioma related to epilepsy and cognition. PLoS ONE. 2012;7(11):e50122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chandra PS, Vaghania G, Bal CS, et al. Role of concordance between ictal‐subtracted SPECT and PET in predicting long‐term outcomes after epilepsy surgery. Epilepsy Res. 2014;108(10):1782‐1789. [DOI] [PubMed] [Google Scholar]
- 28. Lim DA, Tarapore P, Chang E, et al. Safety and feasibility of switching from phenytoin to levetiracetam monotherapy for glioma‐related seizure control following craniotomy: a randomized phase II pilot study. J Neurooncol. 2009;93(3):349‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iuchi T, Kuwabara K, Matsumoto M, Kawasaki K, Hasegawa Y, Sakaida T. Levetiracetam versus phenytoin for seizure prophylaxis during and early after craniotomy for brain tumours: a phase II prospective, randomised study. J Neurol Neurosurg Psychiatry. 2015;86(10):1158‐1162. [DOI] [PubMed] [Google Scholar]
- 30. Rosati A, Buttolo L, Stefini R, Todeschini A, Cenzato M, Padovani A. Efficacy and safety of levetiracetam in patients with glioma: a clinical prospective study. Arch Neurol. 2010;67(3):343‐346. [DOI] [PubMed] [Google Scholar]
- 31. Rossetti AO, Jeckelmann S, Novy J, Roth P, Weller M, Stupp R. Levetiracetam and pregabalin for antiepileptic monotherapy in patients with primary brain tumors. A phase II randomized study. Neuro Oncol. 2014;16(4):584‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Breemen MS, Rijsman RM, Taphoorn MJ, Walchenbach R, Zwinkels H, Vecht CJ. Efficacy of anti‐epileptic drugs in patients with gliomas and seizures. J Neurol. 2009;256(9):1519‐1526. [DOI] [PubMed] [Google Scholar]
- 33. Happold C, Gorlia T, Chinot O, et al. Does Valproic Acid or Levetiracetam Improve Survival in Glioblastoma? A Pooled Analysis of Prospective Clinical Trials in Newly Diagnosed Glioblastoma. J Clin Oncol. 2016;34(7):731‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maschio M, Zarabla A, Maialetti A, et al. Quality of life, mood and seizure control in patients with brain tumor related epilepsy treated with lacosamide as add‐on therapy: A prospective explorative study with a historical control group. Epilepsy Behav. 2017;73:83‐89. [DOI] [PubMed] [Google Scholar]
- 35. Weston J, Greenhalgh J, Marson AG. Antiepileptic drugs as prophylaxis for post‐craniotomy seizures. Cochrane Database Syst Rev. 2015;3:CD007286. [DOI] [PubMed] [Google Scholar]
- 36. Dewan MC, White‐Dzuro GA, Brinson PR, et al. The Influence of Perioperative Seizure Prophylaxis on Seizure Rate and Hospital Quality Metrics Following Glioma Resection. Neurosurgery. 2017;80(4):563‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sirven JI, Wingerchuk DM, Drazkowski JF, Lyons MK, Zimmerman RS. Seizure prophylaxis in patients with brain tumors: a meta‐analysis. Mayo Clin Proc. 2004;79(12):1489‐1494. [DOI] [PubMed] [Google Scholar]
- 38. Wu AS, Trinh VT, Suki D, et al. A prospective randomized trial of perioperative seizure prophylaxis in patients with intraparenchymal brain tumors. J Neurosurg. 2013;118(4):873‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54(10):1886‐1893. [DOI] [PubMed] [Google Scholar]
- 40. Dewan MC, Thompson RC, Kalkanis SN, Barker FG 2nd, Hadjipanayis CG. Prophylactic antiepileptic drug administration following brain tumor resection: results of a recent AANS/CNS Section on Tumors survey. J. Neurosurg. 2017;126(6):1772‐1778. [DOI] [PubMed] [Google Scholar]
- 41. Zhang J, Yao L, Peng S, Fang Y, Tang R, Liu J. Correlation between glioma location and preoperative seizures: a systematic review and meta‐analysis. Neurosurg Rev. 2018. 10.1007/s10143-018-1014-5. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42. Della Puppa A, Denaro L, Rossetto M, et al. Postoperative seizure in high grade glioma patients treated with BCNU wafers. A mono‐institutional experience. J Neurooncol. 2011;105(2):275‐280. [DOI] [PubMed] [Google Scholar]
- 43. Kerkhof M, Benit C, Duran‐Pena A, Vecht CJ. Seizures in oligodendroglial tumors. CNS Oncol. 2015;4(5):347‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kerkhof M, Koekkoek J, Vos MJ, et al. Withdrawal of antiepileptic drugs in patients with low grade and anaplastic glioma after long‐term seizure freedom: a prospective observational study. J Neurooncol. 2019;142(3):463‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koekkoek J, Kerkhof M, Dirven L, et al. Withdrawal of antiepileptic drugs in glioma patients after long‐term seizure freedom: design of a prospective observational study. BMC Neurol. 2014;14:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koekkoek JA, Dirven L, Taphoorn MJ. The withdrawal of antiepileptic drugs in patients with low‐grade and anaplastic glioma. Expert Rev Neurother. 2017;17(2):193‐202. [DOI] [PubMed] [Google Scholar]
- 47. Yang K, Nath S, Koziarz A, et al. Biopsy versus subtotal versus gross total resection in patients with low‐grade glioma: a systematic review and meta‐analysis. World Neurosurg. 2018;120:e762‐e775. [DOI] [PubMed] [Google Scholar]
- 48. Still M, Roux A, Huberfeld G, et al. Extent of resection and residual tumor thresholds for postoperative total seizure freedom in epileptic adult patients harboring a supratentorial diffuse low‐grade glioma. Neurosurgery. 2018. 10.1093/neuros/nyy481. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 49. Yordanova YN, Moritz‐Gasser S, Duffau H. Awake surgery for WHO Grade II gliomas within "noneloquent" areas in the left dominant hemisphere: toward a "supratotal" resection. Clinical article. J Neurosurg. 2011;115(2):232‐239. [DOI] [PubMed] [Google Scholar]
- 50. Yao PS, Zheng SF, Wang F, Kang DZ, Lin YX. Surgery guided with intraoperative electrocorticography in patients with low‐grade glioma and refractory seizures. J Neurosurg. 2018;128(3):840‐845. [DOI] [PubMed] [Google Scholar]
- 51. Nossek E, Matot I, Shahar T, et al. Intraoperative seizures during awake craniotomy: incidence and consequences: analysis of 477 patients. Neurosurgery. 2013;73(1):135‐140; discussion 40. [DOI] [PubMed] [Google Scholar]
- 52. Eseonu CI, Rincon‐Torroella J, Lee YM, ReFaey K, Tripathi P, Quinones‐Hinojosa A. Intraoperative seizures in awake craniotomy for perirolandic glioma resections that undergo cortical mapping. J Neurol Surg A Cent Eur Neurosurg. 2018;79(3):239‐246. [DOI] [PubMed] [Google Scholar]
- 53. Whiting BB, Lee BS, Mahadev V, et al. Combined use of minimal access craniotomy, intraoperative magnetic resonance imaging, and awake functional mapping for the resection of gliomas in 61 patients. J Neurosurg. 2019;1–9. [DOI] [PubMed] [Google Scholar]
- 54. Nossek E, Matot I, Shahar T, et al. Failed awake craniotomy: a retrospective analysis in 424 patients undergoing craniotomy for brain tumor. J Neurosurg. 2013;118(2):243‐249. [DOI] [PubMed] [Google Scholar]
- 55. Gonen T, Grossman R, Sitt R, et al. Tumor location and IDH1 mutation may predict intraoperative seizures during awake craniotomy. J Neurosurg. 2014;121(5):1133‐1138. [DOI] [PubMed] [Google Scholar]
- 56. Boetto J, Bertram L, Moulinie G, Herbet G, Moritz‐Gasser S, Duffau H. Low rate of intraoperative seizures during awake craniotomy in a prospective cohort with 374 supratentorial brain lesions: electrocorticography is not mandatory. World Neurosurg. 2015;84(6):1838‐1844. [DOI] [PubMed] [Google Scholar]
- 57. Koekkoek JA, Kerkhof M, Dirven L, Heimans JJ, Reijneveld JC, Taphoorn MJ. Seizure outcome after radiotherapy and chemotherapy in low‐grade glioma patients: a systematic review. Neuro Oncol. 2015;17(7):924‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van den Bent MJ, Afra D, de Witte O, et al. Long‐term efficacy of early versus delayed radiotherapy for low‐grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985‐990. [DOI] [PubMed] [Google Scholar]
- 59. Sarmiento JM, Venteicher AS, Patil CG. Early versus delayed postoperative radiotherapy for treatment of low‐grade gliomas. Cochrane Database Syst Rev. 2015;6:CD009229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ruda R, Magliola U, Bertero L, et al. Seizure control following radiotherapy in patients with diffuse gliomas: a retrospective study. Neuro Oncol. 2013;15(12):1739‐1749. [DOI] [PMC free article] [PubMed] [Google Scholar]


