Key Points
Blinatumomab treatment leads to long-term remission and improved median OS for patients with R/R B-NHL.
A dose of 60 µg/m2 per day seems to represent the targeted dose level of blinatumomab required for durable remission in R/R B-NHL.
Abstract
Blinatumomab, the first-in-class CD3/CD19 bispecific T-cell engager antibody construct, has recently been approved for treating patients with relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia. However, the clinical proof of concept of blinatumomab efficacy was initially demonstrated in patients with R/R B-cell non-Hodgkin lymphoma (B-NHL) in the MT103-104 phase 1 dose-escalation and expansion trial (NCT00274742), which defined 60 µg/m2 per day as the maximum tolerated dose (MTD). The clinically most relevant adverse effects were neurologic symptoms and cytokine release syndrome. Currently, there are no data on long-term outcomes and toxicity for B-NHL patients receiving blinatumomab treatment, so we performed a single-center, long-term follow-up analysis of 38 patients who participated in the MT103-104 phase 1 trial. We found no evidence for long-term toxicities, especially no blinatumomab-induced neurocognitive impairments. For the entire study population, the median overall survival (OS) was 4.6 years. Remarkably, patients who had received ≥60 µg/m2 per day and responded to blinatumomab achieved a median OS of 7.7 years. Of note, 6 of the surviving patients treated at the MTD have been treatment-free for more than 7 years. In contrast, patients who were treated at dose levels below the MTD had a median OS of only 1.1 years. These results indicate that 60 µg/m2 per day seems to represent the targeted dose level of blinatumomab required for durable remission in R/R B-NHL. Here, we provide the first clinical evidence that blinatumomab lacks long-term toxicity and has the potential to induce sustained remissions in patients with R/R B-NHL.
Visual Abstract
Introduction
Despite the availability of novel therapeutic options for patients with relapsed or refractory (R/R) B-cell non-Hodgkin lymphoma (B-NHL), duration of response (DOR) and overall survival (OS) rates, especially in mantle cell lymphoma (MCL) or diffuse large B-cell lymphoma (DLBCL), are still relatively short. Therefore, evaluation of new therapies that substantially prolong both DOR and OS is a challenging task.
Blinatumomab (Blincyto) is a bispecific T-cell engager antibody construct consisting of 2 flexibly linked single-chain variable fragments that bind to CD3 on T cells and to CD19 on B cells, leading to cytotoxic T-cell response against both normal and malignant B cells.1 Clinical efficacy of blinatumomab treatment has been demonstrated in various phase 1/2 trials in patients with R/R B-NHL2,3 as well as in Philadelphia chromosome–negative (Ph–) R/R B-cell acute lymphoblastic leukemia (B-ALL) or B-lineage ALL.4,5 In a recently published prospective randomized phase 3 trial in patients with R/R B-lineage ALL, blinatumomab was shown to significantly improve OS compared with conventional salvage chemotherapy.6 Notably, favorable outcome of blinatumomab treatment has been reported in patients with minimal residual disease–positive ALL in hematologic complete remission with a long-term leukemia-free survival rate of 50% to 60%.7 Blinatumomab was approved by the US Food and Drug Administration (FDA) in 2014 and by the European Medicines Agency (EMA) in 2015 for second-line treatment of Ph– R/R B-lineage ALL and later received FDA approval for the treatment of minimal residual disease–positive B-ALL in 2018.
The first phase 1 dose-escalation and expansion trial (MT103-104; NCT00274742) explored blinatumomab in patients with R/R B-NHL.2,3 The dose-escalation part defined the maximum tolerated dose at 60 μg/m2 per day administered as a continuous intravenous infusion over 4 to 8 weeks. Frequent adverse effects (AEs) were flu-like symptoms, including pyrexia, headache, and fatigue, consistent with the mode of action of a T-cell–activating and B-cell–depleting therapy. Grade 3 neurologic AEs were considered as dose-limiting toxicities; 22% of patients experienced grade 1 to 3 neurologic events, all of which were fully reversible and manageable. Among 35 patients treated at 60 µg/m2 per day, the overall response rate (ORR) was 69% across NHL subtypes and 55% for DLBCL (n = 11). Median response duration was 404 days (95% confidence interval [CI], 207-1129 days). Efficacy in patients with R/R DLBCL was confirmed in a phase 2 trial.8 In contrast to data on long-term outcome with blinatumomab treatment for R/R B-lineage ALL, there are no data so far for B-NHL. Here, we report the first long-term follow-up analysis and median OS, progression-free survival (PFS), and treatment-free survival (TFS) as well as long-term toxicity of blinatumomab within a single-center cohort of the MT103-104 phase 1 trial.
Patients and methods
Study design
This single-center follow-up study was designed to assess the long-term safety and efficacy of blinatumomab in the subgroup of all patients with R/R B-NHL (n = 38) who participated in the multicenter, single-agent, open-labeled phase 1 MT103-104 study (n = 76) at the Würzburg trial site. The methods and results of the primary analysis have been previously described.3 All patients gave written informed consent to participate in the long-term follow-up analysis in accordance with the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of the University Hospital Würzburg.
Study procedures
Patients were recruited into the MT103-104 trial between 2004 and 2011 and received blinatumomab as a continuous intravenous infusion at escalating doses of 0.5, 1.5, 5, 15, 30, 60, or 90 µg/m2 per day.3 Each treatment cycle was 4 or 8 weeks. Blinatumomab was administered until a dose-limiting toxicity (DLT; DLT period 21 days) or relevant disease progression occurred, followed by an additional 4-week consolidation treatment at the respective initial dose in case of clinical benefit. Response assessment was performed using the Standardized Response Criteria for Non-Hodgkin’s Lymphomas.9 All patients entered long-term follow-up after they completed an end-of-study visit for the phase 1 blinatumomab dose-escalation trial (4 weeks after cessation of blinatumomab infusion) with safety follow-up visits every 3 months. AEs were collected and graded according to the National Cancer Institute’s Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0. Because infectious and neurologic AEs were the most frequent AEs during the treatment phase, long-term immunologic and neurologic outcomes were evaluated by analyzing the number of hospitalizations and their underlying causes within a time frame between the end of treatment and the start of a new systemic antitumor therapy. Patients’ need for immunoglobulin treatment after blinatumomab was surveyed, as well as the occurrence of secondary malignancies or high-grade transformation. To objectify the patients’ mental performance and cognitive impairment, neurocognitive testing (Montreal Cognitive Assessment [MoCA]10) was used for those patients still alive after blinatumomab treatment. Data were provided from study site files, from patients’ treating oncologists, and from family physicians as well as the tumor registry of the Comprehensive Cancer Center Mainfranken (Würzburg, Germany).
Statistical analysis
The primary end points were OS, PFS, and TFS of responders and patients treated at the target dose (maximum tolerated dose). Safety aspects regarding long-term toxicity were occurrence and type of hospitalizations, secondary malignancies, high-grade transformations, and substitution with immunoglobulins. Descriptive statistics for demographic and baseline parameters are summarized in Table 1. Response duration and survival were analyzed by Kaplan-Meier estimates and Cox regression (OS, PFS, and TFS probability; IBM SPSS Statistics). Patients without any AEs were censored at last follow-up. AEs were categorized by using CTCAE version 4.0. Results of the MoCA tests were evaluated by standardized MoCA score and were listed.10
Table 1.
Patient characteristics and assignment to different dose levels with respect to treatment response
| Characteristic | Responders (n = 17) | Nonresponders (n = 21) |
|---|---|---|
| Median age (range), y | 62 (40-80) | 60 (41-76) |
| Males, n (%) | 15 (88.2) | 15 (71.4) |
| Prior therapy/disease status | ||
| Median no. of prior treatment regimens (range) | 2 (1-4) | 3 (1-6) |
| Refractory to last line of therapy, n (%) | 3 (17.6) | 13 (61.9) |
| Histology, n (%) | ||
| FL | 10 (58.8) | 8 (38.1) |
| MCL | 6 (35.3) | 7 (33.3) |
| DLBCL | 1 (5.9) | 4 (19.0) |
| Other (1 immunocytoma, 1 marginal zone lymphoma) | 0 | 2 (9.5) |
| Dose level, µg/m2 per d | ||
| 5 | 0 | 2 |
| 15 | 1 | 8 |
| 30 | 0 | 2 |
| 60 | 14 | 8 |
| 90 | 2 | 1 |
| Treated at target dose, n (%), µg/m2 per d | ||
| <60 | 1 (5.9) | 12 (57.1) |
| ≥60 | 16 (94.1) | 9 (42.9) |
| Median treatment duration (range), d | 57 (4-85) | 31 (1-87) |
| Median no. of treatment cycles (range) | 1 (1-2) | 1 (1-3) |
Results
Patients’ characteristics
A total of 38 patients were analyzed. The median age was 60 years (range, 40-80 years), and 79% were males (Table 1). Eighteen patients (47%) were diagnosed with FL, 13 (34%) with MCL, 5 (13%) with DLBCL, 1 with marginal zone lymphoma, and 1 with immunocytoma. Patients had previously received a median of 3 systemic antitumor therapies. Twelve patients (31.6%) had prior autologous stem cell transplantations. Sixteen (42%) of 38 patients were determined to be refractory to their last line of therapy.
Treatment and response
The different dose approaches are outlined in Table 1. Of the 38 patients, 22 (58%) were treated at the target dose of 60 µg/m2 per day, 3 (8%) received doses of 90 µg/m2 per day, and 13 (34%) received doses below the target dose of 60 µg/m2 per day. Median treatment duration with blinatumomab was 51 days (range, 1-87 days), and 16 (64%) of 25 patients treated at the target dose or higher experienced a response (complete response [CR], 36%; partial response [PR], 28%). In contrast, with the exception of 1 patient who achieved a CR, all patients treated with blinatumomab below the target dose did not respond to therapy. In this study, patients who achieved a CR or PR are defined as responders, and patients whose best response was stable disease are defined as nonresponders. Among the responders to blinatumomab treatment 3 (18%) of 17 suffered from refractory B-NHL.
Safety
AEs associated with blinatumomab treatment of all 76 patients in the MT103-104 trial have been described in detail.3 During the long-term follow-up analysis of 38 patients, 8 patients (21%) were hospitalized after finalization of the blinatumomab treatment and safety follow-up period, mostly as a result of infections. Reasons for hospitalization were pneumonia (n = 2), infectious diarrhea (n = 2), infection of the central venous port catheter system (n = 2), fever of unknown origin (n = 1), and sepsis (n = 1). The median interval from end of treatment to occurrence of all AEs was 234 days (range, 5-3151 days; supplemental Table 1). Four patients (11%) with recurrent infections were substituted with immunoglobulin to compensate for immunoglobulin deficiency. Three patients were diagnosed with breast cancer, stomach cancer, or cervix carcinoma 2.5, 3, or 6.25 years, respectively, after the end of blinatumomab treatment. Five patients experienced transformation from indolent NHL to DLBCL (1% per year).
All patients underwent repeated routine neurologic examination during follow-up without detection of any neurologic abnormality. In a subgroup of 9 patients still alive and available for neurologic safety follow-up analysis at our center, a MoCA test was performed at a median of 5 years (range, 4.2-7.2 years) after the end of blinatumomab therapy to check for cognitive impairment. Results of the MoCA testing are provided in supplemental Table 2. All of these 9 patients were treated at effective dose levels, and notably, 4 patients experienced neurologic AEs while being treated with blinatumomab. The MoCA test detected no obvious long-term neurocognitive abnormalities. One patient presented with a slight impairment of visuospatial cognition that was considered to be related to age rather than to blinatumomab.
Survival
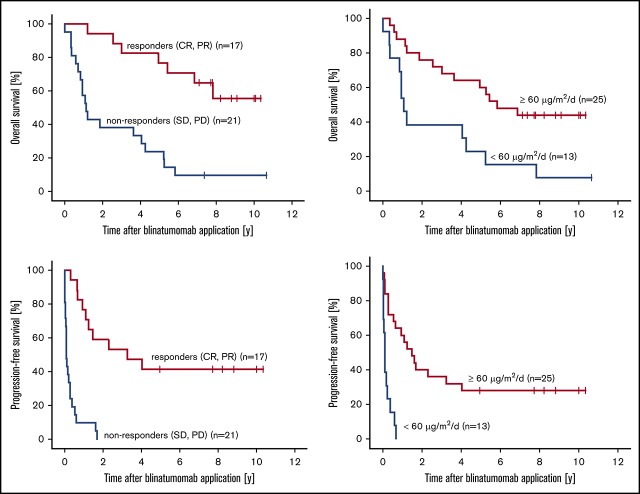
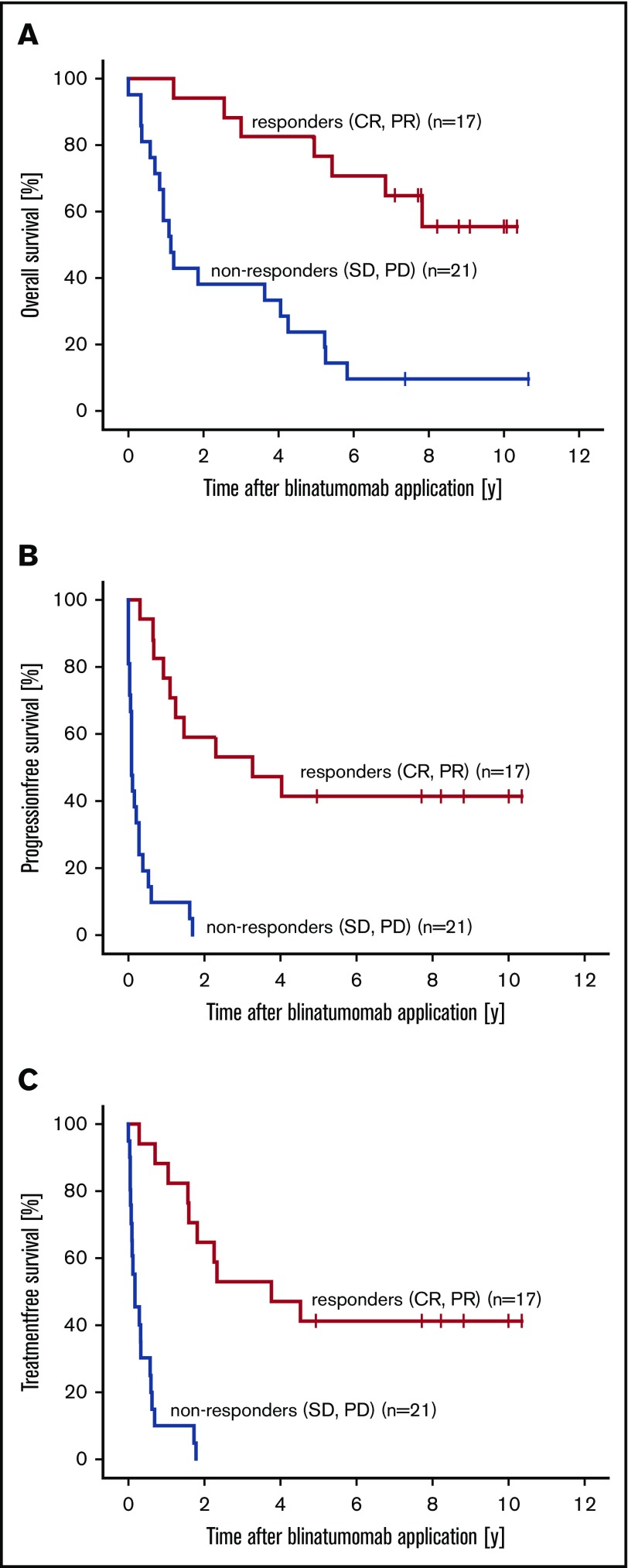
Our follow-up analysis of 38 patients who had received blinatumomab therapy revealed a median OS of 4.6 years (range, 14.6 days to 10.7 years), a median PFS of 6.7 months (range, 0-10.3 years), and a median TFS of 7.6 months (range, 7.3 days to 10.3 years; Table 2). Responders (CR or PR) to blinatumomab (n = 17) had a median OS of 7.7 years (range, 1.1-10.3 years), whereas nonresponders (n = 21) had a median OS of 1.1 years (range, 14.6 days to 10.7 years). According to statistical analyses using Kaplan-Meier estimates, these differences between responders and nonresponders are significant with a hazard ratio (HR) of 0.2 (95% CI, 0.1-0.5; P = .001; Figure 1A). Median PFS in the responder group was 3.2 years (range, 3.2 months to 10.3 years) and 31.8 days (range, 0-1.7 years; HR, 0.1; 95% CI, 0.0-0.3; P < .001; Figure 1B) in the nonresponders group. The median TFS was significantly improved at 3.7 years (range, 3.4 months to 10.3 years) in the responders group compared with a median TFS of 1.9 months (95% CI, 0.0-0.3 months; HR, 0.1; P < .001; Figure 1C) in the nonresponders group.
Table 2.
Correlation between treatment dose levels and response of patients in the Würzburg cohort (N = 38)
| No. of patients treated | Dose | ||
|---|---|---|---|
| ≥60 µg/m2 per d | <60 µg/m2 per d | ||
| Response, n (%) | |||
| CR | 10 (26.3) | 9 (36.0) | 1 (7.7) |
| PR | 7 (18.4) | 7 (28.0) | 0 |
| SD | 7 (18.4) | 2 (8.0) | 5 (38.5) |
| PD | 12 (31.6) | 6 (24.0) | 6 (42.2) |
| NA | 2 (5.3) | 1 (4.0) | 1 (7.7) |
| ORR* n (%) | |||
| FL | 10 (55.6) | 9 | 1 |
| MCL | 6 (46.2) | 6 | 0 |
| DLBCL | 1 (20.0) | 1 | 0 |
| Others | 0 (0) | 0 | 0 |
| Survival | |||
| OS, y | 4.6 | 5.8 | 1.1 |
| PFS | 6.7 mo | 1.5 y | 32 d |
| TFS | 7.6 mo | 3.5 y | 37 d |
Data are shown in absolute numbers and as relative (%) quantities.
NA, not applicable; PD, progressive disease; SD, stable disease.
ORR: CR and PR.
Figure 1.
Combined analysis of patients with R/R FL, MCL, or DLBCL who responded or did not respond to blinatumomab treatment. Kaplan-Meier estimates for OS (A), PFS (B), and TFS (C).
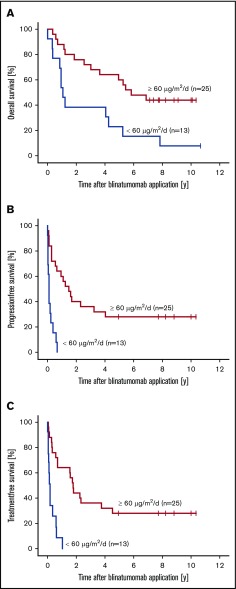
With respect to treatment at the target dose, median OS for patients receiving ≥60 µg/m2 per day (n = 25) was 5.8 years (range, 4.0 months to 10.3 years), whereas patients treated below target dose achieved a median OS of 1.1 years (range, 14.6 days to 10.6 years). Statistical comparison of the OS rates in both dosing groups revealed an HR of 0.3 (95% CI, 0.2-0.8; P = .007), which marked a significant difference (Figure 2A). Further statistical analyses of median PFS and TFS revealed similar statistically significant differences. Thus, patients receiving ≥60 µg/m2 per day had a median PFS of 1.5 years (range, 0-10.3 years), which contrasts to a median PFS of 32 days (range, 0-8.0 months; HR, 0.2; 95% CI, 0.1-0.4; P < .001) for patients treated below the target dose (Figure 2B). Median TFS was 3.5 years (range, 4.8 months to 10.3 years), which was significantly longer for patients at target dose than for patients treated below the target dose whose median TFS was only 37 days (range, 7.3 days to 1.0 years; HR, 0.2; 95% CI, 0.1-0.4; P < .001; Figure 2C). There were no significant differences in median PFS with respect to lymphoma histology (P = .374; supplemental Figure 1).
Figure 2.
Combined analysis of patients with R/R FL, MCL, or DLBCL who received blinatumomab doses of either <60 µg/m2per day or ≥60 µg/m2per day. Kaplan-Meier estimates for OS (A), PFS (B), and TFS (C).
After a median follow-up of 4.6 years, 12 patients (8 with FL, 3 with MCL, and 1 with DLBCL) were still alive (Table 3). All but 1 of these patients received ≥60 µg/m2 per day of blinatumomab. Six patients are in ongoing remission and another 6 relapsed and required salvage treatment (4 autologous stem cell transplantations, 2 allogeneic stem cell transplantations). In the group of patients who were not treated at the effective dose level, only 1 patient was a long-term survivor; however, this patient had received subsequent salvage antitumor therapy. All 6 patients (3 with FL, 2 with MCL, and 1 with DLBCL) in ongoing treatment-free remission after blinatumomab therapy were treated at the effective dose level (5 patients at 60 µg/m2 per day, 1 patient at 90 µg/m2 per day) and responded (3 CR, 3 PR).
Table 3.
Best response and duration of response to blinatumomab in 12 long-term survivors
| Patient | Histology | No. of prior therapies | Dose, µg/m2 per d | Duration of treatment, d | Best response | OS, d | PFS, d | TFS, d |
|---|---|---|---|---|---|---|---|---|
| 47 | FL | 2 | 30 | 55 | SD | 3887 | 73 | 212 |
| 48 | FL | 1 | 60 | 55 | CR | 3677 | 234 | 246 |
| 66 | FL | 3 | 60 | 57 | CR | 2592 | 336 | 576 |
| 67 | MCL | 2 | 60 | 58 | CR | 3320 | 1180 | 1365 |
| 68 | FL | 3 | 60 | 15 | SD | 2690 | 613 | 643 |
| 70 | FL | 3 | 60 | 57 | CR | 2844 | 1466 | 1650 |
| 71* | FL | 3 | 60 | 85 | PR | 3775 | 3775 | 3775 |
| 72* | FL | 1 | 60 | 35 | PR | 3649 | 3649 | 3649 |
| 73* | MCL | 3 | 90 | 57 | CR | 3215 | 3215 | 3215 |
| 74* | FL | 1 | 60 | 57 | PR | 3005 | 3005 | 3005 |
| 75* | DLBCL | 3 | 60 | 56 | CR | 2816 | 2816 | 2816 |
| 76* | MCL | 1 | 60 | 57 | CR | 2816 | 2816 | 2816 |
OS >5 years; patients are in ongoing remission.
Discussion
This single-center evaluation assessed the long-term antitumor efficacy and safety of blinatumomab in 38 R/R B-NHL patients who had been enrolled in the MT103-104 phase 1 trial.3 We observed a median OS of 4.6 years, a median PFS of 6.7 months, and a median TFS of 7.6 months. Comparing patients who had received blinatumomab at the target dose (60 µg/m2 per day) or higher with those who were treated with lower doses, significantly increased median OS (5.8 vs 1.1 years), PFS (1.5 years vs 32 days), and TFS (3.5 years vs 37 days) were observed. Although the number of patients is low, the median OS and PFS are impressive, given the fact that patients were heavily pretreated with a median of 3 prior treatment regimens. Moreover, 47% of the patients suffered from R/R MCL or DLBCL, which are known to respond poorly to salvage treatment approaches.
In light of the fact that treatment of B-NHL is still challenging even though a wide range of innovative targeted treatment modalities have emerged over the past few years, we consider the results of this long-term follow-up for blinatumomab in B-NHL remarkable. Small-molecule inhibitors for R/R NHL treatment such as the proteasome inhibitor bortezomib, the Bruton’s kinase inhibitor ibrutinib, and the mammalian target of rapamycin inhibitor temsirolimus as well as the immunomodulating agent lenalidomide (which all obtained regulatory approval) achieved an ORR between 32% and 68% and a DOR of 9.2 to 17.5 months.11-16 In contrast to blinatumomab, most of these kinase inhibitors or immunomodulators have to be administered until disease progression and require patients’ drug adherence as well as close monitoring of AEs on a regular basis.
Agents that target the immune checkpoint signaling pathways to turn T-cell exhaustion and immune escape into a restored and enhanced immune response against tumor cells are emerging and have led to impressive immunotherapeutic success in the treatment of solid tumors.17 Currently, many clinical trials are under way that evaluate immune checkpoint inhibitors (ICIs) in different lymphoma subtypes as well as their limitations in malignant B-cell lymphoma. Successful use of ICIs has been shown in primary mediastinal B-cell lymphoma (PMBCL) or lymphoma of the central nervous system; groundbreaking efficacy in other B-NHLs is pending. The reasons for divergent efficacy of ICIs in lymphoma patients are not well understood, and efforts are being undertaken to identify predictive biomarkers.
Adoptive cellular immunotherapy with chimeric antigen receptor (CAR) T cells targeting CD19 have changed the treatment landscape of B-cell malignancies and demonstrated impressive efficacy in heavily pretreated patients with DLBCL, FL, and PMBCL.18 In the ZUMA-1 phase 1/2 trial, patients with R/R DLBCL, FL, or PMBCL were treated with the CD19-specific CAR T-cell product axicabtagene ciloleucel, and they achieved high initial responses (CR or PR, 83%; CR, 54%), with 89% of patients still in ongoing remission after a median follow-up of 27.1 months.19,20 The JULIET phase 2 trial investigated the anti-CD19 CAR T-cell product tisagenlecleucel successfully with best ORR of 52%, CR of 40%, and an 18-month PFS of 43%.21,22 As a result, axicabtagene ciloleucel and tisagenlecleucel received approval by the FDA and EMA for treatment of adult patients with R/R DLBCL.
Although initial response rates in patients treated with CAR T cells for B-cell malignancies have been impressive when compared with historical outcomes, about 50% to 60% of patients will not achieve a CR or will relapse. One of the defined mechanisms of resistance to CD19-based immunotherapies is the loss of target antigen.18 Recent clinical data on 50 patients who achieved remission following anti-CD19 CAR therapy with a median follow-up of 10.6 months showed a relapse rate of 40% as a result of the loss of cell surface CD19 in 65% of the total relapses.23 In contrast, CD19-negative relapses were diagnosed in about 10% to 20% of patients with B-cell malignancies after blinatumomab treatment and seem to be less frequent.4,24 A major challenge is the manufacturing process, which has a time frame from lymphocyte apheresis to infusion of CAR T cells ranging between 2 and 6 weeks, dependent on the CAR construct and production capacities. This time might be critical for patients with an otherwise aggressive and refractory disease. Readily available CAR products such as allogeneic donor-derived CAR T cells or CAR natural killer cell products are in clinical development 25,26 and might have the potential to substantially reduce the time delay. Blinatumomab, however, has the advantage of being an immediately available off-the-shelf drug.
A further immunotherapeutic approach in the treatment of B-NHL that needs particular attention are antibody-drug conjugates, some of which are already in late clinical development and are awaiting FDA and EMA Orphan Drug designation in DLBCL. One of those is polatuzumab vedotin, which is composed of a humanized anti-CD79b immunoglobulin G1 antibody that is conjugated to the antimitotic agent monomethyl auristatin E and targets CD79b+ cells in B-NHL. Preliminary data from a randomized phase 2 study (NCT02257567) investigating the antibody-drug conjugate in combination with immunochemotherapy in R/R DLBCL and FL are promising.27 The FDA granted priority review for polatuzumab vedotin in combination with bendamustine and rituximab for the treatment of R/R DLBCL; a decision is expected by August 2019.
In our long-term follow-up analysis, patients who responded to blinatumomab treatment had a significantly longer median OS of 7.7 years (HR, 0.2; P < .001) compared with patients who did not respond to treatment. Likewise, median PFS and TFS were significantly improved, depending on the tumor response. These data support the assumption that a sufficient tumor response after treatment is a prerequisite for long-term remission in heavily pretreated patients.
Six of 38 patients initially treated with blinatumomab are still in ongoing remission after more than 7 years and seem to be long-term survivors (Table 3). All 6 patients had been treated with a target dose of 60 µg/m2 per day. Further analysis supported the significant OS benefit (P = .007) of patients who received the target dose compared with those who did not. Although the analysis of larger patient cohorts is warranted, our study clearly points to some factors that may have influenced long-term outcomes after blinatumomab treatment. All long-term survivors received target doses of at least 60 µg/m2 per day and achieved a blinatumomab-induced CR or PR. The long-term outcomes of these 6 patients illustrate that long-term survival after blinatumomab treatment may be achieved without any subsequent treatment, even in the setting of R/R NHL.
With respect to the safety profile of blinatumomab, no severe long-term AEs became conspicuous. Reasons for hospitalization were mostly infections that presumably occurred as a consequence of temporary lymphopenia, leukopenia, neutropenia, or secondary hypoimmunoglobulinemia. It remains to be clarified whether the necessity of immunoglobulin supplementation was a result of blinatumomab therapy, other antitumor therapies after blinatumomab treatment, or the disease itself. Because the assessment of frequency, degree, and duration of B-cell depletion was not the focus of this follow-up analysis, immunoglobulin deficiency induced by blinatumomab may be underestimated. With this in mind, it seems important to give special attention to a higher susceptibility for infection in patients who received blinatumomab during and after discontinuation of treatment.
Patients with NHL have a 25% increased risk of second primary neoplasms. B-NHL patients have a 25% increased risk for the development of secondary neoplasms.28 Transformations from indolent to aggressive lymphoma range from 2% to 3% per year.29-31 The number of secondary malignancies observed in our cohort as well as the rate of patients who transformed from indolent to aggressive lymphoma were in line with the reported risk for NHL patients.
With respect to neurologic AEs that are among the major acute toxicities during continuous intravenous treatment of blinatumomab neurocognitive function testing should be considered before, during, and shortly after blinatumomab use to objectify the patients’ mental status and to identify neurologic impairments early in the treatment course. The MoCA test that was given to a subgroup of 9 patients proved to be a suitable instrument to monitor and exclude long-term neurotoxicity in all of the 9 tested patients who received the target dose and especially in 4 patients who experienced neurologic AEs during therapy. The MoCA is a relatively simple, brief screening test widely used in clinical practice that is well suited for detection of early and mild cognitive impairment. It assesses executive functions and is more sensitive in this regard than the Mini-Mental State Examination (MMSE). Although the MMSE is the commonly used scale for evaluating cognitive function, it is claimed to be imprecise for detecting early and mild cognition impairment.32
In conclusion, our long-term follow-up analysis of a single-center cohort of the MT103-104 phase 1 trial revealed that blinatumomab is a highly effective CD19-targeting bispecific antibody capable of inducing durable remissions in heavily pretreated patients with advanced-stage R/R B-NHL. This also applies to lymphoma regardless of tumor burden. Although the number of patients is small, the long observation period enables us to point out the ability of blinatumomab potency to induce long-lasting remissions. When compared with standard-of-care treatments in the R/R setting, therapy at target dose as well as response to blinatumomab treatment seem to be associated with duration of response and to be a prerequisite for a prolonged OS. The toxicity profile (including neurologic AEs) is manageable without any long-term toxicities. Thus, blinatumomab may represent an attractive treatment option for R/R NHL, and it holds promise for patients with aggressive NHLs whose diseases are refractory to current standard treatment approaches. The demonstrated benefit and response duration have prompted a series of ongoing phase 1/2 trials to validate the promising efficacy of blinatumomab alone or in combination with chemotherapy, immunomodulators, or ICIs in R/R NHL and to optimize dosing strategies to minimize AEs (NCT02811679, NCT03072771, NCT02568553, NCT03340766, and NCT03605589).
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgment
This study was supported (in part) by research funding from the Graduate School of Life Sciences at the University of Würzburg (V.D.).
Footnotes
Presented in part at the 57th annual meeting of the American Society of Hematology, Orlando, FL, 5-8 December 2015.
Authorship
Contribution: V.D., M.-E.G., and R.C.B. designed the research; V.D., M.-E.G., and C.M.S. performed the research; V.D., M.-E.G., and G.G. analyzed the data; V.D. and M.-E.G. wrote the first draft of the paper; and all authors reviewed and contributed to the final paper.
Conflict-of-interest disclosure: R.C.B. has consulted for and received honoraria from Amgen. R.C.B. and M.-E.G. have served on advisory boards for Amgen. The remaining authors declare no competing financial interests.
Correspondence: Maria-Elisabeth Goebeler, Department of Internal Medicine II, University Hospital Würzburg, Oberdürrbacherstr 6, 97080 Würzburg, Germany; e-mail: goebeler_m@ukw.de.
References
- 1.Löffler A, Kufer P, Lutterbüse R, et al. . A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95(6):2098-2103. [PubMed] [Google Scholar]
- 2.Bargou R, Leo E, Zugmaier G, et al. . Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974-977. [DOI] [PubMed] [Google Scholar]
- 3.Goebeler ME, Knop S, Viardot A, et al. . Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: Final results from a phase I study. J Clin Oncol. 2016;34(10):1104-1111. [DOI] [PubMed] [Google Scholar]
- 4.Topp MS, Gökbuget N, Stein AS, et al. . Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57-66. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H, Jabbour E, Topp MS. Blinatumomab for acute lymphoblastic leukemia. N Engl J Med. 2017;376(23):e49. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Stein A, Gökbuget N, et al. . Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gökbuget N, Dombret H, Bonifacio M, et al. . Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viardot A, Goebeler ME, Hess G, et al. . Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127(11):1410-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheson BD, Horning SJ, Coiffier B, et al. ; NCI Sponsored International Working Group . Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17(4):1244. [DOI] [PubMed] [Google Scholar]
- 10.MoCA, Montreal Cognitive Assessment: MoCA Test. Available at: http://www.mocatest.org. Accessed 12 June 2018.
- 11.Goy A, Younes A, McLaughlin P, et al. . Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23(4):667-675. [DOI] [PubMed] [Google Scholar]
- 12.Goy A, Bernstein SH, Kahl BS, et al. . Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20(3):520-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang ML, Rule S, Martin P, et al. . Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess G, Herbrecht R, Romaguera J, et al. . Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27(23):3822-3829. [DOI] [PubMed] [Google Scholar]
- 15.Witzig TE, Vose JM, Zinzani PL, et al. . An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2011;22(7):1622-1627. [DOI] [PubMed] [Google Scholar]
- 16.Ruan J, Martin P, Shah B, et al. . Lenalidomide plus rituximab as initial treatment for mantle-cell lymphoma. N Engl J Med. 2015;373(19):1835-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merryman RW, Armand P, Wright KT, Rodig SJ. Checkpoint blockade in Hodgkin and non-Hodgkin lymphoma. Blood Adv. 2017;1(26):2643-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavez JC, Bachmeier C, Kharfan-Dabaja MA. CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol. 2019;10:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neelapu SS, Locke FL, Bartlett NL, et al. . Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-Cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locke FL, Ghobadi A, Jacobson CA, et al. . Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster SJ, Svoboda J, Chong EA, et al. . Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svoboda J, Rheingold SR, Gill SI, et al. . Nonviral RNA chimeric antigen receptor-modified T cells in patients with Hodgkin lymphoma. Blood. 2018;132(10):1022-1026. [DOI] [PubMed] [Google Scholar]
- 23.Grupp SA, Maude SL, Shaw PA, et al. . Durable remissions in children with relapsed/refractory ALL treated with T cells engineered with a CD19-targeted chimeric antigen receptor (CTL019) [abstract]. Blood 2015;126(23). Abstract 681. [Google Scholar]
- 24.Braig F, Brandt A, Goebeler M, et al. . Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood. 2017;129(1):100-104. [DOI] [PubMed] [Google Scholar]
- 25.Kochenderfer JN, Dudley ME, Carpenter RO, et al. . Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122(25):4129-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baumeister SH, Murad J, Werner L, et al. . Phase I trial of autologous CAR T cells targeting NKG2D ligands in patients with AML/MDS and multiple myeloma. Cancer Immunol Res. 2019;7(1):100-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sehn LH, Kamdar M, Herrera AF, et al. . Adding polatuzumab vedotin (Pola) to bendamustine and rituximab (BR) treatment improves survival in patients with relapsed/refractory DLBCL: results of a phase II clinical trial. 23rd Congress of the European Hematology Association, June 16, 2018, Stockholm, Sweden. https://www.primeoncology.org/app/uploads/hematology-updates-stockholm−2018-dlbcl-s802-sehn.pdf. Accessed 18 May 2019.
- 28.Chien SH, Liu CJ, Hong YC, et al. . Development of second primary malignancy in patients with non-Hodgkin lymphoma: a nationwide population-based study. J Cancer Res Clin Oncol. 2015;141(11):1995-2004. [DOI] [PubMed] [Google Scholar]
- 29.Al-Tourah AJ, Gill KK, Chhanabhai M, et al. . Population-based analysis of incidence and outcome of transformed non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(32):5165-5169. [DOI] [PubMed] [Google Scholar]
- 30.Link BK, Maurer MJ, Nowakowski GS, et al. . Rates and outcomes of follicular lymphoma transformation in the immunochemotherapy era: a report from the University of Iowa/Mayo Clinic Specialized Program of Research Excellence Molecular Epidemiology Resource. J Clin Oncol. 2013;31(26):3272-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montoto S, Davies AJ, Matthews J, et al. . Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol. 2007;25(17):2426-2433. [DOI] [PubMed] [Google Scholar]
- 32.Sokołowska N, Sokołowski R, Polak-Szabela A, Mazur E, Podhorecka M, Kędziora-Kornatowska K. Comparison of the effectiveness of the Montreal Cognitive Assessment 7.2 and the Mini-Mental State Examination in the detection of mild neurocognitive disorder in people over 60 years of age. Preliminary study [in English and Polish]. Psychiatr Pol. 2018;52(5):843-857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.