Abstract
Mycoleptodiscus includes plant pathogens, animal opportunists, saprobic and endophytic fungi. The present study presents the first molecular phylogeny and revision of the genus based on four loci, including ITS, LSU, rpb2, and tef1. An extensive collection of Mycoleptodiscus cultures, including ex-type strains from the CBS, IMI, MUCL, BRIP, clinical isolates from the USA, and fresh isolates from Brazil and Spain, was studied morphologically and phylogenetically to resolve their taxonomy. The study showed that Mycoleptodiscus sensu lato is polyphyletic. Phylogenetic analysis places Mycoleptodiscus in Muyocopronales (Dothideomycetes), together with Arxiella, Leptodiscella, Muyocopron, Neocochlearomyces, and Paramycoleptodiscus. Mycoleptodiscus terrestris, the type species, and M. sphaericus are reduced to synonyms, and one new species is introduced, M. suttonii. Mycoleptodiscus atromaculans, M. coloratus, M. freycinetiae, M. geniculatus, M. indicus, M. lateralis (including M. unilateralis and M. variabilis as its synonyms) and M. taiwanensis belong to Muyocopron (Muyocopronales, Dothideomycetes), and M. affinis, and M. lunatus to Omnidemptus (Magnaporthales, Sordariomycetes). Based on phylogenetic analyses we propose Muyocopron alcornii sp. nov., a fungus associated with leaf spots on Epidendrum sp. (Orchidaceae) in Australia, Muyocopron zamiae sp. nov. associated with leaf spots on Zamia (Zamiaceae) in the USA, and Omnidemptus graminis sp. nov. isolated from a grass (Poaceae) in Spain. Furthermore, Neomycoleptodiscus venezuelense gen. & sp. nov. is introduced for a genus similar to Mycoleptodiscus in Muyocopronaceae.
Keywords: Ascomycota, Dothideomycetes, fungal pathogen, Muyocopron, mycoses, new taxa, Sordariomycetes
INTRODUCTION
Mycoleptodiscus was proposed to accommodate M. terrestris, a species previously included in the invalid genus Leptodiscus (Gerdemann 1953), and M. sphaericus (Ostazeski 1967). Species of Leptodiscus were originally characterised by sporodochial conidiomata, thick-walled holoblastic conidiogenous cells that produce hyaline, 1-septate conidia with appendages at each end. Subsequently, Sutton & Alcorn (1990) emended the generic concept to include species with 0–2-septate conidia with polar and lateral appendages or lacking appendages, and frequently producing appressoria (Sutton & Hodges 1976, Sutton & Alcorn 1990, Alcorn 1994, Whitton et al. 2012). Currently, Mycoleptodiscus comprises 18 species (Ostazeski 1967, Sutton & Hodges 1976, Sutton & Alcorn 1985, 1990, Matsushima 1987, 1993, Bills & Polishook 1992a, Alcorn 1994, Ando 1996, Whitton et al. 2012, Tibpromma et al. 2018). The sexual morph has not been described for any species of the genus, except for M. affinis, which was introduced as Omnidemptus affinis by Cannon & Alcorn (1994). Omnidemptus is a monotypic genus characterised by having superficial, perithecial ascomata, cylindrical-clavate asci, with an apical pore, and a small ring which stains dark blue with Melzer’s reagent and ascospores that are fusiform, 1–3-septate and hyaline (Cannon & Alcorn 1994).
The first phylogenetic approach of a Mycoleptodiscus species was based on LSU rDNA sequences from M. coloratus (Thongkantha et al. 2009), retaining this genus in Magnaporthales (Sordariomycetes). Later, Luo & Zhang (2013) established that M. affinis (= O. affinis) was also related to Magnaporthaceae. However, Klaubauf et al. (2014) demonstrated that M. coloratus and M. affinis were unrelated, the former affine to Ophioceraceae and the latter affine to Magnaporthaceae.
Recently, Crous et al. (2018) showed that Mycoleptodiscus, based on M. terrestris, the type species, was a member of Muyocopronales, a newly proposed order in Dothideomycetes (Mapook et al. 2016a). Furthermore, analyses of sequences deposited in the GenBank database as M. indicus or Mycoleptodiscus sp. (Dewar & Sigler 2010, Metry et al. 2010, Koo et al. 2012) showed that they are related to Muyocopron (Muyocopronales). Muyocopron was proposed by Spegazzini (1881), re-described by Saccardo (1883), and its taxonomical placement treated initially in Microthyriaceae (Von Arx & Müller 1975, Lumbsch & Huhndorf 2007) and later in Muyocopronaceae (Hyde et al. 2013, Pang et al. 2013, Mapook et al. 2016a). Muyocopron is characterised by pseudothyriothecial, superficial, flattened, carbonaceous, brittle ascomata, with pseudoparaphyses that are longer than the asci and ellipsoidal to ovate, unicellular ascospores (Hyde et al. 2013, Mapook et al. 2016a). No asexual morph has ever been described for any Muyocopron species (Spegazzini 1881, Wu et al. 2011, Mapook et al. 2016a).
Gerdemann (1953), when introducing Mycoleptodiscus (as Leptodiscus), demonstrated M. terrestris to be a pathogen of herbaceous Fabaceae with economic interest, such as Glycine, Lespedeza, Lotus, Medicago, Melilotus, Pisum, and Trifolium in the USA. Other Mycoleptodiscus species have also been reported to cause diseases of economically important plants, such as Alloteropsis, Carpobrotus, Cattleya, Dianella, Panicum, and Stypandra in Australia (Sutton & Alcorn 1985, 1990, Alcorn 1994, Cannon & Alcorn 1994), Eucalyptus, Garcinia, and Vanilla in Brazil (Sutton & Hodges 1976, Bezerra & Ram 1986, Paim et al. 2012), Piper in the Dominican Republic (Watanabe et al. 1997), Ficus in Peru (Matsushima 1993), Arecaceae in Taiwan (Matsushima 1987), Fabaceae (including Lotus) and Zamia in the USA (Ostazeski 1967, Sutton 1973, Vanev 1983). They have also occurred on several plant species in Brunei, Cuba, India, New Zealand, and Nigeria (Sutton & Hodges 1976, Sutton & Alcorn 1990). Mycoleptodiscus species also recorded as putative pathogens of symptomatic tropical forest seedlings in Panama (Spear 2017), and reported on dead leaves of Freycinetia in Australia and the Philippines (Whitton et al. 2012).
Although Mycoleptodiscus species have been widely reported as plant pathogens, some have been isolated as endophytes from Chamaecyparis thyoides (Bills & Polishook 1992a, b), the aquatic Myriophyllum spicatum in the USA (Shearer 2001), Desmotes incomparabilis in Panama (Martínez-Luis et al. 2011, Ortega et al. 2013), Acer truncatum and Cinnamomum camphora in China (Sun et al. 2011, He et al. 2012), Borreria verticillata and Opuntia ficus-indica in Brazil (Andrioli et al. 2012, Bezerra et al. 2012), and Freycinetia sp. in Thailand (Tibpromma et al. 2018). Mycoleptodiscus terrestris has also been isolated as an endophyte from aquatic submersed macrophytes in New Zealand (Hofstra et al. 2009, 2012).
The clinical importance of Mycoleptodiscus was first associated with a subcutaneous infection (phaeohyphomycosis) in the knee of a male with Wegener’s granulomatosis and immunodeficiency in South Carolina (USA) (Padhye et al. 1995) and in a liver transplant patient with human immunodeficiency virus and hepatitis C co-infection (Garrison et al. 2008). The infection caused by Mycoleptodiscus was also reported from a mycotic arthritis of the knee in a healthy Canadian male (Dewar & Sigler 2010). In New Zealand, Koo et al. (2012) reported an association with Mycoleptodiscus from progressive necrotizing fungal cellulitis and myositis in the leg of a patient with glioblastoma multiforme. Domesticated animals were also reported with infections associated with Mycoleptodiscus species, such as a subcutaneous infection in a cat in Australia (Hull et al. 1997), and in an immunosuppressed dog in the USA (Metry et al. 2010). The clinical importance of Mycoleptodiscus species, mainly M. indicus, was discussed by De Hoog et al. (2000).
As scientific research has focused on the discovery of novel microorganisms for biotechnology purposes, some studies have outlined the potential of Mycoleptodiscus. Martínez-Luis et al. (2011) demonstrated the anti-parasitic ability of a Mycoleptodiscus sp. isolated as an endophyte from the leaves of D. incomparabilis. Other studies have established the ability of M. terrestris as a biological control of aquatic plants (Verma & Charudattan 1993, Shearer & Jackson 2006, Nelson & Shearer 2008). Ortega et al. (2013) showed compounds produced by Mycoleptodiscus strains could inhibit the growth of cancer cells in vitro. Furthermore, interesting compounds were identified and isolated from M. indicus, namely eugenitin, four known polyketides, and three new azaphilones, named mycoleptones A–C (Andrioli et al. 2012, 2014). Grandi & Silva (2010) studied the impact of M. disciformis in nutrient cycling in the leaf litter decomposition in Brazil.
The aim of the present study is to clarify the taxonomy and phylogeny of Mycoleptodiscus species within Ascomycota. For this purpose, we used a set of strains of Mycoleptodiscus species isolated from clinical and plant specimens, including the available ex-type strains, and performed the multi-locus analyses of ITS rDNA, LSU, rpb1, rpb2 and tef1 sequences.
MATERIALS AND METHODS
Strains and morphological analysis
A total of 30 strains were examined (Table 1). Specimens of Mycoleptodiscus species, including holotypes and ex-type strains, were obtained from the Westerdijk Fungal Biodiversity Institute (CBS, The Netherlands), the Fungus Testing Laboratory of the University of Texas Health Science Center at San Antonio (UTHSCSA, USA), the Queensland Plant Pathology Herbarium (BRIP, Australia), the Kew Royal Botanic Gardens (IMI, England), and the Mycothèque of the UCL (BCCM/MUCL, Belgium).
Table 1.
GenBank accession numbers included in the alignments of Dothideomycetes and Sordariomycetes.
| Species1 | Old names | Strains2 | Other collections | GenBank accession numbers3 |
References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ITS | LSU | rpb1 | rpb2 | tef1 | ||||||
| Dothideomycetes | ||||||||||
| Acrospermum compressum | M151‡ | EU940161 | EU940084 | – | – | – | Stenroos et al. (2010) | |||
| A. gramineum | M152‡ | EU940162 | EU940085 | – | – | – | Stenroos et al. (2010) | |||
| Arxiella dolichandrae | CBS 138853T | CPC 22951 | NR_137930 | KP004477 | – | MK492710 | MK495954 | Crous et al. (2014), this study | ||
| A. terrestris | CBS 268.65T | MH858565 | MH870201 | – | – | – | Vu et al. (2019) | |||
| Asterodiscus tamaricis | CBS 136918 | L113 | KU234100 | KU234100 | – | KU234115 | KU234132 | Voglmayr et al. (2016) | ||
| Corynespora cassiicola | CBS 100822 | – | GU301808 | – | GU371742 | GU349052 | Schoch et al. (2009) | |||
| Dothidea sambuci | DAOM 231303 | AFTOL-ID 274 | NR_111220 | AY544681 | – | DQ522854 | DQ497606 | Schoch et al. (2014) | ||
| Dyfrolomyces tiomanensis | NTOU 3636‡ | KC692156 | KC692156 | – | – | KC692157 | Pang et al. (2013) | |||
| Jahnula aquatica | R68_1‡ | JN942354 | EF175655 | – | – | – | Campbell et al. (2007) | |||
| J. sangamonensis | A402_1B‡ | JN942349 | EF175661 | – | – | – | Campbell et al. (2007) | |||
| J. seychellensis | SS2113‡ | – | EF175665 | – | – | – | Campbell et al. (2007) | |||
| J. siamensiae | SS81.02‡ | – | EF175666 | – | – | – | Campbell et al. (2007) | |||
| Leptodiscella africana | CBS 400.65T | NR_145359 | MH870275 | – | MK492711 | MK495955 | Madrid et al. (2012), Vu et al. (2019), this study | |||
| L. rintelii | CBS 144927T | LR025180 | LR025181 | – | – | – | Crous et al. (2018) | |||
| Muyocopron alcornii | BRIP 43897T | CBS 141314 | MK487735 | MK487708 | – | MK492712 | MK495956 | This study | ||
| Mu. atromaculans | Mycoleptodiscus atromaculans | MUCL 34983T | BPI GB1369 | MK487736 | MK487709 | – | MK492713 | MK495957 | This study | |
| Mu. castanopsis | MFLUCC 14-1108T | – | KU726965 | – | KY225778 | – | Mapook et al. (2016a, b) | |||
| Mu. coloratum | Mycoleptodiscus coloratus | CBS 720.95T | NR_160197 | MK487710 | – | MK492714 | MK495958 | This study | ||
| Mu. dipterocarpi | MFLUCC 14-1103T | – | KU726966 | – | KY225779 | – | Mapook et al. (2016a, b) | |||
| Mu. garethjonesii | MFLUCC 16-1370T | – | KY070274 | – | – | – | Tibpromma et al. (2016) | |||
| Mu. geniculatum | Mycoleptodiscus geniculatus | CBS 721.95T | MK487737 | MK487711 | – | MK492715 | MK495959 | This study | ||
| Mu. laterale | Mycoleptodiscus lateralis | CBS 141029T | BRIP 16247, ATCC 200213 | MK487738 | MK487712 | – | MK492716 | MK495960 | This study | |
| Mycoleptodiscus unilateralis | IMI 324533T | MK487739 | MK487713 | – | MK492717 | MK495961 | This study | |||
| Mycoleptodiscus variabilis | CBS 719.95T | BRIP 16983, ATCC 96451 | MK487740 | MK487714 | – | MK492718 | MK495962 | This study | ||
| Mycoleptodiscus sp. | CBS 141033 | BRIP 20066 | MK487741 | MK487715 | – | MK492719 | MK495963 | This study | ||
| Mycoleptodiscus sp. | URM 7802 | MK487742 | MK487716 | – | MK492720 | MK495964 | This study | |||
| Mycoleptodiscus sp. | URM 7801 | MK487743 | MK487717 | – | MK492721 | – | This study | |||
| Mycoleptodiscus indicus | CBS 127677 | UAMH 10746 | MK487744 | MK487718 | – | MK492722 | MK495965 | This study | ||
| Mycoleptodiscus sp. | CBS 145310 | UTHSC DI17-18 | MK487745 | MK487719 | – | MK492723 | MK495966 | This study | ||
| Mycoleptodiscus sp. | CBS 145315 | UTHSC DI 17-23 | MK487746 | MK487720 | – | MK492724 | MK495967 | This study | ||
| Mycoleptodiscus sp. | CBS 145313 | UTHSC DI 17-21 | MK487747 | MK487721 | – | MK492725 | MK495968 | This study | ||
| Mycoleptodiscus sp. | CBS 145309 | UTHSC DI 17-17 | MK487748 | MK487722 | – | MK492726 | MK495969 | This study | ||
| Mycoleptodiscus sp. | CBS 145314 | UTHSC DI 17-22 | MK487749 | MK487723 | – | MK492727 | MK495970 | This study | ||
| Mycoleptodiscus sp. | CBS 145311 | UTHSC DI 17-19 | MK487750 | MK487724 | – | MK492728 | – | This study | ||
| Mycoleptodiscus sp. | CBS 145312 | UTHSC DI 17-20 | MK487751 | MK487725 | – | MK492729 | MK495971 | This study | ||
| Mycoleptodiscus sp. | CBS 145316 | UTHSC DI 17-24 | MK487752 | MK487726 | – | MK492730 | MK495972 | This study | ||
| Mycoleptodiscus sp. | FMR 13797 | MK874615 | MK874616 | – | MK875802 | MK875803 | This study | |||
| Mu. lithocarpi | MFLUCC 10-0041 | – | JQ036230 | – | – | – | Wu et al. (2011) | |||
| MFLUCC 14-1106T | – | KU726967 | – | KY225780 | – | Mapook et al. (2016a, b) | ||||
| Mu. zamiae | Mycoleptodiscus sp. | CBS 203.71T | – | MK487727 | – | MK492731 | MK495973 | This study | ||
| ‘Mycoleptodiscus endophyticus’ | MFLUCC 17-0545 | NR_158860 | MG646946 | – | – | MG646985 | Tibpromma et al. (2018) | |||
| Mycoleptodiscus suttonii | Mycoleptodiscus terrestris | CBS 276.72T | MK487753 | MK487728 | – | MK492732 | MK495974 | This study | ||
| Mycoleptodiscus terrestris | CBS 141030 | BRIP 16943, ATCC 200215 | – | MK487729 | – | MK492733 | MK495975 | This study | ||
| M. terrestris | Leptodiscus terrestris | CBS 231.53T | MK487754 | MK487730 | – | MK492734 | MK495976 | This study | ||
| Mycoleptodiscus sphaericus | IMI 159038T | ATCC 18104 | MK487755 | MK487731 | – | MK492735 | MK495977 | This study | ||
| Neocamarosporium betae | CBS 109410 | KY940790 | EU754178 | – | GU371774 | GU349075 | De Gruyter et al. (2009), Schoch et al. (2009), Woudenberg et al. (2017) | |||
| Neomycoleptodiscus venezuelense | Mycoleptodiscus terrestris | CBS 100519T | MK487756 | MK487732 | – | MK492736 | MK495978 | This study | ||
| Paramycoleptodiscus albizziae | CBS 141320T | CPC 27552 | KX228279 | KX228330 | – | MK492737 | MK495979 | Crous et al. (2016), this study | ||
| Saccardoella rhizophorae | JK 5456A | GU479799 | GU479799 | – | – | GU479860 | Suetrong et al. (2009) | |||
| Stemphylium botryosum | CBS 714.68 | AFTOL-ID 934 | AF071345 | DQ678049 | – | DQ677943 | DQ677888 | Berbee et al. (1999), Schoch et al. (2006) | ||
| Stigmatodiscus enigmaticus | L82‡ | KU234112 | KU234112 | – | KU234125 | – | Voglmayr et al. (2016) | |||
| Sydowia polyspora | CBS 116.29 | AFTOL-ID 1300 | – | DQ678058 | – | DQ677953 | DQ677899 | Schoch et al. (2006) | ||
| Sordariomycetes | ||||||||||
| Buergenerula spartinae | ATCC 22848 | JX134666 | DQ341492 | JX134720 | – | JX134692 | Thongkantha et al. (2009), Luo & Zhang (2013) | |||
| Bussabanomyces longisporus | CBS 125232T | KM009166 | KM484951 | KM485046 | – | KM009202 | Klaubauf et al. (2014) | |||
| Falciphoriella oryzae | Harpophora oryzae | CBS 125863T | R-5-6-1 | FJ752606 | KJ026705 | KJ026706 | – | JN857963 | Yuan et al. (2010), Su et al. (2013), Xu et al. (2015) | |
| F. solaniterrestris | Gaeumannomyces sp. | CBS 117.83T | KM484842 | KM484959 | KM485058 | – | – | Klaubauf et al. (2014) | ||
| Gaeumannomyces graminis | Gaeumannomyces graminis var. graminis | CBS 141385 | CPC 26033 | KX306501 | KX306571 | KX306636 | – | KX306704 | Hernández-Restrepo et al. (2016a) | |
| Gaeumannomyces graminis var. graminis | CPC 26045 | KX306505 | KX306575 | KX306640 | – | KX306708 | Hernández-Restrepo et al. (2016a) | |||
| Kohlmeyeriopsis medullaris | Gaeumannomyces medullaris | CBS 117849T | JK5528S | KM484852 | KM484968 | KM485068 | – | – | Klaubauf et al. (2014) | |
| Magnaporthiopsis incrustans | Gaeumannomyces incrustans | M35‡ | JF414843 | JF414892 | JF710437 | – | JF710412 | Zhang et al. (2011) | ||
| Ma. poae | Magnaporthe poae | M48‡ | JF414837 | – | JF710434 | – | JF710416 | Zhang et al. (2011) | ||
| Ma. rhizophila | Magnaporthe poae | M23‡ | JF414834 | JF414846 | JF710432 | – | JF710408 | Zhang et al. (2011) | ||
| Nakataea oryzae | CBS 252.34 | KM484862 | KM484976 | KM485078 | – | – | Klaubauf et al. (2014) | |||
| Neogaeumannomyces bambusicola | MFLUCC 11 0390T | KP744449 | KP744492 | – | – | – | Liu et al. (2015) | |||
| Omnidemptus affinis | BRIP 17195aT | ATCC 200212, IMI 353435, CBS 141031 | JX134674 | JX134686 | JX134728 | – | JX134700 | Luo & Zhang (2013) | ||
| Mycoleptodiscus affinis | BRIP 17195bT | CBS 141032 | MK487757 | MK487733 | – | – | – | This study | ||
| O. graminis | CBS 138107T | FMR 12415 | MK487758 | MK487734 | – | – | MK495980 | This study | ||
| Pseudophialophora eragrostis | CM12m9T | KF689648 | KF689638 | KF689618 | – | KF689628 | Luo et al. (2014) | |||
| Pyricularia grisea | Magnaporthe grisea | BR0029‡ | KM484880 | KM484995 | KM485100 | – | – | Klaubauf et al. (2014) | ||
| CR0024‡ | KM484882 | KM484997 | KM485102 | – | – | Klaubauf et al. (2014) | ||||
| Slopeiomyces cylindrosporus | Gaeumannomyces cylindrosporus | CBS 609.75T | KM484944 | KM485040 | KM485158 | – | – | Klaubauf et al. (2014) | ||
1 New species are in bolditalic.
2 ATCC: American Type Culture Collection, Virginia, USA; BRIP: Queensland Plant Pathology Herbarium, Brisbane, Australia; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CPC: Culture collection of Pedro Crous, housed at Westerdijk Fungal Biodiversity Institute; DAOM: National Mycological Herbarium, Department of Agriculture, Ottawa, Ontario, Canada; IMI: International Mycological Institute, Kew, UK; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Ria, Thailand; MUCL: Mycothèque de l’Université catholique de Louvain, Louvain-la-Neuve, Belgium; URM: Culture collection Prof. Maria Auxiliadora Cavalcanti, Recife, Brazil; UTHSC: Fungus Testing Laboratory of the University of Texas Health Science Center at San Antonio, USA; ‡ for other codes (A, BR, CR, JK, L, M, NTOU, R, SS) see References. T indicate ex-type strains.
3 ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: partial large subunit (28S) of the nrRNA gene operon; rpb1 & rpb2: partial RNA polymerase II largest subunit gene; tef1: partial translation elongation factor 1-alpha gene. Newly generated sequences are in bold.
Fresh specimens were collected in Brazil and Spain. The Brazilian specimens were isolated as endophytes from Opuntia ficus-indica and Poincianella pyramidalis growing in a tropical dry forest. The Spanish specimen was collected in Navarra, Robledal de Orgi natural area, growing on a gramineous plant close to a stream. Pure cultures were obtained from the conidia of the fungi transferred onto water agar (WA; Difco agar 5 g, 1 L tap water, pH 6).
Strains were sub-cultured on malt extract agar (MEA; 40 g malt extract, 15 g agar, 1 L distilled water) and oatmeal agar (OA, filtered oat flakes, 20 g agar, 1 L distilled water), and incubated at 25 °C under daylight conditions for 1–3 wk; UV light conditions were used for some isolates to induce sporulation. After 1–2 wk of incubation, the colony diameters were measured and the colony morphologies described. Colony colours on the surface and reverse of inoculated media were assessed according to the colour charts of Rayner (1970). Micromorphological descriptions and measurements of relevant features were carried out from fungal structures and herbarium specimens mounted in clear lactic acid 90 % v/v. Observations and photomicrographs were made with a Nikon SMZ1500 dissecting microscope and with a Nikon eclipse Ni compound microscope, using a DS-Ri2 digital camera (Nikon, Tokyo, Japan) and NIS-Elements imaging software v. 4.20. Strains and fungarium materials were deposited at the CBS, BRIP, or at the culture collection of Prof. Maria Auxiliadora Cavalcanti from the Federal University of Pernambuco (URM, Recife, Brazil). Taxonomic information and nomenclature for new species were deposited in MycoBank (www.MycoBank.org).
DNA isolation, amplification, sequence alignment and phylogenetic analysis
Genomic DNA was extracted from fungal colonies growing on MEA using the Wizard® Genomic DNA purification kit (Promega, Madison, USA), according to the manufacturer’s protocols. Procedures for amplifying and sequencing the nuclear rDNA, ITS1-5.8S-ITS2 (ITS) and ± 900 bp of the large subunit (LSU), was performed as described in Hernández-Restrepo et al. (2016b). Part of the largest and second largest subunit of the RNA polymerase II gene (rpb1 and rpb2) was amplified and sequenced as described in Hernández-Restrepo et al. (2016b) and Klaubauf et al. (2014), respectively. Translation elongation factor 1-α gene (tef1), corresponding to section 983–1567 bp, was amplified and sequenced as described in Rehner & Buckley (2005). Consensus sequences were assembled in SeqMan Pro (DNASTAR, Madison, WI, USA). The dataset for each gene was aligned using MAFFT v. 7 (Katoh & Standley 2013), using the defaults settings and adjusted by hand in MEGA v. 6.06 (Tamura et al. 2013).
BLASTn searches using ITS and LSU sequences were carried out and sequences of related species, belonging to Dothideomycetes and Sordariomycetes, were obtained from GenBank and listed in Table 1. Two multi-locus phylogenetic analyses were carried out. The first one for Dothideomycetes was based on a concatenated alignment of ITS, LSU, rpb2, and tef1. Another phylogenetic analysis for Magnaporthales (Sordariomycetes) was based on a concatenated alignment of ITS, LSU, rpb1, and tef1. Individual alignment of each locus and the concatenated four-loci dataset were analysed by Maximum Likelihood (ML) using the RAxML HPC BlackBox v. 8.2.10 (Stamatakis 2014) online server of the Cipres Science gateway portal (Miller et al. 2012). The multi-locus datasets were combined using Sequence Matrix v. 1.8 (Vaidya et al. 2011). A Markov Chain Monte Carlo (MCMC) algorithm was used to generate phylogenetic trees with Bayesian probabilities from the concatenated four-locus dataset using MrBayes v. 3.2.6 (Ronquist et al. 2012). The best model of nucleotide substitution for each locus was determined using MrModeltest v. 2.3 (Nylander 2004). Confident branch support was defined as bootstrap values (BS) ≥ 70 % from a ML search with 1 000 replicates and posterior probabilities (PP) ≥ 0.95. The sequences generated during this study and the alignments used in the phylogenetic analyses were deposited in GenBank (Table 1) and TreeBASE (submission number 23523), respectively.
RESULTS
DNA phylogeny
BLASTn searches revealed that the ITS and LSU sequences of M. atromaculans, M. coloratus, M. geniculatus, ‘M.? indicus’, M. lateralis, M. sphaericus, M. terrestris, M. unilateralis, and M. variabilis were similar to sequences of members of Muyocopronales (Dothideomycetes). While M. affinis and the strain CBS 138107, were similar to Magnaporthales (Sordariomycetes).
The first concatenated matrix contained a total of 3 364 characters (761 for ITS, 846 for LSU, 819 for rpb2, and 938 for tef1). For Bayesian analysis, MrModel test proposed a GTR+I+G model for all the loci. The consensus tree obtained from the Bayesian analysis agreed with the topology of the best scoring ML tree (Fig. 1). In the phylogenetic tree, Mycoleptodiscus species were scattered into two well-supported clades in Muyocopronales. The Mycoleptodiscus clade (100 ML BS/0.99 PP) includes the ex-type sequences of M. sphaericus and M.? terrestris. The analysis also revealed the existence of a cryptic species in the current concept of M. terrestris, represented by two strains, CBS 276.72 and CBS 141030, and for which M. suttonii sp. nov. is proposed as a new species in the taxonomy section. The strain CBS 100519, formerly identified as ‘M. terrestris’, was phylogenetically distant and morphologically different from M. terrestris s.str. and formed a subclade with ‘M. endophyticus’ (71/0.98) and Neocochlearomyces chromolaenae (56/0.98), and is therefore proposed here as Neomycoleptodiscus venezuelense gen. & sp. nov. A second clade (100/0.97), including M. atromaculans, M. coloratus, M. geniculatus, ‘M. indicus’, M. lateralis, M. unilateralis, M. variabilis, and numerous unidentified Mycoleptodiscus strains, was placed in a monophyletic lineage together with species of Muyocopron. Furthermore, it is of note that a strain of ‘M. indicus’ CBS 127677 and the ex-type strains of M. lateralis, M. unilateralis, and M. variabilis were grouped in the same clade (89/0.99). Our analysis revealed two undescribed Muyocopron species among the specimens studied, proposed here as Mu. alcornii sp. nov. (BRIP 43897) and Mu. zamiae sp. nov. (CBS 702.71, CBS 703.71).
Fig. 1.
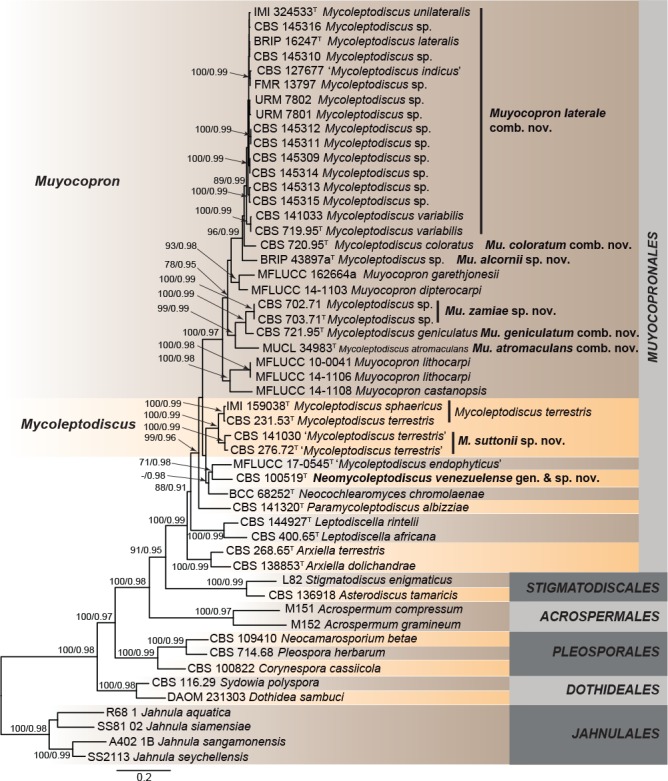
RAxML phylogram obtained from the combined ITS, LSU, rpb2, and tef1 sequences of Dothideomycetes members. The tree was rooted to Jahnulales. Taxonomic novelties described in this study are shown in bold. RAxML bootstrap support (BS) values ≥ 70 % and Bayesian posterior probability (PP) scores ≥ 0.95 are shown at the nodes. GenBank accession numbers are indicated in Table 1. T indicates ex-type.
The Magnaporthales concatenated matrix contained a total of 2 882 characters (589 for ITS, 752 for LSU, 618 for rpb1, and 923 for tef1). For Bayesian analysis, the MrModel test proposed a GTR+I+G model for ITS and LSU and a GTR+G for rpb1 and tef1. The consensus tree obtained from that analysis agreed with the topology of the best scoring ML tree (Fig. 2). In the phylogenetic tree, M. affinis CBS 141031, O. affinis ATCC 200212 and CBS 138107 formed an independent clade (100/1.0) in the Magnaporthaceae. Omnidemptus is circumscribed and M. affinis is formally synonymised under O. affinis, and the strain CBS 138107 is introduced as Omnidemptus graminis sp. nov. based on its phylogenetic and morphological differences.
Fig. 2.
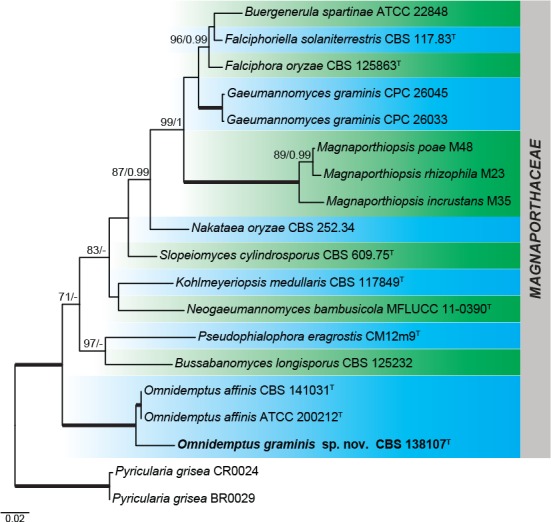
RAxML phylogram obtained from the combined ITS, LSU, rpb1, and tef1 sequences of Magnaporthales members. The tree was rooted to Pyricularia grisea. The new species described in this study is shown in bold. RAxML bootstrap support (BS) values ≥ 70 % and Bayesian posterior probability (PP) scores ≥ 0.95 are shown at the nodes and thickened lines represent nodes with BS = 100 % and PP = 1.00. GenBank accession numbers are indicated in Table 1. T indicates ex-type.
Taxonomy
According to our phylogenetic analyses using DNA sequences from four loci (ITS, LSU, rpb2, and tef1), Mycoleptodiscus and Muyocopron, together with the genera Arxiella, Leptodiscella, Neocochlearomyces, Neomycoleptodiscus, and Paramycoleptodiscus, belong to the Muyocopronaceae (Muyocopronales, Dothideomycetes; Fig. 1), confirming previous results (Crous et al. 2018). Since the relationship of Muyocopron with all of these genera within the Muyocopronales is reported for the first time, the circumscription of the order and family is emended below. Mycoleptodiscus s.str. is restricted to species characterised by cylindrical conidia with appendages at one or both ends. However, one new genus, Neomycoleptodiscus, very similar to Mycoleptodiscus, is recognised based on phylogenetic inferences and subtle morphological differences. Morphological and molecular data revealed that several species with broadly lunate conidia and with variable production of conidial appendages, previously included in Mycoleptodiscus, are part of what we interpret as the core clade of Muyocopron. Following the single-name nomenclature (Hawksworth et al. 2011), new combinations are proposed for M. atromaculans, M. coloratus, M. freycinetiae, M. geniculatus, M. indicus, M. lateralis, and M. taiwanensis, including mycoleptodiscus-like asexual morphs in Muyocopron.
Omnidemptus, which was previously placed in Magnaporthales (Cannon & Alcorn 1994, Luo & Zhang 2013, Klaubauf et al. 2014), is restricted to O. affinis and mycoleptodiscus-like species with falcate conidia lacking appendages.
Dothideomycetes
Muyocopronales Mapook et al., Phytotaxa 265: 230. 2016; emend.
Type family. Muyocopronaceae K.D. Hyde.
Ascomata pseudothyriothecial, superficial, coriaceous, appearing as circular, flattened, with a central ostiole, brown to dark brown, without subiculum, with a poorly developed basal layer; peridium comprising two layers, an outer layer composed of black-brown pseudoparenchymatous cells of textura epidermoidea and an inner layer composed of pale brown cells of textura angularis; hamathecium of pseudoparaphyses. Asci 8-spored, bitunicate, cylindrical to pyriform. Ascospores aseptate, oval to obovoid with obtuse ends, hyaline. Conidiomata if present, sporodochium-like. Conidiophores often reduced to conidiogenous cells. Conidiogenous cells solitary or aggregated, mono- or polyblastic, sympodial and often denticulate (Arxiella, Leptodiscella), or phialidic, globose to ampulliform (Mycoleptodiscus, Neocochlearomyces, Neomycoleptodiscus, Paramycoleptodiscus). Conidia one-celled or septate, lunate, falcate, fusiform or fusoid-ellipsoid, curved to geniculate, usually with terminal and/or lateral appendages, mostly hyaline. Appressoria, if present, ellipsoid to oblong-oval or obovoid, narrowly clavate or subcylindrical, sometimes uncinate, occasionally sigmoid or bluntly bifurcate, straight or curved, brown. Sclerotia often present in culture, black. Pathogenic on plants and opportunistic pathogen on animals, lignicolous, foliicolous and soil-borne.
Muyocopronaceae K.D. Hyde, Fung. Diversity 63: 164. 2013; emend.
Type genus. Muyocopron Speg.
Ascomata pseudothyriothecial, superficial, circular, flattened, lenticular in section, scattered, rarely coalescing, carbonaceous and brittle, black, basal layer slightly developed, with a central ostiole; upper wall comprising irregularly arranged radiating cells; peridium comprising two strata, an outer layer composed of black-brown pseudoparenchymatous cells of compact thick-walled textura epidermoidea, an inner layer comprised of pale brown cells of textura angularis; hamathecium of dense, septate pseudoparaphyses, longer than the asci, immersed in mucilage and inclined towards the centre. Asci 8-spored, bitunicate/fissitunicate, pedicellate, with a small ocular chamber. Ascospores aseptate, ellipsoidal to ovate with obtuse ends, hyaline, with a granular appearance. Conidiomata, if present, sporodochium-like. Conidiogenous cells solitary or aggregated, mono- or polyblastic, sympodial, often denticulate (Arxiella, Leptodiscella), or phialidic, globose or ampulliform (Mycoleptodiscus, Neocochlearomyces, Neomycoleptodiscus, Paramycoleptodiscus), smooth. Conidia one-celled or septate, lunate, falcate, fusiform or fusoid-ellipsoid, curved to geniculate, usually with terminal and/or lateral appendages, hyaline. Appressoria if present ellipsoid to oblong-oval or obovoid, narrowly clavate or subcylindrical, sometimes uncinate, occasionally sigmoid or bluntly bifurcate, straight or curved. Sclerotia often present in culture, black. Pathogenic on plants and opportunistic pathogen on animals, lignicolous, foliicolous, and soil-borne.
Other included genera — Arxiella, Leptodiscella, Mycoleptodiscus, Neocochlearomyces, Neomycoleptodiscus, and Paramycoleptodiscus.
Muyocopron Speg., Anales Soc. Ci. Argent. 12: 113. 1881; emend.
Type species. Muyocopron corrientinum Speg.
Ascomata superficial, solitary or scattered, coriaceous, appearing as circular, flattened, brown to dark brown, without subiculum, with a poorly developed basal layer and an irregular margin, with a central ostiole; peridium comprising two strata, an outer layer composed of dark brown to black pseudoparenchymatous cells of textura epidermoidea, an inner layer comprised of pale brown cells of textura angularis; hamathecium of cylindrical to filiform, septate pseudoparaphyses. Asci 8-spored, bitunicate, saccate or broadly obpyriform, pedicellate, straight or slightly curved, with small ocular chamber. Ascospores aseptate, irregularly arranged, oval to obovoid with obtuse ends, with a granular appearance, hyaline. Conidiomata sporodochium-like, highly irregular. Conidiogenous cells solitary or aggregated, enteroblastic, monophialidic, globose, broadly ellipsoidal to ampulliform, the conidiogenous locus often bordered with a collarette, brown, smooth. Conidia aseptate to septate, falcate, lunate, curved to geniculate, fusiform or fusoid-ellipsoid, with terminal and/or lateral appendages, hyaline, smooth. Appressoria ellipsoid to oblong-oval or obovoid, narrowly clavate or subcylindrical, sometimes uncinate, occasionally sigmoid or bluntly bifurcate, straight or curved, 1–2 inconspicuous or in-visible pores, brown, smooth. Saprobic and pathogenic on plants, opportunistic pathogen on animals.
Notes — Muyocopron is an old name, based on M. corrientinum described from leaves of Oncidium in Argentina (Spegazzini 1881). Luttrell (1951) was the first to include Muyocopron in its own family. However, the family was invalidly published, lacking a Latin diagnosis, and other authors have recognised this genus as a member of the Microthyriaceae (Hawksworth et al. 1995, Lumbsch & Huhndorf 2007). Hyde et al. (2013) provided an English diagnosis and accepted Muyocopronaceae in Dothideomycetes based on molecular data of Mu. castanopsis, Mu. dipterocarpi, and Mu. lithocarpi.
The present study is the first description of an asexual morph for Muyocopron, which is mycoleptodiscus-like. Morphologically, the asexual morph of Muyocopron is distinguished from Mycoleptodiscus by conidiogenous cells with conspicuous flared collarettes, wider lunate conidia, and appressoria being unlobed or rarely with two lobes, with inconspicuous or indistinct pore. In Mycoleptodiscus, the conidiogenous cells lack a collarette, the conidia are usually cylindrical, the appressoria have a visible pore surrounded by dark radial lines, and sclerotia are often present (Ostazeski 1967, Sutton & Hodges 1976, Sutton & Alcorn 1990, Matsushima 1993, Alcorn 1994). No sexual morph is currently known for Mycoleptodiscus as here redefined.
Muyocopron alcornii Hern.-Restr., J.D.P. Bezerra & Y.P. Tan, sp. nov. — MycoBank MB828980; Fig. 3
Fig. 3.
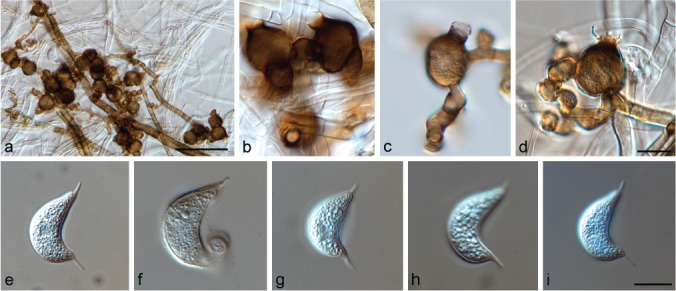
Muyocopron alcornii sp. nov. ex-type BRIP 43897. a–d. Conidiogenous cells; e–i. conidia. — Scale bars: a = 50 μm, others = 10 μm, d applies to b–d, i applies to e–i.
Etymology. Named after the Australian mycologist, John L. Alcorn, who described several species related to Mycoleptodiscus.
Typus. AUSTRALIA, Queensland, Logan (Greenbank), leaf spot (blight) of Epidendrum sp., 2004, L.I Forsberg R9324 (holotype BRIP 43897, culture ex-type BRIP 43897 = CBS 141314).
Hyphae septate, branched, dark brown near the conidiomata, pale brown to hyaline when distant, smooth, 1.5–7 μm wide. Conidiomata sporodochium-like, superficial, mid- to dark brown, varying from a few combined cells to large aggregations, variable in shape and size due to confluence, 36–89×38–60 μm. Conidiogenous cells globose to ampulliform, 12.5–18 × 12–18.5 μm, often with a distinct flared collarette, 3–6×4.5–8 μm, medium to dark brown, smooth. Conidia 0–1(–2)-septate, lunate, fusiform, curved, guttulate, 17.5–20×8–9 μm, with a filiform, unbranched appendage at each end, 3–5.5×0.5–1 μm, hyaline, smooth. Appressoria not observed. Sexual morph unknown.
Culture characteristics — Colonies on OA attaining 75 mm diam after 2 wk at 25 °C; aerial mycelium scarce, zonate, centre hispid, dark brick, periphery glabrous, luteous, margin effuse, diffusible pigment luteous to pale luteous; reverse sienna in the centre, fulvous to the periphery. On MEA attaining 40 mm diam after 1 wk at 25 °C, slightly elevated, cottony to hispid, buff to ochreous, margin effuse, diffusible pigment apricot; reverse dark umber in the centre, paler to the periphery.
Habitat — Epidendrum sp. (Orchidaceae).
Distribution — Australia.
Notes — Muyocopron alcornii can be distinguished from other species by having conidia with up to two septa. Muyocopron alcornii is similar to Mu. sahnii, but differs from it by having larger conidiogenous cells and conidia (Table 2). Phylogenetically, it is placed on an independent branch in the Muyocopron clade.
Table 2.
Morphological features of Muyocopron, Mycoleptodiscus and Omnidemptus species treated in this paper.
| Species | Strains and/or fungarium materials | Sporodochia (μm) | Conidiogenous cells |
Conidia |
Appressoria* (size in μm) | References | |||
|---|---|---|---|---|---|---|---|---|---|
| Size (μm) | Collarette (μm) | Shape & size (μm) | Septa | Appendages length (μm) | |||||
| Muyocopron alcornii | BRIP 43879 | 36–89 × 38–60 | 12.5–18×12–18.5 | 3–6×4.5–8 | Lunate, fusiform; 17.5–20×8–9 | 0–1(–2) | Apical and basal 3–5.5 | – | This study |
| Muyocopron atromaculans | MUCL 34983 | – | 9.5–14.5 diam | 2.5–4×2–7.5 | Broadly falcate or lunate; 15.5–23×6.5–8.5 | 0 | Apical and basal 1–3 | Broadly ellipsoid, outline entire or rarely with 1–2 short broad lobes, 0–1-septate, 10–14×7–10, base 3–4, 1 pore 0.5–1 diam | Bills & Polishook (1992a) |
| Muyocopron coloratum | CBS 720.95 | 25–165×16–125 | 17–25×13–19(–21) | 3–10×5–8 | Broadly lunate to inaequilateral oval-ellipsoidal; 16–27×8–13(–16) | 0 | Apical 2–8; basal 1–6 | Obovoid to broadly clavate, straight or commonly curved or bent, often uncinate, sometimes curved at > 180°, unlobed, aseptate, 14–19×8–13, base 3–5.5, pore not visible in many appressoria | Alcorn (1994) |
| CBS 720.95 | – | 17–29×16.5–26 | 3.5–6×4–6 | Broadly lunate; 22–34×8–15 | 0 | Apical 2–7; basal 0–5 | – | This study | |
| Muyocopron freycineticola | IFRD 8995 | 32–55 diam | 9–18×6–12 | 2–2.5×1.5–3 | Broadly falcate to lunate;11–15×4–4.5 | 0 | Apical and basal 2–3 | – | Whitton et al. (2012) |
| Muyocopron geniculatum | CBS 721.95 | Up to 125 diam | 10–15 diam | 3–12×3.5–6.5 | Lunate to fusiform or fusoid-ellipsoidal; 14–20×5.5–7.5 | 0 | Apical 4–12.5; basal 1–10 | Irregularly obovoid to clavate, curved, sigmoid, unlobed, 1(–2)-septate, 9.5–15×6–7, base 3–5, 1 pore 1 diam or indistinct | Alcorn (1994) |
| Muyocopron laterale | BRIP 16247 Type Mu. lateralis | 25–160×20–150 | 9–16 diam | 4–6 diam | Lunate, fusiform; 15–18×6–8 | 0 | Apical (9–)15–24; basal 5–22.5; lateral 12–26 | Obovoid to clavate or irregular, straight to uncinate, outline rather irregular, (0–)1–2-septate, 9–16×7–11, base 2.5–5, 1 pore 1–2 diam | Sutton & Alcorn (1990) |
| IMI 324533 Type Mu. unilateralis | 27–45×13–32 | 9–12.5 diam | 3–4.5 diam | Lunate, fusiform; 15–20×6–8 | 0 | Apical 7.5–12.5; basal 5–11; lateral 7–9 | – | Sutton & Alcorn (1990) | |
| CBS 719.95 Type Mu. variabilis | 125–370×100–225 | 14–24×9–16 | 4–6.5 diam | Lunate, fusiform; 15–25×5.5–8 | 0 | Apical 3–21; basal 4–15; lateral 11–22 | Obovoid, straight to uncinated, 9–15×7–9, base 3.5–5, pore indistinct | Alcorn (1994) | |
| CBS 719.95 Type Mu. variabilis | – | 12–16×10–15 | 1–2×3–5 | Lunate, fusiform; 16.5–22×8–9.5 | 0 | Apical and basal 2–17; lateral 9–20 | – | This study | |
| CBS 145315 | – | 12–19×7.5–11 | 3–4.5 diam | Lunate, fusiform; 16.5–21×5.5–7 | 0 | Apical, basal and lateral 8–15.5 | – | This study | |
| Muyocopron sahnii | IMI 108220 | 200–300 diam | – | – | Lunate, fusiform; 4–10×1.7–3.3 | 0 | One at each end | – | Sahni (1968) |
| IMI 108220 | 54–71 diam, 56–135×53–256 | 4.5–10.5 diam | – | Lunate, fusiform; 13.5–14.5×5–6 | 0 | Basal 3 | – | This study | |
| Several isolates | 30–100 diam | 7–13×3.5–7 | Up to 3 long | Lunate, fusiform; 11–18.5×4.5–7.5 | 0 | Apical 1–10; basal 0–6 | – | Sutton (1973) | |
| Muyocopron taiwanense | MFC-6T720 | – | 6–12×7–14 | – | Broadly falcate 12–21×5.5–7 | 0 | Apical and basal 1–3 | Clavate-obovoid with a broadly rounded apex, straight or slightly curved, (0–)1(–2)-septate, 9.5–13×5–6.5, base 2.5–3.5, pore indistinct | Matsushima (1987) |
| Muyocopron zamiae | CBS 203.71 | – | 7.5–14×8.5–12 | 1×2.5–3 | Lunate, fusiform; 16–20×5.5–6.5 | 0 | Apical 2.5–6; basal 0.75–5 | – | This study |
| ‘Mycoleptodiscus brasiliensis’ | IMI 196481e | 30–45 diam | 11–17.5×5–11.5 | 3×6 | Cylindrical; 17–19×4–4.5 | 1 | Apical 19–27 | – | Sutton & Hodges (1976) |
| ‘Mycoleptodiscus disciformis’ | MFC-1P143 | 85–430 diam | 4–7×3–5 | – | Cylindrical; 17.5–25×4–5 | 1 | Apical and basal 5–8 | Variable in shape, straight or curved, lobed, 7–17×4.5–7, base 2.5–5, 1(–2) pores, 1–1.5, with short dark radial lines surrounding the pore | Matsushima (1993) |
| ‘Mycoleptodiscus minimus’ | Holotype Discosia minima 5113 Herb. Berk. | 40–85 diam | 5–8.5×3.5–7 | – | Cylindrical; 20–25(–29)×3.5–4 | 0 | Apical and basal, up to 8 | – | Vanev (1983) |
| Mycoleptodiscus terrestris | BPI 403851 (ILL31238) | 200–800 diam | Evanescent | – | Cylindrical; 20–34.8×4.4–7 | 1 | Apical and basal 8.7–18 | Obovoid to clavate or cylindrical, straight or bent, entire or broadly lobed, 0(–1)-septate, 10–28×6–11, base 3–6, 1(–2) pores, 1.5–2(–2.5) diam, circular or sometimes irregular in shape, with dark radial lines surrounding the pore | Gerdemann (1953) |
| ATCC 18104 Type of M. sphaericus | 110–187×86–144 | Obsolete | – | Cylindrical; 28.8–43.2×5–9 | 1(–2) | Apical 0–14 | Clavate to obovoid, straight or curved, entire or incised, 0–1-septate, 10–25×6–11, 1–2 pores, 1.5–2.8 diam, with dark radial lines surrounding the pore | Ostazeski (1967) | |
| Neomycoleptodiscus venezuelense | CBS 100519 | 24–125×17–104 | 5–11×4–6.5 | – | Cylindrical; 18–27×3–5 | 1 | Apical and basal 6.5–13 | – | This study |
| Omnidemptus affinis | BRIP 17195b Type Mycoleptodiscus affinis | 50–160 diam | 7–12 diam [ampuliform]; 11–22×6–10 [elongated] | 3–4×2–3 | Falcate; 21–30×3–4 | 1–2(–3) | – | Obovoid to clavate with sinuate margin, 7.5–14×5–8.5(–11), base 2–4, pore 1.8–2 diam | Cannon & Alcorn (1994) |
| Omnidemptus graminis | CBS 138107 | – | 10–14 diam | 1×3 | Falcate; 11–23×3–4 | 1 | – | Obovoid to clavate sinuate margin, 10–15×7.5–10, 1 pore | This study |
| Omnidemptus lunatus | IMI 271703 | 10.5–13×6.5–11 | 3–4.5 diam | 1.5–2 diam | Falcate; 24.5–32×3.5–4.5 | 1 | – | Obovoid, clavate to irregular in shape, entire or sinuate, some lobed, but not deeply incised, 6–15×4.5–8, base 1.5–3.5, 1 pore 0.8–1.5 | Sutton & Alcorn (1985) |
* Appressoria description taken from Alcorn (1994).
Muyocopron atromaculans (Bills & Polishook) Hern.-Restr., J.D.P. Bezerra & Crous, comb. nov. — MycoBank MB828981
Basionym. Mycoleptodiscus atromaculans Bills & Polishook, Mycotaxon 43: 454. 1992.
Typus. USA, New Jersey, Burlington, Wading River, near State Highway 563, endophytic from leaves of Chamaecyparis thyoides, 1991, H. Pond (holotype BPI GB1369, not seen; culture ex-isotype MUCL 34983).
Illustration — See Bills & Polishook (1992a).
Hyphae septate, branched, hyaline to pale olive grey, 1.5–4 μm diam. Conidiomata sporodochium-like, highly irregular, initially consisting of small groups of conidiogenous cells, in aging irregular forming confluent masses of > 50 conidiogenous cells. Conidiogenous cells solitary, often aggregated, globose, subglobose or irregularly ellipsoid, compressed when aggregated, 9.5–14.5 μm diam, with a cylindrical to flared collarette having ragged margins, 2.5–4×2–7.5 μm, pale brown to blackish brown, usually darkest at the base of the collarette, smooth. Conidia aseptate, broadly falcate or lunate, narrowed at both ends to form terminal appendages, with highly refractive cytoplasm, hyaline, smooth, 15.5–22×6.5–8.5 μm; appendages 1–3 μm long (adapted from Bills & Polishook 1992a). Sexual morph unknown.
Culture characteristics — Colonies on OA attaining 60–65 mm diam after 2 wk at 25 °C, mycelium slightly raised, radially plicate, zonate, with some medium buckling and cracking beneath centre of colony, aerial mycelium moderately abundant to sparse at the margin, floccose to minutely hispid, with black conidiomata scattered beneath aerial mycelium over inner third of colony, margin entire, white to pale or medium grey, pale drab grey, pale smoke grey (adapted from Bills & Polishook 1992a).
Habitat — Endophytic fungi from leaves of Chamaecyparis thyoides (Bills & Polishook 1992a).
Distribution — USA.
Notes — Muyocopron atromaculans morphologically resembles Mu. coloratum and Mu. taiwanense. Muyocopron atromaculans, however, has smaller conidia and shorter appendages than Mu. coloratum, and slightly larger conidia than Mu. taiwanense (Table 2). Although, each species is represented by one strain, they differ in host affinity and geographic distribution. Muyocopron atromaculans was isolated as an endophyte from leaves of Chamaecyparis in the USA (Bills & Polishook 1992a), Mu. coloratum is known only from Cattleya in Australia (Alcorn 1994), while Mu. taiwanense is only known from a decaying leaf rachis of Areca in Taiwan (Matsushima 1987). Phylogenetically, Mu. atromaculans is closely related to Mu. geniculatum and Mu. zamiae (Fig. 1).
Muyocopron coloratum (Alcorn) Hern.-Restr., J.D.P. Bezerra & Crous, comb. nov. — MycoBank MB828982; Fig. 4
Fig. 4.

Muyocopron coloratum ex-type CBS 720.94. a–g. Conidiogenous cells; h–i. appressoria; j–r. conidia. — Scale bars: a = 50 μm, others = 10 μm, r applies to j–r.
Basionym. Mycoleptodiscus coloratus Alcorn, Austral. Syst. Bot. 7: 596. 1994.
Typus. AUSTRALIA, Queensland, Biboohra, on leaf spot of Cattleya sp., 16 Jan. 1992, J. Allen & K.R.E. Grice M6076 (holotype BRIP 19988, culture ex-type BRIP 19988 = CBS 720.95).
Hyphae septate, branched, hyaline to brown, 2–5 μm diam. Conidiomata sporodochium-like, varying from a few cells up to moderately large aggregations, sometimes rounded, but generally irregular in outline, dark brown. Conidiogenous cells ampulliform to broadly ellipsoidal, 17–29×13–26 μm, with a cylindrical to flared collarette having ragged margins, 3–10×4–8 μm, mid- to dark brown, often darker around the neck. Conidia aseptate, broadly lunate to inaequilateral oval-ellipsoidal, strongly convex on one side, concave to flattened to more or less convex on other side which often has a distinct median swelling varying in degree of protrusion, 16–34×8–16 μm, with a filiform, unbranched appendage at each end, apical 2–8 μm long, basal 1–6 μm long, 1 μm wide; the basal appendage often inserted eccentrically on the truncate base. Sexual morph unknown.
Culture characteristics — Colonies on OA attaining 80 mm diam after 2 wk at 25 °C, aerial mycelium cottony to hispid, apricot, margin effuse; reverse apricot. On MEA attaining 30 mm diam after 1 wk at 25 °C, aerial mycelium scarce velvety, rust, margin effuse, diffusible pigment after 2 wk apricot; reverse sienna.
Habitat — Cattleya sp. (Alcorn 1994).
Distribution — Australia.
Notes — The size of the structures observed in this study differs from those described in the protologue of Mu. coloratum. The conidiogenous cells were slightly larger with smaller necks and conidia with reduced appendages were longer (Table 2). The LSU sequence of M. coloratum (DQ341499) previously deposited by Thongkantha et al. (2009) was dissimilar to the sequence generated during the present study. Newly generated sequences of the ex-type strain of Mu. coloratum (CBS 720.95) related it to Muyocopron species, and formed an independent lineage, basal to Mu. laterale.
Muyocopron freycineticola Hern.-Restr. & Crous, nom. nov. — MycoBank MB828983
Basionym. Mycoleptodiscus freycinetiae Whitton et al., Fungal Diversity Research Series 21: 251. 2012. Non Muyocopron freycinetiae (F. Stevens & R.W. Ryan) G. Arnaud, Ann. Cryptog. Exot. 4: 88. 1931.
Etymology. Name refers to Freycinetia, the host genus from which this fungus was collected.
Typus. PHILIPPINES, Luzon Island, Quezon Region, Barangay Llabac Real, on decaying leaves of Freycinetia sp., 22 Oct. 1996, S.R. Whitton HKU(M)12794 (holotype IFRD 8995, not seen).
Illustration — See Whitton et al. (2012).
Hyphae brown to pale brown, smooth, septate, branched, irregular, sparse or sometimes abundant. Conidiomata sporodochium-like, superficial, either solitary or aggregated, typically circular, 32–55 μm diam, brown. Conidiogenous cells ampulliform, 9–18×6–12 μm, with a distinct, sometimes thickened collarette, 2–2.5 μm long, aperture 1.5–3 μm diam, brown, smooth. Conidia aseptate, broadly falcate to lunate, hyaline, smooth, 11–15×4–4.5 μm, with a filiform, unbranched appendage at each end; appendages 2–3 μm long (adapted from Whitton et al. 2012). Sexual morph unknown.
Habitat — Decaying leaves of Freycinetia sp. and F. scadens (Whitton et al. 2012).
Distribution — Australia, Philippines.
Notes — Muyocopron freycineticola is similar to Mu. sahnii, but the former is distinguished by the spherical shape of the conidiomata and wider conidia (Whitton et al. 2012).
Muyocopron geniculatum (Alcorn) Hern.-Restr., J.D.P.
Bezerra & Crous, comb. nov. — MycoBank MB828984
Basionym. Mycoleptodiscus geniculatus Alcorn, Austral. Syst. Bot. 7: 598. 1994.
Typus. AUSTRALIA, Queensland, Girraween National Park, on Stypandra glauca, 13 Oct. 1990, V.P. Cooper 55 (holotype BRIP 17274, culture ex-type BRIP 17274 = CBS 721.95).
Illustration — See Alcorn (1994).
Hyphae immersed, 1.5–7 μm diam, aerial hyphae 1.5–6 μm diam. Conidiomata sporodochium-like, varying from a few conidiogenous cells to irregularly aggregations up to 125 μm diam, or larger by confluence, dark brown. Conidiogenous cells ampulliform, 10–15 μm diam, with a cylindrical to flared collarette having ragged margins, 4–6.5 μm diam, sometimes extended into a thick-walled cylindrical neck 3–12×3.5–6.5 μm, circular pore 2–3.5 μm diam, dark brown, smooth. Conidia aseptate, lunate to fusiform or fusoid-ellipsoidal, curved to geniculate with a distinct change in curvature in the upper part, hyaline, smooth, 14–20×5.5–7.5 μm, with a filiform, unbranched, appendage at each end, measuring 4–12.5 μm long (apical) and 1–10 μm long (basal), 0.5–1 μm wide (adapted from Alcorn 1994). Sexual morph unknown.
Culture characteristics — Colonies on OA attaining 90 mm diam after 2 wk at 25 °C, aerial mycelium scarce, cottony to funiculose, buff, margin effuse; reverse buff. On MEA attaining 45–50 mm diam after 1 wk at 25 °C, elevated, aerial mycelium cottony to funiculose, white, margin effuse; reverse dark brown in the centre paler to the periphery.
Habitat — Stypandra glauca (Alcorn 1994).
Distribution — Australia.
Notes — Muyocopron geniculatum is morphologically similar to Mu. sahnii and Mu. zamiae. However, Mu. geniculatum has slightly larger, geniculate conidia with a distinct change in curvature in the upper part, while conidia in Mu. sahnii and Mu. zamiae are smoothly curved (Table 2).
Muyocopron laterale (Alcorn & B. Sutton) Hern.-Restr., J.D.P.
Bezerra & Crous, comb. nov. — MycoBank MB828986; Fig. 5
Fig. 5.
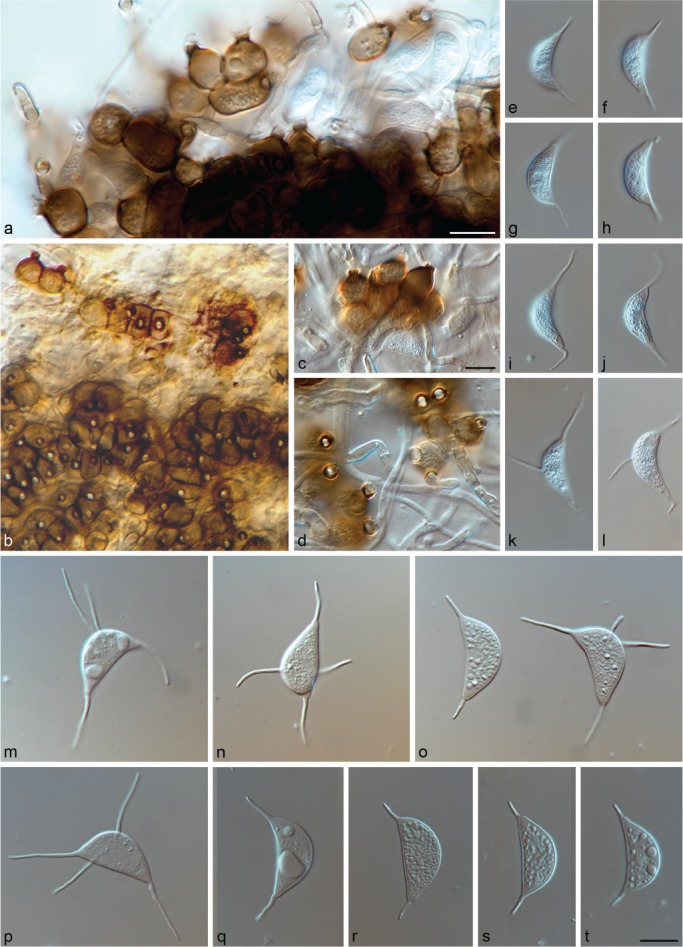
Muyocopron laterale CBS 145315 (a, e–h); CBS 145316 (b–d, i–l), CBS 719.95 (m–t). a–d. Conidiogenous cells; e–t. conidia. — Scale bars: = 10 μm, c applies to b, c; t applies to d–t.
Basionym. Mycoleptodiscus lateralis Alcorn & B. Sutton, Mycol. Res. 94: 564. 1990.
Synonyms. Mycoleptodiscus unilateralis B. Sutton & Alcorn, Mycol. Res. 94: 565. 1990.
Mycoleptodiscus variabilis Alcorn, Austral. Syst. Bot. 7: 599. 1994.
Typus. AUSTRALIA, Queensland, Beerwah, on leaf spot of Alloteropsis semialata, 19 Mar. 1988, J.L. Alcorn 8841a (holotype BRIP 16247, isotype IMI 330181, culture ex-type BRIP 16247 = CBS 141029 = ATCC 200213).
Hyphae thick-walled, septate, branched, dark brown near the conidiomata, pale brown to hyaline when distant, smooth. Conidiomata sporodochium-like, superficial, mid- to dark brown, varying from a few combined conidiogenous cells to large aggregations, sometimes rounded in outline but usually variable in shape and size due to confluence, 25–160(–370)×13–150(–225) μm. Conidiogenous cells ampulliform, 14–24×9–16 μm diam, often with a distinct flared collarette, 3–6.5 μm diam, circular pore 2–3 μm diam, medium to dark brown, smooth. Conidia aseptate, lunate, fusiform, curved, base obtuse to truncate, often guttulate, hyaline, smooth, 15–25.5×5.5–8 μm, with a filiform, unbranched appendage at each end, measuring 1–24 μm long (apical), 4–22.5 μm long (basal), 0.5–1 μm wide; often with 1–2 lateral appendages originate on the convex side of the conidium in a median or slightly supramedian position, 7–26×0.5–1 μm. Sexual morph unknown.
Culture characteristics — Colonies on OA attaining 70–90 mm diam after 2 wk at 25 °C, zonate with aerial mycelium scarce, cottony, or glabrous, in the centre buff, rosy buff to apricot, paler to the periphery, margin effuse; reverse buff, brick, apricot in the centre paler to the periphery. On MEA attaining 30–60 mm diam after 1 wk at 25 °C, elevated, aerial mycelium cottony to hispid, white, buff, pale grey, margin effuse; reverse saffron, apricot, umber, dark umber in the centre paler to the periphery.
Habitat — On leaves of monocotyledons plants such as Alloteropsis semialata, Chlorophytum capense, and Dianella congesta (Sutton & Alcorn 1990, Alcorn 1994), endophyte from Banksia verticillata (Andrioli et al. 2012, as M. indicus) and Opuntia ficus-indica (Bezerra et al. 2012), fresh water foam (Ramesh & Vijaykumar 2005), Homo sapiens (Padhye et al. 1995, Garrison et al. 2008, Dewar & Sigler 2010, Koo et al. 2012, as M. indicus), Canis lupus subsp. familiaris (Metry et al. 2010, as M. indicus), and Felis catus (Hull et al. 1997).
Distribution — America, Australia, India.
Additional materials examined. AUSTRALIA, Queensland, Peregian beach, on leaf spot of Dianella congesta, 28 Jan. 1990, J.L. Alcorn 9007 (BRIP 16983 = CBS 719.95 = ATCC 96451, culture ex-type of M. variabilis); Queensland, Peregian beach, on leaf spot of Dianella congesta, 3 May 1992, J.L. Alcorn 92/1764 (BRIP 20066 = CBS 141033). – BRAZIL, Paraíba, Santa Teresinha, Tamanduá farm (S07°1.524 W037°23.518), as endophyte from Poincianella pyramidalis, May 2013, J.D.P. Bezerra (URM 7802); Pernambuco, Itaíba, Curral Velho farm, as endophyte from Opuntia ficus-indica, May 2013, J.D.P. Bezerra (URM 7801 = isolate PF108). – CANADA, Vancouver General Hospital, R. Rennie, aspirate ex joint from Homo sapiens, unknown date, unknown collector (CBS 127677 = UAMH 10746, as M. indicus). – INDIA, Kerala, Trivandrum, on leaves of Chlorophytum capense, 15 Jan. 1988, K. Nayar (IMI 324533 culture ex-type of M. unilateralis). – USA, Alabama, H. sapiens (tissue-knee wound), Mar. 2012, D.A Sutton (UTHSC DI17-18 = CBS 145310); Florida, H. sapiens (tissue-foot), Dec. 2013, D.A Sutton (UTHSC DI 17-23 = CBS 145315); Florida, H. sapiens (unknown clinical specimen), Sept. 2011, D.A. Sutton (FMR 13797); Louisiana, H. sapiens (tissue toe), Mar. 2013, D.A Sutton (UTHSC DI 17-21 = CBS 145313); Massachusetts, H. sapiens (fascial biopsy), June 2008, D.A Sutton (UTHSC DI 17-17 = CBS 145309); South Carolina, H. sapiens (tissue-foot), May 2013, D.A Sutton (UTHSC DI 17-22 = CBS 145314); Texas, H. sapiens (eye), Oct. 2012, D.A Sutton (UTHSC DI 17-19 = CBS 145311); Texas, H. sapiens (eye), Oct. 2012, D.A Sutton (UTHSC DI 17-20 = CBS 145312); Texas, H. sapiens (tissue-arm), June 2015, D.A Sutton (UTHSC DI 17-24 = CBS 145316).
Notes — Sutton & Alcorn (1990) introduced M. lateralis and M. unilateralis for species similar to M. indicus (Sahni 1968) but distinguished by larger conidia with larger apical appendages and the presence of lateral appendages (Table 2). Later, Alcorn (1994) introduced M. variabilis, another species with lateral appendages, differing from M. lateralis and M. unilateralis mainly by the size of the conidial appendages and conidiomata characteristics. However, these morphological features are not always constant among strains and can be influenced by external factors. In our molecular analyses, the ex-type strains of M. lateralis, M. unilateralis, and M. variabilis grouped in a highly supported subclade (89/0.99) in Muyocopron, together with strains previously identified as ‘M. indicus’ and several clinical isolates of ‘Mycoleptodiscus sp.’ (Fig. 1), from what we concluded that they are conspecific, with M. lateralis having priority.
All the clinical isolates included in this study grouped in Mu. laterale clade. The first report of Muyocopron (as Mycoleptodiscus) as etiologic agent of phaeohyphomycosis was attributed to M. indicus (Padhye et al. 1995). Since then, several authors have reported clinical cases of M. indicus in human and other mammals (Garrison et al. 2008, Dewar & Sigler 2010, Metry et al. 2010). However, the LSU and ITS sequences data from those strains indicated close affinities with Mu. laterale. Additionally, some of those strains showed conidia with lateral appendages (Dewar & Sigler 2010, Metry et al. 2010). Besides M. indicus, only one case of phaeohyphomycosis was reported as caused by M. lateralis in a cat (Hull et al. 1997), while Koo et al. (2012) were unable to identify at the species level one strain isolated from a man with glioblastoma multiforme, due to inconsistencies between the morphological and molecular data.
Muyocopron sahnii Hern.-Restr. & Crous, nom. nov. — MycoBank MB828985; Fig. 6
Fig. 6.

Muyocopron sahnii holotype IMI 108220. a. Holotype details; b–c. leaf spot with sporodochia on the substrate; d–g. sporodochia; d–e. top view; f. down view; g. lateral view; h. conidiogenous cell with collarette; i–l. conidia close to sporodochial; m. conidia. — Scale bars: d = 50 μm, others = 10 μm, f applies to e, f, l applies to i–l.
Basionym. Amerodiscosiella indica V.P. Sahni, Mycopathol. Mycol. Appl. 36: 277. 1968.
Synonyms. Mycoleptodiscus indicus (V.P. Sahni) B. Sutton, Trans. Brit. Mycol. Soc. 60: 528. 1973.
Pucciniopsis guaranitica Speg., Anales Soc. Ci. Argent. 26: 74. 1888. pro parte.
Etymology. Name after the Indian mycologist V.P. Sahni.
Typus. INDIA, Jabalpur, on Ixora parviflora, 1964, V.P. Sahni (IMI 108220 holotype of Amerodiscosiella indica V.P. Sahni).
Hyphae septate, pale brown to brown, smooth. Conidiomata sporodochium-like, superficial, varying from a few combined cells to large aggregations, sometimes rounded in outline 54–71 μm diam, but usually variable in shape and size due to confluence, 56–135×53–256 μm, dark brown, smooth. Conidiogenous cells ampulliform, 4.5–10.5 μm diam, often with a distinct flared collarette, 3×1.5–2.5 μm, brown, smooth. Conidia aseptate, lunate, fusiform, curved, base obtuse to truncate, often guttulate, 13.5–14.5×5–6 μm, hyaline, smooth, often guttulate, with a reduced appendage. Sexual morph unknown.
Habitat — On leaves of several mono- and dicotyledonous plants (Sutton 1973, Sutton & Hodges 1976, Bezerra & Ram 1986), the fruit of Passiflora edulis var. flavicarpa and P. edulis (Sutton & Hodges 1976).
Distribution — America, India, New Zealand (Sutton 1973).
Notes — Amerodiscosciella indica was first described from India causing leaf spots on Ixora parviflora. It was characterised by pycnidial, ostiolate conidiomata, and conidia with appendages at each end (Sahni 1968, Table 2). Sutton (1973) examined the type material (IMI 108220) and additional specimens from different countries (Brunei, Cuba, India, New Zealand, Nigeria, USA, and Venezuela) and host plants (Cocos yatay, Cordyline, Chlorophytum comosum, Grewia asiatica, Hippeastrum, Hymenocallis arenicola, Roupellia grata, and Zamia) and transferred this species to Mycoleptodiscus. However, in the emended description provided, Sutton (1973) described the conidiomata as sporodochial instead of pycnidial and larger conidia, with appendages at one or both ends (Table 2), differing considerably from the protologue of A. indica (Sahni 1968). During the present study, we re-examined the holotype of A. indica and confirmed that the conidiomata were sporodochium-like and the few conidia observed were larger than the measurements provided in the protologue (Sahni 1968) but smaller than those from Sutton (1973), with reduced conidial appendages (Fig. 6).
Since the epithet indicum is preoccupied in Muyocopron, a new name is necessary for this fungus. Unfortunately, no ex-type strain of A. indica was available and the phylogenetic relationships of this species with members of Muyocopron still needs to be assessed. Furthermore, the geographic distribution and host preferences of Mu. sahnii are not completely known.
Muyocopron taiwanense (Matsush.) Hern.-Restr. & Crous,
comb. nov. — MycoBank MB828987
Basionym. Mycoleptodiscus taiwanensis Matsush., Matsushima Mycol. Mem. 5: 21. 1987.
Typus. TAIWAN, Nan-Jen-Shan, on rotten leaf rachis of Areca catechu, 1986 (holotype MFC 6T720, not seen).
Illustration — See Matsushima (1987).
Hyphae branched, septate, hyaline to pale brown, 1.5–4 μm wide. Conidiomata sporodochium-like, irregular, brown. Conidiogenous cells densely aggregated, ampulliform, apex with a protruding neck, 6–12×7–14 μm, brown to dark brown at the neck, smooth. Conidia aseptate, broadly falcate, 12–21×5.5–7 μm long (including appendages), hyaline, smooth, with an unbranched appendage at each end; apical appendage acute, 1–3 μm long; basal appendage inserted obliquely exogenously, 1–3 μm long (adapted from Matsushima 1987). Sexual morph unknown.
Habitat — Rotten leaf rachis of Areca catechu (Matsushima 1987).
Distribution — Taiwan.
Notes — Muyocopron taiwanense is similar to species relocated to Muyocopron rather than Mycoleptodiscus (see Mu. atro-maculans).
Muyocopron zamiae Hern.-Restr. & Crous, sp. nov. — MycoBank MB828988; Fig. 7
Fig. 7.
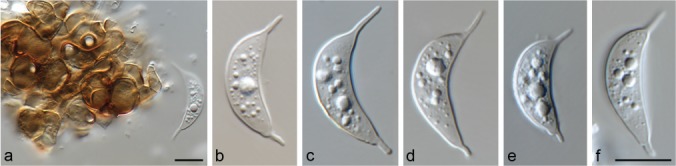
Muyocopron zamiae sp. nov. ex-type CBS 203.71. a. Conidiogenous cells and conidia; b–f. conidia. — Scale bars: = 10 μm, f applies to b–f.
Etymology. Name refers to Zamia, the plant genus from which this fungus was collected.
Typus. USA, Florida, Zamia fisheri, date unknown, isol. S.A. Alfieri Jr., No. 070-2273 (holotype CBS H-23882, culture ex-type CBS 203.71).
Hyphae septate, branched, dark brown near the conidiomata, pale brown to hyaline when distant, smooth, 1.5–4 μm wide. Conidiomata sporodochium-like, superficial, varying from a few combined cells to large aggregations, variable in shape and size due to confluence, mid- to dark brown. Conidiogenous cells globose to ampulliform, 7.5–14×8.5–12 μm, evanescent, sometimes with a flared collarette, 1×2.5–3 μm, medium to dark brown, smooth. Conidia aseptate, lunate, fusiform, curved, 16–20×5.5–6.5 μm, hyaline, smooth, guttulate, with a filiform, unbranched appendage at each end, apical 2.5–6 μm long, basal 0.5–5 μm long. Appressoria not observed. Sexual morph unknown.
Culture characteristics — Colonies on OA attaining 70 mm diam after 2 wk at 25 °C, aerial mycelium scarce, cottony to hispid, smoke grey in the centre greyish sepia to the periphery, margin effuse; reverse olivaceous black. On MEA attaining 65–70 mm diam after 1 wk at 25 °C, elevated, aerial mycelium cottony to hispid, buff, cinnamon close to the agar, margin effuse; reverse sienna in the centre umber to the periphery.
Habitat — Zamia fisheri, Z. integrifolia.
Distribution — USA.
Additional material examined. USA, Florida, leaf spot and necrotic tip on Z. integrifolia (= Z. floridana), date unknown, isol. S.A. Alfieri Jr. No. 070-2288, CBS 202.71.
Notes — Muyocopron zamiae is known from Z. fisheri and Z. integrifolia in the USA, causing oval leaf spots that are more evident in older leaves. Although morphologically similar to Mu. sahnii, conidia of Mu. zamiae are larger (Table 2). Phylogenetically, it formed a subclade (100/0.99) with Mu. atromaculans and Mu. geniculatum (Fig. 1). Muyocopron zamiae can be distinguished from Mu. atromaculans by having slender conidia with longer conidial appendages, and from Mu. geniculatum, its closest relative, by having smoothly curved conidia with shorter conidial appendages (Table 2).
Mycoleptodiscus Ostaz., Mycologia 59: 970. 1968 (1967)
Synonyms. Leptodiscus Gerd., Mycologia 45: 552. 1953.
Pucciniopsis Speg., Anales Soc. Ci. Argent. 26: 74. 1888. pro parte.
Type species. Mycoleptodiscus terrestris (Gerd.) Ostaz.
Conidiomata sporodochium-like, superficial, solitary or confluent, developing radially from central cell to form a thin stroma, circular to irregular in outline, dark brown, comprised of thick-walled phialides. Conidiogenous cells enteroblastic, monophialidic, ampulliform to doliiform, brown, textura globulosa in face view, evanescent; conidiogenous locus circular, perforating the upper cell wall, without a collarette. Conidia 0–2-septate, cylindrical, hyaline, smooth, with an apical and sometimes a basal, unbranched, straight, appendage, base truncated, apex pointed. Sclerotia when present, spherical, black. Sexual morph unknown.
Notes — Mycoleptodiscus is limited to species with sporodochium-like conidiomata, that have conidiogenous cells lacking collarettes, cylindrical conidia with one or two appendages, and sclerotia that are often produced both on the natural substrate and in culture (Ostazeski 1967).
According to our phylogenetic analyses, Mycoleptodiscus includes two phylogenetic species morphologically indistinguishable, namely the type of the genus, M. terrestris, and one new species, M. suttonii (Fig. 1). Other species previously included in the genus, such as M. brasiliensis, M. disciformis, M. minimus, and M. stellatosporus (strictly based on morphological criteria) and M. endophyticus (introduced with only molecular data), are treated here as doubtful species.
Mycoleptodiscus suttonii Hern.-Restr., J.D.P. Bezerra & Crous, sp. nov. — MycoBank MB828989; Fig. 8
Fig. 8.
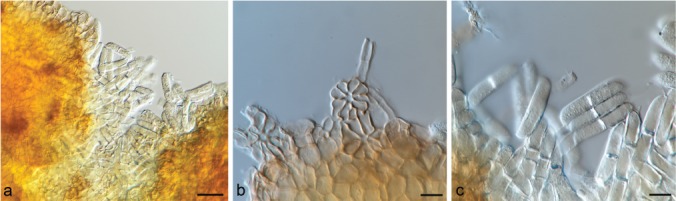
Mycoleptodiscus suttonii sp. nov. holotype CBS H-14851. a. Sporodochia and conidia; b. sporodochia; c. conidia. — Scale bars: a = 50 μm, b–c = 10 μm.
Etymology. Named after the British mycologist, Brian C. Sutton, who described several species related to Mycoleptodiscus.
Typus. BRAZIL, Itabuna, Asha Ram, Centro de Pesquisas do Cacau, on roots of Theobroma cacao, unknown date (dep. 2 Dec. 1971), A. Ram (holotype designated here CBS H-14851, culture ex-type CBS 276.72).
A dry sporulating culture on CMA with a twig consists of scattered, circular conidiomata sporodochia-like and 1-septate, cylindrical conidia, 33–36×7–7.5 μm, pale brown, with appendages at both ends, up to 19 μm, forming an orange mass.
Cultures from ex-type and CBS 141030 remain sterile. Mycoleptodiscus suttonii (CBS 276.72) differs from its closest phylogenetic neighbour, M. terrestris (CBS 231.53), by unique fixed alleles in four loci based on alignments of the separate loci deposited in TreeBASE (23523): ITS positions 171 (A), 192 (T), 197 (C), 201 (T), 287 (–), 299 (T), 719 (A), and 745 (T); LSU positions 506 (T), 514 (T), 541 (T), 697 (T), 698 (T), and 740 (A); rpb2 positions 76 (T), 88 (C), 100 (T), 124 (A), 133 (G), 163 (T), 175 (C), 190 (T), 214 (G), 223 (C), 250 (G), 253 (C), 289 (T), 301 (T), 307 (A), 313 (A), 328 (C), 349 (G), 388 (A), 391 (C), 394 (C), 397(G), 451 (T), 457 (A), 460 (T), 508 (C), 511 (T), 526 (T), 547 (G), 556 (C), 581 (C), 589 (C), 592 (G), 595 (G), 616 (C), 634 (G), 637 (T), 667 (T), 673 (A), 682 (G), 689 (C), 690 (C), 748 (G), 790 (A), 808 (T), 811 (T), 814 (C), 844 (G), and 862 (T); tef1 positions 44 (T), 160 (T), 214 (T), 263 (C), 264 (A), 322 (C), 344 (A), 358 (T), 369 (G), 371 (C), 411 (G), 423 (C), 426 (C), 427 (A), 428 (G), 432 (T), 438 (T), 457 (G), 463 (C), and 472 (G).
Culture characteristics — Colonies on OA attaining 80–90 mm diam after 2 wk at 25 °C, aerial mycelium, cottony to hispid, white, pale olivaceous grey to olivaceous black in the centre buff to the periphery, margin effuse; reverse olivaceous buff, to olivaceous grey in the centre paler to the periphery. On MEA attaining 90 mm diam after 1 wk at 25 °C, slightly elevated, aerial mycelium cottony, white to olivaceous buff, margin effuse; reverse cinnamon to umber.
Habitat — Sannantha (Baeckea) virgata and Theobroma cacao.
Distribution — Australia, Brazil.
Additional material examined. AUSTRALIA, Queensland, Cairns, on stem lesion of Sannantha (Baeckea) virgata, 6 Mar. 1990, J.L Alcorn LF90/985, BRIP 16943a = CBS 141030 = ATCC 200215.
Notes — The conidia observed in the holotype of M. suttonii are very similar to those described in M. terrestris (Table 2). Mycoleptodiscus suttonii is mainly proposed based on the phylogenetic differences using sequences of four loci. The sequences generated from two cultures placed it in a highly supported clade (100/0.99), related to the M. terrestris clade (Fig. 1). After using several culture media, different plant tissues, and incubation conditions, no sporulation was observed in culture.
Mycoleptodiscus terrestris (Gerd.) Ostaz., Mycologia 59:970. 1967
Basionym. Leptodiscus terrestris Gerd., Mycologia 45: 552. 1953.
Synonym. Mycoleptodiscus sphaericus Ostaz., Mycologia 59: 971. 1967.
Typus. USA, Virginia, Illinois, Urbana, Agronomy South Farm of the Illinois Agricultural Experiment Station, isolated from a disease root of Trifolium pratense, 1951, J.W. Gerdemann (lectotype ILL31238 of Leptodiscus terrestris, iso-lectotype BPI 403851, not seen; culture ex-type CBS 231.53).
Illustrations — See Gerdemann 1953, Ostazeski 1967.
Conidiomata sporodochium-like, superficial, developing radially from central cell to form a thin stroma, one cell layer thick, peltate, often fusing to form irregular plates 100–200×86–144 μm, pale to dark brown. Conidiogenous cells evanescent. Conidia 1(–2)-septate, cylindrical, 20–43×4.5–9 μm, hyaline, with pale yellow contents, often becoming brown as the spores age, usually with a filamentous, unbranched appendage at each end, 8–18 μm long, or lacking one appendage, produced in a mucous, pale yellow to brown mass. Sclerotia spherical to fusiform, black, up to 1 mm diam (adapted from Gerdemann 1953, Ostazeski 1967).
Culture characteristics — Colonies on OA attaining 90 mm diam after 2 wk at 25 °C, aerial mycelium scarce, cottony, white, glabrous in the centre, greyish yellow green, if sclerotia are produced the colony is glabrous with olivaceous grey spots, margin effuse, diffusible pigment yellow green; reverse greyish yellow green, or pale grey olivaceous to black. On MEA attaining 90 mm diam after 1 wk at 25 °C, slightly elevated, aerial mycelium cottony, white to mouse grey, margin effuse; reverse cinnamon, umber or olivaceous grey.
Habitat — Root rot of Trifolium pratense (Gerdemann 1953), on decaying roots of Lotus corniculatus (Ostazeski 1967), black pepper roots (Watanabe et al. 1997), endophytic in Myrangium spicatum (Shearer 2001), M. propinquum and Potamogeton cheesemanii (Hofstra et al. 2012).
Distribution — Dominican Republic, New Zealand, USA.
Additional material examined. USA, on decaying roots of Lotus corniculatus, 1967, S.A. Ostazeski (culture ex-type of Mycoleptodiscus sphaericus IMI 159038 = ATCC 18104).
Notes — Mycoleptodiscus terrestris was first introduced as L. terrestris for a fungus causing root rot in red clover and other Fabaceae in the USA (Gerdemann 1953). The genus, however, was invalid because the name had been previously used for a flagellate alga, and Ostazeski (1967) proposed Mycoleptodiscus, to accommodate L. terrestris, and introduced a new species, M. sphaericus. Mycoleptodiscus sphaericus differed from M. terrestris only in its sclerotial size, being larger in M. terrestris, and the presence of only apical conidial appendages in M. sphaericus (Table 2). Phylogenetically, the ex-type strains of both species are grouped in the same clade, suggesting that those subtle morphological differences are intraspecific variations. In our study, both strains remained sterile in culture and the production of sclerotia was only observed in IMI 159038.
Neomycoleptodiscus Hern.-Restr., J.D.P. Bezerra & Crous, gen. nov. — MycoBank MB829829.
Etymology. Name reflects a morphological similarity with the genus Mycoleptodiscus.
Type species. Neomycoleptodiscus venezuelense Hern.-Restr., J.D.P. Bezerra & Crous.
Hyphae smooth, hyaline to pale brown. Conidiomata sporodochium-like, superficial, often fusing to form irregular plates, brown. Conidiogenous cells ampulliform to doliiform, angular in face view, dark brown, smooth, with a circular aperture situated in the upper side. Conidia 1-septate, cylindrical, hyaline, guttulate, with a filamentous appendage at each end, produced in a mucous pale yellow to brown mass. Sclerotia not observed. Appressoria not observed.
Neomycoleptodiscus venezuelense Hern.-Restr., J.D.P.
Bezerra & Crous, sp. nov. — MycoBank MB828990; Fig. 9
Fig. 9.
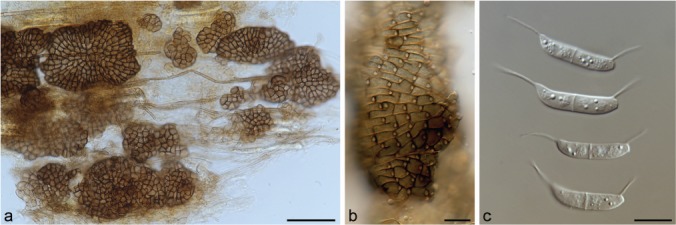
Neomycoleptodiscus venezuelense gen. & sp. nov. ex-type CBS 100519. a–b. Sporodochia and conidiogenous cells; c. conidia. — Scale bars: a = 50 μm, others = 10 μm.
Etymology. Named after the country, Venezuela, where it was found.
Typus. VENEZUELA, on leaf litter of Gyranthera caribensis, 25 Nov. 1997, R.F. Castañeda-Ruiz (holotype designated here CBS H-23881, culture ex-type CBS 100519).
Hyphae septate, smooth, hyaline to pale brown. Conidiomata superficial, often fusing to form irregular plates, 24–125×17–104 μm, brown. Conidiogenous cells ampulliform to doliiform, textura angularis in face view, 5–11×4–6.5 μm, dark brown, smooth, with a circular aperture situated in the upper side, 1–2 μm. Conidia 1-septate, cylindrical, 18–27×3–5 μm, hyaline, guttulate, with a filamentous appendage at each end, straight, 6.5–13 μm long, produced in a mucous pale yellow to brown mass. Sclerotia not observed. Appressoria not observed.
Culture characteristics — Colonies on OA attaining 20 mm diam after 1 wk at 25 °C, aerial mycelium scarce, cottony, zonate centre pale mouse grey, orange, pale luteous to buff to the periphery, margin effuse; reverse orange in the centre, pale luteous to buff to the periphery. On MEA attaining 24–27 mm diam after 1 wk at 25 °C elevated, aerial mycelium velvety, mouse grey in the centre, darker to the periphery, margin effuse; reverse iron grey.
Habitat — Leaf litter of Gyranthera caribensis.
Distribution — Venezuela.
Notes — Based on phylogenetic inference in this study, a strain previously identified as M. terrestris, formed a single lineage, close to ‘M. endophyticus’ and Neocochlearomyces chromolaenae, and distant to the Mycoleptodiscus s.str. clade (Fig. 1). Therefore, the monotypic genus Neomycoleptodiscus is hereby introduced. Neocochlearomyces can be distinguished from Neomycoleptodiscus by the presence of a setiform conidiophore bearing an apical fan-like conidiogenous region with inconspicuous loci and aseptate conidia (Crous et al. 2018). Neomycoleptodiscus is distinguished from Mycoleptodiscus by subtle morphological differences. In Mycoleptodiscus, conidiogenous cells are brown, with textura globulosa in face view with conidia with recurved ends (Hofstra et al. 2012), while in Neomycoleptodiscus conidiogenous cells are dark brown, and conidia are curved at the apex, and truncate at the base. Neomycoleptodiscus venezuelense is similar to M. disciformis, but it has smaller conidial appendages (Table 2).
Sordariomycetes, Magnaporthales, Magnaporthaceae
Omnidemptus P.F. Cannon & Alcorn, Mycotaxon 51: 483. 1994
Type species. Omnidemptus affinis P.F. Cannon & Alcorn.
Hyphae hyaline to pale brown, smooth, septate. Ascomata superficial, perithecial, ostiolate, pyriform, with a long neck; peridium of textura angularis composed of dark brown cells; hamathecium composed of thin-walled, septate paraphyses. Asci bitunicate, 8-spored, cylindric-clavate, short-stalked, with an obtuse apex, apical pore. Ascospores 1–3-septate, biseriate, fusiform, centrally swollen, hyaline. Conidiomata absent or sporodochium-like, irregularly shaped, aggregations, pale to dark brown. Conidiogenous cells phialidic, ampulliform or elongated, cylindrical, clavate or ellipsoid, dark brown, smooth, with a circular aperture enclosed by a cylindrical to flared collarette. Conidia dry, 1–2(–3)-septate, commonly asymmetrically 2-septate, falcate, hyaline, guttulate. Appressoria entire or lobed, mid-olivaceous brown, smooth (adapted from Cannon & Alcorn 1994).
Notes — Cannon & Alcorn (1994) introduced Omnidemptus as the sexual morph of Mycoleptodiscus, and described the asexual morph of M. affinis. Based on similarities of the ascomata and ascus features, the presence of appressoria, and the affinity for monocotyledons host, the authors suggested that Omnidemptus was related to Buergenerula, Gaeumannomyces, and Magnaporthe (Cannon & Alcorn 1994). After the multi-locus phylogenetic analyses, Omnidemptus was shown to belong to the Magnaporthaceae (Luo & Zhang 2013, Klaubauf et al. 2014). Subsequently, many authors assumed that Mycoleptodiscus was also phylogenetically positioned in Magnaporthaceae, because of the connection with Omnidemptus.
In our study phylogenetic inferences clearly showed that Omnidemptus is different from Mycoleptodiscus, the latter is placed in the Muyocopronaceae (Dothideomycetes). The phylogenetic inference using representative sequences of genera in Magnaporthaceae (Fig. 2), resolved Omnidemptus as a fully supported independent lineage. Omnidemptus has ascospores narrowly fusoid and its asexual morph is mycoleptodiscus-like. Omnidemptus is hereby formally separated from Mycoleptodiscus, and M. lunatus is transferred to Omnidemptus.
Omnidemptus affinis P.F. Cannon & Alcorn, Mycotaxon 51: 483. 1994 — Fig. 10
Fig. 10.
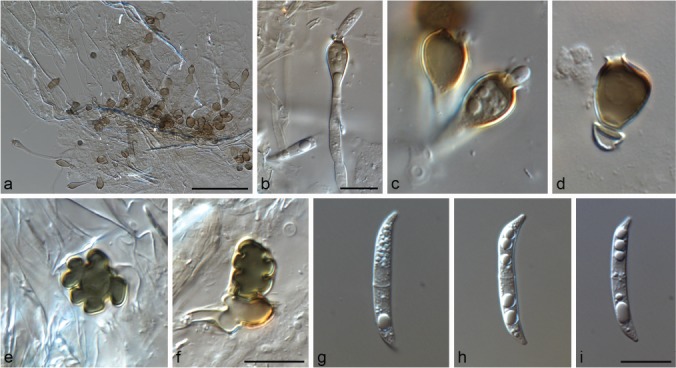
Omnidemptus affinis ex-type CBS 141031. a–d. Conidiogenous cells; e–f. appressoria; g–i. conidia. — Scale bars: a = 50 μm, others = 10 μm, f applies to c–f, i applies to g–i.
Synonym. Mycoleptodiscus affinis P.F. Cannon & Alcorn, Mycotaxon 51: 485. 1994.
Typus. AUSTRALIA, Queensland, Woorford, Stony Creek State Forest, on leaf spot of Panicum effusum, 19 May 1990, V.P. Cooper (holotype BRIP 17195a, isotype IMI 353435, culture ex-type BRIP 17195a = CBS 141031 = ATCC 200212).
Illustration — See Cannon & Alcorn (1994).
Hyphae hyaline to pale brown, smooth, septate, 2–5.5 μm diam. Ascomata superficial perithecial, ostiolate, pyriform, body 145–205 μm diam, dark brown and glabrous, neck 100–250 μm long, 80–110 μm wide at the base and tapering gradually to 40–60 μm wide at the apex, dark brown and densely hairy, with hairs up to 60×2 μm; peridium composed of several layers of dark brown fairly thick-walled, angular cells, 6–10 μm diam; hamathecium with paraphyses 3–6 μm diam, very thin-walled, septate, sometimes deliquescing at maturity, periphyses not observed. Asci cylindric-clavate, short-stalked, sometimes evanescent at the base, fairly thick-walled but without discernible layers, the apex obtuse, with an apical pore 2–3 μm diam and c. 4 μm deep, with a small ring at the base which stains dark blue in Melzer’s reagent, usually 8-spored but not infrequently 4-spored, 54–68×10–13 μm. Ascospores usually 1–3-septate, the medium septum usually placed slightly below the widest point, biseriate, at first narrowly fusiform, sometimes with the end slightly swollen, the central portion usually swelling into an ellipsoidal central bulge, thin-walled, hyaline, without gelatinous sheaths or appendages. Conidiomata absent or sporodochium-like, irregularly shaped, 50–160 μm diam. Conidiogenous cells ampulliform, 7–12 μm diam, or elongated, cylindrical, clavate or ellipsoid, 11–22×6–10 μm, rounded to somewhat angular in face view, dark brown, smooth, with a circular aperture 2–3 μm diam enclosed by a cylindrical to flared collarette 3–4 μm diam. Conidia 1–2(–3)-septate, commonly asymmetrically 2-septate, with the first septum median and the second in the upper half, occasionally in the lower half, falcate, thin-walled, bluntly pointed at the extremities, the base sometimes with an abrupt change in contour to short pedicel-like extension, lacking polar or lateral appendages, 21–30×3–4 μm, hyaline, guttulate, smooth. Appressoria developed by germinating conidia, with outline entire or usually moderately to deeply lobed, 7.5–14×5–8.5(–11) μm and with germ pore 1.8–2 μm diam, mid-olivaceous brown, smooth (adapted from Cannon & Alcorn 1994).
Habitat — On Panicum effusum (Cannon & Alcorn 1994).
Distribution — Australia.
Additional material examined. AUSTRALIA, Queensland, Woodford, Stony Creek State Forest, on leaf spot of Panicum effusum, 19 May 1990, V.P. Cooper (culture ex-type of Mycoleptodiscus affinis BRIP 17195b = CBS 141032).
Notes — Omnidemptus affinis resembles O. lunatus in having septate conidia without appendages. Nevertheless, O. lunatus has conidia with a middle septum, which generally taper gradually towards the base (Sutton & Alcorn 1985), whereas in O. affinis conidia are narrowed more or less abruptly at the extremities and commonly 2-septate (Table 2). Furthermore, they are slightly narrower than those of O. lunatus.
Omnidemptus graminis Hern.-Restr., Gené & Guarro, sp. nov. — MycoBank MB828991; Fig. 11
Fig. 11.
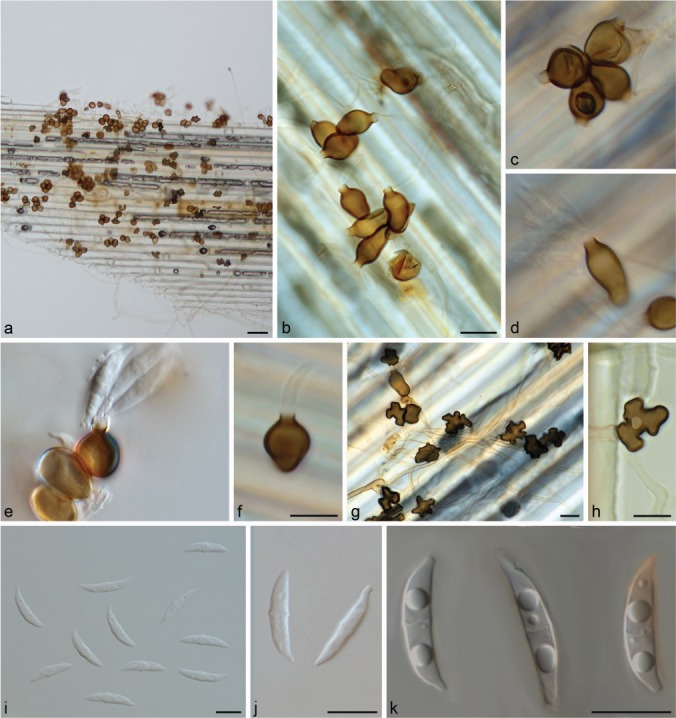
Omnidemptus graminis sp. nov., holotype CBS H-21887. a. Conidiogenous cells and appressoria in natural substrate; b–f. conidiogenous cells; g–h. appressoria; i–k. conidia. — Scale bars: a = 50 μm, others = 10 μm, f applies to c–f.
Etymology. Name refers to the host family, Gramineae (= Poaceae), from which this species was collected.
Typus. SPAIN, Navarra, Robledal de Orgi, leaf of unidentified grass, Mar. 2012, M. Hernández-Restrepo & J. Capilla (holotype designated here CBS H-21887, culture ex-type CBS 138107 = FMR 12415).
Hyphae septate, hyaline to pale brown, smooth, 1–3 μm wide. Conidiomata absent or sporodochium-like, irregularly shaped, effuse, punctiform, dark brown to black. Conidiogenous cells ampulliform, subglobose, angular in face view, 10–14 μm diam, brown, smooth, with a cylindrical collarette, 1×3 μm. Conidia (0–)1-septate, falcate, bluntly pointed at the ends, 11–23×3–4 μm, hyaline, guttulate. Appressoria brown, smooth, multi-lobed, 10–15×7.5–10 μm, with 1–2 germ pores, 1–3 μm diam. Sexual morph unknown.
Culture characteristics — Colonies on OA attaining 30–40 mm diam after 1 wk at 25 °C, zonate, centre cottony, pale mouse grey, periphery glabrous, mouse grey, margin effuse; reverse mouse grey. On MEA attaining 18–20 mm diam after 1 wk at 25 °C, elevated, cottony, vinaceous buff, margin rhizoid; reverse black in the centre, white to the periphery.
Habitat — On grass leaves.
Distribution — Spain.
Notes — Omnidemptus graminis is distinguished from O. affinis and O. lunatus by its smaller conidia (Table 2).
Omnidemptus lunatus (B. Sutton & Alcorn) Hern.-Restr., J.D.P. Bezerra & Crous, comb. nov. — MycoBank MB828992
Basionym. Mycoleptodiscus lunatus B. Sutton & Alcorn, Trans. Brit. Mycol. Soc. 84: 441. 1985.
Typus. AUSTRALIA, Queensland, Peregian Beach, on leaf spot of Carpobrotus glaucescens, 1982, J.L. Alcorn 8231b (holotype IMI 271703, not seen; culture ex-type IMI 271703 = BRIP 13852).
Illustration — See Sutton & Alcorn (1985).
Conidiomata sporodochium-like, flat, effuse, irregular and variable in shape, sometimes linear, consisting of a few to 30 grouped conidiogenous cells, pale to medium brown. Conidiogenous cells ampulliform to doliiform or sometimes lageniform, 10.5–13×6.5–11 μm, pale brown, smooth, the circular aperture 1.5–2 μm diam, surrounded by a distinct, ragged collarette, 3–4.5 μm diam. Conidia medially 1-septate, falcate, 24.5–32×3.5–4.5 μm, tapered gradually to the obtuse to truncate base, apex slightly prolonged but not constituting an appendage, hyaline, often guttulate (adapted from Sutton & Alcorn 1985). Sexual morph unknown.
Habitat — On leaves of Carpobrotus glaucescens (Sutton & Alcorn 1985).
Distribution — Australia.
Notes — Omnidemptus lunatus is morphologically similar to asexual morphs of Omnidemptus in having conidia lacking appendages, and conidiogenous cells with a distinct collarette. The phylogenetic relationship of O. lunatus will require assessment in the future.
EXCLUDED OR DOUBTFUL SPECIES
Mycoleptodiscus brasiliensis B. Sutton & Hodges, Nova Hedwigia 27: 694. 1976
Typus. BRAZIL, Maranhão, São Luís, on dead leaves of Eucalyptus sp., 24 June 1975, C.S. Hodges (holotype IMI 196481e, not seen).
Illustration — See Sutton & Hodges (1976).
Hyphae intracellular, septate, hyaline to pale brown, branched. Conidiomata sporodochium-like, superficial, 30–45 μm diam, dark brown. Conidiogenous cells ampulliform to doliiform or cylindrical, 11–17.5×5–11.5 μm, dark brown, smooth, each with a single distinct circular aperture in the upper wall and a flared or recurved collarette up to 3 μm long, 6 μm wide. Conidia medially 1-septate, cylindrical, straight, base obtuse, apex rounded, 17–19×4–4.5 μm, hyaline, guttulate, with a subapical, unbranched and recurved appendage, 19–27 μm long. Sexual morph unknown (adapted from Sutton & Hodges 1976).
Habitat — On dead leaves of Eucalyptus saligna and Eucalyptus sp. (Sutton & Hodges 1976) and Quercus xalapensis (Abarca et al. 2006).
Distribution — Brazil, Mexico, USA.
Notes — Mycoleptodiscus brasiliensis might belong to a different genus since the conidiogenous cells have a flared collarette and conidia are cylindrical tapering to an obtuse base with only one subapical recurved appendage. These morphological characters do not fit with the Mycoleptodiscus concept.
Mycoleptodiscus disciformis Matsush., Matsushima Mycol. Mem. 7: 58. 1993
Typus. PERU, Loreto, ‘Rio Monanti’, decaying leaf of ‘Oje’ (folk name of Ficus sp.) (Moraceae), 1991 (holotype MFC-1P143, not seen).
Illustration — See Matsushima (1993).
Hyphae smooth, hyaline to pale brown. Conidiomata sporodochium-like, discoid, more or less circular, (85–)100–250(–430) μm diam, solitary, later confluent, composed of two layers, a lower layer of rectangular sterile cells, pale olive, and an upper stratum with rectangular or quadratic, conidiogenous cells, 4–7×3–5 μm wide, dark brown. Conidia medially 1-septate, cylindrical, slightly asymmetrical, 17.5–25×4–5 μm, apex acute, base truncate, hyaline, smooth, with appendages at both ends, unbranched, 5–8 μm long, aggregating in a white mass in the top of the conidiomata (adapted from Matsushima 1993). Sexual morph unknown.
Habitat — Decaying leaves of Moraceae species (Matsushima 1993) and leaf litter (Schoenlein-Crusius et al. 2006, Grandi & Silva 2010).
Distribution — Brazil and Peru.
Notes — Mycoleptodiscus disciformis is comparable with N. venezuelense, in having more or less discoid conidiomata, cylindrical, 1-septate conidia, and conidial appendages at both ends. They can be distinguished by the size of their conidial appendages, which are smaller in M. disciformis (Table 2).
Mycoleptodiscus endophyticus Tibpromma & K.D. Hyde, MycoKeys 33: 49. 2018
Typus. THAILAND, Ranong, Muang, on healthy leaves of Freycinetia sp. (Pandanaceae), 3 Dec. 2016, S. Tibpromma FE101 (holotype MFLU 18-0001, not seen).
Notes — Mycoleptodiscus endophyticus was introduced based on molecular data of LSU, SSU, and tef1 sequences (Tibpromma et al. 2018). Our phylogenetic analysis demonstrates that this taxon does not belong to Mycoleptodiscus s.str. It nested in a subclade (71/0.98) related to CBS 100519, which is described here as a new genus. Given the absence of morphological characters, however, we prefer not to introduce any taxonomic changes until a more accurate study of the available reference material can be carried out.
Mycoleptodiscus minimus (Berk. & M.A. Curtis) Vanev, Proc. Kon. Ned. Akad. Wetensch. C 86: 433. 1983
Basionym. Discosia minima Berk. & M.A. Curtis, Grevillea 25: 7. 1874.
Typus. USA, Alabama, Beaumont, on leaves of Ilex, 1897 (holotype of Discosia minima 5113 Herb. Berk., not seen).
Illustration — See Vanev (1983).
Hyphae immersed, intracellular, brown. Conidiomata sporodochium-like, superficial, generally rounded in outline, 40–85 μm diam, dark brown. Conidiogenous cells radially arranged from the centre towards the periphery, angular (rectangular to irregular), 5–8.5×3.5–7 μm, brown, conidiogenous locus circular, 1–1.5 μm diam, without collarette. Conidia aseptate, cylindrical, tapered at both ends, straight or slightly curved at the ends, 20–25(–29)×3.5–4 μm, hyaline, often guttulate, with appendages at both ends, unbranched, straight, up to 8 μm long (adapted from Vanev 1983). Sexual morph unknown.
Habitat — On leaves of Ilex opaca and Ilex sp. (Vanev 1983).
Distribution — USA.
Notes — Mycoleptodiscus minimus is similar to M. disciformis and M. terrestris in having cylindrical conidia with apical appendages at both ends. Nevertheless, M. minimus is distinguished from both species by its aseptate conidia (Table 2).
Mycoleptodiscus stellatisporus K. Ando, Czech Mycol. 49: 3. 1996
Typus. AUSTRALIA, Queensland, Kuranda, isolated from soil, 1989, K. Ando (holotype TNS-F-180375 as ‘stellatosporus’, not seen).
Illustration — See Ando (1996).
Hyphae septate, dark brown near the conidiomata, pale brown to hyaline when distant, 1.5–4.5 μm diam. Conidiomata sporodochium-like, varying from a few combined conidiogenous cells to large aggregations, sometimes rounded in outline but usually variable in shape and size due to the confluence, mid to dark brown. Conidiogenous cells ampulliform to doliiform, cylindrical or deltoid, 4–9.5×2.5–5.5 μm, dark brown, smooth, with a single distinct circular aperture in the upper wall surrounded by a flared collarette 0.5–1.5(–2.5) μm diam. Conidia aseptate, pentagonal, long isosceles triangular, rhomboid or of irregular shape, with rounded apexes, 4.5–7.5×4–5.5 μm, with a truncate base (c. 1 μm wide), hyaline, smooth, with single appendages at each distal apex, up to 11 μm long, c. 0.5 μm wide (adapted from Ando 1996). Sexual morph unknown.
Habitat — Soil (Ando 1996).
Distribution — Australia.
Notes — The conidial shape of M. stellatisporus is triangular to pentagonal with 3–4 appendages, differing notably from all the species described in Mycoleptodiscus and related genera. The stellate conidia and phialidic conidiogenous cells arranged in conidiomata resembles those of Nawawia malaysiana which is placed in Chaetosphaeriales (Crous et al. 2009).
DISCUSSION
This study provides morphological and molecular data to facilitate the circumscription of Mycoleptodiscus. Phylogenetic analyses confirmed that taxa with mycoleptodiscus-like fungi do not constitute a monophyletic lineage in the Magnaporthales (Sordariomycetes), as previously suggested (Thongkantha et al. 2009, Klaubauf et al. 2014). The core of Mycoleptodiscus resides within Muyocopronales (Dothideomycetes), confirming that Mycoleptodiscus and Omnidemptus are unrelated genera as suggested by Luo & Zhang (2013).
Among Muyocopronaceae, the majority of species previously included in Mycoleptodiscus grouped in a well-supported lineage that we recognise as Muyocopron. This is distinct from the Mycoleptodiscus lineage, which includes the type species of the genus, M. terrestris. Muyocopron, for which the previous description was based only on the sexual morph (Spegazzini 1881, Saccardo 1883, Von Arx & Müller 1975, Lumbsch & Huhndorf 2007, Hyde et al. 2013, Pang et al. 2013), has been redefined in accordance with the one fungus one name principles to accommodate also asexually reproducing species. Therefore, seven new combinations are proposed (Mu. atromaculans, Mu. coloratum, Mu. geniculatum, Mu. laterale, and Mu taiwanense) including two with new names (Mu. freycineticola and Mu. sahnii), and two new species (Mu. alcornii and Mu. zamiae). Furthermore, Muyocopronaceae and Muyocopronales are emended to include the asexual genera: Arxiella, Leptodiscella, Neocochlearomyces, Muyocopron, Mycoleptodiscus, Paramycoleptodiscus, and Neomycoleptodiscus.
Our study shows that conidial morphology, conidiomatal development, and conidiogenous cells are important features in delimiting Mycoleptodiscus and related genera in Muyocopronales. The newly defined Mycoleptodiscus comprises M. suttonii and M. terrestris. Morphologically, Mycoleptodiscus s.str. is characterised by having sporodochium-like conidiomata, conidiogenous cells without or with inconspicuous collarette, and 0–2-septate, cylindrical conidia with appendages at one or both ends. Some species also develop sclerotia in both natural substrate and in culture and appressoria with a pore surrounded by radial lines (Gerdemann 1953, Ostazeski 1967, Alcorn 1994). Species of this genus has been isolated mainly from roots and leaves of plants collected in Australia, Brazil, and the USA (Gerdemann 1953, Ostazeski 1967, this study). The new genus Neomycoleptodiscus is practically indistinguishable from Mycoleptodiscus, however, phylogenetically it was located in a distant lineage.
Species relocated in Muyocopron are characterised by sporodochium-like conidiomata, conidiogenous cells with a flared collarette, and lunate to broadly lunate conidia with appendages at both ends, while in some species lateral appendages are also present, and appressoria entire or with a few lobes and inconspicuous pore. Muyocopron asexual morphs have been isolated mainly from leaf spots, as endophytes or saprobes on plant material, mainly from Australia and the USA (Sutton & Alcorn 1985, 1990, Alcorn 1994, Cannon & Alcorn 1994). Interestingly, clinical isolates causing mycosis in humans were placed in Mu. laterale, suggesting that previous identifications of ‘Mycoleptodiscus indicus’ as the etiological agent of mycosis in cats, dogs, and humans were incorrect (Padhye et al. 1995, Hull et al. 1997, Garrison et al. 2008, Dewar & Sigler 2010, Metry et al. 2010, Koo et al. 2012).
Species that are excluded from Mycoleptodiscus are M. affinis and M. lunatus, which were relocated in Omnidemptus. Omnidemptus retains mycoleptodiscus-like asexual morphs, but they differ from Mycoleptodiscus and Muyocopron in having sporodochium-like conidiomata that are usually less compacted, falcate conidia lacking appendages, and phylogenetically related to Magnaporthaceae (Luo & Zhang 2013, Klaubauf et al. 2014).
Since the original cultures or ex-type strains are not available for M. brasiliensis, M. disciformis, M. minimus, and M. stellatisporus, and since they are morphologically different from the concept of Mycoleptodiscus as here redefined, we prefer to treat them as excluded or doubtful species. Although the generic placement could not be resolved for all Mycoleptodiscus species in this study, the separation of Mycoleptodiscus from Muyocopron and Omnidemptus represents an important step in resolving the taxonomy of these genera. The newly defined mycoleptodiscus-like morphology sheds new light in the evolution of species in these genera. To identify species of Muyocopron, Mycoleptodiscus, and related genera, molecular identification is highly recommended because of the overlapping morphological characters, or poor sporulation with inconsistencies in the production of conidial appendages.
Acknowledgments
We are very grateful to the curators at BRIP, IMI, MUCL, and WI for providing cultures. Angela Bond and the keepers of the fungarium at Kew Royal Botanic Gardens are thanked for the loan of the holotype specimen of Amerodiscosiella indica. J.D.P. Bezerra acknowledges the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001), and the Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE) for financial support and scholarships. This study was in part supported by the Spanish ‘Ministerio de Economía y Competitividad’, grant CGL2017-88094-P. We are very grateful to the reviewers whose suggestions helped improve this manuscript.
REFERENCES
- Abarca GH, Mota RMA, Mena-Portales J, et al. 2006. Adiciones al conocimiento de la diversidad de los hongos conidiales del bosque mesófilo de montaña del estado de Veracruz. II. Acta Botanica Mexicana 77: 15–30. [Google Scholar]
- Alcorn JL. 1994. Appressoria in Mycoleptodiscus species. Australian Systematic Botany 7: 591–603. [Google Scholar]
- Ando K. 1996. A new species of Mycoleptodiscus from Australia. Czech Mycology 49: 1–5. [Google Scholar]
- Andrioli WJ, Conti R, Araújo MJ, et al. 2014. Mycoleptones A–C and polyketides from the endophyte Mycoleptodiscus indicus. Journal of Natural Products 77: 70–78. [DOI] [PubMed] [Google Scholar]
- Andrioli WJ, Silva TM, Silva VB, et al. 2012. The fungal metabolite eugenitin as additive for Aspergillus niveus glucoamylase activation. Journal of Molecular Catalysis B: Enzymatic 74: 156–161. [Google Scholar]
- Berbee ML, Pirseyedi M, Hubbard S. 1999. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 91: 964–977. [Google Scholar]
- Bezerra JDP, Santos MGS, Svedese VM, et al. 2012. Richness of endophytic fungi isolated from Opuntia ficus-indica Mill. (Cactaceae) and preliminary screening for enzyme production. World Journal of Microbiology and Biotechnology 28: 1989–1995. [DOI] [PubMed] [Google Scholar]
- Bezerra JL, Ram A. 1986. A crosta-negra da baunilha (Vanilla fragrans) causada por Mycoleptodiscus indicus (Moniliales, Hyphomycetes). Fitopatologia Brasileira 11: 717–724. [Google Scholar]
- Bills GF, Polishook JD. 1992a. A new species of Mycoleptodiscus from living foliage of Chamaecyparis thyoides. Mycotaxon 43: 453–460. [Google Scholar]
- Bills GF, Polishook JD. 1992b. Recovery of endophytic fungi from Chamaecyparis thyoides. Sydowia 44: 1–12. [Google Scholar]
- Campbell J, Ferrer A, Raja HA, et al. 2007. Phylogenetic relationships among taxa in the Jahnulales inferred from 18S and 28S nuclear ribosomal DNA sequences. Canadian Journal of Botany 85: 873–882. [Google Scholar]
- Cannon PF, Alcorn JL. 1994. Omnidemptus affinis gen. et. sp. nov., teleomorph of Mycoleptodiscus affinis sp. nov. Mycotaxon 51: 483–487. [Google Scholar]
- Crous PW, Groenewald JZ, Lee SS. 2009. Fungal Planet 41 Nawawia malaysiana. Persoonia 23: 194–195. [Google Scholar]
- Crous PW, Luangsaard JJ, Wingfield MJ, et al. 2018. Fungal Planet description sheets: 785–867. Persoonia 41: 238–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Richardson DM. 2014. Fungal Planet description sheets: 281–319. Persoonia 33: 212–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Richardson DM. 2016. Fungal Planet description sheets: 400–468. Persoonia 36: 316–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gruyter J, Aveskamp MM, Woudenberg JH, et al. 2009. Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycological Research 113: 508–519. [DOI] [PubMed] [Google Scholar]
- De Hoog GS, Guarro J, Gené J, et al. 2000. Atlas of Clinical Fungi. 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht. [Google Scholar]
- Dewar CL, Sigler L. 2010. Fungal arthritis of the knee caused by Mycoleptodiscus indicus. Clinical Rheumatology 29: 1061–1065. [DOI] [PubMed] [Google Scholar]
- Garrison AP, Procop GW, Vincek V, et al. 2008. A case of subcutaneous Mycoleptodiscus indicus infection in a liver transplant recipient successfully treated with antifungal therapy. Transplant Infections Disease 10: 218–220. [DOI] [PubMed] [Google Scholar]
- Gerdemann JW. 1953. An undescribed fungus causing a root rot of red clover and other Leguminosae. Mycologia 45: 548–554. [Google Scholar]
- Grandi RAP, Silva P. 2010. Caracterização morfológica de fungos conidiais decompositores de folhedo provenientes de Cubatão, SP, Brasil. Hoehnea 37: 769–775. [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, et al. 2011. The Amsterdam declaration on fungal nomenclature. IMA Fungus 2: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL, Kirk PM, Sutton BC, et al. 1995. Ainsworth & Bisby’s dictionary of the fungi. CABI, Wallingford, England. [Google Scholar]
- He X, Han G, Lin Y, et al. 2012. Diversity and decomposition potential of endophytes in leaves of a Cinnamomum camphora plantation in China. Ecological Research 27: 273–284. [Google Scholar]
- Hernández-Restrepo M, Groenewald JZ, Elliott ML, et al. 2016a. Take-all or nothing. Studies in Mycology 83: 19–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Restrepo M, Schumacher RK, Wingfield MJ, et al. 2016b. Fungal Systematics and Evolution: FUSE 2. Sydowia 68: 193–230. [Google Scholar]
- Hofstra D, Rendle D, Clayton J. 2012. First record of the fungus Mycoleptodiscus terrestris from native milfoil and pondweed in New Zealand. Australasian Mycologist 30: 1–4. [Google Scholar]
- Hofstra DE, Edwards T, Clayton JS, et al. 2009. New record of Mycoleptodiscus terrestris from New Zealand. New Zealand Journal of Botany 47: 411–415. [Google Scholar]
- Hull W, McNamara T, Ireland G. 1997. Subcutaneous phaeohyphomycosis due to Mycoleptodiscus lateralis in a cat. In: Anais do XXV Congresso Brasileiro de Medicina Veterinaria, XIII Congresso Estadual de Medicina Veterinária, II Congresso de Medicina Veterinária do Cone Sul. Gramado, Rio Grande do Sul, Brasil. [Google Scholar]
- Hyde KD, Jones EBG, Liu JK, et al. 2013. Families of Dothideomycetes. Fungal Diversity 63: 1–313. [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT: multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaubauf S, Tharreau D, Fournier E, et al. 2014. Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae). Studies in Mycology 79: 85–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo S, Sutton DA, Yeh WW, et al. 2012. Invasive Mycoleptodiscus fungal cellulitis and myositis. Medical Mycology 50: 740–745. [DOI] [PubMed] [Google Scholar]
- Liu JK, Hyde KD, Jones EBG, et al. 2015. Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Diversity 72: 1–197. [Google Scholar]
- Lumbsch HT, Huhndorf SM. 2007. Outline of Ascomycota – 2007. Myconet 13: 1–58. [Google Scholar]
- Luo J, Walsh E, Zhang N. 2014. Four new species in Magnaporthaceae from grass roots in New Jersey Pine Barrens. Mycologia 106: 580–588. [DOI] [PubMed] [Google Scholar]
- Luo J, Zhang N. 2013. Magnaporthiopsis, a new genus in Magnaporthaceae (Ascomycota). Mycologia 105: 1019–1029. [DOI] [PubMed] [Google Scholar]
- Luttrell ES. 1951. Taxonomy of Pyrenomycetes. University of Missouri Studies 24: 1–120. [Google Scholar]
- Madrid H, Gene J, Cano J, et al. 2012. A new species of Leptodiscella from Spanish soil. Mycological Progress 11: 535–541. [Google Scholar]
- Mapook A, Hyde KD, Dai D-Q, et al. 2016a. Muyocopronales, ord. nov., (Dothideomycetes, Ascomycota) and a reappraisal of Muyocopron species from northern Thailand. Phytotaxa 265: 225–237. [Google Scholar]
- Mapook A, Hyde KD, Hongsanan S, et al. 2016b. Palawaniaceae fam. nov., a new family (Dothideomycetes, Ascomycota) to accommodate Palawania species and their evolutionary time estimates. Mycosphere 7: 1732–1745. [Google Scholar]
- Martínez-Luis S, Cherigo L, Higginbotham S, et al. 2011. Screening and evaluation of antiparasitic and in vitro anticancer activities of Panamanian endophytic fungi. International Microbiology 14: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima T. 1987. Matsushima Mycological Memoirs 7. Kobe, Japan. Published by the author. [Google Scholar]
- Matsushima T. 1993. Matsushima Mycological Memoirs 5. Kobe, Japan. Published by the author. [Google Scholar]
- Metry CA, Hoien-Dalen PS, Maddox CW, et al. 2010. Subcutaneous Mycoleptodiscus indicus infection in an immunosuppressed dog. Journal of Clinical Microbiology 48: 3008–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2012. The CIPRES science gateway: enabling high-impact science for phylogenetics researchers with limited resources. In: Proceedings of the 1st Conference of the Extreme Science and Engineering Discovery Environment: Bridging from the extreme to the campus and beyond: 1–8. Association for Computing Machinery, USA. [Google Scholar]
- Nelson LS, Shearer JF. 2008. Evaluation of triclopyr and Mycoleptodiscus terrestris for control of Eurasian watermilfoil (Myriophyllum spicatum). Invasive Plant Science and Management 1: 337–342. [Google Scholar]
- Nylander J. 2004. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- Ortega HE, Graupner PR, Asai Y, et al. 2013. Mycoleptodiscins A and B, cytotoxic alkaloids from the endophytic fungus Mycoleptodiscus sp. F0194. Journal of Natural Products 76: 741–744. [DOI] [PubMed] [Google Scholar]
- Ostazeski SA. 1967. An undescribed fungus associated with a root and crown rot of birdsfoot trefoil (Lotus corniculatus). Mycologia 10: 970–975. [Google Scholar]
- Padhye AA, Davis MS, Reddick A, et al. 1995. Mycoleptodiscus indicus: a new etiologic agent of phaeohyphomycosis. Journal of Clinical Microbiology 33: 2796–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paim ECA, Silveira AJ, Bezerra JL, et al. 2012. Etiologia do declínio de mangostanzeiros no sul da Bahia. Revista Brasileira de Fruticultura 34: 1074–1083. [Google Scholar]
- Pang K-L, Hyde KH, Alias SA, et al. 2013. Dyfrolomycetaceae, a new family in the Dothideomycetes, Ascomycota. Cryptogamie, Mycologie 34: 223–232. [Google Scholar]
- Ramesh CH, Vijaykumar S. 2005. Studies of fresh water foam fungi of Uttara Kannada, Karnataka. Indian Phytopathology 58: 89–95. [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. Commonwealth Mycological Institute, London, UK. [Google Scholar]
- Rehner SA, Buckley E. 2005. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–89. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccardo PA. 1883. Sylloge Fungorum. Omnium hucusque cognitorum 2: 658–661. [Google Scholar]
- Sahni VP. 1968. Deuteromycetes from Jabalpur III. Mycopathologia Mycologia Applicata 36: 267–288. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, et al. 2009. A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Robbertse B, Robert V, et al. 2014. Finding needles in haystacks: linking scientific names, reference specimens and molecular data for Fungi. Database (Oxford) 2014: bau061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, et al. 2006. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041–1052. [DOI] [PubMed] [Google Scholar]
- Schoenlein-Crusius IH, Milanez AI, Trufem SFB, et al. 2006. Microscopic fungi in the Atlantic rainforest in Cubatão, São Paulo, Brazil. Brazilian Journal of Microbiology 37: 267–275. [Google Scholar]
- Shearer JF. 2001. Recovery of endophytic fungi from Myriophyllum spicatum. ERDC TN-APCRP-BC-03: 1–11. [Google Scholar]
- Shearer JF, Jackson MA. 2006. Liquid culturing of microsclerotia of Mycoleptodiscus terrestris, a potential biological control agent for the management of hydrilla. Biological Control 38: 298–306. [Google Scholar]
- Spear ER. 2017. Phylogenetic relationships and spatial distributions of putative fungal pathogens of seedlings across a rainfall gradient in Panama. Fungal Ecology 26: 65–73. [Google Scholar]
- Spegazzini C. 1881. Fungi argentini additis nonnullis brasiliensibus montevideensibusque. Pugillus quartus (Continuacion). Anales de la Sociedad Científica Argentina 12: 97–117. [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenroos S, Laukka T, Huhtinen S, et al. 2010. Multiple origins of symbioses between ascomycetes and bryophytes suggested by a five gene phylogeny. Cladistics 26: 281–300. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Mao LJ, Li N, et al. 2013. Evidence for biotrophic lifestyle and biocontrol potential of dark septate endophyte Harpophora oryzae to rice blast disease. PLoS One 8: e61332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetrong S, Schoch CL, Spatafora JW, et al. 2009. Molecular systematics of the marine Dothideomycetes. Studies in Mycology 64: 155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Guo LD, Hyde KD. 2011. Community composition of endophytic fungi in Acer truncatum and their role in decomposition. Fungal Diversity 47: 85–95. [Google Scholar]
- Sutton BC. 1973. Pucciniopsis, Mycoleptodiscus and Amerodiscosiella. Transactions of the British Mycological Society 60: 525–536. [Google Scholar]
- Sutton BC, Alcorn JL. 1985. Undescribed species of Crinitospora gen. nov., Massariothea, Mycoleptodiscus and Neottiosporina from Australia. Transactions of the British Mycological Society 84: 437–445. [Google Scholar]
- Sutton BC, Alcorn JL. 1990. New species of Mycoleptodiscus (Hyphomycetes). Mycological Research 94: 564–566. [Google Scholar]
- Sutton BC, Hodges CS. 1976. Eucalyptus microfungi: Mycoleptodiscus species and Pseudotracylla gen. nov. Nova Hedwigia 27: 693–700. [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. 2013. MEGA 6: Molecular Evolutionary Genetics Analysis. Version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongkantha S, Jeewon R, Vijaykrishna D, et al. 2009. Molecular phylogeny of Magnaporthaceae (Sordariomycetes) with a new species, Ophioceras chiangdaoense from Dracaena loureiroi in Thailand. Fungal Diversity 34: 157–173. [Google Scholar]
- Tibpromma S, Hyde KD, Bhat JD, et al. 2018. Identification of endophytic fungi from leaves of Pandanaceae based on their morphotypes and DNA sequence data from southern Thailand. MycoKeys 33: 25–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibpromma S, McKenzie EHC, Karunarathna SC, et al. 2016. Muyocopron garethjonesii sp. nov. (Muyocopronales, Dothideomycetes) on Pandanus sp. Mycosphere 7: 1480–1489. [Google Scholar]
- Vaidya G, Lohman DJ, Meier R. 2011. SequenceMatrix: concatenation software for the fast assembly of multigene datasets with character set and codon information. Cladistics 27: 171–180. [DOI] [PubMed] [Google Scholar]
- Vanev SG. 1983. Mycoleptodiscus minimus (Berk. & Curt.) Vanev, comb. nov. Koninklijke Nederlandse Academie van Wetenschappen, Proceedings C 86: 433–435. [Google Scholar]
- Verma U, Charudattan R. 1993. Host range of Mycoleptodiscus terrestris, a microbial herbicide candidate for Eurasian watermilfoil, Myriophyllum spicatum. Biological Control 3: 271–280. [Google Scholar]
- Voglmayr H, Gardiennet A, Jaklitsch WM. 2016. Asterodiscus and Stigmatodiscus, two new apothecial dothideomycete genera and the new order Stigmatodiscales. Fungal Diversity 80: 271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Arx JA, Müller E. 1975. A re-evaluation of the bitunicate ascomycetes with keys to families and genera. Studies in Mycology 9: 1–159. [Google Scholar]
- Vu D, Groenewald M, De Vries M, et al. 2019. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92: 135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Moya JD, González JL, et al. 1997. Mycoleptodiscus terrestris from black pepper roots in the Dominican Republic. Mycoscience 38: 91–94. [Google Scholar]
- Whitton SR, McKenzie EHC, Hyde KD. 2012. Anamorphic fungi associated with Pandanaceae. In: Whitton SR, McKenzie EHC, Hyde KD. (eds), Fungi associated with Pandanaceae. Fungal Diversity Research Series vol. 21: 125–353. Springer, Dordrecht. [Google Scholar]
- Woudenberg JHC, Hanse B, Van Leeuwen GCM, et al. 2017. Stemphylium revisited. Studies in Mycology 87: 77–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HX, Schoch CL, Boonmee S, et al. 2011. A reappraisal of Microthyriaceae. Fungal Diversity 51: 189–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X-H, Su Z-Z, Wang C, et al. 2015. The rice endophyte Harpophora oryzae genome reveals evolution from a pathogen to a mutualistic endophyte. Scientific Reports 4: 5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan ZL, Zhang CL, Lin FC, et al. 2010. Identity, diversity, and molecular phylogeny of the endophytic mycobiota in the roots of rare wild rice (Oryza granulate) from a nature reserve in Yunnan, China. Applied and Environmental Microbiology 76: 1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Zhao S, Shen Q. 2011. A six-gene phylogeny reveals the evolution of mode of infection in the rice blast fungus and allied species. Mycologia 103: 1267–1276. [DOI] [PubMed] [Google Scholar]


