Abstract
Increased β-adrenergic receptor (β-AR)-mediated activation of adenylyl cyclase (AC) in rat liver during aging has been linked to age-related increases in hepatic glucose output and hepatosteatosis. In this study, we investigated the expression of β-ARs, individual receptor subtypes, and G protein-coupled receptor (GPCR) regulatory proteins in livers from aging rats. Radioligand-binding studies demonstrated that β-AR density increased by greater than threefold in hepatocyte membranes from senescent (24-mo-old) compared with young adult (7-mo-old) rats and that this phenomenon was blocked by food restriction, which is known to retard aging processes in rodents. Competition-binding studies revealed a mixed population of β1- and β2-AR subtypes in liver membranes over the adult life span, with a trend for greater β2-AR density with age. Expression of both β-AR subtype mRNAs in rat liver increased with age, whereas β2- but not β1-AR protein levels declined in livers of old animals. Immunoreactive β2- but not β1-ARs were preferentially distributed in pericentral hepatic regions. Levels of GRK2/3 and β-arrestin 2 proteins, which are involved in downregulation of agonist-activated GPCRs, including β-ARs, increased during aging. Insofar as sympathetic tone increases with age, our findings suggest that, despite enhanced agonist-mediated downregulation of hepatic β-ARs preferentially affecting the β2-AR subtype, increased generation of both receptor subtypes during aging augments the pool of plasma membrane-bound β-ARs coupled to AC in hepatocytes. This study thus identifies one or both β-AR subtypes as possible therapeutic targets involved in aberrant hepatic processes of glucose and lipid metabolism during aging.
Keywords: β-arrestin, food restriction, G protein-coupled receptor, G protein-coupled receptor serine/threonine kinase, hepatocytes
INTRODUCTION
Elevations of hepatic glucose output and lipid accumulation are hallmarks of type 2 diabetes mellitus and nonalcoholic fatty liver disease, two widely occurring metabolic diseases with increasing prevalence during aging (7, 18). Although disordered hepatic glucose and lipid metabolism in these age-related diseases is strongly associated with reduced sensitivity of liver to the actions of insulin (39), altered hepatic responsiveness to counterregulatory factors such as catecholamines might also play a role in the development of liver dysfunction during aging. Sympathetic nervous system activity, as reflected by basal plasma levels of catecholamines noradrenaline (norepinephrine) and adrenaline (epinephrine), increases during aging (9, 17). The responses of many target tissues to adrenergic stimuli are mediated by the classical β-adrenergic receptor (β-AR) coupled adenylyl cyclase (AC)/cAMP cascade (26, 33, 42). Likely in adaptation to increased sympathetic tone with age, β-adrenergic responsiveness of a number of target organs declines during aging, together with decreases in β-AR numbers and/or receptor-linked signaling function(s) (43, 44, 51, 55). We and others, however, established some years ago that membrane content of β-ARs in liver increases during postmaturational or senescent aging in Fischer 344 male rats in association with progressive increases in β-adrenergic sensitive AC stimulation and hepatic glucose output (11, 14, 24, 26, 27). More recent work in our laboratory further suggested that increased hepatic β-AR signaling may also contribute to lipid accumulation in liver during aging (13, 49). Age-related increases in membrane content of β-ARs coupled to AC-mediated functions, with potentially deleterious metabolic consequences, appear to be unique to liver and may reflect a tissue-specific defect in adaptive mechanisms modulating adrenergic responses to increased circulating levels of catecholamines at advanced age. Although ample evidence points to increased β-AR density in whole liver of aging rats, it has not been clarified whether this change with age reflects an increase in receptor content in hepatocytes that may be linked to cellular dysfunction during aging.
β-Adrenergic receptors, which are prototypical members of the guanine nucleotide-binding G protein-coupled receptor (GPCR) superfamily, include β1-, β2-, and β3-AR subtypes (33). β-ARs detected in rat liver by radioligand binding were initially reported to be predominantly, or even exclusively, of the β2-subtype (11, 36). However, subsequent work also documented the presence of β1-ARs in rat liver by competition-binding assays using β-AR subtype selective antagonists and by immunohistochemistry (6, 35, 54). β3-ARs, which play an important role in adipose tissue lipolysis, are undetected or expressed at very low levels in rodent liver (20, 40). β1- and β2- but not β3-AR expression has also been demonstrated in human liver samples by receptor binding, immunoblot, PCR, and/or Southern hybridization experiments (1, 19, 30, 41). Little is known about changes in density or expression of β1- and β2-AR subtypes with age in liver; of the few relevant data published in this area, earlier studies utilizing competition binding described a mixed population of β/β -ARs in rat liver over the lifespan (35, 54), whereas preliminary semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) performed in our laboratory suggested an increase in hepatic β1-AR expression with senescent aging in rats (20).
β-Adrenergic receptors, like most GPCRs, undergo a complex process of desensitization and internalization in response to agonist stimulation. Agonist-bound receptor assumes a conformation allowing phosphorylation by one or more GPCR serine/threonine kinases (GRKs) (50). GRK-induced phosphorylation of the receptor promotes binding of the arrestin family of proteins to the receptor, which in turn interrupts receptor coupling to G proteins (hence, “desensitizing” classical GPCR signaling) and targets the receptor for sequestration/ internalization via clathrin-coated pits. Of the seven members of the GRK family, the GRK2 subfamily members (GRK2 and GRK3, also known as β-ARK1 and β-ARK2) are the most widely expressed and phosphorylate a range of GPCRs. Among the four family members of arrestin molecules, two (visual arrestin and cone arrestin) are expressed exclusively in the retina and two [β-arrestin-1 (βarr1) and β-arrestin-2 (βarr2)] are expressed ubiquitously in other tissues (50). The two β-arrestins do not appear to be functionally redundant, since βarr2 has been shown to be much more effective than βarr1 in supporting internalization of the β2-AR (29). Internalized GPCRs have long been known to undergo degradation or recycling to the plasma membrane, albeit by sorting mechanisms that remain obscure. Altered expression of GRKs and/or β-arrestins in livers of old rats could play a modulatory role in age-related changes of plasma membrane content of β1- and/or β2-AR subtypes and their internalization, but to our knowledge this has not been evaluated.
In the current study, we have extended previous observations of β-ARs in liver during aging by 1) investigating β-AR density, assessed by radioligand binding, in hepatocytes isolated from aging rats; 2) identifying by several methods the β-AR subtype(s) that may be involved in augmenting β-AR-mediated AC activity with age in rat liver; and 3) determining whether hepatic expression levels of GRK2/3 and βarr2 are age dependent. The intent of this study is to begin to identify specific therapeutic targets involved in aberrant β-AR-mediated processes of glucose and lipid metabolism occurring in liver during aging.
MATERIALS AND METHODS
Materials.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. TRI reagent was obtained from Molecular Research Center (Cincinnati, OH). Complete Mini tablets and DNAse I were obtained from Roche Diagnostics (Indianapolis, IN), whereas DTT was purchased from Invitrogen (Carlsbad, CA). TaqMan Gene Expression Assays [β2-AR (Rn00560650), β1-AR (Rn00824536), and 18S (Hs99999901)], TaqMan universal master mix, high-capacity cDNA reverse transcription kit, magnesium chloride, and plasticware required for performing quantitative realtime PCR were obtained from Applied Biosystems (ABI; Foster City, CA). Bicinchoninic acid (BCA) assay and enhanced chemiluminescence (ECL) kit were from Pierce (Rockford, IL). Polyvinylidene fluoride (PVDF) membranes were obtained from GE Osmonics (Minnetonka, MN), whereas collagenase (type II) was purchased from Worthington Biochemical (Lakewood, NJ). Bradford protein assay reagents were purchased from Bio-Rad Laboratories (Hercules, CA). (−)-[125I]iodopindolol (2,200 Ci/mmol) was obtained from Perkin-Elmer (Waltham, MA), whereas Whatman glass fiber filters (GF/C) were purchased from Brandel (Biomedical Research and Development Laboratories, Gaithersburg, MD). In Western blot analyses, antibodies specific for β2-AR (1:500 dilution, no. ab36956) and β1-AR (1:1,000 dilution, no. ab3442) were ordered from Abcam (Cambridge, MA); β-actin (1:5,000 dilution, no. sc-47778), GAPDH (1:5,000 dilution, no. sc-47724), GRK2/3 (1:250 dilution, no. sc-8329), and β-arrestin2 (1:250 dilution, no. sc-13140) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The antibody used in immunohistochemical analysis of β2-AR (1:100 dilution, no. ab36956) was also from Abcam, whereas the β1-AR (1:20 dilution, no. sc-568) antibody was from Santa Cruz Biotechnology. Antibodies utilized in this study were previously validated according to manufacturers’ data sheets and product citations therein [Abcam (www.abcam.com) and Santa Cruz Biotechnology (www.scbt.com)]. Appropriate peroxidase-labeled secondary antibodies and ImmPRESS polymer detection kit were purchased from Vector Laboratories (Burlingame, CA), whereas ICI-118,551 and ICI-89,406 were obtained from Tocris (Bristol, UK). Williams’ E Medium was obtained from GIBCO-BRL (Gaithersburg, MD). Krebs-Henseleit Buffer was from Sigma.
Animals.
For saturation-binding experiments assessing total β-AR content in hepatocyte membranes (Fig. 1), specific pathogen-free (SPF) Fischer 344 male rats were obtained as weanlings from Charles River Laboratories (Wilmington, MA) and were maintained singly in a SPF barrier facility on standard 12-h light-dark cycles at the University of Texas Health Science Center at San Antonio (UTH-SCSA). Rats were fed ad libitum a diet of previously specified composition until 6 wk of age and then continued on the same diet ad libitum or were restricted to 60% of the mean ad libitum intake (i.e., food restricted, FR) until the animals were used at 7, 14, 20, or 24 mo of age (24, 27, 59). For all other experiments young adult (3–6 mo old) and senescent (22–24 mo old) SPF Fischer 344 male rats were obtained from the National Institute on Aging, National Institutes of Health (Bethesda, MD), and housed on standard 12-h light-dark cycles for ≥1 wk in a SPF barrier facility within the Veterinary Medical Unit, Audie L. Murphy Division (ALMD)-South Texas Veterans Health Care System, before use; during the equilibration period, animals were fed ad libitum a diet approximating that used in the experiments of Fig. 1 (59). All animals were treated in accordance with the guidelines approved by the joint Institutional Animal Care and Use Committee at the UTHSCSA/ALMD.
Fig. 1.
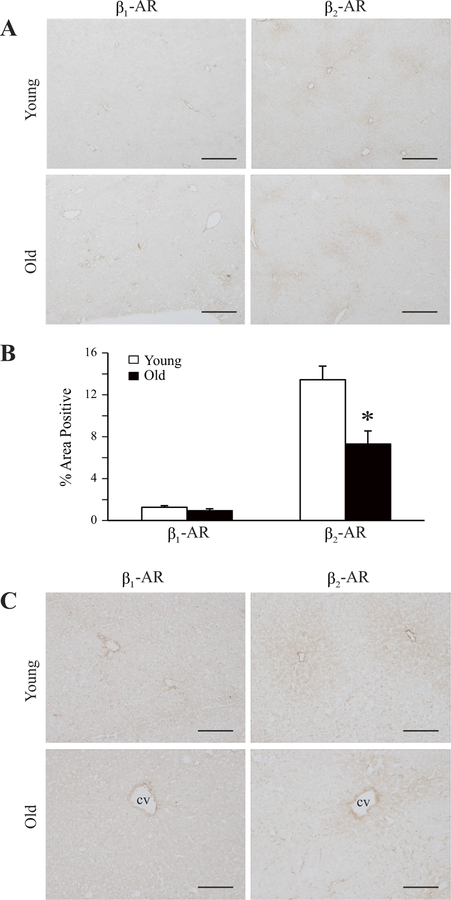
Density of β-adrenergic receptors (β-ARs) in hepatocyte membranes from aging rats: modulation by food restriction. Hepatocytes were isolated from 7-, 14-, 20-, and 24-mo-old Fischer 344 male rats either fed ad libitum (AL) or food restricted (FR) to 60% of the mean ad libitum intake. β-AR density (Bmax) in particulate membrane preparations from freshly isolated hepatocytes was determined by Scatchard analysis of saturation-binding experiments using the β-AR antagonist (−)-[125I]iodopindolol as the radioligand. Values represent means ± SE from 5 to 11 rats in each age/diet group. *P = 0.026 vs. Bmax in 20-mo-old AL-fed rats. β-AR-binding affinity [dissociation constant (Kd)] varied from 50 to 80 pM, with no statistically significant differences among groups.
Preparation of liver samples.
Rats were euthanized by exsanguination after anesthesia, as previously described (23). Livers were rapidly removed, cut into pieces, and quick-frozen in liquid nitrogen for storage at –80°C until further use. For competition-binding studies (Fig. 2 and Table 1), liver pieces were homogenized, followed by filtration and centrifugation of the homogenate to obtain the 4,500-g particulate fraction (25). Particulates were stored at –80°C before use in the binding assay. Unless otherwise specified, protein concentration of liver samples and also hepatocyte membranes (see below) was determined by the method of Bradford.
Fig. 2.
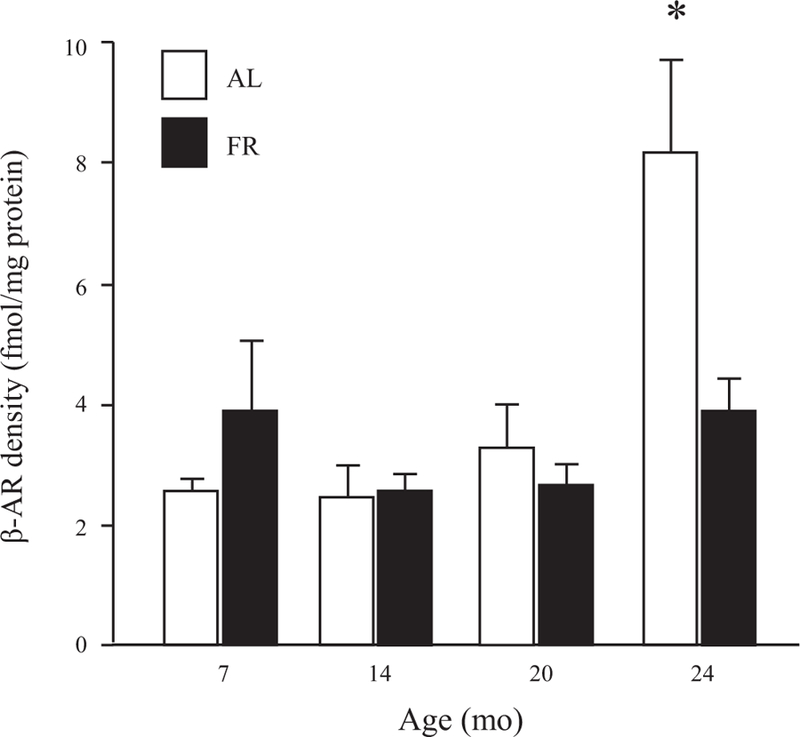
Competition for (−)-[125I]iodopindolol-binding sites in rat liver by ICI-118,551 and ICI-89,406: effects of age. Binding of (−)-[125I]iodopindolol to liver particulates from individual young (6 mo old) vs. old (24 mo old) rats was assayed in the presence of increasing concentrations of unlabeled ICI-118,551 (A) or ICI-89,406 (B), as described previously (57). Results from single representative animals are shown; curve-fitting analysis of competition data from 4 young (3–6 mo old) and 5 old (24 mo old) rats is presented in Table 1.
Table 1.
Effects of age on high- and low-affinity binding sites for ICI-118,551 and ICI-89,406 in rat liver particulates
| Ligand Competing for (—)-[125I]Iodopindolol Binding |
||||||
|---|---|---|---|---|---|---|
| ICI-118,551 |
ICI-89,406 |
|||||
| Young | Old | P value | Young | Old | P value | |
| RH, fmol/mg | 2.34 ± 0.82 | 7.17 ± 2.85 | 0.165 | 2.30 ± 0.30 | 3.24 ± 1.12 | 0.450 |
| RL, fmol/mg | 1.90 ± 0.33 | 2.83 ± 0.83 | 0.346 | 2.48 ± 0.83 | 3.73 ± 1.11 | 0.399 |
| %RH | 51.7 ± 5.8 | 68.1 ± 2.9 | 0.064 | 53.8 ± 11.5 | 44.3 ± 3.0 | 0.478 |
| %RL | 48.3 ± 5.8 | 31.9 ± 2.9 | 0.064 | 46.2 ± 11.5 | 55.7 ± 3.0 | 0.478 |
| KH, nM | 2.13 ± 1.31 | 9.05 ± 5.80 | 0.309 | 0.50 ± 0.09 | 0.86 ± 0.49 | 0.528 |
| KL, µM | 1.14 ± 0.76 | 12.97 ± 9.13 | 0.266 | 7.55 ± 6.19 | 3.72 ± 0.46 | 0.581 |
Values are means ± SE from 4 young and 5 old rats. RH and RL, nos. of sites binding ICI-118,551 or ICI-89,406 with high and low affinity; KH and KL, dissociation constants of high- and low-affinity binding sites. Competition binding curves using liver particulate preparations from young (3–6 mo old) and old (24 mo old) rats were constructed as described in Fig. 2, and the data were analyzed with a weighted, nonlinear, least-squares curve-fitting program (38). ICI-118,551 and ICI-89,406 competition curves were best fit by a 2-site model describing high- and low-affinity binding sites.
Isolation of hepatocytes and preparation of hepatocyte membranes.
Hepatocytes were isolated as previously described (27). Briefly, rats were anesthetized using pentobarbital sodium (65 mg ip injection/kg body wt), and livers were perfused in situ with 0.03% collagenase (type II)-containing Krebs-Henseleit bicarbonate buffer (pH 7.4). For preparation of hepatocyte membranes, filtered and washed hepatocytes were resuspended to a density of 107 cells/ml in ice-cold Krebs-Henseleit buffer and subjected to sequential freeze-thawing and homogenization with a Polytron homogenizer (two 10-s bursts at setting 6). Cell lysates were resuspended (1.8 ml:10 ml) in tissue buffer [0.154 M NaCl and 20 mM HEPES (pH 7.4) with 1 mM MgCl2] and centrifuged at 27,000 g for 15 min at 4°C. Membrane pellets were resuspended in 1.125 ml of tissue buffer, pooled, and homogenized on ice in a 7-ml Dounce homogenizer with 10 strokes using a loose pestle; pellet fractions were then placed in 1-ml aliquots in cryotubes, snap-frozen in liquid nitrogen, and stored at –80° until use in the binding assay.
Receptor-binding assay.
Receptor binding in membrane preparations from freshly isolated hepatocytes or liver homogenates was measured by an equilibrium-binding assay using (−)-[125I]iodopindolol, as described previously (25, 57, 60). About 100 µg of membrane protein was incubated with (−)-[125I]iodopindolol in 125–250 µl of reaction buffer [12.5 mM HEPES (pH 7.5), 115 mM NaCl, and 0.66 mM L-ascorbic acid] for 30 min at 30°C. Reactions were then terminated by adding 4 ml of wash buffer [10 mM Tris (pH 7.5) and 154 mM NaCl] at room temperature, and membrane-bound radioligand was collected on Whatman glass fiber filters (GF/C) with a Brandel Cell Harvester (Gaithersburg, MD). Nonspecific binding of 125I-labeled receptor ligand was defined as the amount of radioligand bound in the presence of an excess (10–4 M) of the unlabeled ligand. Saturation-binding curves were constructed by measuring specific binding of the 125I-labeled receptor ligand at eight different concentrations in the range of 0.01–5 nM. Competition-binding studies were performed by measuring binding of (−)-[125I]iodopindolol (at a concentration approximating the dissociation constant, Kd) in the presence of increasing concentrations of nonlabeled β2-AR antagonist ICI-118,551 or β1-AR antagonist ICI-89,406 in the range of 10–11 to 10–4 M.
Quantitative real-time PCR.
Total RNA was isolated from frozen liver pieces using TRI Reagent according to the manufacturer’s instructions. The RNA samples were treated with DNAse I (RNAse-free) and then reverse transcribed. The complementary DNA (cDNA) synthesis was carried out in a thermocycler at 25°C/10 min, 42°C/50 min, 72°C/10 min, and 4°C. Real-time RT-PCR assay was then performed by the ∆∆CT method with TaqMan Gene Expression Assays for rat β1-AR and β2-AR using an ABI 7900 Sequence Detection System. In each experiment, mRNA levels were normalized to 18S RNA, which did not vary with animal age (data not shown).
Western blotting.
Frozen liver pieces were homogenized in lysis buffer [50 mM NaCl, 1% NP-40, and 50 mM Tris·HCl (pH 7.4)] containing protease and phosphatase inhibitors. Homogenates were rocked at 4°C for 60 min, followed by centrifugation at 10,000 g for 15 min at 4°C. The supernatant proteins were estimated by BCA assay. Protein samples (40–70 µg) were added to 10 µl of 4× sample buffer [150 mM Tris·HCl (pH 8.8), 1% SDS, and 40% glycerol] and β-mercaptoethanol (355 mM) and then diluted with lysis buffer to a total volume of 40 µl. Samples were size-fractionated on SDS-PAGE gels and electroblotted onto 0.2-µm PVDF membranes. Membranes were immunoblotted overnight at 4°C with a primary antibody, followed by a secondary horseradish peroxidase-conjugated antibody for1h at room temperature. Specific proteins were visualized using an enhanced chemiluminescence kit, and immunoblots were quantified with ImageJ software (45) and normalized against β-actin or GAPDH. Molecular weights of target proteins were determined using standard molecular weight markers (Bio-Rad Precision Plus Dual Marker, cat. no. 161–0374).
Immunohistochemistry.
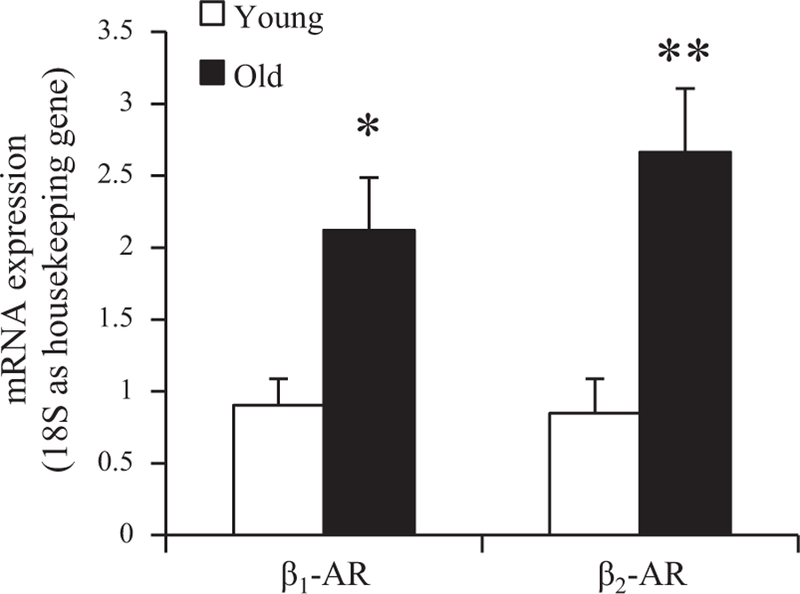
Six-micrometer frozen sections cut from liver pieces were blocked with 0.1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) and then stained with rabbit antibody directed against β1-AR (1:20 dilution, no. sc-568) or β2-AR (1:200 dilution, no. ab36956), followed by incubation with an ImmPRESS polymer detection kit according to the manufacturer’s instructions. The stained tissue was mounted on coverslips, viewed, and photographed using an Olympus AX70 research microscope equipped with a DP-70 digital camera (Olympus America, Melville, NY). For image analysis, photographic images were taken of three to five random fields using a ×4 objective magnification for a total of ≥10.8 sq. mm of each tissue. The area of diaminobenzidine (DAB) reaction product in each image was measured using the segmentation tool of Image-Pro Plus 4.5 imaging software (Media Cybernetics, Silver Spring, MD) calibrated to a stage micrometer. DAB staining in the image was extracted by selecting a lower and upper range of grayscale within the limits of background and the highest intensity of staining (12). The image data were then masked and pseudo-colored for measurement of staining area relative to the total field.
Data analysis.
Data from multiple experiments are expressed as means ± SE. Statistical significance of single comparisons was determined by Student’s t-test. Scatchard analysis of (−)-[125I]iodopindolol saturation-binding data was used to determine β-AR receptor density (Bmax) and Kd. Competition-binding curves describing (−)-[125I]iodopindolol binding in the presence of increasing concentrations of ICI-118,551 or ICI-89,406 were analyzed with a weighted, least-squares curve-fitting program (38).
RESULTS
β-Adrenergic receptor density in rat hepatocyte membranes increases with senescent aging: effect of food restriction.
Earlier radioligand-binding studies in our laboratory and others demonstrated age-related increases in numbers of β-AR-binding sites measured in membrane preparations from whole liver (11, 14, 20, 24, 54). In the present study, we have extended these previous findings in experiments measuring β-AR binding in hepatocytes isolated from rats of increasing age. As shown in Fig. 1, β-AR density in hepatocyte membranes increases more than threefold between 7 and 24 mo of age; interestingly, the increase in receptor number in old rats occurs almost entirely during senescent aging, i.e., after 20 mo of age. In the same experiments, we demonstrated that food restriction, which has long been known to retard aging processes in rodents and other species, blocks the senescent increase of β-AR density in rat hepatocyte membranes (Fig. 1).
β-Adrenergic receptor-binding sites in liver comprise a mixed population of β1- and β2-AR subtypes over the adult lifespan of the rat.
To evaluate whether increased density of β-AR-binding sites in livers of old rats represents changes in the levels of β1- and/or β2-AR subpopulations, the relative proportions of the two receptor subtypes in whole liver particulates from young (3–6 mo old) and old (24 mo old) rats were analyzed in competition-binding experiments using antagonists selective for β2- and β1-ARs (ICI-118,551 and ICI-89,406, respectively). Our results (Fig. 2 and Table 1) reveal high- and low-affinity binding sites for the two antagonists in liver preparations from both young and old animals. The high-affinity binding sites for ICI-118,551 and ICI-89,406 presumably represent authentic β2- and β1-ARs, respectively; in contrast, low-affinity binding by ICI-118,551 and ICI-89,406 is generally believed to occur at β1- and β2-ARs, respectively (41, 54, 57). Inspection of representative binding curves suggests that liver preparations from 24-mo-old rats exhibit a greater degree of binding by both antagonists than do preparations from 6-mo-old animals (Fig. 2). Curve-fitting analysis of competition-binding data may implicate an age-related increase in high-affinity binding for ICI-118,551 but not ICI-89,406, although no apparent age difference reaches the level of statistical significance (Table 1). Overall, these results are consistent with the expression of both β - and β -ARs in rat liver over the adult lifespan, with a trend for an age-related increase in the density of β2-ARs.
β1- and β2-AR subtypes in rat liver demonstrate differential changes in gene and/or protein expression levels during aging.
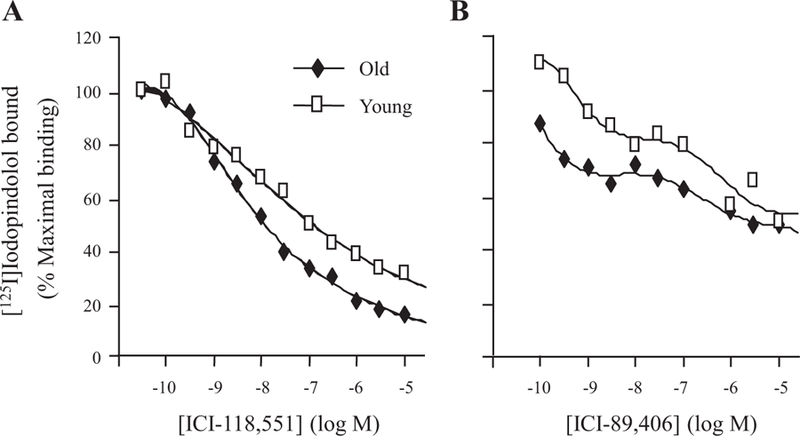
To begin an investigation into the processes by which β-AR expression is regulated in livers of aging rats, we measured the levels of β1- and β2-AR mRNAs and also receptor subtype protein levels in whole liver from young and old rats. Figure 3 shows that hepatic expression of both β-AR subtype mRNAs, determined by quantitative real-time PCR (qRT-PCR), increased ~2.5- to threefold between 6 and 24 mo of age. In contrast to these results, immunoblot analysis revealed no change in hepatic β1-AR levels with age and decreased β2-AR protein expression in livers of senescent rats compared with young animals (Fig. 4). Levels of β-AR subtypes and their distribution within hepatic parenchyma during aging were further evaluated by immunohistochemical staining. Commensurate with the results from immunoblot analysis, β1-AR staining was equivalent in livers of young and old rats, whereas β2-AR staining declined significantly with age (Fig. 5, A and B). Notably, the distribution of staining at both ages appeared to differ between the two isoforms; whereas β1-AR stained diffusely, β2-AR staining tended to concentrate in hepatocytes surrounding the central vein (pericentral region, or zone 3) (Fig. 5C).
Fig. 3.
Effects of age on β1- and β2-AR mRNA levels in rat liver. β1- and β2-AR mRNA levels in liver specimens from young (6 mo old) and old (24 mo old) rats were determined by quantitative RT-PCR, as described in materials and methods. mRNA levels were normalized to 18S RNA and then expressed as fold change relative to the value from a single animal (calibrator). Values shown represent means ± SE from 7 young and 7 old rats. *P = 0.026 vs. β1-AR mRNA level at 6 mo; **P = 0.018 vs. β2-AR mRNA level at 6 mo.
Fig. 4.
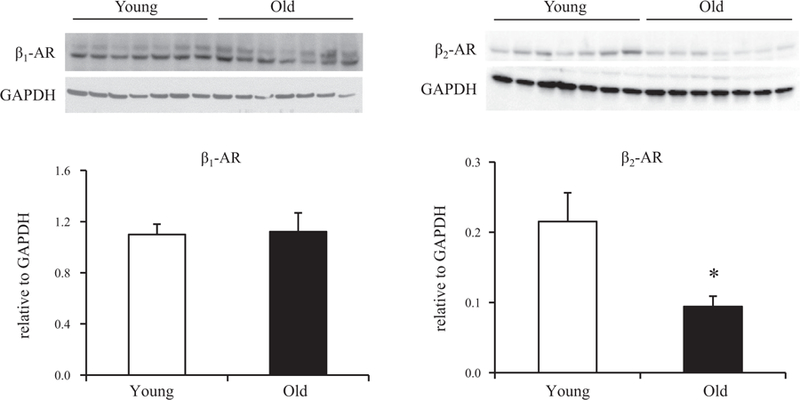
β1- and β2-AR protein levels in rat liver with age. Protein levels of the two β-AR subtypes were measured by Western blotting of lysates prepared from the same liver specimens used for mRNA determinations. All lysates were prepared at the same time and processed in parallel. Top: representative immunoblots depicting β1- and β2-AR protein levels in livers from young (6 mo old) and old (24 mo old) animals. GAPDH was used as loading control. Protein levels were quantified as integrated intensity and then normalized to the loading control. Bottom: bar values represent means ± SE from 7 young and 7 old rats. *P = 0.03 vs. β2-AR protein level at 6 mo. An age-related decline in β2-AR levels was confirmed in additional experiments (not shown) utilizing a different antibody to β2-AR (no. 182136; Abcam) from that employed in the immunoblot shown (no. ab36956; Abcam).
Fig. 5.
β1- and β2-AR immunoreactivity in liver sections from young and old rats. Frozen liver sections were immunostained for β1-AR and β2-AR. A: representative diaminobenzidine (DAB)-stained liver sections from individual young (6 mo old) and old (24 mo old) rats are shown (×4 objective magnification). Scale bar, 300 µm. B: areas of staining for β1- and β2-AR proteins in young vs. old rat liver sections, as in A, determined by quantitation of DAB staining, are represented as bar graphs. Antibody to β1-AR yielded relatively low signal. Data are expressed as means ± SE from 8 to 9 young and 6 to 8 old rats. *P = 0.002. C: representative liver sections exhibiting greater detail are shown at higher magnification (×12.5 objective). Scale bar, 100 µm. cv, Central vein.
Immunoreactive levels of GPCR kinase 2/3 and β-arrestin-2 increase with age in rat liver
In response to agonist stimulation, β-ARs and other GPCRs undergo rapid uncoupling from plasma membrane effectors and internalization mediated by the sequential actions of GPCR kinase (GRK) and arrestin proteins (29, 50). We performed experiments comparing immunoreactive GRK2/3 and βarr2 protein levels in livers of 6- and 24-mo-old rats. In these experiments, livers from old rats exhibited an approximately twofold increase in GRK2/3 protein levels relative to levels in livers from young adult animals (Fig. 6). A significant increase in βarr2 protein levels was also observed with age in rat liver (Fig. 7). These data suggest that increased expression of GRK2/3 and βarr2 may play a role in modifying β-AR internalization during aging.
Fig. 6.
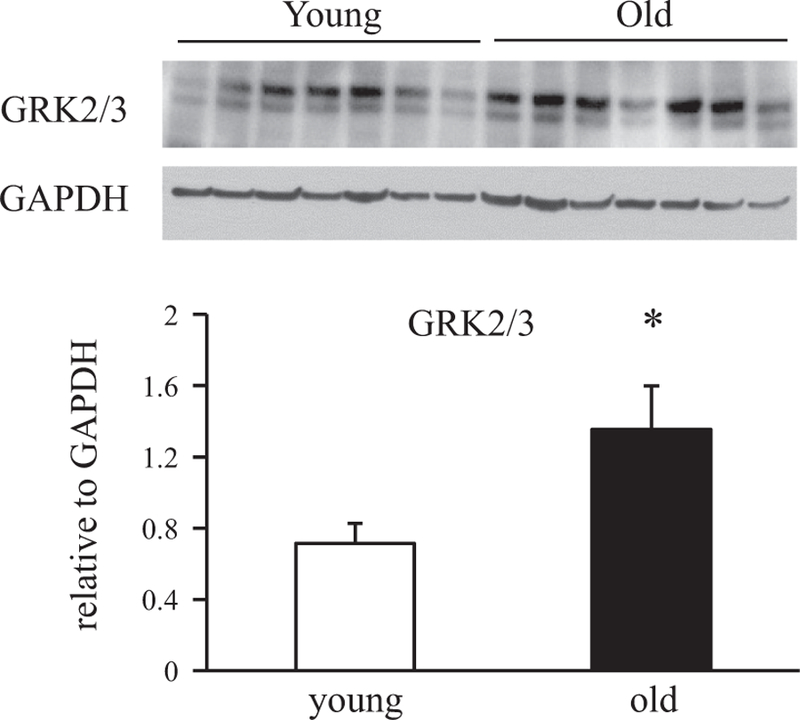
Effects of age on G protein-coupled receptor kinase (GRK) protein expression levels in rat liver. Protein levels of GRK2/3 in whole liver lysates from 6- and 24-mo-old rats were determined by Western blotting. All lysates for Western blotting were prepared at the same time and processed in parallel. Top: immunoblots of GRK2/3 (both bands) in livers from young and old animals. GAPDH was used as loading control. Bottom: proteins levels were quantified as integrated intensity and then normalized to the values of loading controls. Bar values are means ± SE from 7 young and 7 old animals. *P = 0.03 vs. value at 6 mo.
Fig. 7.
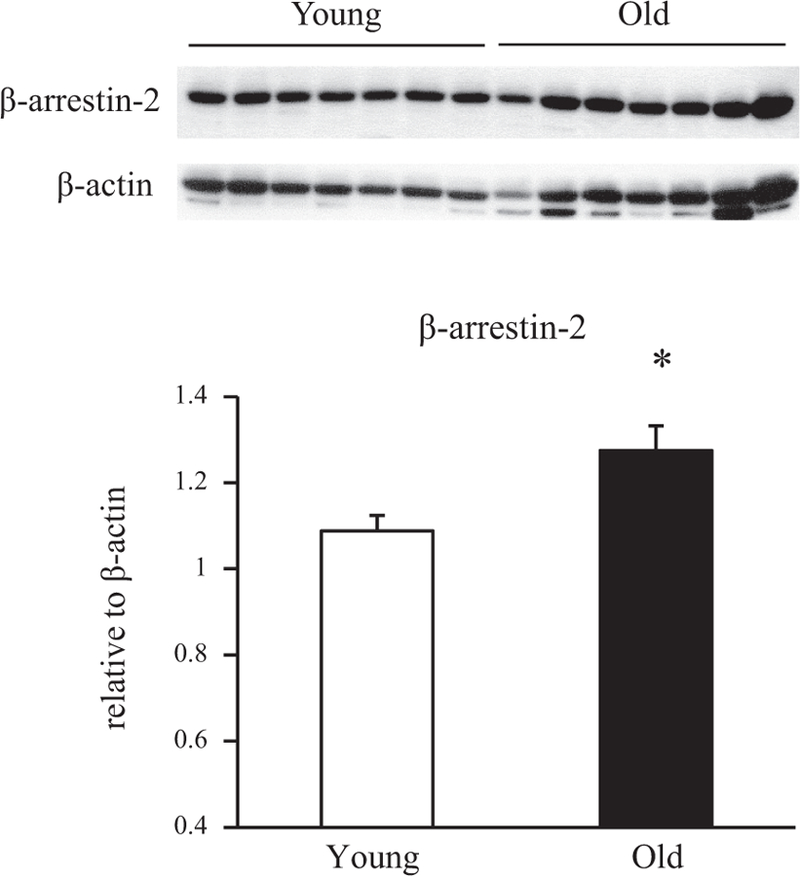
β-Arrestin 2 (βarr2) protein levels in livers from young and old rats. Protein levels of βarr2 in whole liver lysates from 6- and 24-mo-old rats were determined by Western blotting. All lysates were prepared at the same time and processed in parallel. Top: representative immunoblots of βarr2 in livers from young and old animals (n = 7 in each group). Bottom: protein levels were quantified as integrated intensity and then normalized to the values of respective loading controls. Bar values are means ± SE from 7 young and 7 old animals. *P = 0.018 vs value at 6 mo.
DISCUSSION
The results of this study implicate age-related changes in expression of hepatic β-ARs, GRK2/3, and β-arrestin-2 likely underlying previously reported increases in β-AR-responsive signaling, glucose output, and lipid accumulation in livers of aging rats (11, 13, 14, 24, 26, 27, 49). We have demonstrated that β-AR density in membrane preparations from isolated rat hepatocytes increases with senescent aging and that the increase in receptor content in cells from old animals is attenuated by food restriction (Fig. 1). We have further shown by competition-binding studies a mixed population of β1- and β2-AR subtypes in rat livers over the adult lifespan, with a trend for an age-related increase in the density of β2-ARs (Fig. 2 and Table 1). Expression of both β-AR subtype mRNAs in rat liver increases with age (Fig. 3); in apparent contrast to these results, comparison of β-AR subtype protein levels in livers from young and old rats reveals no change with age in β1-AR content yet an age-related decrease in β2-AR levels (Figs. 4 and 5). Finally, we have observed increased expression of GRK2/3 and βarr2 proteins in livers of old rats compared with young animals (Figs. 6 and 7).
To our knowledge, the present investigation is the first to show that the age-related increase in β-AR density previously observed in whole liver preparations from rats (11, 14, 20, 24) reflects a senescent change in the receptor content of parenchymal liver cells, i.e., at the level of hepatocytes. That the increased number of β-AR binding sites observed in hepatocytes from 24-mo-old rats does in fact represent a phenomenon of cellular aging is substantiated by the finding that food restriction, long known to extend the lifespan of rats and delay or prevent age-related changes in hormone action and other physiological processes (24), blocks the increase in β-AR binding occurring at advanced age. Our observations further extend earlier work in the Fischer 344 male rat model of aging by demonstrating that the postmaturational increase in hepatic β-AR binding apparent in previous comparisons of adult (4–6 or 12 mo old) and senescent (24 mo old) animals (14, 20, 24) more precisely corresponds to increased hepatocyte β-AR density occurring only late in the lifespan, with receptor density remaining stable between 7 and 18 mo of age and increasing thereafter by two- to threefold at 24 mo. Moreover, the increase in hepatocyte β-AR binding occurring late in life appears to be closely linked to β-adrenergic-sensitive adenylate cyclase (AC) activation and glycogenolytic functional responses, both of which exhibited equivalent two- to threefold increases between 20 and 24 mo of age in earlier studies of hepatocytes from aging Fischer 344 male rats (10, 27). In these same studies, the late-life increases in AC and glucose output responses to adrenergic stimulations were, like the accompanying increase in β-AR number, also attenuated by food restriction (10).
A primary aim of this study was to investigate the expression of hepatic β1- and β2-ARs in young versus old rats and thereby provide some insight into the relative contributions of the individual receptor subtypes to the age-related increases in β-AR binding linked to AC signaling and downstream cellular functions. The two β-AR subtypes exhibit considerable differences in G protein coupling, AC activation, and feedback regulation. In numerous studies in which the receptor subtypes have been expressed individually in cells lacking intrinsic β-ARs, the β2-AR has exhibited greater high-affinity agonist binding (i.e., formation of the high-affinity agonist-receptor-G protein ternary complex), increased functional coupling to stimulatory G protein (Gs)-mediated AC activation, and a higher degree of agonist-induced regulation via GRK- and/or arrestin-mediated receptor desensitization, sequestration/internalization, and downregulation when compared with the properties displayed by the β1-AR subtype (15, 16, 32, 52, 61). Moreover, although both receptor subtypes appear to be subject to agonist-induced internalization by clathrin-mediated endocytosis, internalized β2- and β1-AR receptors may be sorted into different intracellular compartments (32).
Our findings in competition-binding experiments using receptor subtype-selective antagonists (Table 1 and Fig. 2) confirm and extend earlier results by others (35, 54) showing a mixed population of β1- and β2-AR receptors in livers of young adult and senescent rats. In our experiments, individual competition-binding curves revealed a greater degree of binding by both β1- and β2-AR selective antagonists in liver preparations from old rats compared with young animals (Fig. 2). As in earlier studies, our results are in general consistent with about equal numbers of β1- and β2-AR-binding sites in liver over the adult lifespan of the rat, although we observed a trend toward an age-related increase in the density of β2-ARs (Table 1). Previously, isoproterenol stimulation of hepatic AC activity in liver preparations from old rats was found to be inhibited to a greater extent by β2-AR-selective antagonist than by β1-AR antagonists (54). Overall then, our results and those of others suggest that increased content of both β1- and β2-AR subtypes may contribute to enhanced β-AR binding and signaling in liver of senescent rats but that β2-AR signaling could predominate at advanced age. Definitive conclusions regarding the relative contributions of β1- and β2-AR subpopulations to increased total β-AR density in livers of aging rats would require additional experiments employing more detailed competition-binding curves, i.e., with larger numbers of data points, to improve precision of estimated numbers of the two receptor subtypes. It should also be noted that in early studies comparing coupling of β-ARs (irrespective of subtype) to G protein in liver preparations from young and old rats, we and others found no age-related differences in physical coupling, assessed by high-affinity agonist binding (i.e., KH, or high-affinity dissociation constant for isoproterenol) and guanine nucleotide induced conversion of receptors to a low-affinity binding state (11, 14, 24), or in functional coupling to AC, measured as the concentration of β-adrenergic agonist producing half-maximal AC response (EC50) (22). Moreover, an increase with age in the proportion of hepatic β-ARs in the high-affinity binding state was reported in one study (14) but not in others (11, 24). In general, the binding and functional properties of β-ARs measured in these earlier studies would appear to be of limited sensitivity in distinguishing age-related differences in a mixed population of hepatic β-AR subtypes.
In the present study, steady-state levels of β1- and β2-AR mRNAs in rat liver, measured by qRT-PCR, were found to increase up to threefold during postmaturational aging (Fig. 3), providing evidence that increased numbers of hepatic β-AR binding sites in livers of old animals may well reflect age-related changes in expression of the two receptor subtypes. The current findings extend those of an earlier preliminary report from our laboratory in which increased β1-AR mRNA levels in livers of senescent compared with young adult rats were demonstrable by semiquantitative RT-PCR, whereas Northern analysis was not sufficiently sensitive to detect the age-related increase in hepatic β2-AR mRNA levels revealed in the present work by qRT-PCR (20). The mechanism(s) by which β1- and β2-AR transcript levels increase in livers of aging rats has not been determined. Transcriptional and posttranscriptional processes are known to govern β-AR subtype expression in liver and other tissues. For example, β1- and β2-ARs in multiple species are transcriptionally activated or repressed by hormones and transcription factors acting at response elements in the 5′-flanking promoter regions of the two receptor subtype genes, whereas at the posttranscriptional level, stability, or turnover, of β1- and β2-AR mRNAs appears to be regulated via protein binding to A + U rich elements within the 3′-untranslated regions of the β-AR mRNAs (5, 37). Interestingly, both types of regulatory processes have been invoked to account for changes of β2-AR mRNA levels in rat liver occurring during early postnatal development (2, 3); but whether related modifications of hepatic β-AR transcripts might also occur during postmaturational aging remains to be studied.
In apparent contrast to the observed increases with age in β-AR-binding sites and mRNA levels of both β-AR subtypes in rat liver, immunoblot and immunohistochemistry analyses demonstrated a decrease in hepatic β2-AR protein levels with no change in β1-AR protein levels during aging (Figs. 4 and 5). It should be noted here that in immunohistochemistry experiments, β2- but not β1-ARs were found to be distributed preferentially in pericentral regions (zone 3) of liver parenchyma (Fig. 5C). Although of unclear significance, this finding could be related to the steep oxygen gradient existing from proximal (periportal) to distal (pericentral) regions of the liver acinus. Differences in oxygen tension are recognized to play a key role in regulation of “metabolic zonation,” i.e., hepatic region-specific metabolic functions, under physiological conditions and also in modulation of liver disease (21). For example, low oxygen tension favors optimal glycogenolytic activity in pericentral hepatocytes, and hepatic steatosis in nonalcoholic fatty liver disease, which is most commonly localized to zone 3 (58), has been linked to pericentral hypoxia (21, 34). In separate studies, a pathway of oxygen-responsive β2-AR regulation has been identified, in which hydroxylation and ubiquitination of β2- (but not β1-) ARs by the von Hippel-Lindau tumor suppressor protein (pVHL)-E3 ligase complex target receptors for downregulation by proteasomal degradation; under hypoxic conditions, the activity of this degradative pathway is markedly diminished, thereby increasing β2-AR receptor abundance (56). Our own previous studies have related increased β-AR binding and signaling functions in liver of aging rats to enhanced β-adrenergic responsive glycogenolysis and lipid accumulation (13, 27), with a specific role for the β2-AR subtype implicated in hepatic steatosis with age (49). Evidence of hepatic hypoxia at advanced age is variable; a preliminary immunohistochemical study assessing nitroimidazole-adduct formation under hypoxic conditions failed to detect differences in pericentral or periportal hypoxia between liver specimens from young adult vs. senescent rats, although the nitroimidazole pimonidazole marker utilized in this study was apparently sensitive only to dramatic decreases in intracellular oxygen tension (8). Whether more moderate decreases in oxygen tension of pericentral hepatocytes might play a role in zone-specific increases in β2-AR mediated glucose output and/or fat accumulation during aging is in our view a provocative hypothesis deserving further investigation. Although in the current study pericentral β2-AR staining appeared to decline with age (Fig. 5C), as did total hepatic β2-AR protein levels (Figs. 4 and 5, A and B), the status of β-AR-binding sites coupled with downstream signaling in pericentral hepatocytes from aging animals remains to be determined (also see discussion in the following paragraph).
An approach to reconciling discordant findings from receptor-binding and mRNA experiments with receptor protein level measurements may be suggested in the context of additional experiments (Figs. 6 and 7) showing increases with age in hepatic protein levels of GRK2/3 and βarr2. Rapid signal termination of agonist-activated GPCRs occurs via GRK- and βarr-mediated receptor desensitization and sequestration/internalization, whereas in response to prolonged agonist exposure receptors undergo downregulation by complex processes involving proteolytic degradation within lysosomes (4, 53). In the case of β2-ARs, downregulation appears to be initiated by typical GRK-mediated receptor phosphorylation and binding of the phosphorylated receptor to the βarr2 adaptor protein, followed by β2-AR internalization and degradation requiring ubiquitination of βarr2 and β2-AR, respectively; it should be noted that ubiquitin-dependent lysosomal degradation of β2- ARs is of no clear relationship to the oxygen-dependent process of proteasomal degradation described above (48, 56). Downregulation of β2-ARs might be expected to be amplified in tissues expressing greater levels of GRK2/3 and βarr2, such as in liver of senescent rats. Also in this regard, β-adrenergic responsiveness of a number of tissues (excluding liver) declines with age, likely as a reflection of heightened desensitization or downregulation of β-ARs exposed for prolonged periods to increased circulating concentrations of catecholamines in older animals (9, 17, 43, 44, 51, 55). Moreover, age-related increases in the expression of GRKs and βarr have been invoked as mediators of reduced β-AR-responsive vasorelaxation observed in rats during postmaturational aging (46, 47). Earlier studies by others have demonstrated that, unlike β2-ARs, β1-ARs are resistant to agonist-mediated downregulation via lysosomal degradation (31). In view of the above considerations then, reduced β2-AR but not β1-AR protein levels in livers of senescent rats could relate to an increase with age in receptor-degradative activity preferentially affecting the β2-AR subtype. Similarly, insofar as blood catecholamine levels, like oxygen tension, exhibit a steep zonal concentration gradient in liver (21), preferential distribution of immunoreactive β2-ARs (but not β1-ARs) in pericentral areas of liver from both young and old animals (Fig. 5C) could reflect, at least in part, zone-specific differences in agonist-mediated downregulation differentially affecting the two β-AR subpopulations. It should be emphasized, however, that no prior studies have comprehensively investigated agonist-mediated trafficking and downregulation of β-AR subpopulations in liver during aging.
Notwithstanding an hypothesized increase in hepatic β2-AR downregulation during aging, a relationship between age-related changes in hepatic β-AR-binding sites and immunoreactive receptor levels assessed in the current study remains elusive and has yet to be determined. Immunoblots and immunohistochemical analysis revealing a decline with age in β2- but not β1-AR levels were conducted using whole liver preparations and thus suggest a decrease in the total β-AR pool from livers of old animals. In contrast, increased β-AR binding in hepatocyte membranes prepared from old rats likely reflects an age-related change in a more limited subcellular fraction of β-ARs, i.e., plasma membrane receptors functionally linked to effector signaling. Importantly, it is the finding of increased numbers of β-AR-binding sites coupled with activation of AC-linked signaling during aging that is unique to liver, whereas decreased immunoreactive β2-AR content consistent with augmented agonist-mediated receptor downregulation is an age-related characteristic shared by liver with other tissues demonstrating reduced β-adrenergic responsiveness with age (43, 44, 51, 55). In this context, the increases in steady-state mRNA levels of both β-AR subtypes observed in liver from old rats (Fig. 3) assume significance in possibly resolving the apparent discrepancies in the present data. Agonist-responsive downregulation of β2-ARs is thought to occur not only via proteolytic degradation but also by β2-AR mRNA destabilization or possibly modulated β2-AR gene transcription, leading to reduced steady-state receptor mRNA levels (53). Future studies will be required to determine whether the increase in β-adrenergic responsiveness observed in liver of senescent animals might be related to disrupted downregulation pathways involving β2-AR gene transcription and/or mRNA stability. Of note is that whereas β1-ARs do appear to be resistant to agonist-mediated downregulation characteristic of β2-ARs (31), stability of β1-AR protein content despite increasing steady-state β1-AR mRNA levels in liver during aging suggests an age-related increase in β1-AR turnover by an unclear mechanism.
Perspectives and Significance
The present study, together with earlier findings from our laboratory and by others, strengthens the contention that increased expression of β-ARs in rat liver during aging augments β-adrenergic signaling pathway activities with deleterious consequences on hepatic glucose and lipid metabolism. Our results may implicate a preferential role for age-related changes in expression of the β2-AR subtype, which would appear to be subject to dynamic agonist-mediated regulation in the context of increased circulating concentrations of catecholamines in aging animals. Involvement of the β2-AR in age-related changes in liver metabolism would likely be of relevance to equivalent processes in older humans insofar as β-ARs in human liver plasma membranes are predominantly of the β2-AR subtype (28). Expression changes with age in the hepatic β1-AR, however, are not excluded by the current data in rats. Additional studies are suggested, as discussed above, to determine the mechanisms underlying modulated expression of one or more β-AR subtypes in liver during aging and thereby to identify possible therapeutic targets relevant to metabolic diseases of human aging such as type 2 diabetes mellitus and nonalcoholic fatty liver disease.
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. Robert I. Gregerman, whose leadership in the area of GPCRs and aging has inspired not only the present work but also a generation of gerontological investigations proceeding from his earliest studies in this field beginning more than four decades ago.
GRANTS
This work was supported by a Veterans Administration Merit Review Award (1I01BX001744–01 to A. Kamat) and a Kronos Longevity Research Institute Award (to M. S. Katz).
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Arner P, Engfeldt P, Hellström L, Lönnqvist F, Wahrenberg H, Sonnenfeld T, Brönnegård M. Beta-adrenoreceptor subtype expression in human liver. J Clin Endocrinol Metab 71: 1119–1126, 1990. doi: 10.1210/jcem-71-5-1119. [DOI] [PubMed] [Google Scholar]
- 2.Baeyens DA, Cornett LE. Association of hepatic β2-adrenergic receptor gene transcript destabilization during postnatal development in the Sprague-Dawley rat with a Mr 85,000 protein that binds selectively to the β2-adrenergic receptor mRNA 3′-untranslated region. J Cell Physiol 163: 305–311, 1995. doi: 10.1002/jcp.1041630211. [DOI] [PubMed] [Google Scholar]
- 3.Baeyens DA, McGraw DW, Jacobi SE, Cornett LE. Transcription of the beta2-adrenergic receptor gene in rat liver is regulated during early postnatal development by an upstream repressor element. J Cell Physiol 175: 333–340, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 4.Black JB, Premont RT, Daaka Y. Feedback regulation of G protein-coupled receptor signaling by GRKs and arrestins. Semin Cell Dev Biol 50: 95–104, 2016. doi: 10.1016/j.semcdb.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaxall BC, Pende A, Wu SC, Port JD. Correlation between intrinsic mRNA stability and the affinity of AUF1 (hnRNP D) and HuR for A+U-rich mRNAs. Mol Cell Biochem 232: 1–11, 2002. doi: 10.1023/A:1014819016552. [DOI] [PubMed] [Google Scholar]
- 6.Cardani R, Zavanella T. Immunohistochemical localization of beta 1-adrenergic receptors in the liver of male and female F344 rat. Histochem Cell Biol 116: 441–445, 2001. doi: 10.1007/s00418-001-0340-8. [DOI] [PubMed] [Google Scholar]
- 7.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55: 2005–2023, 2012. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 8.Cheluvappa R, Hilmer SN, Kwun SY, Cogger VC, Le Couteur DG. Effects of old age on hepatocyte oxygenation. Ann NY Acad Sci 1114: 88–92, 2007. doi: 10.1196/annals.1396.007. [DOI] [PubMed] [Google Scholar]
- 9.Cizza G, Pacak K, Kvetnansky R, Palkovits M, Goldstein DS, Brady LS, Fukuhara K, Bergamini E, Kopin IJ, Blackman MR. Decreased stress responsivity of central and peripheral catecholaminergic systems in aged 344/N Fischer rats. J Clin Invest 95: 1217–1224, 1995. doi: 10.1172/JCI117771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dax EM, McNair CL, Partilla JS, Hymer TK, Kohn SR, Gregerman RI, Katz MS. Food restriction (FR) modulates age-related changes in beta-adrenergic stimulated glycogenolysis in hepatocytes of Fischer 344 rats. The Gerontologist 28: 137A–138A, 1988. [Google Scholar]
- 11.Dax EM, Partilla JS, Piñeyro MA, Gregerman RI. Beta-adrenergic receptors, glucagon receptors, and their relationship to adenylate cyclase in rat liver during aging. Endocrinology 120: 1534–1541, 1987. doi: 10.1210/endo-120-4-1534. [DOI] [PubMed] [Google Scholar]
- 12.Faulkner JL, Szcykalski LM, Springer F, Barnes JL. Origin of interstitial fibroblasts in an accelerated model of angiotensin II-induced renal fibrosis. Am J Pathol 167: 1193–1205, 2005. doi: 10.1016/S0002-9440(10)61208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh PM, Shu ZJ, Zhu B, Lu Z, Ikeno Y, Barnes JL, Yeh CK, Zhang BX, Katz MS, Kamat A. Role of β-adrenergic receptors in regulation of hepatic fat accumulation during aging. J Endocrinol 213: 251–261, 2012. doi: 10.1530/JOE-11-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham SM, Herring PA, Arinze IJ. Age-associated alterations in hepatic beta-adrenergic receptor/adenylate cyclase complex. Am J Physiol 253: E277–E282, 1987. doi: 10.1152/ajpendo.1987.253.3.E277. [DOI] [PubMed] [Google Scholar]
- 15.Green SA, Holt BD, Liggett SB. Beta 1- and beta 2-adrenergic receptors display subtype-selective coupling to Gs. Mol Pharmacol 41: 889–893, 1992. [PubMed] [Google Scholar]
- 16.Green SA, Liggett SB. A proline-rich region of the third intracellular loop imparts phenotypic beta 1-versus beta 2-adrenergic receptor coupling and sequestration. J Biol Chem 269: 26215–26219, 1994. [PubMed] [Google Scholar]
- 17.Gruenewald DA, Matsumoto AM. Aging of the endocrine system and selected endrocrine disorders. In: Principles of Geriatric Medicine and Gerontology (edited by Hazzard WR, Blass JP, Halter JB, Ouslander JG, Tinetti ME). Chicago, IL: McGraw-Hill, 2003, p. 819–835. [Google Scholar]
- 18.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 21: 518–524, 1998. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 19.Hellgren I, Sylvén C, Magnusson Y. Study of the beta1 adrenergic receptor expression in human tissues: immunological approach. Biol Pharm Bull 23: 700–703, 2000. doi: 10.1248/bpb.23.700. [DOI] [PubMed] [Google Scholar]
- 20.Jin W Age-related increase of beta1-adrenergic receptor gene expression in rat liver: a potential mechanism contributing to increased beta-adrenergic receptor density and responsiveness during aging. J Recept Signal Transduct Res 30: 24–30, 2010. doi: 10.3109/10799890903358206. [DOI] [PubMed] [Google Scholar]
- 21.Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology 31: 255–260, 2000. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- 22.Kalish MI, Katz MS, Piñeyro MA, Gregerman RI. Epinephrine-and glucagon-sensitive adenylate cyclases of rat liver during aging: Evidence for membrane instability associated with increased enzymatic activity. Biochim Biophys Acta 483: 452–466, 1977. doi: 10.1016/0005-2744(77)90073-0. [DOI] [PubMed] [Google Scholar]
- 23.Kamat A, Ghosh PM, Glover RL, Zhu B, Yeh CK, Choudhury GG, Katz MS. Reduced expression of epidermal growth factor receptors in rat liver during aging. J Gerontol A Biol Sci Med Sci 63: 683–692, 2008. doi: 10.1093/gerona/63.7.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz MS. Food restriction modulates beta-adrenergic-sensitive adenylate cyclase in rat liver during aging. Am J Physiol 254: E54–E62, 1988. doi: 10.1152/ajpendo.1988.254.1.E54. [DOI] [PubMed] [Google Scholar]
- 25.Katz MS, Boland SR, Schmidt SJ. Developmental changes of beta-adrenergic receptor-linked adenylate cyclase of rat liver. Am J Physiol 248: E712–E718, 1985. doi: 10.1152/ajpendo.1985.248.6.E712. [DOI] [PubMed] [Google Scholar]
- 26.Katz MS, Dax EM, Gregerman RI. Beta adrenergic regulation of rat liver glycogenolysis during aging. Exp Gerontol 28: 329–340, 1993. doi: 10.1016/0531-5565(93)90060-Q. [DOI] [PubMed] [Google Scholar]
- 27.Katz MS, McNair CL, Hymer TK, Boland SR. Emergence of beta adrenergic-responsive hepatic glycogenolysis in male rats during postmaturational aging. Biochem Biophys Res Commun 147: 724–730, 1987. doi: 10.1016/0006-291X(87)90990-9. [DOI] [PubMed] [Google Scholar]
- 28.Kawai Y, Powell A, Arinze IJ. Adrenergic receptors in human liver plasma membranes: predominance of beta 2- and alpha 1-receptor subtypes. J Clin Endocrinol Metab 62: 827–832, 1986. doi: 10.1210/jcem-62-5-827. [DOI] [PubMed] [Google Scholar]
- 29.Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci U S A 98: 1601–1606, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krief S, Lönnqvist F, Raimbault S, Baude B, Van Spronsen A, Arner P, Strosberg AD, Ricquier D, Emorine LJ. Tissue distribution of beta 3-adrenergic receptor mRNA in man. J Clin Invest 91: 344–349, 1993. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang W, Austin S, Hoang Q, Fishman PH. Resistance of the human beta 1-adrenergic receptor to agonist-mediated down-regulation. Role of the C terminus in determining beta-subtype degradation. J Biol Chem 278: 39773–39781, 2003. doi: 10.1074/jbc.M304482200. [DOI] [PubMed] [Google Scholar]
- 32.Liang W, Curran PK, Hoang Q, Moreland RT, Fishman PH. Differences in endosomal targeting of human β1- and β2-adrenergic receptors following clathrin-mediated endocytosis. J Cell Sci 117: 723–734, 2004. doi: 10.1242/jcs.00878. [DOI] [PubMed] [Google Scholar]
- 33.Lynch GS, Ryall JG. Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev 88: 729–767, 2008. doi: 10.1152/physrev.00028.2007. [DOI] [PubMed] [Google Scholar]
- 34.Mantena SK, Vaughn DP Jr, Andringa KK, Eccleston HB, King AL, Abrams GA, Doeller JE, Kraus DW, Darley-Usmar VM, Bailey SM. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J 417: 183–193, 2009. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazzeo RS, Podolin DA, Henry V. Effects of age and endurance training on beta-adrenergic receptor characteristics in Fischer 344 rats. Mech Ageing Dev 84: 157–169, 1995. doi: 10.1016/0047-6374(95)01643-0. [DOI] [PubMed] [Google Scholar]
- 36.Minneman KP, Hedberg A, Molinoff PB. Comparison of beta adrenergic receptor subtypes in mammalian tissues. J Pharmacol Exp Ther 211: 502–508, 1979. [PubMed] [Google Scholar]
- 37.Morris AJ, Malbon CC. Physiological regulation of G protein-linked signaling. Physiol Rev 79: 1373–1430, 1999. doi: 10.1152/physrev.1999.79.4.1373. [DOI] [PubMed] [Google Scholar]
- 38.Munson PJ, Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem 107: 220–239, 1980. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 39.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest 126: 12–22, 2016. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanghani MP, Scarpace PJ. Atypical beta-adrenergic receptors in rat liver: evidence for transient expression during aging. J Gerontol 49: B60–B64, 1994. doi: 10.1093/geronj/49.2.B60. [DOI] [PubMed] [Google Scholar]
- 41.Sano M, Yoshimasa T, Yagura T, Yamamoto I. Non-homogeneous distribution of beta 1- and beta 2-adrenoceptors in various human tissues. Life Sci 52: 1063–1070, 1993. doi: 10.1016/0024-3205(93)90199-D. [DOI] [PubMed] [Google Scholar]
- 42.Santulli G, Iaccarino G. Pinpointing beta adrenergic receptor in ageing pathophysiology: victim or executioner? Evidence from crime scenes. Immun Ageing 10: 10, 2013. doi: 10.1186/1742-4933-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santulli G, Lombardi A, Sorriento D, Anastasio A, Del Giudice C, Formisano P, Béguinot F, Trimarco B, Miele C, Iaccarino G. Age-related impairment in insulin release: the essential role of β(2)-adrenergic receptor. Diabetes 61: 692–701, 2012. doi: 10.2337/db11-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scarpace PJ, Tumer N, Mader SL. Beta-adrenergic function in aging. Basic mechanisms and clinical implications. Drugs Aging 1: 116–129, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schutzer WE, Reed JF, Bliziotes M, Mader SL. Upregulation of G protein-linked receptor kinases with advancing age in rat aorta. Am J Physiol Regul Integr Comp Physiol 280: R897–R903, 2001. doi: 10.1152/ajpregu.2001.280.3.R897. [DOI] [PubMed] [Google Scholar]
- 47.Schutzer WE, Xue H, Reed J, Oyama T, Beard DR, Anderson S, Mader SL. Age-related β-adrenergic receptor-mediated vasorelaxation is changed by altering G protein receptor kinase 2 expression. Vascul Pharmacol 55: 178–188, 2011. doi: 10.1016/j.vph.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 294: 1307–1313, 2001. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 49.Shi Y, Shu ZJ, Xue X, Yeh CK, Katz MS, Kamat A. β2-Adrenergic receptor ablation modulates hepatic lipid accumulation and glucose tolerance in aging mice. Exp Gerontol 78: 32–38, 2016. doi: 10.1016/j.exger.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Smith JS, Rajagopal S. The β-arrestins: Multifunctional regulators of G protein-coupled receptors. J Biol Chem 291: 8969–8977, 2016. doi: 10.1074/jbc.R115.713313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Supiano MA, Hogikyan RV. High affinity platelet alpha 2-adrenergic receptor density is decreased in older humans. J Gerontol 48: B173–B179, 1993. doi: 10.1093/geronj/48.5.B173. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki T, Nguyen CT, Nantel F, Bonin H, Valiquette M, Frielle T, Bouvier M. Distinct regulation of beta 1- and beta 2-adrenergic receptors in Chinese hamster fibroblasts. Mol Pharmacol 41: 542–548, 1992. [PubMed] [Google Scholar]
- 53.Tsao P, Cao T, von Zastrow M. Role of endocytosis in mediating downregulation of G-protein-coupled receptors. Trends Pharmacol Sci 22: 91–96, 2001. doi: 10.1016/S0165-6147(00)01620-5. [DOI] [PubMed] [Google Scholar]
- 54.Van Ermen A, Van de Velde E, Vanscheeuwijck P, Fraeyman N. Influence of age on the beta 1- and beta 2-adrenergic receptors in rat liver. Mol Pharmacol 42: 649–655, 1992. [PubMed] [Google Scholar]
- 55.Xiao RP, Tomhave ED, Wang DJ, Ji X, Boluyt MO, Cheng H, Lakatta EG, Koch WJ. Age-associated reductions in cardiac beta1- and beta2- adrenergic responses without changes in inhibitory G proteins or receptor kinases. J Clin Invest 101: 1273–1282, 1998. doi: 10.1172/JCI1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie L, Xiao K, Whalen EJ, Forrester MT, Freeman RS, Fong G, Gygi SP, Lefkowitz RJ, Stamler JS. Oxygen-regulated beta(2)-adrenergic receptor hydroxylation by EGLN3 and ubiquitylation by pVHL. Sci Signal 2: ra33, 2009. doi: 10.1126/scisignal.2000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeh CK, Hymer TK, Sousa AL, Zhang BX, Lifschitz MD, Katz MS. Epidermal growth factor upregulates beta-adrenergic receptor signaling in a human salivary cell line. Am J Physiol Cell Physiol 284: C1164–C1175, 2003. doi: 10.1152/ajpcell.00343.2002. [DOI] [PubMed] [Google Scholar]
- 58.Yeh MM, Brunt EM. Pathological features of fatty liver disease. Gastroenterology 147: 754–764, 2014. doi: 10.1053/j.gastro.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 59.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol 40: 657–670, 1985. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 60.Zhang BX, Yeh CK, Hymer TK, Lifschitz MD, Katz MS. EGF inhibits muscarinic receptor-mediated calcium signaling in a human salivary cell line. Am J Physiol Cell Physiol 279: C1024–C1033, 2000. doi: 10.1152/ajpcell.2000.279.4.C1024. [DOI] [PubMed] [Google Scholar]
- 61.Zhou XM, Pak M, Wang Z, Fishman PH. Differences in desensitization between human beta 1- and beta 2-adrenergic receptors stably expressed in transfected hamster cells. Cell Signal 7: 207–217, 1995. doi: 10.1016/0898-6568(94)00091-O. [DOI] [PubMed] [Google Scholar]