Abstract
Proteoglycans located in basement membranes, the nanostructures underling epithelial and endothelial layers, are unique in several respects. They are usually large, elongated molecules with a collage of domains that share structural and functional homology with numerous extracellular matrix proteins, growth factors and surface receptors. They mainly carry heparan sulfate side chains and these contribute not only to storing and preserving the biological activity of various heparan sulfate-binding cytokines and growth factors, but also in presenting them in a more “active configuration” to their cognate receptors. Abnormal expression or deregulated function of these proteoglycans affect cancer and angiogenesis, and are critical for the evolution of the tumor microenvironment. This review will focus on the functional roles of the major heparan sulfate proteoglycans from basement membrane zones: perlecan, agrin and collagen XVIII, and on their roles in modulating cancer growth and angiogenesis.
INTRODUCTION
Basement membrane proteoglycans comprise a select group of high molecular-weight proteins which are almost universally decorated with heparan sulfate side chains (Yurchenco et al., 2004). Three main basement membrane heparan sulfate proteoglycans (HSPGs) have been well characterized: perlecan, collagen type XVIII and agrin. The first identified HSPG of basement membranes was perlecan, a modular proteoglycan with homology to growth factors and proteins involved in cell growth, lipid metabolism, adhesion, and homo- and heterotypic interactions (Hassell et al., 2003; Iozzo, 1998; Fuki et al., 2000). Collagen XVIII is a hybrid collagen/proteoglycan and a member of the multiplexin gene family together with the closely related collagen XV, which bears chondroitin sulfate side chains instead of HS chains (Oh et al., 1994a). Finally, the HSPG agrin represents an abundant constituent of most basement membranes and possesses a specialized function at the neuromuscular junction (Bezakova and Rüegg, 2003). The genes encoding these three HSPG protein cores are highly conserved and carry disparate biological functions, ranging from maintenance of basement membrane homeostasis to modulation of growth factor activity and angiogenesis. Moreover, some of these gene products are expressed in avascular tissues, such as cartilage, and in musculoskeletal and nervous tissues, where they modulate neuronal transmission and ocular development. Defects in some of these genes cause various human inherited disorders and often the disease phenotypes correlate well with those of mutant mice, flies and worms.
An emerging body of work supports the concept that these HSPGs have a dual function as pro- and anti-angiogenic factors (Iozzo and San Antonio, 2001). Via the HS chains, these proteoglycans can stimulate angiogenic signaling by sequestering, protecting and concentrating HS-binding growth factors such as FGF2, VEGF and PDGF, through which the HSGP-growth factor complex may be presented in a “biologically active” form to the cognate receptors. Alternatively, via proteolytic processing of their C-termini, these gene products can release powerful angiostatic fragments such as endostatin and endorepellin which can act in a paracrine function on sprouting endothelial cells, either locally or distantly.
This review will focus primarily on these three basement membrane HSPGs and will critically assess recent information related to their roles in regulating cancer growth and angiogenesis.
Perlecan, a multimodular and multifunctional proteoglycan
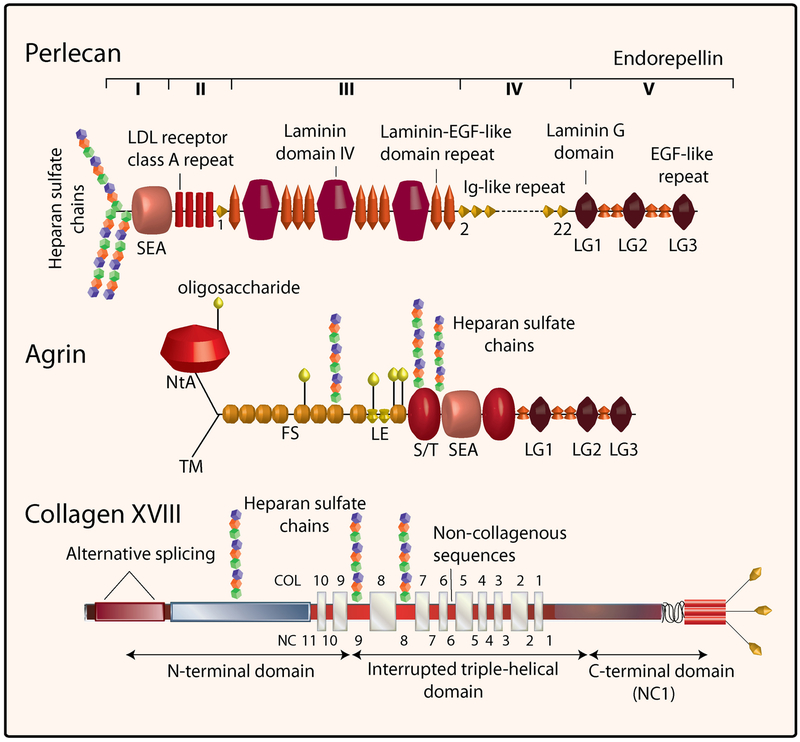
The designation for “perlecan” originates from studies using rotary shadowing electron microscopy which have shown a tortuous linear polymer with interspersed globular domains resembling a “string of pearls” (Hassell et al., 2003). Perlecan is a modular HSPG and is one of the largest single-chain polypeptides found in vertebrate and invertebrate animals (Iozzo, 1994; Iozzo et al., 1994; Iozzo, 1998; Whitelock et al., 2008). The five modules of perlecan are collated from protein units evolutionarily related to molecules involved in cell growth, lipid uptake and metabolism, intercellular interactions and adhesion (Fig.1). The perlecan protein core is ~470 kDa and, together with several O-linked oligosaccharides and as many as four HS chains, three in domain I and one potential in domain V (Hassell et al., 2003), it can reach a molecular weight of over 800 kDa. An intriguing question about perlecan’s evolutionary biology which has been recently raised (Farach-Carson and Carson, 2007) is: What is the advantage of linking 46 protein modules into a gigantic polypeptide over the synthesis of individual protomers? Perhaps, the answer lies in the generation of a protein scaffold, such as a long heterofunctional protein. The large size of perlecan’s protein core which spans ~200 nm in length, together with the three HS chains which could span an additional 60 nm in three dimensions, make perlecan an appropriate linker between extracellular matrix and cell surface receptors or complexes of receptors. The various modules of perlecan and the HS chains have a large repertoire of molecular interactions that include association with numerous HS-binding growth factors such as FGF2, FGF7,VEGF and PDGF, and other proteins which are constituents of basement membranes (Aviezer et al., 1994; Mongiat et al., 2000; Mongiat et al., 2001; Whitelock and Iozzo, 2005; Iozzo, 2005). Perlecan inhibits thrombosis (Nugent et al., 2000), and exhibits adhesive (Whitelock et al., 1999) or anti-adhesive (Klein et al., 1995) properties presumably by differentially affecting surface receptors such as α2β1 integrin (Bix and Iozzo, 2005).
Fig. 1.
Structural domains of human perlecan, agrin and collagen XVIII. The five domains of perlecan are in Roman numerals from the N- to the C-terminus. The other domains of agrin and collagen XVII are listed or detailed in the text.
Our characterization of zebrafish perlecan provided the first genetic evidence linking perlecan function to developmental angiogenesis (Zoeller et al., 2008). By morpholino-mediated perlecan knockdown we found that angiogenic blood vessel development of the intersegmental and sub-intestinal vessels was largely inhibited in the absence of perlecan. Vasculogenesis, the formation of the dorsal aorta and posterior cardinal vein, was observed to proceed normally, suggesting perlecan was only required for angiogenesis. A closer analysis revealed that the morphant vessels were non-functional, as evidenced by the lack of circulatory flow. Combined, these results suggested that perlecan functions at multiple levels during the angiogenic cascade, possibly influencing endothelial cell migration or proliferation and the events of lumen formation. The exact nature of perlecan function within these contexts is the current focus of our investigation.
Preceding the examination of zebrafish perlecan, analysis of murine perlecan expression in wild-type (Handler et al., 1997) and perlecan-deficient animals (Arikawa-Hirasawa et al., 1999; Costell et al., 1999) also supported a role for perlecan in vascular development. The perlecan-null mouse exhibits embryonic lethality associated with hemorrhage in the pericardial cavity or respiratory failure, and vessel modeling defects in the development of the great arteries and coronary artery pattern (Costell et al., 2002; González-Iriarte et al., 2003). The perlecan-null mouse does not exhibit any striking defects in developmental angiogenesis, a surprising observation given the widespread distribution of perlecan throughout the vascular basement membrane. Such observations brought to light the possibility of compensatory mechanisms which, perhaps mediated by collagen XVIII, could counter the lack of perlecan. The generation of a double knockout mouse (collagen XVIII and perlecan HS deficient) (Rossi et al., 2003), which exhibits compounded ocular defects compared to either alone, supports such principles. Furthermore, analysis of the perlecan HS-deficient mouse alone reveals altered growth factor modulation in numerous settings linked to angiogenesis (Tran et al., 2004; Zhou et al., 2004).
Beyond development, the role of perlecan has been defined within the context of tumor growth angiogenesis (Cohen et al., 1994; Iozzo et al., 1997; Mathiak et al., 1997). Structurally, perlecan co-localizes and supports new tumor blood vessel development (Iozzo et al., 1994). In addition to a structural role, perlecan serves a signaling function via the HS chains which are capable of binding and modulating numerous growth factors involved with angiogenesis. Perhaps the best studied are members of the FGF family, including the pro-angiogenic FGF2 (Nugent and Iozzo, 2000). Perlecan binds FGF2 via HS, promotes receptor activation and ultimately downstream signaling which supports mitogenesis and angiogenesis (Iozzo and Murdoch, 1996). Targeted deletion of perlecan-specific HS reduces matrix binding of FGF2 and results in enhanced smooth muscle cell proliferation (Tran et al., 2004), while the lack of HS inhibits wound healing and FGF2-induced angiogenesis and tumor growth (Zhou et al., 2004). Similarly, targeted knockdown of perlecan reduced growth factor response in prostate cancer cells (Savoré et al., 2005) as evidenced by decreased tumor growth and angiogenesis. Accordingly, these findings were similar to those observed in additional tumor cell lines (Aviezer et al., 1997; Sharma et al., 1998; Adatia et al., 1998) and supported by comparable observations in colon cancer cells harboring a somatic mutation in perlecan (Ghiselli et al., 2001). Taken together, these results implicate perlecan as a crucial component of growth factor regulation. The HSPG perlecan could be envisioned to coordinate a matrix gradient, protect growth factors from the environment, concentrate and/or present ligand to receptor, all within a context-specific fashion.
A recent report has further established the role of perlecan HS and tumor angiogenesis, with specific reference to VEGF-VEGFR2 modulation. Using hepatoblastoma xenografts treated with anti-VEGFR therapy, vessel recovery over time was associated with an increase in perlecan and heparanase expression around tumor vessels (Kadenhe-Chiweshe et al., 2008). Accordingly, the potential heparanase-mediated release of HS-bound VEGF (Reiland et al., 2004) or proteolytic protein core processing liberating growth factor-bound fragments (Whitelock et al., 1996), was found to support VEGFR2 activation and downstream survival signaling (Kadenhe-Chiweshe et al., 2008).
Collagen XVIII, an hybrid proteoglycan
Collagen type XVIII possesses features of collagens and proteoglycans and represents a member of the “multiplexin” family (Marneros and Olsen, 2005). The multiplexins, which include collagens XV and XVIII, are characterized by multiple triple-helix domains with interruptions (Oh et al., 1994a). Structurally, collagen type XVIII (Fig. 1) consists of ten interrupted collagenous domains, flanked by noncollagenous domains at the N- and C-termini. Collagen XVIII also harbors three Ser-Gly consensus binding sites for the attachment of HS glycosaminoglycan chains (Dong et al., 2003) and is an HSPG (Halfter et al., 1998). Collagen XVIII expression can be detected throughout the vascular and epithelial basement membranes of human and mouse tissues, with an overall distribution similar to that of perlecan (Marneros and Olsen, 2005).
Collagen XVIII is a homotrimer comprised of three identical α1 chains.The human COL18A1 gene, localized to chromosome 21 and spanning 105 kb, comprises 43 exons (Oh et al., 1994b) which generate three protein variants derived from alternative promoter usage and splicing events (Elamaa et al., 2003; Saarela et al., 1998; Suzuki et al., 2002). The human and mouse α1(XVIII) collagen amino acid sequences exhibit 79% identity (Rehn et al., 1994) and an overall general conservation. Accordingly, the function of collagen XVIII has been explored through the analysis of a murine collagen XVIII knockout. Characterization of the Col18a1−/− mouse revealed that collagen XVIII is not required for viability or fertility but is essential for proper eye development (Fukai et al., 2002). Basement membrane thickening in a sub-line of Col18a1−/− mice also suggests a role for collagen XVIII during maintenance of the basement membrane, and revealed that ~20% of Col18a1−/− mice exhibit hydrocephalus as a consequence of alterations in the epithelial basement membrane of the choroid plexuses (Utriainen et al., 2004).
Ocular defects associated with collagen XVIII-deficient mice include disruption of the iris manifested by rupture of the posterior iris pigment epithelial cell layer (Robinson et al., 2006; Ylikärppä et al., 2003), iris basement membrane thickening (Robinson et al., 2006), improper macrophage-like cell migration (Robinson et al., 2006), abnormal flattening of the ciliary body (Robinson et al., 2006; Ylikärppä et al., 2003), and excessive deposits below the retinal pigment epithelium which compromise function and result in visual impairment (Marneros et al., 2004). Interestingly, these observations are in line with the phenotype associated with Knobloch syndrome caused by a mutation in the human COL18A1 gene (Menzel et al., 2004). Despite the widespread expression of collagen XVIII throughout virtually all vascular basement membranes, evidence of no significant vascular defects in the Col18a1−/− mouse, with the exception of the eye, suggests that collagen XVIII is not essential for vascular development (Fukai et al., 2002). The phenotype associated with the eye vasculature is characterized by delayed regression of the hyaloid vessels and disrupted retinal vascular outgrowth (Fukai et al., 2002). These observations support a central role for collagen XVIII specifically during ocular vessel development and maturation (Hurskainen et al., 2005).
The lack of collagen XVIII enhances angiogenesis in aortic explants derived from the Col18a1−/− mice when compared to wild-type animals which is associated with altered endothelial cell adhesion to the matrix (Li and Olsen, 2004). Collagen XVIII has also been linked to neovascularization and maintenance of vascular permeability during atherosclerosis since targeted deletion of collagen type XVIII increases these processes during disease progression (Moulton et al., 2004). Additionally, the Col18a1−/− animals display enhanced angiogenesis during wound healing (Seppinen et al., 2008), but do not experience enhanced tumor growth (Fukai et al., 2002). Thus, collagen XVIII has been suggested to play a negative regulatory, but not an essential, role during angiogenesis in certain contexts.
Agrin, an assembling proteoglycan
The third basement membrane HSPG, agrin, was first isolated from the electric organs of the Pacific electric ray as an agent responsible for acetylcholine receptor (AChR) clustering, and thus the eponym agrin from the Greek “ageirein” meaning “to assemble” (Nitkin et al., 1987). Subsequently, agrin was shown to be expressed in mammalians with similar AChR-clustering activity. Most work on deciphering agrin function in the mammalian body has hence been focused on agrin’s contribution to the differentiation of the postsynaptic apparatus in neuromuscular junctions. Mice deficient in all agrin forms (Lin et al., 2001), as well as mice lacking nerve-specific agrin and hypomorphic for all other forms of agrin (Gautam et al., 1996), die neonatally due to respiratory failure secondary to improper excitation of the diaphragmatic muscle. Apart from its involvement in neuromuscular synapses, agrin is also important for establishing and maintaining both central nervous system and immunological synapses (Bezakova and Rüegg, 2003; Zhang et al., 2006). However, little is known about agrin’s function outside the synaptic location.
Structurally, agrin shares with perlecan a rather intriguing multimodular organization (Fig. 1), and more complexity to agrin can be added by at least four sites of alternative splicing. The agrin N-terminus can be spliced to generate either a Type II transmembrane form of agrin which is expressed in brain, or a basement membrane-associated form containing the N-terminal-agrin (NtA) domain which is widely expressed throughout the body. The latter form gains via the NtA-domain high affinity for the laminin γ1 chain’s coiled-coil domain. After the initial N-terminal domain lie a stretch of nine follistatin-like (FS) repeats, also known as Kazal-type protein inhibitor domains. The last two repeats are separated by an insertion of two laminin EGF-like (LE) domains. The central part of agrin after the initial FS repeats is comprised of two Ser/Thr (S/T)-rich domains of which the last one can be alternatively spliced to generate an X+/− form (Bezakova and Rüegg, 2003). The two S/T domains are interspersed by a sperm protein enterokinase and agrin (SEA) module (Bezakova and Rüegg, 2003). SEA modules are patterns of secondary structure found in sperm protein, enterokinase, perlecan, agrin, and mucin-like glycoprotein, suggested to be involved in O-glycosylation. The specific function of each unit in the briefly-described domain stretch is largely unknown; however, taken together the agrin N-terminal part can be considered as a structural building block enabling correct presentation of the agrin signaling C-terminus. One important aspect of the N-terminal and central agrin backbone is that it carries HS chains, and rotary shadowing electron microscopy has revealed three attachment sites for HS chains (Denzer et al., 1998). Agrin can also be modified with chondroitin sulfate chains, at least in vitro (Winzen et al., 2003). An agrin fragment comprised of all the above described domains inhibits neuronal outgrowth independently of HS and chondroitin sulfate modifications (Baerwald-De La Torre et al., 2004). A portion containing just the FS-like repeats requires HS modification to inhibit neurite outgrowth, whereas the portion containing the LE repeats does not need to be modified by HS to inhibit neurite outgrowth (Baerwald-De La Torre et al., 2004). With the involvement of the HS-chains agrin has been shown to bind FGF2, thrombospondin, β-amyloid peptide, N-CAM, and the protein tyrosine phosphatase δ (Burgess et al., 2002). It is important to note that the contribution of HS chains from agrin is not essential for life since mice that are deficient in full-length agrin, but muscle-specifically express a genetically-engineered miniaturized form of agrin lacking the HS attachment sites, are viable and fertile (Lin et al., 2008).
The C-terminal portion of agrin contains, in concordance with perlecan and collagen XVIII, the major receptor interaction sites. With regard to its modular organization, the C-terminus of agrin shows close homology to perlecan domain V/endorepellin. Both harbor three LG repeats separated by EGF-like repeats. A structural difference in arrangement between the agrin and perlecan C-terminus is that the agrin LG1 domain is preceded by an EGF-like repeat and only one EGF-like repeat separates the LG2 and LG3 domains. The agrin C-terminus can be alternatively spliced at two different sites producing Y+/− (in chicken, named A+/−) and Z+/− (in chicken B+/−) agrin (Burgess et al., 2002). This splicing generates agrin forms with major differences in receptor affinity. In non-neuronal tissue Y(−)Z(−)-agrin is the predominant form. The agrin receptors will be reviewed below.
The organs richest in agrin content are brain, lung, and kidney (Gesemann et al., 1998; Groffen et al., 1998). Apart from various synapses as mentioned above, agrin is strongly expressed around the blood vessels of the brain, retina (Witmer et al., 2001), lung, and kidney; recently it has become evident that leukocytes also express agrin (Zhang et al., 2006). The low but specific expression seen in other organs can be accounted for by the contribution of agrin from the vasculature (Groffen et al., 1998). Changes in the agrin content of the vasculature can be seen in pathological conditions. Agrin staining of microvessels in Alzheimer’s brains show ragged, punctuated, and irregular patterns and a wider diameter as compared to vessels in non-diseased brains (Verbeek et al., 1999). Furthermore, agrin expression is highly variable in human glioblastomas; it is completely absent in most small blood vessels, but remains in the basement membrane of most larger-vessels (Warth et al., 2004). Interestingly, agrin-negative vessels are predominantly found in the central areas of the tumor (Warth et al., 2004). Agrin can be considered to be a marker of tumor angiogenesis in the liver. In the healthy human liver, agrin can be detected in minor amounts in the basement membranes of blood vessels and bile ducts (Tátrai et al., 2006; Groffen et al., 1998). In contrast, agrin is markedly deposited in proliferating bile ductules, in the newly-formed septal vessels in hepatic cirrhosis and the angiogenic network of malignant hepatocellular carcinomas (Tátrai et al., 2006). Activated myofibroblasts, vascular smooth muscle cells, and epithelial cells all likely contribute to the aberrant production of agrin (Tátrai et al., 2006). Also cholangiocarcinoma, a primary liver carcinoma originating from the bile ductular cells, shows agrin overexpression in the newly-formed blood vessels (Batmunkh et al., 2007). In the early stages of cholangiocarcinoma, agrin is highly expressed but then decreases or is absent from the later stages of poorly-differentiated cholangiocarcinomas (Batmunkh et al., 2007). This suggests that agrin might be crucial in supporting the initial growth of the tumor.
In sum, agrin’s effect on tumor angiogenesis is likely context dependent. Agrin expression seems to be protective against disorganized angiogenesis in glioblastomas, whereas in various hepatic malignancies it seems to support tumor angiogenesis, at least in the initial stages of tumor development.
C-terminal portions of basement membrane proteoglycans with angiostatic activity
The processing of extracellular matrix proteins, especially those derived from basement membrane zone components, generates fragments which possess anti-angiogenic properties (Sund et al., 2005; Nyberg et al., 2005; Clamp and Jayson, 2005; Whitelock et al., 2008). The focus of the next three sections will seek to explore the current literature describing the C-terminal regions of type XVIII collagen, perlecan and agrin.
Endostatin, the C-terminal anti-angiogenic fragment of collagen type XVIII
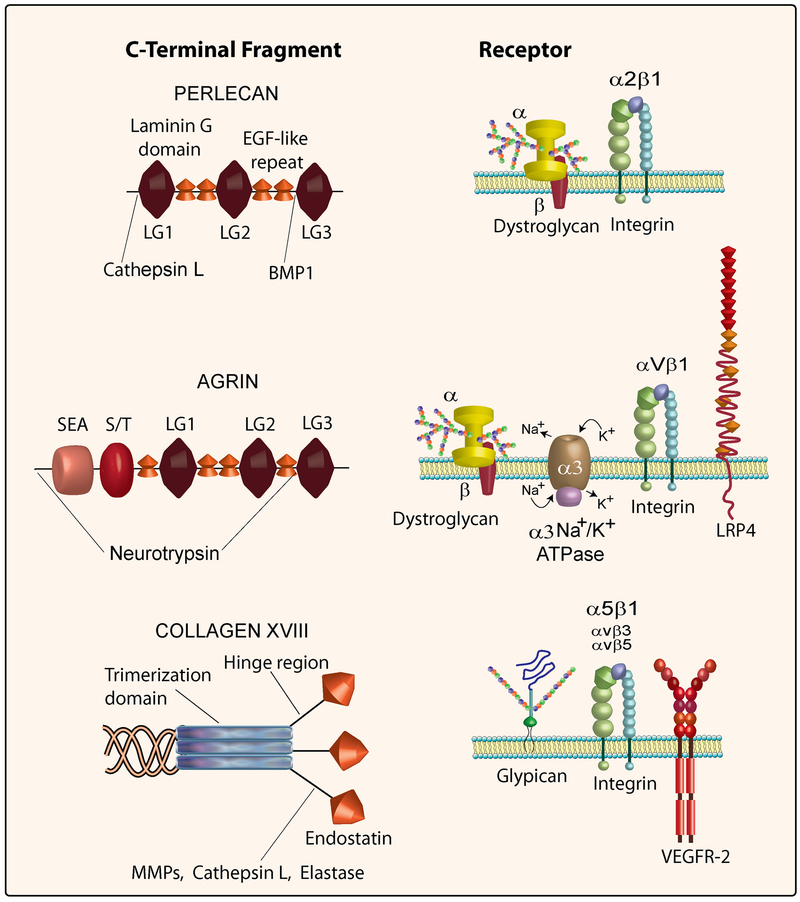
Collagen type XVIII harbors the C-terminal anti-angiogenic fragment, endostatin (Zatterstrom et al., 2000). Endostatin is proteolytically derived from the NC1 domain (Fig. 1) of the collagen type XVIII protein core (Ferreras et al., 2000). The NC1 domain consists of an N-terminal trimerization region, a central hinge region sensitive to proteolytic activity and the C-terminal endostatin domain (Sasaki et al., 1998) (Fig. 2). Endostatin has been linked to cell surface receptors including integrins α5β1, αvβ3 and αvβ5 (Rehn et al., 2001; Sudhakar et al., 2003), glypican (Karumanchi et al., 2001) and VEGFR2 (Kim et al., 2002).
Fig. 2.
Schematic representation of the C-termini of perlecan, agrin and collagen XVIII (left) and their respective cognate receptors (right). Also annotated are the locations of various protease cleavage sites generating intact modules or fragments thereof from the three polypeptides. For additional details see the text.
Endostatin anti-angiogenic properties were originally characterized in a mouse tumor model. Essentially, endostatin treatment significantly reduced tumor growth via interference with tumor angiogenesis (O’Reilly et al., 1997). Subsequent investigations have further explored endostatin within the context of tumor angiogenesis and even expanded to include choroidal neovascularization (Marneros et al., 2007) and wound healing (Seppinen et al., 2008). Taken together, the endostatin anti-angiogenic effect includes a total gene expression reprogramming (Abdollahi et al., 2004) which ultimately disrupts endothelial cell migration (Kuo et al., 2001; Sudhakar et al., 2003) and survival (Dhanabal et al., 1999). Endostatin’s signaling network is vast but clearly shows a linkage to pathways involved in angiogenesis such as those evoked by VEGF signaling and thrombospondin. Specifically, endostatin down-regulates several key components of the VEGF signaling cascade and, at the same time, stimulates the synthesis of thrombospondin, a powerful angiostatic protein (Abdollahi et al., 2004). These findings reveal an integrated role for endostatin during vascular remodeling that, together with endostatin-evoked suppression of c-myc (Shichiri and Hirata, 2001), would ultimately reinforce its angiostatic effects (Wickström et al., 2005). It is not surprising that high levels of circulating endostatin reduce tumor burden and block the formation of pulmonary metastases (Sauter et al., 2001), and that high serum levels of endostatin are found in patients with Down syndrome caused by trisomy of chromosome 21, where collagen XVIII-encoding gene is located (Zorick et al., 2001). The latter can provide an explanation for the relatively decreased incidence of various solid tumors observed in Down syndrome patients.
Endorepellin, the C-terminal anti-angiogenic fragment of perlecan
In a search for novel proteins interacting with perlecan’s domain V using the yeast two-hybrid system, we identified the C-terminal portion of collagen XVIII, including endostatin (Mongiat et al., 2003). It was readily discovered that perlecan’s domain V blocked endothelial cell migration and capillary morphogenesis both in vitro and in vivo, and thus we named this fragment “endorepellin” to signify its repulsive activity against endothelial cells (Mongiat et al., 2003). Endorepellin interacts specifically with the α2β1 integrin (Bix et al., 2007; Bix et al., 2004), a key receptor involved in angiogenesis (Senger et al., 2002; Sweeney et al., 2003). In endothelial cells, endorepellin triggers a signaling cascade that leads to disruption of the actin cytoskeleton and thus to cytostasis (Bix et al., 2004; Iozzo, 2005; Bix and Iozzo, 2005; Bix and Iozzo, 2008). Using a proteomic approach, several key proteins involved in angiogenesis including β-actin were significantly down-regulated by exposing endothelial cells to recombinant endorepellin (Zoeller and Iozzo, 2008). Importantly, systemic delivery of human recombinant endorepellin to tumor xenograft-bearing mice causes a marked suppression of tumor growth and metabolic rate mediated by a sustained down-regulation of the tumor angiogenic network (Bix et al., 2006). Experiments using siRNA-mediated block of endogenous α2β1 integrin or α2β1-null animals have definitively proven that this is a key receptor for endorepellin, and thus for the perlecan protein core, and have further demonstrated that endorepellin targets the tumor xenograft vasculature in an α2β1 integrin-dependent manner (Woodall et al., 2008). Interestingly, endorepellin has been detected as a released product in the upper proliferating zone of fetal growth plate (West et al., 2006), suggesting that cartilage endorepellin might counteract blood vessel invasion in cartilage.
The distal laminin-like globular domain, LG3 (Fig. 2), possesses most of the biological activity (Bix et al., 2004) and can be released from the parent molecule by BMP-1/Tolloid-like metalloproteinases (Gonzalez et al., 2005) which recognize an ND dipeptide, Asn4196 and Asp4197, which is highly conserved across species including human, mouse, Drosophila and zebrafish (Zoeller et al., 2008). This highly-conserved region within the perlecan protein core together with the high conservation of BMP-1/Tolloid-like metalloproteinases suggests that liberation of LG3 might be of physiological importance. Mutations in LG3 producing molecules with lower or no affinity for calcium (Gonzalez et al., 2005) disrupt LG3 angiostatic activity. It is noteworthy that the proximal two globular domains of endorepellin, LG1 and LG2, might be occupied by a number of high-affinity ligands such as α-dystroglycan (Fig. 2) and endostatin within basement membrane zones and on cell surfaces (Bix and Iozzo, 2005; Bix and Iozzo, 2008). In contrast, LG3 might be relatively accessible and thus likely to be released by partial proteolysis, a process that is common to most LG domains of laminin.
Over a decade ago, Oda et al. (Oda et al., 1996) reported the presence of perlecan’s LG3 fragments in the urine of end-stage renal patients. The LG3 fragments had N-terminal residues identical to those found in endothelial cells by us. Following this initial observation, a number of investigators have detected LG3 in several pathological conditions (Table 1). For instance, similar LG3 fragments have been found elevated in the urine of patients with chronic allograft nephropathy (O’Riordan et al., 2008), and in the amniotic fluid of pregnant women (Gianazza et al., 2007) with a marked increase in women with symptoms of premature rupture of fetal membranes (Vuadens et al., 2003; Thadikkaran et al., 2005) and those carrying trisomy 21 (Down syndrome) fetuses (Tsangaris et al., 2006). In addition, endorepellin fragments have been detected in normal human blood (Adkins et al., 2002), in the urine of children with sleep apnea (Krishna et al., 2006), in the secretome of pancreatic and colon carcinoma cells (Grønborg et al., 2006; Gonzalez et al., 2005), and in the media conditioned by apoptotic endothelial cells (Raymond et al., 2004; Laplante et al., 2005). In endothelial cells, the released LG3 interacts with the α2β1 integrin receptor of fibroblasts and triggers a signaling cascade that leads to activation of an anti-apoptotic pathway and potentially to a fibrogenic response (Laplante et al., 2006). Recently, it has been shown that caspase-3 activation triggers extracellular cathepsin L release which in turn cleaves endorepellin (Cailhier et al., 2008). In fibrotic diseases, endothelial cell apoptosis precedes the recruitment of fibroblasts, and thus release of LG3 could affect not only angiogenesis, but also the production of collagen and the overall sclerotic response. This is another example of cell-specific context in which cryptic perlecan fragments might exert diverse effects.
Table 1.
Presence of perlecan’s LG3 module in various tissues and biological fluids, and potential functional implications.
| Location | Condition | Functional Implication | Reference |
|---|---|---|---|
| Urine | End-stage renal disease | Biomarker for vascular injury | (Oda et al., 1996) |
| Urine | Chronic allograft nephropathy | Biomarker for immune-mediated vascular injury | (O’Riordan et al., 2008) |
| Amniotic fluid* | Premature rupture of fetal membranes | Biomarker for fetal ischemia and vascular injury | (Vuadens et al., 2003; Thadikkaran et al., 2005) |
| Amniotic fluid | Mothers carrying Down Syndrome fetuses | Biomarker for abnormal fetal development and vascular injury | (Tsangaris et al., 2006) |
| Blood | Normal subjects and breast cancer patients | Reduced in breast cancer patient: role as anti-angiogenic factor | (Chang et al., 2008) |
| Secreted by endothelial cells | Endothelial cells undergoing apoptosis | Potential function as a paracrine inducer of fibrosis | (Raymond et al., 2004; Laplante et al., 2005; Laplante et al., 2006; Cailhier et al., 2008) |
| Secreted by various cancer cells | Pancreatic and colon carcinoma cells | Potentially linked to the highly turnover rate of transformed cells | (Grønborg et al., 2006; Gonzalez et al., 2005) |
| Urine | Children with sleep apnea | Potential biomarker of transient brain ischemia | (Krishna et al., 2006) |
Agrin LG3 was also found in the amniotic fluid of pregnant women with premature rupture of fetal membranes.
We propose that endorepellin/LG3 is liberated via partial proteolysis during tissue remodeling and cancer growth thereby representing an additional layer of control for angiogenesis (Iozzo, 2005). One possibility is that tumor growth might be enhanced in vivo by a lack of circulating LG3. In line with this idea, circulating LG3 levels are reduced in patients with breast cancer (Chang et al., 2008), suggesting that reduced titers might be a useful biomarker for cancer progression and invasion. Recent studies using α2β1 integrin-null mice have reported an increased angiogenesis in the granulation tissue of wounded animals (Grenache et al., 2006; Zweers et al., 2007). Tumor angiogenesis is dependent on the “cancer cell context” in α2β1 integrin-null animals further corroborating the importance of cross-talk between the invading cancer cells and the tumor microenvironment (Zhang et al., 2008).
An endorepellin-like fragment in agrin
Most of the receptor binding and hence direct biological activity is confined to the C-terminal end of the agrin protein core. Intriguingly, this part can be proteolytically processed and released from the mature protein (Fig. 2). Accountable for this processing is the synaptic serine protease neurotrypsin (Reif et al., 2007). Neurotrypsin is primarily expressed in the pre-synaptic membrane of the neurons of the amygdala, cerebral cortex, and hippocampus, which are also regions with a particular high agrin content (Donahue et al., 1999). This protease has been suggested to be important for synapse reorganization and plasticity since a lack of neurotrypsin leads to mental retardation. Mice overexpressing neurotrypsin in motoneurons mimic agrin deficiency with broad, immature synapse endplates (Reif et al., 2007). Agrin has two cleavage sites for neurotrypsin at its C-terminus: one between the N-terminal S/T domain and the SEA domain and a second found just before the third LG-domain. This generates 110-, 90- and 22-kDa agrin fragments (Matsumoto-Miyai et al., 2009; Reif et al., 2007) (Fig. 2). There is evidence that processing of agrin also occurs outside the brain; agrin in kidney tubular basement membranes does not contain the C-terminal part, whereas in the glomerular basement membranes as well as in larger blood vessels of the kidney agrin is still intact (Groffen et al., 1998). Notably, agrin LG3 as well as perlecan LG3 have been found in the amniotic fluid of women with premature rupture of fetal membranes (Vuadens et al., 2003). The onset of neurotrypsin expression and its maximal expression corresponds to the onset and the maximal release of agrin fragments. In neurotrypsin-deficient mice no cleavage of agrin occurs, whereas released C-terminal agrin fragments can readily be detected in brain and kidney but not in the lungs of wild-type mice (Reif et al., 2007). These results indicate that this enzyme is the main protease responsible for agrin C-terminal processing.
Multiple receptors (Fig. 2) recognize the C-terminal region of agrin, both intact or released, and the specificity for receptor recognition is dependent on the agrin splice variant. Agrin LG2 binds the αvβ1 and an integrin β1 binding site contained in the EGF4-LG3 domains (Burgess et al., 2002). The highly glycosylated receptor α-dystroglycan shows affinity towards a large portion of the C-terminal stretch, and the binding is negatively influenced by the presence of the Y and Z inserts (Scotton et al., 2006). Recently, two novel agrin receptors were identified: the α3 Na+/K+ -ATPase and LRP-4. A 20-kDa agrin fragment at the C-terminus binds to and inhibits the α3 Na+/K+ receptor. Agrin inhibition of α3 Na+/K+ activity leads to membrane depolarization and increased action potential frequency both in vitro and ex vivo. The identification of α3 Na+/K+ as an agrin receptor suggests the possibility that agrin might be involved in cardiac disease since cardiomyocytes express both α3 Na+/K+ and agrin (Hilgenberg et al., 2006). Lrp4 was identified as the long-sought agrin receptor responsible for MuSK phosphorlyation and subsequent AChR clustering in muscles. LRP4 forms a complex with MuSK and the most C-terminal LG domain and the Z-splicing insert is enough for binding Lrp4 and to induce MuSK phosphorylation and the following AChR clustering (Kim et al., 2008). Lrp4 is also present in many developing tissues that do not express MuSK but the outcome of agrin ligation to Lrp4 in such a setting is currently unknown (Kim et al., 2008).
There is still very limited knowledge on how agrin signals through the integrin receptors and how these interactions influence muscle and other organs. More studies are needed to evaluate the role agrin plays in non-muscular tissues and the precise impact and function of the proteolytically-released C-terminal endorepellin-like fragment. Given the structural similarities to endorepellin, the dual processing, the high expression of agrin in blood vessels and its affinity for multiple receptors, it can be speculated that the endorepellin-like domain of agrin might also regulate blood vessel homeostasis.
Conclusions and future directions
The three members of the basement membrane HSPG family described in this review have a long evolutionary history insofar as they are expressed in lifeforms ranging from worms to man, thus encompassing >500 million years of evolution. It is not surprising, therefore, that parts of these ancestral molecules carry intrinsic information and bioactivities that are quite powerful when released from the parent molecule. A common and expanding theme is that endostatin, endorepellin and likely agrin LG3 represent members of a family of cryptic domains residing within larger parent molecules that often act in a dominant negative manner. For example, both endostatin and endorepellin can affect endothelial cell biology via integrin receptors and eventually both lead to endothelial cell dysfunction and inhibition of angiogenesis. Both molecules are found in the blood and in several biological fluids, including urine and amniotic fluid, where they can be indicative of blood vessel damage. These molecules might eventually become biomarkers for diseases such as cancer, as byproducts of the high turnover rate of malignant cells, or perhaps for osteoarthritis where continuous remodeling of bone and cartilage occurs. Another view is that circulating levels of the C-terminal HSPGs might be continuously released to add an additional layer of control for abnormal vessel sprouting during various pathologies including cancer, rheumatoid arthritis and vascular retinopathies.
The discovery of these bioactive fragments has opened new avenues of research and interest in developing antitumor agents. Utilization of HSPG fragments either as protein- or peptide-based pharmacological agents might represent a good therapeutic rationale, especially if provided in combination with other tumor suppressive compounds. Finally, a defined mechanism of action for each of these fragments must be elucidated prior to large clinical trials along with delineation of appropriate biomarkers for efficacy and toxicity testing.
ACKNOWLEDGEMENTS
We thank A. McQuillan for help with the graphics, and C.C. Clark for critical evaluation of the manuscript. This work was supported in part by National Institutes of Health grants RO1 CA39481, RO1 CA47282, and RO1 CA120975 (R.V.I.). J.J.Z. was supported by NIH NRSA Training Grant T32 AA07463.
REFERENCES
- Abdollahi A, Hahnfeldt P, Maercker C, Gröne H-J, Debus J, Ansorge W, Folkman J, Hlatky L, and Huber PE (2004). Endostatin’s antioangiogenic signaling network. Mol. Cell 13, 649–663. [DOI] [PubMed] [Google Scholar]
- Adatia R, Albini A, Carlone S, Giunciuglio D, Benelli R, Santi L, and Noonan DM (1998). Suppression of invasive behavior of melanoma cells by stable expression of anti-sense perlecan cDNA. Ann. Oncol 8, 1257–1261. [DOI] [PubMed] [Google Scholar]
- Adkins JN, Varnum SM, Auberry KJ, Moore RJ, Angell NH, Smith RD, Springer DL, and Pounds JG (2002). Toward a human blood serum proteome. Analysis by multidimensional separation coupled with mass spectrometry. Mol. Cell. Proteom 1, 947–955. [DOI] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Watanabe E, Takami H, Hassell JR, and Yamada Y (1999). Perlecan is essential for cartilage and cephalic development. Nature Genet. 23, 354–358. [DOI] [PubMed] [Google Scholar]
- Aviezer D, Hecht D, Safran M, Eisinger M, David G, and Yayon A (1994). Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell 79, 1005–1013. [DOI] [PubMed] [Google Scholar]
- Aviezer D, Iozzo RV, Noonan DM, and Yayon A (1997). Suppression of autocrine and paracrine functions of basic fibroblast growth factor by stable expression of perlecan antisense cDNA. Mol. Cell. Biol 17, 1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerwald-De La Torre K, Winzen U, Halfter W, and Bixby JL (2004). Glycosaminoglycan-dependent and -independent inhibition of neurite outgrowth by agrin. J. Neurochem 90, 50–61. [DOI] [PubMed] [Google Scholar]
- Batmunkh E, Tátrai P, Szabó E, Lódi C, Holczbauer A, Páska C, Kupcsulik P, Kiss A, Schaff Z, and Kovalszky I (2007). Comparison of the expression of agrin, a basement membrane heparan sulfate proteoglycan, in cholangiocarcinoma and hepatocellular carcinoma. Human Pathol. 38, 1508–1515. [DOI] [PubMed] [Google Scholar]
- Bezakova G and Rüegg MA (2003). New insights into the roles of agrin. Nature Rev. Mol. Cell Biol 4, 295–308. [DOI] [PubMed] [Google Scholar]
- Bix G, Castello R, Burrows M, Zoeller JJ, Weech M, Iozzo RA, Cardi C, Thakur MT, Barker CA, Camphausen KC, and Iozzo RV (2006). Endorepellin in vivo: targeting the tumor vasculature and retarding cancer growth and metabolism. J. Natl. Cancer Inst 98, 1634–1646. [DOI] [PubMed] [Google Scholar]
- Bix G, Fu J, Gonzalez E, Macro L, Barker A, Campbell S, Zutter MM, Santoro SA, Kim JK, Höök M, Reed CC, and Iozzo RV (2004). Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through the α2β1 integrin. J. Cell Biol 166, 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix G, Iozzo RA, Woodall B, Burrows M, McQuillan A, Campbell S, Fields GB, and Iozzo RV (2007). Endorepellin, the C-terminal angiostatic module of perlecan, enhances collagen-platelet responses via the α2β1 integrin receptor. Blood 109, 3745–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix G and Iozzo RV (2005). Matrix revolutions: “tails” of basement-membrane components with angiostatic functions. Trends Cell Biol. 15, 52–60. [DOI] [PubMed] [Google Scholar]
- Bix G and Iozzo RV (2008). Novel interactions of perlecan: Unraveling perlecan’s role in angiogenesis. Microsc. Res 71, 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess RW, Dickman DK, Nunez L, Glass DJ, and Sanes JR (2002). Mapping sites responsible for interactions of agrin with neurons. J. Neurochem 83, 271–284. [DOI] [PubMed] [Google Scholar]
- Cailhier J-F, Sirois I, Raymond M-A, Lepage S, Laplante P, Brassard N, Prat A, Iozzo RV, Pshezhetsky AV, and Hebért M-J (2008). Caspase-3 activation triggers extracellular release of cathepsin L and endorepellin proteolysis. J. Biol. Chem 283, 27220–27229. [DOI] [PubMed] [Google Scholar]
- Chang JW, Kang U-B, Kim DH, Yi JK, Lee JW, Noh D-Y, Lee C, and Yu M-H (2008). Identification of circulating endorepellin LG3 fragment: Potential use as a serological biomarker for breast cancer. Proteomics Clin. Appl 2, 23–32. [DOI] [PubMed] [Google Scholar]
- Clamp AR and Jayson GC (2005). The clinical potential of antiangiogenic fragments of extracellular matrix proteins. Br. J. Cancer 93, 967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IR, Murdoch AD, Naso MF, Marchetti D, Berd D, and Iozzo RV (1994). Abnormal expression of perlecan proteoglycan in metastatic melanomas. Cancer Res. 54, 5771–5774. [PubMed] [Google Scholar]
- Costell M, Carmona R, Gustafsson E, González-Iriarte M, Fässler R, and Munoz-Chápuli R (2002). Hyperplastic conotruncal endocardial cushions and transposition of great arteries in perlecan-null mice. Circ. Res 91, 158–164. [DOI] [PubMed] [Google Scholar]
- Costell M, Gustafsson E, Aszódi A, Mörgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, and Fässler R (1999). Perlecan maintains the integrity of cartilage and some basement membranes. J. Cell Biol 147, 1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer AJ, Sculthess T, Fauser C, Schumacher B, Kammerer RA, Engel J, and Ruegg MA (1998). Electron microscopic structure of agrin and mapping of its binding site in laminin-1. EMBO J. 17, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanabal M, Ramchandran R, Waterman MJ, Lu H, Knebelmann B, Segal M, and Sukhatme VP (1999). Endostatin induces endothelial cell apoptosis. J. Biol. Chem 274, 11721–11726. [DOI] [PubMed] [Google Scholar]
- Donahue JE, Berzin TM, Rafii MS, Glass DJ, Yancopoulos GD, Fallon JR, and Stopa EG (1999). Agrin in Alzheimer’s disease: Altered solubility and abnormal distribution within microvasculature and brain parenchyma. Proc. Natl. Acad. Sci. USA 96, 6468–6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Cole GJ, and Halfter W (2003). Expression of collagen XVIII and localization of its glycosaminoglycan attachment sites. J. Biol. Chem 278, 1700–1707. [DOI] [PubMed] [Google Scholar]
- Elamaa H, Snellman A, Rehn M, Autio-Harmainen H, and Pihlajaniemi T (2003). Characterization of the human type XVIII collagen gene and proteolytic processing and tissue location of the variant containing a frizzled motif. Matrix Biol. 22, 427–442. [DOI] [PubMed] [Google Scholar]
- Farach-Carson MC and Carson DD (2007). Perlecan - a multifunctional extracellular proteoglycan scaffold. Glycobiology 17, 897–905. [DOI] [PubMed] [Google Scholar]
- Ferreras M, Felbor U, Lenhard T, Olsen BR, and Delaisse J (2000). Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 486, 247–251. [DOI] [PubMed] [Google Scholar]
- Fukai N, Eklund L, Marneros AG, Oh SP, Keene DR, Tamarkin L, Niemelä M, Ilves M, Li E, Pihlajaniemi T, and Olsen BR (2002). Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 21, 1535–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuki I, Iozzo RV, and Williams KJ (2000). Perlecan heparan sulfate proteoglycan. A novel receptor that mediates a distinct pathway for ligand catabolism. J. Biol. Chem 275, 25742–25750. [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, and Sanes JR (1996). Defective neuromuscular synaptogenesis in agrin-deficient mice. Cell 85, 525–535. [DOI] [PubMed] [Google Scholar]
- Gesemann M, Brancaccio A, Schumacher B, and Ruegg MA (1998). Agrin is a high-affinity binding protein of dystroglycan in non-muscle tissue. J. Biol. Chem 273, 600–605. [DOI] [PubMed] [Google Scholar]
- Ghiselli G, Eichstetter I, and Iozzo RV (2001). A role for the perlecan protein core in the activation of the keratinocyte growth factor receptor. Biochem. J 359, 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianazza E, Wait R, Begum S, Eberini I, Campagnoli M, Labo S, and Galliano M (2007). Mapping the 5–50-kDa fraction of human amniotic fluid proteins by 2-DE and ESI-MS. Proteomics Clin. Appl 1, 167–175. [DOI] [PubMed] [Google Scholar]
- Gonzalez EM, Reed CC, Bix G, Fu J, Zhang Y, Gopalakrishnan B, Greenspan DS, and Iozzo RV (2005). BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J. Biol. Chem 280, 7080–7087. [DOI] [PubMed] [Google Scholar]
- González-Iriarte M, Carmona R, Pérez-Pomares JM, Macías D, Costell M, and Munoz-Chápuli R (2003). Development of the coronary arteries in a murine model of transposition of great arteries. J. Mol. Cell. Cardio 35, 795–802. [DOI] [PubMed] [Google Scholar]
- Grenache DG, Zhang Z, Wells LE, Santoro SA, Davidson JM, and Zutter MM (2006). Wound healing in the α2β1 integrin-deficient mouse: altered keratinocyte biology and dysregulated matrix metalloproteinase expression. J. Invest. Dermatol 127, 455–466. [DOI] [PubMed] [Google Scholar]
- Groffen AJA, Buskens CAF, van Kuppevelt TH, Veerkamp JH, Monnens LAH, and van den Heuvel LPWJ (1998). Primary structure and high expression of human agrin in basement membranes of adult lung and kidney. Eur. J. Biochem 254, 123–128. [DOI] [PubMed] [Google Scholar]
- Grønborg M, Kristiansen TZ, Iwahori A, Chang R, Reddy R, Sato N, Molina H, Jensen ON, Hruban RH, Goggins MG, Maitra A, and Pandey A (2006). Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol. Cell. Proteom 5, 157–171. [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Schurer B, and Cole GJ (1998). Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J. Biol. Chem 273, 25404–25412. [DOI] [PubMed] [Google Scholar]
- Handler M, Yurchenco PD, and Iozzo RV (1997). Developmental expression of perlecan during murine embryogenesis. Dev. Dyn 210, 130–145. [DOI] [PubMed] [Google Scholar]
- Hassell JR, Yamada Y, and Arikawa-Hirasawa E (2003). Role of perlecan in skeletal development and diseases. Glycoconj. J 19, 263–267. [DOI] [PubMed] [Google Scholar]
- Hilgenberg LGW, Su H, Gu H, O’Dowd DK, and Smith MA (2006). α3Na+/K+-ATPase is a neuronal receptor for agrin. Cell 125, 359–369. [DOI] [PubMed] [Google Scholar]
- Hurskainen M, Eklund L, Hägg PO, Fruttiger M, Sormunen R, IIves M, and Pihlajaniemi T (2005). Abnormal maturation of the retinal vasculature in type XVIII collagen/endostatin deficient mice and changes in retinal glial cells due to lack of collagen types XV and XVIII. FASEB J. 19, 1564–1666. [DOI] [PubMed] [Google Scholar]
- Iozzo RV (1994). Perlecan: a gem of a proteoglycan. Matrix Biol. 14, 203–208. [DOI] [PubMed] [Google Scholar]
- Iozzo RV (1998). Matrix proteoglycans: from molecular design to cellular function. Annu. Rev. Biochem 67, 609–652. [DOI] [PubMed] [Google Scholar]
- Iozzo RV (2005). Basement membrane proteoglycans: from cellar to ceiling. Nature Rev. Mol. Cell Biol 6, 646–656. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Cohen IR, Grässel S, and Murdoch AD (1994). The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem. J 302, 625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV and Murdoch AD (1996). Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 10, 598–614. [PubMed] [Google Scholar]
- Iozzo RV, Pillarisetti J, Sharma B, Murdoch AD, Danielson KG, Uitto J, and Mauviel A (1997). Structural and functional characterization of the human perlecan gene promoter. Transcriptional activation by transforming growth factor-β via a nuclear factor 1-binding element. J. Biol. Chem 272, 5219–5228. [DOI] [PubMed] [Google Scholar]
- Iozzo RV and San Antonio JD (2001). Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J. Clin. Invest 108, 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadenhe-Chiweshe A, Papa J, McCrudden KW, Frischer J, Bae J-O, Huang J, Fisher J, Lefkowitch JH, Feirt N, Rudge J, Holash J, Yancopoulos GD, Kandel JJ, and Yamashiro DJ (2008). Sustained VEGF blockade results in microenvironmental sequestration of VEGF by tumors and persistent VEGF receptor-2 activation. Mol. Cancer. Res 6, 1–9. [DOI] [PubMed] [Google Scholar]
- Karumanchi SA, Jha V, Ramchandran R, Karihaloo A, Tsiokas L, Chan B, Dhanabai M, Hanai J-C, Venkataraman G, Shriver Z, Keiser N, Kalluri R, Zeng H, Mukhopadhyay D, Chen RL, Lander AD, Hagihara K, Yamaguchi Y, Sasisekarharan R, Cantley L, and Sukhatme VP (2001). Cell surface glypicans are low-affinity endostatin receptors. Mol. Cell 7, 811–822. [DOI] [PubMed] [Google Scholar]
- Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML, and Burden SJ (2008). Lrp4 is a receptor for agrin and forms a complex with MuSK. Cell 135, 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-M, Hwang S, Kim Y-M, Pyun B-J, Kim T-Y, Lee S-T, Gho YS, and Kwon Y-G (2002). Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J. Biol. Chem 277, 27872–27879. [DOI] [PubMed] [Google Scholar]
- Klein G, Conzelmann S, Beck S, Timpl R, and Müller CA (1995). Perlecan in human bone marrow: a growth-factor presenting, but anti-adhesive, extracellular matrix component for hematopoietic cells. Matrix Biol. 14, 457–465. [DOI] [PubMed] [Google Scholar]
- Krishna J, Shah ZA, Merchant M, Klein JB, and Gozal D (2006). Urinary protein expression patterns in children with sleep-disordered breathing: preliminary findings. Sleep Med. 7, 221–227. [DOI] [PubMed] [Google Scholar]
- Kuo CJ, LaMontagne KR, Garcia-Cardena G, Ackley BD, Kalman D, Park S, Christofferson R, Kamihara J, Ding Y-H, Lo K-M, Gillies S, Folkman J, Mulligan RC, and Javaherian K (2001). Oligomerization-dependent regulation of motility and morphogenesis by the collagen XVIII NC1/endostatin domain. J. Cell Biol 152, 1233–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante P, Raymond M-A, Labelle A, Abe J-I, Iozzo RV, and Hebért M-J (2006). Perlecan proteolysis induces α2β1 integrin and src-family kinases dependent anti-apoptotic pathway in fibroblasts in the absence of focal adhesion kinase activation. J. Biol. Chem 281, 30383–30392. [DOI] [PubMed] [Google Scholar]
- Laplante P, Raymond MA, Gagnon G, Vigneault N, Sasseville AM, Langelier Y, Bernard M, Raymond Y, and Hebért M-J (2005). Novel fibrogenic pathways are activated in response to endothelial apoptosis: implications in the pathophysiology of systemic sclerosis. J. Immunol 174, 5740–5749. [DOI] [PubMed] [Google Scholar]
- Li Q and Olsen BR (2004). Increased angiogenic response in aortic explants of collagen XVIII/endostatin-null mice. Am. J. Pathol 165, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Maj M, Bezakova G, Magyar JP, Brenner HR, and Ruegg MA (2008). Muscle-wide secretion of a miniaturized form of neural agrin rescues focal neuromuscular innervation in agrin mutant mice. Proc. Natl. Acad. Sci. USA 105, 11406–11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, and Lee K-F (2001). Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 410, 1057–1064. [DOI] [PubMed] [Google Scholar]
- Marneros AG, Keene DR, Hansen U, Fukai N, Moulton K, Goletz PL, Moiseyev G, Pawlyk BS, Halfter W, Dong S, Shibata M, Li T, Crouch RK, Bruckner P, and Olsen BR (2004). Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. EMBO J. 23, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marneros AG and Olsen BR (2005). Physiological role of collagen XVIII and endostatin. FASEB J. 19, 716–728. [DOI] [PubMed] [Google Scholar]
- Marneros AG, She H, Zambarakji H, Hashizume H, Connolly EJ, Kim I, Gragoudas ES, Miller JW, and Olsen BR (2007). Endogenous endostatin inhibits choroidal neovascularization. FASEB J. 21, 3809–3818. [DOI] [PubMed] [Google Scholar]
- Mathiak M, Yenisey C, Grant DS, Sharma B, and Iozzo RV (1997). A role for perlecan in the suppression of growth and invasion in fibrosarcoma cells. Cancer Res. 57, 2130–2136. [PubMed] [Google Scholar]
- Matsumoto-Miyai K, Sokolowska E, Zurlinden A, Gee CE, Lüscher D, Hettwer S, Wölfel J, Ladner AP, Ster J, Gerber U, Rülicke T, Kunz B, and Sonderegger P (2009). Coincident pre- and postsynaptic activation induces dendritic filopodia via neurotrypsin-dependent agrin cleavage. Cell 136, 1161–1171. [DOI] [PubMed] [Google Scholar]
- Menzel O, Bekkeheien RC, Reymond A, Fukai N, Boye E, Kosztolanyi G, Aftimos S, Deutsch S, Scott HS, Olsen BR, Antonarakis SE, and Guipponi M (2004). Knobloch syndrome: novel mutations in COL18A1, evidence for genetic heterogeneity, and a functionally impaired polymorphism in endostatin. Human Mutation 23, 77–84. [DOI] [PubMed] [Google Scholar]
- Mongiat M, Otto J, Oldershaw R, Ferrer F, Sato JD, and Iozzo RV (2001). Fibroblast growth factor-binding protein is a novel partner for perlecan protein core. J. Biol. Chem 276, 10263–10271. [DOI] [PubMed] [Google Scholar]
- Mongiat M, Sweeney S, San Antonio JD, Fu J, and Iozzo RV (2003). Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J. Biol. Chem 278, 4238–4249. [DOI] [PubMed] [Google Scholar]
- Mongiat M, Taylor K, Otto J, Aho S, Uitto J, Whitelock J, and Iozzo RV (2000). The protein core of the proteoglycan perlecan binds specifically to fibroblast growth factor-7. J. Biol. Chem 275, 7095–7100. [DOI] [PubMed] [Google Scholar]
- Moulton KS, Olsen BR, Sonn S, Fukai N, Zurakowski D, and Zeng X (2004). Loss of collagen XVIII enhances neovascularization and vascular permeability in atherosclerosis. Circulation 110, 1330–1336. [DOI] [PubMed] [Google Scholar]
- Nitkin RM, Smith MA, Magill C, Fallon JR, Yao Y-MM, Wallace BG, and McMahan UJ (1987). Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J. Cell Biol 105, 2471–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent MA and Iozzo RV (2000). Fibroblast growth factor-2. Int. J. Biochem. Cell Biol 32, 115–120. [DOI] [PubMed] [Google Scholar]
- Nugent MA, Nugent HM, Iozzo RV, Sanchack K, and Edelman ER (2000). Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc. Natl. Acad. Sci. USA 97, 6722–6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg P, Xie L, and Kalluri R (2005). Endogenous inhibitors of angiogenesis. Cancer Res. 65, 3967–3979. [DOI] [PubMed] [Google Scholar]
- O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, and Folkman J (1997). Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88, 277–285. [DOI] [PubMed] [Google Scholar]
- O’Riordan E, Orlova TN, Mendelev N, Patschan D, Kemp R, Chander PN, Hu R, Hao G, Gross SS, Iozzo RV, Delaney V, and Goligorsky MS (2008). Urinary proteomic analysis of chronic renal allograft nephropathy. Proteomics Clin. Appl 2, 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda O, Shinzato T, Ohbayashi K, Takai I, Kunimatsu M, Maeda K, and Yamanaka N (1996). Purification and characterization of perlecan fragment in urine of end-stage renal failure patients. Clin. Chim. Acta 255, 119–132. [DOI] [PubMed] [Google Scholar]
- Oh SP, Kamagata Y, Muragaki Y, Timmons S, Ooshima A, and Olsen BR (1994a). Isolation and sequencing of cDNAs for proteins with multiple domains of Gly-Xaa-Yaa repeats identify a distinct family of collagenous proteins. Proc. Natl. Acad. Sci. USA 91, 4229–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SP, Warman ML, Seldin MF, Cheng S-D, Knoll JHM, Timmons S, and Olsen BR (1994b). Cloning of cDNA and genomic DNA encoding human type XVIII collagen and localization of the α1(XVIII) collagen gene to mouse chromosome 10 and human chromosome 21. Genomics 19, 494–499. [DOI] [PubMed] [Google Scholar]
- Raymond M-A, Désormeaux A, Laplante P, Vigneault N, Filep JG, Landry K, Pshezhetsky AV, and Hébert M-J (2004). Apoptosis of endothelial cells triggers a caspase-dependent anti-apoptotic paracrine loop active on vascular smooth muscle cells. FASEB J. 18, 705–707. [DOI] [PubMed] [Google Scholar]
- Rehn M, Hintikka E, and Pihlajaniemi T (1994). Primary structure of the α1 chain of mouse type XVIII collagen, partial structure of the corresponding gene, and comparison of the α1(XVIII) chain with its homologue, the α1(XV) collagen chain. J. Biol. Chem 269, 13929–13935. [PubMed] [Google Scholar]
- Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo CR, Pihlajaniemi T, Alitalo K, and Vuori K (2001). Interaction of endostatin with integrins implicated in angiogenesis. Proc. Natl. Acad. Sci. USA 98, 1024–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif R, Sales S, Hettwer S, Dreier B, Gisler C, Wölfel J, Lüscher D, Zurlinden A, Stephan A, Ahmed S, Baici A, Ledermann B, Kunz B, and Sonderegger P (2007). Specific cleavage of agrin by neurotrypsin, a synaptic protease linked to mental retardation. FASEB J. 21, 3468–3478. [DOI] [PubMed] [Google Scholar]
- Reiland J, Sanderson RD, Waguespack M, Barker SA, Long R, Carson DD, and Marchetti D (2004). Heparanase degrades syndecan-1 and perlecan heparan sulfate: functional implications for tumor cell invasion. J. Biol. Chem 279, 8047–8055. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Mulloy B, Gallagher JT, and Stringer SE (2006). VEGF165-binding sites within heparan sulfate encompass two highly sulfated domains and can be liberated by K5 lyase. J. Biol. Chem 281, 1731–1740. [DOI] [PubMed] [Google Scholar]
- Rossi M, Morita H, Sormunen R, Airenne S, Kreivi M, Wang L, Fukai N, Olsen BR, Tryggvason K, and Soininen R (2003). Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 22, 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarela J, Ylikarppa R, Rehn M, Purmonen S, and Pihlajaniemi T (1998). Complete primary structure of two variant forms of human type XVIII collagen and tissue-specific differences in the expression of the corresponding transcripts. Matrix Biol. 16, 319–328. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Fukai N, Mann K, Göhring W, Olsen BR, and Timpl R (1998). Structure, function and tissue forms of the C-terminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin. EMBO J. 17, 4249–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter BV, Martinet O, Zhang W-J, Mandeli J, and Woo SLC (2001). Adenovirus-mediated gene transfer of endostatin in vivo results in high level of transgene expression and inhibition of tumor growth and metastasis. Proc. Natl. Acad. Sci. USA 97, 4802–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoré C, Zhang C, Muir C, Liu R, Wyrwa J, Shu J, Zhau HE, Chung LW, Carson DD, and Farach-Carson MC (2005). Perlecan knockdown in metastatic prostate cancer cells reduces heparin-binding growth factor responses in vitro and tumor growth in vivo. Clin. Exp. Metastasis 22, 377–390. [DOI] [PubMed] [Google Scholar]
- Scotton P, Bleckmann D, Stebler M, Sciandra F, Brancaccio A, Meier T, Stetefeld J, and Ruegg MA (2006). Activation of muscle-specific receptor tyrosine kinase and binding to dystroglycan are regulated by alternative mRNA splicing of agrin. J. Biol. Chem 281, 36835–36845. [DOI] [PubMed] [Google Scholar]
- Senger DR, Perruzzi CA, Streit M, Koteliansky VE, de Fougerolles AR, and Detmar M (2002). The α1β1 and α2β1 integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. Am. J. Pathol 160, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppinen L, Sormunen R, Soini Y, Elamaa H, Heljasvaara R, and Pihlajaniemi T (2008). Lack of collagen XVIII accelerates cutaneous wound healing, while overexpression of its endostatin domain leads to delayed healing. Matrix Biol. 27, 535–546. [DOI] [PubMed] [Google Scholar]
- Sharma B, Handler M, Eichstetter I, Whitelock J, Nugent MA, and Iozzo RV (1998). Antisense targeting of perlecan blocks tumor growth and angiogenesis in vivo. J. Clin. Invest 102, 1599–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichiri M and Hirata Y (2001). Antiangiogenesis signals by endostatin. FASEB J. 15, 1044–1053. [DOI] [PubMed] [Google Scholar]
- Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, and Kalluri R (2003). Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by αvβ3 and α5β1 integrins. Proc. Natl. Acad. Sci. USA 100, 4766–4771. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Sund M, Zeisberg M, and Kalluri R (2005). Endogenous stimulators and inhibitors of angiogenesis in gastrointestinal cancers: basic science to clinical application. Gastroenterology 129, 2076–2091. [DOI] [PubMed] [Google Scholar]
- Suzuki OT, Sertie AL, Der K, V, Kok F, Carpenter M, Murray J, Czeizel AE, Kliemann SE, Rosemberg S, Monteiro M, Olsen BR, and Passos-Bueno MR(2002). Molecular analysis of collagen XVIII reveals novel mutations, presence of a third isoform, and possible genetic heterogeneity in Knobloch syndrome. Am. J. Hum. Genet 71, 1320–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney SM, DiLullo G, Slater SJ, Martinez J, Iozzo RV, Lauer-Fields JL, Fields GB, and San Antonio JD (2003). Angiogenesis in collagen I requires α2β1 ligation of a GFP*GER sequence and possible p38 MAPK activation and focal adhesion disassembly. J. Biol. Chem 278, 30516–30524. [DOI] [PubMed] [Google Scholar]
- Tátrai P, Dudás J, Batmunkh E, Máthé M, Zalatnai A, Schaff Z, Ramadori G, and Kovalszky I (2006). Agrin, a novel basement membrane component in human rat and liver, accumulates in cirrhosis and hepatocellular carcinoma. Lab. Invest 86, 1149–1160. [DOI] [PubMed] [Google Scholar]
- Thadikkaran L, Crettaz D, Siegenthaler MA, Gallot D, Sapin V, Iozzo RV, Queloz PA, Schneider P, and Tissot JD (2005). The role of proteomics in the assessment of premature rupture of fetal membranes. Clin. Chim. Acta 360, 27–36. [DOI] [PubMed] [Google Scholar]
- Tran P-K, Tran-Lundmark K, Soininen R, Tryggvason K, Thyberg J, and Hedin U (2004). Increased intimal hyperplasia and smooth muscle cell proliferation in transgenic mice with heparan sulfate-deficient perlecan. Circ. Res 94, 550–558. [DOI] [PubMed] [Google Scholar]
- Tsangaris GT, Karamessinis P, Kolialexi A, Garbis SD, Antsaklis A, Mavrou A, and Fountoulakis M (2006). Proteomic analysis of amniotic fluid in pregnancies with Down syndrome. Proteomics 6, 4410–4419. [DOI] [PubMed] [Google Scholar]
- Utriainen A, Sormunen R, Kettunen M, Carvalhaes LS, Sajanti E, Eklund L, Kauppinen R, Kitten GT, and Pihlajaniemi T (2004). Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Human Mol. Gen 13, 2089–2099. [DOI] [PubMed] [Google Scholar]
- Verbeek MM, Otte-Höller I, van den Born J, van den Heuvel LPWJ, David G, Wesseling P, and de Waal RM (1999). Agrin is a major heparan sulfate proteoglycan accumulating in Alzheimer’s disease brain. Am. J. Pathol 155, 2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuadens F, Benay C, Crettaz D, Gallot D, Sapin V, Schneider P, Binevenut W-V, Lémery D, Quadroni M, Dastugue B, and Tissot J-D (2003). Identification of biologic markers of the premature rupture of fetal membranes: proteomic approach. Proteomics 3, 1521–1525. [DOI] [PubMed] [Google Scholar]
- Warth A, Kröger S, and Wolburg H (2004). Redistribution of aquaporin-4 in human glioblastoma correlates with loss of agrin immunoreactivity from brain capillary basal laminae. Acta Neuropathol. 107, 311–318. [DOI] [PubMed] [Google Scholar]
- West L, Govindraj P, Koob TJ, and Hassell JR (2006). Changes in perlecan during chondrocyte differentiation in the fetal bovine rib growth plate. J. Orthop. Res 24, 1317–1326. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Graham LD, Melrose J, Murdoch AD, Iozzo RV, and Underwood PA (1999). Human perlecan immunopurified from different endothelial cell sources has different adhesive properties for vascular cells. Matrix Biol. 18, 163–178. [DOI] [PubMed] [Google Scholar]
- Whitelock JM and Iozzo RV (2005). Heparan sulfate: a complex polymer charged with biological activity. Chem. Rev 105, 2745–2764. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Melrose J, and Iozzo RV (2008). Diverse cell signaling events modulated by perlecan. Biochemistry 47, 11174–11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelock JM, Murdoch AD, Iozzo RV, and Underwood PA (1996). The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin and heparanases. J. Biol. Chem 271, 10079–10086. [DOI] [PubMed] [Google Scholar]
- Wickström SA, Alitalo K, and Keski-Oja J (2005). Endostatin signaling and regulation of endothelial cell-matrix interactions. Adv. Cancer Res 94, 197–229. [DOI] [PubMed] [Google Scholar]
- Winzen U, Cole GJ, and Halfter W (2003). Agrin is a chimeric proteoglycan with the attachment sites for heparan sulfate/chondroitin sulfate located in two multiple serine-glycine clusters. J. Biol. Chem 278, 30106–30114. [DOI] [PubMed] [Google Scholar]
- Witmer AN, van den Born J, Vrensen GFJM, and Schlingemann RO (2001). Vascular localization of heparan sulfate proteoglycans in retinas of patients with diabetes mellitus and in VEGF-induced retinopathy using domain-specific antibodies. Current Eye Res. 22, 190–197. [DOI] [PubMed] [Google Scholar]
- Woodall BP, Nyström A, Iozzo RA, Eble JA, Niland S, Krieg T, Eckes B, Pozzi A, and Iozzo RV (2008). Integrin α2β1 is the required receptor for endorepellin angiostatic activity. J. Biol. Chem 283, 2335–2343. [DOI] [PubMed] [Google Scholar]
- Ylikärppä R, Eklund L, Sormunen R, Kontiola AI, Utriainen A, Määttä M, Fukai N, and Olsen BR (2003). Lack of type XVIII collagen results in anterior ocular defects. FASEB J. 17, 2257–2259. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Amenta PS, and Patton BL (2004). Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 22, 521–538. [DOI] [PubMed] [Google Scholar]
- Zatterstrom UK, Felbor U, Fukai N, and Olsen BR (2000). Collagen XVIII/endostatin structure and functional role in angiogenesis. Cell Struct. Funct 25, 97–101. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang Y, Chu Y, Su L, Gong Y, Zhang R, and Xiong S (2006). Agrin is involved in lymphocytes activation that is mediated by α-dystroglycan. FASEB J. 20, 50–58. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Ramirez NE, Yankeelov TE, Li Z, Ford LE, Qi Y, Pozzi A, and Zutter MM (2008). α2β1 integrin expression in the tumor microenvironment enhances tumor angiogenesis in a tumor cell-specific manner. Blood 111, 1980–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Wang J, Cao R, Morita H, Soininen R, Chan KM, Liu B, Cao Y, and Tryggvason K (2004). Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 64, 4699–4702. [DOI] [PubMed] [Google Scholar]
- Zoeller JJ and Iozzo RV (2008). Proteomic profiling of endorepellin angiostatic activity on human endothelial cells. Proteome Sci. 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller JJ, McQuillan A, Whitelock J, Ho S-Y, and Iozzo RV (2008). A central function for perlecan in skeletal muscle and cardiovascular development. J. Cell Biol 181, 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorick TS, Mustacchi Z, Bando SY, Zatz M, Moreira-Filho CA, Olsen B, and Passos-Bueno MR (2001). High serum endostatin levels in Down syndrome: Implications for improved treatment and prevention of solid tumors. Eur. J. Hum. Genet 9, 811–814. [DOI] [PubMed] [Google Scholar]
- Zweers MC, Davidson JM, Pozzi A, Hallinger R, Janz K, Quondamatteo F, Leutgeb B, Krieg T, and Eckes B (2007). Integrin α2β1 is required for regulation of murine wound angiogenesis but is dispensable for reepithelialization. J. Invest. Dermatol 127, 467–478. [DOI] [PubMed] [Google Scholar]