Abstract
Early evidence suggests that provisions of the Food and Drug Administration Safety and Innovation Act of 2012 are associated with reductions in the total number of new national drug shortages. However, drugs frequently used in acute unscheduled care such as the care delivered in emergency departments may be increasingly affected by shortages. Our estimates, based on reported national drug shortages from 2001 to 2014 collected by the University of Utah’s Drug Information Service, show that although the number of new annual shortages has decreased since the act’s passage, half of all drug shortages in the study period involved acute care drugs. Shortages affecting acute care drugs became increasingly frequent and prolonged compared with non–acute care drugs (median duration of 242 versus 173 days, respectively). These results suggest that the drug supply for many acutely and critically ill patients in the United States remains vulnerable despite federal efforts.
The number of national drug shortages, defined as periods when demand or projected demand for a drug in the United States exceeds its supply, has more than tripled over the past decade, 1 increasing risk of patient harm and cost of health care. 2 In response to growing concerns over these shortages, the Food and Drug Administration Safety and Innovation Act (FDASIA) was enacted in 2012, giving the FDA new and expanded regulatory powers to respond to national shortages. These powers include requiring the early reporting of shortages by pharmaceutical manufacturers so that the agency may work with them to resolve production issues; identifying additional manufacturers to compensate for production deficiencies; expediting inspections and reviews of alternative products or manufacturing facilities; and enabling discretion in allowing distribution of products that have quality issues but that do not confer undue risk to patients. 3 Early evidence suggests that the number of new national drug shortages appearing each year has decreased since enactment of the FDASIA. 4,5
However, the overall number of national shortages remains high, and some evidence suggests that shortages may be increasing for drugs that are used for the diagnosis and management of patients in emergency departments (EDs), hospital wards, and intensive care units. 6–15National drug shortages have been reported for many of the most commonly used acute care drugs, including pain medications, sedatives, electrolyte solutions, antibiotics, antidotes and reversal agents, and medications for critical care. 16,17 However, no systematic study of the FDASIA as it relates to drugs used in the acute care setting has been conducted.
Drug shortages are particularly challenging for acute care because of the low tolerance for delays that can occur during the search for therapeutic alternatives. 5,8,18 Emergency clinicians have also expressed concerns about the difficulty of finding alternative medications, 5 which, when found, can be less effective, more costly, or associated with more adverse effects than the preferred drugs. 7,11,19–21 The act of substitution also carries a higher risk of medication errors in the ED than in nonacute settings. 7
Understanding the extent of drug shortages in the acute care setting and any changes since passage of the FDASIA is important to providers and policy makers seeking to ensure safe access to critical medications. This study describes trends in national drug shortages between 2001 and 2014 for drugs used in the ED in comparison to non–acute care drugs. We describe characteristics of national shortages affecting acute and non–acute care drugs, including the frequency, duration, and reported causes of shortages as well as the characteristics of the drugs affected by shortages.
STUDY DATA AND METHODS
DATA SOURCE
We used the University of Utah’s Drug Information Service drug shortage data set from January 1, 2001, to December 31, 2014. The data set includes information on all confirmed national shortages. A critical shortage is defined as a supply issue that affects how a pharmacy prepares or dispenses a drug product or influences patient care when prescribers must use an alternative agent. 22 The Drug Information Service publishes critical drug shortage information on a public website (http://www.ashp.org/shortages) hosted by the American Society of Health-System Pharmacists and receives voluntary reports of drug shortages from manufacturers via a reporting feature on the website. Clinical pharmacists at the service confirm each reported shortage. This verification process includes determining all potential manufacturers of a reported drug, and all drug presentations and National Drug Codes associated with the reported item. Next, each manufacturer is contacted to determine which National Drug Codes are in shortage at the national level. The manufacturers are also asked to provide a reason for the shortage and an estimated date when the shortage would be resolved. A shortage is considered to be resolved when all suppliers have all formulations and dosages available or have permanently discontinued their products, or when the FDA reports on its website that the shortage has been resolved.
Our classification of non–acute care drugs included anticancer medications that may be used in the hospital inpatient setting but not in the ED; oral medications used for the chronic management of medical and psychiatric conditions; birth control; over-the-counter medications that are not time sensitive; all vaccines except those against tetanus, rabies, and postexposure prophylaxis; prescription drugs given in the ED to patients without prescription drug coverage and who have no outpatient access to medication; specialty drugs such as ophthalmic or otic suspensions not used by emergency care providers without specialty consultation; antituberculosis medication; extended release medications; drugs that cannot be delivered by emergency care providers (such as intraocular or intrathecal drugs or inhaled anesthetics); radiologic contrast materials; and nonimmediate anticoagulation such as warfarin.
Institutional Review Board exemption approval was obtained from the Yale University Human Research Committee prior to the initiation of this study.
DISTINGUISHING ACUTE CARE DRUGS FROM NON–ACUTE CARE DRUGS
We defined an acute care drug as one that is used in the ED for the management or diagnosis of acute conditions. This classification is inclusive not only of drugs with an indication for an acute condition but also of drugs commonly used off label in the acute care setting, regardless of efficacy or FDA approval status. We used this definition to best reflect the real-world implications of drug shortages.
To classify whether a drug was used in the acute care setting, we used a small sample of drugs to establish criteria for acute care drugs (details of the criteria are explained in online Appendix 1). 23 The criteria were then used by two emergency medicine physicians (two authors) to independently review the full list of drugs affected by shortages and to assign acute or non–acute care status to them. Disagreements between the reviewers were adjudicated by the author group. We assessed the degree of reliability in acute care designation by having both reviewers conduct a blinded review of all drugs reported to be affected by shortages during the study period.
Overall, 52 percent of shortages (n=1,006 ) involved acute care drugs. The inter-rater agreement upon review using a standardized criteria list was 91 percent, kappa of 89 (95% confidence interval: 87, 90). Ultimately, 170 drugs (9 percent) required adjudication for assignment of acute care classification.
TRENDS AND DURATION OF SHORTAGES AFFECTING ACUTE AND NON–ACUTE CARE DRUGS
We reported the number of shortages affecting acute care and non–acute care drugs by year from 2001 to 2014. We calculated the median duration of shortage for each group of drugs (acute care and non–acute care) using December 31, 2014, as the end observation date in the data set. Shortages that were ongoing as of this date were truncated and assigned December 31, 2014, as the end date. We also conducted a secondary analysis of this assumption by evaluating annual drug shortage durations (the number of days the shortage occurred in a calendar year of 365 days). This secondary analysis tracked changes of duration over time, while avoiding the potential lead-time bias that would result from certain shortages’ starting in an earlier study period. Each shortage was attributed to the calendar year in which the shortage was active. For example, a shortage beginning in June 2008 and lasting until February 2010 would be counted for the years 2008, 2009, and 2010. We reported the number of shortages by year and used a statistical test of trend to assess changes over time. We also reported median duration of shortages by year.
SHORTAGE ETIOLOGY
The primary causes of shortages reported by manufacturers to the University of Utah’s Drug Information Service were supply disruption, regulatory issues, manufacturing problems, raw material shortage, business decision, discontinuation, or unknown. We also characterized differences in the etiology of shortages between acute care and non–acute care drugs.
ANALYSIS
Data processing and merging were performed using Stata software, version 14. Data analysis and graphical presentation were performed in Microsoft Excel, version 14.5.3.
LIMITATIONS
The results of this study should be interpreted in the context of several limitations. First, our study drew from national data; thus, the results do not reflect local or regional shortages 24,25or reveal surpluses that exist in anticipation of shortages. However, examining shortages on the national level is well suited for policy makers and the FDA because drugs are regulated nationally.
Second, it is possible that the effects of the FDASIA might become apparent only over the long term and therefore not reflected in this study, which closely followed the enactment of the legislation. However, the extent of acute care drug shortages and the efficacy of the FDASIA in addressing these shortages are of pressing concern and ought to be assessed in a timely manner. Furthermore, because this study was observational in design, it is also possible that changes observed in drug shortages were not limited to the effects of the FDASIA but may have resulted from other legislation, executive orders, or other nonregulatory factors.
Third, data regarding the causes of shortages were limited because many manufacturers did not disclose the cause of their shortages or did not provide details beyond “manufacturing difficulties.” 26 The reliance on self-reporting from manufacturers may also have led to the underreporting of supply and production disruptions, thus making our estimates more conservative.
STUDY RESULTS
CHARACTERISTICS OF ALL SHORTAGES, 2001–14
We included 1,929 national drug shortages from 2001 to 2014 in the study with an average of 271 active shortages annually and 138 new shortages appearing each year (Appendix 2 lists the drugs that we excluded from the study). 23 The number of new shortages peaked in 2011 at 268 (Exhibit 1 ), and the number of active shortages in a year peaked in 2012 at 489 (Exhibit 2 ). The most common classes of drugs to experience shortages were central nervous system agents, such as drugs to reduce pain and fever ( n=332 ); anti-infectives (278); cardiovascular drugs (151); hormones and synthetic substitutes (130); and electrolyte, caloric, and water balance products (129) (see Appendix 3, Table 1 for a complete list). 23 Overall, 70 percent of the drug products affected by shortages are delivered by injection.
Exhibit 1.
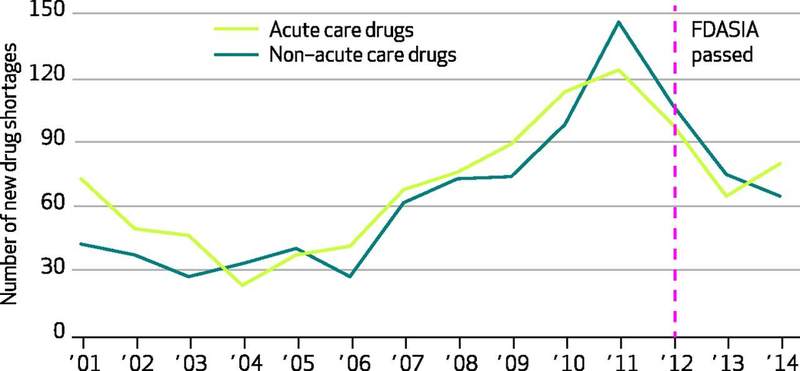
Number of new drug shortages affecting acute care and non–acute care drugs
Authors’ analysis of national critical drug shortage data compiled by the University of Utah’s Drug Information Service.
Acute care drugs include those commonly used in emergency department and critical care settings, including pain medications, sedatives, electrolyte solutions, antibiotics, and antidotes and reversal agents, among others.
FDASIA is Food and Drug Administration Safety and Innovation Act of 2012.
Exhibit 2.
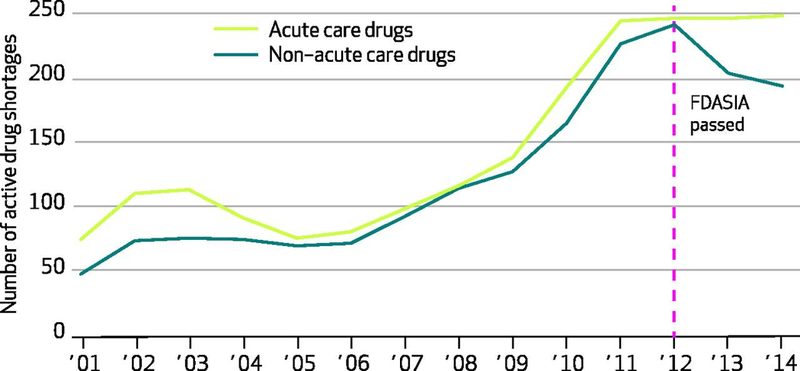
Number of active drug shortages affecting acute care and non–acute care drugs
Authors’ analysis of national critical drug shortage data compiled by the University of Utah’s Drug Information Service.
Acute care drugs include those commonly used in emergency department and critical care settings, including pain medications, sedatives, electrolyte solutions, antibiotics, and antidotes and reversal agents, among others.
FDASIA is Food and Drug Administration Safety and Innovation Act of 2012.
Among the reported causes of national shortages, manufacturing difficulty was consistently the most common, accounting for 25 percent ( n=484 ) of the overall shortages during this period. Sudden changes in demand or the failure of supply to keep up with demand accounted for 12 percent (226). Regulatory issues accounted for 2 percent and 3 percent of shortages in acute care and non–acute care drugs, respectively. The causes of shortages were not reported for 45 percent of acute care and 47 percent of non–acute care drugs. Notably, 47 percent (873) of the shortages did not have a cause reported (see Appendix 3, Table 2, for a complete list). 23
TYPES OF DRUGS AFFECTED
Among acute care drugs, the most common types affected by shortages were anti-infective agents ( n=208, accounting for 21 percent); central nervous system agents ( n=200, 20 percent); autonomic drugs ( n=103, 10 percent); electrolyte, caloric, and water balance products ( n=82, 8 percent); and cardiovascular drugs ( n=81, 8 percent). Among non–acute care drugs, the most common classes were central nervous system agents ( n=137, 15 percent); cancer drugs ( n=124, 13 percent); miscellaneous therapeutic agents ( n=77, 8 percent); hormones and synthetic substitutes (such as testosterone; n=76, 8 percent), and anti-infective agents ( n=70, 8 percent) (see Appendix 3, Table 1 for a complete list). 23Injectables accounted for a statistically higher proportion of acute care than non–acute care drug shortages (69 percent versus 38 percent; p < 0.001 ).
TRENDS IN DRUG SHORTAGES
During 2001–14, acute care and non–acute care drugs showed similar trends in the numbers of new annual shortages ( Exhibit 1 ). New shortages of both drug types had steadily increased since 2004, peaked in 2011, and continued at lower rates since. Exhibit 2 shows the number of active shortages in the 2001–14 period and suggests three important findings. First, the total number of active shortages increased by more than 300 percent from 2001 to 2012. Second, 2012 also represents an inflection point when the number of active shortages of non–acute care drugs notably decreased for the first time since 2004. And third, a similar decrease was not seen among acute care drugs.
DURATION OF DRUG SHORTAGES
The overall median duration of shortages was 210 days (interquartile range [IQR]: 83 to 531 days). The median shortage for non–acute care drugs was 173 days (IQR: 69 to 442), which was significantly shorter than that of acute care drugs with a median of 242 days (IQR: 96 to 624) ( p < 0.001 ) (data not shown). Examining the trends in the median duration of acute care and non–acute care drugs shortages, acute care drug shortages were increasingly prolonged (Exhibit 3 ).
Exhibit 3.
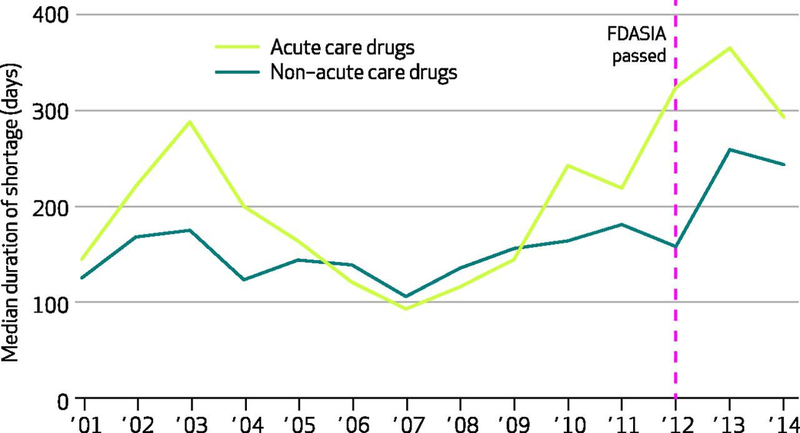
Duration of national drug shortages affecting acute care and non–acute care drugs, in number of days out of 365 days
Authors’ analysis of national critical drug shortage data compiled by the University of Utah’s Drug Information Service.
Acute care drugs include those commonly used in emergency department and critical care settings, including pain medications, sedatives, electrolyte solutions, antibiotics, and antidotes and reversal agents, among others.
FDASIA is Food and Drug Administration Safety and Innovation Act of 2012.
DISCUSSION
We examined drug shortages between 2001 and 2014 and found that the enactment of the FDASIA in 2012 coincided with a decrease in the number of new shortages involving non–acute care but not acute care drugs. Active shortages affecting acute care drugs were also more prolonged compared with non–acute care drugs. Overall, however, shortages have increased in duration for both drug groups, which suggests that shortages are increasingly difficult to resolve despite the FDASIA.
We also found a high number of shortages affecting anticonvulsants, analgesics, anesthetics and sedatives, and cardiovascular drugs—drug classes with heightened risk of patient harm if erroneously administered. Because errors are associated with shortages when providers substitute unfamiliar drugs for more familiar but unavailable drugs, 17,27,28 the prevalence of shortages affecting drugs from these categories is of particular concern for patient safety beyond mere delays in care.
Our results contribute to the current understanding of shortages in several ways. Concerns over shortages in acute care have been noted in the literature and have received media attention. 8,15,29 However, more recent assessments are based on narrative reviews, 7,9 survey results, 30 anecdotal evidence, 9,31 and studies of specific drugs such as antidotes for toxicological emergencies. 17 While one study characterized the overall extent of the issue, 15none examined the FDASIA as it pertains to acute care drugs. Our study extends the body of knowledge by providing quantitative data on the scope of the policy problem. Furthermore, the acute care drugs we found to be most commonly affected by national shortages were also among some of the most commonly prescribed drugs in the ED. 32 For example, intravenous saline—a mainstay of acute management of numerous emergent conditions—has experienced a shortage in nearly every year since 2003 (data not shown) and likely affects millions of patients (an estimated thirteen million visits to the ED alone require intravenous saline every year). 32 Thus, the consequences of shortages on patient care can be significant and widespread.
Several mechanisms may explain why national shortages are increasingly affecting acute care drugs. Drugs used in the acute care setting are more likely to be directly injected into patients instead of taken by mouth; it is well established that injectable drugs are more prone to manufacturing problems, such as contamination and machine breakdown, than non-injectable drugs. 3,26,33,34 Manufacturing difficulties can also be traced to the failures of markets to reward quality and safety. 3,28,35,36 Also, injectables are more prone than oral medications to being underproduced or discontinued because of their low profitability. 36 Injectable drugs are chiefly used in unscheduled inpatient care and EDs, where shorter treatment duration limits the size of the potential market compared to outpatient medications, many of which must be taken daily over the course of years. Shortages may also result from decisions by pharmaceutical companies to focus on developing and manufacturing lucrative brand-name drugs instead of generic but clinically essential drugs. Because these economic realities are not readily addressed by legislation, the FDASIA represents only a temporary measure to deal with shortages for specialty pharmaceuticals and has only limited effects on acute care drugs.
We used data from the University of Utah’s Drug Information Service instead of the FDA because the former is more comprehensive than the latter. 28 The FDA database includes only shortages of “medically necessary drugs” 5,37 and data from that source were difficult to retrieve and analyze until relatively recently. 38 In contrast, the University of Utah’s database includes all reported shortages affecting drugs that affect clinical practice, including vaccines, regardless of whether the drug was deemed medically necessary—only that the shortages require changes in clinical practice. 39 The University of Utah’s database also predates the FDA’s 5 and thus contains more information. 28
POLICY IMPLICATIONS
Our study points to important policy implications. Currently, while manufacturers are required under the FDASIA to report new shortages, the FDA can use a public letter only to compel disclosures of shortages. The voluntary disclosure of shortages also limits data generation and analysis of national shortages. Furthermore, while the Generic Drug User Fee Amendments of 2012 40 requires generic drug facilities to self-identify, additional transparency may help with early identification of generic injectables that are only produced by a single source, allowing for earlier application of preventive strategies to avoid shortages. Finally, the FDA’s approach to shortages is reactive and inadequate for dealing with acute care drugs, as this study shows. Other options previously proposed to stabilize the supply of generic injectables include providing tax credits, rebates, or temporary market exclusivity. 7 In addition, removing price limitations on injectable generic drugs imposed by Medicare could increase profitability to manufacturers, 7 although this strategy likely carries unintended consequences. 41 A recent proposal suggested using long-term contracts with manufacturers to guarantee demand and stabilize long-term prices and supply. 42 Because shortages may have already caused price spikes, long-term contracts may prevent future events such as a recent threefold price increase for acute care drugs like doxycycline from a specific manufacturer. 43,44
CONCLUSION
The recent decline in the overall number of drug shortages coincides with enactment of the FDASIA and has been attributed to the FDA’s ability to prevent drug shortages. 34 However, this study shows that shortages are increasingly affecting drugs commonly used in the management of acute and emergent conditions, despite the FDA’s efforts. More broad-based interventions are necessary to address these persistent drug shortages and help maintain the availability of drugs that are critical for millions of patients every day.
Supplementary Material
ACKNOWLEDGMENTS
An abstract of this article was presented at the October 2015 American College of Emergency Physicians Scientific Assembly, in Boston, Massachusetts. Serene Chen was funded by a scholarship from James D. Hirsch and from the National Institutes of Health–National Heart, Lung, and Blood Institute (NHLBI) Medical Student Research Fellowship. Harlan Krumholz was supported by Grant No. U01 HL105270–05 (Center for Cardiovascular Outcomes Research at Yale University) from the NHLBI. Arjun Venkatesh was funded by the Emergency Medicine Foundation Health Policy Scholar Award. The authors also report that Joseph Ross received research support through Yale University from Medtronic and Johnson & Johnson to develop methods of clinical trial data sharing and from the Food and Drug Administration to develop methods for postmarket surveillance of medical devices. Krumholz is a recipient of research agreements from Medtronic and from Johnson & Johnson, through Yale University, to develop methods of clinical trial data sharing and is chair of a cardiac scientific advisory board for UnitedHealth. Venkatesh, Ross, and Krumholz receive support from the Centers for Medicare and Medicaid Services to develop and maintain performance measures that are used for public reporting.
Footnotes
See Appendices
To access the Appendix, click on the Appendix link in the box to the right of the article online.
Contributor Information
Serene I. Chen, Yale School of Medicine, New Haven, CT
Erin R. Fox, University of Utah, Salt Lake City, UT
M. Kennedy Hall, University of Washington, Seattle, WA.
Joseph S. Ross, Yale School of Medicine, New Haven, CT
Emily M. Bucholz, Boston Children’s Hospital, Boston, MA
Harlan M. Krumholz, Yale School of Medicine, New Haven, CT
Arjun K. Venkatesh, Yale School of Medicine, New Haven, CT
References
- 1.Food and Drug Administration. Strategic plan for preventing and mitigating drug shortages [Internet]. Silver Spring (MD): FDA; 2013. October [cited 2016 Mar 7 ]. p. 40. [Google Scholar]
- 2.Food and Drug Administration. Fact sheet: drug products in shortage in the United States [Internet]. Silver Spring (MD): FDA; ; 2012. [cited 2016 Mar 7]. Available from: http://www.fda.gov/regulatoryinformation/legislation/significantamendmentstothefdcact/fdasia/ucm313121.htm [Google Scholar]
- 3.Woodcock J, Wosinska M. Economic and technological drivers of generic sterile injectable drug shortages. Clin Pharmacol Ther. 2013. ; 93 (2): 170–6. [DOI] [PubMed] [Google Scholar]
- 4.Tucker ME. US drug shortages: a disappearing problem? BMJ. 2012; 345: e8551. [DOI] [PubMed] [Google Scholar]
- 5.Government Accountability Office. Drug shortages: public health threat continues, despite efforts to help ensure product availability[Internet]. Washington (DC) : GAO; ;2014. February [cited 2016 Mar 7]. (Report No. GAO-14–194). p. 94 Available from: http://www.gao.gov/assets/670/660785.pdf [Google Scholar]
- 6.EDs grapple with record-breaking number of drug shortages . ED Manag. 2011. 23 ( 12 ): 133–6. [PubMed] [Google Scholar]
- 7.Mazer-Amirshahi M, Pourmand A, Singer S, Pines JM, van den Anker J. Critical drug shortages: implications for emergency medicine. Acad Emerg Med. 2014. 21 (6): 704–11. [DOI] [PubMed] [Google Scholar]
- 8.Thomas K, Tavernise S. Nitroglycerin, a staple of emergency rooms, is in short supply. New York Times 2014. March 26 Sect. B:1. [Google Scholar]
- 9.Paparella SF . Drug shortages in the emergency department: managing a threat to patient safety . J Emerg Nurs .2012. 38 (5): 466–9. [DOI] [PubMed] [Google Scholar]
- 10.Clune S . Emergency departments struggle with growing drug shortages . PBS News Hour [serial on the Internet].2011. August 29 [cited 2016 Mar 7 ]. Available from: http://to.pbs.org/1kTTysN [Google Scholar]
- 11.McLaughlin M, Kotis D, Thomson K, Harrison M, Fennessy G, Postelnick M, et al. Effects on patient care caused by drug shortages: a survey . J Manag Care Pharm . 2013. ; 19 ( 9 ): 783–8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason MA, Weant KA, Baker SN . Rapid sequence intubation medication therapies: a review in light of recent drug shortages . Adv Emerg Nurs J . 2013. ; 35 ( 1 ): 16–25 . [DOI] [PubMed] [Google Scholar]
- 13.Hvisdas C, Lordan A, Pizzi LT, Thoma BN . US propofol drug shortages: a review of the problem and stakeholder analysis . Am Health Drug Benefits . 2013. ; 6 ( 4 ): 171–5 . [PMC free article] [PubMed] [Google Scholar]
- 14.Balkhi B, Araujo-Lama L, Seoane-Vazquez E, Rodriguez-Monguio R, Szeinbach SL, Fox ER . Shortages of systemic antibiotics in the USA: how long can we wait? J Pharm Health Serv Res . 2013. ; 4 ( 1 ): 13–7 . [Google Scholar]
- 15.Hawley KL, Mazer-Amirshahi M, Zocchi MS, Fox ER, Pines JM . Longitudinal trends in U.S. drug shortages for medications used in emergency departments (2001–2014) . Acad Emerg Med . 2016. ; 23 ( 1 ): 63–9 . [DOI] [PubMed] [Google Scholar]
- 16.Institute for Safe Medication Practices . A shortage of everything except errors: harm associated with drug shortages [Internet]. Horsham (PA) : ISMP; ; 2012. April 19 [cited 2016 Mar 7 ]. Available from: https://www.ismp.org/newsletters/acutecare/showarticle.aspx?id=20 [Google Scholar]
- 17.Mazer-Amirshahi M, Hawley KL, Zocchi M, Fox E, Pines JM, Nelson LS . Drug shortages: implications for medical toxicology . Clin Toxicol (Phila). 2015. 53 ( 6 ): 519–24. [DOI] [PubMed] [Google Scholar]
- 18.Fox ER, Tyler LS . Managing drug shortages: seven years’ experience at one health system. Am J Health Syst Pharm. 2003. ; 60 ( 3 ): 245–53. [DOI] [PubMed] [Google Scholar]
- 19.Metzger ML, Billett A, Link MP . The impact of drug shortages on children with cancer—the example of mechlorethamine . N Engl J Med . 2012. ; 367 ( 26 ): 2461–3. [DOI] [PubMed] [Google Scholar]
- 20.Institute for Safe Medication Practices. Drug shortages: national survey reveals high level of frustration, low level of safety [Internet]. Horsham (PA) : ISMP; ; 2010. September 23 [cited 2016 Mar 28 ]. Available from: http://www.ismp.org/newsletters/acutecare/articles/20100923.asp [Google Scholar]
- 21.Perry JJ, Lee JS, Sillberg VA, Wells GA . Rocuronium versus succinylcholine for rapid sequence induction intubation . Cochrane Database Syst Rev . 2008. ;( 2 ): CD002788 . [DOI] [PubMed] [Google Scholar]
- 22.American Society of Health-System Pharmacists Expert Panel on Drug Product Shortages, Fox ER, Birt A, James KB, Kokko H, Salverson S, et al. ASHP guidelines on managing drug product shortages in hospitals and health systems . Am J Health Syst Pharm . 2009. ; 66 (15): 1399–406 . [DOI] [PubMed] [Google Scholar]
- 25.IMS Institute for Healthcare Informatics . Drug shortages: a closer look at products, suppliers, and volume volatility[Internet]. Parsippany (NJ) : IMS Health Institute; ; 2011. November [cited 2016 Mar 7 ]. Available from: https://www.imshealth.com/files/web/IMSH%20Institute/Reports/Drug%20Shortages%20A%20closer%20look/IHII_Drug_Shortage_Report.pdf [Google Scholar]
- 26.American Hospital Association . AHA survey on drug shortages [Internet]. Washington (DC) : AHA; ; 2011. July 12[cited 2016 Mar 28 ]. Available from: http://www.aha.org/content/11/drugshortagesurvey.pdf [Google Scholar]
- 27.Fox ER, Tyler LS . Call to action: finding solutions for the drug shortage crisis in the United States . Clin Pharmacol Ther . 2013. ; 93 ( 2 ): 145–7 . [DOI] [PubMed] [Google Scholar]
- 28.Ehsani SR, Cheraghi MA, Nejati A, Salari A, Esmaeilpoor AH, Nejad EM . Medication errors of nurses in the emergency department . J Med Ethics Hist Med . 2013. ; 6 : 11 . [PMC free article] [PubMed] [Google Scholar]
- 29.Fox ER, Sweet BV, Jensen V . Drug shortages: a complex health care crisis . Mayo Clinic Proc . 2014. ; 89 ( 3 ): 361–73 . [DOI] [PubMed] [Google Scholar]
- 30.Rubin AJ, Gosselin PG . Shortage of drugs threaten patients . LA Times [serial on the Internet]. 2001. May 6 [cited2016 Mar 7 ]. Available from: http://lat.ms/1OJKp3d [Google Scholar]
- 31.Baumer AM, Clark AM, Witmer DR, Geize SB, Vermeulen LC, Deffenbaugh JH. National survey of the impact of drug shortages in acute care hospitals. Am J Health Syst Pharm . 2004. ; 61 (19): 2015–22 . [DOI] [PubMed] [Google Scholar]
- 32.McKenna M . Hospital pharmacists scrambling amid vast drug shortages: emergency physicians between roc and a hard place . Ann Emerg Med. 2011; 57 ( 2 ): A13–5 . [DOI] [PubMed] [Google Scholar]
- 33.32National Center for Health Statistics . National Hospital Ambulatory Medical Care Survey: 2010 emergency department summary tables [Internet]. Hyattsville (MD) : NCHS; ; 2015. August [cited 2016 Mar 7 ]. (Tables 21 and 22). [Google Scholar]
- 34.Jensen V, Rappaport BA . The reality of drug shortages—the case of the injectable agent propofol . N Engl J Med .2010. ; 363 ( 9 ): 806–7 . [DOI] [PubMed] [Google Scholar]
- 35.Food and Drug Administration . Executive summary: a review of FDA’s approach to medical product shortages[Internet]. Silver Spring (MD) : FDA; ; 2011. November 3 [cited 2016 Mar 7 ] [Google Scholar]
- 36.Gupta DK, Huang SM . Drug shortages in the United States: a critical evaluation of root causes and the need for action . Clin Pharmacol Ther . 2013. ; 93 ( 2 ): 133–5 . [DOI] [PubMed] [Google Scholar]
- 37.Stencel K . Health Policy Brief: drug shortages (updated) . Health Affairs [serial on the Internet]. 2014. September 24 [cited2016 Mar 7 ]. Available from: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=126 [Google Scholar]
- 38.Government Accountability Office . Drug compounding: clear authority and more reliable data needed to strengthen FDA oversight [Internet]. Washington (DC) : GAO; ; 2013. July 31 [cited 2016 Mar 7 ]. (Report No. GAO-13–702). p. 50 . Available for download from: http://www.gao.gov/products/GAO-13-702 [Google Scholar]
- 39.Government Accountability Office . Drug shortages: FDA’s ability to respond should be strengthened [Internet]. Washington (DC) : GAO; ; 2011. December 15 [cited 2016 Mar 7 ]. (Report No. GAO-12–116). p. 63 . Available for download from: http://www.gao.gov/products/GAO-12-116 [Google Scholar]
- 40.Kweder SL, Dill S . Drug shortages: the cycle of quantity and quality . Clin Pharmacol Ther . 2013. ; 93 ( 3 ): 245–51 . [DOI] [PubMed] [Google Scholar]
- 41.Food and Drug Administration . Self-identification of generic drug facilities, sites and organizations [Internet]. Silver Spring (MD) : FDA; ; 2013. [cited 2016 Mar 7 ]. [Google Scholar]
- 42.Chabner BA . Drug shortages—a critical challenge for the generic-drug market . N Engl J Med . 2011. ; 365 ( 23 ): 2147–9 . [DOI] [PubMed] [Google Scholar]
- 43.Wiske CP, Ogbechie OA, Schulman KA . Options to promote competitive generics markets in the United States .JAMA . 2015. ; 314 ( 20 ): 2129–30 . [DOI] [PubMed] [Google Scholar]
- 44.Lazarus D . When a drug costs 30 times what it once did . LA Times [serial on the Internet]. 2013. March 7 [cited 2016 Mar 7 ]. Available from: http://lat.ms/1QndEZS [Google Scholar]
- 45.Rosenthal E . Officials question the rising costs of generic drugs . New York Times [serial on the Internet]. 2014. October 7[cited 2016 Mar 7 ]. Available from: http://nyti.ms/1O7SQ5h [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


