Abstract
Ketogenic diets (KDs) are high fat, low carbohydrate formulations traditionally used to treat epilepsy; more recently, KDs have shown promise for a wide range of other neurological disorders. Drug addiction studies suggest that repeated exposure to drugs of abuse, including cocaine, results in a suite of neurobiological changes that includes neuroinflammation, decreased glucose metabolism, and disordered neurotransmission. Given that KDs positively regulate these factors, we addressed whether administration of a KD has potential as a novel therapy for drug addiction. In this study, male and female Sprague-Dawley rats were placed on a KD or a control diet (CD), beginning at five weeks of age and continuing through the end of behavioral testing. Three weeks after initiation of dietary treatments, rats received daily i.p. injections of cocaine (15 mg/kg) or saline vehicle for one week, were drug free for a subsequent week, and then all animals received a final challenge injection of 15 mg/kg cocaine. In the absence of cocaine injections, stereotyped locomotor responses were minimal and were unaffected by dietary treatment. In contrast, both males and females fed a KD exhibited decreased cocaine-induced stereotyped responses as compared to CD-fed rats. The sensitization of ambulatory responses was also disrupted in KD-fed rats. These results suggest that KDs directly impact dopamine-mediated behaviors, and hence may hold potential as a therapy for drug addiction.
1. Introduction
Addiction to psychostimulants such as cocaine is a complex, multifaceted disorder that to date has proven difficult to prevent or treat. This is in part due to the wide range of changes throughout the central nervous system that occur in addiction, such as decreased glucose metabolism, increased neuroinflammation, and altered neurotransmission. Many of these changes persist for months, if not years, into abstinence. A history of abuse of cocaine or methamphetamines is associated with persistent decreases in glucose metabolism in cortical (orbitofrontal cortex) and subcortical (striatum) brain areas implicated in drug addiction in human addicts (Chang et al., 2007; Volkow et al., 2004), an effect that is also seen in non-human primates treated acutely with cocaine (Lyons et al., 1996). In addition, serum levels of proinflammatory cytokines/chemokines are elevated in cocaine-abusing individuals, and have been put forth as a potential biomarker of the severity of abuse/dependence (Araos et al., 2015; Moreira et al., 2016); these factors, along with reactive oxygen species, are also elevated in the cortex and striatum of rats injected with cocaine (Dietrich et al., 2005; Sorg et al., 2011). Finally, cocaine not only has lasting effects on dopamine neurotransmission within the reward and reinforcement neurocircuitry, but also impacts other neurotransmitters that directly and indirectly interact with dopamine, such as adenosine. For example, there is decreased extracellular availability of adenosine and decreased adenosine A2A receptor (A2AR) expression within the ventral striatum during cocaine withdrawal in rats (Manzoni et al., 1998; Marcellino et al., 2007), and systemic administration of an A2AR agonist diminishes the sensitizing effect of repeated cocaine administration on locomotor responses in rats (Filip et al., 2006). Given this diverse set of factors impacted by psychostimulant addiction, effective therapies will likely need to be multifaceted as well.
Ketogenic diets (KDs) are high fat, low carbohydrate, adequate protein formulations that have a long-standing therapeutic history for the treatment of intractable epilepsy (Masino and Rho, 2010). Individuals on a KD experience a shift in metabolism resulting in the use of ketone bodies (e.g., acetoacetate, β-hydroxybutyrate) as a primary metabolic fuel. This switch occurs due to a decrease in available glucose, leading to a conversion of free fatty acids to ketone bodies within the liver (Hartman et al., 2007). Ketone bodies can readily substitute for glucose in cellular respiratory pathways throughout the body and brain; indeed, ketone bodies are a more efficient energy source, generating more molecules of ATP per unit oxygen vs. glucose (Hartman et al., 2007).
Research into the precise mechanisms underlying the therapeutic effects of KDs on epilepsy and other neurological disorders have revealed that these diets normalize behaviors and neural processes disrupted by disease states. For example, treatment of mice with a KD prior to kainic acid-induced seizures reduces expression of neuroinflammatory factors (e.g., COX-2 and prostaglandin E2) in the hippocampus (Jeong et al., 2011), and administration of the ketone body acetoacetate reduces hippocampal hyperactivity and extracellular glutamate levels in rats induced to have seizures by 4-aminopyridine infusions (Juge et al., 2010). A KD lowers - and perhaps equally important - stabilizes glucose levels (Noakes et al., 2006; Nuttall et al., 2015); furthermore, KD or ketone body treatment elevates ATP in healthy brain tissue (DeVivo et al., 1978; Nakazawa et al., 1983; Pan et al., 1999) and normalizes ATP availability in animal models of brain/spinal cord disorders or damage (Deng-Bryant et al., 2011; Nylen et al., 2009; Zhao et al., 2006). When considering the close inter-relationship between the purines ATP and adenosine as well as the direct link between metabolism and neuronal activity, it is not surprising that purines have been implicated in the therapeutic effects of KDs (Masino et al., 2012; Masino and Geiger, 2008). Specifically, mice lacking the adenosine A1 receptor (A1R) exhibit spontaneous electrographic seizures, and treatment with a KD decreases seizures in mice heterozygous, but not homozygous, for the A1R knockout (Masino et al., 2011). Similarly, recent work demonstrated a ketone ester’s ability to reduce anxiety in a genetic rat model of absence seizures requires activation of A1Rs (Kovács et al., 2018).
Existing research into mechanisms underlying the therapeutic effects of KDs suggests that metabolic therapy can impact or even normalize several factors disrupted or implicated in psychostimulant addiction. To date this hypothesis has not been tested directly. It is well established that rodents exhibit a reliable increase in ambulatory and repetitive, stereotyped locomotor responses following repeated administration of cocaine (Robinson and Berridge, 1993). Here, we examined the impact of KD treatment on these behavioral responses to cocaine in male and female rats. We found that administration of a KD did not alter behaviors in the absence of cocaine injections; in contrast, ambulatory and stereotyped (i.e., rearing) locomotor responses induced by cocaine injections were disrupted in KD-treated male and female rats.
2. Material and methods
2.1. Animals
Male and female Sprague-Dawley rats were first-generation offspring of breeders obtained from Charles River Laboratories. Rats were pair-housed by sex at weaning (21 days) in polycarbonate cages with wire mesh tops. Animals were maintained on a 12:12 hr light:dark cycle (lights on at 7 am), with all behavior testing occurring between the hours of 9 am and 2 pm. Food and water were available ad libitum. Animal procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (8th Ed.) and approved by the Trinity College Institutional Animal Care and Use Committee.
2.2. Diet
At 5 weeks of age, individual cages of male and female rats were randomly assigned to receive either a control diet (CD; LabDiet 5001, Lab Supply, Fort Worth, TX) or a KD (F3666, Bio-Serv, Flemington, NJ) (see Figure 1 for experimental schematic). Rats were weighed prior to placement on the diet, and then at least three times weekly during the five week period that rats remained on their assigned diets. Rodents consuming the F3666 diet self-regulate caloric intake such that it does not differ from control chow (Badman et al., 2009).
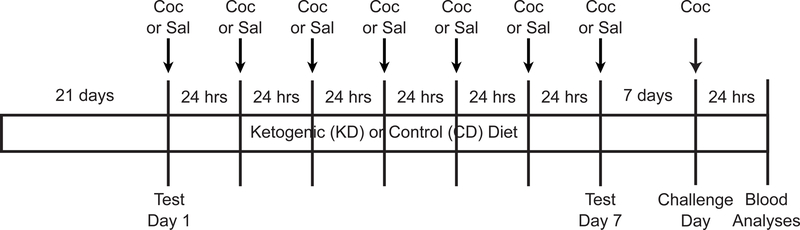
Figure 1.
Timeline of experimental manipulations. Coc = injections of cocaine (15 mg/kg; 1 ml/kg); Sal = injections of saline vehicle (1 ml/kg). Sal-Coc = animals injected with saline on Test Days 1–7; Coc-Coc = animals repeatedly injected with cocaine during this period (all animals injected with cocaine on Challenge Day). Sample sizes per group for females are as follows: CD Sal-Coc (n = 12); CD Coc-Coc (n = 12); KD Sal-Coc (n = 12); KD Coc-Coc (n = 12). Sample sizes per group for males are as follows: CD Sal-Coc (n = 11); CD Coc-Coc (n = 12); KD Sal-Coc (n = 10); KD Coc-Coc (n = 12).
2.3. Drugs and injections
Cocaine hydrochloride (cocaine; C5776, Sigma, St. Louis, MO) was dissolved in sterile saline to the working concentration (15 mg/ml). Starting at eight weeks of age, individual cages of CD and KD male and female rats were randomly assigned to receive daily i.p. injections (1 ml/kg) of either cocaine or sterile saline vehicle. Animals received their assigned injections for seven consecutive days, and then all animals underwent a drug-free period (no injections) for seven consecutive days. All animals then received a final “challenge” injection of cocaine (15 mg/kg) following the conclusion of this drug-free period. All injections occurred in the context of the testing apparatus (see below). The dose and patterning of cocaine injections employed here have previously been shown to induce behavioral sensitization in both male and female rats (Halbout et al., 2016; Kosten et al., 1994; Martinez et al., 2014; Peterson et al., 2016; Sircar and Kim, 1999).
2.4. Behavioral testing
Rats were tested for behavioral responses induced by repeated cocaine or saline injections using an automated system (Kinder Scientific, Poway CA). Each unit of this system was composed of a plexiglas open field chamber (44 × 22 × 20.5 cm) surrounded by a bilevel set of sensing frames. These frames generated an X–Y grid of photobeams within the chamber. Photobeam breaks were transmitted to a Windows computer running MotorMonitor software (Kinder Scientific). This software further discriminated beam breaks into ambulations (change of the animal’s entire body position on the X–Y grid of the lower frame) and time spent rearing (total time spent with any photobeams broken on the upper frame). According to the original scale proposed by Creese and Iversen (1974) and modified for use with cocaine by others (e.g., Daunais and McGinty, 1994; Festa et al., 2004), general ambulatory activity is scored as a low/moderate form of psychostimulant-induced stereotypy, whereas focused/persistent rearing is considered to be a more extreme response.
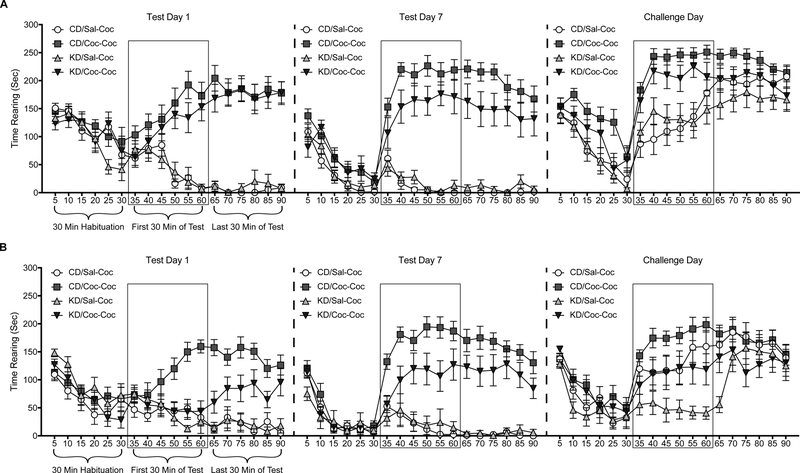
Each testing session began with an initial 30 min habituation period. Rats were then removed from the testing apparatus, injected with either cocaine or saline, and then returned to the testing apparatus for a 60 min test period. Ambulation and rearing data collected by the MotorMonitor software during the test period were summed (by behavior) into individual 5-min bins. As has been reported previously (Boudreau and Wolf, 2005; Shumsky et al., 1997), we observed that ambulatory and rearing responses peaked during the first 30 min following cocaine injections in CD animals (Figure 2); consequently, statistical comparisons were limited to responses that were summed across the first 30 min of the test period.
Figure 2.
Mean (+/− SEM) within-session data for time spent rearing by female (A) and male (B) rats treated with a control (CD) or ketogenic (KD) diet. Within each test (Test Day 1, Test Day 7, and Challenge Day), data were further divided into 5 min bins. The 30 min prior to injection comprised the habituation phase; the first and second 30 min periods following injection comprised the test phase. Note that the labels below each phase for Test Day 1 also apply to Test Day 7 and the Challenge Day. Rectangles enclose the first 30 min of the test phase, to further highlight the subset of the data that were summed for later statistical comparisons. Sal-Coc = animals injected with saline Test Days 1–7, and Coc-Coc = animals repeatedly injected with cocaine during this period (all animals injected with cocaine on Challenge Day).
2.5. Blood analyses
Twenty-four hours following the Challenge Day test, rats were euthanized using isoflurane gas and blood was taken from the lateral tail vein during the procedure. Glucose and β-hydroxybutyrate levels in blood samples were determined using Precision Xtra meters (Abbott Laboratories, Bedford MA).
2.6. Statistical analyses
All data were analyzed using SPSS for Macintosh, version 24.0 (IBM Corp, Armonk, NY USA). Data were first examined for skewness and normality (Shapiro-Wilk test), in order to determine if the assumptions of parametric statistical tests were met. When identified, outliers (scores outside the interquartile range (IQR) by more than 1.5*IQR) were removed from the affected data set. The effects of diet (CD vs. KD), drug history (Sal-Coc vs. Coc-Coc), day (day of testing) and sex (male vs. female) on body weight via mixed-design ANOVAs. Statistically significant three-way interactions were further decomposed for the simple effect of diet on each day, within each sex, using the MANOVA script in SPSS (pooled error term used from overall ANOVA). Challenge Day weight z-scores were tested for their relationship to Challenge Day time spent rearing (separately within each sex) using Pearson’s r. In order to control for between-subject effects of diet condition on weight, z-scores were calculated separately for each treatment group (i.e., each raw score was standardized using its treatment group mean and standard deviation) and then z-scores were pooled within each sex. For the week of daily cocaine or saline injections, analyses were restricted to the first (Test Day 1) and seventh (Test Day 7) injection days in order to simplify the analyses. The effect of diet, sex and time (Test Day 1 vs. Test Day 7) on ambulations and time rearing were examined separately within each drug history group, using mixed-design ANOVAs. Statistically significant interactions were further decomposed for the simple main effect of day within each diet condition using mixed-design ANOVAs. Finally, data from the Challenge Day were examined for the effects of diet, drug history, and sex on ambulations and time rearing using factorial ANOVAs. Statistically significant two-way interactions were further decomposed for the simple main effect of diet within each sex using factorial ANOVAs (pooled error term used from overall ANOVA). In all cases, p-values of less than 0.05 were considered a priori to be significant. Effect sizes are reported as partial η2.
3. Results
3.1. Behavioral measures: Repeated daily cocaine or saline injections
Starting at eight weeks of age, male and female rats maintained on a KD or CD were assigned to receive daily injections of cocaine or saline vehicle for one week. Analyses of ambulations and time spent rearing were restricted to Test Days 1 and 7. Descriptive, within-session data for time rearing on these test days is provided in Figure 2. When examining summed (across the first 30 min following injection) cocaine-induced rearing responses, time spent rearing was 53% higher on Test Day 7 vs. Test Day 1, (F(1,44) = 16.18, p < .001, η2 = 0.27) (Figure 3). Although this sensitizing effect on time spent rearing was seen irrespective of diet condition, the overall levels of cocaine-induced rearing were significantly decreased by 33% in KD vs. CD animals (F(1,44) = 17.67, p < .001, η2 = 0.29). Finally, females spent more time (41%) rearing compared to males, (F(1,44) = 13.072, p < .001, η2 = 0.23). In contrast to animals treated with cocaine, saline-treated animals showed a 51% decrease in rearing over time, (F(1,41) = 15.28, p < .001, η2 = 0.27). Importantly, there was no effect of diet on time spent rearing in these saline-injected animals (F(1,41) = 0.027, p = 0.87, η2 = 0.001).
Figure 3.
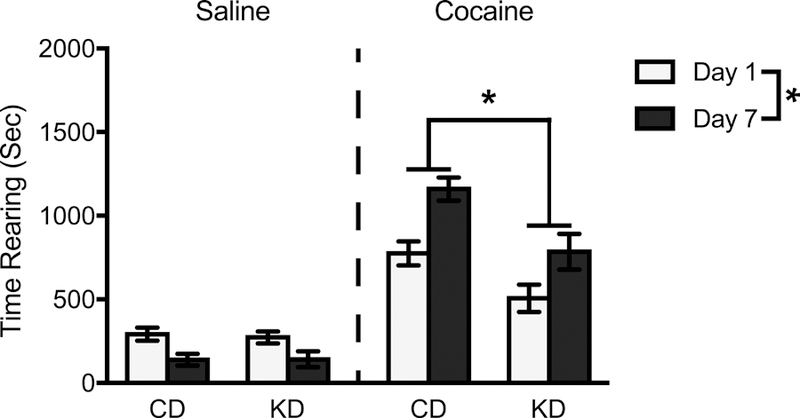
Mean (+/− SEM) time spent rearing summed across the first 30 min of the test phase on Test Day 1 and Test Day 7 by rats on a control (CD) or ketogenic (KD) diet, following injections of either saline or cocaine. Data were collapsed across sex (diet x sex interaction was non-significant). Cocaine-induced rearing increased significantly from Test Day 1 to Test Day 7. Furthermore, rearing time was significantly lower when comparing KD to CD animals. *p < .05, Test Day 1 vs. Test Day 7 for cocaine- and saline-injected animals, as well as CD vs. KD comparisons in cocaine-injected animals.
Cocaine-induced ambulations were also summed across the first 30 min following injections and compared across treatment groups. There were no significant effects of test day (F(1,42) = 1.19, p = 0.28, η2 = 0.028) or diet (F(1,42) = 0.059, p = 0.81, η2 = 0.001) on ambulations; however, there was a significant diet x test day interaction (F(1,42) = 7.77, p = 0.008, η2 = 0.16) (Figure 4). For CD animals, the expected sensitizing effect (increasing ambulations from Day 1 to Day 7) was observed (F(1,23) = 8.57, p = 0.008, η2 = 0.27). In contrast, KD-treated animals failed to show this sensitizing response across test days, (F(1,21) = 1.512, p = 0.23, η2 = 0.067). Similar to rearing results, females engaged in more ambulations (44%) compared to males irrespective of diet condition or test day (F(1,42) = 4.27, p = 0.045, η2 = 0.092). In animals repeatedly injected with saline, ambulations decreased by 52% across test days (F(1,40) = 28.85, p < 0.001, η2 = 0.42). There was no significant effect of diet (F(1,40) = 3.28, p = 0.078, η2 = 0.076), diet x test day interaction (F(1,40) = 1.37, p = 0.25, η2 = 0.033), or effect of sex (F(1,40) = 1.94, p = 0.17, η2 = 0.046) in these animals.
Figure 4.
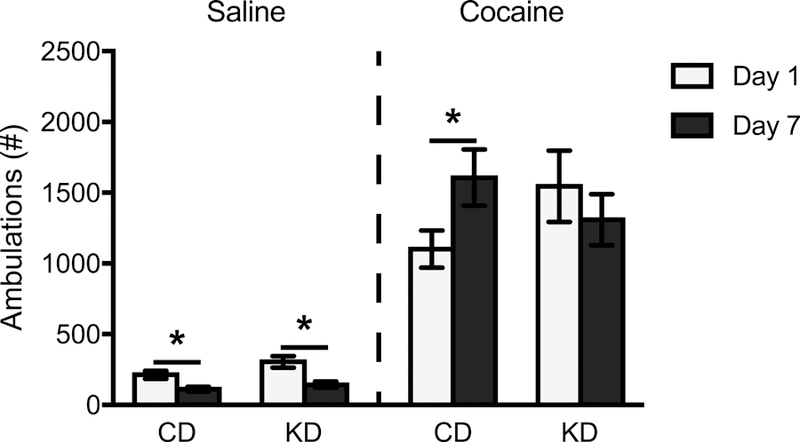
Mean (+/− SEM) ambulations summed across the first 30 min of the test phase on Test Day 1 and Test Day 7, by rats on a control (CD) or ketogenic (KD) diet, following injections of either saline or cocaine. Data were collapsed across sex (diet x sex interaction was non-significant). There was a significant diet x test day interaction, such that the expected increase in cocaine-induced ambulations over time was observed in CD, but not KD, animals. *p < .05, Test Day 1 vs. Test Day 7 for cocaine- (CD only) and saline- (both CD and KD) injected animals.
3.2. Behavioral measures: Challenge day
Following a drug-free (no injections) period of one week, male and female rats were observed for cocaine-induced behaviors on a subsequent challenge day. Descriptive, within-session data for the challenge day rearing can be seen in Figure 2. When comparing animals receiving their first injection of cocaine (Sal-Coc) vs. animals with a history of cocaine injections (Coc-Coc), summed (first 30 min) time spent rearing was 68% higher in Coc-Coc animals (F(1,88) = 33.18, p < 0.001, η2 = 0.27) (Figure 5). Similar to results obtained during daily injections of cocaine, animals treated with the KD exhibited decreased time spent rearing (22%) compared to CD animals (F(1,88) = 7.54, p = 0.007, η2 = 0.079), and females spent 46% more time rearing compared to males (F(1,88) = 18.87, p < 0.001, η2 = 0.18). A significant diet x sex interaction (F(1,88) = 6.70, p = 0.011, η2 = 0.071) indicated that the effect of diet was conditional on sex. Indeed, a significant decrease in time spent rearing (46%) was observed in KD- vs. CD-treated males (F(1,88) = 13.67, p < 0.001, η2 = 0.13), but not females (F(1,88) = 0.013, p = 0.91, η2 < 0.001).
Figure 5.
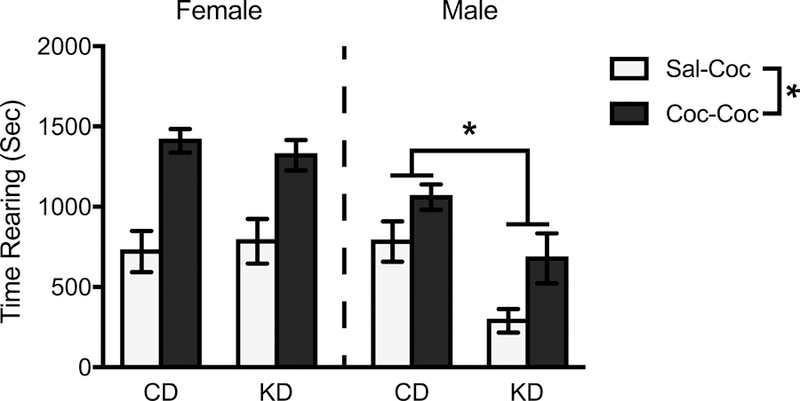
Mean (+/− SEM) time spent rearing during the first 30 min of the test phase on Challenge Day by female and male rats on a control (CD) or ketogenic (KD) diet, following injections of cocaine. Irrespective of sex, animals with a prior history of cocaine injections (Coc-Coc) spent more time rearing vs. those with a prior history of saline injections (Sal-Coc). A significant diet x sex interaction revealed that in males, KD treatment resulted in decreased time rearing compared to CD treatment. This effect was not seen in females. *p < .05, Sal-Coc vs. Coc-Coc comparison, as well as CD vs. KD comparison within males.
Cocaine-induced ambulations on the Challenge Day differed across the sexes (F(1,86) = 9.53, p = 0.003, η2 = 0.10), with females showing 42% more ambulations compared to males irrespective of diet or drug history (Table 1). No significant effects of diet (F(1,86) = 0.005, p = 0.94, η2 < 0.001), drug history (F(1,86) = 0.44, p = 0.51, η2 = 0.005) or interaction effects (all p > 0.05) were observed.
Table 1.
Ambulations Data (Challenge Day Injections)
| Sal-Coc | Coc-Coc | |
|---|---|---|
| Female | ||
| CD | 1909 ± 216 | 2288 ± 230 |
| KD | 2562 ± 306 | 2626 ± 210 |
| Male | ||
| CD | 1776 ± 288 | 1726 ± 187 |
| KD | 1213 ± 149 | 1661 ± 286 |
Mean (± SEM) ambulations during the first 30 min following cocaine injections on the Challenge Day. Female and male rats were maintained on either a control (CD) or ketogenic (KD) diet, and had a prior history of either saline (Sal-Coc) or cocaine (Coc-Coc) injections.
3.3. Physiological measures
Weight of rats assigned to a KD or a CD were assessed at several time points: prior to the diet condition assignment (Initial Weight); prior to their first (Test Day 1) and seventh (Test Day 7) daily injections of cocaine or saline; and prior to cocaine injections on the Challenge Day. Weight increased by 77% over this time period (F(3,258) = 2285.63, p < 0.001, η2 = 0.96) (Table 2). This effect of time varied across diet condition and sex, as evidenced by significant diet x day x sex interaction (F(3,258) = 195.54, p < 0.001, η2 = 0.70). Within each sex, there were no differences in weight between diet groups initially (females: F(1,65.45) = 0.68, p = 0.41, η2 = 0.01; males: F(1, 52.07) = 0.14, p = 0.71, η2 = 0.003); however, weight was significantly decreased in KD vs. CD animals at every other timepoint in both females (Test Day 1: F(1,65.45) = 183.62, p < 0.001, η2 = 0.74; Test Day 7: F(1,65.45) = 196.49, p < 0.001, η2 = 0.75; Challenge Day: F(1,65.45) = 248.34, p < 0.001, η2 = 0.79) and males (Test Day 1: F(1,52.07) = 494.43, p < 0.001, η2 = 0.91; Test Day 7: F(1,52.07) = 602.92, p < 0.001, η2 = 0.93; Challenge Day: F(1,52.07) = 856.48, p < 0.001, η2 = 0.94).
Table 2.
Weight Data
| Baseline | Day 1 | Day 7 | Challenge Day | |
|---|---|---|---|---|
| Females | ||||
| CD | 147.8 ± 3.4 | 241.6 ± 3.4 | 252.5 ± 3.6 | 275.9± 4.0 |
| KD | 142.9 ± 3.5 | 161.2 ± 4.6* | 169.3 ± 4.6* | 182.3 ± 5.1* |
| Males | ||||
| CD | 168.8 ± 5.0 | 358.6 ± 5.2 | 385.1 ± 5.2 | 436.3 ± 5.7 |
| KD | 165.9 ± 4.9 | 184.0 ± 6.2* | 192.3 ± 6.2* | 206.5 ± 7.1* |
Mean (± SEM) weight taken at Baseline (just prior to diet assignment), and just prior to Day 1,7, and Challenge Day injections. Female and male rats were maintained on either a control (CD) or ketogenic (KD) diet.
p < 0.05, within column comparison of diet within each sex.
The effect of diet on weight gain raises the possibility that diet-induced weight changes may be a causal factor driving the reported behavioral effects. To address this issue, we first generated Challenge Day weight z-scores (each score standardized to its treatment group mean and standard deviation). These scores were then pooled within each sex and compared to time spent rearing following cocaine injections on the Challenge Day. We found that there was no correlation between weight z-scores and time spent rearing in either female (r = −0.043, p = 0.77) or male (r = 0.0022, p = 0.99) animals (Figure 6).
Figure 6:
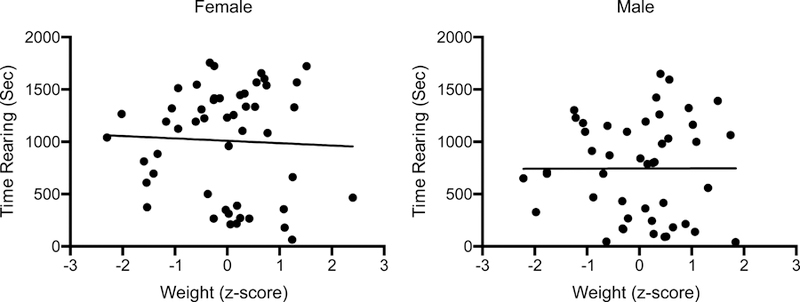
Time spent rearing and standardized (within treatment group) weight of individual female and male rats on the Challenge Day. No significant relationship was observed between these two variables, in either sex. For each graph, the line of best fit (linear) is superimposed.
Twenty-four hours following the Challenge Day test, rats were sacrificed and blood glucose and ketone levels were assessed. Blood ketone levels were 107% higher in animals on a KD vs. those on a CD (F(1,68) = 59.94, p < 0.001, η2 = 0.47) (Figure 7). In contrast, blood glucose levels were 11% lower in KD- vs. CD-treated animals (F(1,68) = 9.39, p = 0.003, η2 = 0.12).
Figure 7:
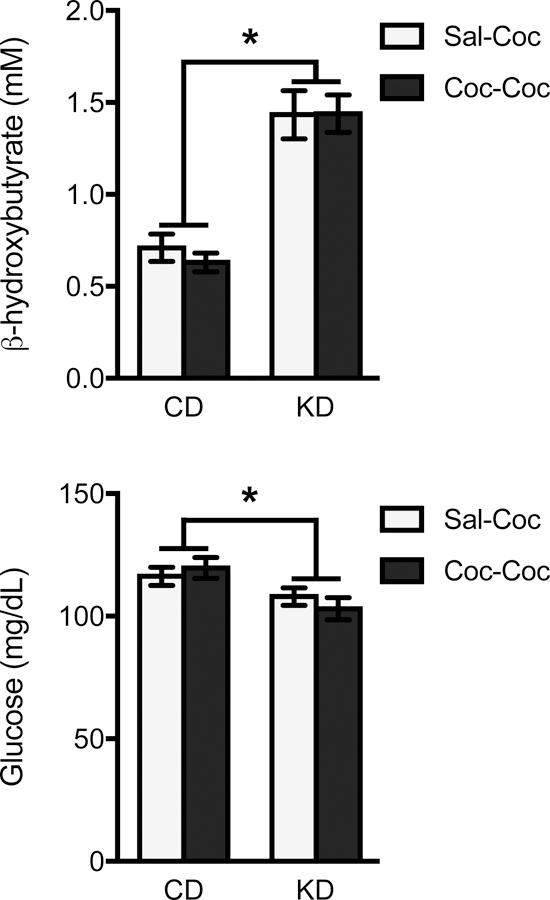
Mean (+/− SEM) blood ketone (β-hydroxybutyrate) and glucose levels taken at the conclusion of the experiment. Data were collapsed across sex (diet x sex interaction was non-significant). Rats treated with a ketogenic diet (KD) had significantly higher blood ketone levels and significantly lower blood glucose levels vs. control diet (CD)-treated rats. *p < .05, CD vs. KD comparison.
4. Discussion
Here we show that ambulatory and stereotyped locomotor responses are disrupted in cocaine-injected male and female rats administered a KD. Sensitization of cocaine-induced ambulatory activity was not observed in KD-fed animals; in addition, animals on a KD showed reduced rearing during the initial week of repeated cocaine injections (both sexes) as well as when challenged with cocaine one week following their last injection (males only). In contrast, there was no effect of KD administration on behavioral responses in animals injected with saline. Considered together, these data are the first to show that KD treatment can modify behavioral responses to psychostimulants, and suggest that KDs hold potential as a novel therapy for the treatment of psychostimulant addiction.
Therapy with a KD may reduce behavioral responses to psychostimulants via multiple mechanisms. These diets are associated with decreased neuroinflammation, stabilized glucose levels, and improved mitochondrial function (Masino and Rho, 2010) – all of which represent positive effects on factors that are negatively impacted by psychostimulant addiction (Chang et al., 2007; de Oliveira and Jardim, 2016; Moreira et al., 2016). Hallmarks of KD administration are increased blood ketones and reduced blood glucose, although these metabolic effects are not necessarily coupled tightly to other beneficial effects or behavioral changes. For example, the KD-induced decrease in stereotyped responses we observed during the week of daily cocaine injections was not maintained on the challenge day in both sexes (i.e., it persisted in males but not females) – despite no differences in the diet-induced increase in ketones or decrease in glucose across the sexes. We and others have found dissociations between metabolic and behavioral results in other models (Dallérac et al., 2017; Ruskin et al., 2013; Schoeler et al., 2017; Simeone et al., 2017; Viggiano et al., 2016), and we have previously identified behavioral effects of this diet that are sex specific (Ruskin et al., 2011; Ruskin, 2016; Ruskin et al., 2017a, 2017b). There are limited other studies on sex differences in KD effects (Chun et al., 2018; Zengin et al., 2016), but to our knowledge, no additional laboratories have published sex differences in behavioral effects of KD. Notably, sex differences in the ability of both diet and non-diet therapies to modulate behavioral responses to cocaine have been reported previously (Collins et al., 2015; Poland et al., 2016; Sershen et al., 1998).
As a link between metabolism, neural activity and behavior, KD-induced increases in the neuromodulator adenosine (Kawamura et al., 2014; Lusardi et al., 2015), potentially consequent to increasing ATP availability in the brain (Masino et al., 2009), may be particularly relevant to psychostimulants and dopaminergic systems. While some adenosine-mediated effects may be receptor-independent (Williams-Karnesky et al., 2013), it is well established that high-affinity adenosine A1R and A2AR receptor subtypes are found within the striatum and form heterodimers with dopamine receptor subtypes that would be activated by cocaine administration (e.g., A2AR with dopamine D2 receptors (D2R); A1R with dopamine D1 receptors (D1R) (Ferré et al., 2008, 1997). Accordingly, KD-mediated changes in adenosine may have therapeutic potential via actions at adenosine-dopamine receptor heterodimers (Filip et al., 2012). In support of this idea, selective disruption of A2AR-D2R heterodimer was found to prevent the reduction in cocaine self-administration normally seen following injections of the A2AR agonist CGS 21680 (Borroto-Escuela et al., 2018).
Some details of the present findings merit discussion. First, the link between treatment with a KD and decreased weight in both sexes potentially complicates the dissociation of KD effects on behavior (e.g., rearing) from its effects on weight. However, our analyses suggest that these effects can indeed be dissociated. KD treatment did not affect cocaine-induced rearing (females) or ambulatory activity (both sexes) on the Challenge Day, despite the significant lower weight observed in KD vs. CD animals at this time point. Importantly, we also found no correlation between Challenge Day rearing and weight (standardized within each treatment group) in either sex. Taken together, these results suggest that weight is unlikely to be a causal factor driving the behavioral differences observed across diet conditions. Second, the present study utilizes behavioral sensitization to cocaine as an initial means of assessing interactions between a KD and psychostimulant-induced behavior. While the cocaine administered in sensitization protocols is non-contingent (i.e., drug delivery is under experimenter, rather than animal, control), this model and the positive effects of the KD may be relevant to addiction for several reasons. Repeated injections of cocaine induce lasting neurobiological responses commonly associated with psychostimulant addiction, including enhanced cocaine-induced dopamine release (Hooks et al., 1994; Kalivas and Duffy, 1990) and structural plasticity (Ferrario et al., 2005; Robinson and Kolb, 1999) within the ventral striatum. Furthermore, a previous history of cocaine injections augments the acquisition of cocaine self-administration (Childs et al., 2006). Additional studies will be required to test directly how the KD specifically impacts the development and progression of psychostimulant addiction, particularly in relation to the rewarding/reinforcing effects of these drugs. Third, all behavioral testing was performed while KD treatment was ongoing. There are indications that the anticonvulsant effects of the KD outlast the diet itself (Bough et al., 2006; Caraballo et al., 2011; Lusardi et al., 2015; Martinez et al., 2007; Patel et al., 2010); whether its impact on cocaine-induced responses are similarly persistent remains to be explored.
In summary, we found that ambulatory and stereotyped locomotor responses induced by cocaine were disrupted in KD-treated male and female rats. This work aligns with a broadening therapeutic scope for metabolic therapies, as well as a significant and growing interest in the relationship among purines, energy homeostasis, and drug addiction (Lindberg et al., 2015). The impact of the KD on acute and chronic dopamine-mediated behaviors observed here suggests that this diet may hold therapeutic potential for neuropsychiatric and neurodegenerative diseases beyond drug addiction. Future work will be required to determine if this is indeed the case.
Highlights.
A ketogenic diet (KD) decreased cocaine-induced stereotypy in male and female rats.
This effect was observed during repeated injections and following abstinence.
Cocaine-induced ambulatory sensitization was also disrupted in KD-fed rats.
KDs may have therapeutic potential for cocaine addiction.
Acknowledgments
This work was supported by the National Institute of Neurological Disease and Stroke (grants numbers NS066392 and NS065957; S.A.M.), the National Center for Complementary and Integrative Health (grant number AT008742; D.N.R.) and Trinity College. The authors would like to thank Julianna Armentano, Amr Arqoub, Kiera Flynn, Madeline Grossman, Bilal Hamzeh, Jonah Meltzer, Lily Russo-Savage, and Momeezah Syed for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None
References
- Araos P, Pedraz M, Serrano A, Lucena M, Barrios V, García-Marchena N, Campos-Cloute R, Ruiz JJ, Romero P, Suárez J, Bai xeras E, Torre R. de la, Montesinos J, Guerri C, Rodríguez-Arias M, Miñarro J, Martínez-Riera R, Torrens M, Chowen JA, Argente J, Mason BJ, Pavón FJ, Fonseca F.R. de, 2015. Plasma profile of pro-inflammatory cytokines and chemokines in cocaine users under outpatient treatment: influence of cocaine symptom severity and psychiatric co-morbidity. Addict. Biol 20, 756–772. 10.1111/adb.12156 [DOI] [PubMed] [Google Scholar]
- Badman MK, Kennedy AR, Adams AC, Pissios P, Maratos-Flier E, 2009. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am. J. Physiol.-Endocrinol. Metab 297, E1197–E1204. 10.1152/ajpendo.00357.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Wydra K, Li X, Rodriguez D, Carlsson J, Jastrzębska J, Filip M, Fuxe K, 2018. Disruption of A2AR-D2R heteroreceptor complexes after A2AR transmembrane 5 peptide administration enhances cocaine self-administration in rats. Mol. Neurobiol 1–11. 10.1007/s12035-018-0887-1 [DOI] [PMC free article] [PubMed]
- Boudreau AC, Wolf ME, 2005. Behavioral Sensitization to Cocaine Is Associated with Increased AMPA Receptor Surface Expression in the Nucleus Accumbens. J. Neurosci 25, 9144–9151. 10.1523/JNEUROSCI.2252-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ, 2006. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol 60, 223–235. 10.1002/ana.20899 [DOI] [PubMed] [Google Scholar]
- Caraballo R, Vaccarezza M, Cersósimo R, Rios V, Soraru A, Arroyo H, Agosta G, Escobal N, Demartini M, Maxit C, Cresta A, Marchione D, Carniello M, Paníco L, 2011. Long-term follow-up of the ketogenic diet for refractory epilepsy: Multicenter Argentinean experience in 216 pediatric patients. Seizure 20, 640–645. 10.1016/j.seizure.2011.06.009 [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N, 2007. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction 102, 16–32. 10.1111/j.1360-0443.2006.01782.x [DOI] [PubMed] [Google Scholar]
- Childs E, Shoaib M, Stolerman IP, 2006. Cocaine self-administration in rats with histories of cocaine exposure and discrimination. Psychopharmacology (Berl.) 186, 168–176. 10.1007/s00213-006-0364-9 [DOI] [PubMed] [Google Scholar]
- Chun K, Ma S-C, Oh H, Rho JM, Kim DY, 2018. Ketogenic diet-induced extension of longevity in epileptic Kcna1-null mice is influenced by gender and age at treatment onset. Epilepsy Res 140, 53–55. 10.1016/j.eplepsyres.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Chen Y, Tschumi C, Rush EL, Mensah A, Koek W, France CP, 2015. Effects of consuming a diet high in fat and/or sugar on the locomotor effects of acute and repeated cocaine in male and female C57BL/6J mice. Exp. Clin. Psychopharmacol 23, 228–237. 10.1037/pha0000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Iversen SD, 1974. The role of forebrain dopamine systems in amphetamine induced stereotyped behavior in the rat. Psychopharmacologia 39, 345–357. 10.1007/BF00422974 [DOI] [PubMed] [Google Scholar]
- Dallérac G, Moulard J, Benoist J-F, Rouach S, Auvin S, Guilbot A, Lenoir L, Rouach N, 2017. Non-ketogenic combination of nutritional strategies provides robust protection against seizures. Sci. Rep 7, 5496 10.1038/s41598-017-05542-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais JB, McGinty JF, 1994. Acute and chronic cocaine administration differentially alters striatal opioid and nuclear transcription factor mRNAs. Synapse 18, 35–45. 10.1002/syn.890180106 [DOI] [PubMed] [Google Scholar]
- de Oliveira MR, Jardim FR, 2016. Cocaine and mitochondria-related signaling in the brain: A mechanistic view and future directions. Neurochem. Int 92, 58–66. 10.1016/j.neuint.2015.12.006 [DOI] [PubMed] [Google Scholar]
- Deng-Bryant Y, Prins ML, Hovda DA, Harris NG, 2011. Ketogenic diet prevents alterations in brain metabolism in young but not adult rats after traumatic brain injury. J. Neurotrauma 28, 1813–1825. 10.1089/neu.2011.1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVivo DC, Leckie MP, Ferrendelli JS, McDougal DB, 1978. Chronic ketosis and cerebral metabolism. Ann. Neurol 3, 331–337. 10.1002/ana.410030410 [DOI] [PubMed] [Google Scholar]
- Dietrich J-B, Mangeol A, Revel M-O, Burgun C, Aunis D, Zwiller J, 2005. Acute or repeated cocaine administration generates reactive oxygen species and induces antioxidant enzyme activity in dopaminergic rat brain structures. Neuropharmacology 48, 965–974. 10.1016/j.neuropharm.2005.01.018 [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE, 2005. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol. Psychiatry 58, 751–759. 10.1016/j.biopsych.2005.04.046 [DOI] [PubMed] [Google Scholar]
- Ferré S, Fuxe K, Fredholm B, B., Morelli M, Popoli P, 1997. Adenosine–dopamine receptor–receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci 20, 482–487. 10.1016/S0166-2236(97)01096-5 [DOI] [PubMed] [Google Scholar]
- Ferré S, Quiroz C, Woods AS, Cunha R, Popoli P, Ciruela F, Lluis C, Franco R, Azdad K, Schiffmann SN, 2008. An update on adenosine A2A-dopamine D2 receptor interactions. Implications for the function of G protein-coupled receptors. Curr. Pharm. Des 14, 1468–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin S-N, Foltz R, Jenab S, Quinones-Jenab V, 2004. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology 46, 672–687. 10.1016/j.neuropharm.2003.11.017 [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Przegaliński E, Műller CE, Agnati L, Franco R, Roberts DCS, Fuxe K, 2006. Involvement of adenosine A2A and dopamine receptors in the locomotor and sensitizing effects of cocaine. Brain Res 1077, 67–80. 10.1016/j.brainres.2006.01.038 [DOI] [PubMed] [Google Scholar]
- Filip M, Zaniewska M, Frankowska M, Wydra K, Fuxe K, 2012. The Importance of the adenosine A2A receptor-dopamine D2 receptor interaction in drug addiction. Curr. Med. Chem 19, 317–355. [DOI] [PubMed] [Google Scholar]
- Halbout B, Liu AT, Ostlund SB, 2016. A closer look at the effects of repeated cocaine exposure on adaptive decision-making under conditions that promote goal-directed control. Front. Psychiatry 7 10.3389/fpsyt.2016.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, Gasior M, Vining EPG, Rogawski MA, 2007. The neuropharmacology of the ketogenic diet. Pediatr. Neurol 36, 281–292. 10.1016/j.pediatrneurol.2007.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Duffy P, Striplin C, Kalivas PW, 1994. Behavioral and neurochemical sensitization following cocaine self-administration. Psychopharmacology (Berl.) 115, 265–272. 10.1007/BF02244782 [DOI] [PubMed] [Google Scholar]
- Jeong EA, Jeon BT, Shin HJ, Kim N, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS, 2011. Ketogenic diet-induced peroxisome proliferator-activated receptor-γ activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Exp. Neurol 232, 195–202. 10.1016/j.expneurol.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, Uneyama H, Edwards RH, Nicoll RA, Moriyama Y, 2010. Metabolic control of vesicular glutamate transport and release. Neuron 68, 99–112. 10.1016/j.neuron.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, 1990. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse 5, 48–58. 10.1002/syn.890050104 [DOI] [PubMed] [Google Scholar]
- Kawamura M, Ruskin DN, Geiger JD, Boison D, Masino SA, 2014. Ketogenic diet sensitizes glucose control of hippocampal excitability. J. Lipid Res 55, 2254–2260. 10.1194/jlr.M046755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Chi S, Nestler EJ, 1994. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J. Pharmacol. Exp. Ther 269, 137–144. [PubMed] [Google Scholar]
- Kovács Z, D’Agostino DP, Ari C, 2018. Anxiol ytic effect of exogenous ketone supplementation is abolished by adenosine A1 receptor inhibition in Wistar albino Glaxo/Rijswijk rats. Front. Behav. Neurosci 12:29, 1–12. 10.3389/fnbeh.2018.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg D, Shan D, Ayers-Ringler J, Oliveros A, Benitez J, Prieto M, McCullumsmith R, Choi D-S, 2015. Purinergic signaling and energy homeostasis in psychiatric disorders. Curr. Mol. Med 15, 275–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusardi TA, Akula KK, Coffman SQ, Ruskin DN, Masino SA, Boison D, 2015. Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats. Neuropharmacology 99, 500–509. 10.1016/j.neuropharm.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons D, Friedman DP, Nader MA, Porrino LJ, 1996. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J. Neurosci 16, 1230–1238. https://doi-org.ezproxy.trincoll.edu/10.1523/JNEUROSCI.16-03-01230.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni O, Pujalte D, Williams J, Bockaert J, 1998. Decreased presynaptic sensitivity to adenosine after cocaine withdrawal. J. Neurosci 18, 7996–8002. 10.1523/JNEUROSCI.18-19-07996.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Roberts DCS, Navarro G, Filip M, Agnati L, Lluís C, Franco R, Fuxe K, 2007. Increase in A2A receptors in the nucleus accumbens after extended cocaine self-administration and its disappearance after cocaine withdrawal. Brain Res 1143, 208–220. 10.1016/j.brainres.2007.01.079 [DOI] [PubMed] [Google Scholar]
- Martinez CC, Pyzik PL, Kossoff EH, 2007. Discontinuing the Ketogenic Diet in Seizure-Free Children: Recurrence and Risk Factors. Epilepsia 48, 187–190. 10.1111/j.1528-1167.2006.00911.x [DOI] [PubMed] [Google Scholar]
- Martinez LA, Peterson BM, Meisel RL, Mermelstein PG, 2014. Estradiol facilitation of cocaine-induced locomotor sensitization in female rats requires activation of mGluR5. Behav. Brain Res 271, 39–42. 10.1016/j.bbr.2014.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino S., Kawamura M, Wasser CD, Pomeroy L., Ruskin D., 2009. Adenosine, ketogenic diet and epilepsy: The emerging therapeutic relationship between metabolism and brain activity. Curr. Neuropharmacol 7, 257–268. 10.2174/157015909789152164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Geiger JD, 2008. Are purines mediators of the anticonvulsant/neuroprotective effects of ketogenic diets? Trends Neurosci 31, 273–278. 10.1016/j.tins.2008.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Kawamura M, Ruskin DN, Geiger JD, Boison D, 2012. Purines and neuronal excitability: Links to the ketogenic diet. Epilepsy Res., Special Issue on Dietary treatments for epilepsy & neurological disorders 100, 229–238. 10.1016/j.eplepsyres.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Li T, Theofilas P, Sandau US, Ruskin DN, Fredholm BB, Geiger JD, Aronica E, Boison D, 2011. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J. Clin. Invest 121, 2679–2683. 10.1172/JCI57813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Rho JM, 2010. Mechanisms of ketogenic diet action. Epilepsia 51, 85–85. 10.1111/j.1528-1167.2010.02871.x20618408 [DOI] [Google Scholar]
- Moreira FP, Medeiros JRC, Lhullier AC, Souza LD de M, Jansen K, Portela LV, Lara DR, Silva R.A. da, Wiener CD, Oses JP, 2016. Cocaine abuse and effects in the serum levels of cytokines IL-6 and IL-10. Drug Alcohol Depend 158, 181–185. 10.1016/j.drugalcdep.2015.11.024 [DOI] [PubMed] [Google Scholar]
- Nakazawa M, Kodama S, Matsuo T, 1983. Effects of ketogenic diet on electroconvulsive threshold and brain contents of adenosine nucleotides. Brain Dev 5, 375–380. 10.1016/S0387-7604(83)80042-4 [DOI] [PubMed] [Google Scholar]
- Noakes M, Foster PR, Keogh JB, James AP, Mamo JC, Clifton PM, 2006. Comparison of isocaloric very low carbohydrate/high saturated fat and high carbohydrate/low saturated fat diets on body composition and cardiovascular risk. Nutr. Metab 3, 7 10.1186/1743-7075-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall FQ, Almokayyad RM, Gannon MC, 2015. Comparison of a carbohydrate-free diet vs. fasting on plasma glucose, insulin and glucagon in type 2 diabetes. Metabolism 64, 253–262. 10.1016/j.metabol.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Nylen K, Velazquez JLP, Sayed V, Gibson KM, Burnham WM, Snead OC, 2009. The effects of a ketogenic diet on ATP concentrations and the number of hippocampal mitochondria in Aldh5a1−/− mice. Biochim. Biophys. Acta BBA - Gen. Subj 1790, 208–212. 10.1016/j.bbagen.2008.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JW, Bebin EM, Chu WJ, Hetherington HP, 1999. Ketosis and epilepsy: 31P spectroscopic imaging at 4.1 T. Epilepsia 40, 703–707. 10.1111/j.1528-1157.1999.tb00766.x [DOI] [PubMed] [Google Scholar]
- Patel A, Pyzik PL, Turner Z, Rubenstein JE, Kossoff EH, 2010. Long-term outcomes of children treated with the ketogenic diet in the past. Epilepsia 51, 1277–1282. 10.1111/j.1528-1167.2009.02488.x [DOI] [PubMed] [Google Scholar]
- Peterson BM, Martinez LA, Meisel RL, Mermelstein PG, 2016. Estradiol impacts the endocannabinoid system in female rats to influence behavioral and structural responses to cocaine. Neuropharmacology 110, Part A, 118–124. 10.1016/j.neuropharm.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland RS, Hahn Y, Knapp PE, Beardsley PM, Bowers MS, 2016. Ibudilast attenuates expression of behavioral sensitization to cocaine in male and female rats. Neuropharmacology 109, 281–292. 10.1016/j.neuropharm.2016.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC, 1993. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev 18, 247–291. 10.1016/0165-0173(93)90013-P [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B, 1999. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur. J. Neurosci 11, 1598–1604. 10.1046/j.1460-9568.1999.00576.x [DOI] [PubMed] [Google Scholar]
- Ruskin DN, 2016. Metabolic Therapy and Pain, in: Ketogenic Diet and Metabolic Therapies: Expanded Roles in Health and Disease Oxford University Press, pp. 196–208. [Google Scholar]
- Ruskin DN, Fortin JA, Bisnauth SN, Masino SA, 2017a. Ketogenic diets improve behaviors associated with autism spectrum disorder in a sex-specific manner in the EL mouse. Physiol. Behav 168, 138–145. 10.1016/j.physbeh.2016.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin DN, Murphy MI, Slade SL, Masino SA, 2017b. Ketogenic diet improves behaviors in a maternal immune activation model of autism spectrum disorder. PLOS ONE 12, e0171643 10.1371/journal.pone.0171643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin DN, Ross JL, Kawamura M, Ruiz TL, Geiger JD, Masino SA, 2011. A ketogenic diet delays weight loss and does not impair working memory or motor function in the R6/2 1J mouse model of Huntington’s disease. Physiol. Behav 103, 501–507. 10.1016/j.physbeh.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin DN, Suter TACS, Ross JL, Masino SA, 2013. Ketogenic diets and thermal pain: dissociation of hypoalgesia, elevated ketones, and lowered glucose in rats. J. Pain 14, 467–474. 10.1016/j.jpain.2012.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeler NE, Bell G, Yuen A, Kapelner AD, Heales SJR, Cross JH, Sisodiya S, 2017. An examination of biochemical parameters and their association with response to ketogenic dietary therapies. Epilepsia 58, 893–900. 10.1111/epi.13729 [DOI] [PubMed] [Google Scholar]
- Sershen H, Hashim A, Lajtha A, 1998. Gender differences in kappa-opioid modulation of cocaine-induced behavior and NMDA-evoked dopamine release. Brain Res 801, 67–71. 10.1016/S0006-8993(98)00546-0 [DOI] [PubMed] [Google Scholar]
- Shumsky JS, Shultz PL, Tonkiss J, Galler JR, 1997. Effects of Diet on Sensitization to Cocaine-Induced Stereotypy in Female Rats. Pharmacol. Biochem. Behav 58, 683–688. 10.1016/S0091-3057(97)00021-X [DOI] [PubMed] [Google Scholar]
- Simeone TA, Matthews SA, Samson KK, Simeone KA, 2017. Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp. Neurol 287, 54–64. 10.1016/j.expneurol.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircar R, Kim D, 1999. Female gonadal hormones differentially modulate cocaine-induced behavioral sensitization in Fischer, Lewis, and Sprague-Dawley rats. J. Pharmacol. Exp. Ther 289, 54–65. [PubMed] [Google Scholar]
- Sorg BA, Krueger JM, Churchill L, Cearley CN, Blindheim K, 2011. Acute cocaine increases interleukin-1β mRNA and immunoreactive cells in the cortex and nucleus accumbens. Neurochem. Res 36, 686–692. 10.1007/s11064-011-0410-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiano A, Stoddard M, Pisano S, Operto FF, Iovane V, Monda M, Coppola G, 2016. Ketogenic diet prevents neuronal firing increase within the substantia nigra during pentylenetetrazole-induced seizure in rats. Brain Res. Bull 125, 168–172. 10.1016/j.brainresbull.2016.07.001 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, 2004. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology 47, 3–13. 10.1016/j.neuropharm.2004.07.019 [DOI] [PubMed] [Google Scholar]
- Williams-Karnesky RL, Sandau US, Lusardi TA, Lytle NK, Farrell JM, Pritchard EM, Kaplan DL, Boison D, 2013. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J. Clin. Invest 123, 3552–3563. 10.1172/JCI65636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin A, Kropp B, Chevalier Y, Junnila R, Sustarsic E, Herbach N, Fanelli F, Mezzullo M, Milz S, Bidlingmaier M, Bielohuby M, 2016. Low-carbohydrate, high-fat diets have sex-specific effects on bone health in rats. Eur. J. Nutr 55, 2307–2320. 10.1007/s00394-015-1040-9 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, Humala N, Thiyagarajan M, Wang J, Pasinetti GM, 2006. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci 7, 29 10.1186/1471-2202-7-29 [DOI] [PMC free article] [PubMed] [Google Scholar]